Abstract
Background
Topical therapies are the mainstay of treatment for psoriasis vulgaris. The fixed combination of calcipotriol (Cal) 50 μg/g plus betamethasone 0.5 mg/g (as dipropionate; BD) is a first‐line topical treatment and available as a gel or ointment. The use of these fixed combination products was compared in PRO‐long, a long‐term noninterventional study, for which interim results (4 and 12 weeks) have previously been reported.
Objective
To describe and compare patients’ perspectives on the fixed combination gel and ointment formulations; to include efficacy, adherence behaviour, treatment satisfaction and health‐related quality of life (HRQoL) aspects during long‐term real‐life psoriasis management.
Methods
PRO‐long was a multicentre, prospective, observational, 52‐week study of patients prescribed fixed combination Cal/BD gel or ointment in clinical practice. For final analysis the following were assessed at weeks 24, 36 and 52: differences in the proportion of patients with ‘mild’/‘very mild’ disease according to patient's global assessment of disease severity, adherence behaviour, treatment satisfaction (nine‐item treatment satisfaction questionnaire for medication) and HRQoL (Skindex‐29).
Results
Patients (n = 328) were prescribed once‐daily Cal/BD gel (n = 152) or ointment (n = 176). At week 52, a higher proportion of patients reported that the severity of their psoriasis was ‘mild’/‘very mild’ vs. baseline (gel: 60.2 vs. 47.1%; ointment: 58.8 vs. 42.4%), with greater treatment satisfaction reported in patients using gel vs. those using ointment. A higher proportion of patients found the gel ‘easy’ to use compared with the ointment (66.7 vs. 45.2%). Daily application of treatment took ≤5 min for 86.1% of patients using gel and 71.0% of patients using ointment.
Conclusion
This real‐life study has demonstrated similar effectiveness between the Cal/BD formulations. However, over a 52‐week treatment period, patients reported greater treatment satisfaction with the gel, which was considered easier to use, faster to apply and overall a more convenient product.
Introduction
Psoriasis is a chronic skin disorder with inflammatory involvement1 with a global incidence of 1–3%.1, 2, 3 Psoriasis vulgaris, the most common form of psoriasis, manifests in sharply demarcated, scaly plaques, usually present on the scalp, limbs and trunk.4 These plaques are the result of epidermal hyperplasia with abnormal keratinocyte differentiation; infiltration of inflammatory cells into the epidermis and dermis; and increased dermal vascularity.5, 6 Psoriasis is associated with a number of comorbidities and psychological disorders,4 and impacts the patient's ability to perform daily activities and form social relationships.7 Psoriasis has been reported to reduce patient health‐related quality of life (HRQoL) to an extent comparable with diseases, such as depression and cancer.8
Treatment nonadherence is a universal medical issue, particularly in chronic disorders. For patients prescribed topical treatment, nonadherence is further exacerbated by the requirement of application, which can be time‐consuming and cumbersome. This is a challenge in psoriasis as topicals are the mainstay of treatment for mild‐to‐moderate disease.9 A real‐life study showed that 46% of psoriasis patients were not fully adherent to their prescribed topical therapy, applying treatment only when they felt it necessary.10 The choice of vehicle is one of the key factors affecting patient adherence to topical psoriasis treatment. A number of formulations are available, including creams, gels and ointments, although some are perceived as less convenient and more difficult to apply than others;11, 12 patients generally prefer a gel, for example, to an ointment.13, 14
Treatment nonadherence impacts the ‘real‐life effectiveness’ of a drug (defined as “a function of drug efficacy [determined by randomized clinical trial results] and patient safety and compliance”).15 Clinical trials provide a stringent environment in which to determine drug efficacy and tolerability, but do not accurately reflect real‐life conditions16 and evidence of patient use from daily clinical practice is limited.
Fixed combination calcipotriol (50 μg/g) plus betamethasone (0.5 mg/g; as dipropionate), available in gel and ointment formulations, is a recommended first‐line treatment for mild‐to‐moderate psoriasis vulgaris.9, 17, 18 PRO‐long (Patient‐Reported Outcomes in a long‐term study) was the first direct ‘real‐life’ study to compare patients’ perspectives on the gel to that of the ointment formulation of this fixed combination, for up to 52 weeks of treatment. From interim analysis (4 and 12 weeks), patients found the gel more convenient, easier and faster to apply than the ointment.19
Here, we report the final analysis of this noninterventional study, including assessments at 24, 36 and 52 weeks after initiation of treatment.
