Abstract
Background
Chronic prostatic inflammation (CPI) could be a cause of symptomatic or complicated benign prostatic hyperplasia (BPH). In previous in vitro and in vivo studies, Hexanic Extract of Serenoa repens (HESr) namely Permixon® has demonstrated potent anti‐inflammatory properties. With the aim to provide new insight onto HESr anti‐inflammatory properties in human we explore its effect on CPI biomarkers in men with lower urinary tract symptoms (LUTS) related to BPH using a non‐invasive method and investigate links between biomarkers and clinical symptoms.
Methods
An international, randomized, double‐blind, parallel‐group, tamsulosin‐controlled study was carried out in 206 men with BPH‐related LUTS. Patients received oral daily HESr 320mg or tamsulosin 0.4 mg during 3 months. The first urine stream after digital rectal examination (DRE) was collected at Day 1 and Day 90 and mRNA was extracted from prostatic epithelial cells desquaming in the lumen of the glands and seminal plasma fluid after DRE. mRNA quantification of the 29 most significant published inflammation markers in BPH and protein detection in urine was performed.
Results
At D90, a decrease in mean gene expression was observed for 65.4% of the markers detected in the HESr group versus 46.2% in the tamsulosin group. In the 15 most frequently expressed genes, this difference was higher (80% vs. 33% respectively). Three proteins (MCP‐1/CCL2, IP‐10/CXCL10, and MIF) were detected. At D90, a decrease in the number of patients who expressed MCP‐1/CCL2 and IP‐10/CXCL10 was observed only in the HESr group. Moreover, MIF expression was significantly reduced by HESr compared with tamsulosin (P = 0.007). Finally, in contrast to tamsulosin, the subgroup of patients treated by HESr and who over expressed MIF at baseline, had a higher response to the International Prostate Symptom Score (I‐PSS) than those who did not over express this protein (mean I‐PSS change: −6.4 vs. −4.5 respectively). As the study is exploratory, results should be confirmed in a powered clinical study.
Conclusions
These results showed for the first time at clinical level the anti‐inflammatory properties of HESr, already indicated in BPH‐related LUTS. Thus, HESr could be of interest to prevent unfavourable evolution in patients with CPI. Prostate 75:1857–1867, 2015. © 2015 The Authors. The Prostate Published by Wiley Periodicals, Inc.
Keywords: benign prostatic hyperplasia, chronic prostatic inflammation, hexanic extract of serenoa repens, lower urinary tract symptoms, permixon®
INTRODUCTION
A growing body of evidence suggests that chronic prostatic inflammation (CPI) leads to symptomatic or complicated benign prostatic hyperplasia (BPH) 1, 2. CPI is a very common condition in men over the age of 50 3: it has been observed in a large proportion of patients treated surgically for lower urinary tract symptoms (LUTS) due to BPH 4 and in the majority of histological BPH tissue obtained from autopsy series 5. Moreover, CPI has been associated with higher prostate volume and a more severe International Prostate Symptom Score (I‐PSS) 3, 4.
Histologically, CPI is characterised by the presence of large confluent inflammatory nodules in prostatic tissue 3, 4. These nodules release multiple inflammatory mediators that have been shown to stimulate prostatic cell growth 6. Nodules also damage the architecture of the gland, resulting in a chain reaction that further sustains the inflammatory response and promotes prostatic cell growth, prostatic enlargement, and bladder outlet obstruction 7.
CPI could be a target for medical treatment in patients with BPH‐related LUTS. A recent review of randomised clinical trials suggested favourable effects of non‐steroidal anti‐inflammatory drugs (NSAIDs) 8, but their side effects related with long‐term use mostly limit their prescription to acute worsening of urinary symptoms. Paubert‐Braquet et al. 9 demonstrated in 1997 that Hexanic Extract of Serenoa repens (HESr), namely Permixon® and already indicated in BPH‐related LUTS could antagonise 5‐lipoxygenase metabolites, leading to an anti‐inflammatory effect. This effect has been recently confirmed by in vitro and in vivo studies, indicating that HESr can modulate the expression of multiple inflammation‐related genes 10, 11, 12.
The primary objective of the study was to assess the effect of HESr on CPI biomarkers in men suffering from BPH‐related LUTS using a non‐invasive method. The secondary objectives were to assess the clinical efficacy of HESr depending on the prostatic inflammation status of the patients.
MATERIALS AND METHODS
Study Participants
The study complied with the principles of the Declaration of Helsinki and was approved by the independent Ethics Committees of participating centres and countries.
Patients were required to understand and sign the informed consent form and understand and fill in questionnaires.
Inclusion criteria
To be included in the study, men were required to be between 45 and 85 years old with BPH‐ related LUTS for over 12 months, have an I‐PSS score ≥12, prostatic volume ≥30 cm3 determined by transrectal ultrasound, maximum flow rate (Qmax) 5–15 ml/s for a voided volume 150–500 ml, and total Prostate‐specific antigen (PSA) ≤4 ng/ml or ≤10 ng/ml with ratio PSA (free)/PSA (total) ≥25% or negative prostate biopsy. Patients were required to be free of anti‐androgens and LH‐RH analog for at least 6 months, 5 alpha‐reductase inhibitors and plant extracts for at least 3 months and alpha blockers and alpha/beta blockers for at least 1 month before screening. Patients taking the following oral medications at screening required a wash‐out of 2 weeks: 5‐PDE inhibitors for BPH treatment, NSAIDs corticosteroids, or antibiotics by systemic route, mepartricine, ACE inhibitors, calcium antagonists, beta blockers, diuretics, sympathomimetics, antihistamines, antidepressants (anticholinergic), atropine, antispasmodic drugs, antiparkinsonism drugs, pseudoephedrine, chlorpheniramine, or spironolactone (if unstable dose or initiated 6 weeks or less prior to selection). Moreover, these medications were prohibited for the duration of the study.
