Abstract
The incretin hormone, glucagon‐like peptide 1 (GLP‐1), regulates gastric emptying, glucose‐dependent stimulation of insulin secretion and glucagon release, and GLP‐1 analogs are therefore approved for treatment of type II diabetes. GLP‐1 receptors are expressed in reward‐related areas such as the ventral tegmental area and nucleus accumbens, and GLP‐1 was recently shown to regulate several alcohol‐mediated behaviors as well as amphetamine‐induced, cocaine‐induced and nicotine‐induced reward. The present series of experiments were undertaken to investigate the effect of the GLP‐1 receptor agonist, liraglutide, on several alcohol‐related behaviors in rats that model different aspects of alcohol use disorder in humans. Acute liraglutide treatment suppressed the well‐documented effects of alcohol on the mesolimbic dopamine system, namely alcohol‐induced accumbal dopamine release and conditioned place preference in mice. In addition, acute administration of liraglutide prevented the alcohol deprivation effect and reduced alcohol intake in outbred rats, while repeated treatment of liraglutide decreased alcohol intake in outbred rats as well as reduced operant self‐administration of alcohol in selectively bred Sardinian alcohol‐preferring rats. Collectively, these data suggest that GLP‐1 receptor agonists could be tested for treatment of alcohol dependence in humans.
Keywords: Addictive behaviours, dependence, reward
Introduction
The incretin hormone, glucagon‐like peptide 1 (GLP‐1), is released into the circulation from the distal small intestine in response to food intake (Brubaker & Anini 2003) and acts thereby as a signal for meal termination (Pannacciulli et al. 2007). The findings that its biological effects include inhibition of gastric emptying (Flint et al. 2001), glucose‐dependent stimulation of insulin secretion (Kreymann et al. 1987) and suppression of glucagon release (Orskov et al. 1988) lead to the approval of GLP‐1 receptor agonists for treatment of type II diabetes(for review, see Holst 2004). Experimental evidence from animals (Tang‐Christensen et al. 1996), healthy subjects (Flint et al. 1998) and patients with type II diabetes (Gutzwiller et al. 1999) shows that circulating GLP‐1 reduces food intake and promotes satiety. Along these lines of investigations, GLP‐1 receptor agonists were found to reduce body weight when administered subcutaneously in humans (Zander et al. 2002) and intracerebroventricularly in animals (Meeran et al. 1999). GLP‐1 regulates food intake via GLP‐1 receptors in the nucleus tractus solitarius (NTS), amygdala and the hypothalamus (Tang‐Christensen et al. 1996; McMahon & Wellman 1998; Hayes et al. 2009). In addition to areas regulating homeostatic feeding, the expression of GLP‐1 receptors is found in reward‐related areas including the ventral tegmental area (VTA) and nucleus accumbens (NAc) (Alvarez et al. 1996; Merchenthaler et al. 1999). Taken together with the findings that both the VTA and NAc are targeted by GLP‐1‐producing neurons from the NTS (Alvarez et al. 1996; Merchenthaler et al. 1999), this provides a pathway through which GLP‐1 may regulate reinforcement.
In contrast to the common view of GLP‐1 in controlling food intake and glucose homeostasis, recent studies pinpoint GLP‐1 as a reward regulator (for review see, Engel & Jerlhag 2014). This was initially reported in a study showing that acute and peripheral administration of the GLP‐1 receptor agonist exendin‐4 (Ex4) blocks the alcohol‐induced conditioned place preference (CPP), locomotor stimulation and accumbal dopamine release in mice (Egecioglu et al. 2013c). Moreover, Ex4 treatment decreased alcohol intake in the intermittent access model as well as alcohol‐seeking behavior, using the progressive ratio schedule of reinforcement, in rats (Egecioglu et al. 2013c). In accordance are the data showing that GLP‐1 as well as Ex4 decreases, whereas GLP‐1 receptor antagonist increases alcohol intake in rats (Shirazi et al. 2013). Ex4 attenuates alcohol‐induced CPP, and GLP‐1‐controlled alcohol intake involves the VTA in rats (Shirazi et al. 2013). Opposed to Ex4, which is metabolized rapidly and extensively (Deacon et al. 1995), the clinically available GLP‐1 receptor agonist, liraglutide, has protracted and maintained biological activity (for review see, Holst 2004). The present series of experiments were undertaken to investigate the effect of liraglutide on several alcohol‐related behaviors in rats that model different aspects of alcohol use disorder (AUD) in humans. Initially, we investigated the effects of acute liraglutide treatment on the rewarding properties of alcohol, as measured by accumbal dopamine release and CPP as well as on blood alcohol concentrations in mice. Thereafter, the ability of acute treatment of liraglutide to influence alcohol intake and relapse‐like drinking in outbred rats were explored. Finally, the effects of repeated liraglutide treatment on alcohol intake in outbred rats as well as on operant and oral self‐administration of alcohol in selectively bred Sardinian alcohol‐preferring (sP) rats were studied. The present experiments, by means of these preclinical models, could therefore elucidate the possibility to use liraglutide as treatment of AUD in humans.
Material and Methods
For further details of animals, experimental protocols and statistic analysis, see Supporting Information.
In vivo microdialysis and dopamine release measurements
For measurements of extracellular dopamine levels, mice were implanted with a microdialysis probe positioned in the NAc shell as described previously (Jerlhag et al. 2006). In brief, mice were anesthetized with isoflurane (Isoflurane Baxter, Univentor 400 Anaesthesia Unit, Univentor Ltd., Zejtun, Malta), placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA), and one hole for the probe and one for the anchoring screw were drilled. Xylocaine Adrenalin (5 µg/ml; Pfizer Inic, New York, NY, USA) was used as local anesthetics and carprofen (Rimadyl®) (Astra Zeneca, Gothenburg, Sweden) at a dose of 5 mg/kg intraperitoneal (ip) to relieve pain. The probe was randomly alternated to either the left or right side of the brain. The coordinates of 1.4 mm anterior to the bregma, ±0.6 lateral to the midline and 4.7 mm below the surface of the brain surface were used (Franklin & Paxinos 1997).
The effect of systemic administration of liraglutide [0.1 mg/kg, subcutaneous (sc)] on alcohol‐induced (1.75 g/kg, ip) accumbal dopamine release was investigated using microdialysis in freely moving mice. On the day of the experiment, the probe was connected to a microperfusion pump (U‐864 Syringe Pump, AgnThós AB, Lidingö, Sweden) and perfused with Ringer solution at a rate of 1.5 µl/minute. After 1 hour of habituation to the microdialysis setup, perfusion samples were collected every 20 minutes. The baseline dopamine level was defined as the average of three consecutive samples before the first alcohol or vehicle (saline, ip) challenge (time 0). This initial alcohol‐challenge was given to establish that all mice included in the experiment would respond with an alcohol‐induced release of accumbal dopamine. The challenge‐induced increase in accumbal dopamine was calculated as the percent increase from baseline. Seven consecutive 20‐minute samples were collected after the initial challenge. At 140 minutes, the mice were injected with liraglutide (0.1 mg/kg, sc) or vehicle (second challenge), and 60 minutes later, vehicle or a second injection of alcohol (1.75 g/kg, ip) was administered (third challenge; 200 minutes) and followed by collection of four 20‐minute samples (experiment terminated at 280 min). Collectively, the following treatment groups (n = 12 in each group) were created: alcohol–vehicle–alcohol (veh–alc), alcohol–liraglutide–alcohol (lir–alc) and vehicle–liraglutide–vehicle (lir–veh). [Correction added on 28 September 2015 after the first online publication: the word ‘in‐veh’ has been changed to ‘lir‐veh’.]
Dopamine was separated and quantified using two different high‐performance liquid chromatography with electrochemical detection as described previously (Clarke et al. 2014). In brief, a pump (UltiMate 3000 Pump, Thermo Scientific, Darmstadt, Germany), an ion exchange column (Nucleosil SA, 2.0 × 150 mm, 5 µm diameter, pore size 100 Å; Phenomenex Scandinavia, Västra Frölunda, Sweden) and a detector (Decade, Kovalent AB, Sweden) operated at 400 mV versus the cell were used. The mobile phase was delivered at 0.3 ml/min and consists of 58 mM citric acid, 135 mM NaOH, 0.107 mM Na2–EDTA and 20 percent methanol. The second system consists of a pump (UltiMate 3000 Pump, Thermo Scientific), a reversed‐phase column (2.0 × 50 mm, 3 µm diameter; pore size 100 Å; Phenomenex Scandinavia) and a detector (Dionex, Västra Frölunda, Sweden) operated at 220 mV versus the cell. The mobile phase was delivered at 0.3 ml/min and consists of 150 mM NaH2PO4, 4.76 mM citric acid, 3 mM sodium dodecyl sulfate, 50 μM EDTA, as well as 10 percent MeOH and 15 percent acetonitrile.
The exact position of the probe was verified by gross observation using light microscopy (Franklin & Paxinos 1997), and only mice with correct placements were used in the statistical analysis.
Conditioned place preference
To evaluate the effects of liraglutide on the rewarding effects of alcohol as well as memory consolidation of a reward, two distinct CPP tests were performed in mice as previously described (Jerlhag et al. 2009). The procedure consisted of preconditioning (day 1), conditioning (days 2–5) and post‐conditioning (day 6).
