Abstract
AGE severity is linked to etiology, and Rotavirus (RV) accounts for most of severe cases. In 2009 the World Health Organization recommended RV vaccination for all children. Worldwide a number of Countries implemented RV vaccination in their pediatric immunisation programmes, but only a limited number in Europe. This study was designed to estimate the proportion of RVGE among children aged <6 y who were diagnosed with AGE and admitted to hospitals in Italy during the years 2005–2012. A total of 334,982 hospital discharge forms were collected, being 79,344 hospitalizations associated with RV. The average hospitalization rate (HR) was 146/100,000 children for RVGE in primary diagnosis (PD) and 150/100,000 children for RVGE in secondary diagnosis (SD). Since 2008 the RVGE hospitalization figures and rates (HRs) in SD exceed those in PD. The majority of RVGE hospitalizations (33.67%) were reported among children aged ≤ 2 years. Despite some limitations due to the hospital discharge database (HDD) synthetic contents and low potential for clinical interpretation, the analysis of national HDD, including PD and SD, documents that RV still represents a consistent cause of pediatric hospitalizations in Italy.
Keywords: children, diagnosis, frequency, gastroenteritis, hospitalization rates, rotavirus, vaccine
Abbreviations
- AGE
acute gastroenteritis
- HDD
hospital discharge database
- HDR
hospital discharge record
- PD
primary diagnosis
- RV
rotavirus
- HR
hospitalization rate
- RVGE
rotavirus gastroenteritis
- SD
secondary diagnosis
- VGE
viral gastroenteritis
Introduction
Acute gastroenteritis (AGE), characterized by the onset of diarrhea with or without vomiting, still represents a major cause of morbidity even in industrialized Countries, being mortality confined in mostly resource-constrained nations. Although generally considered a mild and self-limiting disease, AGE is one of the most common causes of hospitalization and is associated with a substantial disease burden.1-4
Within the European Union, rotavirus gastroenteritis (RVGE) places a high demand on healthcare systems.5-8 Surveillance studies showed that RV accounts for up to 2 thirds of admissions to hospital and emergency room visits and one third of primary care consultations for AGE among children under 5 years, being the greatest burden of disease consistently observed in children aged under 2. RVGE is estimated to occur at a rate of 1 symptomatic infection in every 7 children each year, accounting for 231 deaths, more than 87,000 hospitalizations and almost 700,000 outpatient visits.5
The REVEAL study, carried out in Belgium, France, Germany, Italy, Spain, Sweden, and the UK, reported that RVGE in children under 5 y of age was responsible for between 53.0% and 68.9% of cases presenting to hospitals, 35.4% and 63.3% for those seen in emergency departments, and 7.7% and 41.3% of cases seeking primary care physicians.9
In 2006, 2 new live, oral, attenuated RV vaccines were licensed for infants less than 6 months of age RV vaccination was first recommended to US children in 2006. Subsequently, in 2009 the World Health Organization Strategic Advisory Group of Experts (SAGE) recommended RV vaccination for all children.10 Worldwide a number of countries have adopted this recommendation and implemented RV vaccines in their pediatric immunisation programmes, but only in a limited number of Countries in Europe.
Some countries, such as Austria and Luxembourg (2006), Belgium (2007), Finland (2009), United Kingdom and Germany (2013), Norway and Estonia (2014), Latvia (2015) introduced universal anti-RV vaccination until April 1st 2015, while in other countries, such as, Sweden, Denmark, and Romania the formal assessment for universal vaccination is under consideration.11-17
In Italy, where RVGE is an important cause of pediatric hospitalization, associated with high health care costs, as pointed out by several studies, among them, that of Marocco et al. is the only nationwide but limited only to the primary diagnosis, the National Health care System (NHS) is decentralized i.e Regions are expected to implement – free of charge - all the vaccinations included in the National Immunization Plan (NIP);18-21 further, they are allowed to increase the vaccination offer providing that the additional budget is funded at regional level, either centrally or in co-payment with the citizens. RV in not included in the current National Immunization Plan (NIP) and most of the Regions introduced it in co-payment, sometimes reducing the co-payment to a small amount.22-29 Sicily is the only Region were RV universal mass vaccination (UMV) free of charge was implemented in 2012.30
In order to provide an epidemiological picture that can be helpful in assessing the need to adopt anti-RV universal vaccination also in Italy, this study was designed to estimate the proportion of RVGE among children younger than 6 y of age who were diagnosed with AGE and admitted to hospital in Italy during the years 2005–2012.
Results
In the study time frame, 334,982 AGE hospital discharge records (HDRs) were collected in Italy (average annual number: 41,873 hospitalizations), in children aged <6 years, being AGE the primary diagnosis (PD) in 50.30% (168,509 cases) of these.