Non‐inclusion criteria
Patients were excluded if they had a PVR > 200 ml (by suprapubic ultrasound), previous urological history including urethral stricture disease and/or bladder neck disease, active, or recent (<3 months) or recurrent urinary tract infection, urinary retention, indication of BPH surgery, stone in bladder, or urethra, acute, or chronic prostatitis, prostate, or bladder cancer, interstitial cystitis, active upper tract stone disease causing symptoms, surgery of the prostate, bladder neck or pelvic region. In addition, any local and/or systemic inflammation disorders, orthostatic hypotension, any neurologic or psychiatric disease/disorder interfering with the detrusor or sphincter muscle, insulin‐dependent diabetes mellitus and non‐controlled non‐insulin‐dependent diabetes mellitus, chronic renal insufficiency, history of severe hepatic failure or other severe underlying disease excluded the participation of the patient in the study.
Study Design
This Phase IV trial (https://clinicaltrials.gov/ct2/show/NCT01604811) was conducted as an international, prospective, randomised, double‐blind study in 2 parallel groups. After a 28–42 day wash‐out period, men suffering from BPH‐related LUTS were randomly assigned to receive daily HESr 320 mg (160 mg B.I.D hard capsule) or tamsulosin LP 0.4 mg capsule and were followed up over 90 days. Four visits were planned for each participant: selection visit, baseline visit (Day 1), first assessment visit (Day 30) and end‐of‐study visit (Day 90).
Methods
Urine sample collection
Urine samples were collected from the 203 patients treated with HESr (n = 102) or tamsulosin (n = 101). The first urine stream after digital rectal examination (DRE) was collected at D1, D30, and D90 in preservation tubes (Norgen Biotek Corp., Canada).
RNA isolation and PCR amplification
RNA extraction and PCR quantification were performed using standard methods. Briefly, 10 ml were centrifuged at room temperature for 15 min at 1000g. Two millilitre of the supernatant were removed and stored at −80°C for subsequent protein analyses.
Total RNA was isolated from the pellet by using the RNAble reagent and Qiagen RNeasy mini‐preps according to the manufacturers' instructions (Eurobio and Qiagen, Courtaboeuf, France). The quantity and purity of extracted RNA were assessed with a NanoDrop ND 1000 spectrophotometer (Labtech International, Paris, France).
First‐strand cDNA synthesis was performed with 100 ng of total RNA and SuperScript® VILO™ reverse transcriptase (Invitrogen) in a final volume of 20 µl.
Fourteen multiplex preamplification cycles of 2 pools of TaqMan Gene Expression Assays (n = 16 for each pool including KLK3 reference gene) was performed using the TaqMan PreAmp Master Mix Kit (Life Technologies).
The mRNA expression of the 29 most significant BPH inflammation markers was quantified 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19. Real‐time qPCR was performed with TaqMan Gene Expression Assays (Life Technologie) using the following probes:
ALOX15 Hs00609608_m1, ALOX15B Hs00153988_m1, ALOX5 Hs01095330_m1, CAT Hs00156308_m1, CCL5 Hs00174575_m1, HIF1A Hs00153153_m1, LTC4S Hs00168529_m1 MIF Hs00236988_g1, NFKB1 Hs00765730_m1, PTGES2 Hs00228159_m1, PTGES3 Hs04187821_g1, PTGS2 Hs00153133_m1, PTPRC Hs04189704_m1, SELP Hs00927900_m1, STAT3 Hs00374280_m1, IL17A Hs00174383_m1, ICOS Hs00359999_m1, CCR7 Hs01013469_m1, IL1B Hs01555410_m1, IL6 Hs00985639_m1, IL8 Hs00174103_m1, IL15 Hs01003716_m1, PLA2G2A Hs00179898_m1, CXCL10 Hs01124251_g1, CCL2 Hs00234140_m1, CD40LG Hs00163934_m1, CTLA4 Hs03044418_m1, FGF2 Hs00266645_m1, CXCL6 Hs00605742_g1 and KLK3 Hs02576345_m1
All qPCR reactions were performed with a QuantStudio™ 6 Flex System (Life Technologies, Foster City, CA, USA) and the TaqMan® Gene Expression Master Mix kit (Life Technologies). The thermal cycling conditions comprised an initial denaturation step at 95°C for 10 min, 40 cycles at 95°C for 15 sec and 60°C for 1 min.
Quantification of KLK3 (PSA gene), specific of prostatic cells, was also performed to confirm that the results of markers reflected only the expression of these markers in prostatic cells. The expression of each inflammation marker was therefore normalised to KLK3. In order to have an overview of inflammation markers expression in prostatic cells, we also quantified these markers in the prostate cell line LnCaP and used it as calibrator at baseline.
Enzyme‐linked immunosorbent assays (ELISA)
Protein levels were measured in urine supernatants with Quantikine ELISA kits according to the manufacturer's recommendations (R&D Systems Europe Ltd, Lille, France). Each experiment was repeated at least 3 times.
Efficacy and safety evaluation
The main efficacy criterion was the expression level on each mRNA gene at D90. The secondary efficacy criteria were the assessment of I‐PSS and Quality of life (QoL) at all visits, and sexual function (MSF‐4), Qmax (uroflowmetry), Post void residual urine volume (PVR) (suprapubic ultrasound) and prostate volume (transrectal ultrasound) at D1, D30, and D90.
