Abstract
Therapeutic vaccine research and development are especially important in Chagas disease considering the characteristics of the chronic infection and the number of people in the Americas living with a parasite infection for decades. We have previously reported the efficacy of attenuated Salmonella enterica (S) carrying plasmid encoding cruzipain (SCz) to protect against Trypanosoma cruzi infection. In the present work we investigated whether Cz DNA vaccine immunotherapy could be effective in controlling an ongoing T. cruzi infection in mice. We here report the intramuscular administration of naked Cz DNA or the oral administration of Salmonella as Cz DNA delivery system as therapeutic vaccines in mice during acute or chronic infection. The coadministration of a plasmid encoding GM-CSF improved vaccine performance, indicating that the stimulation of innate immune cells is needed in the event of an ongoing infection. These therapeutic vaccines were able to address the response to a protective and sustained Th1 biased profile not only against Cz but also against a variety of parasite antigens. The combined therapeutic vaccine during the chronic phase of infection prevents tissue pathology as shown by a reduced level of enzyme activity characteristic of tissue damage and a tissue status compatible with normal tissue. The obtained results suggest that immunotherapy with Cz and GM-CSF DNAs, either alone or in combination with other drug treatments, may represent a promising alternative for Chagas disease therapy.
Keywords: chagas disease, cystein protease, DNA-delivery system, GM-CSF, Salmonella enterica, Trypanosoma cruzi, therapeutic DNA vaccine
Introduction
Chagas disease is a zoonotic infection caused by the hemoflagellated protozoan parasite Trypanosoma cruzi. The infection is transmitted to mammalian hosts by a group of hemipteran insects belonging to the family Reduviidae, subfamily Triatominae. In endemic areas, the main transmission mode is vectorial by domestic, peridomestic and sylvatic triatomines. Infection can also be acquired by blood transfusion, organ transplant, congenital infection, and oral transmission from food contaminated with insect feces. Mass migration of chronically infected and asymptomatic persons has caused globalization of Chagas disease, which has now been reported in 19 non-endemic areas including the United States, Canada, Europe, Japan, and Australia.1,2 Currently, Chagas disease causes 12,000 annual deaths, and 100 million people are at risk of infection. In endemic areas of Latin America, 7–10 million people are infected by T. cruzi, with an incidence of 56,000 new cases per year.3
The disease has an initial acute phase characterized by high parasite level in blood. In most instances, this parasitemia is suppressed following the development of a flourishing immune response, although this process does not give rise to sterile immunity. Individuals in this chronic stage remain a source of infection throughout their lives. In about 30% of cases, the disease progresses from the asymptomatic to the symptomatic-chronic stage, sometimes decades after the primary infection,4 leading to clinical outcomes such as cardiomyopathy, alimentary tract pathology (typically, megacolon and megaesophagus) and/or damage to the peripheral nervous system.
For more than 40 years, the nitroheterocyclic agents nifurtimox and benznidazole have been the frontline therapy for Chagas disease.5,6 However, these drugs are far from optimal. They are effective against infections in the acute phase, though their usefulness in preventing or alleviating symptoms in the chronic stage remains controversial.7,8 Both drugs have been reported to be carcinogenic and display a wide range of side effects which include CNS toxicity, leukopenia, muscle weakness and severe dermatitis.5,9
Since T. cruzi treatments are not always effective and possess serious side effects, finding alternatives for controlling Chagas disease demands urgent attention. In this context, parasite proteinases represent an interesting chemotherapeutic target due to the possibility of selective blockage of the key functions performed by these molecules in the parasite life cycle and in host–parasite interactions. In that sense, cruzipain, the major T. cruzi cystein protease has some properties that signal it as a potential target for the generation of an immune response able to block the progression of the parasite inside the host. Among some of these properties, it is worth mentioning that: i- cruzipain is expressed in all developmental stages and strains of the parasite10; ii- it is accumulated in the lysosomes, it is secreted and also present at surface level, being able to hydrolyze immunoglobulins11; iii- it plays an important role in the process of internalization within mammalian cells12; and iv- it is highly immunogenic in natural infection.13
Recent improvements to DNA vaccines and the potential and flexibility of DNA as a potent immunization strategy for inducing both humoral and cell-mediated immune responses, make them an ideal therapeutic approach for treating or eliminating chronic disease, as it was shown in multiple sclerosis,14 human papillomavirus,15,16 hepatitis B17, human immunodeficiency virus,18,19 simian immunodeficiency virus,20 Mycobacterium tuberculosis,21 Leishmania spp.,22,23 Schistosoma mansoni,24 as well as against T. cruzi.25,26
We have previously reported the efficacy of attenuated Salmonella enterica (S) carrying plasmid encoding cruzipain (SCz) to protect against T. cruzi infection.27,28 In the present work we investigated whether Cz DNA vaccine immunotherapy could be effective in controlling an ongoing T. cruzi infection in mice. We administered naked Cz DNA intramuscularly or Salmonella as Cz DNA delivery system via oral route as therapeutic vaccines in mice during acute or chronic infection. Additionally, with the aim of improving the performance of these vaccines, we included the coadministration of a plasmid encoding granulocyte-macrophage colony-stimulating factor (GM-CSF).
Results
Combined treatment with Cz and GM-CSF naked DNAs reduces parasitemia and increases survival of T. cruzi infected mice
To evaluate the immunotherapeutic properties of the Cz DNA, mice infected with a lethal dose of T. cruzi trypomastigotes were treated at 0 and 10 dpi with: GI- Insert-less pCDNA 3.1(+) (nEmpty); GII-pCDNA 3.1(+) encoding GM-CSF (nGM-CSF); GIII-pCDNA 3.1(+) encoding Cz (nCz); and GIV- coadministration of both nCz+nGM−CSF. Control mice (nEmpty) presented high level of parasitemia and 100% mortality at 36 dpi (Fig. 1). In contrast, mice treated with nCz+nGM−CSF elicited an important reduction in the level of circulating trypomastigotes throughout the acute phase of infection compared to control (Fig. 1A). Most importantly, all mice treated with both plasmids survived until the end of the experiment at 100 dpi (Fig. 1B). Although mice monotherapeutically treated with nCz or nGM-CSF did not present any statistically differences in parasitemia compared to control, their survival was 75% and 40%, respectively, in contrast with control mice, which died between 21 and 35 dpi (Fig. 1).
