Abstract
The aim of this study was to clarify the effects of gestational age and birth weight on outcomes of the infants. Medical records of 36 infants with trisomy 18 admitted to Tokyo Women's Medical University Hospital from 1991 to 2012 were reviewed retrospectively. We compared clinical characteristics between term infants (n = 15) and preterm infants (n = 21). There were one very‐low‐birth‐weight (VLBW) term infant (5%) and 12 VLBW preterm infants (80%). Although there were no significant differences in clinical characteristics and provided management between the two groups, none of the preterm infants achieved survival to discharge. On the other hand, 6 of 21 term infants (29%) achieved survival to discharge (P < 0.05). Similar results were obtained for comparisons between the VLBW infants and non‐VLBW infants. Multiple logistic regression analysis revealed that shorter gestational age had a more negative impact than lower birth weight to survival to discharge in infants with trisomy 18. In both preterm and term groups, the infants who died before 30 days commonly died of respiratory failure or apnea. Whereas, the infants who survived more than 30 days mostly died of heart failure. © 2015 The Authors. American Journal of Medical Genetics Part A Published by Wiley Periodicals, Inc.
Keywords: trisomy 18, very‐low‐birth‐weight infant, preterm, mortality, prognosis
INTRODUCTION
Trisomy 18, first described by Edwards et al. [1960]; is the second most commonly diagnosed autosomal trisomy among live‐born infants, with an incidence of between one in 3,000 and one in 10,000 [Carter et al., 1985; Goldstein and Nielsen, 1985; Young et al., 1986; Root and Carey, 1994; Embleton et al., 1996; Rasmussen et al., 2003; Jones, 2006]. Trisomy 18 is characterized by multiple anomalies, and even with medical intervention, the expected lifespan is very short: the median survival duration has been reported to be 2.5–14.5 days [Carter et al., 1985; Goldstein and Nielsen, 1985; Root and Carey, 1994; Young et al., 1986; Root et al., 1994; Embleton et al., 1996; Brewer et al., 2002; Rasmussen et al., 2003; Jones, 2006; Lin et al., 2006; Hsiao et al., 2009; Vendola et al., 2010]. Although McGraw and Periman [2008] reported that attitudes of delivery room management have been changing, management of infants with trisomy 18 is controversial. It is indeed very difficult for caregivers and families to determine whether or not to withdraw resuscitation and treatment of such infants [Carey, 2012].
Recently, Boghossian et al. [2014] reported poor mortality and morbidity of very‐low‐birth‐weight (VLBW) infants with trisomy 18. Other studies reported a difference in the prognosis of infants with trisomy 18 between those born term and preterm, between those with and without cardiac anomaly or esophageal atresia, and between male and female infants [Niedrist and Riegel, 2006]. However, Guon et al. [2013] showed that differences in the plan of care provided to each patient affect the prognosis. Another report from Japan showed a positive effect of intensive care on the prognosis [Kosho et al., 2006]. Carey [2012] reported that to make the better plan for each patient, it is important to provide accurate information to the patients and their families about the outcome. Although there are many reports on the prognosis of infants with trisomy 18, it is still unknown how gestational age, birth weight, and plan of care affect the prognosis of such infants. Our purpose was to clarify the effects of these factors on the prognosis of infants with trisomy 18.
METHODS
Infants who were born from January 1, 1991, through December 31, 2012, and who were admitted to our neonatal intensive care unit at the Tokyo Women's Medical University Hospital were eligible. The infants who were diagnosed with trisomy 18 by chromosomal analysis were enrolled in this study.
Our institute has level III neonatal intensive care units and neonatal surgery and cardiovascular surgery divisions. Our policy for the management of infants with a confirmed or strongly suspected prenatal or postnatal diagnosis of trisomy 18 is to provide information about the natural history of trisomy 18 and discuss the management plan with the parents. After the discussion, we decide a plan of care for each patient. In cases of prenatal diagnosis, we discuss with the parents and decide the management at birth. If the infant can survive the perinatal period, we have another discussion including caregivers and parents and decide a subsequent plan of care. Infants who are more than 37 weeks of corrected gestational age and who need no intravenous drip infusion of any medications are eligible for home medical care. If the patients fulfill these criteria and the parents want home medical care, the patients are discharged from our hospital with respiratory support and enteral tube feeding, as needed.
