Abstract
Background:
The combination and use of multiple drugs in the treatment of tuberculosis (TB) predispose to adverse drug events and reactions. This study evaluated the incidence, frequency, and severity of adverse events to first line anti-tuberculosis (anti-TB) drugs in patients with TB and co-infections with Human Immunodeficiency Virus (HIV).
Objective:
The objective of this study was to determine the effects of HIV status on the risk of developing adverse events to first line anti-TB therapy.
Method:
The study was carried out between 2006 and 2007 when TB therapy was administered without concomitant anti-retroviral therapy. Patients with TB presenting at the chest clinic of a tertiary hospital were sequentially enrolled. Those with TB alone were allocated to the first group while those with TB-HIV infection were allocated to a second group. A checklist of adverse events to the drugs was used to screen for adverse drug events and reactions during the period of anti-TB therapy. Adverse drug events were graded as serious and others (mild-moderate).
Results:
One hundred and three patients completed the study. Thirty one (30.1%) of the patients had TB-HIV co-infection. Majority (70.4%) of the events were detected during the first week of therapy, 92% of these events were mild-moderate. Eight (25.5%) of those with TB-HIV co-infection had serious adverse events. All the serious events occurred in the TB-HIV group. Independent factors for occurrence of ADEs include HIV status, increasing age, and female gender.
Conclusion:
The rate of adverse drug events among patients on first line antituberculosis treatment was higher in HIV co-infected patients.
Keywords: Adverse drug events, Tuberculosis, Anti-TB therapy, HIV co-infection, Nigeria
INTRODUCTION
Tuberculosis (TB) is the leading cause of morbidity and mortality among people living with Human Immunodefi-ciency Virus (HIV), particularly in developing countries1. The disease is a major public health problem in Nigeria, with the country ranking 5th among the 22 high TB burden countries which collectively bear 80% of the global burden of TB 2. The number of TB cases notified in the country increased from 31,264 in 2002 to 90,307 in 2008 and 20 30% of these patients had HIV co-infection 2. The disease has been shown to progress more rapidly and management poses more challenges in those co-infected with HIV3. Thus, there is the need for studies evaluating interactions between the two diseases to guide development of effective treatment policies.
Effective chemotherapy is the mainstay of treatment of tuberculosis and this is largely based on patients' willingness to comply with prescribed regimen. Adverse Drug Events (ADEs) and Adverse Drug Reactions (ADRs) are significant factors in the compliance of patients to medications. These reactions are more common when drugs are taken frequently, in combination, and over prolonged periods. Despite the high level of under-reporting of actual and suspected ADRs 4, it has been shown that adverse drug reactions can be significant factors in the treatment of chronic conditions like tuberculosis 5,6. Studies conducted in developing countries have shown that HIV status is a risk factor for the development of anti-TB drug reactions 7.
Many published reports on anti-TB ADEs are from retrospective studies or are components of studies whose primary objectives are not the detection of ADEs. These studies are likely to have reported lower incidences of ADEs; it has been recommended that prospective observational study designs ought to be used in evaluating adverse drugs events and reactions 8. In addition, studies evaluating the effects of HIV status on ADEs to anti-TB treatment are few, especially in sub-Saharan Africa. This study evaluated the effects of HIV status on ADEs to first line anti-TB treatment using a prospective observational design.
METHODS
Design and treatment protocol
The study was carried out between January 2006 and June 2007, at the chest clinic of the medical out patients' department of the University College Hospital, a tertiary healthcare center located in Ibadan, Nigeria. It was a prospective observational study of patients aged between 18 and 65 years. The study took place when TB-HIV treatment allowed for treatment of TB alone before commencement of anti-retroviral therapy, thus it was possible to evaluate anti-TB therapy without concomitant anti retroviral therapy in patients co-infected with HIV. Consecutive patients who presented with clinical, radiological, histological, and/or microbiological features of pulmonary and/or extra-pulmonary tuberculosis and gave written/verbal informed consent agreeing to adhere to the study protocol were enrolled. HIV diagnosis and voluntary counseling in those with HIV co-infection was done at the HIV-AIDS clinic of the same hospital. Patients on anti-retroviral drugs, other drugs with potential interactions with the TB regimen, women who were pregnant or unwilling to avoid pregnancy during the study period, and those unable to adhere to study protocol were excluded from the study.