Figure 1.
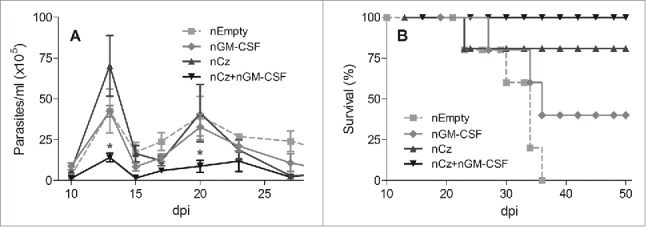
Protection against T. cruzi in animals receiving the immunotherapeutic vaccine during the acute phase of infection. C3H/HeN mice challenged with a lethal dose of RA trypomastigotes were treated by intramuscular route with 2 doses of 100 µg of a naked (n) plasmid encoding the Cz gene (nCz) or the GM-CSF gene (nGM-CSF), or both plasmids simultaneously (nCz+nGM−CSF). Control mice were treated with a plasmid without an insert (nEmpty). (A) Parasitemia determined every 2–3 days in fresh blood. The symbols represent the media ± the standard error. (B) Survival recorded daily. These results are representative of 3 independent experiments. *p < 0.05.
Combined therapy with Cz and GM-CSF increases IgG antibodies with a Th1 profile
To determine the specific antibody response, we evaluated the IgG titers against rCz in sera obtained at 35 and 100 dpi in mice infected with a sublethal dose of T. cruzi and treated as previously described. To assess the immune response against the infecting parasites, we also determined the antibody response against a complex mixture of soluble T. cruzi antigens, F105, containing ∼1% of rCz. The results obtained for the nEmpty group reflect the antibody response elicited by the infecting parasites (Fig. 2A). Thus, the response against F105 at 100 dpi shows a slight increase of antibodies against all parasite antigens compared with 35 dpi (0.7 × 104 vs 0.5 × 104). On the contrary, in the same group, the response against Cz increases 5 times at 100 dpi compared with 35 dpi (2.0 × 103 to 0.4 × 103), pointing to the strong immunogenicity of this antigen, which is also observed in natural infection. The administration of nGM-CSF increases the antibody response elicited by the infecting parasites, against the complex antigen and Cz, as expected for a stimulating cytokine; however, they cannot be significantly distinguished from the control treated with nEmpty. At 35 dpi the antibody titer against rCz in the group immunized with nCz can be attributed mostly to immunization since in nEmpty control mice is lower (1.9 × 103 vs 0.4 × 103). In contrast, we find a strong increase in IgG titers against both rCz and F105 in mice treated with the combination of both plasmids, with respect to the control group (2.5 × 104 vs 0.5 × 104 and 2.2 × 103 vs 0.4 × 103). Similar results were observed at 100 dpi, where mice immunized with the combination of both plasmids showed significant differences with respect to the control group (Fig. 2A). It must be emphasized that immunization with nCz+nGM−CSF not only increased the humoral immune response against the immunizing antigen Cz but also increased more than 11 times the antibody response against the antigens included in F105 compared with nEmpty (8.0 × 104 vs 0.7 × 104).
Figure 2.
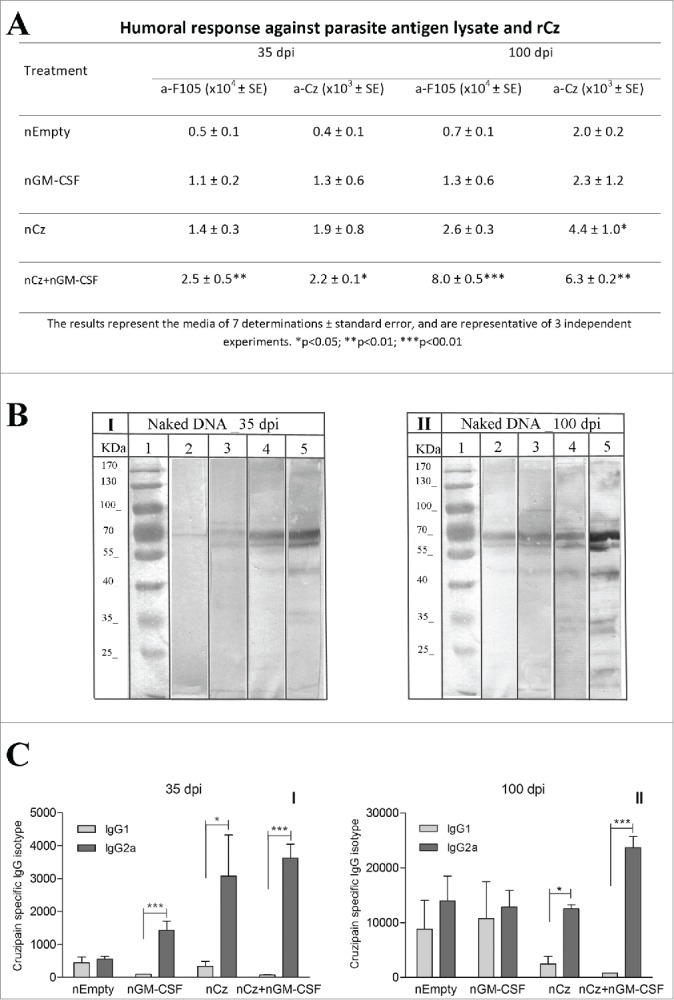
Antibody response at 35 and 100 dpi in mice receiving the immunotherapeutic vaccine during the acute phase of T. cruzi infection. Mice were challenged with RA trypomastigotes and were treated by intramuscular route with 2 doses of nEmpty, nGM-CSF,nCz or nCz+pGM−CSF. Serum samples were taken on day 35 and 100 postinfection. (A) IgG titers against rCz or parasite antigen fraction F105.(B) Immunoblotting of epimastigotes blotted onto nitrocellulose incubated with dilution of mice sera at (I) 35 dpi and (II) 100 dpi; (1) Molecular weight marker, (2 and 3) Control, (4 and 5) Cz+GM−CSF. Strips were incubated with biotinylated rat monoclonal antibodies to mouse IgG1 (2 and 4) or IgG2a (3 and 5) subclasses. (C) Cz specific IgG1 and IgG2a isotypes. The bars represent the media of 7 determinations and are representative of 3 independent experiments.*p < 0.05; **p < 0.01; ***p < 0.001.