We collected both maternal and neonatal clinical data. Gestational age was calculated from the first day of the last menstrual period and confirmed with a crown‐rump length measurement in the first trimester. If the estimated gestational age differed from the calculated one, the scan date was used. Small for gestational age (SGA) was defined as birth weight <10th percentile according to Japanese reference data [Itabashi et al., 2010]. Survival duration was defined as time from each infant's birthdate to the date of the last clinic visit or the end of the study (December 31, 2013). We defined coarctation of the aorta, interrupted aortic arch, and hypoplastic left heart syndrome as patent ductus arteriosus (PDA)‐dependent congenital heart disease. We also defined all gastrointestinal disorders that could affect the prognosis and required treatment as a gastrointestinal (GI) deficit. Congenital diaphragmatic hernia and esophageal atresia were analyzed separately, because of their lethality. We classified mechanical ventilation, when it was used for improvement of a respiratory condition, unless it was used only for perioperative management. We reviewed all of the medical charts of each infant and collected information from the medical records about parents' attitudes toward the management and plan of care. According to the decided plan, we classified the medical management of patients with trisomy 18 at birth and their subsequent neonatal management into three grades: full intensive care (grade A), limited care (grade B), and palliative care (grade C). The grade A treatment plan provides all care needed to manage the complication with the goal of survival and discharge. Operative procedures such as palliative and definitive repair were determined according to the patient's condition. For grade C treatment, all invasive procedures except for oxygen administration are withheld. The infants were given conservative and supportive care. Grade B is an intermediate plan between grades A and C. Namely, the grade B treatment plan provides partial care needed to maintain the patient's condition. For grade B treatment, procedures such as mechanical ventilation and blood transfusion were performed. Surgical intervention was not performed except for gastrostomy for congenital esophageal atresia or repositioning of a hernia into the umbilical cord and omphalocele.
We compared clinical characteristics and outcomes between preterm (gestational age <37 weeks) and term (gestational age ≥37 weeks) infants with trisomy 18. Secondarily, we also compared VLBW (birth weight <1,500 g) and non‐VLBW (birth weight ≥1,500 g) infants with trisomy 18. For comparisons between the two groups, we used Student's t‐test or Wilcoxon‐Mann–Whitney test for continuous variables and χ2 test or Fisher's exact test for categorical variables. For these analyses, a P value <0.05 was considered statistically significant. We also performed stepwise multiple logistic regression analysis to identify the clinical factors contributing to survival to discharge. A P value <0.20 was used for valuable remodel in the stepwise multiple logistic regression analysis. The statistical software used was JMP Pro version 11.2.0 (SAS Institute, Cary, NC).
Ethics approval was obtained from the ethics committee of Tokyo Women's Medical University.
RESULTS
The demographic data of patients and their mothers are shown in Table I. All 36 patients were full trisomy 18 (15 boys and 21 girls). All of them in this study were SGA infants. Median maternal age was 37 years old. One mother got pregnant by intracytoplasmic sperm injection and another by gamete intrafallopian tube transfer. The rest of the mothers conceived naturally. Maternal complications included diabetes mellitus (n = 2), chronic thyroiditis (n = 2), ovarian cyst (n = 2), mild pulmonary stenosis (n = 1), asthma (n = 1), and uterine myoma (n = 1). Antenatal steroid was administered in three mothers, all of whom had preterm labor. A prenatal diagnosis of trisomy 18 was not made in these patients, and their parents wished full intensive care (grade A). Tocolysis, with ritodrine hydrochloride, magnesium sulfate, or both, was induced for threatened preterm labor in 13 patients, but in four of these patients, the medication was discontinued when their babies were diagnosed as trisomy 18 by amniotic fluid tests.
Table I.
