Abstract
Background
Hyperglycemia in premature infants is usually thought to reflect inadequate pancreatic development rather than neonatal diabetes due to a monogenic cause. However, there have been no studies to investigate monogenic forms of permanent and transient neonatal diabetes in pre-term babies.
Methods
We studied an international cohort of 750 patients with diabetes diagnosed before 6 months of age. We compared the genetic etiology and clinical characteristics of 146 patients born prematurely (<37 weeks) and compared them to 604 born ≥37 weeks.
Results
A defined genetic etiology was found in 97/146 (66%) preterm infants compared to 501/604 (83%) born ≥37weeks, p<0.0001. Chromosome 6q24 imprinting abnormalities (27% v 12%, p=0.0001) and GATA6 mutations (9% v 2%,p=0.003) occurred more commonly in pre-term than term infants whilst mutations in KCNJ11 were less common (21 v 34%,p=0.008).
The preterm patients with an identified mutation were diagnosed later than those without an identified mutation (35(34-36) weeks [median(interquartile range)] v 31(28-36) weeks, p<0.0001). No difference was seen in other clinical characteristics of preterm patients with and without an identified mutation including age of presentation, birth weight SDS, and time to referral for genetic testing.
Conclusions
Patients with neonatal diabetes due to a monogenic etiology can be born pre-term, especially those with 6q24 abnormalities or GATA6 mutations. A genetic etiology is more likely in patients with less severe prematurity (32-37 weeks). Prematurity should not prevent referral for genetic testing as 37% have a potassium channel mutation that predicts replacement of insulin with sulphonylurea therapy resulting in improved glycaemic control.
Introduction
Prematurity (gestation <37 weeks) affects up to 12% of all births 1 and hyperglycemia is a common complication usually attributed to an immature pancreas, insulin resistance and abnormal glucose homeostatic mechanisms 2, 3. The risk of hyperglycemia is negatively associated with gestational age and birth weight, with the highest prevalence (up to 88%) seen in extremely low birth weight (<1kg) preterm infants (<30 weeks) and those receiving intravenous glucose or parenteral nutrition, usually within the first week of life 4, 5 6–9.
Neonatal diabetes, a rare (~1:200 000 live births) 10, 11 genetically heterogeneous monogenic form of diabetes, may also present in the days and weeks following birth, and almost always before 6 months of age 12–15. Approximately 50% of patients have heterozygous activating mutations in the KCNJ11 and ABCC8 genes encoding the ATP-sensitive potassium (K-ATP) channel subunits, and in the vast majority of cases insulin injections can be successfully replaced with sulphonylurea tablets, making a correct genetic diagnosis crucial15–17.
The hyperglycemia seen in preterm infants and in patients with neonatal diabetes due to a monogenic cause may be difficult to discriminate. Both groups of patients can present shortly after birth and are associated with reduced birth weight. Neonatal diabetes may affect gestational age and so a percentage of patients could present prematurely, or certain mutations may result in premature birth. No studies have investigated patients with both permanent and transient neonatal diabetes who were born prematurely. We aimed to assess the clinical characteristics and genetic etiology of preterm patients with neonatal diabetes.
Methods
Patients
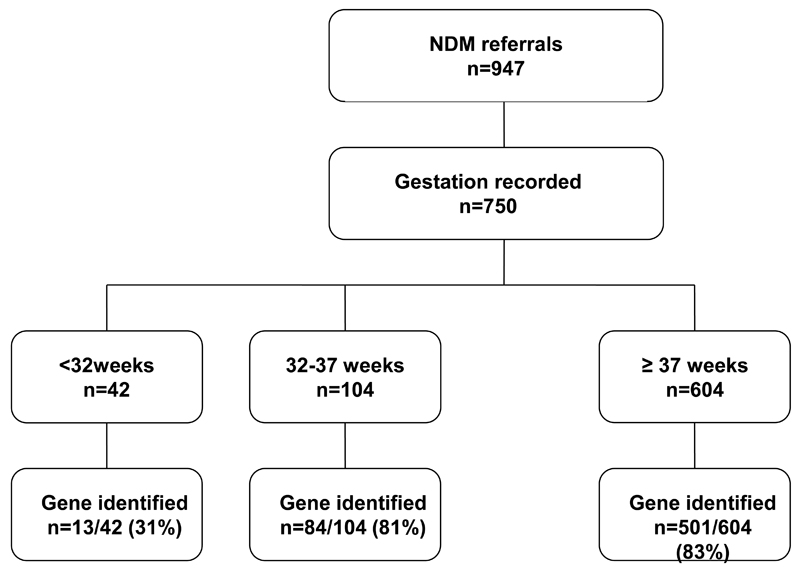
We examined the Exeter International Cohort of 947 patients patients with neonatal diabetes, defined as hyperglycemia presenting <26 weeks of age, who were referred for genetic testing to the Department of Molecular Genetics at the Royal Devon & Exeter NHS Foundation Trust, Exeter, UK, before July 2012. The 750 (79%) patients that had a gestational age provided were included in the analysis (figure 1). A mutation was classified as pathogenic if it was a known cause or highly likely to be pathogenic based on the mutation characteristics, co-segregation in the family and other clinical features consistent with that molecular diagnosis. Patients were recorded as not having an identified mutation if they tested negative for at least the three commonly occurring causes of neonatal diabetes: KCNJ11, ABCC8 and INS mutations.