Table 1 shows the number of hospital admissions only coded as AGE of any etiology in PD: 63.09% of AGE (106,326 cases) presented the code of gastroenteritis of non-specified origin, and 33.75% of cases were viral gastroenteritis (VGE), that were the leading cause of admissions for gastroenteritis of specified origin; RVGE were 39,024 (23.16%), and represented 68.61% of all VGE. Considering the total admissions for AGE (in PD), over the 8-years study period, a significant and consistent reduction of the total number of admissions for AGE, from 27,555 in 2005 to 14,988 in 2012, (a decrease of 45.60% - AGE trend-test: β coefficient=-1,939; p < 0.001) was observed. In parallel, a minor, but statistically significant, decrease for RVGE of 29.72% (from 5,824 in 2005 to 4,093 in 2012; RVGE trend-test: β coefficient = −253; p = 0 .014) was found out.
Table 1.
Number (percentage) of all hospitalizations for gastroenteritis in PD among children <6 y of age in the 2005–2012 period
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2005–2012 | |
|---|---|---|---|---|---|---|---|---|---|
| UNSPECIFIED ETIOLOGY | |||||||||
| Infectious: ICD9CM 009–009.3 | 12,569 (45.61%) | 12,500 (47.57%) | 10,619 (44.96%) | 9,191 (42.55%) | 7,743 (43.47%) | 8,794 (43.46%) | 7,145 (46.92%) | 7,013 (46.79%) | 75,574 (44.85%) |
| Non-infectious: ICD9CM 558.9 | 5,244 (19.03%) | 5,352 (19.51%) | 4,684 (19.83%) | 4,223 (19.55%) | 3,428 (19.25%) | 3,604 (17.81%) | 2,289 (15.03%) | 1,928 (12.86%) | 30,752 (18.25%) |
| SPECIFIED ETIOLOGY | |||||||||
| Viral: ICD9CM 008.62–008.8 (without RV) | 3,055 (11.09%) | 3,275 (11.94%) | 2,524 (10.69%) | 2,235 (10.35%) | 1,930 (10.84%) | 1,987 (9.82%) | 1,455 (9.55%) | 1,392 (9.29%) | 17,853 (10.59%) |
| Rotavirus: ICD9CM 008.61 | 5,824 (21.14%) | 5,439 (19.83%) | 5,144 (21.78%) | 5,324 (24.65%) | 4,146 (23.28%) | 5,301 (26.2%) | 3,753 (24.65%) | 4,093 (27.31%) | 39,024 (23.16%) |
| Bacterial: ICD9CM 001–005, 008–008.5 | 661 (2.4%) | 634 (2.31%) | 475 (2.1%) | 460 (2.13%) | 383 (2.15%) | 406 (2.01%) | 481 (3.16%) | 447 (2.98%) | 3,947 (2.34%) |
| Parasitic: ICD9CM 006–007 | 202 (0.73%) | 228 (0.83%) | 174 (0.74%) | 163 (0.75%) | 181 (1.02%) | 142 (0.70%) | 154 (1.01%) | 115 (0.77%) | 1,359 (0.81%) |
| TOTAL | 27,555 | 27,428 | 23,620 | 21,596 | 17,811 | 20,234 | 15,227 | 14,988 | 168,509 |
AGE trend-test: β coefficient = −1,939; p < 0.001; Number of RVPD hospitalizations, trend-test: β-coefficient = −253; p < 0.014.
Figure 1A shows the trend of hospitalizations for RVGE in children under 6 y of age over the study period, but including secondary diagnosis (SD). SD inclusion leads to a total of 79,344 hospitalizations associated with RV, of which 40,320 (50.81%) in SD.
Figure 1.