In addition, we analyzed by immunoblotting and determined by ELISA the IgG2a/IgG1 relationship against Cz to estimate the T cell profile induced by vaccination. Figure 2B shows the immunodominance of the antibodies against rCz (60–69 kDa bands) that can be observed even in the infected control group immunized with nEmpty. As expected, the immune response against all the antigens increases over time (100 dpi vs 35 dpi) and a stronger IgG2a reaction compared with IgG1 can be observed at both times after infection (lane 4 vs lane 5). This result was confirmed by ELISA when all vaccinated mice showed high levels of the IgG2a isotype against rCz with significant differences with respect to the IgG1 isotype at 35 dpi (Fig. 2CI). These results indicate that the vaccination therapies were able to address the response against Cz to a protective Th1 profile. Most importantly, the Th1 response was sustained over time (100 dpi), only in mice vaccinated with nCz as monotherapy and in mice receiving nCz+nGM−CSF (Fig. 2CII). In addition, this therapeutic vaccine increased and maintained the IgG2a response over time, not only against Cz but also against the variety of parasite antigens (Fig. 2BII, lane 4). It must be emphasized that immunization with nCz alone or with nGM-CSF as adjuvant is able to revert the balanced IgG2a/IgG1 response produced by parasite infection to mostly all IgG2a, as shown in the nEmpty or nGM-CSF groups (Fig. 2CII).
Cz DNA vaccination of infected mice induces an important cellular response in vivo and in vitro
To evaluate whether the protective efficacy of the Cz DNA treatment in mice can be attributed to the elicited cellular response, we conducted an in vivo delayed type hypersensitivity test (DTH) at 100 dpi (Table 1). While mice treated only with nGM-CSF showed similar DTH levels than the control animals, footpad swelling increases significantly after the intradermal injection of the F105 (0.096 mm) or rCz (0.102 mm) in nCz-treated mice compared to controls (0.010 and 0.041 mm, respectively). Importantly, mice vaccinated with nCz+nGM−CSF developed a higher cellular response compared to control (Table 1A). In accordance with these results, when we evaluated the cellular immune response in vitro at 100 dpi in splenocytes stimulated with rCz or F105, we observed a strong proliferative response in both groups of mice vaccinated with nCz. Moreover, the response was even higher in the group that had simultaneously received the combination of plasmids, with values being more than 10-fold higher against rCz and F105 compared to control (Table 1B). These results indicate that immunotherapy with the combination of nCz+nGM−CSF improves the cellular response not only against Cz but also against other parasite antigens contained in F105.
Table 1.
Cellular response in naked DNA-treated mice during the acute phase of T. cruzi infection
| Stimuli | A. In vivo (DTH, mm ± SE) |
B. In vitro (Proliferation, cpm ± SE) |
||
|---|---|---|---|---|
| Treatment | Against F105 | Against Cz | Upon F105 | Upon Cz |
| nEmpty | 0.010 ± 0.004 | 0.041 ± 0.015 | 859 ± 292 | 775 ± 408.4 |
| nGM-CSF | 0.023 ± 0.014 | 0.042 ± 0.014 | 321 ± 111 | 269 ± 64 |
| nCz | 0.096 ± 0.019* | 0.102 ± 0.015* | 5304 ± 1419* | 5214 ± 1785* |
| nCz+nGM−CSF | 0.122 ± 0.013** | 0.106 ± 0.020* | 10209 ± 3143* | 8570 ± 2201** |
C3H/HeN mice challenged with a sublethal dose of RA trypomastigotes were treated by intramuscular route with 2 doses of nCz, nGM-CSF or nCz+nGM−CSF. The results represent the media of 7 determinations ± standard error, and are representative of 3 independent experiments. *p << 0.05; **p < 0.01.
Tissue damage induced by T. cruzi infection is prevented by Cz DNA immunotherapy
We further investigated whether the DNA-based therapeutic regimens that were capable of reducing parasitemia were additionally effective in limiting tissue damage, a pathogenic feature of chronic Chagas disease. Accordingly, sera samples of infected and treated mice were analyzed to determine the enzymatic activity of lactate dehydrogenase (LDH), creatinquinase (CK) and aspartate amino transferase (AST) at 35 and 100 dpi. An important decrease in the serum activity of at least one of these cardiomyopathy-associated enzymes was observed at both times for all vaccinated mice, compared with controls (Fig. 3A). Most importantly, upon treatment with nCz+nGM−CSF, mice displayed serum enzyme levels that were statistically lower for all 3 enzymes with respect to the control. In addition, the activities were similar to the basal values recorded for non-infected mice (28.67±10.41, 1087±240, 10.8±3.5, for CK, LDH and AST, respectively), at least for CK and LDH, both at 35 and 100 dpi (Fig. 3A).
Figure 3.