Materials and methods
Full details of the methods have previously been reported with the interim analysis.19 In brief, psoriasis patients aged ≥18 years and eligible for topical treatment were prescribed fixed combination calcipotriol (50 μg/g) plus betamethasone (0.5 mg/g; as dipropionate) gel or ointment once daily, for up to 52 weeks. Baseline disease severity was evaluated using the physician's global assessment of disease severity (PGA; 5‐point scale: very mild, mild, moderate, severe, very severe), and extent of body surface area affected with psoriasis. Assessments conducted for interim analysis (4 and 12 weeks) were also conducted at weeks 24, 36 and 52. Efficacy was evaluated according to patient's global assessment of disease severity (PaGA; same 5‐point scale as PGA). Adherence behaviour was assessed using a study‐specific questionnaire.19 Treatment satisfaction was assessed using the nine‐item treatment satisfaction questionnaire for medication (TSQM‐9),20 consisting of three domains: effectiveness, convenience and overall satisfaction (overall satisfaction questions were completed at week 52 only). HRQoL was assessed using the Skindex‐29 questionnaire, consisting of three domains: emotions, symptoms and functioning.21
Statistical analysis was performed as for the interim analysis.19 The chi‐squared test was used to compare the proportion of patients with controlled disease between treatments and McNemar's test was used to confirm the statistical significance of changes in the proportion of patients with controlled disease from baseline.19 For patients who had not completed week‐52 questionnaires, PaGA was imputed using the last observation carried forward method. No imputation was applied to the other parameters assessed; missing data were treated as missing completely at random and not replaced.
Final analysis endpoints
The primary endpoint was the difference in the proportion of patients with controlled disease (defined as ‘mild’ or ‘very mild’ according to PaGA) between the gel and ointment at week 52. Secondary endpoints included patients’ perspectives on adherence behaviour, treatment satisfaction and HRQoL at weeks 24, 36 and 52, compared with baseline, as well as differences in the proportion of patients with controlled disease between the gel and the ointment at weeks 24 and 36.
Results
Patients
In total, 328 patients (gel, n = 152; ointment, n = 176) were included in this study. Patient baseline characteristics across treatment groups were generally well‐balanced (Table 1). In this 52‐week, noninterventional study, noncompletion was observed for a large proportion of patients (73%; Fig. 1); post‐hoc analysis revealed that baseline characteristics were similar for completers and noncompleters (Table 2).
Table 1.
Patient demographics and baseline clinical characteristics (by treatment)
| Patients using gel (n = 152) | Patients using ointment (n = 176) | All patients (n = 328) | |
|---|---|---|---|
| Gender, n (%) | |||
| Male | 91 (59.9) | 98 (55.7) | 189 (57.6) |
