Abstract
Background
A possible association between dry eye disease (DED) and irritable bowel syndrome (IBS) has been hypothesized based on the fact that they both share an inflammatory pathogenesis.
Methods
Ninety-five patients with IBS and 276 healthy controls were enrolled in the study. All patients answered a questionnaire regarding DED symptoms and had a complete ophthalmic examination. DED signs were evaluated using Schirmer’s 1 and tear break-up time (tBUT) tests in both groups.
Results
Female IBS participants presented significantly lower Schirmer’s test and tBUT (P=0.002 and P<0.001 respectively) than controls. Both diagnostic tests in male IBS patients were also significantly lower than in controls (P<0.001). 72% of IBS patients gave at least 3 positive answers to the questionnaire compared with 42% of the control group (P<0.01).
Conclusion
Our results suggest a correlation between IBS and DED. DED symptoms can cause further complications in patients with IBS, and should be considered in their management. However, further research is needed to establish a possible pathophysiologic association.
Keywords: Dry eye, irritable bowel syndrome, keratoconjunctivitis sicca
Introduction
Dry eye disease (DED) or keratoconjunctivitis sicca (KCS) is the most common disease of the ocular surface. In 2007, the International Dry Eye Workshop defined it as “a multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability with potential damage to the ocular surface. It is accompanied by increased osmolarity of the tear film and inflammation of the ocular surface” [1]. The prevalence of DED is estimated between 7.4 and 33.7% [2]. Most studies have found an increasing prevalence with aging and a greater prevalence among women probably due to their hormonal status [2,3].
Even though DED is considered as a condition of tear deficiency, either due to decreased lacrimation or excessive evaporation, its pathophysiology seems to be more complex. New concepts of its pathogenesis involve inflammation mechanisms mediated by T-cell lymphocytes [4,5]. It has been suggested that it is an inflammatory disorder, which affects both ocular surface and lacrimal glands. Desiccating stress, hyperosmolarity, proinflammatory cytokines released from the lacrimal glands and blinking abnormalities seem to contribute to inflammation of the ocular surface [6-8]. Loss of peptide growth factors and vitamin A, both necessary for cellular proliferation, migration, normal differentiation, immune modulation and ocular wound healing, might also contribute to ocular surface inflammation [9,10].
In some clinical disorders such as Sjögren’s syndrome, the lacrimal gland becomes an important target of the immune system resulting in DED. The presence of focal lymphocytic infiltrates and the increased local production of proinflammatory cytokines are characteristic findings of lacrimal gland inflammation [11]. This finding has also been demonstrated by studies investigating the role of anti-inflammatory therapies such as topical 0.005% cyclosporine, corticosteroid therapy, and tetracycline in patients with DED [12-14].
Irritable bowel syndrome (IBS) is the most common gastrointestinal disorder [15]. It is defined, according to Rome III criteria, as recurrent abdominal pain or discomfort for at least 3 days per month during the previous 3 months, associated with two or more of the following symptoms: improvement with defecation, change in the frequency and form or appearance of stools [16]. It affects 10-20% of adolescents and adults in western societies and is the most common cause of recurrent abdominal pain in children [17,18].
Despite the growing body of literature, IBS pathophysiology remains poorly understood. Recent studies suggest an inflammatory pathogenesis. Several investigators have demonstrated increased numbers of chronic inflammatory cells in the colonic mucosa of patients with IBS [19-21]. T-lymphocytes of the lamina propria are increased both in post-infectious and non-post-infectious IBS patients compared with controls. Mucosal mast cells are also increased, based on quantification methods [22]. Recent documentation of increased numbers of immune cells expressing CD25, a marker of immune cell activation, provides functional evidence of immune activation in IBS [23]. There is also a change in peripheral cytokine profiles in these patients. Abnormal interleukin (IL) -10/-12 ratio has been observed, suggesting a pro-inflammatory state [24].
In our case-controlled study we aimed to investigate the prevalence of DED in IBS compared with healthy subjects who visited our ophthalmology clinic for a regular visual acuity testing.
Patients and methods
Patients
The study was conducted at the University Hospital of Ioannina Greece, a referral tertiary center for digestive and ocular diseases. Ninety-five patients with IBS, diagnosed according to Rome III criteria from September 2014 to January 2015 in the Hepato-Gastroenterology Unit, were consecutively included. One experienced gastroenterologist diagnosed all IBS patients before they were further tested for DED. 276 age- and gender-matched individuals without IBS, among the outpatients who had a routine visual acuity testing during the same study period in the Department of Ophthalmology, served as controls. Two ophthalmologists performed all ophthalmic examinations, both of whom were blinded to IBS status.