A link between mRNA markers/proteins and BPH clinical symptoms on changes from baseline was investigated as well as the analysis of protein expression profile. Safety criteria included adverse events, physical examination and vital signs at each visit.
Statistical considerations
In the absence of previous information on the markers effect size, a sample size of around 200 patients seemed acceptable to reach the objectives of this exploratory analysis. For the primary criterion, downregulation and upregulation of gene expression were considered to occur when at least a two‐fold change from baseline was observed. Wilcoxon rank‐sum tests were used to compare both groups. Next, values were dichotomised as “expressed“ (value>0) and “not detected“. Values at D30 and D90 were fitted together using a method based on the generalised estimated equations (GEE). Finally, the changes from baseline to D90 were categorised into 4 classes and Cochran‐Mantel‐Haenszel tests based on the rank score were used to compare treatment groups. Protein expression profiles were analysed using the same description in classes and the same test.
To account for multiple testing on RNA markers, we applied a Bonferroni correction, which resulted in an adjusted α level of 0.0033. P‐values larger than α but less than 0.05 were labelled as “nominally significant”.
For the secondary efficacy criteria, I‐PSS score, QoL, MSF‐4 and Qmax, changes from baseline were fitted using a covariance analysis model adjusted for baseline value and treatment. These were also described with respect to the changes of markers.
RESULTS
Baseline Demographics, Disposition, and Disease Characteristics
A total of 206 men were randomised at 36 recruiting centres comprising 26 urologists in 4 countries (Spain, Portugal, Italy and France). The patient flow is illustrated in Figure 1.
Figure 1.
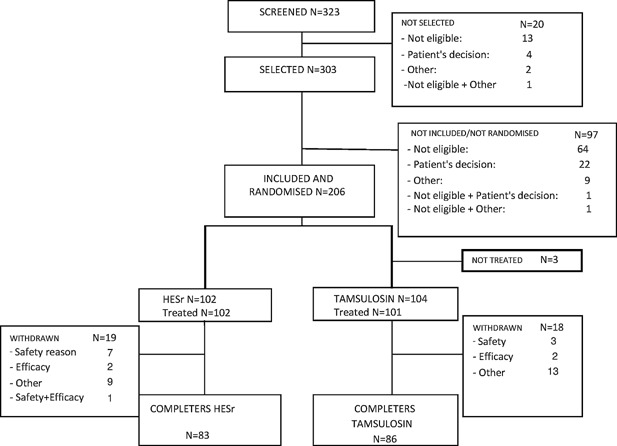
Patient flow chart.
Both treatment groups had similar demographics and other BPH baseline characteristics (Table I). According to the box plots, mRNA gene expression was globally similar in both groups despite the variability observed (data not shown).
Table I.
Evolution of Clinical Symptoms According to Treatment at D90
| HESr n = 102 | Tamsulosin n = 101 | |
|---|---|---|
| I‐PSS: Total score | ||
| Baseline mean (SD) | 17.7 (4.4) | 16.8 (4.5) |
| Min/Median/Max | 8/18.0/28 | 12/16.0/30 |
| Value D90 mean (SD) LOCF ** | 13.2 (6.0) | 10.3 (5.5) |
| Change D90‐baseline LSMean *** (SE) | −4.28 (0.55) | −6.56 (0.55) |
| QoL score | ||
| Baseline mean (SD) | 3.9 (0.9) | 3.8 (0.9) |
| Value D90 mean (SD) LOCF | 3.0 (1.4) | 2.5 (1.2) |
| Change D90‐baseline LSMean *** (SE) | −0.87 (0.12) | −1.29 (0.12) |
| MSF4 score | ||
| Baseline mean (SD) | 7.4 (4.5) | 6.9 (4.5) |
| Value D90 mean (SD) LOCF | 7.7 (4.8) | 7.7 (4.7) |
| Change D90‐baseline LSMean *** (SE) | 0.36 (0.35) | 0.64 (0.35) |
| Qmax | ||
| Baseline mean (SD) | 10.88 (2.69) | 10.60 (3.03) |
| Value D90 mean (SD) LOCF | 12.53 (5.21) | 12.73 (4.42) |
| Change D90‐baseline LSMean *** (SE) | 1.77 (0.46) | 2.09 (0.45) |
| Transrectal prostate volume | ||
| Baseline mean (SD) | 48.82 (20.80) | 46.29 (13.88) |
| Value D90 mean (SD) OC * | 47.95 (20.05) | 46.73 (16.83) |
| Change D90‐baseline LSMean *** (SE) | −0.99 (1.08) | −0.53 (1.05) |
| Supra‐pubic PVR volume (cm3) | ||
| Baseline mean (SD) | 53.82 (57.07) | 42.04 (47.61) |
| Value D90 mean (SD) OC | 64.11 (63.31) | 47.41 (51.29) |
| Change D90‐baseline LSMean *** (SE) | 15.22 (5.80) | 4.04 (5.84) |
Observed case method (OC).
Last Observation Carried Forward method (LOCF).
Adjusted means from the ANCOVA model: Change = Baseline + Treatment.
Primary Endpoints
Out of the 29 genes investigated at mRNA level, 26 were detected at baseline in at least 1 patient; 3 were not detected (CD40LG, CTLA4 and ICOS). At D90, a decrease in mean gene expression was observed for 17/26 (65.4%) markers in the HESr group vs. 12/26 (46.2%) in the tamsulosin group (it should be noted that for 7 of them, a decrease was observed in both groups).