| Female | 61 (40.1) | 78 (44.3) | 139 (42.4) |
| Median age, years (range) | 47 (11–77) | 42 (17–73) | 44 (11–77) |
| Study site, n (%) | |||
| Belgium | 2 (1.3) | 78 (44.3) | 80 (24.4) |
| Netherlands | 119 (78.3) | 40 (22.7) | 159 (48.5) |
| Years suffering from psoriasis, n (%) | |||
| <1 | 10 (6.6) | 20 (11.4) | 30 (9.1) |
| 1–5 | 39 (25.7) | 47 (26.7) | 86 (26.2) |
| 6–10 | 29 (19.1) | 33 (18.8) | 62 (18.9) |
| 11–15 | 18 (11.8) | 23 (13.1) | 41 (12.5) |
| 16–20 | 15 (9.9) | 15 (8.5) | 30 (9.1) |
| >20 | 41 (27.0) | 38 (21.6) | 79 (24.1) |
| Percentage body surface area affected by psoriasis, n (%) | |||
| 1–5 | 42 (27.6) | 80 (45.5) | 122 (37.2) |
| 6–10 | 46 (30.3) | 43 (24.4) | 89 (27.1) |
| 11–15 | 16 (10.5) | 23 (13.1) | 39 (11.9) |
| 16–20 | 14 (9.2) | 14 (8.0) | 28 (8.5) |
| 21–30 | 24 (15.8) | 9 (5.1) | 33 (10.1) |
| 31–40 | 8 (5.3) | 4 (2.3) | 12 (3.7) |
| >40 | 2 (1.3) | 2 (1.1) | 4 (1.2) |
| PGA disease severity, n (%) | |||
| Very mild | 18 (11.8) | 24 (13.6) | 42 (12.8) |
| Mild | 58 (38.2) | 63 (35.8) | 121 (36.9) |
| Moderate | 66 (43.4) | 75 (42.6) | 141 (43.0) |
| Severe | 10 (6.6) | 14 (8.0) | 24 (7.3) |
| Very severe | – | – | – |
| Comorbidity, n (%) | |||
| Hypertension | 23 (15.1) | 30 (17.0) | 53 (16.2) |
| Diabetes | 10 (6.6) | 8 (4.5) | 18 (5.5) |
| Dyslipidaemia | 11 (7.2) | 21 (11.9) | 32 (9.8) |
| None of the above | 125 (82.2) | 135 (76.7) | 260 (79.3) |
| Topicals used during the last year for psoriasis on the body | |||
| Fixed combination calcipotriol plus betamethasone dipropionate, n (%) | 40 (26.3) | 55 (31.3) | 95 (29.0) |
| Vitamin D analogues, n (%) | 14 (9.2) | 19 (10.8) | 33 (10.1) |
| Steroids, n (%) | 60 (39.5) | 66 (37.5) | 126 (38.4) |
| Combination of steroids and vitamin D analogues, n (%) | 2 (1.3) | 13 (7.4) | 15 (4.6) |
| Other, n (%) | 7 (4.6) | 10 (5.7) | 17 (5.2) |
| Prescribed systemic treatment at baseline | |||
| Methotrexate, n (%) | 16 (10.5) | 12 (6.8) | 28 (8.5) |
| Cyclosporine, n (%) | 1 (0.7) | 4 (2.3) | 5 (1.5) |
| Acitretin, n (%) | 4 (2.6) | 6 (3.4) | 10 (3.0) |
| Fumaric acid, n (%) | 12 (7.9) | 1 (0.6) | 13 (4.0) |
| Biologicals, n (%) | 9 (5.9) | 8 (4.5) | 17 (5.2) |
| Other, n (%) | 2 (1.3) | 1 (0.6) | 3 (0.9) |
Figure 1.
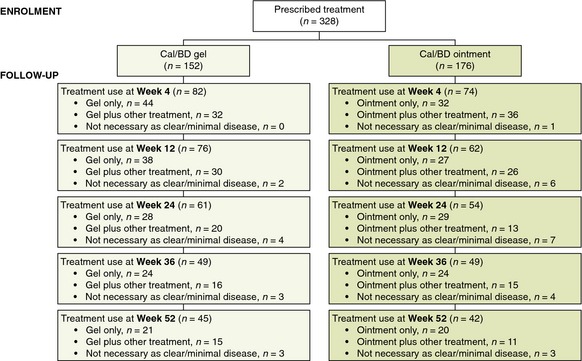
Patient disposition.
Table 2.
Patient demographics and baseline clinical characteristics (completers vs. noncompleters)
| Completers (n = 87) | Noncompleters (n = 241) | |
|---|---|---|
| Gender, n (%) | ||
| Male | 53 (60.9) | 136 (56.4) |
| Female | 34 (39.1) | 105 (43.6) |
| Median age, years (range) | 52 (11–77) | 42 (17–77) |
| Study site, n (%) | ||
| Belgium | 32 (36.8) | 48 (19.9) |
| Netherlands | 55 (63.2) | 104 (43.2) |
| Years suffering from psoriasis, n (%) | ||
| <1 | 11 (12.6) | 19 (7.9) |
| 1–5 | 19 (21.8) | 67 (27.8) |
| 6–10 | 17 (19.5) | 45 (18.7) |
| 11–15 | 8 (9.2) | 33 (13.7) |
| 16–20 | 6 (6.9) | 24 (10.0) |
| >20 | 26 (29.9) | 53 (22.0) |
| Percentage body surface area affected by psoriasis, n (%) | ||
| 1–5 | 33 (37.9) | 89 (36.9) |