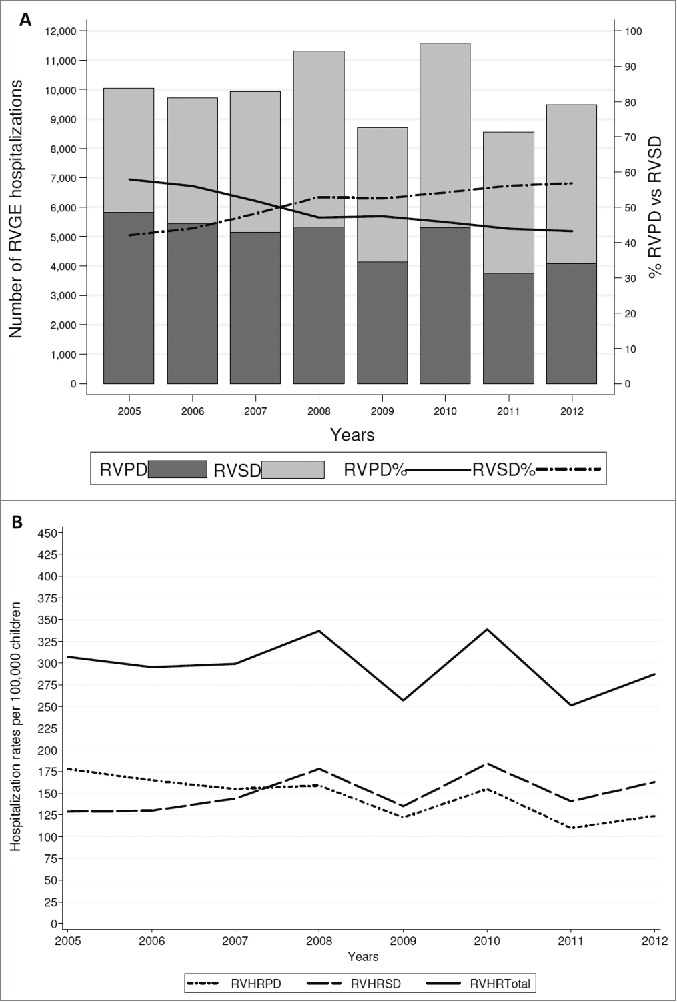
(A) Number of RVGE hospitalizations (code ICD9CM 008.61) in primary (PD) and secondary diagnosis (SD) among children <6 y of age in 2005–2012. Number of RVPD hospitalizations trend-test: β-coefficient = −253; p<0.014; number of RVSD hospitalizations trend-test: β-coefficient =163; p = 0 .184; total number of RV hospitalizations trend-test: β-coefficient =−89; p = 0 .630. RVPD: RVGE hospitalizations in primary diagnosis; RVSD: RVGE hospitalizations in secondary diagnosis; % RVPD: percentage of RVGE hospitalizations in primary diagnosis; % RVSD: percentage of RVGE hopistalizations in secondary diagnosis. (B) Hospitalization rates per 100,000 of RVGE PD and SD among children <6 y of age in 2005-2012. Hospitalization rates per 100,000 of RVGE PD trend-test: β-coefficient=-8.21; p=0.010; hospitalization rates per 100,000 of RVGE SD trend-test: β-coefficient=4 .40; p=0.209. Total HRRV trend-test: β coefficient = −3.80; p = 0.487. RVHRPD: hospitalization rates for RVGE gastroenteritis in primary diagnosis; RVHRSD: hospitalization rates for RVGE gastroenteritis in secondary diagnosis; RVHRTotal: hospitalization rates for RVGE gastroenteritis in any diagnosis (PD and SD).
Overall, the percentage of hospital admissions for RVGE in PD has gradually and significantly decreased from 57.93% in 2005 to 43.18% in 2012. On the contrary, the incidence of RVGE SD admissions has increased from 42.07% in 2005 to 56.82% in 2012.
The annual incidence rates of RV hospitalizations among children <6 y of age are shown in Figure 1 B. The average hospitalization rate (HR) was 296/100,000 children: 146/100,000 children for RVGE in PD and 150/100,000 children for RVGE in SD. Since 2008 the HRs for RVGE in SD exceeds those for RVGE in PD, with the highest peak in 2010 (total RV HR: 339/100,000 children). The decrease of the HRs for RVGE in PD is 8.21 per year (p = 0 .01), while considering the trend of HRs for RVGE in any diagnosis (PD and SD) it is not statistically significant (p = 0 .487).
Most RVGE hospitalizations (80.79%) occurred in children younger than 3 y (Table 2), mainly infants ≤2 years (12–23 months) had the highest number of cases (33.67%), followed by children aged up to 3 y (24–35 months), with 18.45% of annual hospitalizations, then children aged 0–11 months (28.67%).
Table 2.
