ABSTRACT
The 7-valent pneumococcal conjugate vaccine (PCV7) produced a significant herd protection in unvaccinated adult population mostly because of pneumococcus carriage decrease in vaccinated children. It is not known if the 13-valent pneumococcal vaccine can give similar effect on adults. Aims of the work were to evaluate whether the 6 additional serotypes are present in nasopharynx of children and serotype distribution in invasive pneumococcal infections (IPD) in adults. Realtime-PCR was used to evaluate pneumococcal serotypes in adults with confirmed IPD and in nasopharyngeal swabs (NP) from 629 children not vaccinated or vaccinated with PCV7 and resident in the same geographical areas. Two hundred twenty-one patients (116 males, median 67.9 years) with IPD were studied (pneumonia n = 103, meningitis n = 61 sepsis n = 50, other n = 7). Two hundred twelve were serotyped. The most frequent serotypes were 3, (31/212; 14.6%), 19A, (19/212; 9.0%), 12 (17/212; 8.0%), 7F, (14/212; 6.6%). In NP of children, the frequency of those serotypes causing over 50% of IPD in adults was very low, ranging from 0.48% for serotype 7F to 7.9% for serotype 19A. On the other side serotype 5, very frequent in NP (18.7%) caused <1% IPD. In conclusion serotypes causing IPD in adults are very rarely found in children NP. We suggest that herd protection obtainable with the additional 6 serotypes included in PCV13 may be more limited than that demonstrated with PCV7 in the past. In order to reduce the burden of disease in adults, adults should be offered a specific vaccination program with highly immunogenic PCV.
Keywords: pneumococcal serotype, Realtime PCR, invasive pneumococcal disease, vaccine, herd-protection, nasopharyngeal carriage
Abbreviations
- CAP
community acquired pneumonia
- PCV
pneumococcal conjugate vaccine
- IPD
invasive pneumococcal disease
- CSF
cerebrospinal fluid
- RT-PCR
Real Time PCR
- NP swab
Nasopharyngeal swab
- IQR
interquartile range
Introduction
Streptococcus pneumoniae is the most important cause of pneumonia and invasive bacterial infections in any age, with the greatest incidence in children and elderly.1
More than 90 serotypes exist, but only a subset is associated with invasive disease.2
Since its introduction in the United States in 2000, the 7-valent conjugate pneumococcal vaccine (PCV7, including serotypes 4,6B,9V,14,18C,19F,23F) has dramatically reduced invasive pneumococcal disease (IPD) both in vaccinated and in unvaccinated age groups, through induction of herd protection. 3-7 The same effect was present, even though less evident, in Europe where the decrease in adult IPD associated to PCV7 serotypes was counterbalanced by a rapid increase in IPD due to non-PCV7 serotypes.8-10
The herd protection obtained with PCV7 was hypothesized to be due to the reduction in nasopharyngeal carriage of vaccine strains in immunized children, with subsequent interruption of transmission to their non-immunized contacts.11,12
In the pre-PCV7 era, the 7 serotypes included in PCV7, were not only the most frequent serotypes causing IPD in children and adults, but also the most frequently found in healthy carrier children both in USA and in Europe.1-2,12-13 That situation is present today in countries were PCV7 vaccination has never been used.14
In Italy, as in other countries, PCV7 has been used up to 2010 and then substituted by PCV13, which includes the 6 additional serotypes 1, 3, 5, 6A, 7F, 19A. While PCV vaccination is included in the Vaccination Schedule for Italian children and offered to all infants in Italy, no definite suggestion has been given for adults, so that Italian regions follow different strategies, with most regions giving no vaccination; at the same time possible advantages for adults obtainable through herd protection given by infant vaccination are under debate. Actually no data is available to demonstrate whether the 6 additional serotypes included in PCV13 have a large presence in NP of children and whether their elimination through PCV13 may have a significant herd protection effect on adults.
The aim of the present study was therefore to evaluate the distribution of S.pneumoniae serotypes in adults with IPD and compare it with the distribution of serotypes found in a large population of healthy carrier children resident in the same geographical areas in order to evaluate whether PCV13 vaccination of infants and children, reducing nasopharyngeal carriage, may have the potential to reduce IPD burden in adults and offer, with the use of PCV13 the same herd protection we have experienced with PCV7.
Results
Diagnosis of IPD in adults
We identified a total of 221 patients with IPD including pneumococcal pneumonia (n = 103; associated with sepsis in 12/103), meningitis (n = 61; associated with sepsis 14/61); sepsis (n = 50), other IPD (peritonitis, arthritis, otomastoiditis n = 7). Median and interquartile range (IQR) of age was 67.9 (51.9–75.1) years. The gender ratio M/F was 116/105 (1.1).
