Abstract
The glycosphingolipid SSEA‐4 and the glycoprotein YKL‐40 have both been associated with human embryonic and neural stem cell differentiation. We investigated the distribution of SSEA‐4 and YKL‐40 positive cells in proliferative zones of human fetal forebrain using immunohistochemistry and double‐labeling immunofluorescence. A few small rounded SSEA‐4 and YKL‐40 labeled cells were present in the radial glial BLBP positive proliferative zones adjacent to the lateral ganglionic eminence from 12th week post conception. With increasing age, a similarly stained cell population appeared more widespread in the subventricular zone. At midgestation, the entire subventricular zone showed patches of SSEA‐4, YKL‐40, and BLBP positive cells. Co‐labeling with markers for radial glial cells (RGCs) and neuronal, glial, and microglial markers tested the lineage identity of this subpopulation of radial glial descendants. Adjacent to the ventricular zone, a minor fraction showed overlap with GFAP but not with nestin, Olig2, NG2, or S100. No co‐localization was found with neuronal markers NeuN, calbindin, DCX or with markers for microglial cells (Iba‐1, CD68). Moreover, the SSEA‐4 and YKL‐40 positive cell population in subventricular zone was largely devoid of Tbr2, a marker for intermediate neuronal progenitor cells descending from RGCs. YKL‐40 has recently been found in astrocytes in the neuron‐free fimbria, and both SSEA‐4 and YKL‐40 are present in malignant astroglial brain tumors. We suggest that the population of cells characterized by immunohistochemical combination of antibodies against SSEA‐4 and YKL‐40 and devoid of neuronal and microglial markers represent a yet unexplored astrogenic lineage illustrating the complexity of astroglial development. GLIA 2016;64:90–104
Keywords: astrocytes, cerebral cortex, corticogenesis, development, human neural stem cells
Main Points
A newly observed progenitor subtype in human developing subventricular zone is characterized by co‐localization of stem cell marker SSEA‐4 and the glycoprotein YKL‐40.
The results highlight heterogeneity and spatiotemporal diversity of gliogenesis.
Introduction
The human cerebral cortex is generally considered the most complex structure of the human body. The cortical expansion and the enlarged surface area following the highly organized folding into gyri and sulci (cf. Sun and Hevner, 2014), enable extraordinary human cognitive abilities. Although the human brain is not the largest and not the most convoluted brain in the animal kingdom, it has the largest number of neurons. A detailed characterization of the human embryonic and fetal proliferative ventricular and subventricular zones during corticogenesis may aid in defining the properties of human cortical development. The neural progenitor cells underlying the cortical expansion comprise initially neuroepithelial cells, which transform into radial glial cells (RGCs) at the onset of neurogenesis (Götz and Huttner, 2005; Kriegstein and Alvarez‐Buylla, 2009). These neural progenitors, often referred to as neural stem cells (NSCs) (Kriegstein and Alvarez‐Buylla, 2009), give rise to the enormous diversity of neurons and glial cells in the cerebral cortex.
A thorough analysis of the developing monkey cortex showed that the subventricular zone (SVZ) can be further subdivided into an inner SVZ (ISVZ) and outer SVZ (OSVZ) by an inner fibrous layer (Smart et al., 2002). The seminal work of these authors also demonstrated a striking rostral to caudal gradient in differentiation of the cytoarchitectonic compartments of the primate subventricular zone from frontal to occipital cortices (see Discussion). The neocortical SVZ expands even more in the human developing brain (Bayatti et al., 2008; Zecevic et al., 2005), but to a lesser extent in rodents and ferrets (Fietz et al., 2010; Lui et al., 2011; Reillo et al., 2011). At the cellular level recent evidence has shown intermediate progenitor cells (IPCs) and outer radial glial cells (ORGs) in both compartments (Betizeau et al., 2013; Fietz et al., 2010; Hansen et al., 2010; Reillo et al., 2011). They share the expression of classical ventricular RG (VRG) cell markers such as brain lipid‐binding protein (BLBP), glial fibrillary acidic protein (GFAP), vimentin, PAX6, and SOX2, but not IPC markers such as Tbr2 (Fietz et al., 2010; Fietz and Huttner, 2011; Hansen et al., 2010; Reillo et al., 2011). The diversification of RGC progeny in both spatial and temporal aspects is a matter of intense research, and evidence from human and rodent studies point toward distinct subpopulations of RGCs that may be purely gliogenic, purely neurogenic or multipotent (Hartfuss et al., 2001; Howard et al., 2006; Li et al., 2004; Pinto et al., 2008).
The globoseries glycosphingolipid stage‐specific embryonic antigen 4 (SSEA‐4) is widely used in characterization of human embryonic stem cell (hESC) lines (Adewumi et al., 2007), and has been shown in a population of differentiating cells in an intermediate stage between pluripotent hESCs and neural progenitor cells (Noisa et al., 2012). In a previous study of human embryonic central nervous system, SSEA‐4 was found in neural progenitor cells in forebrains of human embryos and early fetuses (Barraud et al., 2007). In spontaneously differentiating hESCs, the highly conserved and secreted glycoprotein YKL‐40 (Bussink et al., 2007), also known as chitinase 3‐like 1 (CHI3L1) was shown to be upregulated when undifferentiated ESCs differentiated, with particular expression in neuroectodermal cells (Brøchner et al., 2012). In the developing human forebrain, YKL‐40 has recently been associated with brain barrier sites such as the radial glial end feet layer at the subpial marginal zone, pericytes of the intermediate and subventricular zones and in the choroid plexus (CHP) epithelium of the lateral ventricles. Furthermore, intriguing small rounded YKL‐40 positive cells in close relation to and occasionally overlapping with GFAP‐positive radial glial fibers in the SVZ was identified (Bjørnbak et al., 2014). The overall distribution of YKL‐40 in the developing human forebrain was noted to be very similar to a study of SSEA‐4 based on the same material collection (Barraud et al., 2007). A wide array of studies have shown overexpression of YKL‐40 in many and diverse pathological conditions. YKL‐40 is overexpressed in glioblastoma multiforme compared to normal tissue, low‐grade gliomas (Nigro et al., 2005; Rousseau et al., 2006; Tanwar et al., 2002) and high‐grade oligodendrogliomas (Nutt et al., 2005; Rousseau et al., 2006). Furthermore, high YKL‐40 expression in glioblastomas is an independent negative prognostic factor (Iwamoto and Hormigo, 2014; Nigro et al., 2005; Pelloski et al., 2005). Many neurological and neurodevelopmental diseases are associated with YKL‐40 (Bonneh‐Barkay et al., 2010; Craig‐Schapiro et al., 2010), however, as to how YKL‐40 mediates its biological effects and its role in normal human developmental biology still lacks some clarification.
In this study, we aimed to investigate the recently described neural progenitor cell marker SSEA‐4 in relation to the newly discovered YKL‐40 positive cell population in the SVZ of developing human forebrain, and examine its proposed astrogenic lineage potential. To this end, we studied human forebrain samples from human embryos and fetuses (8th–21st weeks post conception) by immunohistochemistry and confocal microscopy, with antibodies against SSEA‐4, YKL‐40, and against specific cell types known to reside within the developing human forebrain, for example, RGCs, IPCs, neurons, interneurons, and glial cells including microglial cells.
Materials and Methods
Tissue Samples
Forebrains from one late human embryo, 31 mm crown‐rump length (CRL) and 12 fetuses (38–200 mm CRL) corresponding to 8th–21st weeks post conception (wpc) were examined. The embryo and fetuses were obtained from legal abortions. According to the Helsinki declaration II oral and written information was given and informed consent was obtained from all contributing women, according to and approved by the Research Ethics Committee of the Capital Region (KF–V.100.1735/90). Immediately following operation, the samples were dissected into blocks and fixed for 12–24 h at 4°C in either 10% neutral buffered formalin, 4% Formol‐Calcium, Lillie's or Bouin's fixatives. The specimens were dehydrated with graded alcohols, cleared in xylene and paraffin embedded. Serial sections, 3–10 µm thick, were cut in transverse, sagittal, or horizontal planes, placed on silanized glass slides, and used for single and double immunohistochemical experiments.