| 6–10 | 26 (29.9) | 63 (26.1) |
| 11–15 | 14 (16.1) | 25 (10.4) |
| 16–20 | 5 (5.7) | 23 (9.5) |
| 21–30 | 7 (8.0) | 26 (10.8) |
| 31–40 | 2 (2.3) | 10 (4.1) |
| >40 | – | 4 (1.7) |
| PGA disease severity, n (%) | ||
| Very mild | 11 (12.6) | 31 (12.9) |
| Mild | 40 (46.0) | 81 (33.6) |
| Moderate | 34 (39.1) | 107 (44.4) |
| Severe | 2 (2.3) | 22 (9.1) |
| Very severe | – | – |
| Comorbidity, n (%) | ||
| Hypertension | 15 (17.2) | 38 (15.8) |
| Diabetes | 4 (4.6) | 14 (5.8) |
| Dyslipidaemia | 5 (5.7) | 27 (11.2) |
| None of the above | 70 (80.5) | 190 (78.8) |
| Topicals used for psoriasis of the body during the year prior to start of treatment | ||
| Fixed combination calcipotriol plus betamethasone dipropionate, n (%) | 29 (33.3) | 66 (27.4) |
| Vitamin D analogues, n (%) | 7 (8.0) | 26 (10.8) |
| Steroids, n (%) | 35 (40.2) | 91 (37.8) |
| Combination of steroids and vitamin D analogues, n (%) | 7 (8.0) | 8 (3.3) |
| Other, n (%) | 5 (5.7) | 12 (5.0) |
| Prescribed systemic treatment at baseline | ||
| Methotrexate, n (%) | 5 (5.7) | 23 (9.5) |
| Cyclosporine, n (%) | 1 (1.1) | 4 (1.7) |
| Acitretin, n (%) | 3 (3.4) | 7 (2.9) |
| Fumaric acid, n (%) | 3 (3.4) | 10 (4.1) |
| Biologicals, n (%) | 7 (8.0) | 10 (4.1) |
| Other, n (%) | – | 3 (1.2) |
Efficacy
A significant increase in the proportion of patients with controlled disease (‘mild’ or ‘very mild’ compared with baseline, according to PaGA) was seen with the gel (increase: 13.1%; P = 0.009) and the ointment (increase: 16.4%; P = 0.0003) at week 52 (Fig. 2a,b). This significant increase was also seen at earlier time points (week 24: 16.0 [gel] and 14.7% [ointment]; P = 0.0009 and P = 0.0007; week 36: 12.3 and 18.1%; P = 0.0164 and P < 0.0001; Fig. 2c). There were no statistically significant differences in the proportion of patients with controlled disease between treatment groups at any time point (Fig. 2c).
Figure 2.
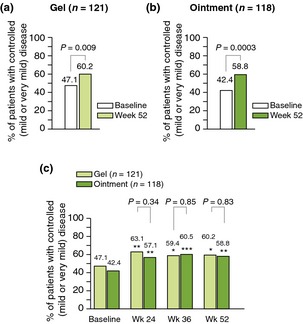
Proportion of patients with controlled disease (PaGA) (a) using gel, week 52 compared with baseline; (b) using ointment, week 52 compared with baseline and (c) using gel or ointment, weeks 24, 36 and 52 compared with baseline. *P < 0.05; **P < 0.001; ***P < 0.0001 vs. baseline. BD, betamethasone 0.5 mg/g (as dipropionate); Cal, calcipotriol 50 μg/g.
Adherence behaviour
Patient adherence to treatment was assessed using a study‐specific questionnaire.19 At week 52, a large proportion of patients (gel: 86.1%; ointment: 67.8%) reported that application of treatment was ‘never’ or ‘rarely’ too great a burden. No patients using gel reported that the burden of application was ‘often’ or ‘always’ too great compared with 16.1% of patients using ointment (Fig. 3a). Daily application of treatment took 5 min or less for 86.1% of patients using gel and 71.0% of patients using ointment at 52 weeks (Fig. 3b).
Figure 3.
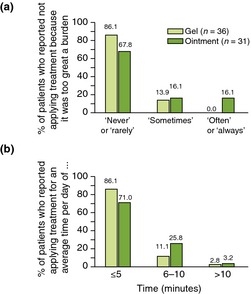
Patient adherence to treatment at week 52 (a) patient‐reported burden of treatment (b) patient‐reported application time.