Diagnosis of IPD was obtained using RT PCR directly on normally sterile fluids (n = 93) or on culture isolates (n = 128).
IPD incidence increased with age as shown in Figure 1a. As for the clinical presentation of IPD, pneumonia was the most frequent IPD in any age group with the exception of the 31–45 y group where sepsis and meningitis were more frequent (35.7% each) (Fig. 1b).
Figure 1.
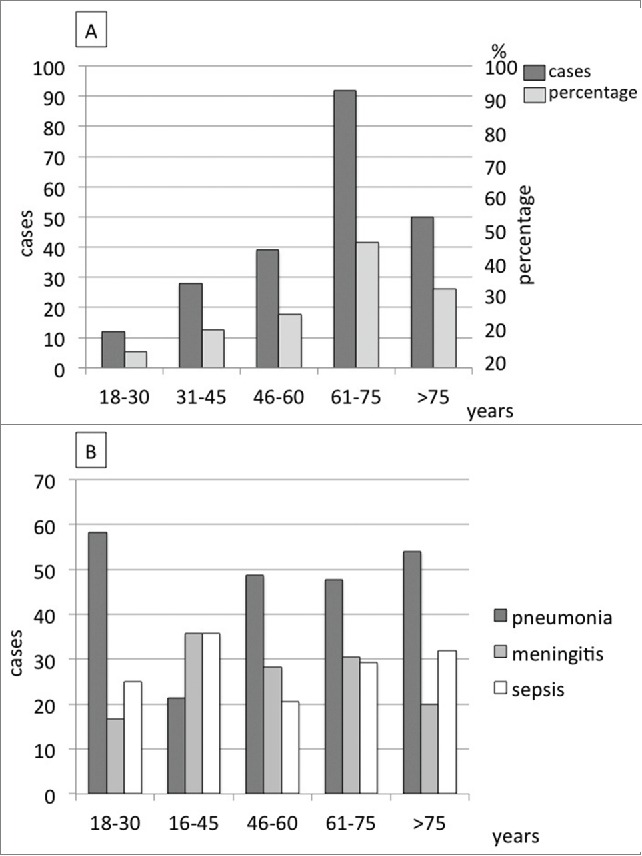
Distribution of IPD cases (n = 221) according to age groups (A) and clinical presentation (B).
Serotype distribution in IPD
Pneumococcal serotyping was obtained in 212/221 (96.0%) patients using RT-PCR. In 128 cases, serotyping was performed on culture isolates while in 93 cases serotyping was performed directly on biological samples. All 128 isolates could be serotyped using RT-PCR; IPD remained non-typeable in 9/93 biological samples (9.7%), because of sample paucity.
The most frequent serotypes were 3 (31/212 14.6%), 19A (19/212, 9.0%), 12 (17/212, 8.0%), 7F (14/212, 6.6%); serotype 12 was the most frequent serotype (7/14, 50.0%) in the class of age 61–75 y.
Serotype distribution according to potential vaccine protection is shown in Figure 2. Percentage of IPDs due to one serotype potentially preventable with PCV13 were respectively 72.7%, 39.3%, 54.1%, 48.1%, 52.6% for the age classes 18–30, 31–45, 46–60, 61–75 or over 75.
Figure 2.
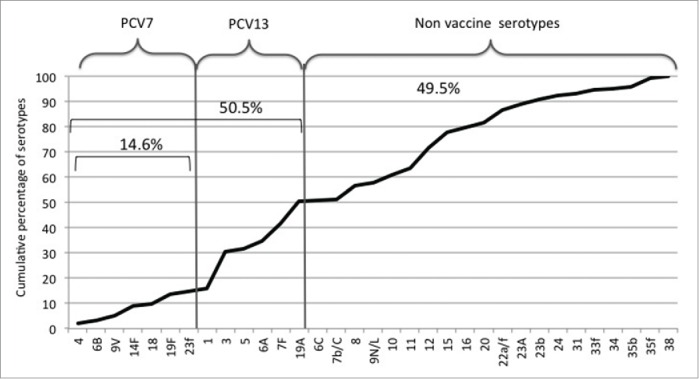
Serotype distribution in 212 adults with invasive pneumococcal disease. PCV7 serotypes accounted for 14.6%, all PCV13 serotypes 50.5%, non vaccine serotypes 49.5%.
Overall, potential serotype coverage on all age IPDs was respectively 14.6% and 50.5% for PCV7 or PCV13. Others serotypes, classified as not vaccine serotypes, caused 49.5% of IPDs. No difference between serotype distributions was found in patients with different clinical presentation.