Temporal distribution of rotavirus infections in Italy. Distribution (absolute and relative) by age groups of the hospitalizations for rotavirus gastroenteritis (code ICD-9 CM 008.61) in PD in children aged <6 y over the 2005–2012 period
| Age groups |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <1 y of age | 1 year | 2 years | 3 years | 4 years | 5 years | ||||||||
| 0–11 months |
12–23 months |
24–35 months |
36–47 months |
48–59 months |
60–71 months |
||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | Total | |
| 2005 | 1,599 | 27.46 | 1,957 | 33.60 | 1,057 | 18.15 | 622 | 10.68 | 367 | 6.30 | 222 | 3.81 | 5,824 |
| 2006 | 1,499 | 27.56 | 1,898 | 34.90 | 994 | 18.28 | 559 | 10.28 | 294 | 5.41 | 195 | 3.59 | 5,439 |
| 2007 | 1,498 | 29.12 | 1,694 | 32.93 | 939 | 18.25 | 524 | 10.19 | 291 | 5.66 | 198 | 3.85 | 5,144 |
| 2008 | 1,514 | 28.44 | 1,733 | 32.55 | 1,011 | 18.99 | 562 | 10.56 | 302 | 5.67 | 202 | 3.79 | 5,324 |
| 2009 | 1,246 | 30.05 | 1,375 | 33.16 | 812 | 19.59 | 393 | 9.48 | 203 | 4.90 | 117 | 2.82 | 4,146 |
| 2010 | 1,557 | 29.37 | 1,845 | 34.80 | 933 | 17.60 | 487 | 9.19 | 290 | 5.47 | 189 | 3.57 | 5,301 |
| 2011 | 1,119 | 29.82 | 1,252 | 33.36 | 670 | 17.85 | 371 | 9.89 | 207 | 5.52 | 134 | 3.57 | 3,753 |
| 2012 | 1,156 | 28.24 | 1,386 | 33.86 | 784 | 19.15 | 385 | 9.41 | 212 | 5.18 | 170 | 4.15 | 4,093 |
| 2005–2012 | 11,188 | 28.67 | 13,140 | 33.67 | 7,200 | 18.45 | 3,903 | 10.00 | 2,166 | 5.55 | 1,427 | 3.66 | 39,024 |
N/% = number/percentage of all hospitalizations in the given year for the specified age group.
RVGE hospitalizations seasonal peak was during December - March every year, with maximum values of 2,850 cases in PD during 2006 and 3,139 cases in SD over 2008 y (data by Italian HDD database; data not shown).
In RVGE SD cases, 26,675 (66.16%) main diagnoses were related to symptoms or conditions attributable to AGE (Fig. 2): the most frequent were dehydration (49.77%) syncopations and convulsions (6.59%) and acidosis and electrolyte fluid disorders (5.35%). The main (52%) DRG code for RVGE SD was 298 (symptoms concerning nutrition and metabolism <18 years).
Figure 2.
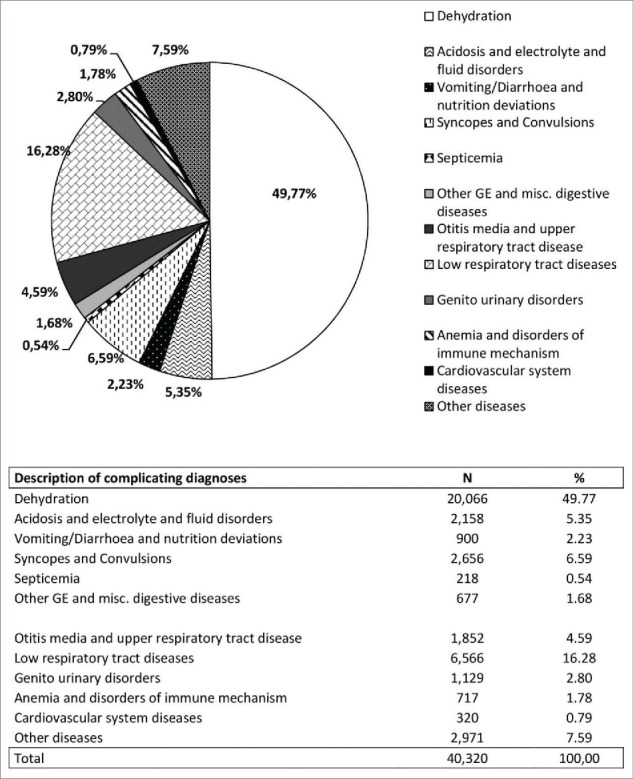
Number (N) and frequency (%) of other diseases in PD in RVGE SD cases.
Total hospital charges for the admissions for RV in the overall period were approximately € 112 million. They, however, decreased from € 7,238,739 in 2005 to € 3,158,220 in 2012, considering only PD.
Discussion
Hospital admission rates for RV AGE in children aged <6 y still remain a relevant topic in Italy. The hospital discharge data (HDD) analysis confirmed that RVGE still represents the greatest proportion of hospitalized VGE, in agreement with previous results either in Italy and in other parts of Europe or USA.7,9,18,21-32
As in the study time frame the RV vaccine coverage (estimated less than 10%) did not reach yet significant levels to affect the overall epidemiology of the disease, the figures reported here can be considered as a pre-vaccination picture.28 A progressive reduction of all hospitalizations for acute diarrhea in children, in the study period, which is more evident for AGE of unspecified etiology and of bacterial and parasitic origin was observed.