Immunohistochemistry
For bright field light microscopy analysis, sections were deparaffinized and rehydrated in xylene following standard protocols. Endogenous peroxidase was quenched using a 0.5% solution of hydrogen peroxide in methanol for 15 min. Following rinses with TRIS buffered saline (TBS, 5 mM Tris‐HCl, 146 mM NaCl, pH 7.6), nonspecific binding was inhibited by incubation for 30 min with blocking buffer (ChemMate antibody diluent S2022, DakoCytomation, Glostrup, Denmark) or 0.2% casein (Sigma, C‐7078) at room temperature. The sections were incubated overnight at 4°C with primary antibodies diluted in blocking buffer and washed with TBS. The REAL EnVision Detection System (Peroxidase/DAB+ rabbit/mouse, code K5007, DakoCytomation, Glostrup, Denmark) was used for detecting mouse and rabbit primaries. The sections were washed with TBS, followed by incubation for 10 min with 3,3′‐diamino‐benzidine chromogen solution. Positive staining was recognized as a brown color. The sections were counterstained with Mayers hematoxylin and dehydrated in graded alcohols followed by xylene and coverslipped with DPX mounting media. For immunofluorescence, sections were prepared as for bright field light microscopy including incubation of the first primary antibody overnight at 4°C. Sections were then incubated for 30 min at room temperature with Labeled Polymer –HRP anti‐mouse (DakoCytomation, EnVisionTM+ System/HRP K4007) followed by Tyramid Signal Amplification (TSA) with Alexa Fluor 488 Tyramide (Invitrogen, Molecular Probes, T20912) for 7 min at room temperature. Subsequently, the sections were incubated for 30 min at room temperature with biotin‐SP‐conjugated F(ab')2 fragment donkey anti‐rabbit antibodies (Jackson ImmunoResearch, 711‐066‐152, 1:200) followed by streptavidin‐conjugated DyLight 594 (Vector Laboratories, SA5594, 1:200). Finally, a nuclear counterstain with DAPI (4′,6‐diamidino‐2‐phenylindole, Invitrogen, Molecular Probes, D1306, 1:1,000) was added for 3 min, before sections were coverslipped. For double labeling of YKL‐40 and SSEA‐4, nonspecific binding was inhibited by incubation in blocking reagent (Invitrogen, Molecular Probes, T20912) for 1 h at room temperature. The sections were then incubated with YKL‐40 diluted in blocking reagent for 48 h at 4°C and washed with TBS. Following this, the secondary antibody was added (Jackson biotin anti‐rabbit 1:100), then HRP‐conjugated streptavidin (Perkin Elmer, NEL 700, 711‐066‐152, 1:100) followed by Tyramid Signal Amplification for 10 min at room temperature. Endogenous peroxidase was quenched with hydrogen peroxide for 30 min prior to the second primary antibody, SSEA‐4, overnight at 4°C followed by Labeled Polymer –HRP anti‐mouse (DakoCytomation, EnVisionTM+ System/HRP K4007) followed by Tyramid Signal Amplification (TSA) with Dylight 594 for 7 min at room temperature.
Monoclonal antibodies against YKL‐40 and SSEA‐4 were used for identification and characterization of the cell population in question; a polyclonal anti‐YKL‐40/anti‐CHI3L1 antibody was used for double labeling with SSEA‐4. RGCs were labeled with GFAP, nestin and BLBP antibodies. IPCs, neurons, and interneurons were identified using antibodies against Tbr2, the neuronal‐specific NeuN and doublecortin (DCX) and the calcium‐binding protein calbindin, respectively. Glial cells were labeled with GFAP, S100, Olig2, and the proteoglycan NG2 antibodies, and microglial cells were distinguished with antibodies against Iba1 and CD68. Details of the primary antibodies including dilutions and suppliers are listed in Table 1. Staining specificity of YKL‐40 was tested on the same material in a recent study (see Fig. 1 in Bjørnbak et al., 2014), and further controls were performed by omitting primary antibodies. For laser scanning confocal microscopy a Carl Zeiss LSM 780 was used. During image acquisition, a sequential scan procedure through the z‐axis of the double‐labeled sections was performed when appropriate, covering in total 9–11 µm in depth. Confocal images were acquired and analyzed, and individual optical sections were stored as TIFF files using Zeiss ZEN Vision v10. Representative images for figure editing were chosen from the analyzed samples and processed in Adobe Photoshop CS6.
Table 1.
List of Primary Antibodies
| Primary antibodies | Host IgG | Dilution | HIER | Producer | Code number |
|---|---|---|---|---|---|
| Stem Cells and Progenitors | |||||
| BLBP | Rabbit IgG | 1:4,000 | – | Millipore | ABN14 |
| Nestin | Rabbit IgG | 1:1,500 | – | Millipore | A5922 |
| GFAP | Rabbit IgG | 1:1,000 | – | Dako | Z0334 |
| GFAP | Rabbit IgG | 1:2,000 | – | Abcam | Ab7260 |
| Tbr2 | Rabbit IgG | 1:200 | – | Millipore | AB2283 |
| Neurons and interneurons | |||||
| NeuN | Mouse IgG1 | 1:500 | TEG, pH9 | Millipore | MAB377 |
| Calbindin | Rabbit IgG | 1:400 | Citrate, pH6 | Abcam | ab25085 |
| DCX | Rabbit IgG | 1:3,000 | – | Cell signaling | 4604 |
| Glial cells | |||||
| NG2 | Rabbit IgG | 1:200 | – | Millipore | AB5320 |
| S100 | Rabbit IgG | 1:800 | Citrate, pH6 | Dako | Z0311 |
| Olig2 | Rabbit IgG | 1:500 | Citrate, pH6 | Abcam | ab81093 |
| Microglial cells | |||||
| CD68 | Mouse IgG1 | 1:400 | – | Dako | M0814 |
| Iba1 | Rabbit IgG | 1:250 | – | Wako | 019‐19741 |
| Other | |||||
| SSEA‐4 | Mouse IgG3 | 1:50/1:100 | – | Millipore | MAB4304 |
| YKL‐40 | Mouse IgG2kb | 1:50/1:100 | – | * | 201.F9 |
| CHI3L1 | Rabbit IgG | 1:10 | – | Proteintech | 12036‐1‐AP |
HIER: Heat Induced Epitope Retrival. TEG: TRIS + ethylene glycol tetraacetic acid buffer. Producers: Abcam, Cambridge, United Kingdom. Cell Signaling, Danvers, MA. Dako, Glostrup, Denmark. Millipore, Merck Life Science, Hellerup, Denmark. Proteintech, Manchester, United Kingdom. Wako, Richmond, VA. *Kind gift from Prof. Paul A. Price, UCSD.
Figure 1.
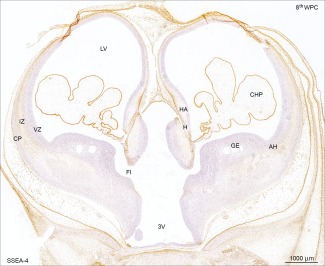
Distribution of SSEA‐4 immunoreactivity in a coronal section of developing forebrain at the level of foramen interventriculare (FI) from a late 8th wpc human embryo (CRL: 31 mm). Note the reactivity of leptomeninges and in particular the choroid plexus (CHP) epithelium compared with that of the telencephalic wall. Immunoreactivity was absent from the ganglionic eminence (GE), antihem (AH) and ventricular zone at this stage. A strikingly similar pattern of YKL‐40 immunoreactivity in a parallel section from the same embryo has recently been demonstrated (Fig.2B in Bjørnbak et al. 2014). Abbreviations: AH: antihem; CHP: choroid plexus; CP: cortical plate; FI: foramen interventriculare; GE: ganglionic eminence; HA: hippocampal anlage; H: hem; IZ: intermediate zone; LV: lateral ventricle; VZ: ventricular zone; 3V: third ventricle. Scale bar: 1,000 µm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Cell Counts/Quantification and Qualification
A total of 16 different sections from occipital cortices of 21 wpc fetuses were doublelabeled with fluorophore‐labeled antibodies against SSEA‐4 or YKL‐40 and BLBP or Tbr2, respectively, and used for counting total and labeled cells of the subventricular zone from occipital cortex. The images were captured with a Carl Zeiss LSM 780 confocal microscope with a 20× objective and imported into the open source software Fiji. The Cell Counter plugin was used for counting SSEA‐4 and YKL‐40 immunopositive cells out of total number of nuclear counterstained DAPI‐positive cells, BLBP‐immunoreactive cells in relation to doublelabeled BLBP and SSEA‐4 or YKL‐40 immunopositive cells and the number of YKL‐40 or SSEA‐4 positive cells that were also Tbr2‐immunolabeled. The different proportions were then calculated.
Results
The characterization of the recently described YKL‐40 positive cell population within the SVZ of developing human forebrain was based on immunohistochemistry and confocal microscopy. We used antibodies against known cell types, which have been associated with the SVZ, such as RGCs (BLBP, GFAP, nestin), IPCs (Tbr2), neurons and migrating interneurons and neurons (DCX, calbindin, NeuN), glial cells (GFAP, S100, NG2, Olig2), and microglial cells (CD68, Iba1). By immunostaining and doublelabeling adjacent sections with antibodies against SSEA‐4 and YKL‐40 we investigated the apparently similar distribution of YKL‐40 and SSEA‐4 in the developing human forebrain, supplied by counting SSEA‐4 and YKL‐40 immunolabeled cells in adjacent sections.