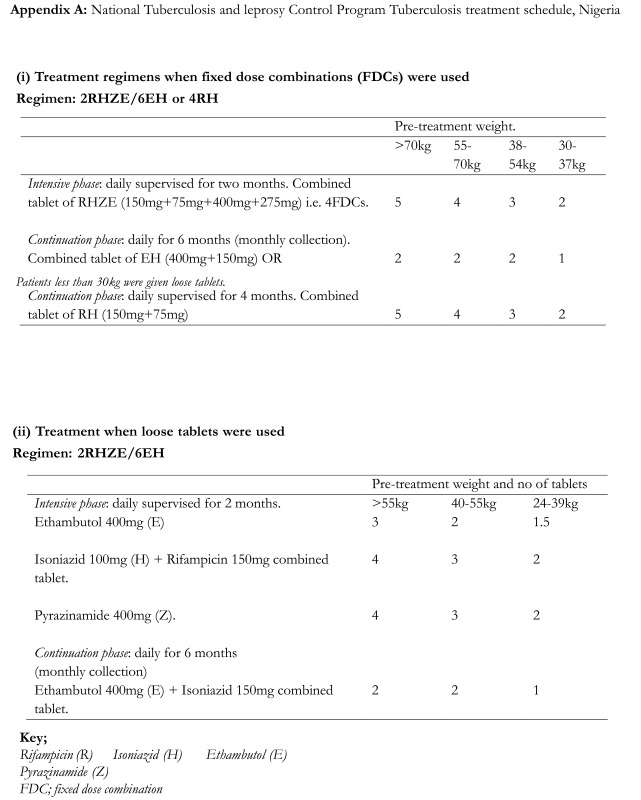
Once enrollment criteria were fulfilled, the patients were allocated to two groups. Patients who had TB mono-infection were allocated to group 1 while those who had TB and HIV co-infections were allocated to group 2. The patients received first line TB therapy in accordance to the National TB and Leprosy treatment guidelines (appendix Ai and Aii)9. Patient demographic information, findings of clinical examination, positive and negative responses were entered into individual case record forms. This process was carried out before the commencement of treatment and during all clinic visits. Laboratory evaluations were not part of the protocol for the study.
Search for Adverse Drug Events
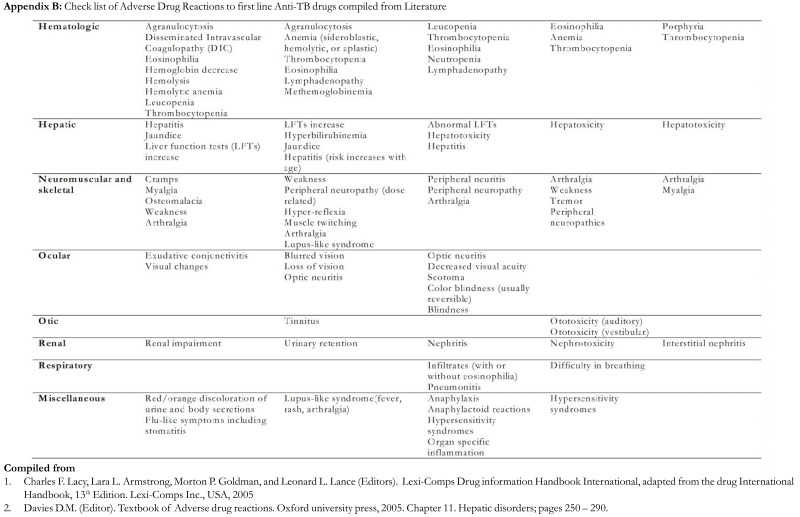
All enrolled patients had detailed clinical examination (history and physical) of all the systems followed by an interview guided by a checklist of reported Adverse Drug Events to first line anti-TB medications. The checklist was compiled from reported literature on anti-TB drugs side effects and adverse drug reactions (appendix B). The checklist was used to screen for adverse events at every clinic visit during the duration of treatment (eight months). In this report, all detected reactions and side effects have been categorized under the term adverse drug events. This is largely because we did not conduct causality testing in this study.
Grading of adverse drug eventss
Identified adverse drug events (ADEs) were graded as follows; Serious: for adverse drug events causing inability to carry out usual activities. These reactions were usually more prolonged and required significant modification or discontinuation of anti-TB medications. Others; for all other events that do not fall into the above category. Orange - red discoloration of urine attributable to rifampicin was not considered an adverse drug event in this study and patients were informed and reassured of this occurrence. This is a modification of conventional classification of adverse drug reactions into mild, moderate, and serious(which includes severe grades)10.