Figure 1.
Number of patients with neonatal diabetes diagnosed < 26 weeks referred for genetic testing according to gestation.
Genetic analysis
In all patients the KCNJ11, ABCC8 and INS genes were sequenced as previously described 13, 18. Methylation analysis of the chromosome 6q24 region, that causes transient neonatal diabetes, was undertaken in all patients whose diabetes had entered remission and in patients who were less than 6 months at the time of referral for genetic testing. The EIF2AK3, NEUROD1, RFX6, GCK, FOXP3, SLC19A2, GLIS3, PDX1, PTF1A, NEUROG3, HNF1B, and BSCL2 genes were sequenced using standard methods in patients when testing was indicated by the phenotype or genetic information showing homozygosity over the region of the gene (methods available on request).
Ethical considerations
This study was conducted in accordance with the Declaration of Helsinki and informed consent was obtained from all patients, with parental consent given on behalf of children.
Statistical analyses
Data were not normally distributed so non-parametric analysis was used. Patients were coded as preterm (born <37 completed weeks gestation) or term (≥37 completed weeks gestation). Birth weight was converted into gestation and gender adjusted standard deviation scores (SDS) according to UK-preterm growth charts (ref).
Characteristics of preterm and term infants with a confirmed monogenic cause for their diabetes, and preterm infants with and without an identified mutation were assessed using the Mann-Whitney U test for continuous variables not normally distributed (age of diagnosis, birth weight, gestation, time to referral) and the Chi2 test for categorical data (gender, gene affected). Where expected frequencies of mutations were <5, the Fisher’s exact test was used.
Gestation was split into the following four groups based on the degree of prematurity: very preterm (<32 completed weeks, moderately preterm (33-36 completed weeks,), term (37-40 completed weeks,) and term plus (>40 weeks)19. The association between gestation and both birth weight and birth weight SDS was assessed using the Jonckheere-Terpstra Test for trend.
Statistical analysis was performed using SPSS version 15 software and a probability level of p<0.05 was assumed statistically significant.
Results
Patient characteristics
146/750 (19.4%) patients were born preterm, of which 104/750 (13.8%) patients were moderately preterm (33-36 completed weeks) and 42/750 (5.6%) very preterm (<32 completed weeks) (Figure 1). A genetic cause was identified in 598/750 (79.7%) infants, of which 97/598 (16%) were preterm.
Characteristics of preterm and term infants with a monogenic cause for their diabetes are summarised in Table 1A. 97/146 (66%) preterm infants had a defined genetic etiology for their diabetes which was a lower percentage than those born ≥37weeks (501/604 (83%), p<0.0001), Table 1B. This was predominantly because of 42 patients referred with gestation <32 completed weeks, of which only 13/42 (31%) had a monogenic cause for their diabetes. If these very preterm patients were removed from the analysis, there was no difference in the proportion of preterm and term patients with a monogenic cause for their diabetes (84/104(81%) v 501/604(83%), p=0.59.
Preterm infants with a mutation compared to patients born at term, presented earlier (age of diagnosis: 1(0.1-4) v 6(2-11) weeks, p<0.0001), had a higher birth weight SDS (-1.27(-2.27, -0.43) v -1.76(-2.59, -0.98), p<0.001) and were referred for genetic testing earlier (19(4, 212) v 73(7, 473), weeks p=0.003), Table 1A.