Indeed, we provided evidence that there was a switch in the position of RV AGE diagnosis from PD to SD. No explicit reasons justifying such a switch could be found out. Vitale et al, using the Markov model and considering a cohort of 555,791 births in 2011 in Italy, in the absence of vaccination, estimated an average of 14,000 hospitalizations per year by using the RVGE hospital rates collected within the REVEAL study;19 in our study, an average of 9,918 hospitalizations per year for RV in any diagnosis was found out. Whereas an underestimation of hospitalizations through the HDD by around 40–50% was reported, even if calculated from a different setting, our findings are consistent with those reported. RV HRs variations were in line with the switch of PD with SD. Our findings support the need of including both PD and SD, which also includes nosocomial infection forms and the incidence of which was estimated in Italy by 5.3% in children under 30 months, in RV hospitalizations analysis.18-33
The most frequent PD in cases of SD RVGE were: dehydration, acidosis and vomiting (54%), infections of the upper and lower respiratory tract (respectively 11% and 13%), seizures or other neurological symptoms (7%) and urinary tract infection (6%), in agreement with previous reports.34 Although further studies would be needed to confirm the hypothesis, a consistent part of admissions such as dehydration, gastrointestinal disorders, febrile seizures and electrolyte abnormalities reported in main diagnosis could be associated with the RV etiology; this would also partially justify the under estimation of RVGE hospitalization figures. It can be assumed that variations in coding RV hospitalizations in the studied years might be related to the introduction of strategies of containment of the sanitary expense, being some ICD9-CM codes leading to a more specialized management and reimbursement.20
Even though the study was not intended as an economic analysis of RVGE hospitalizations, some calculations of RVGE costs were derived from the codes available in the database. However, figures obtained were just a rough indicator of real healthcare costs when compared to more accurate calculations.35
Limitations of the Study. The data shown are not linked to RV uptakes that are not searched. Indeed, regional data are not detailed even if the regional differences in the payment of RVGE hopitalizations may influence the coverage of RV vaccines and the AGE hospitalizations.
In conclusion, RVGE hospitalization figures in Italy are still relevant and generate significant costs to the NHS. As observed in other Countries, the introduction of RV UMV in Italy might consistently reduce morbidity and associated medical costs.
Materials and Methods
This is a retrospective population-based study, conducted among all pediatric patients aged <6 y hospitalized for AGE in Italy, between January 1st 2005 and December 31st 2012.
The data source was the Italian HDD obtained from Ministry of Health (Processing National HDD, Ministry of Health, General Directorate for Health Planning, VI Office). This database contains administrative and health data regarding hospital admissions, that all public and privately-owned hospitals in Italy are legally required to report. For each admission, a PD is reported; this represents the clinical condition which took up the greatest amount of resources and therefore involved the greatest cost for the hospital. Up to 5 additional SD may be listed.
The clinical information is coded by the international ICD9-CM system (International Classification of Diseases, 9th revision, Clinical Modification), currently used in Italy. Gastroenteritis codes include the following:
- unspecified etiology gastroenteritis of presumed infectious etiology (009–009.3) and presumed noninfectious etiology (558.9);
- gastroenteritis with specified etiology: VGE (008.61–69), bacterial gastroenteritis (001–005 and 008–008.5) and parasitic gastroenteritis (006–007). RVGE discharges were identified by the code 008.61.
In this study we included all admissions with at least one gastroenteritis related main or secondary discharge diagnosis. For each of these hospitalizations, we obtained the following data: age, month of admission, costs related to admissions.
Each hospitalization cost, on average, has been estimated, according to the theoretical remuneration of admission reported in each HDR provided by Diagnosis Related Group (DRG) system. Even if a specific DRG rates for RVGE is not available, the 3 possible DRG codes to which the disease can be referred to (184: esophagitis, gastroenteritis etc., € 785; 298: symptoms concerning nutrition and metabolism <18 years, € 1,190; 422: diseases of viral origin and fever of unknown origin, € 1,660) were considered.36,37
Data provided by the Ministry of the Health did not contain any patient identifiers and was therefore completely anonymous. Hence notification of the study to Ethics Committees was not applicable, nor was informed consent of patients required.
The frequency of hospitalization with a PD of gastroenteritis was calculated as the ratio between patients with any AGE code in PD over the total aggregate observed in the database. As the RVGEs are the only vaccine-preventable, for these frequencies and hospitalization rates also in SD were analyzed, to obtain their overall epidemiological picture.