Treatment satisfaction
Treatment satisfaction was assessed using TSQM‐9 (comprising convenience, effectiveness and overall satisfaction [at week 52 only] domains). At week 52, patients found that the gel was significantly more convenient (P = 0.033) and effective (P = 0.003) than the ointment (Fig. 4a). At weeks 24 and 36, significant differences in convenience between the gel and ointment were also seen (P = 0.027 and P < 0.001, respectively). At week 52, a greater proportion of patients using the gel compared with those using ointment found it ‘easy’ to use (66.7 vs. 45.2%), ‘easy’ to plan treatment (61.1 vs. 38.7%), or ‘easy’ to apply (72.2 vs. 45.2%), although this led to a significant difference in TSQM‐9 mean scores for ease of application only (P = 0.019; Fig. 4b). Significantly favourable responses to the gel over ointment were seen in all TSQM‐9 components for effectiveness at week 52 (Fig. 4c) and patient responses to ‘application time’ were also significantly more positive for the gel compared with ointment at weeks 24 and 36 (P = 0.009 and P = 0.026, respectively). For week‐52 overall satisfaction, patients rated the gel significantly greater at providing benefit than the ointment (P = 0.031; Fig. 4d) and were in favour of the gel, although not significantly so, when asked whether the positives of their treatment outweighed the negatives (P = 0.296). However, a numerical difference in favour of ointment was observed when patients evaluated their global satisfaction with treatment (P = 0.726), although the TSQM‐9 mean overall satisfaction domain score was similar for both treatments (P = 0.192; Fig. 4a).
Figure 4.
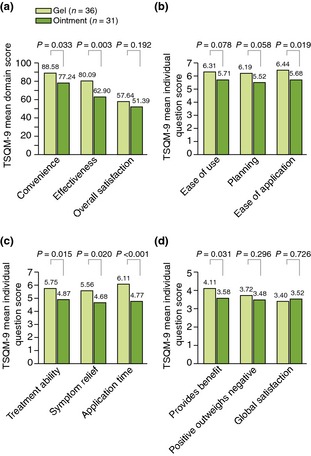
Treatment satisfaction at week 52 (a) total convenience, effectiveness, and overall satisfaction domain scores (b) individual convenience scores (c) individual effectiveness scores (d) individual overall satisfaction scores. TSQM‐9, nine‐item treatment satisfaction questionnaire for medication.
HRQoL
According to Skindex‐29 assessment of HRQoL, improvements from baseline in mean scores at week 52 were greater for patients treated with the gel vs. ointment (Fig. 5), although these differences did not reach significance.
Figure 5.
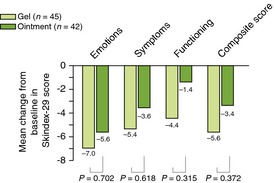
Patient‐reported quality of life, week 52 compared with baseline.
Discussion
This 52‐week, noninterventional study compared patients’ perspectives on the use of calcipotriol (50 μg/g) plus betamethasone (0.5 mg/g; as dipropionate) gel with ointment for the management of psoriasis vulgaris. Patients who completed this study reported greater treatment satisfaction with the gel compared with ointment: it was considered easier to use, faster to apply and overall more convenient than the ointment. This supports the findings of the interim analysis, where a difference in convenience was observed in favour of the gel by the first assessment (week 4).19
Treatment adherence in chronic diseases remains a challenge and adherence rates to topical treatments are low, with over a third of patients not using their medication as prescribed.13 Even for short‐term topical psoriasis treatment (up to 8 weeks) in clinical trials, patient adherence is still poor, ranging between 51 and 58%.22, 23, 24 Furthermore, in a 52‐week, randomized study, the withdrawal rate of patients using the fixed combination gel was 21.4 and 39.8% of those using calcipotriol gel.25 For PRO‐long, being a 52‐week, noninterventional study, high rates of noncompletion were expected; this was found to be the case, regardless of the overall preference for the gel; however, post‐hoc analysis indicated that patient demographics and baseline characteristics were generally similar for completers compared to noncompleters and a sensitivity analysis supported the final analysis.
