Abstract
Background
A growing number of studies have investigated delay discounting, a behavioral economic index of impulsivity, and its relevance to attention deficit hyperactivity disorder (ADHD), but with mixed findings. The current meta-analysis synthesizes the literature on the relationship between monetary delay discounting and ADHD in studies using case-control designs. Specifically, the objectives were: 1) to characterize the aggregated differences in monetary delay discounting between individuals with ADHD (cases) and controls in studies using categorical case-control designs; 2) to examine potential differences based on sample age (<18 vs. >18), reward outcome (real vs. hypothetical), and prevalence of conduct disorder and oppositional defiant disorder in the sample; and 3) to evaluate potential small-study (publication) bias in the literature.
Methods
From 567 candidate articles, 21 independent investigations yielded 25 case-control comparisons (total N=3,913). Random effects meta-analysis was conducted using Cohen's d as the common effect size. Publication bias was evaluated using fail-safe N, Begg-Mazumdar and Egger tests, and metaregression of publication year and effect size.
Results
Across studies, a statistically significant difference of medium magnitude effect size was present for the case-control comparisons (d=0.43; p < 10−15). No significant differences based on sample age, reward outcome, or comorbid status was detected. Minimal heterogeneity and evidence of publication bias was present.
Conclusions
These findings provide robust evidence that delay discounting is significantly elevated among individuals with ADHD compared to controls. Gaps in the literature and the importance of characterizing the neural and genetic bases of this relationship are discussed.
Keywords: Attention deficit hyperactivity disorder, delay discounting, impulsivity, intertemporal choice, decision making, meta-analysis
Introduction
Since the earliest clinical descriptions of attention deficit hyperactivity disorder (ADHD), high levels of impulsivity have been described as a cardinal feature of the disorder (1). However, although impulsivity can be broadly defined as a person's capacity to inhibit or regulate arising impulses, it is operationalized in a variety of different ways (2; 3), including both self-report questionnaires and behavioral tasks. Importantly, these diverse measures are often not highly intercorrelated, undermining the notion of a single underlying process. A recent meta-analysis of the interrelationships among self-report and behavioral task measures of impulsivity indicated little overlap between the two domains (4). Recent factor analyses suggest that when multiple measures of impulsivity are examined concurrently, latent aggregations of measures emerge (5-7), suggesting that impulsivity appears to be a psychological genus that subsumes a number of species.
One discrete form of impulsivity is delay discounting, reflecting how much a person devalues a reward based on its delay in time. Delay discounting is an index of impulsivity from behavioral economics, a hybrid discipline that integrates principles and methods from psychology and economics to study choice behavior. It is commonly measured using decision making tasks that pose a variety of decisions, such as “Would you rather have $40 today or $100 in a month?,” with systematic variation of smaller immediate rewards and delays in time, while keeping the larger delayed reward consistent. Then, across an array of smaller (discounted) immediate rewards and future delays, an individual's overall devaluation of the larger delayed reward can be generated. Often, this is via examination of where an individual switches preferences from smaller-sooner to large-later rewards and then using those points of indifference across delays to model the individual's temporal discounting function. A widely used model uses a hyperbolic function, vd = V/(1+kd) (8), where vd is the discounted value of the delayed reward, V is the objective value of the delayed amount, d is the delay duration, and k is the derived parameter that characterizes the degree of future reward discounting. Alternatively, the ‘switchpoints’ can be used to generate an individual's discounting curve and area under the curve (AUC) can be used as a measure of future discounting (9). Of note, larger k values reflect more precipitous devaluation of future rewards (reflecting a larger denominator in the equation), whereas the opposite is true for AUC values (reflecting a smaller space beneath the curve). Common to all methods is that if a delayed reward loses value more rapidly, the individual is considered more impulsive. Of note, delay discounting tasks are related to delay of gratification paradigms (10), such as the “marshmallow test” (11), but differ insofar as the latter typically have a real-time component in which participants can alter their preference at any point, incorporating an element of in vivo temptation.
There has been longstanding experimental and theoretical interest in steep discounting of delayed rewards as a feature of ADHD. Empirically, early studies used simple choice tasks and found support for intertemporal choice deficits among children with ADHD (12; 13). Subsequently, studies using iterative behavioral tasks systematically examining preferences for monetary rewards have revealed similar patterns in children, adolescents, and adults (14-18). Theoretically, steep delay discounting is considered a hallmark deficit of ADHD (19-21), akin to deficits in response inhibition and sustained attention(22; 23). The underlying deficit has been theorized to be hypoactivity in mesocortical dopamine neurotransmission based on preclinical and functional magnetic resonance imaging (fMRI) studies (19; 24), although characterizing these processes remains an active area of inquiry. In addition, steep temporal discounting may be a cause of the high comorbidity between ADHD and substance use disorders (SUDs) (25; 26). For example, individuals with ADHD have, depending on the drug type, a twofold-to-eightfold higher prevalence of substance use disorder (25). Conversely, in a large recent study of treatment-seeking individuals with SUDs, over 40% screened positive for ADHD (27). This substantial overlap suggests some level of common etiological causality, one form of which may be delay discounting. Behavioral studies have found evidence of more precipitous temporal discounting in individuals with alcohol use disorders (28), nicotine dependence (29), cocaine dependence (30), and opioid dependence (31) compared to matched control participants. Thus, steep discounting of delayed rewards may be a common risk process in ADHD and addiction.
As a proliferation of studies on delay discounting and ADHD has emerged over the last five years, several have not reported significant associations (32; 33), suggesting the link may be weaker or more ambiguous than initially believed. Furthermore, the accumulating literature has not been systematically examined to characterize overall patterns of findings and possible bias. This was the focus of the current meta-analysis. Specifically, the present study had three aims: 1) to characterize the relationship between monetary delay discounting and ADHD in previously published case-control comparisons; 2) to examine three potential moderators of effects, namely, sample age (<18 vs. >18), reward outcome (real vs. hypothetical), and prevalence of conduct disorder/ oppositional defiant disorder in the sample; and 3) to investigate the presence of small-study bias, reflecting the probability of publication bias.