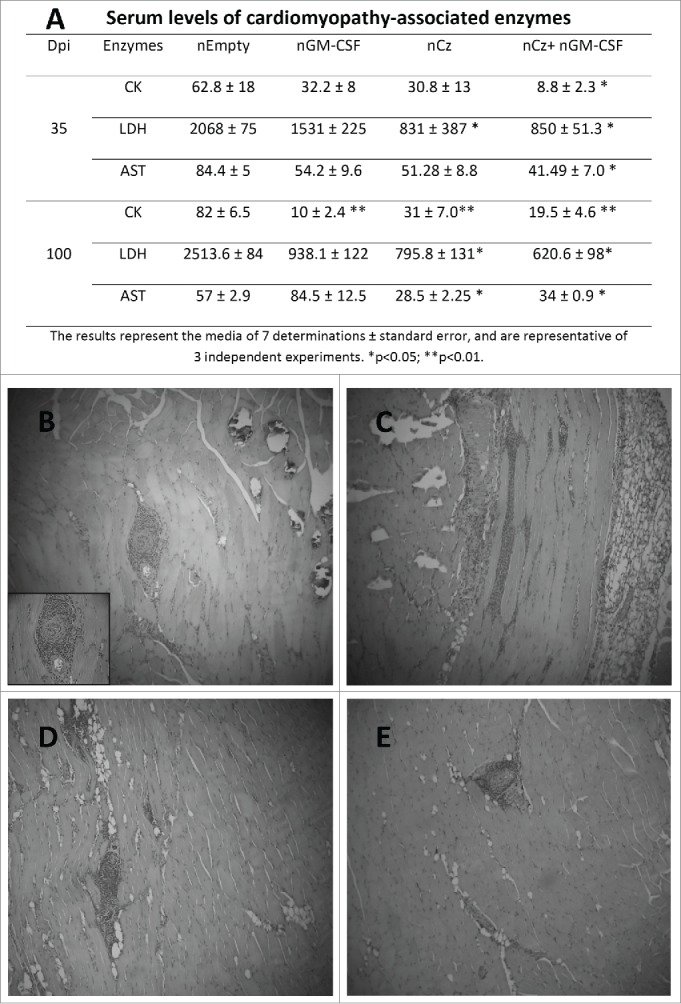
Tissue damage in T. cruzi-infected and treated mice. Mice infected with a sublethal dose of trypomastigotes were vaccinated by intramuscular route with the naked DNA of Cz and GM-CSF the day of the infection, and also 7 days later. (A) Serum levels of cardiomyopathy-associated enzymes. Blood was collected at 35 and 100 dpi and assays were performed to determine the levels of CK, LDH and AST. The results represent the media of 7 determinations ± standard error, and are representative of 3 independent experiments. *p < 0.05; **p < 0.01. Serum levels of cardiomyopathy-associated enzymes in non-infected mice are: 30.02 5± 10.41, 1,080 ± 420, and 7.80 ± 6.0 IU/liter for CK, LDH, and AST, respectively. Histopathological analysis of skeletal muscle in T. cruzi- infected and treated mice at 100 dpi. A blind histological test was performed. Magnification 10X (B) nEmpty, (C) nGM-CSF, (D) nCz, and (E) nCz+nGM−CSF.
To further characterize tissue damage, we performed histological analyses of heart and skeletal muscle in T. cruzi-infected and treated mice at 100 dpi. Based on the serum levels of the cardiomyopathy-associated enzymes, infected and nEmpty-treated mice exhibited calcifications, intense perivascular mononuclear cell infiltrate, as well as skeletal muscle fiber infiltration (Fig. 3B). By contrast, animals treated with nCz+nGM−CSF, presented reduced inflammatory lymphocyte foci in muscle tissue, minimal perivascular infiltrate, scarce infiltrated and dystrophic calcifications, and absence of interfiber infiltration (Fig. 3E). T. cruzi-infected animals treated with nCz and nGM-CSF as mono-therapeutic vaccine showed only a few inflammatory infiltrates, without a general increase in tissue cellularity (Fig. 3C, D). We were unable to identify any abnormalities in histopathological cardiac tissues at 100 dpi, neither in treated mice nor in the controls.
Cz and GM-CSF DNAs delivered by attenuated Salmonella during the acute phase of T. cruzi challenge reduces parasitemia and increases survival of infected mice
To overcome the requirement of high dosages of purified DNA needed in traditional naked DNA vaccines, we explored Salmonella enterica serovar Typhimurium aro A, which has been characterized as a successful delivery system for orally administered Chagas DNA vaccines.29,30,31 C3H/HeN groups of mice lethally infected with T. cruzi were treated orally at 0 and 10 dpi with 2 doses of 109 UFC of Salmonella transfected with plasmid lacking an insert (SEmpty), plasmid encoding Cz (SCz) or GM-CSF (SGM-CSF). Another group was treated simultaneously with SCz+SGM−CSF. We observed that SGM-CSF was not able to reduce parasitemia compared with control, but an important decrease in the number of blood parasites was found in mice treated with SCz as well as with SCz+SGM−CSF (Fig. 4A). Additionally, we found that 75% of the animals under the vaccination therapy with SCz+SGM−CSF survived to T. cruzi challenge until the end of the experiment (100 dpi). By contrast, all mice monotherapeutically treated with SCz or SGM-CSF died at 24 dpi, showing similar mortality than control animals (Fig. 4B).
Figure 4.
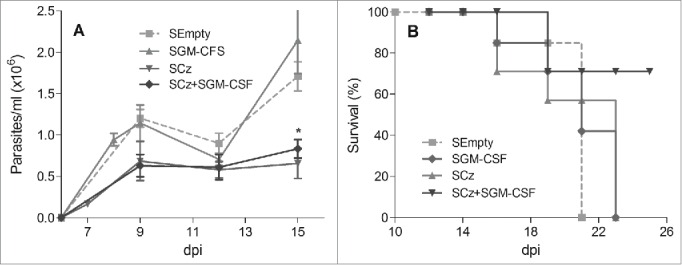
Protection against T. cruzi, in mice treated during the acute phase of infection with Cz and GM-CSF DNAs delivered by attenuated Salmonella. C3H/HeN mice infected with a lethal dose of RA trypomastigotes were treated at 0 and 10 dpi with 2 oral doses of 109 UFC of SCz, SGM-CSF or both combined SCz+SGM−CSF. As control, a group of mice was treated with Salmonella transfected with a plasmid without insert (SEmpty). (A) Parasitemia determined every 2–3 d in fresh blood. The symbols represent the media ± the standard error. (B) Survival recorded daily. These results were representative of 3 independent experiments. *p < 0.05.
Immunotherapy with SCz+SGM−CSF increases specific antibodies with predominance of the IgG2a isotype
We evaluated the development of the humoral response at 35 and 100 dpi in mice treated during the acute phase of T.cruzi infection with Salmonella carrying the different genes. Mice treated with SGM-CSF or SCz presented slightly higher antibodies against Cz and F105 at 35 dpi, with respect to the control. By contrast, in mice vaccinated simultaneously with SCz+SGM−CSF, the humoral response against rCz significantly enhanced with respect to the control (15.8 × 103 vs 1.2 × 103). At 100 dpi, all treated mice exhibited an increase in the titers of IgG antibodies against rCz and F105 compared to control; however, the differences were statistically significant only in the mice treated with SCz+SGM−CSF (Fig. 5A). These results showed that immunotherapy with SCz+SGM−CSF is able to elicit a specific response as good as with naked DNA and, more importantly, this humoral immune response persists in the chronic phase of infection (100 dpi).