Demographic Data of Study Population
| N = 36 | |||
|---|---|---|---|
| Maternal age, median (range) | 37 (24–44) | ||
| Primipara, n (%) | 16 (44%) | ||
| Gestational age, median (range) | 37w5d (28w4d–42w1d) | ||
| Birth weight (g), median (range) | 1659 (787–2314) | ||
| SGA, n (%) | 36 (100%) | ||
| Sex, n (%) | |||
| Male | 21 (58%) | ||
| Female | 15 (42%) | ||
| Mode of delivery, n (%) | |||
| Vaginal | 18 (50%) | ||
| Cesarean section | 18 (50%) | ||
| Prenatal diagnosis, n (%) | 16 (44%) | ||
| Apgar score (1 min), median (range) | 2.5 (1–8) | ||
| Apgar score (5 min), median (range) | 5.5 (1–9) | ||
| Congenital heart disease, n (%) | 35 (97%) | ||
| GI deficit, n (%) | 13/34 (36%) |
| Delivery room management | Grade A n = 17 | Grade B n = 12 | Grade C n = 7 |
|---|---|---|---|
| Intubation, n (%) | 9 (53%) | 0 | 0 |
| Bag mask ventilation, n (%) | 4 (24%) | 10 (83%) | 0 |
| O2 administration, n (%) | 0 | 0 | 2 (29%) |
| Stimulation, n (%) | 0 | 2 (17%) | 0 |
| None, n (%) | 4 (24%) | 0 | 5 (71%) |
| Neonatal management | Grade A n = 9 | Grade B n = 16 | Grade C n = 11 |
| Mechanical ventilation, n (%) | 5 (56%) | 4 (25%) | 1 (9%) |
| Cardiac surgery, n (%) | 2 (22%) | 0 | 0 |
| GI tract surgery, n (%) | 3 (33%) | 2 (13%) | 0 |
| Tracheostomy, n (%) | 1 (11%) | 0 | 0 |
| Blood transfusion, n (%) | 6 (67%) | 1 (6%) | 0 |
| Hospitalization, median (range) | 8.5 (0–404) | ||
| Survival days, median (range) | 12.5 (0–898) | ||
d, days; GI, gastrointestinal; SGA, small for gestational age; w, weeks.
The median birth weight of the study infants was 1,659 g, and because all infants were SGA, we divided the study infants into two groups based on a cutoff birth weight of 1,500 g. Mean standard deviation scores of birth weight, height, and head circumference were −3.32, −2.72 (n = 34), and −1.48 (n = 34), respectively. Two patients had no record of height and head circumference. Although some kind of congenital syndrome was suspected in 27 of 36 cases, amniotic fluid was tested in only 18 cases. In two of these cases, the infants were born before the results of the test became clear, and a prenatal diagnosis was made in 16 cases. Among the 18 cases whose amniotic fluid was not tested, three infants were born before the parents decided to have the fluid tested; in six cases, the parents did not wish to have the fluid tested; and in the remaining nine fetuses, there was no suspicion of any chromosomal anomalies. These nine infants were suspected of having trisomy 18 after birth, because of characteristic facial appearance, cardiac anomaly, cerebellar hypoplasia, or other anomalies. We performed chromosomal analyses on peripheral blood of the infants after getting informed consents from the parents.
All but one of the infants had congenital heart disease, including ventricular septal defect (VSD) (n = 17), double outlet right ventricle (DORV) (n = 6), tetralogy of Fallot (TOF) (n = 3), hypoplastic left heart syndrome (HLHS) (n = 3), atrioventricular septal defect (AVSD) (n = 2), single ventricle (n = 1), Ebstein's anomaly (n = 1), DORV with AVSD (n = 1), and aortic coarctation and DORV with AVSD (n = 1). GI disorders of the infants included esophageal atresia (n = 6), omphalocele (n = 3), congenital diaphragmatic hernia (n = 1), imperforate anus (n = 1), intestinal malformation (n = 1), and pyloric stenosis (n = 1).
Of the 36 patients, 20 (56%) survived the first week, 16 (44%) the first month, 11 (31%) the third month, and four (11%) the first year of life. The median survival was 12.5 days.
The study flowcharts and main outcomes are shown in Figure 1. The parents of 17 infants wished full intensive care (grade A). In the grade A group, four infants did not need any resuscitation. In the grade C group, two infants received oxygen administration, but none received stimulation. Prenatal diagnosis of trisomy18 was made in two (11.8%), eight (66.7%), and six (85.7%) infants in grade A, B, and C groups, respectively. In subsequent management, two patients underwent cardiac surgery in the grade A group: pulmonary artery banding (n = 1); and pulmonary artery banding and VSD closure (n = 1). Three patients in the grade A group underwent GI surgery: surgical repair of the omphalocele (n = 1), gastrostomy and surgical repair of the GI perforation (n = 1), and gastrostomy and esophageal atresia repair (n = 1). Two patients in the grade B group received manual repositioning of an omphalocele as a GI surgery. In the grade B group, all four infants who received mechanical ventilation had not been diagnosed prenatally with trisomy 18. Therefore, they received grade A management at birth and were intubated. In the grade C group, one patient with diaphragmatic hernia, VSD, and atrial septal defect received mechanical ventilation at birth. The diagnosis of trisomy 18 in the infant was made after birth.
Figure 1.