Cosmetically elegant products, such as gels and creams, are generally considered less greasy, taking less time to apply than ointments.13, 26 As such, patients perceive gels and creams to be more acceptable than ointments for topical treatment.11 PRO‐long supports the difference in application time between topical preparations, as more patients using the gel (86.1%) compared with the ointment (71.0%) could apply their treatment within 5 min. Treatment effectiveness is a key aspect of adherence, as patients are more likely to continue with treatments that they judge to be beneficial.27 In our study, there were patients using the ointment who reported good treatment satisfaction and completed the study. This finding is in agreement with previously reported incidental evidence that some patients like using the ointment and do not notice a cosmetic difference between formulations.26 Furthermore, this finding demonstrates that resolving treatment adherence is not as simple as developing a more cosmetically elegant vehicle. Each patient has a different experience of previous treatments and their own expectations of the current course of treatment, and involving patients in treatment decisions may increase rates of adherence.26 A recent expert review of the management of topical psoriasis treatment, using the fixed combination calcipotriol plus betamethasone dipropionate gel as a working example, highlighted the importance of tailoring treatment to the needs of each patient.26 On the basis of our findings, as well as the recommendations presented within the recent expert review,26 we suggest that patients are given a choice between gel and ointment formulations.
A noninterventional study design is important to gain insight into real‐life effectiveness, although this design has its limitations. Patients were prescribed gel or ointment at the dermatologists’ discretion and therefore bias may have been introduced during the selection process. This bias may have been augmented by the limited availability of the gel formulation in Belgium. Furthermore, incomplete follow‐up is common in noninterventional studies and limits the power of these studies for comparator analysis. However, as previously discussed, post‐hoc analysis showed no difference in baseline characteristics between completers and noncompleters, and the sample sizes for both groups were similar at week 52, indicating similar rates of noncompletion for both treatments.
This study has drawn on the perspectives of patients managing their psoriasis with the use of topical therapy, over a long treatment period. It provides a valuable insight into the behaviour of psoriasis patients and their perspectives on the fixed combination gel and ointment formulations. Patients found the gel to be more convenient than the ointment, as it was easier to use, faster to apply and had similar efficacy. Both formulations are effective in daily clinical practice and decisions on which formulation to prescribe should be made on a per‐patient basis.
Acknowledgements
This study was sponsored by LEO Pharma. We would like to thank Mirjam Griekspoor and Patricia Dockx (LEO Pharma), who facilitated the conduct of the study. Additionally, writing assistance was provided by Carly Hayes, PhD, from Mudskipper Business Ltd, funded by LEO Pharma.
Conflicts of interest
C.W. Hol is an employee of LEO Pharma BV. J. Lambert and J. Vink have no conflicts of interest to declare.
Funding sources
This research was funded by LEO Pharma BV.
Previous presentations and publications: Poster presentation of interim analysis at EADV 2013; Poster presentation of final analysis at EADV 2014; Interim analysis original article in JEADV 2014.19
References
- 1. Schön MP, Boehncke W‐H. Psoriasis. N Engl J Med 2005; 352: 1899–1912. [DOI] [PubMed] [Google Scholar]
- 2. Gelfand JM, Weinstein R, Porter SB, Neimann AL, Berlin JA, Margolis DJ. Prevalence and treatment of psoriasis in the United Kingdom: a population‐based study. Arch Dermatol 2005; 141: 1537–1541. [DOI] [PubMed] [Google Scholar]
- 3. Schäfer T. Epidemiology of psoriasis. Review and the German perspective. Dermatology 2006; 212: 327–337. [DOI] [PubMed] [Google Scholar]
- 4. Menter A, Gottlieb A, Feldman SR et al Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol 2008; 58: 826–850. [DOI] [PubMed] [Google Scholar]
- 5. Elder JT, Bruce AT, Gudjonsson JE et al Molecular dissection of psoriasis: integrating genetics and biology. J Invest Dermatol 2010; 130: 1213–1226. [DOI] [PubMed] [Google Scholar]
- 6. Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet 2007; 370: 263–271. [DOI] [PubMed] [Google Scholar]