Figure 5.
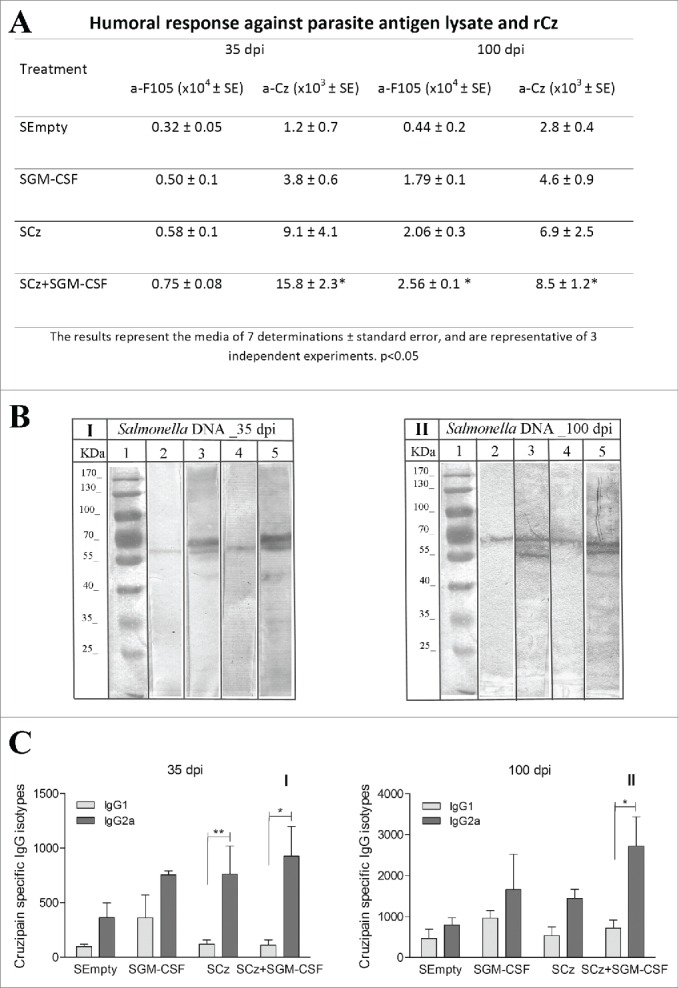
Antibody response at 35 and 100 dpi in mice receiving the Salmonella carrying the Cz and GM-CSF genes during the acute phase of T. cruzi infection. Mice were challenged with a sublethal amount of RA trypomastigotes and were treated orally with 2 doses of SCz, SGM-CSF or SCz+SGM−CSF. Serum samples were taken on 35 and 100 dpi. (A) IgG titers against F105 or rCz. (B) Immunoblotting of epimastigotes incubated with dilution of mice sera at (I) 35 dpi and (II) 100 dpi; (1) Molecular weight marker, (2 and 3) Control, (4 and 5) Cz+GM−CSF. Strips were incubated with biotinylated rat monoclonal antibodies to mouse IgG1 (2 and 4) or IgG2a (3 and 5) subclasses. (C) Cz specific IgG1 and IgG2a isotypes. The bars represent the media of 8 determinations and are representative of 3 independent experiments.*p < 0.05; ** p< 0.01.
To further characterize the humoral response elicited by the therapeutic vaccine, we analyzed isotypes IgG1 and IgG2a against Cz. Coadministration of Salmonella carrying both plasmids was the only therapy able to increase IgG2a in the acute phase of T. cruzi infection and also to maintain it during the chronic phase (Fig. 5B, C).
Immunotherapy with both Cz and GM-CSF DNA delivered by attenuated Salmonella increases in vivo and in vitro specific cellular responses
C3H/HeN mice challenged with a sublethal dose of RA trypomastigotes were treated orally with 2 doses of SCz, SGM-CSF or SCz+SGM−CSF. We found significant differences in the DTH test with respect to the control mice only in the SCz+SGM−CSF group (p < 0.05 for F105 and Cz). In contrast, no differences were found in mice vaccinated with SCz or SGM-CSF administered as a single therapy (Table 2).
Table 2.
Cellular response in Salmonella-treated mice during the acute phase of T. cruzi infection
| Stimuli | A. In vivo (DTH, mm ± SE) |
B. In vitro (Proliferation, cpm ± SE) |
||
|---|---|---|---|---|
| Treatment | Against F105 | AgainstCz | Upon F105 | UponCz |
| SEmpty | 0.018 ± 0.011 | 0.021 ± 0.021 | 85.4 ± 50.7 | 8.2 ± 4.4 |
| SGM-CSF | 0.048 ± 0.024 | 0.020 ± 0.015 | 185.5 ± 99.6 | 5.4± 3.4 |
| SCz | 0.037 ± 0.018 | 0.046 ± 0.019 | 133.1 ± 36.62 | 64.1 ± 51.6 |
| SCz+SGM−CSF | 0.074 ± 0.013* | 0.098 ± 0.018* | 1508 ± 557.2* | 847.2 ± 331.6*** |
C3H/HeN mice challenged with a sublethal dose of RA trypomastigotes were treated by oral route with 2 doses of SCz, SGM-CSF or SCz+SGM−CSF. The results represent the media of 7 determinations ± standard error, and are representative of 3 independent experiments.
When splenocytes from infected and treated mice were stimulated in vitro with F105 or recombinant Cz, we observed similar results to those obtained in vivo. Only the animals treated with both plasmids showed a significant proliferative response upon restimulation (Table 2).
Salmonella carrying Cz+GM−CSF DNAs prevents tissue damage characteristic of T. cruzi infection
We measured serum activity of CK, LDH and AST in mice treated with the different plasmids carried by Salmonella at 35 and 100 dpi. The activity of the 3 myopathy-linked enzymes was significantly lower with respect to the control only in infected mice treated with SCz+SGM−CSF, at both 35 and 100 dpi (Fig. 6A).
Figure 6.