Which genetic mutations are seen in preterm infants?
The genetic mutations seen in premature and term patients are shown in Table 1B. Methylation defects at chromosome 6q24 and GATA6 mutations occurred more commonly in preterm than term infants (6q24: 27% v 12%, p=0.0001; GATA6: 9% v 2%, p=0.003). Mutations in KCNJ11 were less common in preterm infants (21% v 34%, p=0.008) but there was no difference in ABCC8 mutations 16% v 18%, p=0.73. No difference was seen between preterm and term infants for all other mutations, p>0.1).
Can clinical characteristics identify which preterm infants are more likely to have neonatal diabetes?
The clinical characteristics seen in premature patients <37 completed weeks gestation with and without mutations are shown in Table 2. Preterm infants with a monogenic form of diabetes were born later than those without an identified mutation (gestation 35(33-36) v 31(29-36) weeks, p<0.0001). In keeping with this the birth weight was greater (1730(1450-2200) v (1340(820-1770), p<0.0001) but there was no difference after correction for gestational age birth weight SDS (-1.28(-2.27, -0.43) v -1.06(-1.98, -0.20), p=0.48). There was no difference in the age at presentation (1(0.1-4) v 0.7(0.1-3.5) weeks, p=0.99), or gender (% male 54 v 63, p=0.27). There was an insignificant trend for time to referral to be longer in preterm patients with a monogenic cause compared to those where a mutation was not found (19(4, 212) v 8(4, 42)weeks, p=0.10). Only one patient was diagnosed with a mutation that at the time of referral for genetic testing were under a corrected gestational age of 34 completed weeks. There were 12 patients without a mutation who were referred with a corrected gestational age less than 34 completed weeks.
Impact of gestation on birth weight in patients with neonatal diabetes
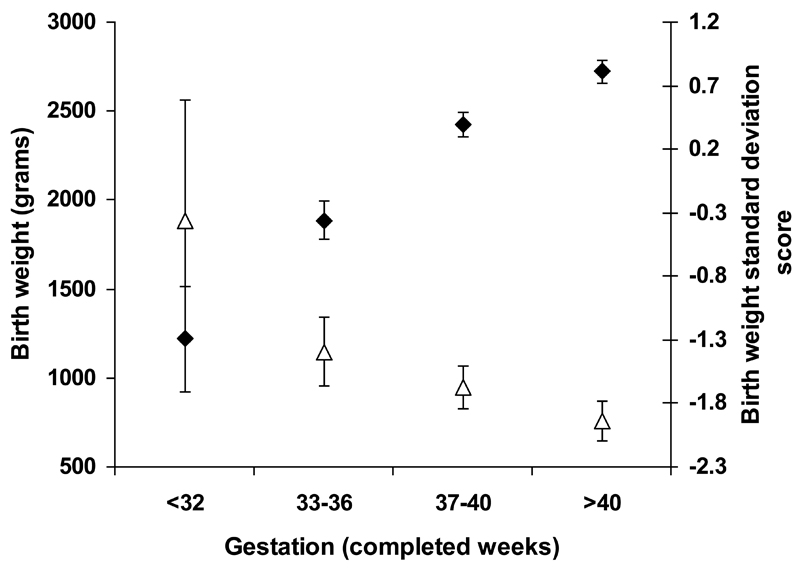
In the preterm patients with an identified mutation gestation-adjusted birth weight SDS was higher than in those born at term (-1.27(-2.27, -0.43) v -1.76(-2.59, -0.98), p<0.0001). The impact of gestational age on both birth weight and birth weight adjusted for gestation and gender, (birth weight SDS) in patients with an identified mutation is shown in Figure 2. Birth weight increased with gestational age (p<0.0001) but birth weight SDS decreased with gestational age (p<0.0001), Figure 2.
Figure 2.
Relationship between gestation and birth weight in patients with a proven genetic etiology of their neonatal diabetes.
Discussion
In this study of 750 patients with neonatal diabetes, we have demonstrated that patients with a monogenic etiology can be born premature. Although mutations were less common in children with severe prematurity (less than 32 completed week’s gestation), probably due to pancreatic immaturity causing hyperglycaemia, it is important to note that a monogenic diagnosis was still made on 31% of patients in this group.