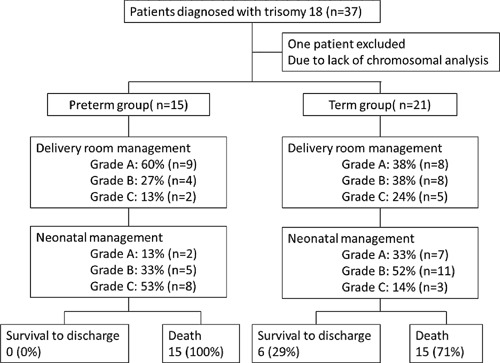
Flowchart of study patients and main outcomes between preterm and term groups. VLBW, very low birth weight.
Regarding the relationship between neonatal management and prognosis, of six infants who survived to discharge, two infants received grade A neonatal management and four infants received grade B management. Our results show a tendency for more infants with grade A or grade B management to survive to discharge compared with grade C (P = 0.099 and 0.08, respectively), but the differences were not statistically significant and there was no difference between grade A and B. However, infants receiving grade A management survived significantly longer (median, 152 days) than infants receiving grade C management (median, 0 days) (P < 0.01), and infants receiving grade B management survived longer (median, 29 days) than infants receiving grade C management (P < 0.01). Infants in the grade A group also tended to survive longer than those in the grade B group, but the difference was not significant (P = 0.06). There were also no significant differences in the incidence of survival to discharge and survival duration between the groups in regard to delivery room management.
The comparisons of clinical characteristics between the preterm and term groups are shown in Table II. Of 15 infants in the preterm group, 12 (80%) were VLBW infants, whereas of 21 infants in the term group, 20 (95%) were non‐VLBW infants. The rates of delivery room management and neonatal management in each group were not significantly different between the two groups. The infants who underwent cardiac surgery were included only in the term group. However, the ratios of cardiac surgery and other medications or treatments, including mechanical ventilation, GI surgery, and blood transfusion, were not significantly different between the two groups. The incidence of survival to discharge was significantly higher in the term group (29%) than in the preterm group (0%) (P = 0.02). Survival duration was also significantly longer in the term group (82 days) than in the preterm group (2 days) (P < 0.01).
Table II.
Comparison Between Preterm and Term Groups
| Preterm group (n = 15) | Term group (n = 21) | P value | |
|---|---|---|---|
| Gestational age, median (range) | 33w6d (28w4d–36w6d) | 39w2d (37w0d–42w1d) | <0.0001 |
| Birth weight (g), median (range) | 1216 (787–1902) | 1920 (1387–2314) | <0.0001 |
| VLBW, n (%) | 12 (80%) | 1 (5%) | <0.0001 |
| Male, n (%) | 9 (60%) | 6 (29%) | 0.059 |
| Prenatal diagnosis, n (%) | 5 (33%) | 11 (52%) | 0.256 |
| Apgar score (1 min), median (range) | 2 (1–5) | 4 (1–8) | 0.010 |
| Apgar score (5 min), median (range) | 4 (2–7) | 7 (1–9) | 0.0087 |
| Delivery room management, n (%) | 0.49 | ||
| Grade A | 9 (60%) | 8 (38%) | |
| Grade B | 4 (27%) | 8 (38%) | |
| Grade C | 2 (13%) | 5 (24%) | |
| Neonatal management, n (%) | 0.059 | ||
| Grade A | 2 (13%) | 7 (33%) | |
| Grade B | 5 (33%) | 11 (52%) | |
| Grade C | 8 (53%) | 3 (14%) | |
| CHD, n (%) | 15 (100%) | 20 (95%) | 0.39 |
| PDA‐dependent CHD, n (%) | 1 (7%) | 4 (19%) | 0.29 |
| Cardiac surgery, n (%) | 0 (0%) | 2 (10%) | 0.22 |
| GI deficit, n (%) | 4 (29%) | 3 (15%) | 0.34 |
| CDH or EA, n (%) | 7/14 (50%) | 6/20 (30%) | 0.24 |
| GI surgery, n (%) | 2 (13%) | 3 (14%) | 0.94 |
| Sepsis, n (%) | 0 (0%) | 3 (14%) | 0.13 |
| Blood transfusion, n (%) | 2 (13%) | 5 (24%) | 0.43 |
| Mechanical ventilation, n (%) | 6 (40%) | 4 (19%) | 0.17 |
| Survival to discharge, n (%) | 0 (0%) | 6 (29%) | 0.023 |
| Days of survival, median (range) | 2 (0–274) | 82 (0–898) | 0.0075 |
w, weeks; d, days; VLBW, very‐low‐birth‐weight; CDH, congenital diaphragmatic hernia; CHD, congenital heart disease; EA, esophageal atresia, GI, gastrointestinal; PDA, patent ductus arteriosus.