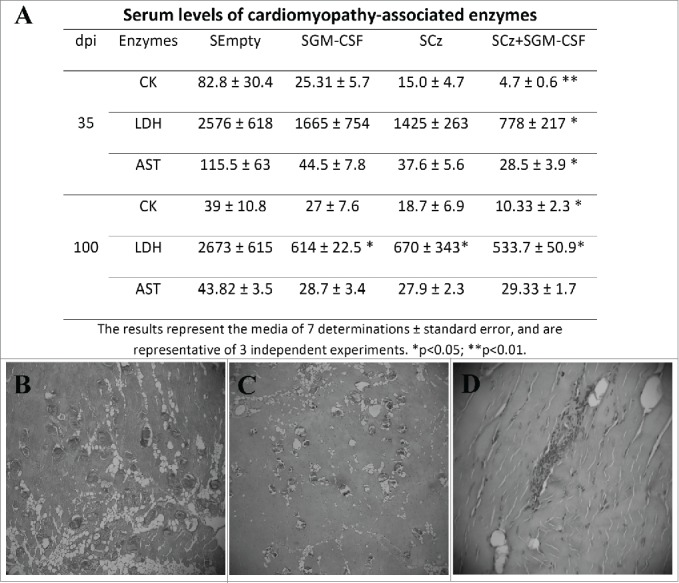
Tissue damage in T. cruzi infected mice receiving the Salmonella-based therapeutic vaccine during the acute phase of infection. C3H infected mice were treated by oral route with 2 doses of SCz, SGM-CSF or SCz+SGM−CSF. (A) Serum levels of myopathy-linked enzyme in blood collected at 35 and 100 dpi. The results represent the media ± standard error and are representative of 3 independent experiments. *p < 0.05. Serum levels of cardiomyopathy-associated enzymes in non infected mice are: 30.02 5 ± 10.41, 1,080 ± 420, and 7.80 ± 6.0 IU/liter for CK, LDH, and AST, respectively. The sections of quadriceps were stained with H/E and observed by microscopy (40X) one hundred days after the last immunizations. (B) Skeletal muscle of control mice showing important necroses, calcifications and perivasculitis. (C) Mice treated with SCz exhibited some zones of muscular necrosis, and (D) mice treated with SCz+SGM−CSF displayed an important reduction in mononuclear cell infiltration.
We were unable to identify any abnormalities in histopathological cardiac tissues at 100 dpi either in treated mice or in control. By contrast, when we analyzed the skeletal muscles, we observed perivasculitis with degenerative alterations of the muscular fibers, calcifications, and diffuse interfiber infiltrations in control animals (Fig. 6B, C). Mice treated with SCz alone or coadministered with SGM-CSF showed scarce calcifications. Most importantly, the treatment with both plasmids prevents inflammation and infiltration of mononuclear cells (Fig. 6D). Taken together, these results indicate that immunotherapy with SCz+SGM−CSF during the acute phase of infection is able to reduce muscle damage in the chronic phase of T. cruzi infection.
The immunotherapeutic vaccine with Cz plus GM-CSF DNAs administered during the chronic phase of infection prevents Chagas disease
We tested the efficacy of both naked and Salmonella-delivered DNA therapeutic vaccines during the chronic phase of infection. Mice infected with a sublethal challenge of trypomastigotes were treated 3 months after the infection by intramuscular route with 2 doses of naked DNA vaccines nCz or nCz+nGM−CSF. As controls, mice were treated with nEmpty. Another group of T. cruzi-infected mice were orally vaccinated on days 90 and 100 with SCz, SCz+SGM−CSF or SEmpty as control. As a marker of prevention of tissue damage, we analyzed the activity of serum enzymes and observed an important decrease in the serum activity of enzymes LDH, CK and AST in the mice treated with Cz+GM−CSF, with respect to the control in both naked and Salmonella-delivered DNA vaccines. Although a reduction in serum enzyme levels was observed in the animals treated with Cz monotherapy, the differences were not statistically significant with respect to the control (Fig. 7A).
Figure 7.
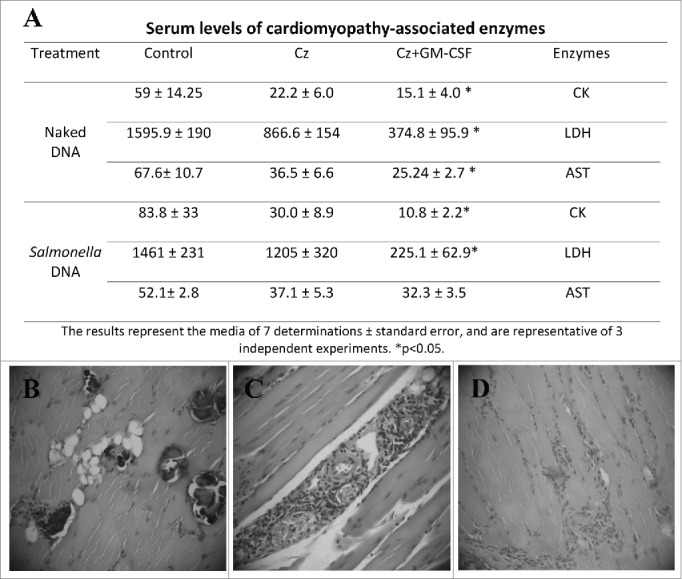
Tissue damage in T. cruzi infected and treated mice during the chronic phase of parasite infection. (A) Serum levels of myopathy-linked enzyme from T. cruzi-infected mice, vaccinated during the chronic phase of infection. CK, LDH and AST were determined by UV spectrophotometry in blood sample. The results represent the media of 7 determinations and are representative of 3 independent experiments. *p < 0.05. (B, C, and D). Serum levels of cardiomyopathy-associated enzymes in non infected mice are: 30.02 5 ± 10.41, 1,080 ± 420, and 7.80 ± 6.0 IU/liter for CK, LDH, and AST, respectively. Histopathology sections of skeletal muscle. Infected animals treated with 2 doses of Salmonella without plasmid showed calcifications, degenerative muscle alterations, perivasculitis and lymphomonocytary infiltrates (B). Mice treated with SCz, exhibited scarce inflammatory infiltration (C). Mice vaccinated with 2 doses of SCz+SGM−CSF presented little perivasculitis and scarce interstitial infiltrates (D). B and C magnification: 100X and D: 40X.