Methods and Materials
Meta-analysis sample
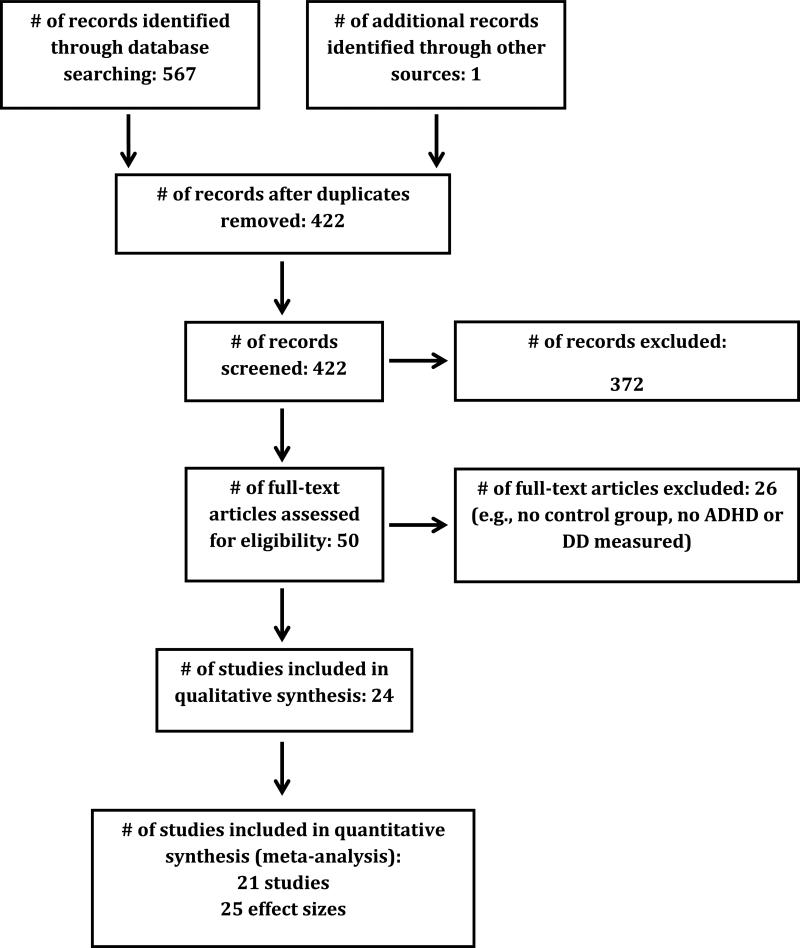
The initial inclusion criterion was any peer-reviewed published study or unpublished dissertation reporting comparisons of delay discounting between a group meeting ADHD diagnostic criteria and a control group. To minimize substantial methodological variability, studies were restricted to delay discounting of monetary rewards (e.g., simple choice tasks using golden donkeys, spaceships, etc., were excluded) and studies of probability discounting and social discounting were excluded. Studies were identified via a literature search using the PubMed and PsyclNFO databases as of September 25, 2015. The specific Boolean terms entered were (“discounting” OR “delay of gratification”) AND (“attention” OR “ADHD”). The search term “ADHD” is automatically expanded to include “attention deficit disorder with hyperactivity” OR (“attention” AND “deficit” AND “disorder” AND “hyperactivity”). A total of 567 records were generated, of which 422 were unique after eliminating duplicates from the two databases, and 49 were relevant. Full-text reviews were conducted on the relevant studies, yielding 21 viable studies with 25 distinct comparisons. Records were excluded if they used rodent models, were review papers, or if they did not collect data on either ADHD or delay discounting. Two of the relevant studies reported overlapping data. After contacting the authors, only the more extensive of these two papers was included. The meta-analysis was performed on the 25 viable comparisons of delay discounting and ADHD. Meta-analysis of continuous associations was considered but ultimately not pursued because of a paucity of studies (k = 3). A PRISMA flow diagram that is consistent with meta-analyses guidelines is provided in Figure 1 (34).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) inclusion flow diagram.
Sample characteristics
Effect sizes reflecting differences between an ADHD-positive group and a control group were available for 21 of the 422 uniquely identified articles, yielding 25 comparisons. Fifteen of these relevant comparisons reported statistically significant differences in DD performance between ADHD and control groups and ten reported non-significant differences. Three individual papers provided results for delay discounting at multiple magnitudes. All reported comparisons were included in order to maximize representation of the literature, but a follow-up analysis included a single meta-analyzed effect size from studies using multiple measures.
Individual study characteristics are available in Table 1 and illustrate the wide variation of study protocol. Sample sizes range considerably, from N=36 to N=1,298 (total N=3,913), with an average sample size of 157. The average age within studies ranged from 7.9 to 36.9 years old, with a total sample average age of 21.0 and median study age of 16.0. The delayed reward for various discounting tasks were primarily for hypothetical outcomes, ranging from $0.10 to $5000, with an average of $380 and a median of $42.50. The vast majority of studies used k or AUC as the index of discounting, but one study reported individual points of indifference and one reported impulsive choice ratio and both included an aggregated F statistic for a group contrast; the latter was used as an overall index of discounting.
Table 1.
All case-control effect sizes (k=24), listed in order of recency.
| Study | Mean Age | Group Ns (ADHD vs. Control) | ADHD Comorbidities | Stimulant Medication Status | Reward Type | Delayed Amount | Temporal Delays | Discounting Index |
|---|---|---|---|---|---|---|---|---|
| Mostert et al. 2015a (81) | 35.93 | 109 vs. 123 | 1 or more comorbidity | 76% medicated, no abstinence | Hypothetical | € 10 | NA | k |
| Mostert et al. 2015b (81) | 35.93 | 133 vs. 132 | 1 or more comorbidity | 76% medicated, no abstinence | Hypothetical | € 30 | NA | k |
| Mostert et al. 2015c (81) | 35.93 | 133 vs. 132 | 1 or more comorbidity | 76% medicated, no abstinence | Hypothetical | € 100 | NA | k |
| Onnink et al. 2015 (82) | 35.55 | 107 vs. 109 | 49% 1+ MDD episodes | 60% medicated, no abstinence | Hypothetical | € 100 | 2, 30, 180, 365, and 730 days | k |
| Yu et al. 2015 (83) | 8.93 | 19 vs. 25 | None | None | Real | $ 0.10 | 0, 5, 10, 20, 30, and 60 secs | AUC |
| Costa Dias et al. 2015 (84) | 9.28 | 36 vs. 54 | 39% 1+ comorbid diagnosis | 42% medicated, abstinence of 5 half-lives (24-48hr) | Hypothetical | $ 10 | 7, 30, 90, and 180 days | k |
| Castellanos-Ryan et al. 2014 (85) | 16.00 | 41 vs. 1257 | 27% CD | NA | Hypothetical | $ 55† | 7-186 days (23 unique delays) | k |
| Chantiluke et al. 2014 (53) | 14.79 | 31 vs. 18 | 42% ASD | 50% medicated, 48hr abstinence | Hypothetical | £ 100 | l wk, 1 mo, and 1 yr | k |
| Fassbender et al. 2014 (86) | 17.99 | 25 vs. 40 | None | None | Real | $ 33 | 7, 14, 21, 28, 35, 42, 49, and 56 days | k |
| Dai et al. 2013a (32) | 33.20 | 31 vs. 28 | Significantly higher rate of MDD and SP | NA | Hypothetical | NZ$ 50 | 1 and 6 mos, 1, 3, 5, and 10 yrs | AUC |
| Dai et al. 2013b (32) | 33.20 | 31 vs. 28 | Significantly higher rate of MDD and SP | NA | Hypothetical | NZ$ 5000 | 1 and 6 mos, 1, 3, 5, and 10 yrs | AUC |
| Costa Dias et al. 2013 (87) | 9.34 | 35 vs. 64 | None | Abstinence of 5 half-lives (24-48hr) | Hypothetical | $ 10 | 1, 7, 30, 90, 180, and 365 days | k |
| Crunelle et al. 2013 (88) | 32.98 | 28 vs. 17 | 39% cocaine dependence | Medication naive | Hypothetical | NA | 5 days, 1 and 3 mos, 1, 3 and 10 yrs | k |
| Demurie et al. 2013 (16) | 11.82 | 72 vs. 130 | None | 24hr abstinence | Hypothetical | € 30 | 1 and 2 days, 1 and 2 wks | AUC |
| Scheidel 2013 (89) | 26.46 | 25 vs. 25 | None | 100% medicated, no abstinence | Hypothetical | $ 1000 | 1 and 3 days, 1 wk, 6mos, and 5 yrs | AUC |
| Demurie et al. 2012 (15) | 12.30 | 38 vs. 46 | 11% ODD/CD | 79% medicated, 24hr abstinence | Hypothetical | € 30 | 1 and 2 days, 1 and 2 wks | AUC |
| Wilbertz et al. 2012 (33) | 36.91 | 28 vs. 28 | 25% current diagnosis, 32% lifetime | ≥ two months abstinence | Hypothetical | € 200 | 1, 3, 9, 24, 60, 120, and 240 mos | k |