Prematurity in neonatal diabetes
We found 66% of preterm patients with neonatal diabetes in our cohort with a genetic diagnosis and conversely 16% of patients with a genetic diagnosis were born prematurely. This suggests that premature delivery may be more frequent in patients with monogenic neonatal diabetes than the 12% seen in the normal population1. There are only 2 large independent series to compare this with and both have limited information on gestational age. In an American series of neonatal diabetes in patients diagnosed with diabetes <12 months of age (n=77), at least one of the 23 patients was born prematurely20. In a series by the French Neonatal Diabetes Study Group (n=79), 2 of the 15 patients with ABCC8 mutations were born premature 21, 22, whereas none of the 9 patients with KCNJ11 mutations or 7 patients with INS mutations (where recorded) were born premature 22. It is unclear whether the 5 preterm patients included in the original French series had abnormalities in chromosome 6q 23. More recently, in a small study of 15 patients born under 32 weeks with transient hyperglycemia, 2 patients were identified as having a monogenic etiology (KCNJ11 mutation, 6q24 abnormality) 24. Similar to our study, these two patients presented earlier than those without a mutation. It is unclear whether the 13 patients without an identified mutation had a monogenic etiology because genetic testing was limited to 6q24 abnormalities, INS, KCNJ11, and ABCC8 gene mutations. In our study, we identified 12 different causes of monogenic etiology in preterm infants born under 37 weeks.
Genetic etiology of neonatal diabetes in preterm infants
The high proportion of premature infants with 6q24 abnormalities is similar to recent reports by Temple and colleagues25 . Our finding of a higher proportion of GATA6 mutations in preterm infants has not been reported previously, but is not unexpected. One feature common to both is severe intra uterine growth retardation 26 27which may result from a reduction in third trimester insulin mediated foetal growth 18, 28. Prematurity may result from early elective delivery due to poor fetal growth. Furthermore, GATA6 is a key transcription factor involved in multiple organ development, including the endocrine and exocrine pancreas 26. Critical cardiac anomalies have been reported in patients with mutations in GATA6 and it is possible that prematurity is the result of spontaneous or elective delivery due to concerns with cardiac functioning, although we do not have any data to test this hypothesis26. This may explain the finding in our data that preterm patients who have a genetic etiology identified are referred for testing sooner than term patients.
Clinical differences to aid genetic referral
It is not possible to accurately identify patients who are born prematurely with monogenic neonatal diabetes based on clinical features alone. Although hyperglycemia in the very preterm infant (<32 completed weeks) makes a monogenic cause less likely it cannot be excluded as 31% of patients born after this date referred for genetic testing for neonatal diabetes had a potassium channel mutation which would radically alter the optimum treatment. This means that prematurity should not deter clinicians from referring preterm patients with hyperglycemia for genetic testing.
Although in our cohort, preterm infants with an identified mutation were referred earlier than those born at term, we do not have any data about the duration of hyperglycemia prior to genetic referral, or the reason for referral. Hyperglycemia secondary to prematurity would not be expected to persist as the immature pancreas, coupled with abnormal glucose homeostasis, the need for parenteral nutrition, steroid administration and stress, including sepsis should resolve with time. Remitting hyperglycemia may also occur in neonatal diabetes due to a genetic cause (transient neonatal diabetes mellitus), whereas in permanent neonatal diabetes mellitus the hyperglycemia will persist. Resolution of hyperglycemia therefore may reflect improved maturity or natural remission due to a monogenic cause but persistence of hyperglycemia is likely to reflect a monogenic cause of neonatal diabetes. However patients with an identified mutation did not have a longer time to referral.
Impact of gestation on birth weight
Our results showing reduced birth weight in patients with neonatal diabetes is well-described 29. This is attributed to hypoinsulinemia during the third trimester of pregnancy when insulin-stimulated foetal growth should normally occur. This is supported by our data which demonstrates that birth weight SDS is lower with increasing gestational age.