Patients with pre-existing ocular disease such as glaucoma or uveitis, disorders of the lid or nasolacrimal pathway, previous ocular surgery, and subjects using contact lenses were excluded from the study. We also excluded patients with ocular allergies, pterygium or blepharitis, because these factors may have an influence on dry eye test results (Fig. 1) [3,25].
Figure 1.
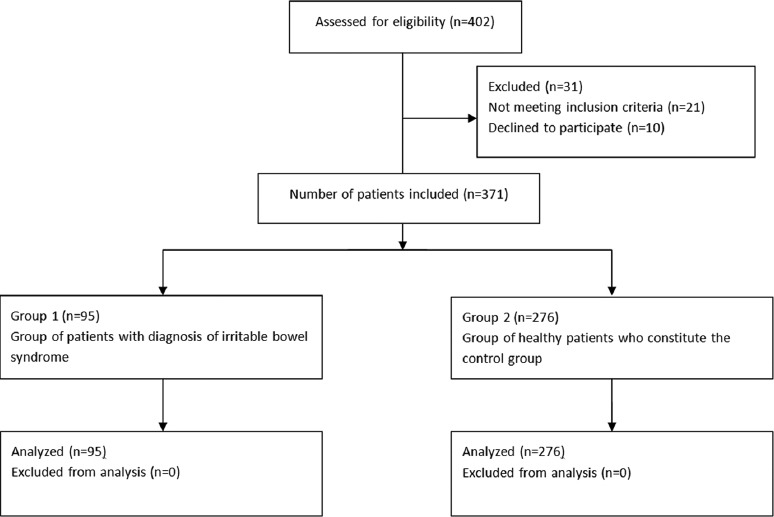
Consort flow diagram of patients enrolled
For each patient we recorded gender and age. All patients had a routine ophthalmic examination, which included visual acuity measurement, external examination and slit lamp biomicroscopy. All patients were asked to answer a questionnaire regarding dry eye-related ocular symptoms, in the form of oral interview. It comprised 6 questions about itching, sand and burning sensations, red eye appearance, secretions, and discomfort in dry environments [26,27] (Table 1). Each question could be answered with either a “Yes” or a “No”. Positive answers to at least three questions of ocular symptoms questionnaire were considered as presence of DED. Additional diagnostic tests for DED, such as Schirmer’s test (ST) 1 and tear break-up time (tBUT), were used in this order.
Table 1.
Questionnaire of ocular symptoms related to dry eye disease

The study was approved from the Institutional Review Board. Informed consent was obtained from all participating patients.
Diagnostic tests
ST was performed under natural light without topical anesthesia. Standardized filter paper was placed in the lateral canthus away from the cornea and left in place for 5 min, while the eye was closed. The distance moistened was measured in mm. A reading of less than 5 mm was considered abnormal. It was only performed once in each patient.
Tear film stability was assessed using tBUT via a commercially available fluorescein test paper that came in contact with the bottom conjunctiva. The subjects were instructed to blink 3 times, and then to look straight-forward without any blink. Tear film was observed using a blue cobalt filter under wide lighting. The interval between the last blink and the appearance of the first corneal dry spot was measured. The last procedure was repeated 3 times and the mean value was recorded. A tBUT value less than 10 sec was accepted as abnormal.
Statistical analysis
We presented data separately for IBS patients and controls. Continuous variables are presented as mean ± standard deviation (SD, normal distribution), while non-continuous variables are presented as percentages. Normality was assessed with both the graphical inspection of the distribution and with the Shapiro-Wilk normality test. Statistical comparisons between groups were performed using the unpaired t-test or x2 test, as indicated. In case of unequal variances between subgroups the unequal variance assumption was employed, with the use of Welch t-test. Statistical significance for all comparisons was achieved if the P values from the corresponding statistical tests were below 0.05. All statistical analyses were conducted using the Stata Statistical Software Release 13 for Windows (College Station, TX, Stata Corp LP).