| Wilson et al. 2011 (18) | 7.93 | 27 vs. 31 | 26% ODD | 30% medicated, 24-48hr abstinence | Hypothetical | $ 10 | 7, 30, 90, and 180 days | k |
| Hurst et al. 2011 (17) | 20.00 | 21 vs. 218 | None | NA | Hypothetical | $ 1000 | 1 and 2 wks, 1 and 6 mos, 1, 3, and 10 yrs | AUC |
| Scheres et al. 2010 (77) | 11.81 | 45 vs. 37 | None | Unmedicated | Real | $ 0.10 | 5, 10, 20, 30 and 60 secs | AUC |
| Paloyelis et al. 2010 (73) | 15.42 | 36 vs. 32 | None | 72% medicated, 48hr abstinence | Hypothetical | £ 17† | 1, 2, 7, 14, 30, 60, 120, and 180 days | AUC |
| Plichta et al. 2009 (54) | 23.45 | 14 vs. 14 | None | 50% medicated, 96hr abstinence | Real | € 60 | 0, 2, and 4 wks | ICR |
| Scheres et al. 2006 (90) | 11.42 | 22 vs. 24 | 14% psychotic or PDD symptoms | 81% medicated, 36% abstinent | Real | $ 0.10 | 0, 5, 10, 20, and 30 secs | AUC |
| Barkley et al. 2001a (14) | 14.73 | 101 vs. 39 | 100% ODD | 58% medicated, 24hr abstinence | Hypothetical | $ 100 | 1 mo, 1, 5, and 10 yrs | POI |
| Barkley et al. 2001b (14) | 14.73 | 101 vs. 39 | 100% ODD | 58% medicated, 24hr abstinence | Hypothetical | $ 1000 | 1 mo, 1, 5, and 10 yrs | POI |
Notes: Hypothetical = hypothetical delay discounting task; NA = not available; k = hyperbolic discounting function; MDD = major depressive disorder; Real = real delay discounting task; secs = seconds; AUC = area under the curve; hr = hour; CD = conduct disorder
indicates a mean value; ASD = autism spectrum disorder; wk = week; yr = year; SP = Social Phobia; mo = month; ODD = oppositional defiant disorder; ICR = intertemporal choice ratio; PDD = pervasive developmental disorder; POI = points of indifference
Meta-analytic approach
Both fixed and random effects meta-analytic approaches were considered, but a random effects approach was selected as the primary method in light of methodological heterogeneity. The effect size used in the meta-analysis of case-control studies was Cohen's d (35). This value was either identified directly from the publication or generated from reported statistics. Effect sizes generated using AUC were inverted to be consistent with those generated using k values. In three viable studies, sufficient information to generate effect sizes was not provided. The authors of these studies were contacted and all were able to supply the necessary data. A fixed effects approach was applied as a follow-up strategy to characterize heterogeneity of effect size. Cochran's Q and I2 are two common indices that determine heterogeneity of effect size. Cochran's Q statistic reflects the sum of square differences among the individual weighted study effects and the overall mean. Q tests the significance of heterogeneity using a χ2 test. I2 reflects the percentage of study effect size variation that is explained by true heterogeneity. Values of I2 ≤ 25% indicate low heterogeneity, ~ 50% indicate moderate heterogeneity, and ≥75% indicate high heterogeneity across studies (36). Following the analysis of all included studies, a ‘jackknife’ analysis systematically excluding each effect size was conducted. In addition, because active ADHD medication could suppress observed differences between groups, a follow-up analysis was conducted excluding studies including medicated participants with no washout period. Participant average age (below 18 versus above 18), task outcome (hypothetical versus actual), and percentage of comorbid conduct disorder and conduct disorder or oppositional defiant disorder were examined as moderators. For the latter, studies were coded based on the reported prevalence of the comorbidities, with absent information coded as zero. Age and task outcome were tested using the Q statistic associated with the between groups difference in a mixed effects analysis. Percent comorbidity was examined using meta-regression. Four indices were used to evaluate publication bias: the classic fail-safe N approach; examination of the funnel plots of sample size and effect size via the two-tailed Begg-Mazumdar test (37), measuring the interdependence of variance and effect size; and the one-tailed Egger's test (38), measuring asymmetry of the funnel plot away from negative findings (assuming no publication bias for positive findings); and meta-regression to examine the relationship between year of publication and effect size. Overall evidence for bias was based on consideration of all four indices. Using Duval and Tweedie's trim and fill approach (39) adjusted estimates of effect size were generated based on imputed unpublished studies. Meta-analytic computations were conducted using Comprehensive Meta-analysis 2.0 (40). The methods of this meta-analysis conformed to the preferred reporting items for systematic reviews and meta-analysis (PRISMA) standards (34).
Results
Primary Meta-Analysis Findings
Overall, the difference between groups was of medium effect size (d = 0.43) and highly statistically significant (p < 10−15). The forest plot of the aggregated effect is presented in Figure 2. Although a random effects model was the primary analytic strategy, a fixed effects analysis was conducted to characterize heterogeneity, which was not significantly present across the sample of studies (Q = 25.91, p = .36; I2 = 7.56), meaning that both models produce virtually identical results. Re-running the primary analysis and systematically excluding each study generated very similar effect sizes and significance levels (ds = .42-.45, all ps < 10−15), suggesting a limited influence of any single study on the results. Re-running the primary analysis with a consolidation of effect sizes from studies using multiple measures revealed a similar effect size (d = 0.50, p < 10−15), suggesting a modest influence. The majority of studies indicated that participants had been abstinent from medication for at least 48hrs, were medication naïve, or did not discuss medication status. However, after removing three studies from the analysis that indicated participants had been medicated during the study, the effect-size increased slightly (d = 0.45, p < 10−15).
Figure 2.