Cure
A patient who met the inclusion criteria for this study and whose symptoms, signs and laboratory findings were significantly improved in the last month of treatment and on at least one previous study time point (and signed as cured by managing chest physician). Patients who were smear-positive at diagnosis, who completed eight months of treatment and who are smear negative at the end of seven months of treatment were signed as cured 9.
Data analysis
Data analysis was done using STATA (version 12, USA). Continuous variables were expressed as means and standard deviation; categorical variables were expressed as proportions. Comparison of categorical variables was done using chi-square and means using the student "t" test. Where the assumption of normality was not satisfied, Mann-Whitney's test was used to compare the two groups. A p value of <0.05 was taken as significant. Univariate analysis was done for all outcome variables to determine estimates of incidence rate ratios. Multivariate analysis was done by Poisson regression model to determine factor associations. Manuscript writing was facilitated by use of EndNotes Reference Manager, version 7.0,
Ethical approval
Approval was obtained from the University of Ibadan/University College Hospital Institutional Review Committee before commencement of the study.
RESULTS
One hundred and seventeen of 326 (35.9%) patients who were diagnosed and scheduled for TB treatment at the chest clinic, between January 2006 and February 2007, were enrolled into the study.
Of the 117 eligible patients, 103 (88%) patients completed the study and constituted the patients evaluated for adverse drug reactions. Sixty three (61.28%) of the patients were male while 40 (38.8%) were females. The duration of illness was similar in both groups. Seventy-two (69.9%) of these patients had TB mono-infection while 31 (30.1%) had TB-HIV co-infection (group 2). Extra-pulmonary tuberculosis was more common in those in group 2 compared to group 1. Table 1 shows the distribution of baseline characteristics of the subjects based on HIV status.
Table 1:
Distribution of baseline characteristics of the study subjects based on HIV status.
| Study Variable | HIV Positive | HIV | Statistical Test* |
|---|---|---|---|
| (N = 31 ) | Negative | (P value) | |
| (N = 72) | |||
|
| |||
| Age (median) | 27 years | 37 years | Kruskal-Wallis = 5.12 |
| (0.024) | |||
| Gender | |||
| Male | 15 (48.4%) | 48 (66.7%) | χ2= 3.05 |
| Female | 16 (51.6%) | 24 (33.3%) | |
| (0.081) | |||
| Weight(median) | 51 kg | 55 kg | Kruskal-Wallis = 2.02 |
| (0.155) | |||
| Duration of TB illness (median) | 4.5 months | 5.0 months | Kruskal-Wallis = 2.02 |
| (0.155) | |||
| Extrapulmonary TB | |||
| Yes | 18 (58.1%) | 16 (22.2%) | χ2= 12.59 |
| No | 13 (41.9%) | 56 (77.8%) | |
| (<0.0001) | |||
Kruskal-Wallis test implemented to compare medians because of heterogeneity of variances or non-normal distribution of the two comparison groups
Adverse drug events were reported in both groups of patients. In absolute patient numbers, 32 (44.4%) patients in group1 reported at least one adverse drug event while 23 (74.2%) patients in group 2 reported an adverse drug event (ñ-value = 0.01). Cumulatively, 124 adverse drug events were reported; (63 in group1 patients and 62 in group 2 patients). Analysis of these adverse events (ADEs) showed that the pattern of systemic affectation of ADEs was similar in both groups. The majority (70.4%) of the adverse drug events was detected during the first week; 93.5% of these ADEs were mild-moderate and resolved within 3 to 14 days of commencement of therapy.
Peripheral neuropathy occurred relatively later (1-2 months of commencing therapy) and lasted for more than 4 months. Burning and tingling sensation of the extremities were the commonest manifestations of neuropathy. Cutaneous reactions were second to neuropathy in terms of frequency. They were reported by both groups of patients but were more extensive in patients who had TB-HIV co-infection compared with those patients who had TB alone. Generalized skin rash occurred in 7 of the 9 cases of skin rash reported among group2 patients as against 3 of the 10 cases reported among those who had TB alone.
These were fine pruritic papular rashes affecting different regions of the body, especially the trunk and extremities. Table 2 shows the list of all the ADEs reported during the study.
