Summary
Liposomal bupivacaine is a prolonged‐release local anaesthetic, the neurotoxicity of which has not yet been determined. We used quantitative histomorphometric and immunohistochemical analyses to evaluate the neurotoxic effect of liposomal bupivacaine after perineural and intraneural (extrafascicular) injection of the sciatic nerve in pigs. In this double‐blind prospective randomised trial, 4 ml liposomal bupivacaine 1.3% was injected either perineurally (n = 5) or intraneurally extrafascicularly (n = 5). Intraneural–extrafascicular injection of saline (n = 5) was used as a control. After emergence from anaesthesia, neurological examinations were conducted over two weeks. After harvesting the sciatic nerves, no changes in nerve fibre density or myelin width indicative of nerve injury were observed in any of the groups. Intraneural injections resulted in longer sensory blockade than perineural (p < 0.003) without persistent motor or sensory deficit. Sciatic nerve block with liposomal bupivacaine in pigs did not result in histological evidence of nerve injury.
Introduction
Prolonged‐release local anaesthetic formulations have been developed to extend the duration of sensory block and to reduce the risk of systemic and local tissue toxicity/inflammatory reactions 1. In the last two decades, a wide variety of formulations have been reported to extend the duration of brachial plexus 2, intercostal 3 and epidural blocks 4. However, their adoption into clinical practice has been slow because of concerns over potential neurological and tissue toxicity 5.
Liposomal bupivacaine (DepoFoam bupivacaine; Exparel, Pacira Pharmaceuticals, Inc., San Diego, CA, USA) is a recent formulation that contains bupivacaine encapsulated in multivesicular liposomes. The liposomes consist of non‐concentric bilayer lipids that increase the drug's stability and extend its duration of action 6. To date, liposomal bupivacaine has been used extensively for soft tissue infiltration 7, 8, 9. A recent pharmacokinetic study showed that liposomal bupivacaine exhibited bimodal kinetics with rapid uptake during the first few hours and prolonged release over 96 h 10. Such drug characteristics can be useful in peripheral nerve blocks as an alternative to indwelling catheters.
Liposomal bupivacaine's neurotoxicity is relevant to clinical practice since new pharmacological agents must be subjected to toxicity testing before their widespread use. In recent studies of brachial plexus blockade in rabbit and dog models, neurotoxicity has not been demonstrated 11; however, the haematoxylin‐eosin staining used did not allow a detailed evaluation of nerve anatomy. A study using a unifascicular rat nerve model 12, in spite of using high‐resolution light microscopy, was suboptimal because of a paucity of connective tissue within the epineurium. Therefore, intraneural injections into these nerves could have a higher chance of intrafascicular injection. In contrast, most intraneural injections in humans appear to occur between the fascicles, as the advancing needle is more likely to traverse the nerve through the connective tissue between the fascicles of a multifascicular nerve 13. To come as close as possible to clinical settings with standard equipment for regional anaesthesia, we have chosen a porcine nerve model 14, similar to humans with respect to its polyfascicular pattern and its ratio of neural (i.e. axonal tissues, fascicles) to non‐neural (i.e. connective) tissue and consequent susceptibility to injury.
Our primary aim was to assess the neurotoxic effect of liposomal bupivacaine on the sciatic nerve after perineural and intraneural (extrafascicular) injection, using quantitative histomorphometric and immunohistochemical analyses. Our secondary aim was to evaluate sensory and motor dysfunction from injection of liposomal bupivacaine.
Methods
After approval of the Review Board for Animal Research (No. U34401‐28/2013/77) and in accordance with Slovenian Governmental regulations and the ARRIVE guidelines, 15 pigs (13 female and two castrated male hybrids from landrace and large white, with mean (SD) weight 20 (2) kg, purchased from a local farm) were included in the study. All had been vaccinated against mycoplasmosis 4–6 weeks before the experiment and were free of swine fever, Aujeszky disease, porcine respiratory and reproductive syndrome, and salmonellosis. The pigs were fed twice a day with a commercial pig diet. Water was provided ad libitum. A 12–h light–dark cycle was provided.
The animals were premedicated with intramuscular midazolam 0.5 mg.kg−1, butorphanol 0.5 mg.kg−1 and ketamine 10 mg.kg−1 in a warmed, straw‐bedded pen. All procedures were performed between 08.00 and 15.00. An intravenous catheter was placed, and anaesthesia was induced in an operating theatre with propofol. After jaw relaxation, the trachea was intubated and connected to the breathing system. Anaesthesia was maintained with isoflurane in 50:50 oxygen:air mixture, and non‐invasive blood pressure, oxygen saturation, end‐tidal CO2 concentration, inspiratory and expiratory isoflurane concentration, oesophageal temperature and ECG were continuously monitored.