Results
Patients
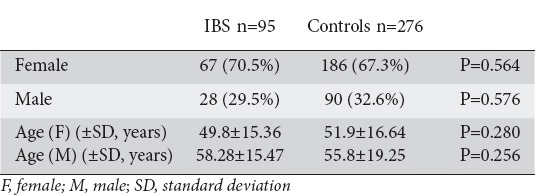
The study group comprised 95 IBS patients and the control group 276 healthy participants. Demographic characteristics for IBS patients and controls are summarized in Table 2. Mean age was 49.8±15.36 for women and 58.28±15.47 years for men in the IBS group versus 51.9±16.64 and 55.8±19.25 years respectively in the control group. Both groups matched in gender (70.5% females in the IBS group vs. 67.3% in the control group). However, age difference between the sexes of the same group was greater in IBS patients (8.5 years) than in controls (3.9 years) (P=0.280 for female age, P=0.256 for male age, t-test).
Table 2.
Comparison of demographic data between patients with irritable bowel syndrome (IBS) and controls
Dry eye symptoms in IBS and healthy controls
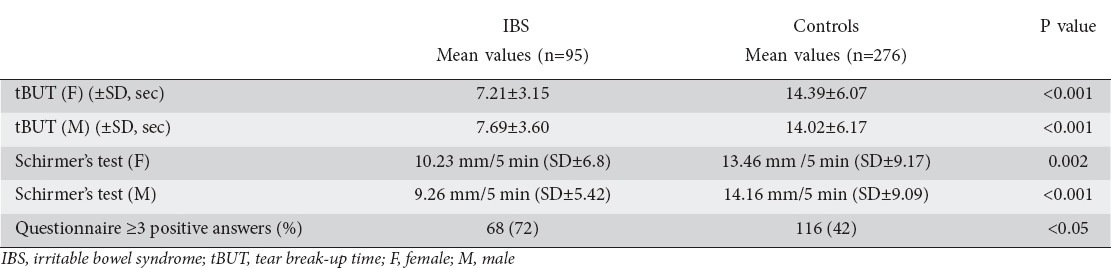
More IBS patients gave three or more positive answers than healthy controls (Table 3). Specifically, 68 (72%) IBS patients gave at least three positive answers to the questionnaire compared with 116 (42%) healthy subjects (P<0.01, x2 test).
Table 3.
Schirmer’s 1 test, tBUT test, and questionnaire in IBS patients compared with healthy controls
Tear production in IBS patients compared with healthy control participants
All clinical tests showed significant differences between groups. Patients with IBS were more likely to have an abnormal ST than healthy controls (9.26 mm/5 min ± 5.42 for males with IBS vs. 14.16±9.09 for male controls, P<0.001, t-test; and 10.23 mm/5 min ± 6.8 for females with IBS vs. 13.46±9.17 for female controls, P=0.002, t-test). Likewise, IBS patients were more likely to have abnormal tBUT (7.69±3.60 for males and 7.21±3.15 for females with IBS vs. 14.02±6.17 for male and 14.39±6.07 for female controls; both with P<0.001, t-test).
Discussion
DED is an extremely common disorder that in various grades of severity affects a large part of the general population at one time or another. Millions of people experience symptoms of IBS each year. Both DED and IBS have an unclear pathogenesis, including a common theory of inflammatory pathogenesis.
Over the past few years several studies have shown that dry eye seems to be caused by inflammation mediated by T-cell lymphocytes [5]. A dangerous vicious cycle of inflammation develops on the ocular surface that may lead to ocular surface disease. The role of inflammatory cytokines and matrix metalloproteinases (MMPs) in the pathogenesis of dry eye seems to be very important for both the easier understanding of KCS and for the discovery of new therapeutic agents [7,28]. Increased production and activation of pro-inflammatory cytokines (IL-1, IL-6, IL-8, and transforming growth factor-β1), RNA transcripts, and tumor necrosis factor have been reported in dry eye [29]. MMP-9 appears to play a physiological role in regulating corneal epithelial desquamation. In systemic vitamin A deficiency, there is a reduced expression of MMP-9 and hyperstratification of the corneal epithelium. In contrast, the increased MMP-9 activity in KCS is associated with deranged corneal epithelial barrier function (increased fluorescein permeability), increased corneal epithelial desquamation (punctate epithelial erosions), and corneal surface irregularity [30,14].