The choroid plexus showed a uniform strong SSEA‐4 reactivity toward the end of 8th wpc and the subpial layer of radial glial end feet was clearly immunoreactive with the strongest reactivity corresponding to the outer surface of the hippocampal anlage and the lateral part of the dorsolateral wall (Fig. 1). Leptomeningeal cells in the pia‐arachnoid and many of the small vessels in the meninges also showed a strong SSEA‐4 immunostaining. These brain barrier‐related recently published findings (Brøchner et al., 2015) will not be dealt with here where the focus is on the subpopulation of SSEA‐4 and YKL‐40 positive cells in SVZ. No positive staining reactions were seen corresponding to the ganglionic eminence, the antihem or the early developing subventricular zone (SVZ). The first indication of cell bodies immunoreactive for SSEA‐4 and YKL‐40 were found in the antihem adjacent to the lateral ganglionic eminence in 12th wpc fetuses. At 15th wpc there was a pronounced reactivity in the antihem (Fig. 2) but scattered positive cells particularly in more rostral parts of the SVZ were also observed. The SSEA‐4 and YKL‐40 positive cell populations in the antihem were not stained with markers for NG2 or calbindin (Fig. 2). Immunoreactivity for the interneuron marker calbindin was particularly prominent in the lateral ganglionic eminence (LGE) adjacent to the antihem, whereas BLBP staining was characteristic for RGCs. Many of the small SSEA‐4 positive cells in the intermediate zone (IZ) were pericytes and thus not a part of the proposed astrogenic subpopulation. The entire end feet layer which was strongly SSEA‐4 and BLBP positive was indicative of all RGCs, which terminate at the subpial basement membrane, and therefore not specifically associated with any subgroup of RGCs (not shown).
Figure 2.
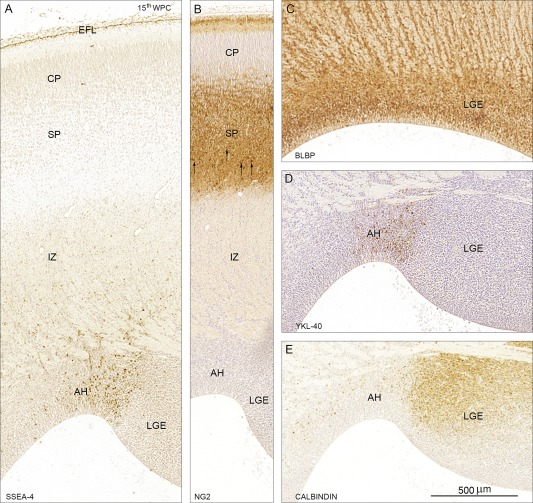
SSEA‐4 (A), NG2 (B), BLBP (C), YKL‐40 (D), and calbindin (E) immunoreactivity in coronal sections of frontal cortex from a 15th wpc human fetus (CRL: 111 mm). A distinct population of small rounded cells in the antihem (AH) is stained for SSEA‐4 (A) and YKL‐40 (D). NG2 positive cells are not associated with the proliferative zones but predominantly found in the lower part of the subplate (SP) indicated by arrows in (B). Immunoreactivity for the interneuron marker calbindin is particularly prominent in the lateral ganglionic eminence (LGE) adjacent to the antihem, whereas BLBP staining is characteristic for all RGCs (C). Many of the small SSEA‐4 positive cells in the intermediate zone (IZ) in (A) are pericytes and thus not a part of the proposed astrogenic subpopulation, and the entire end feet layer (EFL) which is also strongly SSEA‐4 positive and BLBP positive (not shown) is indicative of all RGCs which terminate at the subpial basement membrane and therefore not specifically associated with the astrogenic subpopulation. Abbreviations: AH: antihem; CP: cortical plate; EFL: end feet layer; LGE: lateral ganglionic eminence; IZ: intermediate zone; SP: subplate. Scale bar: 500 µm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Through thorough examination of embryos and fetuses up until midgestation, it was evident that the population of cells in question was most prominent at later stages. To avoid the major interneuron‐producing parts of the SVZ—the ganglionic eminences—we chose to focus our investigations from this point onward to midterm occipital cortices, also a major focus for many developmental studies (see e.g., Smart et al., 2002). In a 21st wpc fetus, the YKL‐40 positive cells were distributed within the inner subventricular zone, and few had migrated through the inner fibrous layer to the outer subventricular zone (Fig. 3). No reactivity was seen in corresponding cells in intermediate zone or cortical plate, apart from pericytic reactivity. The many unstained cells in the SVZ probably belonged to groups of other progenitor cells, interneurons or microglia, harbored in the subventricular zone.
Figure 3.
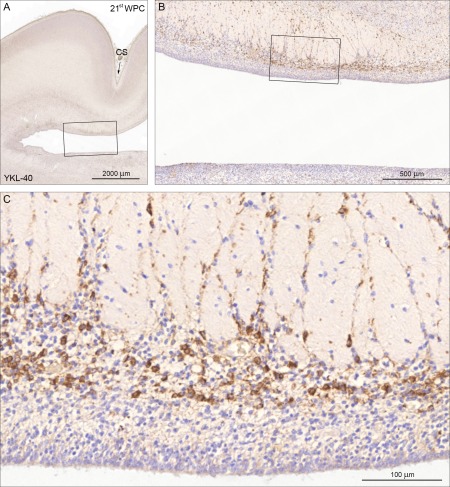
YKL‐40 immunoreactivity in a coronal section of visual cortex from a 21st wpc fetus (CRL: 200 mm). A low power overview is shown in (A) where the calcarine sulcus (CS) is indicated with an arrow. The boxed area is shown in higher magnification in (B) which provides an overview of the distribution of the YKL‐40 positive cells in the inner and outer subventricular zones separated by the inner fibrous layer with migrating positive cells. The boxed area in (B) is shown in higher magnification in (C) where it is obvious that the YKL‐40‐immunoreactive population is not present in the ventricular zone whereas immunostained cells occupy a substantial part of the inner subventricular zone of the visual cortex. The many unstained cells might belong to groups of other progenitor cells, interneurons or microglia. Scale bars: A: 2,000 µm; B: 500 µm; C: 100 µm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Several previous studies have shown that no antibody specifically labels all RGCs throughout development (Noctor et al., 2002; Pinto and Götz, 2007). The population is very heterogeneous, and may change expression profile in a spatiotemporal manner. To further characterize the cell population, we used the radial glial cell marker BLBP double labeled with SSEA‐4 or YKL‐40. In all midterm cases investigated, there was no ventricular SSEA‐4 or YKL‐40 immunoreactivity (Fig. 4). Both SSEA‐4 and YKL‐40 positive cells were present in the SVZ often distributed in small clusters along with or in close vicinity to radial glial cell fibers, rendering the impression that they were migrating along these fibers. The distribution of SSEA‐4 and YKL‐40 positive cells was strikingly similar. Some cells in the innermost part of the SVZ seemed to be only BLBP‐positive and others seemingly only SSEA‐4 or YKL‐40‐positive. A proportion of BLBP immunoreactive cells co‐localized with SSEA‐4 or YKL‐40 immunopositive cells (ranging from 11.6 to 49.6%, averaging 30.8%) and the co‐localization of SSEA‐4 and BLBP was accordingly similar to that of YKL‐40 and BLBP (Fig. 4). The proportion of SSEA‐4 or YKL‐40 positive cells that were not BLBP positive out of all SSEA‐4 or YKL‐40 labeled cells were ranging from 36.3 to 93.2%, averaging 61.1%.
Figure 4.
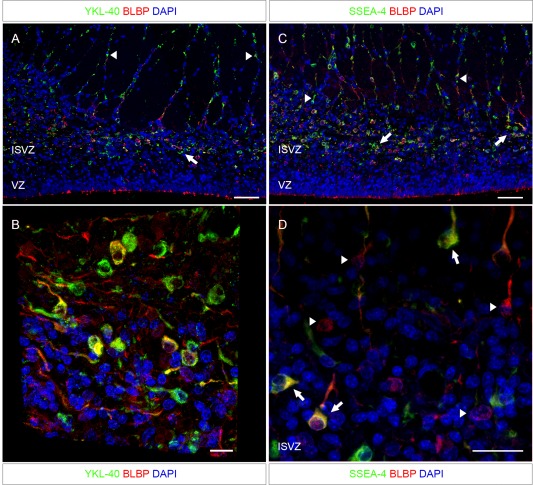
Adjacent sections to that shown in Fig. 3 from the same fetus (21st wpc, CRL: 200 mm) double immunostained for the radial glial cell marker BLBP and YKL‐40 or SSEA‐4. The ventricular zone is lined with BLBP‐reactivity, but shows no YKL‐40 or SSEA‐4 staining. Both YKL‐40 and SSEA‐4 positive cells are apparently migrating along radial glial cell fibers [arrowheads in (A) and (C)]. Some cells in the innermost part of the SVZ seem to be only BLBP‐positive and others seemingly only YKL‐40‐positive. The YKL‐40 and SSEA‐4 positive cells are found in small clusters close to their sites of migration (arrows in (A) and (C)). The distribution of SSEA‐4 positive cells is apparently similar to that of YKL‐40 described in (A), and the co‐localization of SSEA‐4 and BLBP is similar to that of YKL‐40 and BLBP. Higher magnification through the z‐axis of the sections are shown in (B) and (D). (B) A three‐dimensional view of the SVZ, stained for BLBP and YKL‐40, shows overlap between a subset of BLBP positive RGC fibers and YKL‐40 throughout the z‐axis of the section. (D) A maximum projection intensity image of the z‐axis of the ISVZ shows SSEA‐4 positive cells co‐localized with BLBP‐positive cells (arrows). However, some BLBP‐positive cells do not express SSEA‐4 (arrowheads). Abbreviations: ISVZ: inner subventricular zone; VZ: ventricular zone. Scale bars: A, C: 50 µm; B: 10 µm; D: 20 µm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
To examine the distribution in the SVZ of SSEA‐4 and YKL‐40 immunoreactive cells, sections were counted with respect to either SSEA‐4 or YKL‐40 and DAPI as a denominator of all cell nuclei or total cells. Within the given area of occipital cortex at 21 wpc, the proportions were strikingly similar with an average of 11.99% of YKL‐40 immunopositive cells out of all nuclei compared to an average of 10.68% of SSEA‐4 immunopositive cells out of all nuclei (Fig. 5). Using a different, polyclonal antibody against YKL‐40/CHI3L1 we performed double immunolabeling of the same occipital cortical area of a 21st wpc fetus, and found co‐localization of SSEA‐4 and YKL‐40 in the SVZ cell population (Fig. 6).