The first CPP test was designed to investigate the effect of liraglutide on the rewarding properties of alcohol. Mice at preconditioning were placed in the chamber with free access to both compartments during 20 minutes to determine the initial place preference. Conditioning (20 minutes per session) was performed using a biased procedure in which alcohol (1.75 g/kg, ip) was paired with the least preferred compartment and vehicle with the preferred compartment. In these experiments, liraglutide (0.1 mg/kg, sc) or vehicle was administered 60 minutes prior to the alcohol injection on each of the four conditioning days (creating the following treatment groups: veh–alc or lir–alc). At post‐conditioning, the mice were untreated and were placed on the midline between the two compartments with free access to both compartments for 20 minutes. In a control experiment for liraglutide, separate mice were subjected to the same procedure but received vehicle injections instead of alcohol throughout the conditioning (non‐alcohol‐conditioned control group; creating the following treatment groups: vehicle–vehicle and liraglutide–vehicle).
The second experiment in separate mice was designed to investigate the effect of liraglutide on memory consolidation of a reward. In this experiment, mice during preconditioning were injected sc with vehicle and were placed in the chamber with free access to both compartments during 20 minutes to determine the initial place preference. Conditioning (20 minutes per session) was performed using a biased procedure in which alcohol (1.75 g/kg, ip) was paired with the least preferred compartment and vehicle with the preferred compartment. All mice received one alcohol and one vehicle injection every day, and the injections were altered between morning and afternoon in a balanced design. At post‐conditioning, mice were injected with liraglutide (0.1 mg/kg, sc) or an equal volume of vehicle solution and 60 minutes later, placed on the midline between the two compartments with free access to both compartments for 20 minutes (creating the following treatment groups: alc–veh and alc–lir).
Conditioned place preference was calculated as the difference in percent of total time spent in the drug‐paired (i.e. less preferred) compartment during the post‐conditioning and the preconditioning sessions.
Blood alcohol concentration
The mice received an acute injection of liraglutide (0.1 mg/kg, sc) or an equal volume of vehicle. Sixty minutes later all mice were injected with alcohol (1.75 g/kg, ip). The mice were decapitated 20 minutes later and trunk blood was collected in micro tubes (Vacuette; Greiner Bio‐one, Florence, Italy). The analysis of the blood alcohol concentration from experiment one and two was outsourced to Sahlghrenska University Hospital (Gothenburg, Sweden; study agreement BML‐NEURO).
Intermittent access 20 percent alcohol two‐bottle‐choice drinking paradigm
Ten to twelve weeks of intermittent access 20 percent alcohol two‐bottle‐choice drinking paradigm induces voluntary intake of high amounts of alcohol (Wise 1973; Simms et al. 2008; Loi et al. 2010) and pharmacological relevant blood alcohol concentrations (Simms et al. 2008; Carnicella et al. 2009) in rodents. In addition, the expression of ghrelin receptors, which mediate alcohol reinforcement, is increased in reward‐related areas in high‐alcohol‐consuming compared with low‐alcohol‐consuming rats following 10 weeks of voluntary alcohol intake (Landgren et al. 2011). In brief, the rats were given free access to one bottle of 20 percent alcohol and one bottle of water during three 24‐hour sessions per week (Mondays, Wednesdays and Fridays). The rats had unlimited access to two bottles of water between the alcohol‐access periods. Bottles were weighed at 24 hours after the fluids were presented to the rats. The body weight of each rat was measured daily prior to bottle presentation, to allow for calculating the grams of alcohol intake per kilogram of body weight (g/kg). The preference for alcohol over water (the ratio of alcohol to total fluid intake) was calculated at all time points. In addition, water and food intake was measured. Three separate drinking experiments in outbred rats were conducted after a period of 10–12 weeks of intermittent access to alcohol.
Effects of acute treatment of liraglutide on alcohol intake in outbred rats
In the first drinking experiment, the effects of acute administration of liraglutide (0.1 mg/kg, sc, n = 7) or vehicle (n = 8) on alcohol, water and food intake as well as body weight in outbred Wistar rats (n = 15) that had voluntarily consumed 20 percent alcohol for 12 weeks were investigated. The injection was given 60 minutes before the rats were given access to alcohol and water, and the intakes were measured 24 hours following treatment. Thereafter, these rats were repeatedly administered with liraglutide (0.1 mg/kg, sc) or vehicle for another 7 days, allowing us to investigate the effects of repeated liraglutide treatment on the daily alcohol intake (in the succeeding text).
In a separate experiment, the effects of acute administration of liraglutide (0.05 mg/kg) or vehicle on alcohol, water and food intake as well as body weight were evaluated in outbred Wistar rats (n = 28) that had voluntarily consumed 20 percent alcohol for 12 weeks. Based on the baseline alcohol consumption (cut‐off 2.5 g/kg), outbred rats were divided into high‐alcohol‐consuming and low‐alcohol‐consuming rats.
Effects of acute liraglutide treatment on the alcohol deprivation effect in outbred rats
The alcohol deprivation model is based on the observation that voluntary alcohol intake will increase temporarily when compared with baseline drinking conditions following forced abstinence in alcohol‐experienced rats (Spanagel 2000). Rats (n = 11) were subjected to the intermittent access 20 percent alcohol two‐bottle‐choice drinking paradigm (as described earlier) for 10 weeks, and a stable baseline alcohol intake (g/kg/day) was obtained. Alcohol, water and food intake was measured at 1 and 24 hours during baseline as well as following drug treatment. The rats were deprived of alcohol for 10 days, and alcohol was thereafter reintroduced. Sixty minutes before the reintroduction of alcohol, the rats were treated with either liraglutide (0.1 mg/kg, sc, n = 5) or vehicle (n = 6) in a balanced design. Thereafter, bottles and food were weighed at 24 hours after the fluids were presented.
Effects of repeated treatment of liraglutide on alcohol intake using the intermittent access model in outbred rats
Following acute sc treatment of 0.1 mg/kg liraglutide, these outbred Wistar rats (n = 15) were subjected to an additional 7 days of liraglutide (0.1 mg/kg, sc) (n = 7) or vehicle (n = 8) treatment. The effect of repeated administration of liraglutide on the daily alcohol, water, total fluid and food intake was investigated at test session 1 (Monday), test session 2 (Wednesday) and at test session 3 (Friday), which correspond to alcohol drinking days in the intermittent access model. In addition to the alcohol consumption days, the rats received treatment on water consumption days, i.e. Saturday, Sunday, Tuesday and Thursday. All injections were given 60 minutes before the rats were given access to alcohol and water. In addition, water intake was measured the three following days after discontinuation of the liraglutide treatment.
Operant alcohol self‐administration in selectively bred alcohol‐preferring sP rats
Self‐administration sessions were conducted in modular chambers (Med Associates, St. Albans, VT, USA) as described previously (Maccioni et al. 2012) (for detailed information, see Supporting Information).
Effects of repeated liraglutide treatment on operant alcohol self‐administration in selectively bred alcohol‐preferring sP rats
Test sessions started immediately after termination of the 20‐day maintenance phase. In test sessions, response requirement on the alcohol and water lever was kept at FR4 and FR1, respectively. Test sessions lasted for 30 minute and were conducted for 5 consecutive days. Vehicle or liraglutide, at the doses of 0.05, and 0.1 mg/kg, was administered sc to independent groups of n = 9 rats. After completion of the treatment phase, rats were exposed to four additional daily self‐administration sessions (posttreatment phase). Sessions of the treatment and posttreatment phases were conducted with no weekend interruption.
Results
Liraglutide attenuates alcohol‐induced accumbal dopamine release and CPP, but does not change the blood alcohol concentration in mice
Accumbal microdialysis measurements of dopamine in mice revealed an overall main effect of treatment [F(16, 187) = 1.98, P = 0.0162], time [F(32, 374) = 92.35, P < 0.0001] and a significant interaction of treatment × time [F(32, 374) = 2.236, P = 0.0002]. In the first part of the experiment, the responsiveness to alcohol (1.75 g/kg) per se was investigated (alcohol injection at time point 0 minute). Initial injections of alcohol caused a significant increase in accumbal dopamine release compared with vehicle treatment in both groups treated with alcohol (veh–alc and lir–alc). Specifically, in the veh–alc group, alcohol increased accumbal dopamine at time points 40 (P < 0.01), 60–100 (P < 0.001) and 120–140 minutes (P < 0.01). In addition, alcohol increased NAc dopamine in the lir–alc group at time points 60 and 1000 minutes (P < 0.01) and 120 minutes (P < 0.05) (Fig. 1a). In the subsequent part of the experiment, administration of liraglutide (0.1 mg/kg, at 160 minutes) 60 minutes prior to the second alcohol injection (1.75 g/kg, at 200 minutes) significantly attenuated the alcohol‐induced accumbal dopamine release (alc–lir–alc) compared with vehicle pretreatment (alc–veh–alc) at time points 200 (P < 0.05) and 240–280 minutes (P < 0.01) (Fig. 1a).
Figure 1.