The animals were placed in the right lateral recumbent position, then their left sciatic nerves were exposed between the superficial gluteus and the biceps femoris muscles (Fig. 1). They were randomly assigned to one of three groups (5 per group) using a computer‐generated sequence with sealed envelopes. The first group received an injection of 4 ml liposomal bupivacaine 1.3% perineurally, the second group received 4 ml liposomal bupivacaine 1.3% intraneurally–extrafascicularly, whereas the third group (control) received 4 ml saline intraneurally and extrafascicularly. The injections were performed under direct vision. For perineural injections, the needle bevel was placed outside the external epineurium to inject liposomal bupivacaine around the nerve, whereas for intraneural injections, the needle was inserted under the external epineurium. To decrease the risk of intrafascicular injection and to prevent needle‐nerve trauma with consequent inflammatory reaction 15, injection pressure monitoring and electrical nerve stimulation were employed. If, during needle insertion, an evoked motor response was elicited at < 0.3 mA 16 or injection could not commence with injection pressure < 104 kPa 17, 18, 19, the injection was re‐attempted after the needle was repositioned.
Figure 1.
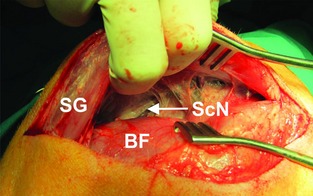
Surgical exposure of the sciatic nerve (ScN) using blunt dissection between the superficial gluteus (SG) and the biceps femoris (BF) muscles.
Insulated 22‐G, 5‐cm‐long, short‐bevelled, nerve block needles (Stimuplex A; B. Braun Melsungen AG, Melsungen, Germany) were used. Drugs were injected by an automated infusion pump (PHD 2000; Harvard Apparatus, Holliston, MA, USA), at a speed of 10 ml.min−1 20. Data were acquired with an in‐line manometer (PG5000; PSI Tronics Technologies, Inc., Tulare, CA, USA) coupled to the computer via an analogue‐digital conversion board and placed proximal to the needle in line with a non‐distensible high‐durometer polyvinylchloride injection tubing (2.1‐m arterial pressure tubing, Abbott Critical Care Systems; Abbott Laboratories, North Chicago, IL, USA). The site of injection was marked with a suture, the muscle tissue was approximated, and the wound was closed. Time from induction to extubation was approximately 60 min. Pre‐surgical antibiotic prophylaxis with amoxicillin‐clavulanic acid 9 mg.kg−1 was given intramuscularly 30 min before the procedure and then orally once a day for the following 10 days. Postoperative analgesia was provided with butorphanol 0.5 mg.kg−1 administered intramuscularly 4 h, 8 h and 12 h after the end of anaesthesia. Recovery was in a warmed, straw‐bedded pen. Veterinary doctors did not recognise any signs of discomfort and pain (agitation, vocalisation, strange behaviour) in the postoperative period.
Gross motor and sensory functions were evaluated before and after the experiment (at 2‐h intervals for the first 12 h after the injections and daily thereafter). Neurological examinations were conducted by a blinded observer using the modified Thalhammer's neurological examination 21, including presence and severity of: paresis (0 absent; 1 slight paresis; 2 moderate paresis; 3 severe paresis; 4 flaccid extremity); ataxia (0 no ataxia; 1 slight ataxia; 2 moderate ataxia; 3 severe ataxia); and nociception (0 no withdrawal reaction of the pinched extremity (performed with haemostatic clamp), no vocalisation; 1 barely perceptible withdrawal reaction, no vocalisation; 2 slow withdrawal reaction, no vocalisation; 3 slower (weaker) withdrawal reaction, vocalisation; 4 normal (brisk) withdrawal reaction, vocalization).
After two weeks, the pigs were killed with T‐61 euthanasia solution (1 ml containing embutramid 200 mg, mebezonium iodide 50 mg and tetracaine hydrochloride 5 mg; Hoechst GmbH, Munich, Germany) under general anaesthesia. Their left sciatic nerves were excised proximal and distal to the injection site to obtain 1.5‐cm‐long specimens. The nerve specimens were divided into three sections taken: (1) from the injected region (marked with the suture) and fixed for high resolution light microscopy; (2) more distally and placed in liquid nitrogen (at −80°C) for immunohistochemical analysis; and (3) from the most distal region and placed in RNA Stabilization Reagent (RNAlater; Qiagen, Hilden, Germany) for quantitative polymerase chain reaction (PCR). The contralateral right sciatic nerves were also resected (1.5‐cm‐long section) to yield nerve tissue that was neither exposed to drug nor traumatised by the needle (negative control). These were also divided into three parts and prepared in the same way. All specimens were numbered, and key numbers were kept in sealed envelopes to ensure blinding.