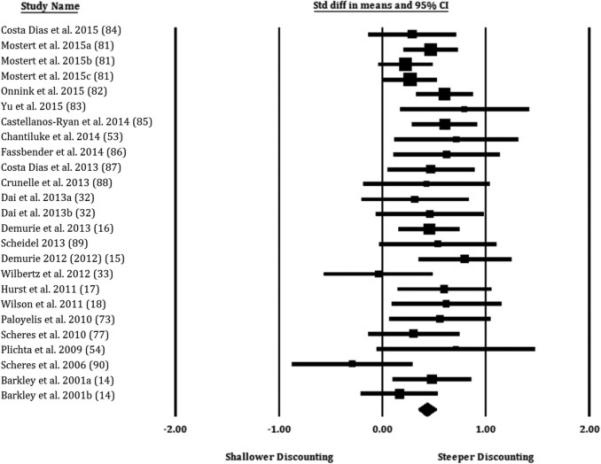
Forest plot providing effect sizes (standard differences in means; d) and 95% confidence intervals by study for comparisons of individuals with ADHD to control individuals. Effect size symbols are proportional to study sample size. Effects to the right of zero reflect greater delay discounting (steeper devaluation of future rewards) in the ADHD group compared to the control group. Study subscripts refer to multiple comparisons within the same study; numbers in parentheses refer to the references.
Relationship to Age, Task Outcome, and Comorbidity with Conduct Disorder/Oppositional Defiant Disorder
Although there was no significant heterogeneity, the studies differed noticeably in the age of the samples (Table 1) and were further examined by studies using child/adolescent participants (i.e., mean age <18) and adult participants (i.e., mean age >18). In studies that focused on children and adolescents with ADHD, the difference between groups was again highly statistically significant and of medium effect size (d = .47; p < 10−12). Similarly, in studies that used adult participants, the difference was also statistically significant and the effect size was of medium magnitude (d = 0.40; p < 10−11), albeit somewhat smaller. However, the difference in effect size was not statistically significant (Q = .69, p = .41). In addition, a meta-regression of age and effect size was non-significant (slope = −.004, p = .25). Thus, the magnitude of difference in delay discounting between individuals with ADHD and controls appears to be generally consistent across age.
With regard to tasks using hypothetical versus actual rewards, tasks using hypothetical outcomes exhibited somewhat larger effect sizes (d = 0.44, p < 10−15 versus d = 0.41; p < 0.03), but the difference was not statistically significant (Q = 0.016, p = 0.90). However, it is notable that there were many more effect sizes for tasks with hypothetical outcomes than actual outcomes (k=20 versus k=5; Table 1).
The meta-regression analysis of percentage of participants with conduct disorder was nonsignificant (slope = .84, p = .17), as was the meta-regression of percentage of participants with conduct disorder and/or oppositional defiant disorder (slope = −.05, p = .72).
Publication Bias
For the primary meta-analysis findings, none of the four indices indicated small-study bias. The classic fail-safe N indicated that there would need to be 676 unpublished studies to raise the p-value to above the threshold for statistical significance. With regard to the funnel plot (Figure 3), Kendall's τ = 0.12 (p = 0.40) and the Egger's test intercept = 0.43 (p = 0.53), do not suggest publication bias. Finally, a meta-regression indicated no statistically significant relationship between year of publication and effect size (slope = 0.011; p = 0.29). Duval and Tweedie's trim and fill method (39) indicated the possibility of four unpublished studies and, if they were included in the meta-analysis, then the effect size would change from 0.43 to 0.40 (Figure 3).
Figure 3.
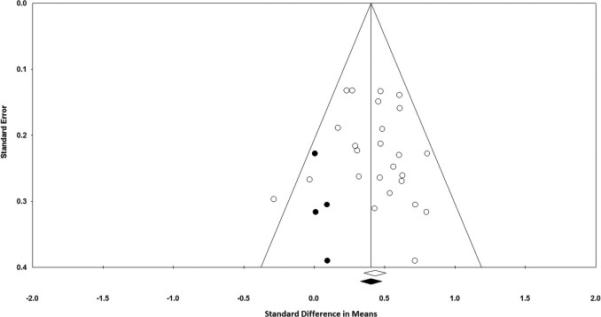
Funnel plot of the relationship between effect sizes (d) and standard errors (open circles), with imputation of unpublished studies (filled circles) using Duval and Tweedie's trim and fill approach.
Discussion
The primary aim of the present meta-analysis was to quantitatively aggregate the findings across published studies comparing monetary delay discounting in individuals with ADHD to healthy control individuals. The results provide very strong evidence of significantly higher discounting of future rewards in ADHD individuals. Indeed, the level of statistical significance observed (p < 10−15) would meet the highest evidentiary thresholds in statistics. In terms of the magnitude of the difference, the aggregated effect size was of medium magnitude, reflecting approximately half a standard deviation. Additional analyses suggested that there was relatively little heterogeneity of effect size and no single study had a substantial effect on the aggregated effect size. Notably, approximately half of the studies focused on children and the other half focused on adults, but neither categorical nor continuous analyses suggested that the effect sizes were systematically different by age. Similarly, no significant difference was present between studies using tasks with hypothetical outcomes and those using actual outcomes, although the lopsided numbers of studies makes this conclusion somewhat more tentative. Meta-regressions of the relationship effect size and percent of participants with conduct disorder and oppositional defiant disorder did not suggest systematic differences, although caution should be applied to this finding because many of the included studies did not fully characterize the sample for these conditions. For the primary meta-analysis, a further goal of the meta-analysis was to characterize the potential for publication bias in the literature on delay discounting and ADHD. In this case, the bias indicators suggest a large number of null findings would need to be in the ‘file-drawer’ (41) to render the overall conclusion null and that the interrelationships between effect size magnitude and variance or year of publication are not suggestive of small study bias. Of course, it is worth noting that these are imperfect measures, but the consistent absence of evidence for publication bias is supportive of the overall conclusions. Collectively, these findings suggest that elevated monetary delay discounting is a robust distinguishing feature of ADHD in studies using case-control designs and that this is consistent in the context of various collateral variables.
These findings have a number of potentially important implications. To start, a clear implication is the need to understand how the observed difference is instantiated neurally. In general, fMRI studies have identified several domains of abnormal functionality in ADHD, including reward, attention, inhibition, timing, and default mode activity (42-48). Individuals with ADHD have been found to exhibit reduced ventral striatal activity in response to reward, opposite to their healthy control counterparts (47; 48). Scheres et al. suggest that neural hypo-responsiveness to anticipated reward may result in compensatory increases of reward-seeking behavior - a key characteristic of ADHD and an elemental process within delay discounting decision making (47). Indeed, studies of delay discounting using fMRI have directly implicated the ventral striatum in delay discounting, among a number of other regions, including subunits of prefrontal cortex, posterior cingulate cortex, anterior insula, and anterior cingulate (24; 49). Among these active regions, multiple accounts for both integrated and opposing relationships have been proposed, although the data do not support any account definitively at this point (24). However, the regions implicated in neuroimaging studies, particularly the ventral striatum and prefrontal cortex, are highly compatible with findings from preclinical studies using animal models of delay discounting (50; 51).