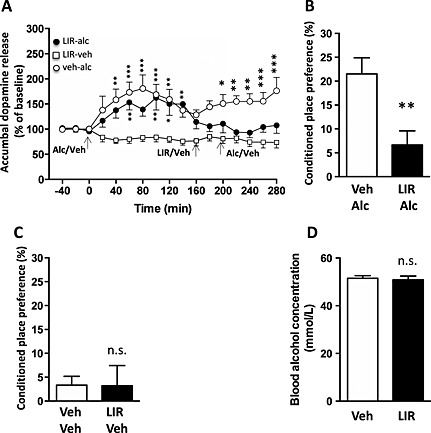
Acute administration of liraglutide attenuates accumbal dopamine release and conditioned place preference (CPP) but does not alter blood alcohol concentrations in mice. (a) Initial injections of alcohol (1.75 g/kg) caused a significant increase in accumbal dopamine release compared with vehicle treatment liraglutide–vehicle (lir–veh) in the alcohol–vehicle–alcohol (veh–alc) and alcohol–liraglutide–alcohol (lir–alc) group. In the subsequent part of the experiment, administration of liraglutide (0.1 mg/kg, at 160 minutes) 60 minutes prior to the second alcohol injection (at 200 minutes) significantly attenuated the alcohol‐induced accumbal dopamine release (lir–alc) compared with vehicle pretreatment (veh–alc). (b) The alcohol‐induced (1.75 g/kg) (veh–alc) CPP was significantly attenuated by concomitant injection of liraglutide (0.1 mg/kg) (lir–alc) on each conditioning day compared with vehicle injection. (c) The control experiment showed that there was no difference between lir–veh (0.1 mg/kg) and vehicle–vehicle treatment on CPP. (d) Compared with vehicle injection, acute treatment of liraglutide (0.1 mg/kg) did not affect the blood alcohol concentrations induced by a peripheral injection of alcohol (1.75 g/kg). Data are presented as mean (g/kg) ± SEM (*P < 0.05, **P < 0.01, ***P < 0.001, n.s. P > 0.05). [Correction added on 28 September 2015 after the first online publication: the legends of Figure 1a has been updated]
During a drug‐free test session (post‐conditioning), alcohol‐induced (1.75 kg/kg) (veh‐alc) CPP was significantly attenuated by concomitant injection of liraglutide (0.1 mg/kg) (lir–alc) on each conditioning day compared with vehicle injection (P = 0.0065, n = 7 in each group; Fig. 1b). The control experiment showed that there was no difference between liraglutide–vehicle and vehicle–vehicle treatment on CPP (P = 0.9788, n = 8 in each group; Fig. 1c) during the drug‐free test session (post‐conditioning). However, the alcohol‐induced (1.75 kg/kg) (alc–veh, 12 ± 6 percent) CPP was not affected by an acute single injection of liraglutide (0.1 mg/kg) (alc–lir, 14 ± 7 percent) on the post‐conditioning day compared with vehicle injection (P = 0.7903, n = 8 in each group).
Acute administration of liraglutide (n = 7) did not alter the blood alcohol concentration induced by an injection of alcohol (1.75 g/kg, ip) in mice compared with vehicle‐treated (n = 7) mice (P = 0.7288) (Fig. 1d).
Acute treatment of liraglutide decreases alcohol intake in outbred rats
The effect of acute liraglutide (0.1 mg/kg, sc) treatment on alcohol intake was investigated in a group of rats (n = 15). After 12 weeks of intermittent alcohol intake, there was no significant difference in 24‐hour baseline consumption of alcohol intake (vehicle 4.0 ± 0.8 g/kg, n = 8; liraglutide 3.4 ± 0.7 g/kg, n = 7; P = 0.5889) between the two groups of rats. Liraglutide treatment significantly reduced alcohol intake (g/kg) compared with vehicle (Fig. 2a, P < 0.01), alcohol preference (Fig. 2d, P < 0.05) as well as food intake (Fig. 2e, P < 0.001). There was a tendency in increased water intake (ml) by liraglutide (Fig. 2b, P = 0.0936), but there was no effect on total fluid intake (ml) (Fig. 2c P = 0.6119) or on body weight (g) (Fig. 2f, P = 0.3901).
Figure 2.
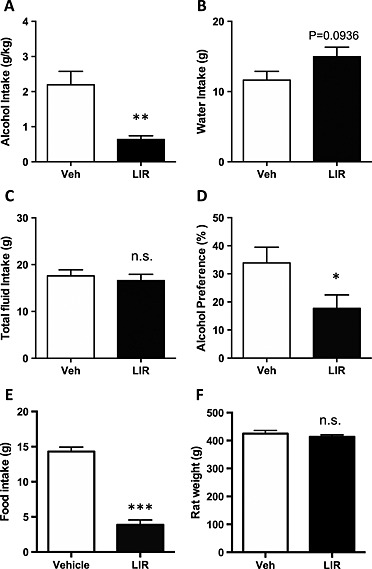
Acute administration of liraglutide (lir) (0.1 mg/kg) decreases alcohol intake in high‐alcohol‐consuming outbred rats. (a) Acute administration of liraglutide (0.1 mg/kg) reduced alcohol intake (g/kg) in outbred rats. Liraglutide had a tendency of increasing water intake (g) (b) and had no effect on total fluid intake (g) (c). Liraglutide reduced alcohol preference ( percent) (d) as well as food intake (g) (e) and did not affect body weight (g) (f). All values represent mean ± SEM (*P < 0.05, **P < 0.01, ***P < 0.001, n.s. P > 0.05). Veh, vehicle
Acute treatment of liraglutide decreases alcohol intake in outbred rats
The rats were divided into high‐alcohol‐consuming and low‐alcohol‐consuming rats based on baseline alcohol intake. There was no difference (P = 0.7635) in baseline alcohol intake (g/kg/24 hours) in high‐alcohol‐consuming rats later treated with vehicle (3.9 ± 0.2, n = 9) or liraglutide (4.0 ± 0.2, n = 10). Liraglutide (0.05 /kg, sc) significantly decreased alcohol intake (g/kg) (Fig. 3a, P = 0.0056) compared with vehicle treatment in these rats. There were no differences in water intake (g, Fig. 3b, P = 0.5891), total fluid intake (g, Fig. 3c, P = 0.0878) or preference (percent, Fig. 3d, P = 0.1936) between liraglutide and vehicle treatments. Liraglutide decreased food intake (g, Fig. 3e, P = 0.0084), but there was no effect on body weight (g, Fig. 3f, P = 0.4022) compared with vehicle treatment.
Figure 3.
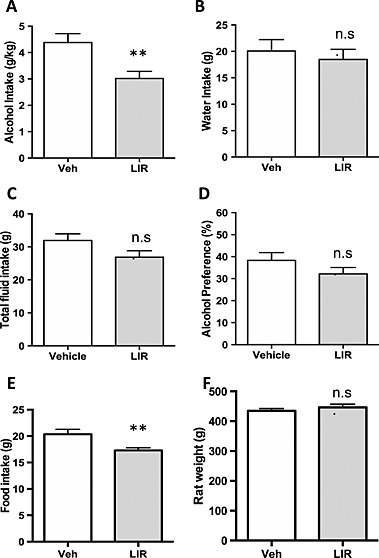
Acute administration of liraglutide (lir) (0.05 mg/kg) decreases alcohol intake in high‐alcohol‐consuming outbred rats. (a) Acute administration of liraglutide (0.05 mg/kg) reduced alcohol intake (g/kg) in high‐alcohol‐consuming outbred rats. (b) Liraglutide had no effect on water intake (g), (c) total fluid intake (g) or (d) preference ( percent). (e) Liraglutide reduced food intake (g) and (f) had no effect on body weight (g). All values represent mean ± SEM (**P < 0.01, n.s. P > 0.05). Veh, vehicle
There was no difference (P = 0.3235) in baseline alcohol intake (g/kg/24 hours) in low‐alcohol‐consuming rats later treated with vehicle (1.7 ± 0.3, n = 5) or liraglutide (2.2 ± 0.4, n = 4). In these low‐alcohol‐consuming rats, liraglutide (0.05 mg/kg) had no significant effect on alcohol intake (g/kg, P = 0.1100), water intake (g, P = 0.3886), total liquid intake (g, P = 0.1016), preference (percent, P = 0.5405), food intake (g, P = 0.8597) or body weight (g, P = 0.2614) compared with vehicle treatment (data not shown).
Acute treatment with the GLP‐1 receptor agonist liraglutide prevents the alcohol deprivation effect and reduces alcohol intake in outbred rats
The effect of acute liraglutide treatment on relapse‐like drinking in the alcohol deprivation paradigm was investigated in a separate group of rats (n = 11). After 10 weeks of intermittent alcohol intake, there was no significant difference in 24‐hour baseline consumption of alcohol (vehicle 2.3 ± 0.3 g/kg; liraglutide 2.8 ± 0.2 g/kg; P = 0.2066) intake between the two groups of rats.
After 10 days of forced alcohol abstinence, the rats were treated with liraglutide (n = 5) or vehicle (n = 6) 60 minutes before given access to one bottle of water and one bottle of 20 percent alcohol. After 24 hours of alcohol access, there was an overall main effect of treatment [F(1, 9) = 20.44, P = 0.0014] and an significant interaction of treatment × time [F(1, 9) = 11.39, P = 0.0082]. However, no effect of time was observed [F(1, 9) = 1.247, P = 0.2930]. Post hoc analysis revealed a significant alcohol deprivation effect (i.e. significant increase in alcohol intake compared with respective baseline) in vehicle‐treated (P < 0.05) but not in liraglutide‐treated (P > 0.05) rats and that the alcohol intake was significantly higher in vehicle‐treated compared with liraglutide‐treated rats (P < 0.001) (Fig. 4). In addition, the liraglutide treatment significantly reduced alcohol intake (g/kg) (vehicle 2.6 ± 0.4; liraglutide 0.6 ± 0.1; P < 0.01) and alcohol preference (vehicle 41 ± 7; liraglutide 12 ± 3; P < 0.01) compared with vehicle. No effect on water (ml) (vehicle 14 ± 5; liraglutide 17 ± 6; P = 0.6180), total fluid (ml) (vehicle 21 ± 4; liraglutide 19 ± 6; P = 0.7530) nor on food (vehicle 13 ± 3; liraglutide 7 ± 2; P = 0.1941) intake was observed.