Study Limitations
Our data has a strong referral bias. We were only able to include patients who were referred to our center, so we are unable to say how often the hyperglycemia seen in premature infants is due to a monogenic cause. It is likely that a large number of patients with hyperglycemia due to prematurity were not referred for genetic testing. It is likely that, in the absence of sepsis, term infants are referred for genetic testing more commonly as there is no underlying prematurity to explain the hyperglycemia. In addition, some term as well as preterm infants with transient hyperglycemia may not have been referred for genetic testing. This group of patients may represent at a later stage with diabetes mellitus, and depending on the age at presentation, they may be misdiagnosed as having either type 1 diabetes if they are young and/or slim, or type 2 diabetes, if they are older or obese. This would result in inapprorpriate management. In addition, it may not raise suspicion of monogenic diabetes within other family members. These limitations mean that the prevalence of monogenic diabetes in preterm infants is unknown, and is likely to be overestimated in this cohort. Prospective studies are needed to determine the prevalence of neonatal diabetes in preterm infants with hyperglycemia.
We are only able to test for known genetic causes of neonatal diabetes and it is likely that there are more unknown causes. In the patients without a genetic cause, we have not systematically screened for all known genetic causes of neonatal diabetes so it is possible that there are more monogenic cases in the cohort currently without a genetic diagnosis
We were unable to determine the duration and severity of hyperglycemia in our cohort. Further studies are needed to determine whether the duration or degree of hyperglycemia can identify when hyperglycemia is likely to be due to a monogenic cause.
Implications
Our results indicate that prematurity should not exclude a diagnosis of neonatal diabetes. As improved survival of extremely low birth weight preterm neonates continues, hyperglycemia will become more common in this age group. It is likely that there is an under referral of preterm hyperglycemic infants for genetic testing, particularly as some early genetic studies excluded patients born prematurely.
The 37% of preterm patients with K-ATP channel mutations is clinically important as these patients are likely to be successfully transferred from insulin onto oral sulphonylureas with an improvement in glycaemic control16, 17. Identifying neonatal diabetes due to other genetic causes is also recommended to provide prognostic information on disease progression, screening for co-morbidities and genetic counselling for affected future pregnancies and .
Recommendations
Since only 13/750 (<2%) severely preterm patients referred for genetic testing have a monogenic cause identified, we recommend genetic referral of preterm patients with hyperglycemia if they are born after 32 completed weeks in whom septicemia is not present. Testing before this gestational age should probably be limited to those patients in whom hyperglycemia persists until their corrected gestational age is 32 weeks.
In conclusion, we report that patients with monogenic neonatal diabetes are frequently premature and hence genetic testing should not be discouraged based on prematurity alone.
Supplementary Material
What’s Known on This Subject.
Neonatal diabetes that presents in the first 6 months should be correctly diagnosed as the molecular genetic etiology defines the optimum lifelong management. Hyperglycemia is common in markedly-premature neonates and usually rapidly remits without long term treatment.
What This Study Adds.
Patients with neonatal diabetes due to a monogenic etiology can be born preterm. A genetic cause is more likely in those born with less severe prematurity (gestation >32 weeks), in whom genetic testing should be routinely offered.
Funding source
REJB was funded by Diabetes UK, through funding from a Clinical Training Fellowship, and the National Institute of Health Research from an Academic Clinical Lectureship award. IKT and DJGM are supported by the Wessex CRN, NIHR Wellcome Trust Clinical Research Facility, Southampton, and Diabetes UK. BS and MS are supported by the NIHR Exeter Clinical Research Facility. The genetic testing was funded by the Wellcome Trust. ATH and SE are Wellcome Trust Senior Investigators. ATH is an NIHR Senior Investigator.
Abbreviations
- SDS
Standard deviation score
- IQR
Interquartile range
- NDM
Neonatal diabetes
- K-ATP
ATP-sensitive potassium channel
Footnotes
Financial Disclosure: None of the authors have any financial relationships relevant to this article to disclose.
Conflict of Interest. None of the authors have any conflicts of interest to disclose.
Contributors’ Statement:
Rachel EJ Besser: Dr. Besser conceptualized and designed the study, performed the statistical analysis and drafted and revised the initial manuscript as submitted.
Sarah E Flanagan, Deborah JG Mackay and Professor IK Temple: Dr Flanagan, Dr Mackay and Professor Temple performed mutational analysis of samples, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Maggie H Shepherd. Professor Shepherd coordinated the clinical data collection, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Beverley M Shields: Dr. Shields performed statistical analysis for the paper and reviewed and revised the manuscript, and approved the final manuscript as submitted.