Figure 5.
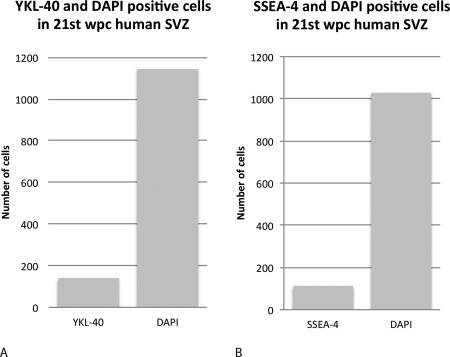
Adjacent sections of occipital cortex subventricular zone from a 21st wpc human fetus stained for SSEA‐4 or YKL‐40 and processed with z‐stacks of equivalent representative areas. Maximum intensity projections are applied, and using Fiji Cell Counter, SSEA‐4, YKL‐40, and DAPI‐positive cells are manually counted on 8 adjacent representative sections. A total of 4,584 and 4,121 DAPI‐positive nuclei are counted from 4 YKL‐40 and 4 SSEA‐4 labeled sections, respectively. A total of 554 YKL‐40 and 442 SSEA‐4 positive cells are counted. Of all the counted cells, in the four YKL‐40 stained sections 11,99% of total cells are YKL‐40 immunopositive and in the other four SSEA‐4‐stained sections 10,68% are SSEA‐4 positive. Mean total values of YKL‐40 and SSEA‐4 out of total cells are shown in A and B, respectively.
Figure 6.
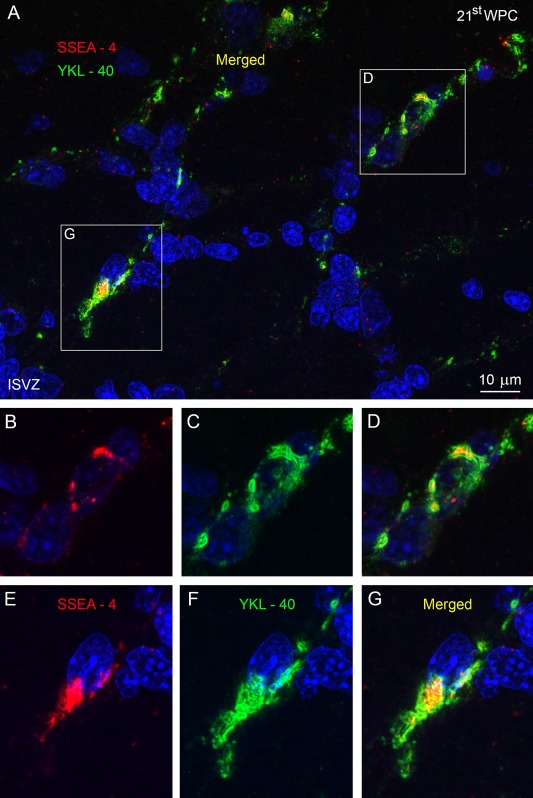
The inner subventricular zone (ISVZ) of occipital cortex from a 21st wpc human fetus (CRL: 200 mm). Double immunolabeling with antibodies against SSEA‐4 (red) and YKL‐40 (green), with nuclei labeled with DAPI (blue). (A) The SSEA‐4 and YKL‐40 positive cells are oriented in the same direction on a migratory trajectory. Merged images of double‐immunolabeled cells are boxed in (A) and shown at higher magnification in (D) and (G), with individual channels in (B), (C) and (E), (F), respectively. The double‐labeled cells show immunoreactivity within the same cell compartments (ER/Golgi apparatus) in close apposition to the nucleus [(D), (G)]. Abbreviations: ISVZ: inner subventricular zone. Scale bar: 10 µm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The previously briefly described relation between this YKL‐40 positive population and GFAP (Bjørnbak et al., 2014) led us to a closer examination of GFAP and YKL‐40. It has been stated, that only a fraction of RGCs express GFAP in humans (and none in mice during development). In occipital cortex, in a 21st wpc fetus, GFAP positive processes from RGCs were situated apically, as a ventricular surface lining much like BLBP (Fig. 7). In contrast, however, hardly any cells were coexpressing GFAP and YKL‐40, and when they did the cells were situated in the apical part of the inner subventricular zone.
Figure 7.
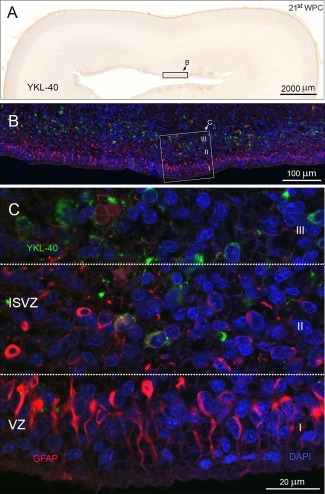
A different area of occipital cortex from a 21st wpc human fetus (CRL: 200 mm) outside the calcarine sulcus. The entire section stained for YKL‐40 is shown in (A) at low magnification. The subventricular zone contains a large pool of YKL‐40‐ immunoreactive cells. Reactivity is also observed in the subpial layer, as described previously. The boxed area corresponds to a similar area of a neighboring section used for double immunolabeling. (B) Examination of YKL‐40 immunoreactivity with RGCs labeled with antibodies against GFAP shows a GFAP‐lined ventricular zone and an YKL‐40 positive population in the SVZ. The boxed area, seen in (C) at higher magnification, shows a differential pattern of GFAP positive cells compared to YKL‐40 positive cells. This is highlighted in three compartments, marked I–III. The ventricular zone (I) contains GFAP positive radial fibers anchoring at the ventricular surface. No YKL‐40 reactivity is observed in this compartment. The basal part of the VZ (II) is a GFAP/YKL‐40 positive zone with hardly any double‐labeled cells, while the deeper layer of the inner SVZ only contains YKL‐40 positive and GFAP negative cells (III). Abbreviations: ISVZ: inner subventricular zone; VZ: ventricular zone. Scale bars: A: 2,000 µm; B: 100 µm; C: 20 µm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In an attempt to narrow down the lineage of these SSEA‐4/YKL‐40 and BLBP positive cells, we used antibodies against astrocytes (S100) and the pan‐neuronal marker NeuN. IPCs were examined with Tbr2 (Fig. 8). The S100 positive astrocytes were scattered through the entire cortical wall although somewhat more abundant in the lower cortical plate/upper subplate and in the inner subventricular zone. The density and distribution of the S100 positive cells were clearly different from those of YKL‐40 stained cells. Double immunolabeling of NeuN together with YKL‐40 demonstrated a lack of colocalization in the SVZ and in the cortical plate. To elucidate whether the cell population was related to IPCs, we used the IPC‐marker Tbr2, and double‐labeled sections for SSEA‐4 and YKL‐40 with Tbr2, respectively. In general the Tbr2 immunoreactive cells were not SSEA‐4 or YKL‐40 positive. However, in a very few cases, SSEA‐4 and YKL‐40 did evidently co‐localize with Tbr2. In four counted sections of 21st wpc occipital cortex, we found 171 SSEA‐4 or YKL‐40 positive cells and only 13 of these were co‐labeled with Tbr2. These cells might be involved in the transition from newborn IPC to fully mature IPC in the subventricular zone. SSEA‐4 and YKL‐40 positive cells possessed a basal process, and we noted that most cells appeared to migrate in the same radial direction, though a minor fraction appeared to be migrating tangentially. Apart from their role in phagocytosis, microglial cells also function as important modulators of neurogenesis (Cunningham et al., 2013). As expected, abundant CD68 and Iba1 positive cells were found in the SVZ. We showed, however, that Iba1 positive cells did not co‐localize with YKL‐40 in the proliferative subventricular zone (Fig. 8).
Figure 8.