Figure 4.
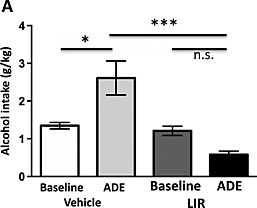
Acute administration of liraglutide (lir) prevents the alcohol deprivation effect in outbred rats. There was an alcohol deprivation effect (ADE) in vehicle‐treated rats but not in rats treated with lir compared with corresponding baseline. The alcohol intake was higher in vehicle‐treated rats compared with liraglutide treated rats. All values represent mean ± SEM (*P < 0.05, ***P < 0.001, n.s. P > 0.05)
Repeated treatment of liraglutide reduces alcohol intake in outbred rats
When liraglutide (0.1 mg/kg) was given repeatedly, in total for 8 days from which three are alcohol drinking days, there was an overall main effect of treatment [F(1, 13) = 5.838, P = 0.031] and of time [F(2, 26) = 4.642, P = 0.019], and a tendency for the interaction of treatment × time [F(2, 26) = 2.912, P = 0.072] on alcohol intake. Post hoc test revealed that liraglutide significantly reduced alcohol intake at test session 1 (P < 0.05) and test session 2 (P < 0.05), but not at test session 3 (Fig. 5a). There was an overall effect on water intake following repeated liraglutide treatment [F(1, 13) = 11.77, P = 0.0045]. There was no overall effect of time [F(2, 26) = 0.4355, P = 0.6516] nor of treatment × time interaction [F(2, 26) = 1.555, P = 0.2301]. Post hoc test revealed that liraglutide significantly increased water intake at test session 1 (P < 0.01) and test session 3 (P < 0.01), but not at test session 2 (Fig. 5b). There was an overall effect on total fluid intake of treatment × time interaction [F(2, 26) = 3.752, P = 0.0370] and tendency of treatment effect [F(1, 13) = 3.894, P = 0.0701]. There was no overall effect of time [F(2, 26) = 0.7541, P = 0.4804]. Post hoc test revealed that liraglutide significantly increased total fluid intake at test session 3 (P < 0.05), but not at test session 1 and test session 2 (Fig. 5c). There was an overall effect on alcohol preference of treatment [F(1, 13) = 11.40, P = 0.0050]. There was no overall effect of time [F(2, 26) = 0.1893, P = 0.8286] and of treatment × time interaction [F(2, 26) = 1.312, P = 0.2865]. Post hoc test revealed that liraglutide significantly decreased alcohol preference at test session 1 (P < 0.01) and test session 2 (P < 0.01), but not at test session 3 (Fig. 5d). There was an overall main effect of treatment [F(1, 13) = 38.36, P < 0.0001], but not of time [F(2, 26) = 1.931, P = 0.1652], nor of the treatment × time interaction [F(2, 26) = 0.9202, P = 0.4110] on food intake. Post hoc test revealed that liraglutide significantly reduced food intake at test session 1 (P < 0.001), test session 2 (P < 0.001) and test session 3 (P < 0.05) (Fig. 5e). There was an overall main effect of treatment [F(1, 13) = 5.625, P = 0.0338], but not of time [F(2, 26) = 0.4649, P = 0.6333], nor of treatment × time interaction [F(2, 26) = 0.9091, P = 0.4153] on body weight. Post hoc test revealed that liraglutide did not reduce body weight at any treatment day (Fig. 5f).
Figure 5.
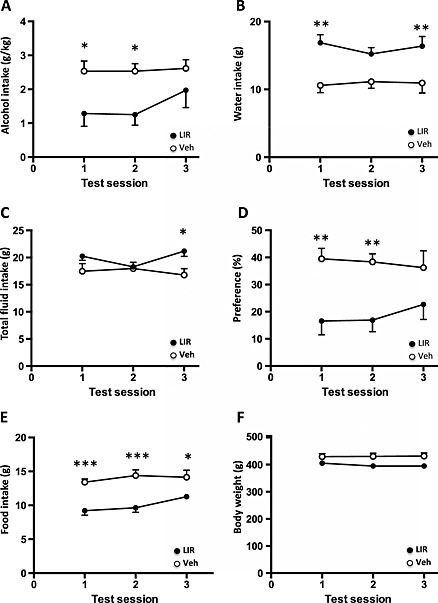
Repeated administration of a low dose of liraglutide decreases alcohol intake in outbred rats. (a) Compared with vehicle (veh) treatment (unfilled circle), repeated liraglutide (lir) (filled circle) reduced alcohol intake (g/kg) at test sessions 1 and 2, (b) increased water intake (g) at test sessions 1 and 3 (c), increased total fluid intake (g) at test session 3 (d) and decreased preference for alcohol ( percent) at test sessions 1 and 2. (e) Repeated liraglutide treatment reduced food intake at test sessions 1, 2 and 3, (e) and there was an overall effect on body weight by repeated liraglutide treatment, but not any specific test session. All values represent mean ± SEM (*P < 0.05, **P < 0.01, ***P < 0.001). Test session represents the alcohol consumption days
Following discontinuation of liraglutide treatment, there was a tendency to an overall effect on water intake of treatment [F(1, 13) = 3.29, P = 0.0928], or of treatment × time interaction [F(2, 26) = 2.932, P = 0.0711]. There was an overall effect of time [F(2, 26) = 16.26, P < 0.0001] (vehicle 17, 22 and 26 g; liraglutide 23, 25 and 25 g). Post hoc test revealed the water intake was higher in previous liraglutide treatment group at posttreatment day 1, but not at day 2 or day 3, compared with vehicle.
Repeated treatment of liraglutide reduces operant self‐administration of alcohol in alcohol‐preferring sP rats
There was an overall effect of treatment [F(2, 24) = 5.47, P = 0.0110], of time [F(4, 96) = 3.15, P = 0.0176] and of treatment × time interaction [F(8, 96) = 3.24, P = 0.0026] on the number of lever responses for alcohol during the 5‐day treatment phase. There was also an overall effect of treatment [F(2, 24) = 3.46, P = 0.0479], of time [F(4, 96) = 3.92, P = 0.0054] and of treatment × time interaction [F(8, 96) = 4.15, P = 0.0003] on amount of self‐administered alcohol during the 5‐day treatment phase. Post hoc analysis shows that treatment with either 0.1 (n = 9) or 0.05 (n = 9) mg/kg of liraglutide was totally ineffective on day 1 on the number of lever responses for alcohol (Fig. 6a) and the amount of self‐administered alcohol (Fig. 6b) compared with vehicle (n = 9). Conversely, on day 2, treatment with both doses of liraglutide produced a similar reduction (40–50 percent in comparison with vehicle‐treated rats) in both variables (Fig. 6a & 6b). In the subsequent three daily self‐administration sessions (days 3–5), number of lever responses for alcohol and amount of self‐administered alcohol progressively returned to control values in the rat group treated with 0.05 mg/kg liraglutide, while both variables remained relatively stable and reduced in the rat group treated with 0.1 mg/kg liraglutide (Fig. 6a & 6b).
Figure 6.
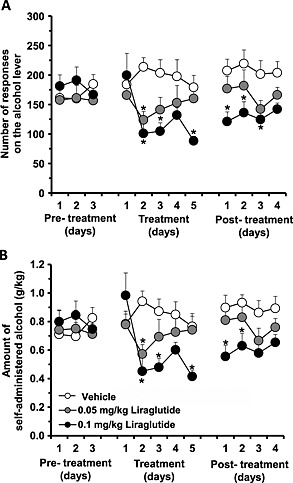
Repeated treatment of low doses of liraglutide reduces operant self‐administration of alcohol and alcohol intake in alcohol‐preferring sP rats. Repeated treatment of two doses of liraglutide (0.1 or 0.05 mg/kg) reduced (a) the number of lever responses for alcohol as well as (b) the amount of self‐administered alcohol during the 5‐day treatment phase in sP rats. After treatment discontinuation, the number of lever responses for alcohol and amount of self‐administered alcohol were lower in the rat group treated with 0.1 mg/kg liraglutide in comparison with control values. All values represent mean ± SEM (*P < 0.05)
An overall effect of treatment [F(2, 24) = 6.52, P = 0.0055] but not of time [F(3, 72) = 1.60, P = 0.1964], and no significant treatment × time interaction [F(6, 72) = 0.59, P = 0.7347], on number of lever responses for alcohol during the 4‐day posttreatment phase was observed (Fig. 6a). In addition, an overall effect of treatment [F(2, 24) = 4.56, P = 0.0209] but not of time [F(3, 72) = 1.42, P = 0.2441], and no significant treatment × time interaction [F(6, 72) = 0.53, P = 0.7866], on amount of self‐administered alcohol during the 4‐day posttreatment phase was obtained (Fig. 6b). After treatment discontinuation, number of lever responses for alcohol (Fig. 6a) and amount of self‐administered alcohol (Fig. 6b) in the rat group treated with 0.1 mg/kg liraglutide remained reduced, in comparison with control values, during the first 2–3 days, subsequently tending to return to control values.