Since in the areas under study PCV13 was included in the vaccination schedule in July 2010 in substitution of PCV7, we analyzed the 2 intervals of time as “pre-PCV13” (IPDs diagnosed in the 4 y interval 2007–2010) and “post-PCV13” (IPDs diagnosed in 4 y time, 2011–2014). The percentage of IPDs due to one serotype potentially covered by PCV13, was 55.5% in “pre-PCV13” era and 48.9% in “post-PCV13” era (−6.6%), while non vaccine serotypes represented respectively 44.4% and 51,0% (+6.6%) in the 2 periods; the difference was not statistically different (p=0.47; 95% CL 0.69–2.46). As for the single serotypes, incidence of serotype 19A was 6/63 (9.5%) in pre-PCV13 and 13/149 (8.7%) in post-PCV13; serotype 7F incidence was respectively 8/63 (12.7%) and 6/149 (4.0%) while serotype 3 increased from 6/63 (9.5%) in pre-PCV13 to 25/149 (38.9%) in post-PCV13.
Nasopharyngeal carriage of streptococcus pneumoniae in healthy children
Six-hundred and twenty-nine healthy children were included in the study (262 from Friuli and 367 from Tuscany); mean age was 24.4 months (median 20, IQR 9–37 months); 564/629 (89.7%) had been fully vaccinated, 3/629 (0.48%) were incompletely vaccinated and 62/629 (9.9%) were not vaccinated. Among the 564 fully vaccinated children, in 555/629 (88.2%) vaccination was completed with 3 doses in the first year of life, in 6/629 (0.95%) vaccination started in the second year of life, in 3/629 (0.48%) after the second year of life.
In 315/629 NP swabs (50.1%) Streptococcus pneumoniae was found and serotype distribution was evaluated. Two-hundred and ninety-seven (297/315, 94.3%) NP samples were serotyped while 18/315 (5.7%) were found positive for Streptococcus pneumoniae but the serotype was not identified and were defined as non-typeable. Multiple serotype carriage was found in 247/297 (83.2%). Serotype distribution is shown in Figure 3a. Serotype 3, the most frequently found in adults with IPD, was revealed in 7/315 (2.2%) Streptococcus pneumoniae positive NP swabs, or 7/629 (1.1% ) of the entire pediatric population studied; serotype 19A was found respectively in 50/315 (15.9%) of the pneumococcus positive NP swab or 50/629 (7.9%) of the pediatric population; as for serotype 12, third in frequency, causing 7.8% of adult IPD, it was found only in 1 NP swab (1/315; 0.3% or 1/629; 0.16% of the pediatric population) while serotype 7F, fourth in frequency with 6.6% of IPD, was found in 3/315 (0.95%) pneumococcus positive NP swabs or 3/629 (0.48%) of the entire pediatric population studied. Overall, serotype causing over 50% of IPD in adults (3,19A, 12, 7F, 15, 22, 8, on the whole 54.7%) were found in NP of healthy children in 12.9% of cases.
Figure 3.
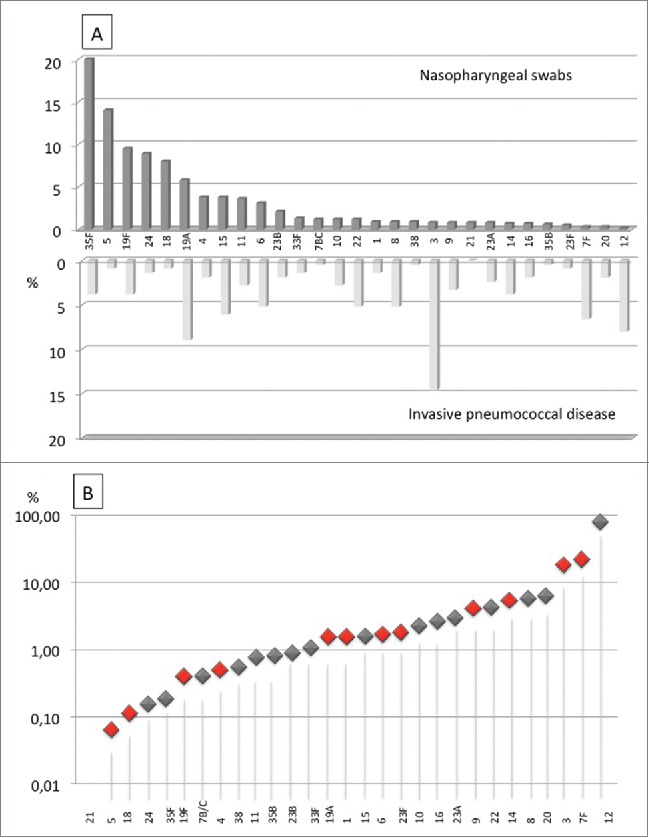
Comparison between pneumococcal serotypes found in nasopharyngeal swabs obtained from healthy carrier children and adults with invasive pneumococcal disease expressed as percentages (A) and case/carrier ratio (B).