The nerve samples were processed for Epon‐embedding and fixed in Karnovsky's KII Solution (glutaraldehyde 2.5%, paraformaldehyde 4.0% in 0.1 M sodium cacodylate buffer, pH 7.4), post‐fixed in 1:1 solution of aqueous osmium tetroxide 2% and potassium ferrocyanide 3%, dehydrated in graded ethanol solutions and propylene oxide, and infiltrated with Epon mixtures. Cross‐sections (0.5 μm) were cut and stained with toluidine blue and captured by a digital camera (DXM1200F; Nikon, Tokyo, Japan) connected to the microscope (Eclipse E800; Nikon).
Morphometric analysis was performed using the Ellipse program (ViDiTo, version 2.0.7.1, 2004, Kosice, Slovakia). The entire nerve image and the outer border of the fascicles were delineated at low magnification. Next, the inner areas of three randomly selected fascicles were analysed. The outer border of the nerve fibres and the inner border of myelin were circumscribed (Fig. 2a and b). Morphometric software assessed: (1) percentage of fascicle area per nerve; (2) number and density of nerve fibres; (3) percentage of large fibres per nerve; (4) nerve fibre diameter; (5) axon diameter; and (6) myelin width. A single histologist blinded to group assignment analysed the images.
Figure 2.
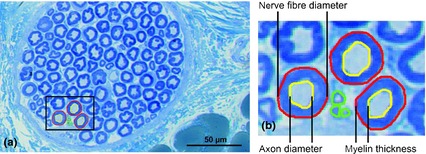
Histomorphometric analysis of the sciatic nerve. Staining with toluidine blue. Scale bar: 50 μm. (a) Cross‐section of the sciatic nerve 14 days after intraneural (extrafascicular) injection of liposomal bupivacaine demonstrating no pathological changes of nerve structure. The outer border of large (red) and small (green) nerve fibres were circumscribed. (b) In large fibres, the inner border of myelin was circumscribed (yellow). Using morphometric software, several parameters were assessed: (1) percentage of fascicle area per nerve; (2) number and density of nerve fibres; (3) percentage of large fibres per nerve; (4) nerve fibre diameter; (5) axon diameter; and (6) myelin width.
Frozen specimens were used to study lymphocytes with CD45 antibody (CD45RA; AbDSerotec, Oxford, UK), macrophages with CD14 antibody (MCA1218; AbDSerotec), and monocytes and granulocytes with Anti‐Monocyte + Granulocyte antibody (ab24991; Abcam, Cambridge, UK). Cross‐sectional nerve areas were captured from all stained serial sections. For each specimen, inflammatory cells were counted in five sections separated by 1 mm, and expressed as mean of immunopositive cells per mm2. The numbers of immunopositive cells from the three stainings were summed (overall count).
Total RNA, extracted with the TRIzol Reagent (Ambion, Life Technologies, Paisley, UK), was reversely transcribed with a High‐Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Warrington, UK). Quantitative PCR was performed on an ABI PRISM SDS 7500 (Applied Biosystems) using TaqMan chemistry (TaqMan Universal PCR Master Mix) and the following Gene Expression Assays: TNF‐α (Ss03391318_g1), IL‐6 (Ss03384604_u1) and β‐actin (Ss03376081_u1) for the internal control. Starting concentrations of target and reference (β‐actin) mRNA were calculated with respect to efficiency of PCR with the aid of the LinRegPCR computer program 22.
Statistical analysis was performed with the Statistical Package for the Social Sciences (SPSS, version 19, 2011, Chicago, IL, USA). Four animals per group were required for power 0.80 to detect a decrease in fibre density of more than 30% (6000 fibres per mm2) with a standard deviation of 2000 fibres per mm2 23. All histomorphometric parameters, the overall count of immunopositive cells, and duration of blockade were compared between the groups using ANOVA. Statistically significant differences in sample means were further evaluated by Dunnett tests, and the corrected p values were reported. Due to the small sample size, a stringent value of < 0.01 was considered as statistically significant in all comparisons. The extent of neurological impairment after nerve blockade was presented with descriptive statistics only.