Directly speaking to this question for ADHD, a small number of studies have examined the neural correlates of delay discounting in individuals with ADHD compared to controls using fMRI. In a pediatric sample, boys with ADHD exhibited significantly less activation in inferior prefrontal cortex, orbitofrontal cortex, and inferior parietal lobule compared to controls during a contrast of larger delayed choices minus smaller immediate choices (52). In another pediatric study, three clinical groups - individuals with ADHD, individuals with autism spectrum disorder, and individuals with both - were contrasted with each other and control participants (53). In this case, the comorbid group exhibited the most pronounced differences in terms of activation, specifically in areas of ventromedial prefrontal cortex, anterior cingulate, inferior frontal cortex and ventral striatum, among others. Interestingly, the ADHD-only group exhibited some of these differences but also exhibited more robust associations between inferior frontal cortex activity and temporal discounting functions. One study has investigated the neural correlates of delay discounting in adults with ADHD versus matched controls, reporting reduced ventral striatal activity during both choices for immediate rewards and delayed rewards (54), which is consistent with the hypoactive reward system hypothesis. In addition, Plichta et al. (54) detected greater activity in the dorsal caudate and amygdala in ADHD participants during delayed reward choices and, in turn, this activity was associated with ADHD symptoms, a pattern that may reflect delay aversion in ADHD.
Notably, these findings are highly compatible with a meta-analysis of monetary delay discounting in studies of drug addiction (55). That study similarly found evidence of a medium effect size difference in delay discounting between addiction criterion groups and control participants. Interestingly, most studies on delay discounting and addiction do not report ADHD status or symptoms, raising the question of whether observed elevations are in part a function of unidentified ADHD. This is necessarily speculation, but the results of this meta-analysis provide further support for the need to understand the intersection of ADHD and addictive disorders. It is also notable that similar general patterns of striatal hypoactivity are seen in substance users (56), indicating the possibility that reduced ventral striatal activity may be a shared trait between the two populations. In addition, fMRI studies on delay discounting in individuals with SUDs compared to control participants, have revealed similar findings to those on ADHD. For example, alcohol misuse has been implicated with distinct patterns of neural activity in subunits of the prefrontal cortex, parietal cortex, anterior cingulate and anterior insula, among others, during delay discounting decision making (57-59). Future imaging studies have the potential to disentangle commonalities and differences between individuals with addictive disorders and ADHD at an even finer level.
Finally, a more oblique implication of these findings is that they provide further support for studies investigating delay discounting in efforts to understand genetic influences on ADHD. There is extensive evidence of the heritability of ADHD (42), including family, twin, and adoption studies (60-62). However, attempts to determine the molecular basis for genetic influences have been less successful than anticipated, potentially because of the heterogeneity of the diagnosis. Endophenotypes, or genetically-influenced mechanistic variables that are putatively simpler than clinical phenotypes (63; 64), are hoped to inform psychiatric genetics by providing a simpler genetic architecture, leading to more robust and reliable associations, and revealing the etiological processes that confer risk and protection. With regard to delay discounting, there is evidence that delay discounting preferences are heritable from studies using both non-human animals (65; 66) and twin cohorts (67; 68). In addition, a small number of molecular genetic studies have implicated dopamine- related polymorphisms and delay discounting (58; 69-72) and one has investigated this question among individuals with ADHD (73). Paloyelis et al. (73) found evidence that rs4680 genotype in COMT and DAT1 haplotype predicted discounting rates in a case-control study of adolescents with ADHD. Specifically, A-allele COMT homozygotes exhibited higher discounting of the future across both groups, and an interaction was present between DAT1 haplotype status and diagnosis such that the group without ADHD who were carriers of the DAT1 risk haplotype exhibited discounting levels that were similarly steep to the group with ADHD. These links to dopamine-related loci are consistent with preclinical studies that implicate dopamine neurotransmission with delay discounting. Of note, however, serotonin and norepinephrine also appear to play important roles (50; 51; 74) and two studies have found associations between polymorphisms in serotonin and noradrenergic genes and delay discounting preferences (75; 76). Although genetic studies are at an early stage, the current findings support the need for further attempts to examine whether delay discounting can be used to understand one pathway for genetic influences on ADHD.
Despite generally robust findings, it is important to note that the current study does not address a number of issues. Most studies did not make a distinction between ADHD subtypes and it is important to note that, when treated as different groups, some studies have reported that ADHD subtypes exhibit significantly different results for delay discounting (77) and Scheres et al. reported that symptoms of these different ADHD subtypes correlated with delay discounting differently (78). Future studies will be needed to tease out potentially meaningful differences by ADHD subtype. In addition, due to the small number of studies identified, this meta-analysis could not address continuous relationships between delay discounting and ADHD symptoms. Clearly, there is a need for more research examining this relationship and when more studies are present, it will be important to determine if parallel findings are present or whether any systematic differences emerge (e.g., larger effect sizes in continuous studies). Finally, from a methodological standpoint, the study focused on monetary delay discounting tasks, not simple choice tasks that use points or other outcomes and substantially different procedures (e.g., 71). These tasks have revealed important findings in fractionating elemental deficits in ADHD (80) but were considered qualitatively different from the typical monetary delay discounting tasks. Thus, the findings cannot speak to that domain of the literature.
These considerations notwithstanding, the current meta-analysis nonetheless provides robust validation for steep discounting of future monetary rewards as a feature of individuals with ADHD, with minimal heterogeneity of effect size or publication bias. As such, these findings support further focus on understanding the role of delay discounting in the etiology of ADHD in general, and the dissection of the neural and genetic underpinnings of this relationship in particular.