Similar results were observed for the comparisons between the VLBW and non‐VLBW groups. The median Apgar score at 1 min was significantly higher in the non‐VLBW group (P = 0.02). However, there was no significant difference in other clinical factors, delivery room and neonatal management grades, and all treatments including respiratory care, cardiac surgery, GI surgery, and blood transfusion between the two groups. No infants in the VLBW group survived to discharge, but 6 of 23 infants in the non‐VLBW group survived to discharge (P = 0.04). The median survival duration was also significantly longer in the non‐VLBW group (57 days) than in the VLBW group (4 days) (P = 0.03).
In our population, both preterm birth and VLBW had a negative effect to survival to discharge. We categorized the study infants to Preterm VLBW group, Preterm Non‐VLBW group, Term VLBW group, and Term Non‐VLBW group. The populations of each group were 12, 3, 1, and 20, respectively (Table III). The median survival duration in each group was four (range, 0–274), one (range, 1–2), two (range, 2–2), and 86 (range, 0–898), respectively. An infant who was preterm and Non‐VLBW was provided intermediate care (grade B). He had teratology of fallot and malrotation of small intestine. He died of apnea. Another infant who was term and VLBW received grade B neonatal management. She had esophageal atresia and coarctation of aorta. She also died of apnea. The other infant who was term and VLBW had congenital diaphragmatic hernia. She was not diagnosed as trisomy 18 prenatally. Although her parents wished palliative care to the infant (grade C), she died of PPHN. The sample sizes were too small to compare statistically the effect of prematurity or that of birth weight to survival to discharge.
Table III.
The Survival Time in Different Groups of Combination of Prematurity and Birth Weight
| Preterm | Term | |||
|---|---|---|---|---|
| VLBW (n = 12) | Non‐VLBW (n = 3) | VLBW (n = 1) | Non‐VLBW (n = 2) | |
| Median (range) | 4 (0–274) | 1 (1–2) | 2 | 86 (0–898) |
VLBW, very‐low‐birth‐weight.
To clarify which factor has a stronger effect on the outcomes, we applied multiple logistic regression analysis to our data. We designed a model that contained survival to discharge as response variable, sex, standard deviation of birth weight, Apgar score, mechanical ventilation, any medication, any surgery, PDA dependent cardiac anomaly, congenital diaphragmatic hernia or esophageal atresia, and preterm birth as explanatory variables. The other model contained VLBW infant as explanatory variable, instead of preterm birth. A comparison with a likelihood ratio test of 34 infants (data were incomplete for two infants) revealed that the model contained preterm birth had superior fitting to explain the survival to discharge. Furthermore, we analyzed whether the survival to discharge could be affected by each of the following clinical factors: male sex, gestational age, birth weight, extremely SGA (<4 standard deviations), severe asphyxia (Apgar score at 5 min <4), PDA‐dependent congenital heart disease, congenital diaphragmatic hernia or esophageal atresia, sepsis, mechanical ventilation, any surgery, and any medications. We adopted gestational age and birth weight as continuous variables instead of preterm and VLBW‐ infants, because we had no preterm and VLBW‐infants who could survive to discharge in our population. Univariate logistic regression analysis revealed male sex, gestational age, birth weight, severe asphyxia, and mechanical ventilation were negatively associated with survival to discharge (P < 0.20). Stepwise logistic regression analysis revealed that shorter gestational age was the only independent factor to negatively affect the outcome (P = 0.02) (Table IV).
Table IV.
Parameter Affecting Survival to Hospital Discharge
| Parameter | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Gestational age (/week) | 1.53 | (1.05–2.72) | 0.02 |
CI, confidence interval.
Survival analysis using a Kaplan–Meier survival curve is shown in Figure 2. The survival rate was significantly higher in the term group than in the preterm group (P < 0.05). Similarly, the survival rate was significantly higher in the non‐VLBW group than in the VLBW group (P < 0.05).
Figure 2.
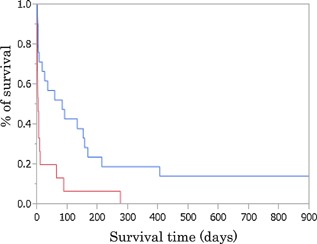
Kaplan–Meier survival curves for patients with trisomy 18 in preterm (red line) and term (blue line) groups. The survival time of the preterm group was significantly shorter than that of the term group (P < 0.05).
We also examined the causes of death in the preterm and term groups (Table V). One infant was excluded, because the cause of death was not confirmed. In both groups, the infants who died before 30 days commonly died of respiratory failure or apnea. The ratio of heart failure as the cause of death was increased in infants who survived more than 30 days compared with infants who died before 30 days.