Sian Ellard. Professor Ellard acquired the neonatal diabetes dataset, performed mutational analysis of samples, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Andrew T Hattersley. Professor Hattersley acquired the neonatal diabetes dataset, designed the study, interpreted the data, reviewed and revised the manuscript, and approved the final manuscript as submitted.
References
- 1.Martin JA, Hamilton BE, Ventura SJ, et al. Births: final data for 2009. Natl Vital Stat Rep. 2011;60(1):1–70. [PubMed] [Google Scholar]
- 2.Mitanchez-Mokhtari D, Lahlou N, Kieffer F, Magny JF, Roger M, Voyer M. Both relative insulin resistance and defective islet beta-cell processing of proinsulin are responsible for transient hyperglycemia in extremely preterm infants. Pediatrics. 2004;113(3 Pt 1):537–541. doi: 10.1542/peds.113.3.537. [DOI] [PubMed] [Google Scholar]
- 3.Ogilvy-Stuart AL, Beardsall K. Management of aemia in the preterm infant. Arch Dis Child Fetal Neonatal Ed. 2010;95(2):F126–131. doi: 10.1136/adc.2008.154716. [DOI] [PubMed] [Google Scholar]
- 4.Dweck HS, Cassady G. Glucose intolerance in infants of very low birth weight. I. Incidence of hyperglycemia in infants of birth weights 1,100 grams or less. Pediatrics. 1974;53(2):189–195. [PubMed] [Google Scholar]
- 5.Louik C, Mitchell AA, Epstein MF, Shapiro S. Risk factors for neonatal hyperglycemia associated with 10% dextrose infusion. Am J Dis Child. 1985;139(8):783–786. doi: 10.1001/archpedi.1985.02140100045025. [DOI] [PubMed] [Google Scholar]
- 6.Falcao MC, Ramos JL. [Hyperglycemia and glucosuria in preterm infants receiving parenteral glucose: influence of birth weight, gestational age and infusion rate] J Pediatr (Rio J) 1998;74(5):389–396. doi: 10.2223/jped.463. [DOI] [PubMed] [Google Scholar]
- 7.Blanco CL, Baillargeon JG, Morrison RL, Gong AK. Hyperglycemia in extremely low birth weight infants in a predominantly Hispanic population and related morbidities. J Perinatol. 2006;26(12):737–741. doi: 10.1038/sj.jp.7211594. [DOI] [PubMed] [Google Scholar]
- 8.Hays SP, Smith EO, Sunehag AL. Hyperglycemia is a risk factor for early death and morbidity in extremely low birth-weight infants. Pediatrics. 2006;118(5):1811–1818. doi: 10.1542/peds.2006-0628. [DOI] [PubMed] [Google Scholar]
- 9.Ng SM, May JE, Emmerson AJ. Continuous insulin infusion in hyperglycaemic extremely-low- birth-weight neonates. Biology of the neonate. 2005;87(4):269–272. doi: 10.1159/000083863. [DOI] [PubMed] [Google Scholar]
- 10.Stanik J, Gasperikova D, Paskova M, et al. Prevalence of permanent neonatal diabetes in Slovakia and successful replacement of insulin with sulfonylurea therapy in KCNJ11 and ABCC8 mutation carriers. The Journal of clinical endocrinology and metabolism. 2007;92(4):1276–1282. doi: 10.1210/jc.2006-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanakatti Shankar R, Pihoker C, Dolan LM, et al. Permanent neonatal diabetes mellitus: prevalence and genetic diagnosis in the SEARCH for Diabetes in Youth Study. Pediatric diabetes. 2013;14(3):174–180. doi: 10.1111/pedi.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iafusco D, Stazi MA, Cotichini R, et al. Permanent diabetes mellitus in the first year of life. Diabetologia. 2002;45(6):798–804. doi: 10.1007/s00125-002-0837-2. [DOI] [PubMed] [Google Scholar]
- 13.Edghill EL, Dix RJ, Flanagan SE, et al. HLA genotyping supports a nonautoimmune etiology in patients diagnosed with diabetes under the age of 6 months. Diabetes. 2006;55(6):1895–1898. doi: 10.2337/db06-0094. [DOI] [PubMed] [Google Scholar]
- 14.Rubio-Cabezas O, Flanagan SE, Damhuis A, Hattersley AT, Ellard S. KATP channel mutations in infants with permanent diabetes diagnosed after 6 months of life. Pediatric diabetes. 2012;13(4):322–325. doi: 10.1111/j.1399-5448.2011.00824.x. [DOI] [PubMed] [Google Scholar]