The most frequent serotypes in NP swabs were serotype 35F and 5, found respectively in 171/315 (54.3%) and in 114/315 (36.2%) of pneumococcus positive NP swab that is in 171/629 (27.2%) and in 114/629 (18.1% ) of all children studied. Those serotypes were detected respectively in 8 cases (3.6%) and 2 cases (0.9%) of IPD in adults (1.1%). Case/carriage ratio is shown in Figure 3b.
Evaluating separately serotypes that could be eliminated through PCV13 vaccination, we found that serotypes 1,3,6,7F and 19A, which represent 36.8% of IPD in adults, are not found in more than 15% of NP. On the other side serotype 5, which is very frequent in NP, reaching alone 18.7%, was found in IPD in 0.9% of cases.
Discussion
The present study, conducted on a large population of adults in 2 Italian areas with high coverage of infant pneumococcal vaccination (PCV7 since 2006 and PCV13 since July 2010), is, for our knowledge, the largest study on IPD in adults conducted in Italy in the last 10 years, that is during the PCV7 and PCV13 era. The study confirms the progressive increase of IPD incidence with increasing age in adults, with a peak in the age group of 61–75 y. When evaluated in the whole period under study (2007–2014) serotype distribution demonstrated a low proportion, less than 15%, of IPDs due to PCV7 serotypes in all the age groups. That figure is significantly lower than that demonstrated in Italy in the pre-PCV7 era,15 so suggesting a herd protection for the PCV7 serotypes obtained through infant vaccination.
The 6 additional serotypes included in PCV13 accounted for a further 35%, while “non- vaccine” serotypes were found in about 50% of cases. Serotypes 3 and 19A for the PCV13 group and serotype 12 for the “non-vaccine serotypes” group were the most frequent.
NP swabs were found positive for Streptococcus pneumoniae in over 50% or healthy children included in the study, confirming our previous results.16 Over 80% children carried multiple serotypes. That proportion is higher than previously described by our group;16 the main reason is that 29 primers/probes were used in the present work compared to 21 primer/probes in our previous works16,17 so allowing individuation of a large number of serotypes. When comparison is done with studies performed by other groups using cultural methods18 the difference is even greater. Actually, since the serotyping methods based on culture commonly require that only one or 2 colonies be tested, the chance to find more than one serotype is limited.18 For the same reason culture-based methods probably overesteem replacement by non vaccine serotypes after vaccination.18 Our present and previous16,17 studies, performed using serotyping methods based on RT-PCR suggest that multiple non vaccine serotypes are commonly present in nasopharynx of healthy carriers
Our study suggests a low correspondence between serotypes found in IPD in adults and serotypes found in nasopharyngeal swabs of healthy carrier children with a very low incidence in NP of those serotypes such as 1,3,7F and 19A causing most IPD in adults. According to the design of the study, nasopharyngeal swabs had been collected from children who could have been vaccinated with PCV7 but not PCV13, in order to avoid possible decrease in the incidence of the 6 additional serotypes included in PCV13 because of vaccination. Nonetheless, even though evaluated in the pre-PCV13 era, the most invasive serotypes found in adults, such as serotypes 3, 7F and 19A, included in PCV13, were very rarely found in nasopharyngeal swabs from children.
That was not completely unexpected since it's well known that some serotypes have more frequently or exclusively an invasive behavior while other are more commonly or exclusively found in carriers.13 However this phenomenon assumes an important role when carried serotypes are considered in relationship with herd protection effect. Vaccine coverage was not even all over Italy at the time the study started, in 2007, so that our results, obtained in 2 regions with high vaccine coverage could not fit perfectly for other regions. However, vaccine coverage has rapidly increased over the following years exceeding 70% in the large majority of Regions in the mid-term and reaching a mean coverage over 80% at the end of the study.19 Therefore we suggest the study results can we applicable to all Italian Regions.
In the present study IPD in adults were evaluated in a larger timeframe (2007–2014) compared to nasopharyngeal carriage (2009–2010); however that difference does not affect study conclusions since serotype distribution in adult IPD is homogenous all over the study period.
Experience on PCV7 in many countries in the world demonstrated a strong herd-protection of adults when vaccine was administered to children in the first year of life3,7,8,11,12,20 and it has been suggested that herd protection is mainly due to the reduction of NP carriage obtainable in children through vaccination.11,12 Actually the demonstrated herd protection effect of PCV7 could be expected, since in the pre-PCV7 era, the 7 serotypes 4,6B,9V,14,18C,19F and 23F were at the same time the most commonly found in adult IPD and in NP of healthy children13,14,15 so that their elimination trough vaccination could significantly reduce bacterial diffusion and disease in non-immunized cohorts.