Table 2:
Detected adverse drug reactions during the study period.
| ADR | TB group | TB-HIV group | Total Complaints |
|---|---|---|---|
|
| |||
| Neuropathy | 12 | 12 | 24 |
| Skin rash | 10 | 9 | 19 |
| Body weakness | 9 | 6 | 15 |
| Headache | 9 | 9 | 18 |
| Pruritus | 8 | 9 | 17 |
| Vomiting | 5 | 2 | 7 |
| Insomnia | 3 | 2 | 5 |
| Anorexia | 2 | 2 | 4 |
| Arthragia | 1 | 3 | 4 |
| Mood change | 1 | 0 | 1 |
| Diarrhea | 1 | 3 | 4 |
| Dizziness | 1 | 0 | 1 |
| Stomatitis | 1 | 0 | 1 |
| Delayed menstruation | 0 | 1 | 1 |
| Anemia | 0 | 2 | 2 |
| Jaundice | 0 | 1 | 1 |
| Total | 63 | 61 | 124 |
Serious ADEs occurred in 8/31 (25.8%) of the subjects that had TB-HIV co-infection; none occurred in the TB alone group. Four patients developed florid generalized skin rash (between the second week to 3rd month of therapy), four other patients developed persistent vomiting (2nd week of therapy), severe diarrhea (3rd week of therapy), jaundice (1st week of therapy), and anemia (2nd week of therapy) respectively.
These patients had their medications discontinued and were admitted to the wards for medical care. No death was recorded during the study.
Table 3 summarizes the relationship between the risks for ADEs and characteristic features of the patients, using multivariate analyses. HIV positive status increased the risk of developing an ADE to TB medications by about 1.7 fold. Increase in age and female gender also increased the risk of developing an ADE. Higher weight appeared to decrease the risk of developing an ADE.
Table 3:
Crude and adjusted incidence rate ratio estimates of the effects of HIV status and other characteristics on adverse drug reactions from tuberculosis medications
| Study Variable | Crude Incidence | Adjusted Incidence |
|---|---|---|
| Rate Ratio | Rate Ratio* | |
| (95% CI) | (95% CI) | |
|
| ||
| HIV status (positive versus negative) | 2.87 (1.89 - 4.31) | 1.67 (1.04 - 2.68) |
| Age (26 - 39 years versus 15 - 25 years) | 0.82 (0.48 - 1.40) | 1.12 (0.64 - 1.97) |
| Age (40 - 75 years versus 15 - 25 years) | 4.88 (2.97 - 8.14) | 6.40 (3.57 - 11.49) |
| Gender (female versus male) | 1.66 (1.10 - 2.47) | 2.10 (1.36 - 3.25) |
| Weight (35 - 49 kg versus 50 - 59 kg) | 0.522 (0.33 - 0.83) | 0.64 (0.40 - 1.02) |
| Weight (35 - 49 kg versus 60 - 89 kg) | 0.62 (0.35 - 1.07) | 0.37 (0.20 - 0.71) |
| Duration of TB illness in months (0 - 4 versus 5 - 14) | 1.14 (0.77 - 1.70) | 0.82 (0.54 - 1.24) |
| Extra-pulmonary TB (yes versus no) | 1.93 (1.28 - 2.88) | 1.38 (0.84 - 2.27) |
The regression model mutually adjusted all the study variables by included terms as for each subject characteristic as described above.
The treatment outcome was different between the two groups; almost all (90.3%) the patients with TB alone met the definition for cure at the end of the study as against 61.3% of those with TB-HIV (p-value <0.0001).
DISCUSSION
The management of tuberculosis has continued to evolve, largely on account of drug resistance and HIV-AIDS co-infection. There is increased need for accurate TB diagnosis and prompt effective treatment of infected patients. Effective chemotherapy is dependent on the willingness and ability of patients to adhere to chemotherapy for the required six to eight months. This long duration of therapy and the multiple drugs involved are predisposing factors to adverse drug events and reactions11. The directed search for adverse drug events carried out in the study we are reporting resulted in relatively higher rates than commonly reported by some studies 6.
