As I sat writing this ‘personal reflections’ manuscript in the spring of 2015, I was seeing press reports related to the use of tobacco to make an Ebola therapeutic called ZMapp. For several months newspaper articles, radio shows and hour‐long TV documentaries have given the public unprecedented exposure to the fact that ‘plant‐made pharmaceuticals’ (PMP) can be life‐saving drugs. I have been asked by many nonspecialists – why tobacco? How can this work? After spending over twenty years doing research in this field and many, many hours in public policy meetings promoting PMPs as an important tool of public health, I do not tire of hearing the same questions. Although there is an increasing pipeline of new protein drugs that will come from plants for both human and animal health, the general public has little knowledge of these specialized tools and therefore limited support for the field. ZMapp has given us free advertising on an international scale that I could never have anticipated.
On 4 August 2014, I opened an email from Larry Zeitlin – the President of Mapp Biopharmaceutical, Inc. in San Diego, California, USA. It was a short message that alerted me to the fact that ZMapp had been used to treat two missionaries in Liberia, and they were recovering from severe Ebola infection. By that afternoon, the newswires were alive with stories with titles such as ‘Secret serum likely saved Ebola patients’. It was hardly a secret to Larry and me, or our colleagues. Arizona State University (ASU) had received substantial grant funds in 2002, including a subcontract to our colleagues in San Diego. (Mapp Biopharmaceutical was incorporated the following year.) We had proposed to the U.S. Army that plants (specifically tobacco) could be used for rapid production of vaccines or monoclonal antibodies (mAbs) that might be necessary to protect us from bioterrorism. This was shortly after terrorist attacks on New York and the Pentagon in 2001, and the use of anthrax spores for a bioterror attack in the U.S. Senate building; funding for countermeasures was quickly becoming available. Our 2005 final report for the Army contract ended with the statement: ‘we have demonstrated that Ebola virus‐specific “humanized” monoclonal antibodies can be produced using plant biotechnology’.
Research on our plant‐made vaccines and mAbs for biodefence has continued as collaborative programmes between Mapp and ASU, gradually involving a wider range of multidisciplinary participants. Chief among these were scientists at Kentucky Bioprocessing LLC, founded in 2006, Herta Steinkellner and her colleagues in Vienna who contributed host plant glycoengineering skills, Yuri Gleba and his colleagues at ICON Gentics and a skilled team at the Army Medical Research Institute for Infectious Diseases (USAMRIID) who conducted preclinical studies of our Ebola‐related products. Back‐to‐back ASU/Mapp publications in 2011 described our success in protecting animal models from Ebola infection using plant‐made vaccine or antibodies (Phoolcharoen et al., 2011; Zeitlin et al., 2011). Subsequent demonstration of protection of nonhuman primates using a cocktail of three tobacco‐made anti‐Ebola mAbs as a postexposure therapeutic paved the way for ZMapp's use in human volunteers. I am sure all of us who participated even in some small part of ZMapp development remain amazed that in only 12 years this project could go from a hypothesis to a drug that is currently the leading Ebola therapeutic under evaluation. We are also amazed this happened with modest monetary support – only a fraction of what is normally spent on development of a new drug in the pharmaceutical industry. Early parts of my career had not seen such clearly defined outcomes.
Events leading up to ‘Edible Vaccines’
The first half of my scientific career had been entirely devoted to plant biology, with a strong interest in agricultural improvements from a basic research perspective. I began with studies of chloroplast structure and the factors which regulate/limit the quantum efficiency of photosynthesis in higher plants. After providing evidence that photosystems I and II exist in discrete polyprotein complexes, we were the first to show that proteins are phosphorylated in plants – a newly emerging concept at that time – and showed further that the kinase activation was controlled by membrane redox potential – also a novel idea. This phosphorylation altered chloroplast thylakoid surface charge and thereby regulated spatial positioning of photosystem reaction centres and light harvesting pigment–protein complexes to modulate and optimize solar energy capture (Staehelin and Arntzen, 1983). My studies of chloroplast membrane organization led to a collaboration with agronomists who had discovered herbicide‐resistant weeds. Knowing that some of these herbicides acted by blocking photosynthetic electron transport, my team showed that triazine herbicides bind to a specific protein in the photosystem II complex; this was the first molecular target known for a commercial herbicide (Steinback et al., 1981). We learned that a mutation of a single amino acid in this binding pocket could completely block herbicide binding and action. This discovery was at the time when genetic transformation was just being developed for plants, and our team was influential in pioneering the concept of engineering a crop for herbicide resistance.