Table V.
Causes of Death Among Different Survival Groups
| Preterm group | Term group | ||||
|---|---|---|---|---|---|
| Survival (days) | <30 (n = 12) | ≥30 (n = 3) | <30 (n = 8) | ≥30 (n = 12) | Total (n = 35) |
| Heart failure | 0 (0%) | 2 (67%) | 1 (13%) | 4 (33%) | 7 (20%) |
| Respiratory failure or apnea | 8 (75%) | 1 (33%) | 5 (63%) | 4 (33%) | 18 (51%) |
| Infection | 0 (0%) | 0 (0%) | 1 (13%) | 1 (8%) | 3 (9%) |
| Others | 4 (25%) * | 0 (0%) | 1 (13%) a | 2 (17%) b | 6 (17%) |
| Unknown | 0 (0%) | 0 (0%) | 0 (0%) | 1 (8%) | 1 (3%) |
Others include the following:
Gastrointestinal perforation (n = 2), nonreactive to resuscitation (n = 1), and persistent pulmonary hypertension of the newborn (n = 1).
Ductal shock (n = 1).
Pulmonary hypertension (n = 1) and acute pharyngitis (n = 1).
DISCUSSION
Our results show that it was difficult for infants with trisomy 18 born less than 37 weeks of gestational age to achieve survival to discharge, as the infants born at such a stage of prematurity do not have a favorable prognosis and medical treatments for neonates are still being developed. Thus, the parents of preterm infants with trisomy 18 should be informed of the potential poor outcomes.
Several reports have stated that gestational age or birth weight affects the outcome of infants with trisomy 18. Kosho et al. [2013] demonstrated that mean gestational age was older and mean birth weight was greater in infants with trisomy 18 who survived more than 1 year compared with those who died less than 1 year after birth. According to the influence of gestational age, Wu et al. [2013] reported a difference in survival duration between term and preterm infants with trisomy 18. In this report, median survival duration was 35 days for term infants, 9 days for infants born at 32–36 weeks of gestational age, and 2 days for infants born at <32 weeks of gestational age. Niedrist and Riegel [2006] also showed gestational age at delivery was a strong predictor of survival duration in such subjects. Boghossian et al. [2014] reported a 9% survival to discharge of VLBW infants with trisomy 18 using data from the Neonatal Research Network in the United States. Our findings were compatible with those of the previous reports. During the study period, there was no survival to discharge in preterm infants with trisomy 18, although they received the same management as the term infants. Because the VLBW and preterm categories overlapped in almost all infants in our population, it was difficult to distinguish the effects on outcomes of birth weight from those of gestational age. Therefore, we performed multivariate analysis using both birth weight and gestational age as some of covaluables. The result revealed that the effect of shorter gestational age was stronger than that of lower birth weight. The same as other infants, in the infants of trisomy 18, their gestational age had a great impact on the survival rate to discharge.
It is important to recognize the effect of the management plan on the prognosis. Guen et al. [2013] reported the plan of care for each patient with trisomy 18 strongly affected the patient's prognosis. Kosho et al. [2006] also reported improved survival of infants with trisomy 18 who received intensive care management. In this study, we took management into account in our comparisons between the groups. Our data showed that for trisomy18 patients, even if the same management is provided, it is more difficult for preterm infants to survive to discharge than term infants. Except for a tendency that more intensive care in neonatal management was provided to the term group than the preterm group, no significant difference was seen in either delivery room or neonatal management.
In this study, the median survival duration was 12.5 days. Survival rates at 1 week, 1 month, 3 month, and 1 year were 55.6, 44.4, 30.6, and 11.1%, respectively. These results are compatible with previous studies [Carter et al., 1985; Goldstein and Nielsen, 1985; Young et al., 1986; Root and Carey, 1994; Embleton et al., 1996; Naguib et al., 1999; Nembhard et al., 2001; Brewer et al., 2002; Rasmussen et al., 2003; Lin et al., 2006; Hsiao et al., 2009; Vendola et al., 2010; Irving et al., 2011; Guon et al., 2013; Wu et al., 2013].