HRs were calculated for every year as the ratio between the number of hospital discharges and the resident populations aged <6 y per 100,000. Population data for 2005–2012 period was obtained from Italian Institute of Statistics (ISTAT), which registers the National population, by age group, as of the January 1st for each year.38
The statistical significance of temporal trend of HRs was determined using the analysis of the slope of the regression line between HRs and years of observation. p < 0.05 was the criterion for statistical significance. Data analysis was performed using STATA/IC 12.1.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors are grateful to Professor Flavia Carle, General Directorate for Health Planning, VI Office, Ministry of Health, for providing data useful to carry out the analysis.
Funding
This research was conducted independently and publishing costs only were supported by an unrestricted grant from GlaxoSmithKline S.p.A.
References
- 1.Pieścik-Lech M, Shamir R, Guarino A, Szajewska H. Review article: the management of acute gastroenteritis in children. Aliment Pharmacol Ther 2013; 37:289-303; PMID:23190209; http://dx.doi.org/ 10.1111/apt.12163 [DOI] [PubMed] [Google Scholar]
- 2.Guarino A, Dupont C, Gorelov AV, Gottrand F, Lee JK, Lin Z, Lo Vecchio A, Nguyen TD, Salazar-Lindo E. The management of acute diarrhea in children in developed and developing areas: from evidence base to clinical practice. Expert Opin Pharmacother 2012; 13:17-26; PMID:22106840; http://dx.doi.org/ 10.1517/14656566.2011.634800 [DOI] [PubMed] [Google Scholar]
- 3.Parashar UD, Gibson CJ, Breese JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerg Infect Dis 2006; 12: 304-6; PMID:16494759; http://dx.doi.org/ 10.3201/eid1202.050006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parashar UD, Burton A, Lanata C, Boschi-Pinto C, Shibuya K, Steele D, Birmingham M, Glass RI. Global mortality associated with rotavirus disease among children in 2004. J Infect Dis 2009; 200:S9-15; PMID:19817620; http://dx.doi.org/ 10.1086/605025 [DOI] [PubMed] [Google Scholar]
- 5.Soriano-Gabarró M, Mrukowicz J, Vesikari T, Verstraeten T. Burden of rotavirus disease in European Union countries. Pediatr Infect Dis J 2006; 25:S7-11; http://dx.doi.org/ 10.1097/01.inf.0000197622.98559.01 [DOI] [PubMed] [Google Scholar]
- 6.Van Damme P, Giaquinto C, Huet F, Gothefors L, Maxwell M, Van der Wielen M. Multicenter prospective study of the burden of rotavirus acute gastroenteritis in Europe, 2004-2005: the REVEAL study. J Infect Dis 2007; 195:S4-16; PMID:17387650; http://dx.doi.org/ 10.1086/516714 [DOI] [PubMed] [Google Scholar]
- 7.Forster J, Guarino A, Parez N, Moraga F, Román E, Mory O, Tozzi AE, L∫pez de Aguileta A, Wahn U, Graham C et al.. Hospital-based surveillance to estimate the burden of rotavirus gastroenteritis among European children younger than 5 years of age. Pediatrics 2009; 123:e393-400; PMID:19254975; http://dx.doi.org/ 10.1542/peds.2008-2088 [DOI] [PubMed] [Google Scholar]
- 8.Diez-Domingo J, Baldo JM, Patrzalek M, Pazdiora P, Forster J, Cantarutti L, Pirçon JY, Soriano-Gabarró M, Meyer N; SPRIK Rotavirus Study Group . Primary care-based surveillance to estimate the burden of rotavirus gastroenteritis among children aged less than 5 years in six European countries. Eur J Pediatr 2011; 170:213-22; PMID:20842379; http://dx.doi.org/ 10.1007/s00431-010-1289-1 [DOI] [PubMed] [Google Scholar]
- 9.Giaquinto C, Van Damme P, Huet F, Gothefors L, Maxwell M, Todd P, da Dalt L; REVEAL Study Group. Clinical consequences of rotavirus acute gastroenteritis in Europe, 2004-2005 : the REVEAL study. J Infect Dis 2007; 195:S26-35; PMID:17387649; http://dx.doi.org/ 10.1086/516717 [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization . Rotavirus vaccines. Rotavirus vaccines: an update. Wkly Epidemiol Rec 2009; 84:533-40; PMID:20034143 [PubMed] [Google Scholar]