The present situation, during the PCV13 era in Italy is quite different, as suggested by our data. The 6 additional serotypes included in PCV13 are very rarely found in NP. Therefore, even though mass vaccination would succeed in eliminating completely all the 6 additional serotypes from NP of healthy carrier children, it would, altogether eliminate only the most rare serotypes. It's not clear how elimination of very rare serotypes from NP might impact on disease diffusion in non-immunized cohorts.
Moreover recent studies have demonstrated16,21,22 that PCV is not able to eliminate carriage state forever probably because of the physiological decrease in antibody titers, which remain high enough to prevent invasive infections but not enough to prevent carriage state. However even a transient elimination or a significant decrease of vaccine serotypes from NP of carriers might have an important impact on diffusion of the disease of some serotypes and therefore on burden of disease in adults if the frequency of those serotypes is high, as was the case of PCV7 serotypes in pre-PCV7 era.13,14,15
Our results, obtained in a large cohort of healthy children, confirm16,23,24 that serotypes causing today IPD in adults in Italy are very rarely found in carrier children. Serotype distribution is not a stable state and modifies over decades. However, at present it seems unlikely that vaccination of children with PCV13 can strongly impact on disease burden of adults through carriage reduction in Italy. Herd protection of adults through PCV13 might be therefore more limited than what previously described for PCV7.
As for serotype 5, the second in frequency in nasopharyngeal carriage in children, it has been demonstrated to be very rarely cause of IPD in adults;13 our study confirm that over 10 y after previous studies performed in Italy and Europe,3,15 serotype 5 is still implicated in less than 1% of IPD in adults.
Therefore, even if vaccination could completely eliminate serotype 5 from NP, IPD burden in adults in Italy would not be influenced much by that change.
We are aware that, in the present cross-sectional study, we considered serotype distribution in carriers as the only factor affecting disease diffusion to unvaccinated population, however, other variants such as duration of carriage25 seems to produce a small impact on IPD rate.
In essence, the herd protection effect may result from a reduction in the transmission rate because of lowering the rates of colonization or infection or both in the vaccinated population.26 Therefore, even if a partial herd protection will help reduce the burden of adult IPDs in Italy through the reduction of IPD in PCV13 vaccinated children, it is unlikely that herd protection will occur because of a significant reduction of invasive serotypes from NP of healthy children.
A limitation of the study is that IPD cases included may be only a part of all IPD really occurred in the regions studied. However we believe that cases we included may be representative of serotype distribution in those geographical areas, since our data set is the largest available in Italy, being even larger than that obtained by the National Institute of Health through IPD surveillance.
Cases in adults and elderly are undoubtedly the highest burden for the society and cause the highest number of deaths due to IPD, in Italy as in many other countries in the world. Therefore, according to our results, in order to decrease disease burden, adults should be offered a specific vaccination program with available highly immunogenic conjugate vaccines since herd protection obtainable with the 6 additional serotypes included in PCV13 could be more limited than that obtained with PCV7.
Patients and Methods
Adult Patients with IPD
The study included, within an active hospital-based surveillance program, all adults >18 y with a confirmed diagnosis of invasive pneumococcal disease in the period March 2007-February 2014. The study was performed in 2 Italian areas (Friuli-Venezia Giulia and Tuscany respectively in the North-East and Center of Italy) where pneumococcal vaccine is offered to all infants and vaccine coverage is >70% with a coverage of 74.9% for Friuli and 93.5% for Tuscany in the most recent survey, in 2011.19
Invasive pneumococcal disease was defined as clinical suspicion of bacterial disease (pneumonia, meningitis, sepsis, other ) and laboratory confirmation of the presence of S. pneumoniae in a normally sterile fluid (blood, cerebrospinal fluid, pleural fluid, peritoneal fluid) by Realtime-PCR and/or culture methods as previously described.15,27
Healthy streptococcus pneumoniae carrier children
Retrospective analysis of nasopharyngeal carriage of Streptococcus pneumoniae was evaluated in 629 nasopharyngeal swabs obtained from healthy children 0–60 months of age from the same geographical areas. Nasopharyngeal samples had been collected within a 9 month-period in 2009–2010, immediately before the introduction of PCV13.
Informed consent was obtained from all patients or guardians before initiation of the study. The study was approved by the local ethical committee.
Sample handling
Whole blood, and/or cerebrospinal fluid (CSF), and/or pleural fluid were collected from all patients as soon as possible after hospital admission; isolates from blood or CSF-cultures were also collected. Nasopharyngeal (NP) swabs were obtained as previously described.16
For culture purposes, 4–6 mL of blood samples (up to 3 sets) were drawn and immediately sent to the local laboratory. Standardized procedures were used for collection and shipment of blood samples to local laboratories. Samples for molecular tests were sent at room temperature to the central Laboratory (Immunology Laboratory, Anna Meyer Children's University Hospital, Florence, Italy) using an overnight freepost carrier; molecular tests were performed within 2 h of delivery; for both diagnosis and serotyping by Real Time PCR (RT-PCR), 200 µL of whole blood, or CSF, or pleural fluid, or isolate (1 colony sampled in 1 mL of 0.9% NaCl) or liquid obtained from NP swab were used.