In 1984, I joined the DuPont Company to help create their programme in crop biotechnology, with a strong focus on creation of herbicide and insect resistance via introduction of new proteins into the crop plants. This was my first intensive interaction with scientists working at the forefront of gene expression in plants. DuPont was also a setting where I could learn the discipline needed for product development and management of multidisciplinary projects that have dominated my research since that time.
While industrial research was one of the most enjoyable periods of my research career, an offer from Texas A&M University and the opportunity to lead their creation of a research centre in the Texas Medical Center in Houston caused me to again change directions. As the doors opened to this new Institute, my goal was to find a project area that built upon my prior expertise but which would have a very direct medical focus. I was greatly influenced by the launch of The Children's Vaccine Initiative (CVI) in September of 1990 (Douglas, 1993). The CVI purpose was to harness new technologies in the quest for the ultimate vaccine – including being affordable, heat‐stable and orally administered candidates. This was the era when subunit vaccines were just being accepted; in 1986, a vaccine to prevent Hepatitis B infection was licensed in which the antigen was the viral surface protein (HBsAg) produced in yeast using recombinant DNA technology. This vaccine was very highly effective and a dramatic breakthrough in vaccinology, but it was an injected vaccine and still comparatively expensive. My prior experience in DuPont made me ask – could plants make HBsAg? And next, to ask whether it or other subunit antigens could be orally delivered?
While at Texas A&M, I had the wonderful good fortune to meet Hugh S. Mason, who was a postdoc in Biochemistry. I was and still am impressed by his skills in plant molecular biology. Moreover, we hit it off personally – he accepted my naïve notions about producing vaccines in plants and tempered the ideas with sound experimental planning. He was also willing to create a team with me, and live through early years of financial uncertainty as we tried to capture funding for PMP research – a concept that grant panels found somewhat zany at the time. Our team has moved among three separate academic institutions over the last 25 years, and we have jointly written a large number of grant proposals and publications. I am very grateful for his continued friendship and collegiality.
Achieving HBsAg expression in plants in 1992 was our first joint publishable success (Mason et al., 1992). Hugh and I were delighted to see that the antigen we recovered from transgenic plants was organized as virus‐like particles (VLP), which had the same properties as the antigen in the yeast‐produced hepatitis B vaccine (Figure 1). While this may seem unsurprising today, it was a key step for us in convincing a skeptical vaccine research community of the PMP concept. In the next few years, we also created transgenic plants that expressed other antigens, including the binding subunit of E. coli enterotoxin (LTB) and the capsid protein of norovirus genotype 1 (or Norwalk virus, as it was known then) which formed virus‐like particles (NV‐VLP). Another necessary step for gaining credibility for PMP was to show that oral delivery of tissue from transgenic plants would induce an immune response. In a series of publications, we reported that feeding “animals” plant tissue expressing HBsAg, LTB or NV‐VLP would induce antigen‐specific antibodies. Using these data, we filed Investigational New Drug (IND) applications with the U.S. Food and Drug Administration (FDA) and gained approval for three human clinical trials. In all cases, the volunteers ate raw potato tubers peeled and cut into bite‐sized chunks, and in all cases, we saw antibody responses indicating mucosal immunization (Arntzen et al., 2005).
Figure 1.
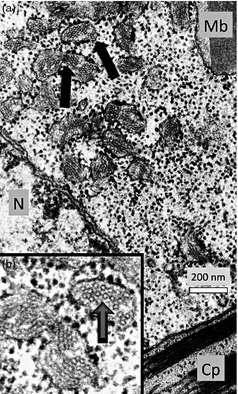
We used electron microscopy and immunocytochemistry to demonstrate that HBsAg accumulated in plant tissue as either tubular structures or virus‐like particles (Smith et al., 2003). (a) In this electron micrograph, arrows indicate dilated portions of rough endoplasmic reticulum (RER) containing circular particles or cross sections of tubular structures. (b) Enlargement of the RER. (N = Nucleus; Cp = Chloroplast; Mb = Microbody) (Thanks to Lizabeth Richter for these electron micrographs.).