As it is widely recognized that inflammation plays a significant role in the etiopathogenesis of dry eye, promoting ocular surface disruption and symptoms of irritation, a number of anti-inflammatory treatments are currently in use for its management. These agents inhibit the expression of inflammatory mediators on the ocular surface, thereby restoring the secretion of a healthy tear film and reducing signs and symptoms. Cyclosporine reduces the inflammation via inhibition of T-cell activation and down-regulation of inflammatory cytokines in the conjunctiva, and lacrimal gland is thus thought to enhance tear production. Topical cyclosporine also increases goblet cell density and decreases epithelial cell apoptosis [31]. Corticosteroids have been reported to decrease the production of a number of inflammatory cytokines [IL-1, -6, -8, tumor necrosis factor (TNF)-α, GM-CSF] and MMP-9 by the corneal epithelium [32]. Tetracyclines also have a variety of anti-inflammatory properties such as inhibition of MMPs and IL-1 production. In ophthalmic practice, doxycyclines have been reported to be effective in dry eye especially induced by acne rosacea and severe blepharitis by reducing irritation symptoms, improving tear film stability, and decreasing the severity of ocular surface disease [33,34].
Similar inflammatory pathogenesis has been reported for IBS. Increased numbers of T cells in various lymphoid compartments of the small or large intestine in IBS patients have been described. Pro-inflammatory cytokines, such as IL-1β, IL-6 and TNF-α, in peripheral blood, and mononuclear cells, IL-6, and IL-8 in serum, were reported to be increased in IBS patients. Low-grade inflammation can be detected both through biopsies from intestine and blood testing. Subsequent studies in IBS patients revealed increased numbers of mast cells in the lamina propria of the terminal ileum and mucosa of the colon [35].
To our knowledge, this is the first study investigating the association between IBS and DED based on their inflammatory pathogenesis. Our study indicates that patients with IBS should be considered at increased risk of having DED. ST scores were significantly affected compared with healthy controls. tBUT test also yielded significantly lower results in IBS patients than in controls. In addition, all abnormalities in tear film production resulted in more common ocular complaints of dry eyes (more than 3 positive answers to the dry eye symptoms questionnaire) in IBS patients. Our results are supported by previous studies that demonstrated a relation between IBS and dry eye syndrome. Barton et al in 1999 [36] assessed the frequency of fibromyalgia and sicca complex (dry eyes and mouth) in secondary care patients with IBS. 33% of 46 IBS patients presented with sicca syndrome compared with only 6% in the control group, thus adding it to the list of extracolonic manifestations of IBS [36]. In a recent study concerning the prevalence and risk factors of DED in a female cohort, a high association between DED and IBS was found (P<0.0005). Vehof et al showed that four chronic pain syndromes including pelvic pain, chronic widespread pain, IBS, and dry eye were heritable and shared genetic etiologic factors in common biologic pathways, thus introducing the concept of pain hypersensitivity being a common pathogenetic mechanism [37,38].
Data concerning ocular changes in patients with IBS are limited, and dry eye is often overlooked in the context of IBS. Our IBS patients presented a higher prevalence of DED compared with healthy controls. The fact that the score of all tests used for the evaluation of DED was worse in IBS patients than in controls, makes us believe that DED should be considered when treating IBS patients. However, further investigation should be done to establish their pathophysiologic association.
Summary Box.