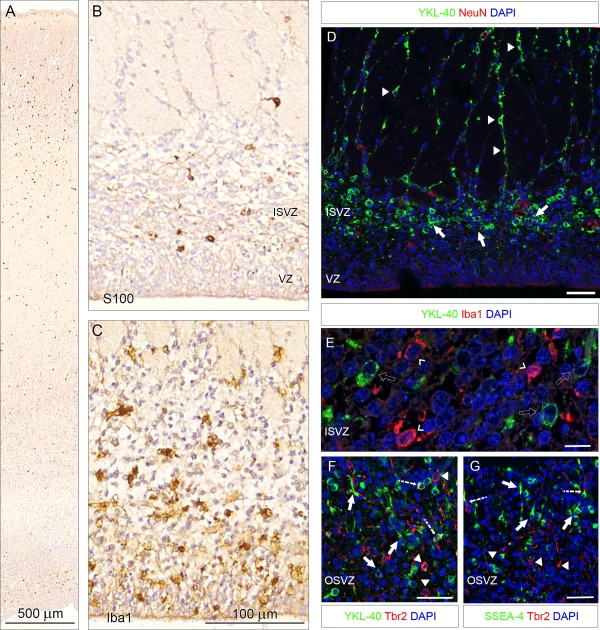
Distribution of S100 (A, B) and Iba1 (C) immunoreactivity in occipital cortex from a 21st wpc fetus (CRL: 200 mm), same fetus as in Figs. 3 and 4. S100 positive astrocytes are scattered through the entire cortical wall although somewhat more abundant in the lower cortical plate/upper subplate and in the inner subventricular zone (A) which is shown in higher magnification in (B). Note that the density and distribution of the S100 positive cells in (B) is different from that of YKL‐40 stained cells in (D). Iba1 stained microglial cells are present in both ventricular and inner subventricular zones in (C) but do not co‐localize with YKL‐40 positive cells (see E). D–G show double immunostainings. The pan‐neuronal marker NeuN together with YKL‐40 (D) demonstrate no YKL‐40 immunoreactive neurons in the SVZ. The YKL‐40 positive population seems to cluster in small groups (arrows) prior to migration on a fiber scaffold (arrowheads). Note the simultaneous migration of NeuN‐positive cells (open arrowheads) and YKL‐40 positive cells on the same radial glial fiber (arrowheads). In (E), double immunolabeling for regulatory microglia in the inner SVZ with the microglia marker Iba1 and YKL‐40 (open arrow), showed no crossreactivity, indicating that the YKL‐40 positive cell population is not of microglial origin. In adjacent sections (F, G), staining for YKL‐40 and SSEA‐4 and intermediate progenitor cells with Tbr2, respectively, depicts distinct populations in the OSVZ. Virtually no co‐localization is observed, however in a very few cases, YKL‐40 and SSEA‐4 do co‐localize with Tbr2 (dashed arrows). Solid arrows indicate monolabeled YKL‐40 and SSEA‐4 positive cells with a basal process. Arrowheads indicate IPCs that are only immunoreactive to Tbr2. Note that most cells appear to migrate in the same radial direction though tangential migration is also observed. Abbreviations: ISVZ: inner subventricular zone; OSVZ: outer subventricular zone; VZ: ventricular zone. Scale bars: A: 500 µm; B, C: 100 µm; E: 10 µm; D, F, G: 50 µm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Finally we performed double immunolabeling and compared the distribution of the co‐localized SSEA‐4 and YKL‐40 immunoreactive cell population in basal VZ and SVZ with cell populations immunostained for the progenitor marker nestin, the oligodendrocyte progenitor marker Olig2 and the marker for migrating neuronal precursors DCX. These markers have all been described in human midgestation ventricular and subventricular zones (e.g., Messam et al., 2002; Jakovcevski and Zecevic, 2005a,b; Meyer et al., 2002). Most of the VZ cells but very few cells in ISVZ at midgestation of occipital cortex showed immunoreactivity for nestin in marked contrast to the abundant SSEA‐4 immunopositive cells in ISVZ and lack of SSEA‐4 staining in the VZ (Fig. 9A). Olig2 and YKL‐40 immunoreactivity in midgestation parietal cortex showed a pattern of distribution similar to that of nestin and SSEA‐4 double immunolabeling. Very few cell bodies in the outer VZ were positively stained for YKL‐40 and the merged images showed no overlap (Fig. 9B). Double immunolabeling of medial temporal cortex with antibodies against DCX and SSEA‐4 demonstrated many SSEA‐4 immunopositive cell bodies in ISVZ and many DCX positive fibers in both VZ and ISVZ but without overlap in merged images (Fig. 9C). Nestin, Olig2 and DCX positive cells were as expected present in many developmental regions where we found no trace of SSEA‐4/YKL‐40 reactivity.
Figure 9.
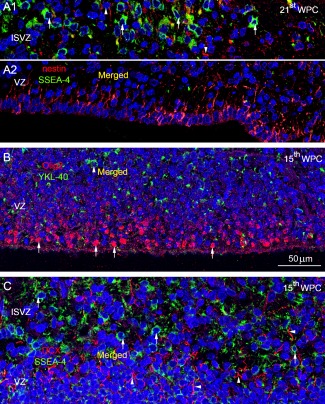
Distribution of nestin (A), Olig2 (B), and DCX (C) in relation to SSEA‐4/YKL‐40 shown in merged DAPI stained images. The inner subventricular zone (ISVZ) (A1) and ventricular zone (VZ) (A2) of occipital cortex from a 21st wpc human fetus (CRL: 200 mm), double‐immunolabeled with antibodies against nestin (red) and SSEA‐4 (green), and nuclei labeled with DAPI (blue). The outer VZ which showed no immunoreactivity was left out. Note the abundant SSEA‐4 immunopositive cells in ISVZ (arrows) and the very few nestin‐positive cells (arrow‐heads). In VZ adjacent to the lateral ventricle the majority of the cells are nestin‐positive whereas no SSEA‐4 positive cells are present (A1). The few yellow strokes in (A1) probably represent closely apposed fibers belonging to SSEA‐4 and nestin positive cells in the merged image. In (B) many nuclei of cells in the inner VZ of parietal cortex from a 15th wpc human fetus (CRL: 110 mm), are positive for Olig2 (arrows) whereas very few cell bodies are immunoreactive for YKL‐40 (arrowhead) in the outer VZ. The merged images show no overlap. In (C) the ISVZ and outer VZ in the medial wall of the temporal cortex from the same fetus are double immunolabeled with antibodies against DCX (red) and SSEA‐4 (green). Note the abundant SSEA‐4 immunopositive cells in ISVZ (arrows) and the DCX labeled fibers in both VZ and ISVZ (arrowheads). The merged images show no overlap. Abbreviations: DCX: doublecortin; ISVZ: inner subventricular zone; SVZ: subventricular zone; VZ: ventricular zone. A–C, same magnification. Scale bar: 50 µm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Discussion
So far YKL‐40 has been associated with various pathological conditions and promoted as a factor with profound implications for both diagnostic and therapeutic applications (Prakash et al., 2013), whereas the general role of YKL‐40 in developmental biology has been largely ignored with a few exceptions (Brøchner et al., 2012; Johansen et al., 2007). A very recent investigation of YKL‐40 in the developing human forebrain showed marked YKL‐40 immunoreactivity in neuroepithelial cells, radial glial end feet, leptomeninges and choroid plexus epithelial cells in early stages of development. Later developmental features included strong YKL‐40 immunoreactivity in astrocyte‐resembling cells in the developing hippocampus, and a so far unknown population of small rounded YKL‐40 positive cells in close relation to and occasionally overlapping with GFAP‐positive radial glial fibers in the SVZ at midgestation (Bjørnbak et al., 2014). In an earlier study partly based on the same material, SSEA‐4 was depicted as a marker for some NSCs and showed immunoreactivity very similar to YKL‐40 in the early human forebrain (Barraud et al., 2007). Furthermore SSEA‐4 has been proposed as a potential therapeutic target in glioblastoma multiforme (Lou et al., 2014), much in the same way as YKL‐40 has. An important part of the present study is a novel characterization of SSEA‐4 in the late embryonic human forebrain where we found a marked SSEA‐4 immunoreactivity in radial glial end feet, leptomeninges, and choroid plexus epithelial cells in early stages of development. Later developmental features included a population of small rounded SSEA‐4 positive cells, first in the antihem and later scattered in different parts of the SVZ, with the same spatiotemporal distribution as the YKL‐40 positive cells.
RGCs show a characteristic apicobasal polarity, with an apical process lining the ventricle and a basal process spanning the neuroepithelium and anchoring at the pial border. The cell bodies occupy the ventricular zone (VZ), and through rounds of asymmetric divisions, they give rise to IPCs, which in turn produce two post‐mitotic neurons or in some cases an additional pair of progenitors (Englund et al., 2005; Molnár et al., 2014; Noctor et al., 2004; Pontious et al., 2008; Vasistha et al., 2014).