Serious adverse drug events pose a challenge in the management of TB especially in HIV co-infected subjects. The rate of serious adverse drug events recorded in this study was unexpected, however, HIV co-infection is known to worsen the progression of tuberculosis and to increase the incidence and severity of adverse drug reactions 12. During the period that this study was carried out, awareness of availability of free diagnosis, counseling and treatment of HIV-AIDS was low. These in part, culminated into many of the patients presenting rather late at late stages of the disease. Late presentation is a recognized risk factor for adverse reactions to antimicrobial agents including anti-TB drugs13. In addition, the HIV state is also known to be associated with high risk of hypersensitivity reactions, up to a hundred times more common in some reports 14,15. In a study conducted at Canada in 2003, Yee and colleagues reported that HIV positive status was associated with occurrence of major side-effects to first line anti-TB drugs (adjusted hazards ratio of 3.8)16.
Another factor that may account for the rate of serious adverse drug events recorded in this study is the attribution of these findings to drug events on account of the study design which had the objective of detecting adverse drug events. There is a huge proportion of adverse drug events that go unnoticed, undetected, and unreported in general medical practice 10. This may not be unconnected to general poor knowledge about them, the difficulty in differentiating between disease process and drug reactions, and general disinterest by physicians in searching for adverse drug reactions and events. Increasing age was found to be an independent risk factor for development of ADEs in this study. While chronological age may not be an independent risk factor for the development of adverse drug reactions, it has been reported that age-dependent factors such as poly-pharmacy, multiple diseases and changes in pharmacokinetics and pharmacodynamics seem to be responsible for the risk of developing more adverse drug reactions 17,18. These factors may account for the same association between age and ADEs reported by other studies 19-22.
Other independent factors found to be associated with ADEs in this study include female gender and extra-pulmonary TB. The association between female gender and the risk of adverse drug reactions is not recent and has been previously reported by many workers20. Extra-pulmonary TB is not an uncommon finding in advanced HIV disease, which in this study was correlated with higher incidence of adverse drug events and reactions.
Skin rash was the second most common adverse events in this study although thiacetazone was not used in the regimen. This is in keeping with previous reports that the HIV state is associated with high incidence of drug allergies. Skin rash is also a reported adverse drug reaction to all the anti-TB medications used in this study; however, severe skin rash is commoner with thiacetazone therapy on account of which the use of the drug is commonly avoided12,23,24. The overall incidence of severe skin rash in our study was low.
It is well recognized that most of the anti-TB drugs are hepatotoxic, however, only a single case of clinical jaundice was recorded in our study. Studies have reported similar low rates of clinical jaundice but with relatively higher rates of asymptomatic elevation of liver enzymes 25. A better evaluation of hepatotoxicity would include the assessment of Liver function tests, which was not done in our study. This was a major limitation of our study. Another limitation was not conducting a causality assessment for the specific drugs that may have led to the detected ADEs and confirmed them as specific adverse drug reactions. Our study was also limited by small sample size, especially in the arm with TB-HIV co-infection.
The occurrence of majority of the ADEs (48.2%) during the first week of therapy is consistent with previous studies which reported that most ADEs occur within the first few days to weeks of starting therapy and generally clear within a few days11. This is the period when patients need closer monitoring as therapy-related ADEs may lead to poor compliance and to discomfort. The directed search for ADEs in our study led to detection of cases that normally may have gone unreported because in many instances, patients do not complain about them and they resolve within days of commencing treatment. However, some adverse drug events e.g. peripheral neuropathies occurred after one or more months of starting therapy and lasted much longer. It is advisable that the directed search for adverse drug reactions should be diligently done throughout the period of anti-TB therapy.
In an almost similar design to this study, Chhetri et al evaluated ADRs caused by first line anti-tuberculous drugs on directly observed (DOTs) therapy. Their study showed an ADR prevalence of 54.7%. Almost three-quarters (70.67%) of the ADRs in that series were categorized as 'possibly' due to the administered drugs and 93.33% were classified as 'mild. They concluded that occurrence of ADRs from anti-tuberculous drugs was high in the population of Western Nepal 26.
CONCLUSIONS
The study was limited by lack of laboratory data for liver function test, not conducting causality testing, and a small sample size in the arm with HIV-co-infection, however, our findings suggest that the rate of adverse drug events among patients on first line anti tuberculosis treatment may be higher in those with HIV co-infection. Factors found to be associated with occurrence of ADEs were HIV positive status, older age, female gender, and extra-pulmonary TB. Routine monitoring for identification and treatment of ADEs is essential especially during the first weeks of therapy and in the follow-up evaluation of patients on treatment for TB, especially in those co-infected with HIV. Where anti-TB drugs and other medications are to be combined e.g. in patients with HIV co-infection, monitoring of ADEs especially during the first weeks of commencement of anti-TB regimen becomes more pertinent. Using a checklist to search for ADEs may improve the chances of detecting more of them.