- 11.Braeckman T, Van Herck K, Raes M, Vergison A, Sabbe M, Van Damme P. Rotavirus vaccines in Belgium policy and impact. Pediatr Infect Dis J 2011; 30:S21-4; PMID:21183836; http://dx.doi.org/ 10.1097/INF.0b013e3181fefc51 [DOI] [PubMed] [Google Scholar]
- 12.Parez N, Giaquinto C, Du Roure C, Martinon-Torres F, Spoulou V, Van Damme P, Vesikari T. Rotavirus vaccination in Europe: drivers and barriers. Lancet Infect Dis 2014; 14:416-25; PMID:24758998; http://dx.doi.org/ 10.1016/S1473-3099(14)70035-0 [DOI] [PubMed] [Google Scholar]
- 13.Paulke-Korinek M, Kollaritsch H, Aberle SW, Zwazl I, Schmidle-Loss B, Vécsei A, Kundi M. Sustained low hospitalization rates after four years of rotavirus mass vaccination in Austria. Vaccine 2013; 31:2686-91; PMID:23597718; http://dx.doi.org/ 10.1016/j.vaccine.2013.04.001 [DOI] [PubMed] [Google Scholar]
- 14.Nohynek H, Salo H, Renko M, Leino T. Finland introduces rotavirus vaccine into the national vaccination programme in September 2009. Euro Surveill 2009; 14:pii=19322 [PubMed] [Google Scholar]
- 15.NHS England Important changes to the national immunisation programme in 2013-14. URL:https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/193055/130429_Rotavirus_tripartite_letter_FINAL.pdf [Google Scholar]
- 16.STIKO Recommendation for routine rotavirus vaccination of infants in Germany. 2013; 56:955-6 [DOI] [PubMed] [Google Scholar]
- 17.URL: http://sites.path.org/rotavirusvaccine/country-introduction-maps-and-spreadsheet/ [Google Scholar]
- 18.Marchetti F, Assael B, Gabutti G, Guarino A, Lopalco PL, Marocco A, Ruggeri F, Titone L, Tozzi A, Vitali Rosati G, Zotti C, Franco E. Monitoring the rate of hospitalization before rotavirus immunization in Italy utilizing ICD9-CM regional databases. Hum Vaccin 2009; 5:172-6; PMID:18802404; http://dx.doi.org/ 10.4161/hv.5.3.6764 [DOI] [PubMed] [Google Scholar]
- 19.Vitale F, Barbieri M, Dirodi B, Vitali Rosati G, Franco E. A full economic evaluation of extensive vaccination against rotavirus with RIX4414 vaccine at National and Regional level in Italy. Ann Ig 2013; 25:43-56; PMID:23435779 [DOI] [PubMed] [Google Scholar]
- 20.Saia M, Giliberti A, Callegaro G, Baldovin T, Busana MC, Pietrobon F, Bertoncello C, Baldo V. Hospitalisation for rotavirus gastroenteritis in the paediatric population in the Veneto Region, Italy. BMC Public Health 2010; 10:636; PMID:20969755; http://dx.doi.org/ 10.1186/1471-2458-10-636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marocco A, Assael B, Gabutti G, Guarino A, Lopalco PL, Marchetti F, Ruggeri FM, Titone L, Tozzi AE, Vitali Rosati G et al.. Hospitalisation associated with Rotavirus gastroenteritis in Italy, 2001-2003, evaluated by means of ICD9-CM diagnostic codes. Ig Sanita Pubbl. 2006; 62:215-44; PMID:17206191 [PubMed] [Google Scholar]
- 22.Alfonsi V, D'Ancona F, Giambi C, Nacca G, Rota MC. Current immunization policies for pneumococcal, meningococcal C, varicella and rotavirus vaccinations in Italy. Health Policy 2011; 103:176-83; PMID:22030308; http://dx.doi.org/ 10.1016/j.healthpol.2011.10.002 [DOI] [PubMed] [Google Scholar]
- 23.Regione Liguria. Deliberazione della giunta regionale 19 luglio 2013, n. 891. Piano Regionale Prevenzione Vaccinale. Bollettino Ufficiale della Regione Liguria n. 33 del 14.08.2013. Italian [Google Scholar]
- 24.Regione Calabria Decreto 29 luglio 2010, n. 11096 del. Approvazione calendario vaccinale regionale per l'età evolutiva (0-18 anni). Bollettino Ufficiale della Regione Calabria n. 34 del 27.08.2010. Italian. [Google Scholar]
- 25.Regione Veneto Deliberazione della giunta regionale 15 novembre 2011, n. 1873. Tariffario Unico Regionale relativo alle prestazioni rese dalle strutture del Dipartimento di Prevenzione delle Aziende Ulss del Veneto per l'esecuzione delle vaccinazioni: modifica ed integrazione alla D.G.R. n. 1664 del 9/06/2009. Bollettino Ufficiale della Regione Veneto n. 91 del 6.12.2011. Italian. [Google Scholar]