Nasopharyngeal (NP) swabs were obtained from children as previously described.16 The swabs were placed in tubes containing liquid Amies medium (Becton, Dickinson, Sparks, MD), stored at 4°C and sent to the laboratory on a weekly basis on ice. NP samples were than aliquoted and stored at −20°C. Only one sample was obtained from each child so that there were not multiple samples from the same individual.
Diagnosis and serotyping of IPD
Diagnosis of laboratory confirmed IPD was based on RT-PCR results for the lytA gene as previously described.17,27 A sample was considered negative if there was no increase in fluorescent signal before cycle 40. Isolates already classified as S.pneumoniae by cultural methods were also sampled and analyzed for the lytA gene in order to confirm the diagnosis.
For serotyping, 33 primer/probe sets targeting different regions of cpsA gene were used, specific for 33 different serotypes. Twenty-nine primer-probes set were previously published by our group;17,27,28 the sequences of the 4 additional primer-probe sets are available upon request. Same procedure was used for NP swabs, as previously described.16,17
If no increase in fluorescent signal was observed after 40 cycles for any of the serotype specific primer/probe sets in spite of a positive result with both RT PCR (lytA gene) and end-point PCR (cpsA gene), the sample was considered non-typeable.
Pneumococcal serotypes were classified as PCV-7 or PCV13 serotypes if they were included in the 7-valent (4, 6B, 9V, 14, 18, 19F, 23F) or 13-valent (7-valent with the addition of 1, 3, 5, 6A, 7F, 19A) conjugate vaccine, respectively.
Definition of vaccination status
None of the IPD adult patients had received pneumococcal vaccines (either conjugate or polysaccharide). As for carrier children, data on pneumococcal vaccination status, including the number of doses received and the timing, were taken from the vaccination chart. The Italian vaccination schedule includes 3 doses of PCV in the first year of life or 2 doses between the first and the second year of life, or a single dose after the second year of life. Vaccine shift from PCV7 to PCV13 was done in July 2010. None of the children included in the study had received PCV13.
As previously described16 children were considered completely vaccinated if at the time of enrolling they had received 3 doses of PCV7 and vaccination had been completed in the first year of life or if they had received 2 doses of PCV7 in the second year of life or one dose of PCV7 after the second year of life. Children were considered incompletely vaccinated if they had started but not completed the vaccination schedule.
Statistical analysis
Two tailed p values were used and p values <0.05 were considered statistically significant. Results were expressed as means and standard deviations (SD) or as median and interquartile range (IQR) as appropriate. χ2 test was used to assess group differences in categorical variables. Data were processed with the SPSSX statistical package (SPSS 11.0, SPSS Inc., Chicago, IL).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
The study was partly supported by the Center for Disease Control in Italy (CCM) through a fund dedicated to “Improving diagnosis of invasive bacterial disease through molecular methods” and partly funded by the University of Florence.
References
- 1.Bridy-Pappas AE, Margolis MB, Center KJ. Streptococcus pneumoniae: description of the pathogen, disease epidemiology, treatment and prevention. Pharmacotherapy 2005; 25:1193-212; PMID:16164394; http://dx.doi.org/ 10.1592/phco.2005.25.9.1193 [DOI] [PubMed] [Google Scholar]
- 2.Hausdorff WP, Feikin DR, Klugman KP. Epidemiology differences among pneumococcal serotypes. Lancet Infect Dis 2005; 5:83-93; PMID:15680778; http://dx.doi.org/ 10.1016/S1473-3099(05)70083-9 [DOI] [PubMed] [Google Scholar]