Lever responding for water was negligible (≤3 responses) and was not affected by liraglutide administration during both treatment and posttreatment phases (data not shown).
Repeated treatment of liraglutide reduces food intake and body weight in alcohol‐preferring sP rats
An overall effect of treatment [F(2, 24) = 3.30, P = 0.0587], of time [F(4, 96) = 7.03, P < 0.0001] and of treatment × time interaction [F(8, 96) = 3.09, P < 0.0039] on daily food intake in the home cage during the 5‐day treatment phase was obtained. Post hoc analysis indicated that treatment with 0.05 and 0.1 mg/kg liraglutide reduced daily food intake on day 1 and on days 1 and 2, respectively (Fig. 7a). On continuing treatment, the magnitude of the anorectic effect of both doses of liraglutide tended to decrease (Fig. 7a). Daily food intake was virtually identical among the three rat groups during the 4‐day posttreatment phase [treatment: F(2, 24) = 0.08, P = 0.9194; time: F(3, 72) = 40.47, P < 0.0001; treatment × time interaction: F(6, 72) = 0.40, P = 0.8774] (Fig. 7a).
Figure 7.
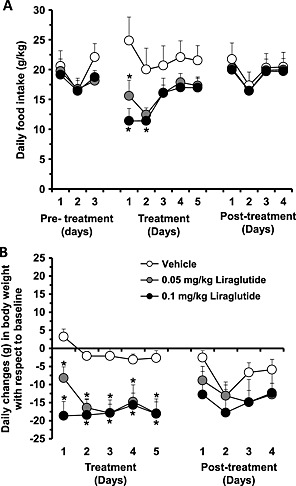
Repeated treatment of low doses of liraglutide reduces food intake and body weight in alcohol‐preferring sP rats. (a) Repeated treatment with 0.05 and 0.1 mg/kg liraglutide reduced daily food intake during the 5‐day treatment phase in sP rats. Daily food intake was identical among the three rat groups during the 4‐day posttreatment phase. (b) Repeated treatment with both doses of liraglutide resulted in an immediate and relatively stable reduction in body weight in comparison with vehicle‐treated rats. Daily changes in rat body weight were similar among the three rat groups during the 4‐day posttreatment phase
An overall effect of treatment [F(2, 24) = 9.67, P = 0.0008], of time [F(4, 96) = 4.95, P = 0.0011] and of treatment × time interaction [F(8, 96) = 2.26, P = 0.0293] on daily changes in rat body weight during the 5‐day treatment phase was observed. Post hoc analysis indicated that treatment with both doses of liraglutide resulted in an immediate and relatively stable reduction in body weight of 15–20 g in comparison with saline‐treated rats (Fig. 7b). Daily changes in rat body weight were similar among the three rat groups during the 4‐day posttreatment phase [treatment: F(2, 24) = 0.82, P = 0.4522; time: F(3, 72) = 7.76, P < =0.0002; treatment × time interaction: F(6, 72) = 1.42, P = 0.2198] (Fig. 7b).
Discussion
Alcohol use disorder is a major healthcare challenge, causing an enormous cost to society, and novel treatment strategies are warranted. The present study provides evidence that the GLP‐1 receptor agonist liraglutide have an important role in regulating alcohol‐mediated behaviors in rodents, suggesting that this clinically available medication could be used for treatment of AUD in humans. Indeed, we found that liraglutide suppresses the well‐documented effects of alcohol on the mesolimbic dopamine system, namely alcohol‐induced accumbal dopamine release and CPP in mice (Engel et al. 1988; Spanagel 2000). Acute liraglutide treatment reduced alcohol intake and prevented the alcohol deprivation effect in outbred rats. In addition, repeated treatments of liraglutide decreased alcohol intake in outbred rats as well as reduced the operant self‐administration of alcohol in alcohol‐preferring sP rats.
Given that liraglutide was administered peripherally, the mechanisms through which the GLP‐1 receptor ligand attenuates alcohol‐mediated behavior cannot be determined. However, GLP‐1 receptors are distributed throughout the mesolimbic dopamine system (Alvarez et al. 1996; Merchenthaler et al. 1999), a reward pathway intimately associated with development of AUD (for review, see Engel & Jerlhag 2014 and Soderpalm et al. 2009). The possibility should therefore be considered that liraglutide attenuates the alcohol‐mediated behaviors through NAc and VTA GLP‐1 receptor. This is further substantiated by the findings that liraglutide passes the blood brain barrier (Hunter & Holscher 2012) and that local administration of a GLP‐1 receptor agonist into the VTA reduces alcohol intake as well as abolishes the ability of alcohol to cause a CPP in rats (Shirazi et al. 2013). In addition, GLP‐1 receptors are distributed throughout the brain, suggesting that GLP‐1 receptors outside of limbic areas may also control alcohol reinforcement. For instance, GLP‐1 receptors in the lateral septum, which is highly interconnected with the mesolimbic dopamine system, regulate the activity of VTA‐dopamine neurons as well as stress‐induced drug relapse (Highfield et al. 2000; Luo et al. 2011; Harasta et al. 2015). The recent data showing that GLP‐1 receptors within the lateral septum regulate cocaine‐induced behaviors in mice raise the possibility that these receptors may be involved in mediating the ability of liraglutide to attenuate alcohol‐induced behaviors in rodents (Harasta et al. 2015). Moreover, GLP‐1 receptors within the NTS are known to regulate food intake (Tang‐Christensen et al. 1996; McMahon & Wellman 1998; Hayes et al. 2009) as well as food reward behavior in rats (Richard et al. 2015) and may thus be involved in mediating the rewarding properties of alcohol. The findings that liraglutide activates GLP‐1 receptors in the arcuate nucleus of the hypothalamus, regulating liraglutide‐dependent weight loss (Secher et al. 2014), and that there are hypothalamic projections to the VTA provide another possible indirect pathway through which GLP‐1 may regulate alcohol‐mediated behaviors. In support for an indirect regulation of the mesolimbic dopamine system by liraglutide are the present data showing that liraglutide has no effect on accumbal dopamine release per se. Liraglutide may also regulate alcohol‐mediated behaviors via peripheral mechanisms because circulating GLP‐1 acts through activation of vagal afferents to GLP‐1 containing neurons in the NTS (Larsen & Holst 2005), which project directly to the VTA and NAc (Alvarez et al. 1996; Merchenthaler et al. 1999). To explore the role of GLP‐1 receptors within various brain areas, studies examining the effect of intra‐nuclei infusion of GLP‐1 receptor ligands on alcohol‐mediated behaviors are warranted.
In the present study, we firstly showed that acute administration of liraglutide, with no effect per se on accumbal dopamine releases or CPP, attenuated the rewarding properties of alcohol as measured by accumbal dopamine release as well as the CPP. The CPP experiments showed that liraglutide was able to block the rewarding properties of alcohol, but not the memory consolidation of alcohol reward. Moreover, acute administration of liraglutide did not influence the blood alcohol concentrations in mice. Secondly, acute administration of two different doses of liraglutide reduced alcohol intake in high‐alcohol‐consuming rats. We also showed that acute liraglutide treatment of a low dose in rats prevented the alcohol deprivation effect, an important characteristics of AUD. The alcohol deprivation effect in rodents has been suggested to reflect relapse caused by craving in the clinical setting (Spanagel 2000). Indeed, two currently available agents for treatment of alcohol dependence, naltrexone and acamprosate, prevent the alcohol deprivation effect in rats (Spanagel & Zieglgansberger 1997; Heyser et al. 2003) as well as craving‐induced relapse in humans (Soyka & Rosner 2008). Thirdly, repeated administration of a liraglutide reduced alcohol intake as well as alcohol preference in high‐consuming Wistar rats. Most importantly, the effect of liraglutide is long‐lasting and is pronounced during 24 hours. Repeated administration of liraglutide increased the water intake and did not affect total fluid intake, suggesting that the effects of liraglutide are selective for alcohol. Fourthly, repeated liraglutide treatment reduced the reinforcing effects of alcohol in alcohol‐preferring sP rats exposed to a standard procedure of operant self‐administration of alcohol. This set of data are of relevance as they extend the reducing effect of liraglutide on alcohol intake in unselected Wistar rats to (i) an operant procedure of oral alcohol self‐administration, providing therefore a first line of evidence on liraglutide potential on the reinforcing properties of alcohol (beside on its mere consumption), and (ii) a rat line selectively bred for high alcohol preference and intake, whose alcohol‐seeking and alcohol‐taking behaviors have been proposed to model several aspects of excessive alcohol consumption in humans (Colombo et al. 2006). Finally, we showed that the GLP‐1 receptor agonist did not induce a rebound increase in alcohol self‐administration in sP rats after the treatment was terminated. Indeed, we actually showed that the number of lever responses for alcohol and amount of self‐administered alcohol are lower, over the 4‐day posttreatment period, in sP rats previously treated with liraglutide compared with vehicle treatment. Of clinical interest is that we have used lower doses of liraglutide than in studies showing that liraglutide affects body weight and blood glucose levels in rodents (Raun et al. 2007; Secher et al. 2014). Collectively, these data show that the physiological role of GLP‐1 extends outside regulation of food intake and glucose homeostasis (Flint et al. 1998) to include reinforcement mediation.