- 26.Regione Piemonte Deliberazione della giunta regionale 29 luglio 2013, n. 17. Approvazione del Piano Piemontese di Prevenzione Vaccinale (PPPV) 2013-2015. Bollettino Ufficiale della Regione Piemonte n. 36 del 05.09.2013. Italian. [Google Scholar]
- 27.Regione Autonoma Friuli Venezia Giulia Decreto del Presidente della Regione 21 agosto 2012, n. 0163. Estensione dell'offerta vaccinale nella Regione Friuli Venezia Giulia. Bollettino Ufficiale della Regione Autonoma Friuli Venezia Giulia n. 36 del 05.09.2012. Italian. [Google Scholar]
- 28.Prato R. La vaccinazione anti-rotavirus in Europa ed in Italia - Le esperienze italiane. In: proceedings Vaccinazioni in Italia: obiettivi raggiunti e strategie per il futuro. Genova, 12-13 giugno 2014. Available from: http://www.etagamma.it/wp-content/uploads/Prato.pdf. Italian. [Google Scholar]
- 29.Regione Puglia Deliberazione della giunta regionale 20 maggio 2014, n. 958. Commissione Regionale Vaccini. Modifica Calendario Regionale per la vita 2012 - DGR 241/2013. Approvazione nuovo Calendario Vaccinale per la vita 2014. Bollettino Ufficiale della Regione Puglia n. 74 del 11.06.2014. Italian. [Google Scholar]
- 30.Regione Sicilia Decreto 7 maggio 2012. Calendario vaccinale per la vita. Modifica ed integrazione del calendario vaccinale regionale. Gazzetta Ufficiale della Regione Sicilia n. 23 del 8.06.2012. Italian. [Google Scholar]
- 31.Guarino A, Albano F, Ashkenazi S, Gendrel D, Hoekstra JH, Shamir R, Szajewska H, ESPGHAN/ESPID Evidence-Based Guidelines for the Management of Acute Gastroenteritis in Children in Europe Expert Working Group . European Society for Paediatric Gastroenterology, Hepatology, and Nutrition/European Society for Paediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: executive summary. J Pediatr Gastroenterol Nutr 2008; 46:619-21; PMID:18493225; http://dx.doi.org/ 10.1097/01.mpg.0000319064.93905.44 [DOI] [PubMed] [Google Scholar]
- 32.Fischer TK, Viboud C, Parashar U, Malek M, Steiner C, Glass R, Simonsen L. Hospitalizations and deaths from diarrhea and rotavirus among children. J Infect Dis 2007; 195:1117-25; PMID:17357047; http://dx.doi.org/ 10.1086/512863 [DOI] [PubMed] [Google Scholar]
- 33.Festini F, Cocchi P, Mambretti D, Tagliabue B, Carotti M, Ciolfi D, Biermann KP, Schiatti R, Ruggeri FM, De Benedictis FM et al.. Nosocomial Rotavirus Gastroenteritis in pediatric patients: a multicenter prospective cohort study. BMC Infect Dis 2010; 10:235; PMID:20696065; http://dx.doi.org/ 10.1186/1471-2334-10-235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mattei A, Angelone AM, Michetti M, Sbarbati M, Ceci R, Murgano A, di Orio F. Epidemiological impact of RV gastroenteritis in the Abruzzo Region: SDO analysis. Ann Ig. 2009; 21:41-9; PMID:19385333 [PubMed] [Google Scholar]
- 35.Capri S, Veneziano MA. La vaccinazione anti-rotavirus in Italia: valutazione economica. In: Health technology assessment della vaccinazione anti-rotavirus con il vaccino Rotarix. QIJPH 2014; 3:55-67 [Google Scholar]
- 36.Gabutti G, Lazzara C, Marsella M, Bergamini M, Malaventura C, Borgna-Pignatti C. Burden of hospitalizations due to Rotavirus infection in Emilia Romagna, Italy. Acta Biomed, 2007; 78:176-81 [PubMed] [Google Scholar]
- 37.DRG, Ministero della Salute Decreto 18 ottobre 2012. GU n. 23 del 28 gennaio 2013. Remunerazione delle prestazioni di assistenza ospedaliera per acuti, assistenza ospedaliera di riabilitazione e di lungodegenza post acuzie e di assistenza specialistica ambulatoriale. Available from: http://www.crob.it/crob/files/docs/10/63/33/DOCUMENT_FILE_106333.pdf. Italian. [Google Scholar]
- 38.Istituto Italiano di Statistica (ISTAT) URL: http://www.demo.istat.it. [Google Scholar]