The histological analysis of cardiac tissue shows a small increase in cellularity and scarce infiltration, aggregates and dystrophic calcifications in animals receiving SEmpty. By contrast, these calcifications were not observed in mice treated with SCz, although some increase in the number of cells and infiltrations were observed. Importantly, mice simultaneously vaccinated with both plasmids exhibited a general status compatible with normal tissue, with some minor calcifications proximal to the endocardium. Moreover, the skeletal muscle of SCz+SGM−CSF-vaccinated mice showed light perivasculitis while controls showed severe perivasculitis with degenerative alterations of the muscular fibers and calcifications, also presenting scarce interfiber infiltrations (Fig. 7B-D). Similar results were observed in naked DNA-vaccinated mice (data not shown).
Overall, these results support the efficacy of the treatment with Cz+GM−CSF in the chronic phase of Chagas disease with both protocols using naked DNA and a DNA delivery system.
Discussion
Because T. cruzi has evolved many unique and clever mechanisms to evade, modulate and even exploit host immune responses, to develop a highly efficient and safe therapeutic vaccine still remains a challenge. Recent highlights on the biochemical pathways of kinetoplastids and the availability of the completion of genome sequencing have enabled the rational design of a large number of potential new lead compounds and drug candidates against kinetoplastid diseases. In that sense, the development of Cz inhibitors has shown to be a promising drug discovery avenue for Chagas disease. Recently, identified inhibitors of this protease have blocked the proliferation of both extracellular epimastigotes and intracellular amastigotes and arrested metacyclogenesis.30-33 Previously, we had also targeted the parasitic enzyme with the effector molecules and cells of the immune response as bullets. Thus, prophylactic Cz-based vaccines demonstrated strong protection against deadly challenges with trypomastigotes.27,34-37 Here we analyzed the ability of Cz and haematopoietic cytokine GM-CSF DNAs to protect already infected mice. Research on and developments of therapeutic vaccines are especially important in Chagas disease considering the characteristic mammal chronic infection and the number of people living in the Americas with a parasite infection for decades. In experimental T. cruzi infection, Dumonteil et al.25,26 demonstrated that an intramuscular injection of plasmid DNA encoding TSA-1 or Tc24 protected infected mice.
The use of live attenuated organisms as foreign DNA-delivery systems offers several advantages as compared to the administration of naked DNA. First, the vaccine can be administered by the mucosal route, facilitating its widespread development in pathogen eradication campaigns. In addition, the mucosa-associated lymphoid tissue facilitates the capture of antigen by specialized immune cells: macrophages and dendritic cells, initiating a strong humoral and cellular immune response, both locally and systemically. Thus, we recently obtained promising results in prophylactic Salmonella-based monocomponent27,38 or multicomponent29 vaccines against experimental infection with T. cruzi.
Numerous clinical studies have evaluated recombinant GM-CSF as monotherapy, as adjuvant with or without cancer vaccines, or in combination with chemotherapy.39,40 In the present work, although we have observed some degree of protection in monotherapeutically-treated mice with Cz or GM-CSF DNA, we found that mice treated during the acute phase of T. cruzi infection with the combination Cz+GM−CSF exhibited a significant reduction in parasitemia compared with controls, indicating that the stimulation of innate immune response cells is needed for a more efficient immune response in the event of an ongoing infection. Cz+GM−CSF combination as a therapeutic vaccine was effective in both naked plasmid and Salmonella- based DNA delivery system strategies. These results contrast with those obtained when both plasmid were used as a prophylactic vaccine, since the addition of GM-CSF to Cz DNA did not improve protection.27
Since lethal infection kills 100% of the Control group (nEmpty), 60% of the nGM-CSF group as well as 20% of the nCz group by day 36 (Fig. 1), to show the immune response differences between the 4 groups at 35 and 100 dpi we performed a sublethal infection. In addition, we evaluated the immune response to a sublethal inoculum of trypomastigotes with the aim of assessing what happens in humans, where the inoculum in the vector-borne disease is considered to be small and most patients overcome the acute phase of infection progressing to a chronic infection. The assayed therapeutic vaccines were able to address the response to a protective and sustained Th1 biased profile not only against Cz but also against the variety of parasite antigens. We found that GM-CSF co-administered with Cz contributes to the early generation and persistence of Cz specific IgG antibodies, particularly IgG2a, in both naked and Salmonella DNA-delivery system approaches. In addition, the cellular immune response against Cz and F105 is significantly higher in animals treated with Cz with respect to the control; and this response even increased when co-administered with GM-CSF, mainly for splenocyte proliferation. Lymphocyte proliferation after stimulation with Cz or F105 using SCz+SGM−CSF increased 10 times, compared with SCz as monotherapy (Table 2). These results emphasize that GM-CSF co-administration is necessary for enhancing cellular response, which is essential in eradicating intracellular pathogens such as T. cruzi.
Considering that Cz is highly immunogenic in natural infection,13,36 it is not surprising that nEmpty as well as SEmpty-treated mice showed specific IgG and activated lymphocytes. The humoral and cellular immune response is elicited against the native Cz present in the infecting parasites. Moreover, these responses were not only directed against Cz but also against other proteins present in a complex mixture of soluble T. cruzi antigens (F105) (Figs. 2A and 5A). These results can also be observed in the immunoblotting assay, where control mice at 35 dpi have a small amount of IgG2a against Cz whereas at 100 dpi antibodies recognizing other antigens can also be observed. However, in both nEmpty and SEmpty groups there were no significant differences between IgG1 and IgG2, indicating that the immune response is not addressed to Th1 (Fig. 2C and 5C). By contrast, in Cz+GM−CSF-treated mice, IgG2a was high at 35 and increased at 100 dpi against a broad range of antigens (Figs. 2 and 5). This Th1-driven immune response is the most protective against T. cruzi infection, as previously demonstrated.36,41,42 In addition, the critical importance of specific antibody-producing B cells for controlling systemic T. cruzi infection was recently reported by Sullivan et al.43
The robust humoral immune response and intense cellular response observed would explain the decreased parasitemia found in mice treated with the combination of both plasmids. These results become even more important since serum levels of enzymes that are markers of muscle damage in chronic Chagas disease (CK, AST and LDH) achieved significantly lower activity levels with respect to controls. The decline is mainly evident in CK serum levels, which would be more specific for cell injury in both skeletal and cardiac muscles. We also analyzed the effect of the therapeutic vaccine on day 100 after infection by microscopic examination of the tissues most affected by Chagas disease. We observed that mice vaccinated with Cz+GM−CSF only exhibited scarce inflammatory infiltrates in both cardiac and skeletal muscle, in contrast with the intense mononuclear cell infiltration in skeletal muscle found in control mice (Figs. 3 and 6). Recently, Pereira et al.44 have highlighted the importance of reprogramming the immune response to preserve and recover tissue injury in Chagas heart disease induced by parasite infection.