In the present series of experiment, we show that liraglutide attenuates the ability of a second injection of alcohol to increase accumbal dopamine. Therefore, the possibility should be considered that we investigate a priming effect, rather than an acute reward effect, of alcohol. It should be considered a limitation that only one single dose of liraglutide was used to investigate the effects on alcohol‐induced activation of the mesolimbic dopamine system. In addition, we investigated the effect of alcohol in NAc shell (for schematic placements, see Figure S1), because we obtain a robust increase in accumbal dopamine in NAc shell as compared with NAc core (unpublished data). In addition, ghrelin increases NAc dopamine in shell but not in the core (Quarta et al. 2009). Even though we showed that acute administration or liraglutide did not influence the blood alcohol concentrations in mice, we cannot rule out that repeated liraglutide treatment affects the metabolism of alcohol. In addition, the blood alcohol levels were investigated following peripheral injections of alcohol, rather than following alcohol consumption. We also showed that co‐adjuvant, in contrast to acute, treatment of liraglutide attenuates alcohol‐induced CPP, suggesting that GLP‐1 receptor agonist interfere with the primary motivational properties of alcohol rather than the expression of CPP (Sanchis‐Segura & Spanagel 2006). The findings might be somewhat surprising because GLP‐1 receptor ligands, including liraglutide, previously has been attributed profound effects on memory formation, synaptic plasticity as well as hippocampal neuroprotection in a mouse model of Alzheimer disease (Holscher 2014). However, the possibility that memory consolidation of alcohol‐induced CPP and synaptic plasticity involves various brain circuits should therefore be considered. In the present experiment, we showed that liraglutide reduced both food and alcohol intake, raising the possibility that the GLP‐1 receptor agonist reduced ingestive behaviors of reinforcers with caloric value. However, repeated administration of liraglutide increases, and acute administration has a tendency of increasing water intake in rats. Moreover, this appears less likely because GLP‐1 receptor ligands attenuate drug‐induced reward (Erreger et al. 2012; Graham et al. 2013; Egecioglu et al. 2013a,2013b). Investigation of the possibility that liraglutide alters ingestive behavior and tasting of the effects of liraglutide on intravenous alcohol self‐administration in rodents or humans should be explored. In corroboration, intravenous ghrelin administration increases the craving for alcohol in alcohol‐dependent individuals (Leggio et al. 2014).
The direct involvement of GLP‐1 receptors for reinforcement is further supported by the data showing that another GLP‐1 receptor agonist, Ex4, reduces alcohol intake, attenuates alcohol‐seeking behavior as well as blocks the alcohol‐induced reward in mice (Egecioglu et al. 2013c). These data were extended and corroborated by others showing that a GLP‐1 receptor antagonist increases alcohol intake in rats (Shirazi et al. 2013). In addition, Ex4 reduced food reward and motivation to consume palatable food, and a GLP‐1 antagonist blocked these effects in rats (Dickson et al. 2012). Taken together with the data showing that liraglutide reduced weight and fat gains, decreased calorie intake as well as shifted food preference from candy to regular chow in candy‐fed rats (Raun et al. 2007), it may be suggest that GLP‐1‐sensitive mechanisms regulate reward processes in general. In corroboration, Ex4 attenuate nicotine‐induced, amphetamine‐induced and cocaine‐induced reward as measured by accumbal dopamine release, CPP and locomotor stimulation in rodents (Erreger et al. 2012; Graham et al. 2013; Egecioglu et al. 2013a,2013b). It was recently found that withdrawal‐induced anxiety as well as tolerance to the anxiolytic effects of alcohol is delayed by liraglutide or by inhibition of the enzyme, dipeptidyl‐peptidase IV, responsible for degradation of endogenous GLP‐1 (Sharma et al. 2014a,2014b). The findings that this gut‐brain peptide reduces alcohol as well as nicotine reinforcement in rodents and that there is a co‐morbidity between smoking and alcohol dependence (for review, see Soderpalm et al. 2009) hold potentially important clinical implications because both addictions might be affected by GLP‐1 receptor ligands.
Acute treatment with high doses of liraglutide has previously been shown to induce a condition taste aversion (Kanoski et al. 2012), raising the possibility that a reduction in alcohol intake is due to nausea rather than reduced alcohol reward. However, this appears less likely because we evaluated the effects of a low dose of liraglutide on conditioned place aversion, which has been suggested to correlate to conditioned taste aversion (Cagniard & Murphy 2012). We showed that repeated liraglutide treatment did not induce a conditioned place aversion in vehicle‐paired mice during a drug‐free session, indicating that the selected doses of liraglutide do not induce aversion. Moreover, we used lower doses than those causing a taste aversion (Kanoski et al. 2012), and we followed up the acute studies with repeated administration of liraglutide, a design with documented tolerance for nausea. This is further substantiated by the findings that liraglutide, in contrast to Ex4, does not condition a taste avoidance to saccharine in mice (McKay & Daniels 2013).
The present study show that repeated administration of the GLP‐1 analog decreases food intake as well as body weight. Rodent and human studies collectively show that liraglutide induces weight loss by reducing appetite and energy intake (Tang‐Christensen et al. 1996; Flint et al. 1998; Gutzwiller et al. 1999; ). The findings showing that liraglutide does not alter the intake of chow nor high‐fat diet in neuronal GLP‐1 knockout mice, but still affects glucose levels, suggest that central GLP‐1 receptors are required for regulation of food intake in mice (Sisley et al. 2014). Previous studies show that the anorexigenic properties of GLP‐1 are mediated via GLP‐1 receptors in the NTS, amygdala and hypothalamus (Tang‐Christensen et al. 1996; McMahon & Wellman 1998; Hayes et al. 2009) as well as NAc (Dossat et al. 2011). In addition, intra‐VTA or intra‐NAc administration of Ex4 decreased the motivation for sucrose, reduced the intake of palatable food as well as attenuated CPP for palatable food (Alhadeff et al. 2012; Dickson et al. 2012). The liraglutide‐induced weight loss, at least in part, is mediated via pro‐opiomelanocortin (POMC)/cocaine‐ and amphetamine‐regulated transcript (CART) neurons in the hypothalamic arcuate nucleus in mice (Secher et al. 2014). We therefore suggest that the obtained reduction in food intake and body weight following peripheral administration of liraglutide is due to GLP‐1 signaling within as well as outside of brain reward areas. Given that liraglutide reduced food consumption in rats and humans (Tang‐Christensen et al. 1996; Flint et al. 1998; Gutzwiller et al. 1999; ), the possibility that the reduction in alcohol intake is due to calories rather than reward should be considered. This appears less likely because we showed that liraglutide did not induce a CPP in vehicle‐paired mice. Moreover, other GLP‐1 receptor agonists attenuate calorie‐independent reward, namely nicotine‐induced, amphetamine‐induced and cocaine‐induced CPP, locomotor stimulation and accumbal dopamine releases (Erreger et al. 2012; Graham et al. 2013; Egecioglu et al. 2013a,2013b).
In addition to GLP‐1, several other neuropeptides modulate the responses to alcohol. Ghrelin increases, whereas ghrelin receptor antagonists reduce alcohol intake, the rewarding properties of alcohol as well as the motivational properties of alcohol in rodents (for review, see Engel & Jerlhag 2014). It was initially shown that the alpha‐melanocyte‐stimulating hormone–melanocortin (MC)‐4 receptor system reduces alcohol intake and ease alcohol withdrawal symptoms (for review, see Olney et al. 2014). In addition, a recent study showed that intra‐VTA injection of alpha‐melanocyte‐stimulating hormone or an MC4 agonists increased lever presses for alcohol in rats (Shelkar et al. 2015). Cocaine‐regulated and amphetamine‐regulated transcript, which interacts prominently with the mesolimbic dopamine system, inhibits context‐induced reinstatement of alcohol‐seeking behaviors, and cocaine‐regulated and amphetamine‐regulated transcript knockout mice consume and prefer alcohol less than wild‐type mice (King et al. 2010; Salinas et al. 2014). In comparison with wild‐type mice, neuropeptide Y (NPY)‐deficient mice display an increased alcohol intake whereas NPY‐overexpressing mice have suppressed alcohol consumption (for review, see Thorsell 2007). Moreover, an NPY2 antagonist reduces alcohol intake in alcohol‐naïve as well postdependent animals, and NPY blocks yohimbine‐induced reinstatement of alcohol seeking in rats (Thorsell 2007; Cippitelli et al. 2010). The anorexigenic peptides leptin, cholecystokinin and galanin decrease preference for as well as intake of alcohol in rodents (for review, see Engel & Jerlhag 2014).
The present study shows that acute administration of a low dose of liraglutide attenuates the ability of alcohol to release accumbal dopamine, to cause a CPP in mice and to reduce alcohol consumption as well as prevents the alcohol deprivation effect in rats. Moreover, repeated administration of a low dose of liraglutide reduces alcohol intake as well as decreases the reinforcing properties of alcohol in rats. Liraglutide is currently used as treatments for type II diabetes because it enhances glucose‐dependent insulin secretion (Holst & Seino 2009), reduces gastric emptying as well as decreases glucagon secretion (Kreymann et al. 1987; Orskov et al. 1988; Flint et al. 2001). We therefore argue that GLP‐1 and its receptor play a role in the pathophysiology of alcohol‐mediated behavior and that GLP‐1 receptor agonists, such as liraglutide, deserve to be evaluated as potential therapeutics for AUD.