What is already known:
Inflammation plays a significant role in the etiopathogenesis of dry eye disease (DED)
Many studies also suggest an inflammatory pathogenesis for irritable bowel syndrome (IBS)
What the new findings are:
We found a possible association between IBS and DED
A higher prevalence of DED was found in IBS patients than in controls
DED should be considered when treating IBS patients
Biography
University of Ioannina, Ioannina, Greece
Footnotes
Conflict of Interest: None
References
- 1.The definition and classification of dry eye disease: report of the definition and classification subcommittee of the international dry eye workshop (2007) Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 2.Lin PY, Tsai SY, Cheng CY, Liu JH, Chou P, Hsu WM. Prevalence of dry eye among an elderly Chinese population in Taiwan: The Shihpai eye study. Ophthalmology. 2003;110:1096–1101. doi: 10.1016/S0161-6420(03)00262-8. [DOI] [PubMed] [Google Scholar]
- 3.Moss SE, Klein R, Klein BE. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol. 2000;118:1264–1268. doi: 10.1001/archopht.118.9.1264. [DOI] [PubMed] [Google Scholar]
- 4.Gao J, Schwalb TA, Addeo JV, Ghosn CR, Stern ME. The role of apoptosis in the pathogenesis of canine keratoconjunctivitis sicca: the effect of topical cyclosporin A therapy. Cornea. 1998;7:654–663. doi: 10.1097/00003226-199811000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Stern ME, Gao J, Schwalb TA, et al. Conjunctival T-cell subpopulations in Sjogren’s and non-Sjφgren’s patients with dry eye. Invest Ophthalmol Vis Sci. 2002;43:2609–2614. [PubMed] [Google Scholar]
- 6.Corrales RM, Villarreal A, Farley W, Stern ME, Li DQ, Pflugfelder SC. Strain-related cytokine profiles on the murine ocular surface in response to desiccating stress. Cornea. 2007;26:579–584. doi: 10.1097/ICO.0b013e318033a729. [DOI] [PubMed] [Google Scholar]
- 7.Li DQ, Pflugfelder SC. Matrix metalloproteinases in corneal inflammation. Ocul Surf. 2005;3:198–202. doi: 10.1016/s1542-0124(12)70255-0. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura S, Shibuya M, Nakashima H, et al. Involvement of oxidative stress on corneal epithelial alterations in a blink-suppressed dry eye. Invest Ophthalmol Vis Sci. 2007;48:1552–1558. doi: 10.1167/iovs.06-1027. [DOI] [PubMed] [Google Scholar]
- 9.Sommer A. Effects of vitamin A deficiency on the ocular surface. Ophthalmology. 1983;90:592–600. doi: 10.1016/s0161-6420(83)34512-7. [DOI] [PubMed] [Google Scholar]
- 10.Van Setten GB, Viinikka L, Tervo T, Pesonen K, Tarkkanen A, Perheentupa J. Epidermal growth factor is a constant component of normal human tear fluid. Graefes Arch Clin Exp Ophthalmol. 1989;227:184–187. doi: 10.1007/BF02169794. [DOI] [PubMed] [Google Scholar]
- 11.Zoukhri D. Effect of inflammation on lacrimal gland function. Exp Eye Res. 2006;82:885–898. doi: 10.1016/j.exer.2005.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahin A, Bozkurt B, Irkec M. Topical cyclosporine A in the treatment of superior limbic keratoconjunctivitis: a long-term follow-up. Cornea. 2008;27:193–195. doi: 10.1097/ICO.0b013e318033bd25. [DOI] [PubMed] [Google Scholar]
- 13.Hong S, Kim T, Chung SH, Kim EK, Seo KY. Recurrence after topical nonpreserved methylprednisolone therapy for keratoconjunctivitis sicca in Sjögren’s syndrome. J Ocul Pharmacol Ther. 2007;23:78–82. doi: 10.1089/jop.2006.0091. [DOI] [PubMed] [Google Scholar]
- 14.De Paiva CS, Corrales RM, Villarreal AL, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp Eye Res. 2006;83:526–535. doi: 10.1016/j.exer.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Olden KW. Diagnosis of irritable bowel syndrome. Gastroenterology. 2002;122:1701–1704. doi: 10.1053/gast.2002.33741. [DOI] [PubMed] [Google Scholar]
- 16.Drossman DA, Dumitrascu DL. Rome III: New standard for functional gastrointestinal disorders. J Gastrointestin Liver Dis. 2006;15:237–241. [PubMed] [Google Scholar]
- 17.Ringel Y, Drossman DA. Irritable bowel syndrome: classification and conceptualization. J Clin Gastroenterol. 2002;35:S7–S10. doi: 10.1097/00004836-200207001-00003. [DOI] [PubMed] [Google Scholar]