ACKNOWLEDGEMENTS
We appreciate the cooperation of all patients, physicians and nurses at the Medical Out-patients' Department, of the University College Hospital, Ibadan. We acknowledge invaluable contributions of Jim Kyraicou, Chad Achenbach, Shannon Galvin, Rebecca Wurtz, and Angela Fought, during the preparation of this manuscript. We acknowledge Damien Foundation Belgium (DFB) for technical support and donation of drugs. Data analysis and writing of this paper was supported by the Medical Education Partnership Initiative in Nigeria (MEPIN) project funded by Fogarty International Center, the Office of AIDS Research, and the National Human Genome Research Institute of the National Institute of Health, the Health Resources and Services Administration (HRSA) and the Office of the U.S. Global AIDS Coordinator under Award Number R24TW008878. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding organizations. Support for this work was provided in part by the Medical Education Partnership Initiative Nigeria grant 5R24TW008878.
Appendix
Additional data
Conflict of Interest
Authors declare no conflict of Interest
Authors' contributions
Michael S.O., conceived the study and wrote the first draft of the manuscript. Other authors participated actively in the running and supervision of the project. Drs. Ige O.M., and Sogaolu O.M., are Consultant chest physicians. Prof Catherine O. Falade, consultant clinical pharmacologist, was the senior Supervisor for the project.
REFERENCES
- 1.Lawson L, Yassin MA, Thacher TD, et al. Clinical presentation of adults with pulmonary tuberculosis with and without HIV infection in Nigeria. Scand J Infect Dis. 2008;40(1):30–35. doi: 10.1080/00365540701509899. [DOI] [PubMed] [Google Scholar]
- 2.WHO. AIDS, TB and Malaria http://www.afro.who.int/en/nigeria/country-programmes/aidstb-and-malaria.html . WHO. 2009. [website accessed on 29 June 2012].
- 3.Cahn P, Perez H, Ben G, Ochoa C. Tuberculosis and HIV: a partnership against the most vulnerable. J Int Assoc Physicians AIDS Care (Chic) 2003;2(3):106–123. doi: 10.1177/154510970300200303. [DOI] [PubMed] [Google Scholar]
- 4.Oshikoya KA, Awobusuyi JO. Perceptions of doctors to adverse drug reaction reporting in a teaching hospital in Lagos, Nigeria. BMC Clin Pharmacol. 2009;9:14. doi: 10.1186/1472-6904-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinderaker SG, Ysykeeva J, Veen J, Enarson DA. Serious adverse reactions in a tuberculosis programme setting in Kyrgyzstan. Int J Tuberc Lung Dis. 2009;13(12):1560–1562. [PubMed] [Google Scholar]
- 6.Zierski M, Bek E. Side-effects of drug regimens used in short-course chemotherapy for pulmonary tuberculosis. A controlled clinical study. Tubercle. 1980;61(1):41–49. doi: 10.1016/0041-3879(80)90060-4. [DOI] [PubMed] [Google Scholar]
- 7.Sharma SK, Mohan A, Kadhiravan T. HIV-TB co-infection: epidemiology, diagnosis & management. Indian J Med Res. 2005;121(4):550–567. [PubMed] [Google Scholar]
- 8.Erik von Elm, Douglas GA, Mattias E, et al. The Strengthening the reporting of Observational Studies in Epidemiology (STROBE). Guidelines for reporting observational studies. Preventive Medicine. 2007;45:247–251. doi: 10.1016/j.ypmed.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Federal Ministry of Health. National Tuberculosis and Leprosy Control Programme (NTBLCP) Workers Manual. 4th Edition. Nigeria: Department of Public Health, Federal Ministry of Health; 2004. [Google Scholar]
- 10.NAFDAC. Safety of Medicines in Nigeria, a Guide for detecting and reporting Adverse drug Reactions. Why Health Practitioners need to act. Nigeria: National Pharmacovigilance Center; 2004. [Google Scholar]