- 15.De Franco E, Flanagan SE, Houghton JA, et al. The effect of early, comprehensive genomic testing on clinical care in neonatal diabetes: an international cohort study. Lancet. 2015;386(9997):957–963. doi: 10.1016/S0140-6736(15)60098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pearson ER, Flechtner I, Njolstad PR, et al. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. The New England journal of medicine. 2006;355(5):467–477. doi: 10.1056/NEJMoa061759. [DOI] [PubMed] [Google Scholar]
- 17.Rafiq M, Flanagan SE, Patch AM, Shields BM, Ellard S, Hattersley AT. Effective treatment with oral sulfonylureas in patients with diabetes due to sulfonylurea receptor 1 (SUR1) mutations. Diabetes care. 2008;31(2):204–209. doi: 10.2337/dc07-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flanagan SE, Patch AM, Mackay DJ, et al. Mutations in ATP-sensitive K+ channel genes cause transient neonatal diabetes and permanent diabetes in childhood or adulthood. Diabetes. 2007;56(7):1930–1937. doi: 10.2337/db07-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawn JE, Gravett MG, Nunes TM, Rubens CE, Stanton C, Group GR. Global report on preterm birth and stillbirth (1 of 7): definitions, description of the burden and opportunities to improve data. BMC pregnancy and childbirth. 2010;10(Suppl 1):S1. doi: 10.1186/1471-2393-10-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stoy J, Greeley SA, Paz VP, et al. Diagnosis and treatment of neonatal diabetes: a United States experience. Pediatric diabetes. 2008;9(5):450–459. doi: 10.1111/j.1399-5448.2008.00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaxillaire M, Dechaume A, Busiah K, et al. New ABCC8 mutations in relapsing neonatal diabetes and clinical features. Diabetes. 2007;56(6):1737–1741. doi: 10.2337/db06-1540. [DOI] [PubMed] [Google Scholar]
- 22.Babenko AP, Polak M, Cave H, et al. Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. The New England journal of medicine. 2006;355(5):456–466. doi: 10.1056/NEJMoa055068. [DOI] [PubMed] [Google Scholar]
- 23.Metz C, Cave H, Bertrand AM, et al. Neonatal diabetes mellitus: chromosomal analysis in transient and permanent cases. J Pediatr. 2002;141(4):483–489. doi: 10.1067/mpd.2002.127089. [DOI] [PubMed] [Google Scholar]
- 24.Busiah K, Auger J, Fauret-Amsellem AL, et al. Differentiating Transient Idiopathic Hyperglycaemia and Neonatal Diabetes Mellitus in Preterm Infants. Horm Res Paediatr. 2015;84(1):68–72. doi: 10.1159/000381621. [DOI] [PubMed] [Google Scholar]
- 25.Docherty LE, Kabwama S, Lehmann A, et al. Clinical presentation of 6q24 transient neonatal diabetes mellitus (6q24 TNDM) and genotype-phenotype correlation in an international cohort of patients. Diabetologia. 2013;56(4):758–762. doi: 10.1007/s00125-013-2832-1. [DOI] [PubMed] [Google Scholar]
- 26.Allen HL, Flanagan SE, Shaw-Smith C, et al. GATA6 haploinsufficiency causes pancreatic agenesis in humans. Nat Genet. 2012;44(1):20–22. doi: 10.1038/ng.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Franco E, Shaw-Smith C, Flanagan SE, Shepherd MH, Hattersley AT, Ellard S. GATA6 mutations cause a broad phenotypic spectrum of diabetes from pancreatic agenesis to adult-onset diabetes without exocrine insufficiency. Diabetes. 2013;62(3):993–997. doi: 10.2337/db12-0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Temple IK, Shield JP. Transient neonatal diabetes, a disorder of imprinting. J Med Genet. 2002;39(12):872–875. doi: 10.1136/jmg.39.12.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Muhlendahl KE, Herkenhoff H. Long-term course of neonatal diabetes. The New England journal of medicine. 1995;333(11):704–708. doi: 10.1056/NEJM199509143331105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.