- 3.Simonsen L, Taylor RJ, Young-Xu Y, Haber M, May L, Klugman KP. Impact of pneumococcal conjugate vaccination of infants on pneumonia and influenza hospitalization and mortality in all age groups in the United States. MBio 2011; 2(1):e00309-10; PMID:21264063; http://dx.doi.org/ 10.1128/mBio.00309-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, Reingold A, Cieslak PR, Pilishvili T, Jackson D, et al. Decline in invasive pneumococcal disease after the introduction of protein- polysaccharide conjugate vaccine. N Engl J Med 2003; 348:1737-46; PMID:12724479; http://dx.doi.org/ 10.1056/NEJMoa022823 [DOI] [PubMed] [Google Scholar]
- 5.Poehling KA, Talbot TR, Griffin MR, Craig AS, Whitney CG, Zell E, Lexau CA, Thomas AR, Harrison LH, Reingold AL, et al.. Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine. JAMA 2006; 295:1668-74; PMID:16609088; http://dx.doi.org/ 10.1001/jama.295.14.1668 [DOI] [PubMed] [Google Scholar]
- 6.Kellner JD, Vanderkooi OG, MacDonald J, Church DL, Tyrrell GJ, Scheifele DW. Changing epidemiology of invasive pneumococcal disease in Canada, 1998–2007: update from the Calgary-area Streptococcus pneumoniae research CASPER) study. Clin Infect Dis 2009; 49: 205-12; PMID:19508165; http://dx.doi.org/ 10.1086/599827 [DOI] [PubMed] [Google Scholar]
- 7.Griffin MR, Zhu Y, Moore MR, Whitney CG, Grijalva CG. US hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med 2013; 369(2):155-63; PMID:23841730; http://dx.doi.org/ 10.1056/NEJMoa1209165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ardanuy C, Tubau F, Pallares R, Calatayud L, Domínguez MA, Rolo D, Grau I, Martín R, Liñares J. Epidemiology of invasive pneumococcal disease among adult patients in Barcelona before and after pediatric 7-valent pneumococcal conjugate vaccine introduction, 1997–2007. Clin Infect Dis 2009; 48: 57-64; PMID:19035779; http://dx.doi.org/ 10.1086/594125 [DOI] [PubMed] [Google Scholar]
- 9.Rose M, Zielen S. Impact of infant immunization programs with pneumococcal conjugate vaccine in Europe. Expert Rev Vaccines 2009; 8:1351-64; PMID:19803758; http://dx.doi.org/ 10.1586/erv.09.78 [DOI] [PubMed] [Google Scholar]
- 10.Isaacman DJ, McIntosh ED, Reinert RR. Burden of invasive pneumococcal disease and serotype distribution among Streptococcus pneumoniae isolates in young children in Europe: impact of the 7-valent pneumococcal conjugate vaccine and considerations for future conjugate vaccines. Int J Infect Dis 2010; 14: e197-209; PMID:19700359; http://dx.doi.org/ 10.1016/j.ijid.2009.05.010 [DOI] [PubMed] [Google Scholar]
- 11.Isaacman DJ, Strutton DR, Kalpas EA, Horowicz-Mehler N, Stern LS, Casciano R, Ciuryla V. The impact of indirect (herd) protection on the cost-effectiveness of pneumococcal conjugate vaccine. Clin Ther 2008; 30(2):341-57; PMID:18343273; http://dx.doi.org/ 10.1016/j.clinthera.2008.02.003 [DOI] [PubMed] [Google Scholar]
- 12.O'Brien KL, Dagan R. The potential indirect effect on conjugate pneumococcal vaccines. Vaccine 2003; 21: 1815-25; PMID:12706665; http://dx.doi.org/ 10.1016/S0264-410X(02)00807-1 [DOI] [PubMed] [Google Scholar]
- 13.Sandgren A, Sjostrom K, Olsson-Liljequist B, Christensson B, Samuelsson A, Kronvall G, et al.. Effect of clonal and serotype-specific properties on the invasive capacity of Streptococcus pneumoniae. J Infect Dis. 2004; 189(5):785-96; PMID:14976594; http://dx.doi.org/ 10.1086/381686 [DOI] [PubMed] [Google Scholar]
- 14.Khoshdel A, Rastabi RI, Doosti A, Askari S, Hafizi M. Prevalence of Heptavalent Vaccine-related Pneumococcal Serotypes in Nasopharyngeal carrier in children under five years old in Shahrekord, Iran by Multiplex-PCR during 2010-2011. J Clin Diagn Res 2014; 8(11):PC01-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pantosti A, Boccia D, D'Ambrosio F, Recchia S, Orefici G, Moro ML. Inferring the potential success of pneumococcal vaccination in Italy: serotypes and antibiotic resistance of Streptococcus pneumoniae isolates from invasive diseases. Microb Drug Resist 2003; 9 Suppl 1:S61-8; PMID:14633369; http://dx.doi.org/ 10.1089/107662903322541919 [DOI] [PubMed] [Google Scholar]
- 16.Pasinato A, Indolfi G, Marchisio P, Valleriani C, Cortimiglia M, Spanevello V, Chiamenti G, Buzzetti R, Resti M, Azzari C. Pneumococcal serotype distribution in 1315 nasopharyngeal swabs from a highly vaccinated cohort of Italian children as detected by RT-PCR. Vaccine 2014; 32:1375-81; PMID:24486364; http://dx.doi.org/ 10.1016/j.vaccine.2014.01.023 [DOI] [PubMed] [Google Scholar]