Acknowledgments
The authors are grateful to data provided by the following individuals: Anouk Scheres, PhD; Nathalie Castellanous-Ryan, PhD; Gregor Wilbertz, PhD; and Damien Brevers, PhD. This work was partially supported by NIH grants P30 DA027827 (JM) and R01 DA032015 (JM). Dr. MacKillop is the holder of the Peter Boris Chair in Addictions Research, which partially supported his role.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Still GF. Some abnormal psychical conditions in children: excerpts from three lectures. J Atten Disord. 2006 doi: 10.1177/1087054706288114. [DOI] [PubMed] [Google Scholar]
- 2.Evenden JL. Varieties of impulsivity. Psychopharmacol. 1999;146:348–361. doi: 10.1007/pl00005481. 1999/11/07. [DOI] [PubMed] [Google Scholar]
- 3.Dick DM, Smith G, Olausson P, Mitchell SH, Leeman RF, O'Malley SS, Sher K. Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addict Biol. 2010;15:217–26. doi: 10.1111/j.1369-1600.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cyders MA, Coskunpinar A. Measurement of constructs using self-report and behavioral lab tasks: Is there overlap in nomothetic span and construct representation for impulsivity? Clin Psychol Rev. 2011;31:965–982. doi: 10.1016/j.cpr.2011.06.001. 2011/07/08. [DOI] [PubMed] [Google Scholar]
- 5.Reynolds B, Ortengren A, Richards JB, de Wit H. Dimensions of impulsive behavior: Personality and behavioral measures. Pers Individ Dif. 2006;40:305–315. [Google Scholar]
- 6.Meda SA, Stevens MC, Potenza MN, Pittman B, Gueorguieva R, Andrews MM, et al. Investigating the behavioral and self-report constructs of impulsivity domains using principal component analysis. Behav Pharmacol. 2009;20:390–399. doi: 10.1097/FBP.0b013e32833113a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mackillop J, Miller JD, Fortune E, Maples J, Lance CE, Campbell WK, Goodie AS. Multidimensional examination of impulsivity in relation to disordered gambling. Exp Clin Psychopharmacol. 2014;22:176–85. doi: 10.1037/a0035874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazur JE. An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. Quant Anal Behav. Vol. 5. Hillsdale, NJ, England: 1987. p. 5573. [Google Scholar]
- 9.Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. J Exp Anal Behav. 2001;76:235–243. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamilton KR, Mitchell MR, Wing VC, Balodis IM, Bickel WK, Fillmore M, et al. Choice impulsivity: Definitions, measurement issues, and clinical implications. Personal Disord. 2015;6:182–98. doi: 10.1037/per0000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mischel W, Ebbesen EB. Attention in delay of gratification. J Pers Soc Psychol. 1970;16:329–337. doi: 10.1037/h0032198. [DOI] [PubMed] [Google Scholar]
- 12.Rapport Hyperactivity and Frustration: The influence of Control Over and Size of Rewards in Delaying Gratification. 1986 doi: 10.1007/BF00915440. [DOI] [PubMed] [Google Scholar]
- 13.Sonuga-Barke EJ, Taylor E, Sembi S, Smith J. Hyperactivity and delay aversion--I. The effect of delay on choice. J Child Psychol Psychiatry. 1992;33:387–398. doi: 10.1111/j.1469-7610.1992.tb00874.x. [DOI] [PubMed] [Google Scholar]
- 14.Barkley RA, Edwards G, Laneri M, Fletcher K, Metevia L. Executive Functioning, Temporal Discounting, and Sense of Time in Adolescents With Attention Deficit Hyperactivity Disorder ( ADHD ) and Oppositional Defiant Disorder ( ODD ) 1. 2001;29:541–556. doi: 10.1023/a:1012233310098. [DOI] [PubMed] [Google Scholar]
- 15.Demurie E, Roeyers H, Baeyens D, Sonuga-Barke E. Temporal discounting of monetary rewards in children and adolescents with ADHD and autism spectrum disorders. Dev Sci. 2012;15:791–800. doi: 10.1111/j.1467-7687.2012.01178.x. [DOI] [PubMed] [Google Scholar]
- 16.Demurie E, Roeyers H, Baeyens D, Sonuga-Barke E. Domain-general and domain-specific aspects of temporal discounting in children with ADHD and autism spectrum disorders (ASD) a proof of concept study. Res Dev Disabil. 2013;34:1870–80. doi: 10.1016/j.ridd.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Hurst RM, Kepley HO, McCalla MK, Livermore MK. Internal consistency and discriminant validity of a delay-discounting task with an adult self-reported ADHD sample. J Atten Disord. 2011;15:412–22. doi: 10.1177/1087054710365993. [DOI] [PubMed] [Google Scholar]
- 18.Wilson VB, Mitchell SH, Musser ED, Schmitt CF, Nigg JT. Delay discounting of reward in ADHD: application in young children. J Child Psychol Psychiatry. 2011;52:256–64. doi: 10.1111/j.1469-7610.2010.02347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sagvolden T, Johansen EB, Aase H, Russell VA. A dynamic developmental theory of attention-deficitJhyperactivity disorder (ADHD) predominantly hyperactiveJimpulsive and combined subtypes. Behav Brain Sci. 2005;28:397–419. doi: 10.1017/S0140525X05000075. discussion 419-68. [DOI] [PubMed] [Google Scholar]
- 20.Sonuga-Barke EJS, Wiersema JR, van der Meere JJ, Roeyers H. Context-dependent dynamic processes in attention deficit/hyperactivity disorder: differentiating common and unique effects of state regulation deficits and delay aversion. Neuropsychol Rev. 2010;20:86–102. doi: 10.1007/s11065-009-9115-0. [DOI] [PubMed] [Google Scholar]
- 21.Tripp G, Wickens JR. Neurobiology of ADHD. Neuropharmacology. 2009;57:579–589. doi: 10.1016/j.neuropharm.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 22.Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 23.Nigg JT. Neuropsychologic theory and findings in attention-deficit/hyperactivity disorder: the state of the field and salient challenges for the coming decade. Biol Psychiatry. 2005;57:1424–35. doi: 10.1016/j.biopsych.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 24.Scheres A, de Water E, Mies GW. The neural correlates of temporal reward discounting. Wiley Interdiscip Rev Cogn Sci. 2013;4:523–45. doi: 10.1002/wcs.1246. [DOI] [PubMed] [Google Scholar]
- 25.Groenman AP, Oosterlaan J, Rommelse N, Franke B, Roeyers H, Oades RD, et al. Substance use disorders in adolescents with attention deficit hyperactivity disorder: a 4-year follow-up study. Addiction. 2013;108:1503–11. doi: 10.1111/add.12188. [DOI] [PubMed] [Google Scholar]
- 26.Van Emmerik-van Oortmerssen K, van de Glind G, van den Brink W, Smit F, Crunelle CL, Swets M, Schoevers R a. Prevalence of attention-deficit hyperactivity disorder in substance use disorder patients: a meta-analysis and meta-regression analysis. Drug Alcohol Depend. 2012;122:11–9. doi: 10.1016/j.drugalcdep.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Van de Glind G, Van Emmerik-van Oortmerssen K, Carpentier PJ, Levin FR, Koeter MWJ, Barta C, et al. The International ADHD in Substance Use Disorders Prevalence (IASP) study: background, methods and study population. Int J Methods Psychiatr Res. 2013;22:232–44. doi: 10.1002/mpr.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacol. 2001;154:243–250. doi: 10.1007/s002130000638. 2001/05/16. [DOI] [PubMed] [Google Scholar]
- 29.Baker F, Johnson MW, Bickel WK. Delay discounting in current and never-before cigarette smokers: similarities and differences across commodity, sign, and magnitude. J Abnorm Psychol. 2003;112:382–392. doi: 10.1037/0021-843x.112.3.382. 2003/08/29. [DOI] [PubMed] [Google Scholar]