The IPCs create the second proliferative layer just basal to the VZ, namely the subventricular zone (SVZ) (Bystron et al., 2008; Møllgård and Jacobsen, 1984), which subdivides into an inner and outer subventricular zone, separated by a fibrous layer (Bayatti et al., 2008; Smart et al., 2002; Zecevic et al., 2005) as mentioned in the Introduction. Based on coronal and sagittal sections through the developing monkey neocortex Smart et al. (2002) clearly distinguished between cytoarchitectonic compartments (VZ, ISVZ, and OSVZ) and their rostrocaudal gradient of histogenesis showing a decreasing depth of all layers except the VZ from prefrontal to occipital cortex. The ISVZ is composed of cells oriented in various directions whereas the OSVZ exhibits a columnar structure composed of radially oriented nuclei (Smart et al., 2002). Although the OSVZ cells (or outer radial glial‐like cells—ORGCs) were first thought to be a unique cell type in fetal primate neocortex, counterparts have also been observed in species such as ferrets (Fietz et al., 2010) and mice (Shitamukai et al., 2011; Wang et al., 2011). Thus, although mice do not possess the same cytoarchitectonic compartments as primates they have the same progenitor populations but in different proportions. As summarized very recently by Hoerder‐Suabedissen and Molnár (2015): To date, all proliferative regions and modes of cell division identified in primate brains have also been documented in rodent brains and other large and small, lissencephalic and gyrencephalic brains (Garcia‐Moreno et al., 2012), although the SVZ is disproportionately smaller in mice than in large primates (Cheung et al., 2010). Thus, not only are ORGs not primate specific, but their presence in large numbers is not the cause of brain folding as it was originally suggested (Garcia‐Moreno et al., 2012).
The recent seminal work by several groups that led to the identification of ORGCs within the OSVZ has primarily focused on the neurogenic potential of these cells (Betizeau et al., 2013; Fietz et al., 2010; Hansen et al., 2010; Kelava et al., 2012; Reillo et al., 2011). The diversification of RGC progeny in both spatial and temporal aspects is a matter of intense research, and evidence from human and rodent studies point toward distinct subpopulations of RGCs that may be purely gliogenic, purely neurogenic or multipotent (Hartfuss et al., 2001; Howard et al., 2006; Li et al., 2004; Pinto et al., 2008). This suggestion is very much supported by our findings that Tbr2 is not expressed in the majority of these SSEA‐4/YKL‐40 immunopositive progenitors. A Tbr2 expressing lineage has been recently studied in the developing mouse brain by Vasistha et al. (2014) who found no glial cell lineage arising from Tbr2‐positive intermediate progenitors using the CLoNe method. In humans, so far no solid setup exists, that enables the segregation of ORGCs into neuronal, multipotent, or gliogenic subpopulations. The ORGCs differ in morphology from ventricular RGCs in that they are delaminated from the adherens junctions of the RGC epithelium, and they lack an apical process, but, however, retain a basal anchor (Betizeau et al., 2013; Fietz et al., 2010; Hansen et al., 2010).
When examining for RGC markers (BLBP, GFAP, nestin), neuronal and known glial markers, a subgroup of SSEA‐4 and YKL‐40 positive cells only co‐localize with BLBP within the ISVZ and not with the other markers used. This indicates that the SSEA‐4 YKL‐40 cell population is non‐neuronal descendants from RGCs. Interestingly, cell culture studies of human midgestation dissociated VZ/SVZ showed many diverse subtypes of RGCs among which a subtype of dividing cells not labeled with 4A4 or neuronal markers were suggested to represent a multipotent precursor or restricted progenitor line identified by a another yet unused antibody (Howard et al., 2006). The microtubule‐associated protein DCX, that is expressed by migrating neuronal precursors show no overlap with the investigated cell population. Based on cell counts of immunoreactive SSEA‐4 and YKL‐40 positive cells in adjacent sections and in equal regions of a 21 wpc occipital cortical area, we show that cell numbers between these two are strikingly equivalent within the SVZ. On a morphological basis, the cells express the same characteristics, with approximately the same nuclear size and a migrating profile, and co‐localization from double labeling studies of SSEA‐4 and YKL‐40 confirms that the cell populations marked by SSEA‐4 or YKL‐40 do correspond to one another.
Both GFAP and nestin are structural filaments found in various cell types, and also in subsets of glial cells and RGCs. Double labeling with GFAP and YKL‐40 and with nestin and SSEA‐4 revealed that hardly any cells co‐labeled in the basal part of the ISVZ. A very recent analysis of the clonal dispersion of astrocytes in mouse cerebral cortex indicates that heterogeneous astroglial populations arise from specialized progenitor cells (García‐Marqués and López‐Mascaraque, 2013). In a study by Singh et al. (Singh et al., 2011), the expression of YKL‐40 during in vitro differentiation of human neural progenitors into astrocytes was dramatically increased during astrocyte differentiation. Moreover, YKL‐40 was easily detected in primary cultures of human embryonic astrocytes. In mice, the stem cell marker SSEA‐1, the SSEA‐4 counterpart in hESC lines, has interestingly been associated with a subpopulation of astrocytes in the adult SVZ progenitor cells and developmental studies of rat brain showed SSEA‐1 in telencephalic germinal zones (Capela and Temple, 2002). It should be noted that SSEA‐4 is associated with human pluripotent stem cells of the inner cell mass, while the murine counterpart associated with pluripotent stem cells is SSEA‐1 (Henderson et al., 2002).
The nonpolarized IPCs of the ISVZ are neuronal descendants of ventricular RGCs. A commonly used IPC marker is the T‐box transcription factor Tbr2, and in our study we found that the majority of Tbr2‐positive IPCs did not co‐localize with either SSEA‐4 or YKL‐40. However, a few SSEA‐4 or YKL‐40 positive cells did co‐express Tbr2, and these double‐labeled cells possessed a leading process uncharacteristic for IPCs. They might depict an intermediate differentiated stage, as the IPCs migrate to the final location in the ISVZ and OSVZ. As gliogenesis progresses particularly from midgestation, appearing astrocytes are appreciated as an ultimately very heterogeneous population of cells, with distinct progenitors and diverse important functions in both normal and diseased brain. We examined glial markers such as S100 (astrocytes) and NG2 and Olig2 (oligodendrocytes). The sequence of oligodendrocyte development in human fetal forebrain from early oligodendrocyte progenitor cells to mature oligodendrocytes was described by Jakovcevski and Zecevic (2005a) and the distribution of Olig2 from second trimester (15th gestational week) was elucidated in a following paper (Jakovcevski and Zecevic, 2005b). At midgestation Olig2 positive nuclei were mainly positioned close to the VZ surface, see also Fig. 1 in Mo and Zecevic (2009). No cells were found to express these glial markers in combination with SSEA‐4 or YKL‐40. However, this does not rule out that the identified population is in fact part of the astroglial lineage, as the panel of astrocyte markers is by now not sufficiently extensive. YKL‐40 and SSEA‐4 may prove to be functionally relevant in this matter.
Another important cell type within the subventricular zone is the microglial cell. Blood monocytes are known to enter the early human forebrain via the cortical plate and meninges to become amoeboid microglial cells (Aguzzi et al., 2013). Microglial cells have been shown to be important modulators of neurogenesis, and during early human development they are localized to the ISVZ, subplate, lower cortical plate, and restricted laminar bands at the axonal crossroads in the white matter (Cunningham et al., 2013; Rezaie et al., 2005; Verney et al., 2010). Studies indicate that microglia do not show YKL‐40 staining in vivo (Bonneh‐Barkay et al., 2010; Craig‐Schapiro et al., 2010). In concert with these findings, the abundant population of Iba1 positive cells within the ISVZ in 21st wpc fetuses did not co‐localize with YKL‐40, indicating that the SSEA‐4 and YKL‐40 positive population is not of microglial origin. We did not observe any co‐localization throughout the examined fetuses.
The NSCs and neural progenitor cells, that is, neuroepithelial cells and RGCs, are as previously described characterized by a panel of molecular markers as well as ultrastructural and morphological features. SSEA‐4 and YKL‐40 have so far not been a part of this panel, but our results suggest that these may be future important markers for a particular fraction of RGCs with astrogenic potential.
In summary, we characterized this SSEA‐4 and YKL‐40 positive cell population, by means of a range of molecular markers for cell types found within the subventricular zone. Co‐localization for both SSEA‐4 and YKL‐40 was only evident for a subset of BLBP positive RGCs, and not for the other markers used. The late appearance of this intriguing population of SSEA‐4 and YKL‐40 positive cells in the SVZ corresponds to initiation of gliogenesis (Rakic, 1988), and several studies have linked YKL‐40 expression to astrocytes in vitro and in vivo (Bonneh‐Barkay et al., 2012; Singh et al., 2011). The previous description based on the same material, of late appearing YKL‐40 positive astrocyte‐resembling cells within the fimbria, a neuron‐free region of the hippocampus, strongly supports this notion (see Fig. 10 in Bjørnbak et al., 2014). In line with these observations, we suggest that our newly identified subgroup of SSEA‐4 and YKL‐40 positive cells constitute a fraction of astroglial progenitors, and suggest the name SYPAP (SSEA‐4 and YKL‐40 positive astroglial progenitors) for this particular population. Immunoreactivity of SYPAPs depends critically on the stage of development and the region of the forebrain studied and follows the rostrocaudal gradient of histogenesis described by Smart et al. (2002). We hypothesize that this population has a developmental important role and may prove to be important for understanding human cortical development and glial functioning. Identification of an astroglial progenitor population based on the same molecular markers including SSEA‐4 and YKL‐40 in a rodent model has to our knowledge not yet been performed. The subventricular zone at mid‐gestation harbors many different cell types including interneurons, microglia and combinations of neurons, glial, and RGCs. Subclassification of SVZ progenitors based on single cell gene analysis may be an ultimate way to shed more light onto the diverse behavior of progenitors within the germinal zones, and thus aiding our understanding of late corticogenesis. Whether these cells also play a role in origin or maintenance of glioblastoma multiforme, or if they contribute to the population of adult human NSCs in the subependymal zone (Alvarez‐Buylla et al., 2002), warrants further examination.