We then focused on the most frequently expressed markers; 15 markers (ALOX5, ALOX15B, CAT, CCL2, HIF1A, IL1b, IL8, MIF, NFKB1, PLA2G2A, PTGES2, PTGES3, PTGS2, PTPRC, and STAT3) were expressed at baseline and D90 in at least 30 patients per group, a subgroup considered sufficient to be analysed. At D90, the decrease in the mean gene expression was observed in 80% of these markers in the HESr group versus 33% in the tamsulosin group (data not shown). Moreover, for 9/15 markers (60%) downregulation was observed more frequently in the HESr group compared with the tamsulosin group (5/15 markers—33.3%), No difference was observed between the groups for the fifteenth marker. In addition, for 11/15 markers (73.3%), upregulation was observed in fewer patients in HESr group compared with the tamsulosin group (4/15 markers—26.6%) (Fig. 2). Thus, combining higher decrease and lower increase of these markers resulted in a favourable effect of HESr in 73.3% of mRNA markers (including a nominally significant difference for HIF1A: P = 0.008 and PTGES3: P = 0.0038) compared with 26.6% for tamsulosin (Fig. 3).
Figure 2.
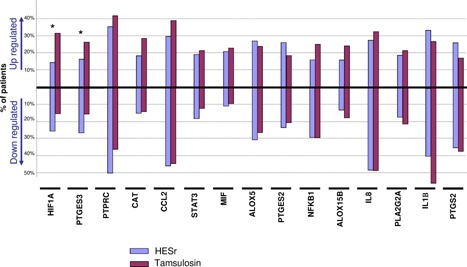
Evolution of the 15 most frequently expressed inflammation genes (at least in 30 patients per group at baseline and D90). mRNA expression was quantified by qPCR. Percentage of patients for whom mRNA expression level was downregulated or upregulated between baseline and end of treament period (D90). Downregulation and upregulation were considered to occur when a change of at least twofold between baseline and D90 was observed (see Section 3.5: 'Statistical considerations'). Asterix * denoted nominally significant at P < 0.05.
Figure 3.
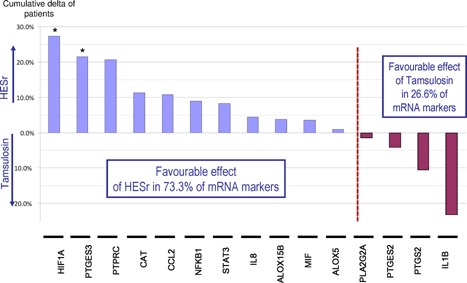
Cumulative favourable effect by mRNA gene at the end of treatment (D90). For each mRNA gene, the global favourable effect corresponding to the sum of the delta of patients between treatment groups for downregulation and delta of patients for less upregulation was calculated. This was followed by a classification by group. Asterix * denoted nominally significant at P < 0.05.
Secondary Endpoints
Protein expression profile in urine
Based on these mRNA results, 10 proteins were selected, comprising 5 cellular proteins (ALOX5, ALOX15B, HIF1A, NFKB, and PTPRC) and 5 proteins potentially excreted in urine (MCP‐1/CCL2, IL1B, IL8, MIF, and IP‐10/CXCL10). Three proteins (MCP‐1/CCL2, IP‐10/CXCL10, and MIF) were detected.
At D90, a decrease was observed in the number of patients who expressed MCP‐1/CCL2 and IP‐10/CXCL10 in the HESr group (mean from 54.8% and 74.0% at baseline to 35.6% and 63.0% at D90 respectively) in contrast to the tamsulosin group (mean from 46.5% and 64.8% at baseline to 47.9% and 67.6% at D90 respectively). Moreover, in the HESr group, MCP‐1/CCL2 and IP‐10/CXCL10 were downregulated for a higher percentage of patients (37.0% and 39.7% respectively) and upregulated for a lower percentage of patients (20.5% and 34.3%) compared with the tamsulosin group (28.2% and 31% respectively for downregulation; 25.4% and 43.7% respectively for upregulation) (Fig. 4). It is interesting to note that in the HESr group, MCP‐1/CCL2 and IP‐10/CXCL10 were switched off for 27.4% and 20.5% of patients respectively compared with 15.5% and 12.7% of patients respectively in the tamsulosin group (data not shown).
Figure 4.
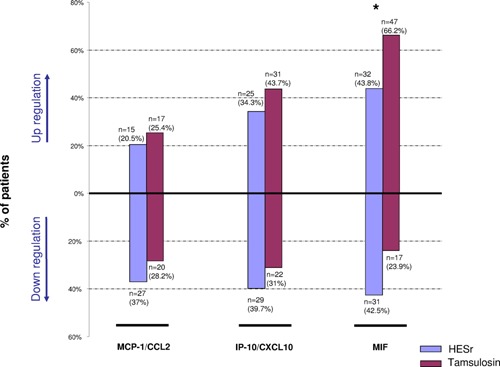
Protein downregulation and upregulation at the end of treatment (D90). Downregulation and upregulation were considered to occur when a change of at least 25% was observed between D90 and baseline. Treatments were compared using a Cochran‐Mantel‐Haenszel test based on the rank score. Asterik * denoted P < 0.05.
MIF protein was expressed in all urine samples at D1 and D90 (Table II). A statistical significance was observed at D90 in favour of HESr with a higher percentage of patients for whom MIF expression was downregulated (42.5%) compared with the tamsulosin group (23.9%), and a lower percentage of patients for whom the MIF expression was upregulated (43.8% vs. 66.2% respectively) (P = 0.007) (Fig. 4).
Table II.