- 7. Kimball AB, Jacobson C, Weiss S, Vreeland MG, Wu Y. The psychosocial burden of psoriasis. Am J Clin Dermatol 2005; 6: 383–392. [DOI] [PubMed] [Google Scholar]
- 8. Rapp SR, Feldman SR, Exum ML, Fleischer AB Jr, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol 1999; 41: 401–407. [DOI] [PubMed] [Google Scholar]
- 9. Menter A, Korman NJ, Elmets CA et al Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol 2009; 60: 643–659. [DOI] [PubMed] [Google Scholar]
- 10. Bewley A, Burrage DM, Ersser SJ, Hansen M, Ward C. Identifying individual psychosocial and adherence support needs in patients with psoriasis: a multinational two‐stage qualitative and quantitative study. J Eur Acad Dermatol Venereol 2013; 28: 763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hol K. Patient preference for topical psoriasis formulations. Poster presented at the EADV Congress, Gothenburg, Sweden, 6–10 October 2010; abst: P572. [Google Scholar]
- 12. Housman TS, Mellen BG, Rapp SR, Fleischer AB Jr, Feldman SR. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis 2002; 70: 327–332. [PubMed] [Google Scholar]
- 13. Bewley A, Page B. Maximizing patient adherence for optimal outcomes in psoriasis. J Eur Acad Dermatol Venereol 2011; 25(Suppl. 4): 9–14. [DOI] [PubMed] [Google Scholar]
- 14. Sandoval LF, Huang KE, Harrison J, Clark A, Feldman SR. Calcipotriene 0.005%‐betamethasone dipropionate 0.064% ointment versus topical suspension in the treatment of plaque psoriasis: a randomized pilot study of patient preference. Cutis 2014; 94: 304–309. [PubMed] [Google Scholar]
- 15. Stein Gold L, Corvari L. The roles of safety and compliance in determining effectiveness of topical therapy for psoriasis. Cutis 2007; 79: 32–38. [PubMed] [Google Scholar]
- 16. Devaux S, Castela A, Archier E et al Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol 2012; 26(Suppl. 3): 61–67. [DOI] [PubMed] [Google Scholar]
- 17. Murphy G, Reich K. In touch with psoriasis: topical treatments and current guidelines. J Eur Acad Dermatol Venereol 2011; 25(Suppl. 4): 3–8. [DOI] [PubMed] [Google Scholar]
- 18. Paul C, Gallini A, Archier E et al Evidence‐based recommendations on topical treatment and phototherapy of psoriasis: systematic review and expert opinion of a panel of dermatologists. J Eur Acad Dermatol Venereol 2012; 26(Suppl. 3): 1–10. [DOI] [PubMed] [Google Scholar]
- 19. Lambert J, Hol CW, Vink J. Real‐life effectiveness of once‐daily calcipotriol and betamethasone dipropionate gel vs. ointment formulations in psoriasis vulgaris: 4‐ and 12‐week interim results from the PRO‐long study. J Eur Acad Dermatol Venereol 2014; 28: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 20. Bharmal M, Payne K, Atkinson MJ, Desrosiers M‐P, Morisky DE, Gemmen E. Validation of an abbreviated Treatment Satisfaction Questionnaire for Medication (TSQM‐9) among patients on antihypertensive medications. Health Qual Life Outcomes 2009; 7: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chren MM, Lasek RJ, Flocke SA, Zyzanski SJ. Improved discriminative and evaluative capability of a refined version of Skindex, a quality‐of‐life instrument for patients with skin diseases. Arch Dermatol 1997; 133: 1433–1440. [PubMed] [Google Scholar]
- 22. Carroll CL, Feldman SR, Camacho FT, Balkrishnan R. Better medication adherence results in greater improvement in severity of psoriasis. Br J Dermatol 2004; 151: 895–897. [DOI] [PubMed] [Google Scholar]
- 23. Carroll CL, Feldman SR, Camacho FT, Manuel JC, Balkrishnan R. Adherence to topical therapy decreases during the course of an 8‐week psoriasis clinical trial: commonly used methods of measuring adherence to topical therapy overestimate actual use. J Am Acad Dermatol 2004; 51: 212–216. [DOI] [PubMed] [Google Scholar]
- 24. Feldman SR, Camacho FT, Krejci‐Manwaring J, Carroll CL, Balkrishnan R. Adherence to topical therapy increases around the time of office visits. J Am Acad Dermatol 2007; 57: 81–83. [DOI] [PubMed] [Google Scholar]
- 25. Luger TA, Cambazard F, Larsen FG et al A study of the safety and efficacy of calcipotriol and betamethasone dipropionate scalp formulation in the long‐term management of scalp psoriasis. Dermatology 2008; 217: 321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dauden E, Bewley A, Lambert J, Girolomoni G, Cambazard F, Reich K. Expert recommendations: the use of the fixed combination calcipotriol and betamethasone dipropionate gel for the topical treatment of psoriasis. J Eur Acad Dermatol Venereol 2014; 28(Suppl. 2): 22–32. [DOI] [PubMed] [Google Scholar]
- 27. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005; 353: 487–497. [DOI] [PubMed] [Google Scholar]