Results
All animals successfully completed the experiment and had uneventful post‐surgical recovery and weight gain. There were no signs of local or systemic infection in any of the animals.
No changes in nerve fibre density or myelin width indicative of nerve injury were observed (Fig. 2a). The groups did not differ in any of the histomorphometric variables (Table 1). The calculated axon‐myelin ratio (G‐ratio) was approximately 0.4 in all groups.
Table 1.
Percentage of fascicle area per nerve, total fibres per nerve, fibre density in fascicles, fibre area in fascicles, percentage of large fibres per nerve, large fibre diameter, axon diameter and myelin width 14 days after intraneural (extrafascicular) and perineural injections of liposomal bupivacaine and intraneural (extrafascicular) injections of saline. Values are mean (SD)
| Type of injection | Percentage of fascicle area per nerve | Total number of fibres per nerve | Fibre density in fascicles; fibres.mm2 | Fibre area in fascicles; μm2 | Percentage of large fibres per nerve | Large fibre diameter; μm | Axon diameter; μm | Myelin width; μm |
|---|---|---|---|---|---|---|---|---|
| Intraneural liposomal bupivacaine | 47.5 (10.6) | 29817 (10838) | 15074 (7018) | 34.1 (16.2) | 50.8 (17.1) | 8.9 (2.1) | 3.7 (2.0) | 2.6 (0.5) |
| Perineural liposomal bupivacaine | 54.5 (8.3) | 34527 (14516) | 15015 (5572) | 24.1 (4.0) | 65.4 (19.1) | 8.3 (1.6) | 3.1 (0.3) | 2.6 (0.7) |
| Intraneural saline | 56.3 (7.6) | 33051 (20870) | 12254 (3008) | 29.1 (9.3) | 67.7 (15.7) | 9.0 (0.9) | 3.5 (0.6) | 2.7 (0.6) |
| Negative control (non‐injected) | 54.2 (3.7) | 37218 (20870) | 15212 (7267) | 30.2 (13.4) | 61.4 (21.9) | 8.8 (1.4) | 3.5 (1.0) | 2.6 (0.4) |
| p value | 0.059 | 0.843 | 0.214 | 0.100 | 0.450 | 0.905 | 0.844 | 0.990 |
There was no statistically significant difference among groups.
The inflammatory response was minimal (Fig. 3). The predominant types of cells were lymphocytes (Table 2) observed only interfascicularly with no signs of perineural infiltration.
Figure 3.
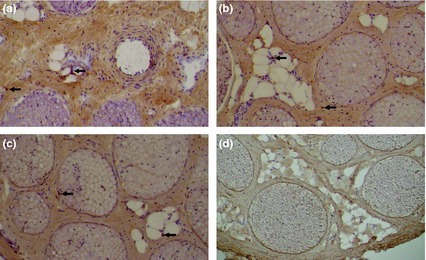
Cross‐section of the left sciatic nerve 14 days after (a) intraneural (extrafascicular) and (b) perineural injection of liposomal bupivacaine and (c) intraneural (extrafascicular) injection of saline, demonstrating minimal lymphocytes (arrows) infiltration. (d) no lymphocyte infiltration was demonstrated in cross‐section of right sciatic nerve (negative control). Immunoreactivity for CD45 (lymphocytes) is presented. Scale bar: 100 μm.
Table 2.
Number of immunopositive cells 14 days after intraneural (extrafascicular) and perineural injections of liposomal bupivacaine and intraneural (extrafascicular) injection of saline. Values are mean (SD)
| Total number of immunopositive cells; per mm2 | Lymphocytes; per mm2 | Macrophages; per mm2 | Granulocytes; per mm2 | |
|---|---|---|---|---|
| Intraneural liposomal bupivacaine | 23 (6) | 12 (5) | 7 (3) | 5 (3) |
| Perineural liposomal bupivacaine | 23 (7) | 13 (4) | 8 (3) | 3 (2) |
| Intraneural saline | 10 (6) | 8 (5) | 2 (1) | 1 (1) |
| p valuea | 0.012 | 0.158 | 0.011 | 0.008 |
| p valueb | 0.008 | 0.135 | 0.003 | 0.190 |
Comparison between intraneural liposomal bupivacaine and intraneural saline.
Comparison between perineural liposomal bupivacaine and intraneural saline.
The groups did not differ in cytokine mRNA content (Fig. 4).
Figure 4.