Number of Patients who Expressed the Proteins at Baseline and D90
| HESr n=102 | Tamsulosin n=101 | |||||
|---|---|---|---|---|---|---|
| Protein detected | Number of available data | 73 | 71 | |||
| MCP 1/CCL2 | Baseline | Not detected | 33 | (45.2 %) | 38 | (53.5 %) |
| Expressed | 40 | (54.8 %) | 33 | (46.5 %) | ||
| Value at V4 (D90) | Not detected | 47 | (64.4 %) | 37 | (52.1 %) | |
| Expressed | 26 | (35.6 %) | 34 | (47.9 %) | ||
| IP 10/CXCL10 | Baseline | Not detected | 19 | (26.0 %) | 25 | (35.2 %) |
| Expressed | 54 | (74.0 %) | 46 | (64.8 %) | ||
| Value at V4 (D90) | Not detected | 27 | (37.0 %) | 23 | (32.4 %) | |
| Expressed | 46 | (63.0 %) | 48 | (67.6 %) | ||
| MIF | Baseline | Not detected | — | — | ||
| Expressed | 73 | (100.0 %) | 71 | (100.0 %) | ||
| Value at V4 (D90) | Not detected | — | — | |||
| Expressed | 73 | (100.0 %) | 71 | (100.0 %) | ||
Clinical outcomes
Compliance to the study treatment was very good and similar in both groups (>95%). At D90, an improvement in I‐PSS, QoL, Qmax and prostate volume was observed in both groups. Results are summarised in Table I. The curves for I‐PSS over time in both groups are shown in Figure 5.
Figure 5.
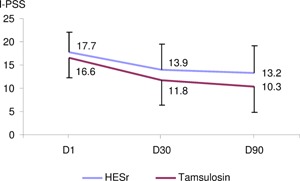
I‐PSS overtime in both treatment groups. I‐PSS: International Prostate Symptom Score; D1: baseline, D30: follow‐up visit Day 30; D90: end of treatment visit Day 90.
Link between mRNA expression level and clinical symptoms
No apparent relationship was identified between changes in clinical symptoms and changes in these markers, but, it should be noted the variability observed within groups and the small number of patients in subgroups).
Protein expression profile and clinical outcomes
For the 3 proteins detected, we examined at the subgroup of patients who over expressed the protein at baseline (protein value >3rd quartile) compared with the other patients in the same group and analysed the I‐PSS changes between baseline and D90 by treatment group.
No difference was observed between subgroups for MCP‐1/CCL2 and IP‐10/CXCL10.
In contrast, for patients treated by HESr and who over expressed MIF at baseline, a higher response to I‐PSS was observed compared with the other patients in the same group (mean I‐PSS change: −6.4 vs. −4.5 respectively). This improvement was not observed in the tamsulosin subgroup (mean I‐PSS change: −6.5 vs. −6.3) (Fig. 6).
Figure 6.
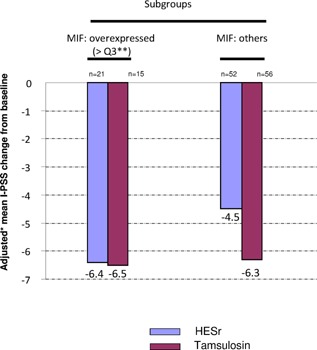
I‐PSS adjusted* mean change from baseline to end of treatment (D90). In HESr group, response to I‐PSS at D90 was evaluated in patients who over expressed MIF protein at baseline (>3rd quartile) to those who did not over express this protein. * using Ancova model change=baseline+treatment. **>Q3 corresponds to the 25% of patients who expressed MIF at the highest level.
Safety Assessments
Eight serious adverse events (SAE) were reported during the study including 4 during treatment administration. One (a bilateral gynecomastia), declared in the tamsulosin group, was suspected by the investigator to be in relationship with the treatment. The percentage of patients with at least one adverse event (AE) was 29.4% in the HESr group (41 AEs reported) versus 30.7% in the tamsulosin group (50 AEs reported). A total of 10.8% patients in the HESr group vs. 8.9% in the tamsulosin group had at least one related treatment‐emergent AE. The most frequent (>2% of patients) treatment‐emergent AE (preferred term) were retrograde ejaculation (4% of patients), constipation (3%) and back pain (3%) with tamsulosin while no adverse event occurred at a frequency of more than 2% with HESr. No related treatment‐emergent AE had a frequency over 1% in the HESr group compared with the tamsulosin group in which ejaculation failure (2%), retrograde ejaculation (2%) and asthenia (2%) were reported.
With respect to treatment discontinuation, 7.8% of patients in the HESr group vs. 3.0% in the tamsulosin group had at least one adverse event (AE) leading to study drug discontinuation (Table III).
Table III.
List of Patients who Withdrew From the Study for Safety Reasons (Reported Terms)
| Group | Subject‐Sex‐age | Adverse event reported term | Severity |
|---|---|---|---|
| HESr | M‐57 | Feeling stuffy nose | Mild |
| Palpitation | Moderate | ||
| M‐69 | Rash | Moderate | |
| M‐74 | Dizziness sensation | Moderate | |
| Persistant tiredness | Moderate | ||
| M‐73 | Abdominal pain | Moderate | |
| Dry mouth | Moderate | ||
| Insomnia | Moderate | ||
| Nightmare | Moderate | ||
| M‐60 | Erectile dysfunction | Mild | |
| M‐68 | Groin testicular. the patient suffered from pubic pain/ache | Mild | |
| M‐67 | Diarrhea | Moderate | |
| Joint swelling of both hands | Moderate | ||
| Hypertension | Mild | ||
| M‐46 | Epigastric pain | Mild | |
| Tamsulosin | M‐53 | Bilateral gynecomastia | Moderate |
| M‐61 | Anejaculation | Mild | |
| M‐62 | Weight loss (between v2‐w3) | Moderate |
DISCUSSION
To the best of our knowledge, this study is the largest randomised clinical trial specifically designed to investigate the anti‐inflammatory effects of medical treatments in patients presenting BPH‐related LUTS, and the only to use a non‐invasive method. The study design was rigorous and followed the standards of quality clinical research: wash‐out period, double‐blind protocol and comparison between different active treatments. The baseline characteristics of the study population were in accordance with the required selection criteria. It should be noted the high mean I‐PSS at baseline in both groups (mean I‐PSS=17.7 in HESr group and 16.8 in tamsulosin group) which corresponds to moderate to severe LUTS.