Conflict of Interest
EJ has for another project received financial support from the Novo Nordisk Foundation. This does not alter the authors' adherence to any of the journals policies on sharing data and materials. The remaining authors declare no conflict of interest.
Authors Contribution
JAE designed the study, managed literature search and wrote the manuscript; DV, PM and GC designed and performed part or the hands‐on work, analyzed the data and wrote the manuscript; MM and JWJ performed parts or the hands‐on work; EE wrote the manuscript; EJ designed the study, wrote the protocol, managed literature search, analyzed and undertook statistical analysis and wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Supporting information
Supporting Information
Supporting Information
Acknowledgements
Britt‐Mari Larsson, Kenn Johannessen and Carla Acciaro are gratefully acknowledged for their expert and valuable technical assistance. The GLP‐1 receptor agonist, liraglutide, was supplied by Novo Nordisk. The study is supported by grants from the Swedish Research Council (2009‐2782 and 2011‐4646), Swedish Society for Medical Research, Swedish Brain Foundation, LUA/ALF (grant no. 148251) from the Sahlgrenska University Hospital, Ragnar Söderberg, Alcohol Research Council of the Swedish Alcohol Retailing Monopoly and the Foundations of Adlerbertska, Fredrik and Ingrid Thuring, Tore Nilsson, Längmanska, Wilhelm and Martina Lundgren, Knut and Alice Wallenberg, Magnus Bergvall, Anérs, Jeansons and Åke Wiberg, and the Swedish Society of Medicine.
Vallöf, D. , Maccioni, P. , Colombo, G. , Mandrapa, M. , Jörnulf, J. W. , Egecioglu, E. , Engel, J. A. , and Jerlhag, E. (2016) The glucagon‐like peptide 1 receptor agonist liraglutide attenuates the reinforcing properties of alcohol in rodents. Addiction Biology, 21: 422–437. doi: 10.1111/adb.12295.
References
- Alhadeff AL, Rupprecht LE, Hayes MR (2012) GLP‐1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology 153:647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez E, Roncero I, Chowen JA, Thorens B, Blazquez E (1996) Expression of the glucagon‐like peptide‐1 receptor gene in rat brain. J Neurochem 66:920–927. [DOI] [PubMed] [Google Scholar]
- Brubaker PL, Anini Y (2003) Direct and indirect mechanisms regulating secretion of glucagon‐like peptide‐1 and glucagon‐like peptide‐2. Can J Physiol Pharmacol 81:1005–1012. [DOI] [PubMed] [Google Scholar]
- Cagniard B, Murphy NP (2012) Affective taste responses in the presence of reward‐ and aversion‐conditioned stimuli and their relationship to psychomotor sensitization and place conditioning. Behav Brain Res 236:289–294. [DOI] [PubMed] [Google Scholar]
- Carnicella S, Amamoto R, Ron D (2009) Excessive alcohol consumption is blocked by glial cell line‐derived neurotrophic factor. Alcohol 43:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Damadzic R, Hansson AC, Singley E, Sommer WH, Eskay R, Thorsell A, Heilig M (2010) Neuropeptide Y (NPY) suppresses yohimbine‐induced reinstatement of alcohol seeking. Psychopharmacology (Berl) 208:417–426. [DOI] [PubMed] [Google Scholar]
- Clarke RB, Adermark L, Chau P, Soderpalm B, Ericson M (2014) Increase in nucleus accumbens dopamine levels following local ethanol administration is not mediated by acetaldehyde. Alcohol Alcohol 49:498–504. [DOI] [PubMed] [Google Scholar]
- Colombo G, Lobina C, Carai MA, Gessa GL (2006) Phenotypic characterization of genetically selected Sardinian alcohol‐preferring (sP) and ‐non‐preferring (sNP) rats. Addict Biol 11:324–338. [DOI] [PubMed] [Google Scholar]
- Deacon CF, Johnsen AH, Holst JJ (1995) Degradation of glucagon‐like peptide‐1 by human plasma in vitro yields an N‐terminally truncated peptide that is a major endogenous metabolite in vivo . J Clin Endocrinol Metab 80:952–957. [DOI] [PubMed] [Google Scholar]
- Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP (2012) The glucagon‐like peptide 1 (GLP‐1) analogue, exendin‐4, decreases the rewarding value of food: a new role for mesolimbic GLP‐1 receptors. The Journal of neuroscience: the official journal of the Society for Neuroscience 32:4812–4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dossat AM, Lilly N, Kay K, Williams DL (2011) Glucagon‐like peptide 1 receptors in nucleus accumbens affect food intake. The Journal of neuroscience: the official journal of the Society for Neuroscience 31:14453–14457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egecioglu E, Engel JA, Jerlhag E (2013a) The glucagon‐like peptide 1 analogue exendin‐4 attenuates the nicotine‐induced locomotor stimulation, accumbal dopamine release, conditioned place preference as well as the expression of locomotor sensitization in mice. PLoS One 8:e77284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egecioglu E, Engel JA, Jerlhag E (2013b) The glucagon‐like peptide 1 analogue, exendin‐4, attenuates the rewarding properties of psychostimulant drugs in mice. PLoS One 8:e69010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egecioglu E, Steensland P, Fredriksson I, Feltmann K, Engel JA, Jerlhag E (2013c) The glucagon‐like peptide 1 analogue exendin‐4 attenuates alcohol mediated behaviors in rodents. Psychoneuroendocrinology 38:1259–1270. [DOI] [PubMed] [Google Scholar]
- Engel JA, Fahlke C, Hulthe P, Hard E, Johannessen K, Snape B, Svensson L (1988) Biochemical and behavioral evidence for an interaction between ethanol and calcium channel antagonists. J Neural Transm 74:181–193. [DOI] [PubMed] [Google Scholar]
- Engel JA, Jerlhag E (2014) Role of gut‐brain hormones in the pathophysiology of alcoholism: implications for pharmacotherapy. CNS Drugs, Epub ahead of print. [DOI] [PMC free article] [PubMed]
- Erreger K, Davis AR, Poe AM, Greig NH, Stanwood GD, Galli A (2012) Exendin‐4 decreases amphetamine‐induced locomotor activity. Physiol Behav 106:574–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint A, Raben A, Astrup A, Holst JJ (1998) Glucagon‐like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest 101:515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint A, Raben A, Ersboll AK, Holst JJ, Astrup A (2001) The effect of physiological levels of glucagon‐like peptide‐1 on appetite, gastric emptying, energy and substrate metabolism in obesity. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity 25:781–792. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G (1997) The Mouse Brain in Stereotaxic Coordinates. Academic Press: San Diego. [Google Scholar]
- Graham DL, Erreger K, Galli A, Stanwood GD (2013) GLP‐1 analog attenuates cocaine reward. Mol Psychiatry 18:961–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutzwiller JP, Drewe J, Goke B, Schmidt H, Rohrer B, Lareida J, Beglinger C (1999) Glucagon‐like peptide‐1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol 276:R1541–1544. [DOI] [PubMed] [Google Scholar]
- Harasta AE, Power JM, von Jonquieres G, Karl T, Drucker DJ, Housley GD, Schneider M, Klugmann M (2015) Septal glucagon‐like peptide 1 receptor expression determines suppression of cocaine‐induced behavior. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MR, Bradley L, Grill HJ (2009) Endogenous hindbrain glucagon‐like peptide‐1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology 150:2654–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyser CJ, Moc K, Koob GF (2003) Effects of naltrexone alone and in combination with acamprosate on the alcohol deprivation effect in rats. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 28:1463–1471. [DOI] [PubMed] [Google Scholar]
- Highfield D, Clements A, Shalev U, McDonald R, Featherstone R, Stewart J, Shaham Y (2000) Involvement of the medial septum in stress‐induced relapse to heroin seeking in rats. Eur J Neurosci 12:1705–1713. [DOI] [PubMed] [Google Scholar]
- Holscher C (2014) Central effects of GLP‐1: new opportunities for treatments of neurodegenerative diseases. J Endocrinol 221:T31–41. [DOI] [PubMed] [Google Scholar]
- Holst JJ (2004) Treatment of type 2 diabetes mellitus with agonists of the GLP‐1 receptor or DPP‐IV inhibitors. Expert Opin Emerg Drugs 9:155–166. [DOI] [PubMed] [Google Scholar]
- Holst JJ, Seino Y (2009) GLP‐1 receptor agonists: targeting both hyperglycaemia and disease processes in diabetes. Diabetes Res Clin Pract 85:1–3. [DOI] [PubMed] [Google Scholar]