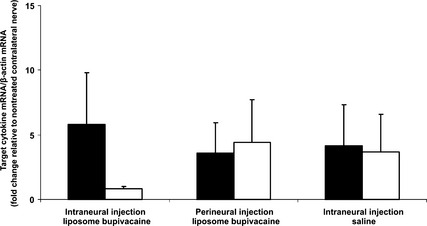
Mean transcript levels (qPCR) of TNF‐α (black bars) and IL‐6 (white bars) following intraneural (extrafascicular) and perineural injection of liposomal bupivacaine and intraneural injection (extrafascicular) of saline. β‐Actin was used as the endogenous control. Error bars = SD. No statistically significant difference between groups.
Mean (SD) duration of sensory blockade was longer after intraneural than after perineural injection of liposomal bupivacaine (12.2 (0.4) h vs 9.2 (1.8) h, respectively, p = 0.003). Intraneural injection of saline resulted in sensory deficit lasting 1.6 (0.9) h. Duration of motor blockade did not differ between intraneural (10.0 (2.0) h) and perineural injection of liposomal bupivacaine (7.2 (1.8) h). In one animal that received an injection of liposomal bupivacaine intraneurally, slight paresis without sensory deficit was observed longer than 24 h, with full recovery within 48 h. Thus, no injections resulted in a persistent motor or sensory deficit throughout the study period (Fig. 5).
Figure 5.
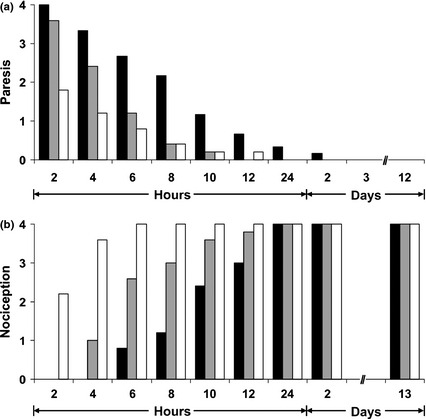
(a) Motor block after intraneural (extrafascicular) (black bars) and perineural injection (grey bars) of liposomal bupivacaine and intraneural (extrafascicular) injection of saline (white bars). 0 no paresis; 1 slight paresis; 2 moderate paresis; 3 severe paresis; 4 flaccid extremity. (b) Sensory block after intraneural (extrafascicular) (black bars) and perineural injection (grey bars) of liposomal bupivacaine and intraneural (extrafascicular) injection of saline (white bars). 0 no withdrawal reaction of the pinched extremity, no vocalisation; 1 barely perceptible withdrawal reaction, no vocalisation; 2 slow withdrawal reaction, no vocalisation; 3 slower (weaker) withdrawal reaction, vocalisation; 4 normal (brisk) withdrawal reaction, vocalisation.
Discussion
Our study found no histological evidence of neurotoxicity following sciatic nerve block with liposomal bupivacaine. The G‐ratio is species‐specific and does not indicate nerve injury, especially since there was no difference among the groups. In a rodent model, for instance, its normal value is 0.6 24, 25. A higher value indicates a decreased amount of myelin and subsequent demyelisation as a predominant type of injury, while a lower value indicates axon atrophy or axonotmesis 25.
A pronounced inflammatory response after intraneural injection can occur independently of any structural lesion 14, which may alter neurological function by a release of toxic mediators via macrophages 26. A slight interfascicular accumulation of inflammatory cells was observed only in the liposomal bupivacaine samples; however, cytokines mRNA (TNF‐α, IL6) were also detected in non‐injected specimens where no inflammatory cells were observed, perhaps the result of systemic inflammation or surgery 27. Additional studies are indicated using higher liposomal bupivacaine dosages, as these may be relevant for patients with poor peripheral circulation where longer exposure to higher concentrations may result in more inflammatory changes.
We found that sensory block was longer for intraneural injections compared with perineural injections; however, none of the animals developed persistent neurological deficit. This is consistent with previous results using a canine model 28, 29, where prolonged sensory block without residual functional impairment was observed after intraneural injection under low injection pressure. Longer duration of neural blockade after intraneural injections 30, 31, 32, 33 can be explained by longer exposure of nerve fascicles to a relatively higher concentration of local anaesthetic 34 distributed in anatomical space with lower systemic absorption and slower dilution by interstitial fluid.