With regard to the lack of placebo arm, given that the results showed the superior anti‐inflammatory activity of HESr over tamsulosin, it was considered that any potential effect of a placebo would have been even lower than that of tamsulosin. A placebo arm would therefore not have provided any additional value regarding the primary endpoint.
With regards clinical outcomes, the improvement in the I‐PSS observed in both groups (−4.3 and −6.6) was in line with already published data on active LUTS/BPH treatments, thereby confirming the reliability of the clinical findings.
Although prostatic biopsies remain the reference method for investigating CPI 3, some urinary biomarkers were already successfully proposed to assess CPI in BPH with a reliable link between urine and tissue samples 14. It was therefore decided that the study participants should not be exposed to any risk of complication related to an invasive procedure such as prostatitis. In this protocol, inflammatory status was monitored with a non‐invasive method, which allowed for the collection of prostatic epithelial cells desquaming in the lumen of the glands and seminal plasma fluid after DRE.
Considering all detected markers, a higher decrease in mean mRNA expression was observed in HESr group compared to tamsulosin (65% vs. 46% respectively).
Regarding the 15 most frequently expressed genes at baseline, a nominally significant difference in favour of HESr was observed for HIF1A (P = 0.008) and PTGES3 (P = 0.038), though none remained significant after Bonferroni correction (P < 0.0033) (Fig. 2). Moreover, combining reduced, switched‐off, and lower‐increased genes, favourable anti‐inflammatory activity of HESr was observed in 73.3% of the genes compared with 26.6% of the genes after tamsulosin treatment (Fig. 3). Thus, the trend clearly favoured the anti‐inflammatory activity of HESr compared with tamsulosin.
The favourable effect of tamsulosin observed in some genes and/or patients could be explained by the obstruction relief associated with alpha‐blocker therapy.
MCP‐1/CCL2 and IP‐10/CXCL10 mean protein expressions were clearly reduced after HESr treatment, whereas they were slightly raised after tamsulosin treatment (Table II). MIF protein expression was expressed in all samples and almost 50% of patients receiving HESr experienced a significant reduction in MIF (Fig. 6).
CCL2 encodes MCP‐1 (monocyte chemoattractant protein‐1), a chemotactic protein that plays a critical role in the recruitment and activation of monocytes and macrophages in inflammatory diseases 20. MCP‐1/CCL2 downregulation by HESr is in line with a previous in vitro study which demonstrated that HESr was able to reduce MCP‐1/CCL2 expression in epithelial and stromal cell lines 10; MCP‐1/CCL2 was previously described as the most elevated protein secreted in the prostatic fluid of large prostatic glands 21. The stimulation of prostatic epithelial cells by MCP‐1/CCL2 resulted in increased cell proliferation, potentially leading to prostatic enlargement 21.
CXCL10 encodes IP‐10 protein (interferon γ inducible protein 10), which plays an important role in the trafficking of monocytes and activated T cells. When activated CD4+T cells, common prostate‐infiltrating cells in BPH patients 7, were co‐cultured with BPH cells, a significant increase in IP‐10/CXCL10 was observed 22.
Human prostate stromal fibroblastic cells can secrete MCP‐1/CCL2 and IP‐10/CXCL10 cytokines 13, 23 that are able to recruit and activate CD4+T cells into the inflamed prostate, thereby generating an immune response leading to the development of chronic immune‐mediated tissue destruction and fibromyomatosus growth, as observed in the pathogenesis of BPH 13. Downregulation of MCP‐1/CCL2 and IP‐10/CXCL10 by HESr in patients with high CPI could therefore prevent LUTS/BPH progression.
MIF is a long‐known T cell cytokine that has been recognised to be a key mediator of innate immunity and pleiotropic inflammatory cytokine 24. MIF has a direct chemokine‐like function, promotes “directed” cell migration (i.e. chemotaxis) and plays a prominent role in inflammatory and atherogenic leukocyte recruitment 25. Another physiological function of MIF is to counter‐regulate glucocorticoid suppression of immune cell responses 26.
MIF plays a pivotal role in the pathogenesis of acute and chronic inflammatory diseases by promoting and amplifying involved inflammatory reactions such as monocyte/macrophage survival or inflammatory cytokine release. Therefore, a direct action of HESr on MIF expression makes it an additional benefit in the management of BPH. However, MIF expression may locally result in higher inflammatory microenvironment 27, suggesting that the decrease in MIF expression could be the consequence of the overall anti‐inflammatory effect of HESr treatment; in this way, we observed that HIF1A is downregulated under HESr treatment and it has been proved that HIF1A knockdown led to a reduction of MIF protein level in primary human CD4+T cells 28.
Several clinical studies have indicated the usefulness of MIF as a biomarker for different diseases possessing an inflammatory component 29, it could be also considered as a biomarker of particular interest in LUTS/BPH treatment.
No link could be found between mRNA expression and the clinical outcomes. This was probably due largely to the low number of patients and the very high variability of gene expression at baseline.
This study was not designed to compare functional outcomes between groups, but significant improvement of I‐PSS was observed in both groups at D90. However, a better response of −6.4 points I‐PSS improvement was observed in the subgroup of patients under HESr with higher baseline MIF protein expression, compared with −4.5 points I‐PSS observed in the other HESr subgroup.