Acknowledgment
We thank H. Hadberg, P. S. Froh, and K. Ottosen, Department of Cellular and Molecular Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark for excellent technical assistance. We thank Professor Paul A Price, Department of Biology, University of California, San Diego, for providing the YKL‐40 antibody, and Professor Julia S. Johansen, M.D., and Camilla Bjørnbak, M.D. for thorough discussions of the manuscript. The authors declare no conflict of interest.
References
- Adewumi O, Aflatoonian B, Ahrlund‐Richter L, Amit M, Andrews PW, Beighton G, Bello PA, Benvenisty N, Berry LS, Bevan S, Blum B, Brooking J, Chen KG, Choo ABH, Churchill GA, Corbel M, Damjanov I, Draper JS, Dvorak P, Emanuelsson K, Fleck RA, Ford A, Gertow K, Gertsenstein M, Gokhale PJ, Hamilton RS, Hampl A, Healy LE, Hovatta O, Hyllner J, Imreh MP, Itskovitz‐Eldor J, Jackson J, Johnson JL, Jones M, Kee K, King BL, Knowles BB, Lako M, Lebrin F, Mallon BS, Manning D, Mayshar Y, McKay RDG, Michalska AE, Mikkola M, Mileikovsky M, Minger SL, Moore HD, Mummery CL, Nagy A, Nakatsuji N, O'Brien CM, Oh SKW, Olsson C, Otonkoski T, Park K‐Y, Passier R, Patel H, Patel M, Pedersen R, Pera MF, Piekarczyk MS, Pera RaR, Reubinoff BE, Robins AJ, Rossant J, Rugg‐Gunn P, Schulz TC, Semb H, Sherrer ES, Siemen H, Stacey GN, Stojkovic M, Suemori H, Szatkiewicz J, Turetsky T, Tuuri T, van den Brink S, Vintersten K, Vuoristo S, Ward D, Weaver TA, Young LA, Zhang W. 2007. Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat Biotechnol 25:803–816. [DOI] [PubMed] [Google Scholar]
- Aguzzi A, Barres BA, Bennett ML. 2013. Microglia: scapegoat, saboteur, or something else? Science 339:156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez‐Buylla A, Seri B, Doetsch F. 2002. Identification of neural stem cells in the adult vertebrate brain. Brain Res Bull 57:751–758. [DOI] [PubMed] [Google Scholar]
- Barraud P, Stott S, Møllgård K, Parmar M, Björklund A. 2007. In vitro characterization of a human neural progenitor cell coexpressing SSEA4 and CD133. J Neurosci Res 85:250–259. [DOI] [PubMed] [Google Scholar]
- Bayatti N, Moss JA, Sun L, Ambrose P, Ward JFH, Lindsay S, Clowry GJ. 2008. A molecular neuroanatomical study of the developing human neocortex from 8 to 17 postconceptional weeks revealing the early differentiation of the subplate and subventricular zone. Cereb Cortex 18:1536–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betizeau M, Cortay V, Patti D, Pfister S, Gautier E, Bellemin‐Ménard A, Afanassieff M, Huissoud C, Douglas RJ, Kennedy H, Dehay C. 2013. Precursor diversity and complexity of lineage relationships in the outer subventricular zone of the primate. Neuron 80:442–457. [DOI] [PubMed] [Google Scholar]
- Bjørnbak C, Brøchner CB, Larsen LA, Johansen JS, Møllgård K. 2014. Brain barriers and a subpopulation of astroglial progenitors of developing human forebrain are immunostained for the glycoprotein YKL‐40. J Histochem Cytochem 62:369–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneh‐Barkay D, Bissel SJ, Kofler J, Starkey A, Wang G, Wiley CA. 2012. Astrocyte and macrophage regulation of YKL‐40 expression and cellular response in neuroinflammation. Brain Pathol 22:530–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneh‐Barkay D, Wang G, Starkey A, Hamilton RL, Wiley CA. 2010. In vivo CHI3L1 (YKL‐40) expression in astrocytes in acute and chronic neurological diseases. J Neuroinflammation 7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brøchner CB, Holst CB, Møllgård K. 2015. Outer brain barriers in rat and human development. Front Neurosci 9:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brøchner CB, Johansen JS, Larsen LA, Bak M, Mikkelsen HB, Byskov AG, Andersen CY, Møllgård K. 2012. YKL‐40 is differentially expressed in human embryonic stem cells and in cell progeny of the three germ layers. J Histochem Cytochem 60:188–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussink AP, Speijer D, Aerts JMFG, Boot RG. 2007. Evolution of mammalian chitinase(‐like) members of family 18 glycosyl hydrolases. Genetics 177:959–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystron I, Blakemore C, Rakic P. 2008. Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci 9:110–122. [DOI] [PubMed] [Google Scholar]
- Capela A, Temple S. 2002. LeX/ssea‐1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron 35:865–875. [DOI] [PubMed] [Google Scholar]
- Cheung AF, Kondo S, Abdel‐Mannan O, Chodroff RA, Sirey TM, Bluy LE, Webber N, DeProto J, Karlen SJ, Krubitzer L, Stolp HB, Saunders NR, Molnár Z. 2010. The subventricular zone is the developmental milestone of a 6‐layered neocortex: Comparisons in metatherian and eutherian mammals. Cereb Cortex 20:1071–1081. [DOI] [PubMed] [Google Scholar]
- Craig‐Schapiro R, Perrin RJ, Roe CM, Xiong C, Carter D, Cairns NJ, Mintun MA, Peskind ER, Li G, Galasko DR, Clark CM, Quinn JF, D'Angelo G, Malone JP, Townsend RR, Morris JC, Fagan AM, Holtzman DM. 2010. YKL‐40: A novel prognostic fluid biomarker for preclinical Alzheimer's disease. Biol Psychiatry 68:903–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Martínez‐Cerdeño V, Noctor SC. 2013. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci 33:4216–4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund C, Fink A, Lau C, Pham D, Daza RAM, Bulfone A, Kowalczyk T, Hevner RF. 2005. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J Neurosci 25:247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fietz SA, Huttner WB. 2011. Cortical progenitor expansion, self‐renewal and neurogenesis ‐ a polarized perspective. Curr Opin Neurobiol 21:23–35. [DOI] [PubMed] [Google Scholar]
- Fietz SA, Kelava I, Vogt J, Wilsch‐Bräuninger M, Stenzel D, Fish JL, Corbeil D, Riehn A, Distler W, Nitsch R, Huttner WB. 2010. OSVZ progenitors of human and ferret neocortex are epithelial‐like and expand by integrin signaling. Nat Neurosci 13:690–699. [DOI] [PubMed] [Google Scholar]
- García‐Marqués J, López‐Mascaraque L. 2013. Clonal identity determines astrocyte cortical heterogeneity. Cereb Cortex 23:1463–1472. [DOI] [PubMed] [Google Scholar]
- Garcia‐Moreno F, Vasistha NA, Trevia N, Bourne JA, Molnár Z. 2012. Compartmentalization of cerebral cortical germinal zones in a lissencephalic primate and gyrencephalic rodent. Cereb Cortex 22:482–492. [DOI] [PubMed] [Google Scholar]
- Götz M, Huttner WB. 2005. The cell biology of neurogenesis. Nat Rev Mol Cell Biol 6:777–788. [DOI] [PubMed] [Google Scholar]
- Hansen DV, Lui JH, Parker PRL, Kriegstein AR. 2010. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature 464:554–561. [DOI] [PubMed] [Google Scholar]
- Hartfuss E, Galli R, Heins N, Götz M. 2001. Characterization of CNS Precursor Subtypes and Radial Glia. Dev Biol 229:15–30. [DOI] [PubMed] [Google Scholar]
- Henderson J, Draper J, Baillie H, Fishel S, Thomson J, Moore H, Andrews P. 2002. Preimplantation human embryos and embryonic stem cells show comparable expression of stage‐specific embryonic antigens. Stem Cells 20:329–337. [DOI] [PubMed] [Google Scholar]
- Hoerder‐Suabedissen A, Molnár Z. 2015. Development, evolution and pathology of neocortical subplate neurons. Nat Rev Neurosci 16:133–46. [DOI] [PubMed] [Google Scholar]
- Howard B, Chen Y, Zecevic N. 2006. Cortical progenitor cells in the developing human telencephalon. Glia 53:57–66. [DOI] [PubMed] [Google Scholar]
- Iwamoto FM, Hormigo A. 2014. Unveiling YKL‐40, from Serum Marker to Target Therapy in Glioblastoma. Front Oncol 4:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakovcevski I, Zecevic N. 2005a. Sequence of oligodendrocyte development in the human fetal telencephalon. Glia 49:480–491. [DOI] [PubMed] [Google Scholar]