Some factors related to survival have been reported in previous studies. Kosho et al. [2006] reported that being female could positively affect survival but having esophageal atresia or a prenatal diagnosis could negatively affect survival. GI anomalies, especially esophageal atresia and congenital diaphragmatic hernia, would also negatively affect outcomes in trisomy 18 infants. In the univariate analysis, these complications were not factors that contributed to the prognosis of our population. This is because our sample size and number of complications were too small to investigate the effects. Rasmussen et al. [2003] reported that congenital heart disease was also a negative factor for survival. However, except one patient in term group, all other patients had congenital heart disease in our study. In regards to cardiac surgery, only two patients in the non‐VLBW group underwent surgery. Although we do not have a clear standard of birth weight and gestational age for cardiac surgery, it is often difficult for surgeons to perform pulmonary artery banding in low‐birth‐weight infants.
The most common cause of death in infants with trisomy 18 is thought to be apnea, which is what we found for the infants who died before 30 days. However, for infants who died after 30 days, heart failure was the most common cause of death. This result indicates that the main cause of death of trisomy 18 may vary by age. Further studies are needed to clarify this idea.
In this study, there are several limitations. First, although the same explanation and discussion was provided to the families of trisomy 18 patients, for the preterm infants with trisomy 18, parents and caregivers tended to choose more limited neonatal management. As a result, many of these infants were classified into the grade B or C group. However, actual management, including cardiac surgery, GI surgery, and mechanical ventilation, was not different between the term and the preterm group. Second, because our study period was 20 years, we could not exclude the possibility of a change in therapeutic strategy over the course of the study. There have been some changes in medical management during the long study period, but we reviewed all of the treatment plans in the medical chart of each patient and confirmed that our basic policy of trisomy 18 management, which is that medical treatment decisions are made after discussion between caregivers and families, has not changed. Third, our results showed no infants in preterm group survived to discharge, which seems somewhat immoderate. Actually, other reports [Boghossian et al., 2014; Niedrist and Riegel, 2006] showed survival to discharge in preterm infants with trisomy 18. These differences could result from differences in the population of infants or their management. Finally, this is a single‐center study with a small sample size and not a population‐based study. For further investigation, a multiple‐center or population‐based study is needed to clarify how gestational age or birth weight affects the outcome of infants with trisomy 18.
CONCLUSION
In infants with trisomy 18, both preterm and VLBW‐birth negatively affect survival to discharge and survival duration. Gestational age has more strongly impact than birth weight to survival to hospital discharge in such infants. For the infants who survived over 30 days, heart failure was increased as a cause of death, added to respiratory failure or apnea in both preterm and term infants.
ACKNOWLEDGMENT
The authors thank Yumi Nakagawa for assistance in collecting clinical data.
Imai K, Uchiyama A, Okamura T, Ago M, Suenaga H, Sugita E, Ono H, Shuri K, Masumoto K, Totsu S, Nakanishi H, Kusuda S. 2015. Differences in mortality and morbidity according to gestational ages and birth weights in infants with trisomy 18. Am J Med Genet Part A 167A:2610–2617.
Conflict of interest: none.
REFERENCES
- Boghossian NS, Hansen NI, Bell EF, Stoll BJ, Murray JC, Carey JC, Adams‐Chapman I, Shankaran S, Walsh MC, Laptook AR, Faix RG, Newman NS, Hale EC, Das A, Wilson LD, Hensman AM, Grisby C, Collins MV, Vasil DM, Finkle J, Maffett D, Ball MB, Lacy CB, Bara R, Higgins RD. 2014. Mortality and morbidity of VLBW infants with trisomy 13 or trisomy 18. Pediatrics 133:226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer CM, Holloway SH, Stone DH, Carothers AD, FitzPatrick DR. 2002. Survival in trisomy 13 and trisomy 18 cases ascertained from population based registers. J Med Genet 39:e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey JC. 2012. Perspectives on the care and management of infants with trisomy 18 and trisomy 13: Striving for balance. Curr Opin Pediatr 24:672–678. [DOI] [PubMed] [Google Scholar]