These results suggest that HESr could be more effective in patients with higher MIF expression and higher CPI.
CONCLUSIONS
In this double‐blind clinical study, HESr showed for the first time the anti‐inflammatory activity in men with BPH‐related LUTS. HESr, already well‐known as a safe product indicated in the management of symptomatic BPH patients, could be particularly useful as an early treatment to prevent unfavourable evolution in patients with CPI.
ACKNOWLEDGMENTS
The authors thank all the investigators involved in the study: Arcangelo Pagliarulo, Rocco Damiano, Matthieu Durand, Emanuele Belgrano, Antonio Alcaraz, Hakim Fassi‐Fehri, Bernard Malavaud, Philippe Igigabel, Xavier Durand, Raul Martos Calvo, Luis Campos Pinheiro, Cesare Selli, Riccardo Bartoletti, Henri Chaussade, Jean‐Paul Boyes, Richard Yvon, Miguel Unda, Aurélien Descazeaud, Giorgio Carmignani, Régis Soulie, Jacques Tondut, Juan Morote, Alain Palomba, Massimo Porena, Philippe Remaud, Francesco Montorsi, Jean‐François Foucault, Rafael Medina, Alfredo Rodriguez Antolin, Avelino Fraga, José Palma Dos Reis, Francisco Gomez Veiga, Benoît Daguzan, Gérard Tatareau, Bernard Boillot, Francisco Javier Burgos, Joaquin Carballido, José Manuel Cozar, Dario Garcia Rojo, Gilles Karsenty. The authors thank Pierre Bunouf for fruitfull collaboration in statistical analyses.
[The copyright line was changed in October 2015 after original online publication.]
Conflicts of interest: Including specific financial interests and relationships and affiliations relevant to the subject matter discussed in the manuscript are the following: Alain Latil, Marie‐Thérèse Pétrissans and Jérôme Rouquet are employees of Pierre Fabre. Grégoire Robert is Investigator in the study and Scientific Advisor. Alexandre de la Taille is the International Study Coordinating Investigator.
REFERENCES
- 1. Ficarra V, Rossanese M, Zazzara M, Giannarini G, Abbinante M, Bartoletti R, Mirone V, Scaglione F. The role of inflammation in lower urinary tract symptoms (LUTS) due to benign prostatic hyperplasia (BPH) and its potential impact on medical therapy. Curr Urol Rep 2014; 15:463–469. [DOI] [PubMed] [Google Scholar]
- 2. Gandaglia G, Briganti A, Gontero P, Mondeni N, Novara G, Salonia A, Sciarra A, Montorsi F. The role of chronic prostatic inflammation in the pathogenesis and progression of benign prostatic hyperplasia (BPH). BJU Int 2013; 112:432–441. [DOI] [PubMed] [Google Scholar]
- 3. Nickel JC, Roehrborn CG, O'Leary MP, Bostwick DG, Somerville MC, Rittmaster RS. The relationship between prostate inflammation and lower urinary tract symptoms: examination of baseline data from the REDUCE trial. Eur Urol 2008; 54:1379–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Robert G, Descazeaud A, Nicolaiew N, Terry S, Sirab N, Vacherot F, Maillé P, Allory Y, de la Taille A. Inflammation in benign prostatic hyperplasia: A 282 patients' immunohistochemical analysis. The Prostate 2009; 69:1774–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zlotta AR, Egawa S, Pushkar D, Govorov A, Kimura T, Kido M, Takahashi H, Kuk C, Kovylina M, Aldaoud N, Fleshner N, Finelli A, Klotz L, Lockwood G, Sykes J, Kwast Tv.. Prevalence of inflammation and benign prostatic hyperplasia on autopsy in Asian and Caucasian men. Eur Urol 2014; 66:619–622. [DOI] [PubMed] [Google Scholar]
- 6. Begley LA, Kasina S, MacDonald J, Macoska JA. The inflammatory microenvironment of the aging prostate facilitates cellular proliferation and hypertrophy. Cytokine 2008; 43:194–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kramer G, Mitteregger D, Marberger M. Is benign prostatic hyperplasia (BPH) an immune inflammatory disease?. Eur Urol 2007; 51:1202–1216. [DOI] [PubMed] [Google Scholar]
- 8. Kahokehr A, Vather R, Nixon A, Hill AG. Non‐steroidal anti‐inflammatory drugs for lower urinary tract symptoms in benign prostatic hyperplasia: Systematic review and meta‐analysis of randomized controlled trials. BJU Int 2013; 111:304–311. [DOI] [PubMed] [Google Scholar]
- 9. Paubert‐Braquet M, Mencia Huerta JM, Cousse H, Braquet P. Effect of the lipidic lipidosterolic extract of Serenoa repens (Permixon) on the ionophore A23187‐stimulated production of leukotriene B4 (LTB4) from human polymorphonuclear neutrophils. Prostaglandins Leukot Essent Fatty Acids 1997; 57:299–304. [DOI] [PubMed] [Google Scholar]
- 10. Latil A, Libon C, Templier M, Junquero D, Lantoine‐Adam F, Nguyen T. Hexanic lipidosterolic extract of Serenoa repens inhibits the expression of two key inflammatory mediators, MCP‐1/CCL2 and VCAM‐1, in vitro. BJU Int 2012; 110:301–307. [DOI] [PubMed] [Google Scholar]