The average duration of sensory block after liposomal bupivacaine injection of approximately 12 h was shorter in our study than in preliminary clinical studies 35, 36. In a recent case report, a prolonged intercostal block duration of 96 h was observed after liposomal bupivacaine administration 37. A dose‐dependent study of femoral blocks revealed partial sensory and motor blocks lasting longer than 24 h after the highest doses of liposomal bupivacaine used, with sizeable inter‐subject variability 35. Epidurally administrated liposomal bupivacaine in doses up to 266 mg resulted in a sensory block of 69 h 36 with an acceptable mean plasma concentration of < 250 ng.l−1 38, 39. As our focus was on neurotoxicity and not on the characteristics of the sensory‐motor blockade, gross sensory‐motor testing was performed to detect overt neurologic deficit rather than subtle sensory blockade. Also, we used a lower dose of liposomal bupivacaine (approximately 2.6 mg.kg−1 or 53 mg) compared with doses used in studies in humans 35, 36. Most importantly, we used an open surgical model where retraction of the surrounding tissue for adequate nerve exposure resulted in some spillage of liposomal bupivacaine into the surrounding tissue. Longer exposure of the nerve in a closed model may be essential for prolonged blockade.
Our study has several important limitations. First, although our surgical approach was minimised to prevent nerve tissue trauma, disruptions of anatomical relationships after dissection were inevitable. Second, the open model we used resulted in drug leakage around the surrounding muscles and fascia, decreasing the exposure to liposomal bupivacaine, which could also account for the shorter block duration. Third, subtle neurological impairment and/or symptoms such as transient paresthesia/dysesthesia in animals may be difficult to detect. Fourth, avoidance of intrafascicular injection was essential in our study to avoid iatrogenic fascicular injury. To decrease the risk of an intrafascicular injection, we avoided injections when nerve stimulation occurred at < 0.3 mA (0.1 ms) or when injection could not be initiated with opening injection pressure < 104 kPa. However, nerve stimulation may not be entirely predictable in defining intraneural versus extraneural injection 40. Likewise, the sensitivity of the opening injection pressure to detect an intrafascicular injection in this model is now known. Finally, our data do not mean that higher doses of liposomal bupivacaine do not carry a risk of neurological damage, or that similar doses will not cause neurological damage in a much larger study.
Competing interests
AH has consulted for Pacira Pharmaceuticals. Research funding: Glaxo Smith‐Kline Industries, Pacira, Baxter. AH receives royalty income from BBraun Medical. Other authors have no competing interests to declare.
Acknowledgements
We thank Prof. Mark Van de Velde from the Department of Anaesthesiology, UZ Leuven for his advice and support of this study. For their assistance with the study, we also thank: Prof. Miha Pintaric from Faculty of Arts, University of Ljubljana; Ivan Blazinovic, Stane Kristl, Majda Crnak‐Maasarani, Marko Slak, Natasa Pollak, Friderik Stendler, Milan Stevanec and Andreja Vidmar, from the Institute of Anatomy, Faculty of Medicine; Ksenja Babic‐Benedik from the Institute of Pathophysiology, Faculty of Medicine, University of Ljubljana; and Jerneja Serdensek DMV from Clinic for Small Animal Medicine and Surgery, Veterinary Faculty, University of Ljubljana.
The study was sponsored by the European Society of Regional Anaesthesia (ESRA Research Grant 2014); the North‐American Institute of Continuous Education (NAICE Research Grant) New York, NY; the Slovenian Research Agency (P3‐0043‐0381, P40053) and the Tertiary funding of the Clinical Department of Anaesthesiology and Intensive Therapy.