- 30.Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp Clin Psychopharmacol. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. 2003/03/08. [DOI] [PubMed] [Google Scholar]
- 31.Madden GJ, Bickel WK, Jacobs EA. Discounting of delayed rewards in opioid-dependent outpatients: exponential or hyperbolic discounting functions? Exp Clin Psychopharmacol. 1999;7:284–293. doi: 10.1037//1064-1297.7.3.284. 1999/09/03. [DOI] [PubMed] [Google Scholar]
- 32.Dai Z, Harrow S-E, Song X, Rucklidge J, Grace R. Gambling, Delay, and Probability Discounting in Adults With and Without ADHD. J Atten Disord. 2013 doi: 10.1177/1087054713496461. doi: 10.1177/1087054713496461. [DOI] [PubMed] [Google Scholar]
- 33.Wilbertz G, van Elst LT, Delgado MR, Maier S, Feige B, Philipsen A, Blechert J. Orbitofrontal reward sensitivity and impulsivity in adult attention deficit hyperactivity disorder. Neuroimage. 2012;60:353–61. doi: 10.1016/j.neuroimage.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 34.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 35.Cohen J. Statistical power analysis for the behavioral sciences. Lawrence Erlbaum Associates; Mahwah: 1998. [Google Scholar]
- 36.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Begg CB, Mazumdar M. Biometrics. Vol. 50. Begg and Madhuchhanda Mazumdar; Published by: International Biometric Society Stable: 1994. Operating Characteristics of a Rank Correlation Test for Publication Bias Author ( s ) Colin B. pp. 1088–1101. URL : http://www.jstor.org/stable/2533446. [PubMed] [Google Scholar]
- 38.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duval S, Tweedie R. Trim and Fill: A Simple Funnel-Plot-Based Method. 2000:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 40.Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta-analysis Version 2. 2005 [Google Scholar]
- 41.Rosenthal R. The file drawer problem and tolerance for null results. Psychol Bull. 1979;86:638–641. [Google Scholar]
- 42.Matthews M, Road JP, Portland L, Fair DA. NIH Public Access. Curr topi. 2014 doi: 10.1007/7854. [Google Scholar]
- 43.Hart H, Radua J, Mataix-Cols D, Rubia K. Meta-analysis of fMRI studies of timing in attention-deficit hyperactivity disorder (ADHD). Neurosci Biobehav Rev. 2012;36:2248–56. doi: 10.1016/j.neubiorev.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA psychiatry. 2013;70:185–98. doi: 10.1001/jamapsychiatry.2013.277. [DOI] [PubMed] [Google Scholar]
- 45.Posner J, Park C, Wang Z. Connecting the dots: a review of resting connectivity MRI studies in attention-deficit/hyperactivity disorder. Neuropsychol Rev. 2014;24:3–15. doi: 10.1007/s11065-014-9251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Plichta MM, Scheres A. Ventral-striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: a meta-analytic review of the fMRI literature. Neurosci Biobehav Rev. 2014;38:125–34. doi: 10.1016/j.neubiorev.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scheres A, Milham MP, Knutson B, Castellanos FX. Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61:720–4. doi: 10.1016/j.biopsych.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 48.Ströhle A, Stoy M, Wrase J, Schwarzer S, Schlagenhauf F, Huss M, et al. Reward anticipation and outcomes in adult males with attention-deficit/hyperactivity disorder. Neuroimage. 2008;39:966–72. doi: 10.1016/j.neuroimage.2007.09.044. [DOI] [PubMed] [Google Scholar]
- 49.Peters J, Büchel C. The neural mechanisms of inter-temporal decision-making: understanding variability. Trends Cogn Sci. 2011;15:227–39. doi: 10.1016/j.tics.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 50.Cardinal RN. Neural systems implicated in delayed and probabilistic reinforcement. Neural Netw. 2006;19:1277–301. doi: 10.1016/j.neunet.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 51.Dalley JW, Mar AC, Economidou D, Robbins TW. Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav. 2008;90:250–60. doi: 10.1016/j.pbb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 52.Rubia K, Halari R, Christakou A, Taylor E. Impulsiveness as a timing disturbance: neurocognitive abnormalities in attention-deficit hyperactivity disorder during temporal processes and normalization with methylphenidate. Philos Trans R Soc Lond B Biol Sci. 2009;364:1919–31. doi: 10.1098/rstb.2009.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chantiluke K, Christakou A, Murphy CM, Giampietro V, Daly EM, Ecker C, et al. Disorder-specific functional abnormalities during temporal discounting in youth with Attention Deficit Hyperactivity Disorder (ADHD), Autism and comorbid ADHD and Autism. Psychiatry Res. 2014;223:113–20. doi: 10.1016/j.pscychresns.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 54.Plichta MM, Vasic N, Wolf RC, Lesch K-P, Brummer D, Jacob C, et al. Neural hyporesponsiveness and hyperresponsiveness during immediate and delayed reward processing in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2009;65:7–14. doi: 10.1016/j.biopsych.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 55.MacKillop J, Amlung M, Few L, Ray L, Sweet L, Munafó M. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology (Berl) 2011;216:305–321. doi: 10.1007/s00213-011-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci USA. 2010;107:14811–6. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amlung M, Sweet LH, Acker J, Brown CL, Mackillop J. Dissociable brain signatures of choice conflict and immediate reward preferences in alcohol use disorders. Addict Biol. 2012 doi: 10.1111/adb.12017. doi: 10.1111/adb.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boettiger CA, Mitchell JM, Tavares VC, Robertson M, Joslyn G, D'Esposito M, Fields HL. Immediate reward bias in humans: fronto-parietal networks and a role for the catechol-O-methyltransferase 158(Val/Val) genotype. J Neurosci. 2007;27:14383–14391. doi: 10.1523/JNEUROSCI.2551-07.2007. 2007/12/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Claus ED, Kiehl KA, Hutchison KE. Neural and behavioral mechanisms of impulsive choice in alcohol use disorder. Alcohol Clin Exp Res. 2011;35:1209–19. doi: 10.1111/j.1530-0277.2011.01455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren M a, Sklar P. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1313–23. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 61.Burt SA. Rethinking environmental contributions to child and adolescent psychopathology: A meta-analysis of shared environmental influences. Psychol Bull. 2009;135:608637. doi: 10.1037/a0015702. [DOI] [PubMed] [Google Scholar]
- 62.Sprich S, Biederman J, Crawford MH, Mundy E, Faraone S V. Adoptive and biological families of children and adolescents with ADHD. J Am Acad Child Adolesc Psychiatry. 2000;39:1432–7. doi: 10.1097/00004583-200011000-00018. [DOI] [PubMed] [Google Scholar]
- 63.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 64.MacKillop J, Munafó M. An intermediate phenotype approach to addiction genetics. In: MacKillop J, Munafó M, editors. Genet Influ Addict An Intermed Phenotype Approach. MIT Press; Cambridge, MA, US: 2013. [Google Scholar]