- 18.El-Matary W, Spray C, Sandhu B. Irritable bowel syndrome: the commonest cause of recurrent abdominall pain in children. Eur J Pediatr. 2004;163:584–588. doi: 10.1007/s00431-004-1503-0. [DOI] [PubMed] [Google Scholar]
- 19.Spiller RC, Jenkins D, Thornley JP, et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804–811. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chadwick VS, Chen W, Shu D, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778–1783. doi: 10.1053/gast.2002.33579. [DOI] [PubMed] [Google Scholar]
- 21.Dunlop SP, Jenkins D, Neal KR, Spiller RC. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology. 2003;125:1651–1659. doi: 10.1053/j.gastro.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 22.Dunlop SP, Jenkins D, Spiller RC. Distinctive clinical, psychological, and histological features of postinfective irritable bowel syndrome. Am J Gastroenterol. 2003;98:1578–1583. doi: 10.1111/j.1572-0241.2003.07542.x. [DOI] [PubMed] [Google Scholar]
- 23.Collins SM, Barbara G. East meets West: infection, nerves, and mast cells in the irritable bowel syndrome. Gut. 2004;53:1068–1069. doi: 10.1136/gut.2004.038893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541–551. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 25.Lee AJ, Lee J, Saw SM, et al. Prevalence and risk factors associated with dry eye symptoms: a population based study in Indonesia. Br J Ophthalmol. 2002;86:1347–1351. doi: 10.1136/bjo.86.12.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bandeen-Roche K, Muñoz B, Tielsch JM, West SK, Schein OD. Self-reported assessment of dry eye in a population based setting. Invest Ophthalmol Vis Sci. 1997;38:2469–2475. [PubMed] [Google Scholar]
- 27.Schein OD, Tielsch JM, Munõz B, Bandeen-Roche K, West S. Relation between signs and symptoms of dry eye in the elderly-a population-based perspective. Ophthalmology. 1997;104:1395–1401. doi: 10.1016/s0161-6420(97)30125-0. [DOI] [PubMed] [Google Scholar]
- 28.Gumus K, Cavanagh DH. The role of inflammation and antiinflammation therapies in keratoconjunctivitis sicca. Clin Ophthalmol. 2009;3:57–67. [PMC free article] [PubMed] [Google Scholar]
- 29.Niederkorn JY, Stern ME, Pflugfelder SC, et al. Desiccating stress induces T cell-mediated Sjögren’s Syndrome-like lacrimal keratoconjunctivitis. J Immunol. 2006;176:3950–3957. doi: 10.4049/jimmunol.176.7.3950. [DOI] [PubMed] [Google Scholar]
- 30.Luo L, Li DQ, Doshi A, Farley W, Corrales RM, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45:4293–4301. doi: 10.1167/iovs.03-1145. [DOI] [PubMed] [Google Scholar]
- 31.Hessen M, Akpek EK. Dry eye: an inflammatory ocular disease. J Ophthalmic Vis Res. 2014;9:240–250. [PMC free article] [PubMed] [Google Scholar]
- 32.Djalilian AR, Nagineni CN, Mahesh SP, Smith JA, Nussenblatt RB, Hooks JJ. Inhibition of inflammatory cytokine production in human corneal cells by dexamethasone, but not cyclosporine. Cornea. 2006;25:709–714. doi: 10.1097/01.ico.0000208815.02120.90. [DOI] [PubMed] [Google Scholar]
- 33.Akpek EK, Merchant A, Pinar V, Foster CS. Ocular rosacea: patient characteristics and follow-up. Ophthalmology. 1997;104:1863–1867. [PubMed] [Google Scholar]
- 34.Gilbard JP. Dry eye, blepharitis and chronic eye irritation: divide and conquer. J Ophthalmic Nurs Technol. 1999;18:109–115. [PubMed] [Google Scholar]
- 35.Akiho H, Ihara E, Nakamura K. Low-grade inflammation plays a pivotal role in gastrointestinal dysfunction in irritable bowel syndrome. World J Gastrointest Pathophysiol. 2010;15:97–105. doi: 10.4291/wjgp.v1.i3.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barton A, Pal B, Whorwell PJ, Marshall D. Increased prevalence of sicca complex and fibromyalgia in patients with irritable bowel syndrome. Am J Gastroenterol. 1999;94:1898–1901. doi: 10.1111/j.1572-0241.1999.01146.x. [DOI] [PubMed] [Google Scholar]
- 37.Vehof J, Kozareva D, Hysi PG, Hammond CJ. Prevalence and risk factors of dry eye disease in a British female cohort. Br J Ophthalmol. 2014;98:1712–1717. doi: 10.1136/bjophthalmol-2014-305201. [DOI] [PubMed] [Google Scholar]
- 38.Vehof J, Zavos HM, Lachance G, Hammond CJ, Williams FM. Shared genetic factors underlie chronic pain syndromes. Pain. 2014;155:1562–1568. doi: 10.1016/j.pain.2014.05.002. [DOI] [PubMed] [Google Scholar]