- Hunter K, Holscher C (2012) Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci 13:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Dickson SL, Andersson M, Svensson L, Engel JA (2006) Ghrelin stimulates locomotor activity and accumbal dopamine‐overflow via central cholinergic systems in mice: implications for its involvement in brain reward. Addict Biol 11:45–54. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Landgren S, Salome N, Heilig M, Moechars D, Datta R, Perrissoud D, Dickson SL, Engel JA (2009) Requirement of central ghrelin signaling for alcohol reward. Proc Natl Acad Sci U S A 106:11318–11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski SE, Rupprecht LE, Fortin SM, De Jonghe BC, Hayes MR (2012) The role of nausea in food intake and body weight suppression by peripheral GLP‐1 receptor agonists, exendin‐4 and liraglutide. Neuropharmacology 62:1916–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BJ, Furlong TM, McNally GP (2010) Cocaine and amphetamine related transcript (CART) inhibits context induced reinstatement of reward seeking. Behav Neurosci 124:423–427. [DOI] [PubMed] [Google Scholar]
- Kreymann B, Williams G, Ghatei MA, Bloom SR (1987) Glucagon‐like peptide‐1 7‐36: a physiological incretin in man. Lancet 2:1300–1304. [DOI] [PubMed] [Google Scholar]
- Landgren S, Engel JA, Hyytia P, Zetterberg H, Blennow K, Jerlhag E (2011) Expression of the gene encoding the ghrelin receptor in rats selected for differential alcohol preference. Behav Brain Res 221:182–188. [DOI] [PubMed] [Google Scholar]
- Larsen PJ, Holst JJ (2005) Glucagon‐related peptide 1 (GLP‐1): hormone and neurotransmitter. Regul Pept 128:97–107. [DOI] [PubMed] [Google Scholar]
- Leggio L, Zywiak WH, Fricchione SR, Edwards SM, de la Monte SM, Swift RM, Kenna GA (2014) Intravenous ghrelin administration increases alcohol craving in alcohol‐dependent heavy drinkers: a preliminary investigation. Biol Psychiatry . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loi B, Lobina C, Maccioni P, Fantini N, Carai MA, Gessa GL, Colombo G (2010) Increase in alcohol intake, reduced flexibility of alcohol drinking, and evidence of signs of alcohol intoxication in Sardinian alcohol‐preferring rats exposed to intermittent access to 20% alcohol. Alcohol Clin Exp Res 34:2147–2154. [DOI] [PubMed] [Google Scholar]
- Luo AH, Tahsili‐Fahadan P, Wise RA, Lupica CR, Aston‐Jones G (2011) Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science 333:353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccioni P, Zaru A, Loi B, Lobina C, Carai MA, Gessa GL, Capra A, Mugnaini C, Pasquini S, Corelli F, Hyytia P, Lumeng L, Colombo G (2012) Comparison of the effect of the GABABeta receptor agonist, baclofen, and the positive allosteric modulator of the GABAB receptor, GS39783, on alcohol self‐administration in 3 different lines of alcohol‐preferring rats. Alcohol Clin Exp Res 36:1748–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay NJ, Daniels D (2013) Glucagon‐like peptide‐1 receptor agonist administration suppresses both water and saline intake in rats. J Neuroendocrinol 25:929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon LR, Wellman PJ (1998) PVN infusion of GLP‐1‐(7‐36) amide suppresses feeding but does not induce aversion or alter locomotion in rats. Am J Physiol 274:R23–29. [DOI] [PubMed] [Google Scholar]
- Meeran K, O'Shea D, Edwards CM, Turton MD, Heath MM, Gunn I, Abusnana S, Rossi M, Small CJ, Goldstone AP, Taylor GM, Sunter D, Steere J, Choi SJ, Ghatei MA, Bloom SR (1999) Repeated intracerebroventricular administration of glucagon‐like peptide‐1‐(7‐36) amide or exendin‐(9‐39) alters body weight in the rat. Endocrinology 140:244–250. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Lane M, Shughrue P (1999) Distribution of pre‐pro‐glucagon and glucagon‐like peptide‐1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol 403:261–280. [DOI] [PubMed] [Google Scholar]
- Olney JJ, Navarro M, Thiele TE (2014) Targeting central melanocortin receptors: a promising novel approach for treating alcohol abuse disorders. Front Neurosci 8:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov C, Holst JJ, Nielsen OV (1988) Effect of truncated glucagon‐like peptide‐1 [proglucagon‐(78‐107) amide] on endocrine secretion from pig pancreas, antrum, and nonantral stomach. Endocrinology 123:2009–2013. [DOI] [PubMed] [Google Scholar]
- Pannacciulli N, Le DS, Salbe AD, Chen K, Reiman EM, Tataranni PA, Krakoff J (2007) Postprandial glucagon‐like peptide‐1 (GLP‐1) response is positively associated with changes in neuronal activity of brain areas implicated in satiety and food intake regulation in humans. Neuroimage 35:511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarta D, Di Francesco C, Melotto S, Mangiarini L, Heidbreder C, Hedou G (2009) Systemic administration of ghrelin increases extracellular dopamine in the shell but not the core subdivision of the nucleus accumbens. Neurochem Int 54:89–94. [DOI] [PubMed] [Google Scholar]
- Raun K, von Voss P, Gotfredsen CF, Golozoubova V, Rolin B, Knudsen LB (2007) Liraglutide, a long‐acting glucagon‐like peptide‐1 analog, reduces body weight and food intake in obese candy‐fed rats, whereas a dipeptidyl peptidase‐IV inhibitor, vildagliptin, does not. Diabetes 56:8–15. [DOI] [PubMed] [Google Scholar]
- Richard JE, Anderberg RH, Goteson A, Gribble FM, Reimann F, Skibicka KP (2015) Activation of the GLP‐1 receptors in the nucleus of the solitary tract reduces food reward behavior and targets the mesolimbic system. PLoS One 10:e0119034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas AG, Nguyen CT, Ahmadi‐Tehrani D, Morrisett RA (2014) Reduced ethanol consumption and preference in cocaine‐ and amphetamine‐regulated transcript (CART) knockout mice. Addict Biol 19:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis‐Segura C, Spanagel R (2006) Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol 11:2–38. [DOI] [PubMed] [Google Scholar]
- Secher A, Jelsing J, Baquero AF, Hecksher‐Sorensen J, Cowley MA, Dalboge LS, Hansen G, Grove KL, Pyke C, Raun K, Schaffer L, Tang‐Christensen M, Verma S, Witgen BM, Vrang N, Bjerre Knudsen L (2014) The arcuate nucleus mediates GLP‐1 receptor agonist liraglutide‐dependent weight loss. J Clin Invest 124:4473–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma AN, Pise A, Sharma JN, Shukla P (2014a) Dipeptidyl‐peptidase IV (DPP‐IV) inhibitor delays tolerance to anxiolytic effect of ethanol and withdrawal‐induced anxiety in rats. Metab Brain Dis. [DOI] [PubMed] [Google Scholar]
- Sharma AN, Pise A, Sharma JN, Shukla P (2014b) Glucagon‐like peptide‐1 (GLP‐1) receptor agonist prevents development of tolerance to anti‐anxiety effect of ethanol and withdrawal‐induced anxiety in rats. Metab Brain Dis. [DOI] [PubMed] [Google Scholar]
- Shelkar GP, Kale AD, Singh U, Singru PS, Subhedar NK, Kokare DM (2015) Alpha‐melanocyte stimulating hormone modulates ethanol self‐administration in posterior ventral tegmental area through melanocortin‐4 receptors. Addict Biol 20:302–315. [DOI] [PubMed] [Google Scholar]
- Shirazi RH, Dickson SL, Skibicka KP (2013) Gut peptide GLP‐1 and its analogue, exendin‐4, decrease alcohol intake and reward. PLoS One 8:e61965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE (2008) Intermittent access to 20% ethanol induces high ethanol consumption in Long‐Evans and Wistar rats. Alcohol Clin Exp Res 32:1816–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisley S, Gutierrez‐Aguilar R, Scott M, D'Alessio DA, Sandoval DA, Seeley RJ (2014) Neuronal GLP1R mediates liraglutide's anorectic but not glucose‐lowering effect. J Clin Invest 124:2456–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderpalm B, Lof E, Ericson M (2009) Mechanistic studies of ethanol's interaction with the mesolimbic dopamine reward system. Pharmacopsychiatry 42:S87–94. [DOI] [PubMed] [Google Scholar]
- Soyka M, Rosner S (2008) Opioid antagonists for pharmacological treatment of alcohol dependence ‐ a critical review. Curr Drug Abuse Rev 1:280–291. [DOI] [PubMed] [Google Scholar]
- Spanagel R (2000) Recent animal models of alcoholism. Alcohol Res Health 24:124–131. [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Zieglgansberger W (1997) Anti‐craving compounds for ethanol: new pharmacological tools to study addictive processes. Trends Pharmacol Sci 18:54–59. [PubMed] [Google Scholar]
- Tang‐Christensen M, Larsen PJ, Goke R, Fink‐Jensen A, Jessop DS, Moller M, Sheikh SP (1996) Central administration of GLP‐1‐(7‐36) amide inhibits food and water intake in rats. Am J Physiol 271:R848–856. [DOI] [PubMed] [Google Scholar]
- Thorsell A (2007) Neuropeptide Y (NPY) in alcohol intake and dependence. Peptides 28:480–483. [DOI] [PubMed] [Google Scholar]
- Wise RA (1973) Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia 29:203–210. [DOI] [PubMed] [Google Scholar]
- Zander M, Madsbad S, Madsen JL, Holst JJ (2002) Effect of 6‐week course of glucagon‐like peptide 1 on glycaemic control, insulin sensitivity, and beta‐cell function in type 2 diabetes: a parallel‐group study. Lancet 359:824–830. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