- 11. Sirab N, Robert G, Fasolo V, Descazeaud A, Vacherot F, de la Taille A, Terry S. Lipidosterolic extract of serenoa repens modulates the expression of inflammation related‐genes in benign prostatic hyperplasia epithelial and stromal cells. Int J Mol Sci 2013; 14:14301–14320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bernichtein S, Pigat N, Camparo P, Latil A, Viltard M, Friedlander G, Goffin V. Anti‐inflammatory properties of Lipidosterolic Extract of Serenoa Repens (Permixon®) in a mouse model of prostate hyperplasia. The Prostate 2015; 75:706–722. [DOI] [PubMed] [Google Scholar]
- 13. Penna G, Fibbi B, Amuchastegui S, Cossetti C, Aquilano F, Laverny G, Gacci M, Crescioli C, Maggi M, Adorini L. Human benign prostatic hyperplasia stromal cells as inducers and targets of chronic immuno‐mediated inflammation. J Immunol 2009; 182:4056–4064. [DOI] [PubMed] [Google Scholar]
- 14. Robert G, Smit F, Hessels D, Jannink S, Karthaus HF, Aalders T, Jansen K, de la Taille A, Mulders PF, Schalken JA. Biomarkers for the diagnosis of prostatic inflammation in benign prostatic hyperplasia. The Prostate 2011; 71:1701–1709. [DOI] [PubMed] [Google Scholar]
- 15. Siejka A, Schally AV, Block NL, Barabutis N. Mechanisms of inhibition of human benign prostatic hyperplasia in vitro by the luteinizing hormone‐releasing hormone antagonist cetrorelix. BJU Int 2010; 106:1382–1388. [DOI] [PubMed] [Google Scholar]
- 16. Stephan C, Xu C, Brown DA, Breit SN, Michael A, Nakamura T, Diamandis EP, Meyer H, Cammann H, Jung K. Three new serum markers for prostate cancer detection within a percent free PSA‐based artificial neural network. The Prostate 2006; 66:651–659. [DOI] [PubMed] [Google Scholar]
- 17. Tagaya M, Oka M, Ueda M, Takagaki K, Tanaka M, Ohgi T, Yano J. Eviprostat suppresses proinflammatory gene expression in the prostate of rats with partial bladder‐outlet obstruction: A genome‐wide DNA microarray analysis. Cytokine 2009; 47:185–193. [DOI] [PubMed] [Google Scholar]
- 18. Theyer G, Kramer G, Assmann I, Sherwood E, Preinfalk W, Marberger M, Zechner O, Steiner GE. Phenotypic characterization of infiltrating leukocytes in benign prostatic hyperplasia. Lab Invest 1992; 66:96–107. [PubMed] [Google Scholar]
- 19. Fan Y, Hu S, Liu J, Xiao F, Li X, Yu W, Cui Y, Sun M, Lv T, He Q, Jin J. Low intraprostatic DHT Promotes the Infiltration of CD8+ T Cells in BPH tissues via modulation of CCL5 secretion. Mediators Inflamm 2014; 2014:397815 DOI: 10.1155/2014/397815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein‐1 (MCP‐1): An overview. J Interferon Cytokine Res 2009; 29:313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fujita K, Ewing CM, Getzenberg RH, Parsons JK, Isaacs WB, Pavlovich CP. Monocyte chemotactic protein‐1 (MCP‐1/CCL2) is associated with prostatic growth dysregulation and benign prostatic hyperplasia. The Prostate 2010; 70:473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vignozzi l, Cellai I, Santi R, Lombardelli L, Morelli A, Comeglio P, Filippi S, Logiodice F, Carini M, Nesi G, Gacci M, Piccinni MP, Adorini L, Maggi M. Antiinflammatory effect of androgen receptor activation in human benign prostatic hyperplasia cells. J Endocrinol 2012; 214:31–43. [DOI] [PubMed] [Google Scholar]
- 23. McDowell KL, Begley LA, Mor‐Vaknin N, Markovitz DM, Macoska JA. Leukocytic promotion of prostate cellular proliferation. The Prostate 2010; 70:377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Greven D, Leng L, Bucala R. Autoimmune diseases: MIF as a therapeutic target. Expert Opin Ther Targets 2010; 14:253–264. [DOI] [PubMed] [Google Scholar]
- 25. Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, Dewor M, Georgiev I, Schober A, Leng L, Kooistra T, Fingerle‐Rowson G, Ghezzi P, Kleemann R, McColl SR, Bucala R, Hickey MJ, Weber C. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med 2007; 13:587–596. [DOI] [PubMed] [Google Scholar]
- 26. Calandra T, Bernhagen J, Metz CN, Spiegel LA, Bacher M, Donnelly T, Cerami A, Bucala R. MIF as a glucocorticoid‐induced modulator of cytokine production. Nature 1995; 377:68–71. [DOI] [PubMed] [Google Scholar]
- 27. Bucala R. MIF rediscovered: Cytokine, pituitary hormone, and glucocorticoid‐induced regulator of the immune response. FASEB J 1996; 10:1607–1613. [DOI] [PubMed] [Google Scholar]
- 28. Gaber T, Schellmann S, Erekul KB, Fangradt M, Tykwinska K, Hahne M, Maschmeyer P, Wagegg M, Stahn C, Kolar P, Dziurla R, Löhning M, Burmester GR, Buttgereit F. Macrophage migration inhibitory factor counterregulates dexamethasone‐mediated suppression of hypoxia‐inducible factor‐1 alpha function and differentially influences human CD4+ T cell proliferation under hypoxia. J Immunol 2011; 186:764–774. [DOI] [PubMed] [Google Scholar]
- 29. Grieb G, Merk M, Bernhagen J, Bucala R. Macrophage migration inhibitory factor (MIF): a promising biomarker. Drug News Perspect 2010; 23:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]