Presented in part at the 33rd Annual ESRA Congress, Seville, Spain, September 2014
You can respond to this article at http://www.anaesthesiacorrespondence.com
References
- 1. Epstein‐Barash H, Shichor I, Kwon AH, et al. Prolonged duration local anesthesia with minimal toxicity. Proceedings of the National Academy of Sciences of the United States of America 2009; 106: 7125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lafont ND, Boogaerts JG, Legros FJ. Use of liposome‐associated bupivacaine for the management of a chronic pain syndrome. Anesthesia and Analgesia 1994; 79: 818. [DOI] [PubMed] [Google Scholar]
- 3. Kopacz DJ, Lacouture PG, Wu D, Nandy P, Swanton R, Landau C. The dose response and effects of dexamethasone on bupivacaine microcapsules for intercostal blockade (T9 to T11) in healthy volunteers. Anesthesia and Analgesia 2003; 96: 576–82. [DOI] [PubMed] [Google Scholar]
- 4. Boogaerts JG, Lafont ND, Declercq AG, et al. Epidural administration of liposome‐associated bupivacaine for the management of postsurgical pain: a first study. Journal of Clinical Anesthesia 1994; 6: 315–20. [DOI] [PubMed] [Google Scholar]
- 5. Kohane DS, Langer R. Biocompatibility and drug delivery systems. Chemical Science 2010; 1: 441–6. [Google Scholar]
- 6. Chahar P, Cummings KC 3rd. Liposome bupivacaine: a review of a new bupivacaine formulation. Journal of Pain Research 2012; 5: 257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Richard BM, Rickert DE, Newton PE, et al. Safety evaluation of EXPAREL (DepoFoam bupivacaine) administered by repeated subcutaneous injection in rabbits and dogs: species comparison. Journal of Drug Delivery 2011; 2011: 467429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Golf M, Daniels SE, Onel E. A phase 3, randomized, placebo‐controlled trial of DepoFoam (R) bupivacaine (extended‐release bupivacaine local analgesic) in bunionectomy. Advances in Therapy 2011; 28: 776–88. [DOI] [PubMed] [Google Scholar]
- 9. Bramlett K, Onel E, Viscusi ER, Jones K. A randomized, double‐blind, dose ranging study comparing wound infiltration of DepoFoam bupivacaine, an extended‐release liposome bupivacaine, to bupivacaine HCl for postsurgical analgesia in total knee arthroplasty. Knee 2012; 19: 530–6. [DOI] [PubMed] [Google Scholar]
- 10. Hu D, Onel E, Singla N, Kramer WG, Hadzic A. Pharmacokinetic profile of liposome bupivacaine injection following a single administration at the surgical site. Clinical Drug Investigation 2013; 33: 109–15. [DOI] [PubMed] [Google Scholar]
- 11. Richard BM, Newton P, Ott LR, et al. The safety of EXPAREL (R) (bupivacaine liposome injectable suspension) administered by peripheral nerve block in rabbits and dogs. Journal of Drug Delivery 2012; 2012: 962101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McAlvin JB, Padera RF, Shankarappa SA, et al. Multivesicular liposome bupivacaine at the sciatic nerve. Biomaterials 2014; 35: 4557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sala‐Blanch X, Ribalta T, Rivas E, et al. Structural injury to the human sciatic nerve after intraneural needle insertion. Regional Anesthesia and Pain Medicine 2009; 34: 201–5. [DOI] [PubMed] [Google Scholar]
- 14. Steinfeldt T, Nimphius W, Werner T, et al. Nerve injury by needle nerve perforation in regional anaesthesia: does size matter? British Journal of Anaesthesia 2010; 104: 245–53. [DOI] [PubMed] [Google Scholar]
- 15. Steinfeldt T, Graf J, Schneider J, et al. Histological consequences of needle‐nerve contact following nerve stimulation in a pig model. Anesthesiology Research and Practice 2011; 2011: 591851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Voelckel WG, Klima G, Krismer AC, et al. Signs of inflammation after sciatic nerve block in pigs. Anesthesia and Analgesia 2005; 101: 1844–6. [DOI] [PubMed] [Google Scholar]
- 17. Gadsden JC, Choi JJ, Lin E, Robinson A. Opening injection pressure consistently detects needle‐nerve contact during ultrasound‐guided interscalene brachial plexus block. Anesthesiology 2014; 120: 1246–53. [DOI] [PubMed] [Google Scholar]
- 18. Orebaugh SL, Mukalel JJ, Krediet AC, et al. Brachial plexus root injection in a human cadaver model: injectate distribution and effects on the neuraxis. Regional Anesthesia and Pain Medicine 2012; 37: 525–9. [DOI] [PubMed] [Google Scholar]
- 19. Krol A, Szarko M, Vala A, Andres JD. Pressure monitoring of intraneural an perineural injections into the median, radial, and ulnar nerves; lessons from a cadaveric study. Anesthesilolgy and Pain Medicine 2015; 5: e22723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Claudio R, Hadzic A, Shih H, et al. Injection pressures by anesthesiologists during simulated peripheral nerve block. Regional Anesthesia and Pain Medicine 2004; 29: 201–5. [DOI] [PubMed] [Google Scholar]