- Carter P, Pearn J, Bell J, Martin N, Anderson N. 1985. Survival in trisomy 18. Clin Genet 27:59–61. [DOI] [PubMed] [Google Scholar]
- Edwards JH, Harnden DG, Cameron AH, Cross BM, Wolff OH. 1960. A new trisomic syndrome. Lancet 1:787. [DOI] [PubMed] [Google Scholar]
- Embleton ND, Wyllie JP, Wright MJ, Burn J, Hunter S. 1996. Natural history of trisomy 18. Arch Dis Child Fetal Neonatal Ed 75:F38–F41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein H, Nielsen KG. 1985. Rates and survival of individuals with trisomy 13 and 18. Clin Genet 27:59–61. [DOI] [PubMed] [Google Scholar]
- Guon J, Wilfond BS, Farlow B, Brazg T, Janvier A. 2013. Our children are not a diagnosis: The experience of parents who continue their pregnancy after a prenatal diagnosis of trisomy 13 or 18. Am J Med Genet Part A 164A:308–318. [DOI] [PubMed] [Google Scholar]
- Hsiao CC, Tsao LY, Chen HN, Chiu HY, Chang WC. 2009. Changing clinical presentations and survival pattern in trisomy 18. Pediatr Neonatal 50:147–151. [DOI] [PubMed] [Google Scholar]
- Irving C, Richmond S, Wren C, Longster C, Embleton ND. 2011. Changes in fetal prevalence and outcome for trisomies 13 and 18: A population‐based study over 23 years. J Matern Fetal Neonatal Med 24:137–141. [DOI] [PubMed] [Google Scholar]
- Itabashi K, Fujimura M, Kusuda S, Tamura M, Hayashi T, Takahashi T, Goishi K, Futamura M, Takahashi Y, Isobe K, Iida K, Uetani Y, Kondo Y, Shirahata S, Sugiura M, Takahashi N, Funado M, Horiuchi A, Yamaguchi K. 2010. Introduction of new standard values according to gestation age. Japan Pediatr Soc 114:1271–1293. [Google Scholar]
- Jones KL. 2006. Trisomy 18 syndrome. In: Smith's recognizable patterns of human malformation, 6e. Philadelphia: WB Saunders, p 13–17. [Google Scholar]
- Kosho T, Nakamura T, Kawame H, Baba A, Tamura M, Fukushima Y. 2006. Neonatal management of trisomy 18: Clinical details of 24 patients receiving intensive treatment. Am J Med Genet Part A 140A:937–944. [DOI] [PubMed] [Google Scholar]
- Kosho T, Kuniba H, Tanikawa Y, Hashimoto Y, Sakurai H. 2013. Natural history and parental experience of children with trisomy 18 based on a questionnaire given to a Japanese trisomy 18 parental support group. Am J Med Genet Part A 161A:1531–1542. [DOI] [PubMed] [Google Scholar]
- Lin HY, Lin SP, Chen YJ, Hung HY, Kao HA, Hsu CH, Chen MR, Chang JH, Ho CS, Huang FY, Shyur SD, Lin DS, Lee HC. 2006. Clinical characteristics and survival of trisomy 18 in a medical center in Taipei, 1988–2004. Am J Med Genet Part A 140A:945–951. [DOI] [PubMed] [Google Scholar]
- McGraw MP, Periman JM. 2008. Attitudes of neonatologists toward delivery room management of confirmed trisomy 18: Potential factors influencing a changing dynamic. Pediatrics 121:1106–1110. [DOI] [PubMed] [Google Scholar]
- Naguib KK, Al‐Awadi SA, Moussa MA, Bastaki L, Gouda S, Redha MA, Mustafa F, Tayel SM, Abulhassan SA, Murthy DS. 1999. Trisomy 18 in Kuwait. Int J Epidemiol 28:711–716. [DOI] [PubMed] [Google Scholar]
- Nembhard WN, Waller DK, Sever LE, Canfield MA. 2001. Patterns of first‐year survival among infants with selected congenital anomalies in Texas, 1995–1997. Teratology 64:267–275. [DOI] [PubMed] [Google Scholar]
- Niedrist D, Riegel M. 2006. Survival with trisomy 18‐data from Switzerland. Am J Med Genet Part A 959A:952–959. [DOI] [PubMed] [Google Scholar]
- Rasmussen SA, Wong LYC, Yang QY, May KM, Friedman JM. 2003. Population‐based analysis of mortality in trisomy 13 and trisomy 18. Pediatrics 111:777–784. [DOI] [PubMed] [Google Scholar]
- Root S, Carey JC. 1994. Survival in trisomy 18. Am J Med Genet 49:170–174. [DOI] [PubMed] [Google Scholar]
- Vendola C, Canfield M, Daiger SP, Gambello M, Hashmi SS, King T, Noblin SJ, Waller DK, Hecht JT. 2010. Survival of Texas infants born with trisomies 21, 18, and 13. Am J Med Genet Part A 152A:360–366. [DOI] [PubMed] [Google Scholar]
- Wu J, Springett A, Morris JK. 2013. Survival of trisomy 18 (Edwards syndrome) and trisomy 13 (Patau syndrome) in England and Wales: 2004–2011. Am J Med Genet Part A 161A:2512–2518. [DOI] [PubMed] [Google Scholar]
- Young ID, Cook JP, Mehta L. 1986. Changing demography of trisomy 18. Arch Dis Child 61:1035–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]