In 2004, I was delighted to help organize a scientific conference on PMP, supported by the Fondation Mérieux and held in Veyrier‐du‐Lac, France. I believe this was the first time that an international group had assembled to discuss ‘Plant‐Derived Vaccines and Antibodies: Potential and Limitations’. We noted that in the decade following our initial 1992 publication, at least 100 publications on the subject of plant‐derived vaccines were in journals, describing at least 45 antigens produced in plants. In a summary figure from that meeting (Arntzen et al., 2005) we identified three research strategies then in use: transgenic plants with stable expression of genes in the nucleus, or in the chloroplast genome, and transient expression using virus‐derived vectors. The overriding focus of the time was on transgenic plants. The only nod given to transient expression was modified viruses, such as tobacco mosaic viruses that could infect plants and produce virions with epitope fusions to the coat protein. In that context, it is amazing that a decade later international meetings such as PBVAB‐15 (held in June of 2015 in Switzerland) are dominated by protein expression using Agrobacterium‐mediated transient expression, and deconstructed viral genomes delivered via Agrobacterium (Agro) to plant leaves (see the superb review on this subject by G. Lomonossoff and H. Peyret in this volume). Although the research relating to Agro‐delivered reconstructed viral genomes had started in 2004, it was too immature to be treated as something necessary to include in a conference! Seeing where that area is today emphasizes the beauty of ‘disruptive technical innovation’.
In 1992, my research concept had been entirely focused on low‐cost vaccines for oral delivery in the developing world, and for the next several years, I actively promoted the idea of ‘edible vaccines’ (Figure 2). As I have said in recent years that may have attracted attention in the plant biology realm, but it probably has been much more counterproductive in the traditional vaccine industry. I have come to regret coining the term. In my naiveté, I was ignoring the rigorous regulatory requirements that government agencies and the vaccine industry follow to give us today's highly effective and wonderfully safe vaccines. I still believe that using plant tissue for low‐cost delivery of unpurified (or partially purified) antigens, perhaps as a dry powder, is technically feasible for mucosal vaccination. I see progress in this direction for animal vaccines, which is needed. However, plant‐to‐plant variations will require careful (and expensive) analytical controls to determine exact dosages of antigen if it is not purified. As we noted in 2004, ‘While it was frequently mentioned that plant‐based protein production is highly cost effective, participants at the conference were reminded that production costs may represent only a small part of the cost of a vaccine. GMP (Good Manufacturing Practice) requirements, purification, quality controls for vaccine approval are major cost factors in (human) vaccine production (Arntzen et al., 2005)’. My ‘edible vaccine’ notion also had an unspecified need for high levels of antigen accumulation in plant tissues to ensure that only a small amount of plant tissue would be needed to deliver one dose of vaccine. This issue has been addressed in chloroplast transformation systems (see the excellent commentary on this topic by K‐C Kwon and H. Daniell in this volume), but current deconstructed viral systems delivered by Agro have shown orders of magnitude higher antigen accumulation than we could achieve in transgenic plants with nuclear gene insertion. By 2015, I have come to appreciate that the costs of purifying an antigen (or antibody or human enzyme) may not be an impediment to development of a new vaccine or drug if the desired protein is produced at high levels in plant tissue. Additionally, purified antigens can follow the regulatory pathways now well established for subunit vaccines or other protein drugs.
Figure 2.

In the 1990s, we expressed antigens in multiple crop species with a goal of creating a minimally processed oral vaccine. The concept of using banana fruit attracted extensive interest in the press, although the low expression of antigen in the fruit, and the 3 + year cycle needed to create a transgenic fruiting plant caused us to abandon this area of research. (Photograph from the Boyce Thompson Institute files, 1999).
The recent past and looking ahead
In 1997, I joined the Advisory Board of the ‘Agbio Capital’ venture capital fund. We listened to many business plan presentations, but one particular plan had a great impact on me. It was by Dr. Yuri Gleba, who had founded ICON Genetics AG in Halle, Germany in 1999. He described ‘deconstructed viral vectors’ which could be delivered by Agro infiltration into plant leaves. In my view, it was the ‘disruptive technology’ that the PMP field needed. But the board did not approve funding for ICON because they thought it was ‘a platform technology that needed a product focus’. After determining that it would not be a conflict of interest, I made personal contact with Dr. Gleba (or Yuri as most of us call him) to discuss collaboration. He agreed, and Luca Santi travelled from our ASU laboratory to Germany to work for a few months with the ICON scientists. Besides bringing additional viral vector skills back to our team in Arizona, this lead to our first ASU/ICON collaborative publication relating to a Yersinia pestis biothreat vaccine that protected animal models (Santi et al., 2006).