- 17.Azzari C, Moriondo M, Indolfi G, Cortimiglia M, Canessa C, Becciolini L, Lippi F, de Martino M, Resti M. Realtime PCR Is More Sensitive than Multiplex PCR for Diagnosis and Serotyping in Children with Culture Negative Pneumococcal Invasive Disease. PLoS One 2010; 5(2):e9282. doi: 10.1371/journal.pone.00092822010; http://dx.doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet 2011; 378: 1962-73; PMID:21492929; http://dx.doi.org/ 10.1016/S0140-6736(10)62225-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ISS Dati e evidenze disponibili per l'utilizzo dei vaccini anti-pneumococcici nei soggetti a rischio di qualsiasi eta´ e per l'eventuale ampliamento dell'offerta ai soggetti anziani. 2013. http://www.epicentro.iss.it/temi/vaccinazioni/pdf/Dati%20e%20evidenze%20vaccini%20antipneumococcici.pdf; accessed 2015.September.07. [Google Scholar]
- 20.Miller E, Nicholas JA, Waight PA, Slack MPE, George RC. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis 2011; 11: 760-8; PMID:21621466; http://dx.doi.org/ 10.1016/S1473-3099(11)70090-1 [DOI] [PubMed] [Google Scholar]
- 21.Ansaldi F, de Florentiis D, Canepa P, Zancolli M, Martini M, Orsi A, Durando P, Icardi G. Carriage of Streptoccoccus pneumoniae 7 years after implementation of vaccination program in a population with very high and long-lasting coverage, Italy. Vaccine 2012; 30(13):2288-94; PMID:22306795; http://dx.doi.org/ 10.1016/j.vaccine.2012.01.067 [DOI] [PubMed] [Google Scholar]
- 22.Dagan R, Juergens C, Trammel J, Patterson S, Greenberg D, Givon-Lavi N, Porat N, Gurtman A, Gruber WC, Scott DA. Efficacy of 13-valent pneumococcal conjugate vaccine (PCV13) versus that of 7-valent PCV (PCV7) against nasopharyngeal colonization of antibiotic-nonsusceptible Streptococcus pneumoniae. J Infect Dis 2015; 211(7):1144-53; PMID:25355940; http://dx.doi.org/ 10.1093/infdis/jiu576 [DOI] [PubMed] [Google Scholar]
- 23.van Hoek AJ, Sheppard CL, Andrews NJ, Waight PA, Slack MP, Harrison TG, Ladhani SN, Miller E. Pneumococcal carriage in children and adults two years after introduction of the thirteen valent pneumococcal conjugate vaccine in England. Vaccine 2014; 32(34):4349-55; PMID:24657717; http://dx.doi.org/ 10.1016/j.vaccine.2014.03.017 [DOI] [PubMed] [Google Scholar]
- 24.Flasche S, Van Hoek AJ, Sheasby E, Waight P, Andrews N, Sheppard C, George R, Miller E. Effect of pneumococcal conjugate vaccination on serotype-specific carriage and invasive disease in England: a cross-sectional study. PLoS Med 2011; 8(4):e1001017; PMID:21483718; http://dx.doi.org/ 10.1371/journal.pmed.1001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sleeman KL, Griffiths D, Shackley F, Diggle L, Gupta S, Maiden MC, Moxon ER, Crook DW, Peto TE. Capsular serotype-specific attack rates and duration of carriage of Streptococcus pneumoniae in a population of children. J Infect Dis 2006; 194(5):682-8; PMID:16897668; http://dx.doi.org/ 10.1086/505710 [DOI] [PubMed] [Google Scholar]
- 26.Isaacman DJ, Fletcher MA, Fritzell B, Ciuryla V, Schranz J. Indirect effects associated with widespread vaccination of infants with heptavalent pneumococcal conjugate vaccine (PCV7; Prevnar). Vaccine 2007; 25(13):2420-7; PMID:17049677; http://dx.doi.org/ 10.1016/j.vaccine.2006.09.011 [DOI] [PubMed] [Google Scholar]
- 27.Resti M, Moriondo M, Cortimiglia M, Indolfi G, Canessa C, Becciolini L, Bartolini E, de Benedictis FM, de Martino M, Azzari C. Community-acquired bacteremic pneumococcal pneumonia in children: diagnosis and serotyping by Real-time Polymerase Chain Reaction using blood samples. Clin Infect Dis 2010; 51(9):1042-9; PMID:20883110; http://dx.doi.org/ 10.1086/656579 [DOI] [PubMed] [Google Scholar]
- 28.Azzari C, Moriondo M, Cortimiglia M, Valleriani C, Canessa C, Indolfi G, Ricci S, Nieddu F, de Martino M, Resti M. Potential serotype coverage of three pneumococcal conjugate vaccines against invasive pneumococcal infection in Italian children. Vaccine 2012; 30:2701-5; PMID:22178097; http://dx.doi.org/ 10.1016/j.vaccine.2011.12.008 [DOI] [PubMed] [Google Scholar]