- 65.Richards JB, Lloyd DR, Kuehlewind B, Militello L, Paredez M, Solberg Woods L, Palmer AA. Strong genetic influences on measures of behavioral-regulation among inbred rat strains. Genes Brain Behav. 2013;12:490–502. doi: 10.1111/gbb.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilhelm CJ, Mitchell SH. Strain differences in delay discounting using inbred rats. Genes Brain Behav. 2009;8:426–34. doi: 10.1111/j.1601-183X.2009.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Anokhin AP, Golosheykin S, Grant JD, Heath AC. Heritability of delay discounting in adolescence: a longitudinal twin study. Behav Genet. 2011;41:175–83. doi: 10.1007/s10519-010-9384-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anokhin AP, Grant JD, Mulligan RC, Heath AC. The Genetics of Impulsivity: Evidence for the Heritability of Delay Discounting. Biol Psychiatry. 2014:1–8. doi: 10.1016/j.biopsych.2014.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eisenberg DT, Mackillop J, Modi M, Beauchemin J, Dang D, Lisman SA, et al. Examining impulsivity as an endophenotype using a behavioral approach: a DRD2 TaqI A and DRD4 48-bp VNTR association study. Behav Brain Funct. 2007;3:2. doi: 10.1186/1744-9081-3-2. 2007/01/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smith CT, Boettiger CA. Age modulates the effect of COMT genotype on delay discounting behavior. Psychopharmacology (Berl) 2012;222:609–617. doi: 10.1007/s00213-012-2653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gray JC, MacKillop J. Genetic basis of delay discounting in frequent gamblers: examination of a priori candidates and exploration of a panel of dopamine-related loci. Brain Behav. 2014;4:812–21. doi: 10.1002/brb3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.MacKillop J, Gray JC, Bidwell LC, Bickel WK, Sheffer CE, McGeary JE. Genetic influences on delay discounting in smokers: examination of a priori candidates and exploration of dopamine-related haplotypes. Psychopharmacology (Berl) 2015;232:3731–9. doi: 10.1007/s00213-015-4029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Paloyelis Y, Asherson P, Mehta M a, Faraone SV, Kuntsi J. DAT1 and COMT effects on delay discounting and trait impulsivity in male adolescents with attention deficitJhyperactivity disorder and healthy controls. Neuropsychopharmacology. 2010;35:2414–26. doi: 10.1038/npp.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jentsch JD, Ashenhurst JR, Cervantes MC, Groman SM, James AS, Pennington ZT. Dissecting impulsivity and its relationships to drug addictions. Ann N YAcad Sci. 2014;1327:1–26. doi: 10.1111/nyas.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sonuga-Barke EJS, Kumsta R, Schlotz W, Lasky-Su J, Marco R, Miranda A, et al. A functional variant of the serotonin transporter gene (SLC6A4) moderates impulsive choice in attention-deficit/hyperactivity disorder boys and siblings. Biol Psychiatry. 2011;70:230–6. doi: 10.1016/j.biopsych.2011.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Havranek MM, Hulka LM, Tasiudi E, Eisenegger C, Vonmoos M, Preller KH, et al. α2A - Adrenergic receptor polymorphisms and mRNA expression levels are associated with delay discounting in cocaine users. Addict Biol. 2015 doi: 10.1111/adb.12324. doi: 10.1111/adb.12324. [DOI] [PubMed] [Google Scholar]
- 77.Scheres A, Tontsch C, Thoeny AL, Kaczkurkin A. Temporal reward discounting in attention-deficit/hyperactivity disorder: the contribution of symptom domains, reward magnitude, and session length. Biol Psychiatry. 2010;67:641–8. doi: 10.1016/j.biopsych.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 78.Scheres a, Lee a, Sumiya M. Temporal reward discounting and ADHD: task and symptom specific effects. J Neural Transm. 2008;115:221–6. doi: 10.1007/s00702-007-0813-6. [DOI] [PubMed] [Google Scholar]
- 79.Marco R, Miranda A, Schlotz W, Melia A, Mulligan A, Muller U, et al. Delay and reward choice in ADHD: an experimental test of the role of delay aversion. Neuropsychology. 2009;23:36780. doi: 10.1037/a0014914. [DOI] [PubMed] [Google Scholar]
- 80.Willcutt E, Sonuga-Barke EJ, Nigg JT, Sergeant JA. Recent developments in neuropsychological models of childhood psychiatric disorders. In: Banaschewski T, Rhode L, editors. Biol Child Psychiatry Recent Trends Dev. Karger; Basel, Switzerland: 2008. pp. 195–226. [Google Scholar]
- 81.Mostert JC, Onnink AMH, Klein M, Dammers J, Harneit A, Schulten T, et al. Cognitive heterogeneity in adult attention de fi cit / hyperactivity disorder : A systematic analysis of neuropsychological measurements. Eur Neuropsychopharmacol. 2015:1–13. doi: 10.1016/j.euroneuro.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Onnink AMH, Zwiers MP, Hoogman M, Mostert JC, Dammers J, Kan CC, et al. Progress in Neuro-Psychopharmacology & Biological Psychiatry Deviant white matter structure in adults with attention-de fi cit / hyperactivity disorder points to aberrant myelination and affects neuropsychological performance. Prog Neuropsychopharmacol Biol Psychiatry. 2015;63:14–22. doi: 10.1016/j.pnpbp.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu X, Sonuga-Barke E, Liu X. Preference for Smaller Sooner Over Larger Later Rewards in ADHD: Contribution of Delay Duration and Paradigm Type. J Atten Disord. 2015 doi: 10.1177/1087054715570390. doi: 10.1177/1087054715570390. [DOI] [PubMed] [Google Scholar]
- 84.Costa Dias TG, Iyer SP, Carpenter SD, Cary RP, Wilson VB, Mitchel SH, et al. Characterizing heterogeneity in children with and without ADHD based on reward system connectivity. Dev Cogn Neurosci. 2015;11:155–74. doi: 10.1016/j.dcn.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Castellanos-ryan N, Ph D, Struve M, Whelan R, Banaschewski T, Bokde ALW, et al. Neural and Cognitive Correlates of the Common and Speci fi c Variance Across Externalizing Problems in Young Adolescence. 2014:1–10. doi: 10.1176/appi.ajp.2014.13111499. [DOI] [PubMed] [Google Scholar]
- 86.Fassbender C, Houde S, Silver-Balbus S, Ballard K, Kim B, Rutledge KJ, et al. The decimal effect: behavioral and neural bases for a novel influence on intertemporal choice in healthy individuals and in ADHD. J Cogn Neurosci. 2014;26:2455–68. doi: 10.1162/jocn_a_00642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Costa Dias TG, Wilson VB, Bathula DR, Iyer SP, Mills KL, Thurlow BL, et al. Reward circuit connectivity relates to delay discounting in children with attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol. 2013;23:33–45. doi: 10.1016/j.euroneuro.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Crunelle CL, Veltman DJ, van Emmerik-van Oortmerssen K, Booij J, van den Brink W. Impulsivity in adult ADHD patients with and without cocaine dependence. Drug Alcohol Depend. 2013;129:18–24. doi: 10.1016/j.drugalcdep.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 89.Scheidel G. 2013. ATTENTION-DEFICIT / HYPERACTIVITY DISORDER ( ADHD ) by Gretchen Scheidel A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in Psychology Approved : Gretchen Peacock, Ph. D . Major Professor Amy Od.
- 90.Scheres A, Dijkstra M, Ainslie E, Balkan J, Reynolds B, Sonuga-Barke E, Castellanos FX. Temporal and probabilistic discounting of rewards in children and adolescents: effects of age and ADHD symptoms. Neuropsychologia. 2006;44:2092–103. doi: 10.1016/j.neuropsychologia.2005.10.012. [DOI] [PubMed] [Google Scholar]