For the last 14 years, since moving to ASU, my major contribution to science has been in the design and management of multidisciplinary and multicomponent projects, especially those that link academia and industry. It has been highly rewarding to see the emergence of PMP‐based companies begin commercial introduction of products. The success of Protalix Biotherapeutics in gaining FDA approval for an enzyme therapeutic for human use was a milestone for the field, and ongoing product introduction by them, and by Medicago and Ventria (just to name two of the larger efforts) and others will ensure the successful future of the PMP field.
As for me, I will return this discussion to ZMapp, which I used to introduce this manuscript. Although I have only played a small role in the development of the product, it is incredibly rewarding to see that something that started as a hypothesis has actually been used in life‐saving treatments. I have been fortunate to work with a wonderful team of people at ASU, and with companies with extraordinary and complementary skills – and especially Mapp, ICON, and KBP. I am not yet ready to quit, as I believe that a plant‐made norovirus vaccine will only come if we can find a formulation that will work as a robust mucosal vaccine (Velasquez et al., 2011). And, we have ongoing research with that goal in mind.
Acknowledgements
The people who deserve my thanks are too numerous to mention. As to the companies I have mentioned, I have no conflict of interest to cite for any of them.
The copyright line for this article was changed on 15 December 2015 after original publication.
References
- Arntzen, C. , Plotkin, S. and Dodet, B. (2005) Plant‐derived vaccines and antibodies: potential and limitations. Vaccine, 23, 1753–1756. [DOI] [PubMed] [Google Scholar]
- Douglas, R.G. Jr . (1993) The Children's Vaccine Initiative–will it work? J. Infect. Dis. 168, 269–274. [DOI] [PubMed] [Google Scholar]
- Mason, H.S. , Lam, D.M. and Arntzen, C.J. (1992) Expression of hepatitis B surface antigen in transgenic plants. Proc. Natl Acad. Sci. USA, 89, 11745–11749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoolcharoen, W. , Dye, J.M. , Kilbourne, J. , Piensook, K. , Pratt, W.D. , Arntzen, C.J. , Chen, Q. , Mason, H.S. and Herbst‐Kralovetz, M.M. (2011) A nonreplicating subunit vaccine protects mice against lethal Ebola virus challenge. Proc. Natl Acad. Sci. USA, 108, 20695–20700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi, L. , Giritch, A. , Roy, C.J. , Marillonnet, S. , Klimyuk, V. , Gleba, Y. , Webb, R. , Arntzen, C.J. and Mason, H.S. (2006) Protection conferred by recombinant Yersinia pestis antigens produced by a rapid and highly scalable plant expression system. Proc. Natl Acad. Sci. USA, 103, 861–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, M.L. , Richter, L. , Arntzen, C.J. , Shuler, M.L. and Mason, H.S. (2003) Structural characterization of plant‐derived hepatitis B surface antigen employed in oral immunization studies. Vaccine, 21, 4011–4021. [DOI] [PubMed] [Google Scholar]
- Staehelin, L.A. and Arntzen, C.J. (1983) Regulation of chloroplast membrane function: protein phosphorylation changes the spatial organization of membrane components. J. Cell Biol. 97, 1327–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinback, K.E. , McIntosh, L. , Bogorad, L. and Arntzen, C.J. (1981) Identification of the triazine receptor protein as a chloroplast gene product. Proc. Natl Acad. Sci. USA, 78, 7463–7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez, L.S. , Shira, S. , Berta, A.N. , Kilbourne, J. , Medi, B.M. , Tizard, I. , Ni, Y. , Arntzen, C.J. and Herbst‐Kralovetz, M.M. (2011) Intranasal delivery of Norwalk virus‐like particles formulated in an in situ gelling, dry powder vaccine. Vaccine, 29, 5221–5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitlin, L. , Pettitt, J. , Scully, C. , Bohorova, N. , Kim, D. , Pauly, M. , Hiatt, A. , Ngo, L. , Steinkellner, H. , Whaley, K.J. and Olinger, G.G. (2011) Enhanced potency of a fucose‐free monoclonal antibody being developed as an Ebola virus immunoprotectant. Proc. Natl Acad. Sci. USA, 108, 20690–20694. [DOI] [PMC free article] [PubMed] [Google Scholar]