- 21. Thalhammer JG, Vladimirova M, Bershadsky B, Strichartz GR. Neurologic evaluation of the rat during sciatic nerve block with lidocaine. Anesthesiology 1995; 82: 1013–25. [DOI] [PubMed] [Google Scholar]
- 22. Ruijter JM, Ramakers C, Hoogaars WM, et al. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Research 2009; 37: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Whitlock EL, Brenner MJ, Fox IK, Moradzadeh A, Hunter DA, Mackinnon SE. Ropivacaine‐induced peripheral nerve injection injury in the rodent model. Anesthesia and Analgesia 2010; 111: 214–20. [DOI] [PubMed] [Google Scholar]
- 24. Farber SJ, Saheb‐Al‐Zamani M, Zieske L, et al. Peripheral nerve injury after local anesthetic injection. Anesthesia and Analgesia 2013; 117: 731–9. [DOI] [PubMed] [Google Scholar]
- 25. Mazzer PY, Barbieri CH, Mazzer N, Fazan VP. Morphologic and morphometric evaluation of experimental acute crush injuries of the sciatic nerve of rats. Journal of Neuroscience Methods 2008; 173: 249–58. [DOI] [PubMed] [Google Scholar]
- 26. Kiefer R, Kieseier BC, Stoll G, Hartung HP. The role of macrophages in immune‐mediated damage to the peripheral nervous system. Progress in Neurobiology 2001; 64: 109–27. [DOI] [PubMed] [Google Scholar]
- 27. Zhang JM, An J. Cytokines, inflammation, and pain. International Anesthesiology Clinics 2007; 45: 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kapur E, Vuckovic I, Dilberovic F, et al. Neurologic and histologic outcome after intraneural injections of lidocaine in canine sciatic nerves. Acta Anaesthesiologica Scandinavica 2007; 51: 101–7. [DOI] [PubMed] [Google Scholar]
- 29. Hadzic A, Dilberovic F, Shah S, et al. Combination of intraneural injection and high injection pressure leads to fascicular injury and neurologic deficits in dogs. Regional Anesthesia and Pain Medicine 2004; 29: 417–23. [DOI] [PubMed] [Google Scholar]
- 30. Robards C, Hadzic A, Somasundaram L, et al. Intraneural injection with low‐current stimulation during popliteal sciatic nerve block. Anesthesia and Analgesia 2009; 109: 673–7. [DOI] [PubMed] [Google Scholar]
- 31. Sala‐Blanch X, Pome′s J, Matute P, et al. Intraneural injection during anterior approach for sciatic nerve block. Anesthesiology 2004; 101: 1027–30. [DOI] [PubMed] [Google Scholar]
- 32. Bigeleisen PE. Nerve puncture and apparent intraneural injection during ultrasound‐guided axillary block does not invariably result in neurologic injury. Anesthesiology 2006; 105: 779–83. [DOI] [PubMed] [Google Scholar]
- 33. Schafhalter‐Zoppoth I, Zeitz ID, Gray AT. Inadvertent femoral nerve impalement and intraneural injection visualized by ultrasound. Anesthesia and Analgesia 2004; 99: 627–8. [DOI] [PubMed] [Google Scholar]
- 34. Lambert L, Lambert D, Strichartz G. Irreversible conduction block in isolated nerve by high concentrations of local anesthetics. Anesthesiology 1994; 80: 1082–93. [DOI] [PubMed] [Google Scholar]
- 35. Ilfeld BM, Malhotra N, Furnish TJ, Donohue MC, Madison SJ. Liposome bupivacaine as a single‐injection peripheral nerve block: a dose‐response study. Anesthesia and Analgesia 2013; 117: 1248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Viscusi ER, Candiotti KA, Onel E, Morren M, Ludbrook GL. The pharmacokinetics and pharmacodynamics of liposome bupivacaine administered via a single epidural injection to healthy volunteers. Regional Anesthesia and Pain Medicine 2012; 37: 616–22. [DOI] [PubMed] [Google Scholar]
- 37. Yin C, Matchett G. Intercostal administration of liposome bupivacaine as a prognostic nerve block prior to phenol neurolysis for intractable chest wall pain. Journal of Pain and Palliative Care Pharmacotherapy 2014; 28: 33–6. [DOI] [PubMed] [Google Scholar]
- 38. Scott DB, Lee A, Fagan D, Bowler GM, Bloomfield P, Lundh R. Acute toxicity of ropivacaine compared with that of bupivacaine. Anesthesia and Analgesia 1989; 69: 563–9. [PubMed] [Google Scholar]
- 39. Bardsley H, Gristwood R, Baker H, Watson N, Nimmo W. A comparison of the cardiovascular effects of levobupivacaine and rac‐bupivacaine following intravenous administration to healthy volunteers. British Journal of Clinical Pharmacology 1998; 46: 245–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Perlas A, Niazi A, McCartney C, Chan V, Xu D, Abbas S. The sensitivity of motor response to nerve stimulation and paresthesia for nerve localization as evaluated by ultrasound. Regional Anesthesia and Pain Medicine 2006; 31: 445–50. [DOI] [PubMed] [Google Scholar]