- Jakovcevski I, Zecevic N. 2005b. Olig transcription factors are expressed in oligodendrocyte and neuronal cells in human fetal CNS. J Neurosci 25:10064–10073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JS, Høyer PE, Larsen LA, Price PA, Møllgård K. 2007. YKL‐40 protein expression in the early developing human musculoskeletal system. J Histochem Cytochem 55:1213–1228. [DOI] [PubMed] [Google Scholar]
- Kelava I, Reillo I, Murayama AY, Kalinka AT, Stenzel D, Tomancak P, Matsuzaki F, Lebrand C, Sasaki E, Schwamborn JC, Okano H, Huttner WB, Borrell V. 2012. Abundant occurrence of basal radial glia in the subventricular zone of embryonic neocortex of a lissencephalic primate, the common marmoset Callithrix jacchus. Cereb Cortex 22:469–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez‐Buylla A. 2009. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci 32:149–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Babiarz J, Woodbury J, Kane‐Goldsmith N, Grumet M. 2004. Spatiotemporal heterogeneity of CNS radial glial cells and their transition to restricted precursors. Dev Biol 271:225–238. [DOI] [PubMed] [Google Scholar]
- Lou Y‐W, Wang P‐Y, Yeh S‐C, Chuang P‐K, Li S‐T, Wu C‐Y, Khoo K‐H, Hsiao M, Hsu T‐L, Wong C‐H. 2014. Stage‐specific embryonic antigen‐4 as a potential therapeutic target in glioblastoma multiforme and other cancers. Proc Natl Acad Sci USA 111:2482–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui JH, Hansen DV, Kriegstein AR. 2011. Development and evolution of the human neocortex. Cell 146:18–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messam CA, Hou J, Berman JW, Major EO. 2002. Analysis of the temporal expression of nestin in human fetal brain derived neuronal and glial progenitor cells. Brain Res Dev Brain Res 134:87–92. [DOI] [PubMed] [Google Scholar]
- Meyer G, Perez‐Garcia CG, Gleeson JG. 2002. Selective expression of doublecortin and LIS1 in developing human cortex suggests unique modes of neuronal movement. Cereb Cortex 12:1225–1236. [DOI] [PubMed] [Google Scholar]
- Mo Z, Zecevic N. 2009. Human fetal radial glia cells generate oligodendrocytes in vitro. Glia 57:490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnár Z, Kaas JH, de Carlos JA, Hevner RF, Lein E, Němec P. 2014. Evolution and development of the mammalian cerebral cortex. Brain Behav Evol. 83:126–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møllgård K, Jacobsen M. 1984. Immunohistochemical identification of some plasma proteins in human embryonic and fetal forebrain with particular reference to the development of the neocortex. Dev Brain Res 315:49–63. [DOI] [PubMed] [Google Scholar]
- Nigro JM, Misra A, Zhang L, Smirnov I, Colman H, Griffin C, Ozburn N, Chen M, Pan E, Koul D, Yung WKA, Feuerstein BG, Aldape KD. 2005. Integrated array‐comparative genomic hybridization and expression array profiles identify clinically relevant molecular subtypes of glioblastoma. Cancer Res 65:1678–1686. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Wong WS, Clinton BK, Kriegstein AR. 2002. Dividing precursor cells of the embryonic cortical ventricular zone have morphological and molecular characteristics of radial glia. J Neurosci 22:3161–3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor SC, Martínez‐Cerdeño V, Ivic L, Kriegstein AR. 2004. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci 7:136–144. [DOI] [PubMed] [Google Scholar]
- Noisa P, Ramasamy TS, Lamont FR, Yu JSL, Sheldon MJ, Russell A, Jin X, Cui W. 2012. Identification and characterisation of the early differentiating cells in neural differentiation of human embryonic stem cells. PLoS One 7:e37129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt CL, Betensky RA, Brower MA, Batchelor TT, Louis DN, Stemmer‐Rachamimov AO. 2005. YKL‐40 is a differential diagnostic marker for histologic subtypes of high‐grade gliomas. Clin Cancer Res 11:2258–2264. [DOI] [PubMed] [Google Scholar]
- Pelloski CE, Mahajan A, Maor M, Chang EL, Woo S, Gilbert M, Colman H, Yang H, Ledoux A, Blair H, Passe S, Jenkins RB, Aldape KD. 2005. YKL‐40 expression is associated with poorer response to radiation and shorter overall survival in glioblastoma. Clin Cancer Res 11:3326–3334. [DOI] [PubMed] [Google Scholar]
- Pinto L, Götz M. 2007. Radial glial cell heterogeneity—The source of diverse progeny in the CNS. Prog Neurobiol 83:2–23. [DOI] [PubMed] [Google Scholar]
- Pinto L, Mader MT, Irmler M, Gentilini M, Santoni F, Drechsel D, Blum R, Stahl R, Bulfone A, Malatesta P, Beckers J, Götz M. 2008. Prospective isolation of functionally distinct radial glial subtypes ‐ lineage and transcriptome analysis. Mol Cell Neurosci 38:15–42. [DOI] [PubMed] [Google Scholar]
- Pontious A, Kowalczyk T, Englund C, Hevner RF. 2008. Role of intermediate progenitor cells in cerebral cortex development. Dev Neurosci 30:24–32. [DOI] [PubMed] [Google Scholar]
- Prakash M, Bodas M, Prakash D, Nawani N, Khetmalas M, Mandal A, Eriksson C. 2013. Diverse pathological implications of YKL‐40: Answers may lie in “outside‐in” signaling. Cell Signal 25:1567–1573. [DOI] [PubMed] [Google Scholar]
- Rakic P. 1988. Specification of cerebral cortical areas. Science 241:170–176. [DOI] [PubMed] [Google Scholar]
- Reillo I, de Juan Romero C, García‐Cabezas MÁ, Borrell V. 2011. A role for intermediate radial glia in the tangential expansion of the mammalian cerebral cortex. Cereb Cortex 21:1674–1694. [DOI] [PubMed] [Google Scholar]
- Rezaie P, Dean A, Male D, Ulfig N. 2005. Microglia in the cerebral wall of the human telencephalon at second trimester. Cereb Cortex 15:938–949. [DOI] [PubMed] [Google Scholar]
- Rousseau A, Nutt CL, Betensky RA, Lafrate AJ, Han M, Ligon KL, Rowitch DH, Louis DN. 2006. Expression of oligodendroglial and astrocytic lineage markers in diffuse gliomas: Use of YKL‐40, ApoE, ASCL1, and NKX2‐2. J Neuropathol Exp Neurol 65:1149–1156. [DOI] [PubMed] [Google Scholar]
- Shitamukai A, Konno D, Matsuzaki F. 2011. Oblique radial glial divisions in the developing mouse neocortex induce self‐renewing progenitors outside the germinal zone that resemble primate outer subventricular zone progenitors. J Neurosci 31:3683–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Bhardwaj R, Wilczynska KM, Dumur CI, Kordula T. 2011. A complex of nuclear factor I‐X3 and STAT3 regulates astrocyte and glioma migration through the secreted glycoprotein YKL‐40. J Biol Chem 286:39893–39903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart IH, Dehay C, Giroud P, Berland M, Kennedy H. 2002. Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey. Cereb Cortex 12:37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Hevner RF. 2014. Growth and folding of the mammalian cerebral cortex: from molecules to malformations. Nat Rev Neurosci 15:217–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanwar MK, Gilbert MR, Holland EC. 2002. Gene expression microarray analysis reveals YKL‐40 to be a potential serum marker for malignant character in human glioma. Cancer Res 62:4364–4368. [PubMed] [Google Scholar]
- Vasistha NA, García‐Moreno F, Arora S, Cheung AFP, Arnold SJ, Robertson EJ, Molnár Z. 2014. Cortical and clonal contribution of Tbr2 expressing progenitors in the developing mouse brain. Cereb Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verney C, Monier A, Fallet‐Bianco C, Gressens P. 2010. Early microglial colonization of the human forebrain and possible involvement in periventricular white‐matter injury of preterm infants. J Anat 217:436–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Tsai JW, LaMonica B, Kriegstein AR. 2011. A new subtype of progenitor cell in the mouse embryonic neocortex. Nat Neurosci 14:555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecevic N, Chen Y, Filipovic R. 2005. Contributions of cortical subventricular zone to the development of the human cerebral cortex. J Comp Neurol 491:109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]