- 11.Yamada H, Yasuoka A, Sasayama K, et al. Adverse reactions of antituberculous agents, especially about its onset and duration. Kekkaku. 1990;65(9):563–568. [PubMed] [Google Scholar]
- 12.Okwera A, Johnson JL, Vjecha MJ, et al. Risk factors for adverse drug reactions during thiacetazone treatment of pulmonary tuberculosis in human immunodeficiency virus infected adults. Int J Tuberc Lung Dis. 1997;1(5):441–445. [PubMed] [Google Scholar]
- 13.Harb GE, Alldredge BK, Coleman R, Jacobson MA. Pharmacoepidemiology of adverse drug reactions in hospitalized patients with human immunodeficiency virus disease. J Acquir Immune Defic Syndr. 1993;6(8):919–926. [PubMed] [Google Scholar]
- 14.Phillips E, Mallal S. Drug hypersensitivity in HIV. Curr Opin Allergy Clin Immunol. 2007;7(4):324–30. doi: 10.1097/ACI.0b013e32825ea68a. [DOI] [PubMed] [Google Scholar]
- 15.Pirmohamed M, Drummond NS, Naisbitt DJ, Park BK. Drug hypersensitivity reactions in patients with HIV disease. Expert Rev Clin Immunol. 2007;3(3):395–410. doi: 10.1586/1744666X.3.3.395. [DOI] [PubMed] [Google Scholar]
- 16.Yee D, Valiquette C, Pelletier M, et al. Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am J Respir Crit Care Med. 2003;167(11):1472–1477. doi: 10.1164/rccm.200206-626OC. [DOI] [PubMed] [Google Scholar]
- 17.Ciorciaro C, Hartmann K, Kuhn M. Differences in the relative incidence of adverse drug reactions in relation to age? An evaluation of the spontaneous reporting system of SANZ (Swiss Drug Monitoring Center) Schweiz Med Wochenschr. 1998;128(7):254–258. [PubMed] [Google Scholar]
- 18.Corsonello A, Pedone C, Incalzi RA. Age-related pharmacokinetic and pharmacodynamic changes and related risk of adverse drug reactions. Curr Med Chem. 17(6):571–584. doi: 10.2174/092986710790416326. [DOI] [PubMed] [Google Scholar]
- 19.Gurwitz JH, Avorn J. Old age is it a risk for adverse drug reactions? Agents Actions Suppl. 1990;29:13–25. doi: 10.1007/978-3-0348-7292-8_3. [DOI] [PubMed] [Google Scholar]
- 20.Klein U, Klein M, Sturm H, et al. The frequency of adverse drug reactions as dependent upon age, sex and duration of hospitalization. Int J Clin Pharmacol Biopharm. 1976;13(3):187–195. [PubMed] [Google Scholar]
- 21.Martin RM, Biswas PN, Freemantle SN, et al. Age and sex distribution of suspected adverse drug reactions to newly marketed drugs in general practice in England: analysis of 48 cohort studies. Br J Clin Pharmacol. 1998;46(5):505–511. doi: 10.1046/j.1365-2125.1998.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pahor M, Guralnik JM, Gambassi G, et al. The impact of age on risk of adverse drug reactions to digoxin. For The Gruppo Italiano di Farmacovigilanza nell' Anziano. J Clin Epidemiol. 1993;46(11):1305–1314. doi: 10.1016/0895-4356(93)90099-m. [DOI] [PubMed] [Google Scholar]
- 23.Ipuge YA, Rieder HL, Enarson DA. Adverse cutaneous reactions to thiacetazone for tuberculosis treatment in Tanzania. Lancet. 1995;346(8976):657–660. doi: 10.1016/s0140-6736(95)92278-4. [DOI] [PubMed] [Google Scholar]
- 24.Wirima JJ, Harries AD. Stevens-Johnson syndrome during anti-tuberculosis chemotherapy in HIV-seropositive patients: report on six cases. East Afr Med J. 1991;68(1):64–66. [PubMed] [Google Scholar]
- 25.Gulbay BE, Gurkan OU, Yildiz OA, et al. Side effects due to primary antituberculosis drugs during the initial phase of therapy in 1149 hospitalized patients for tuberculosis. Respir Med. 2006;100(10):1834–1842. doi: 10.1016/j.rmed.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 26.Chhetri AK, Saha A, Verma SC, et al. Study of adverse drug reactions caused by first line antitubercular drugs used in directly observed treatment, short course (DOTS) therapy in Western Nepal, Pokhara. J Pak Med Assoc. 2008;58(10):531–536. [PubMed] [Google Scholar]