Due to the lack of an effective treatment during the chronic phase of T. cruzi infection, an important finding reported here is that mice treated with Cz plus GM-CSF DNAs as a combined therapeutic vaccine during the chronic phase of infection prevents tissue pathology, as shown by a reduced level of enzyme activity characteristic of tissue damage and a tissue status compatible with normal tissue (Fig. 7).
In this manuscript we demonstrated that several specific components of the immune system are involved in cruzipain-induced T. cruzi control. However, a deeper explanation of the mechanisms as to how immunotherapy using Cz and GM-CSF DNAs mediate disease control is needed. Unfortunately, we were not able to deplete only CD4+ and CD8+ T cells that were amplified by cruzipain to properly answer this question. We previously tried to approach the issue by depleting CD4+ or CD8+ T cells with rat IgG anti-CD4 and anti-CD8 monoclonal antibodies. Normal IgG-treated mice survived infection, whereas 100% of CD4- and CD8-T-cell-depleted animals died between days 17 and 20 after trypomastigote challenge. These results showed that both CD4- and CD8-T cells are involved in the control of parasitemia achieved through cruzipain vaccination.27 In addition, selective depletion of NK cells and adoptive transfer of sera containing anti-Cz antibodies should be tested in order to better identify the therapeutic mechanisms.
We were unable to find significant advantages of Salmonella as a DNA delivery system compared with the naked DNA vaccine. Both nCz+nGM−CSF and SCz+SGM−CSF treatments showed similar parameters of protection such as parasitemia, survival and cardiomyopathy-associated enzyme activity. However, surprisingly, when we compared the humoral response at 35 and 100 dpi in SCz+SGM−CSF and nCz+nGM−CSF we found that the Salmonella delivery system was able to increase the specific IgG with respect to the naked vaccine (IgG titers: 15.8 vs 2.2 × 103 and 8.5 vs 6.3 × 103 at 35 and 100 dpi, in SCz+SGM−CSF and nCz+nGM−CSF, respectively). In contrast, the response against the whole parasites (F105) was not higher in the Salmonella-delivered vaccine with respect to the naked DNA vaccine in Cz+GM−CSF-treated mice. Considering the drawbacks associated to the naked DNA immunization and the advantage of attenuated organisms, it seems that further investigation must be conducted in order to improve protection using the Salmonella DNA delivery system.
In addition, in the fight against kinetoplastid disease, consensus has grown over the past few years toward the use of combination therapies allowing reducing treatment duration or total drug doses, resulting in fewer toxic effects and less burden on the health system.45 Recently, it has been demonstrated that combinatorial therapy with benznidazole and granulocyte colony-stimulating factor (G-CSF) was beneficial in arresting inflammatory processes and tissue damage in Chagas heart disease.45 Exogenous administration of G-CSF partially repaired the damage provoked by T. cruzi due to the high level of inflammation that persisted in this experimental model. Immunotherapy with Cz and the GM-CSF DNAs may represent a promising alternative for Chagas disease therapy, both alone or in combination with other drug treatments. Thus, Salmonella as a DNA delivery system of Cz+GM−CSF might help reduce doses, delay the emergence of resistance and increase the therapeutic lifespan of benznidazole or nifurtimox. Moreover, the DNA therapeutic vaccine can overcome the challenges that affect the “druggability” of a compound, such as poor solubility or in vivo instability, inadequate pharmacokinetic and distribution tissue profile, poor therapeutic index (ratio toxicity/efficacy) or limited oral bioavailability, which could also reduce overall costs and provide a more cost-effective option.
Future therapeutic strategies should focus upon mode of administration, antigen selection, adequate immune stimulation and the potential combination of therapeutic vaccines with antiparasitic drug therapies in order to achieve improved control or clearance of chronic parasitic infection.
Materials and Methods
Parasites and antigens
T. cruzi bloodstream trypomastigotes (RA strain) were isolated from acutely infected mice at the peak of parasitemia. Epimastigotes of the same parasite strain were grown in biphasic medium as previously described.29 Parasites were harvested during exponential growth phase, re-suspended in 0.25M sucrose and 5mMKCl and broken by freezing/thawing and sonication in the presence of protease inhibitors as described.34 After centrifugation at 105000 × g, the supernatant obtained, called F105, was conserved at −20°C. F105 was used as a representative fraction of the parasite antigens.
Recombinant cruzipain (rCz) purification was previously described.28 Purity was assessed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Protein concentration was determined by the Bradford assay (Bio-Rad, Hercules, CA), using bovine serum albumin (Sigma) as standard.
Eukaryotic expression plasmids pCDNA 3.1(+) encoding Cz and murine GM-CSF were constructed as mentioned in Cazorla28 and purified from transformed E. coli XL2-Blue, using the Megaprep kit (Qiagen). The corresponding expression vectors were then transferred into the attenuated Salmonella enterica serovar Typhimurium aroA 7207 strain (an S. enterica serovar Typhimurium 2337–65 derivative, hisG46 DEL407 [aroA::Tn105Tc-s6]).
Animals and T.cruzi challenge
All studies were carried out in inbred female 6 to 8-week old C3H/HeN mice bred at the University of Buenos Aires, using 7 animals in each group, which were maintained under standard conditions. Experiments using animals are in accordance with the guidelines established by the National Research Council (CONICET). Mice were infected intraperitoneally with a lethal dose of 500 or a sub-lethal dose of 50 bloodstream trypomastigote forms. Parasitemia was monitored by counting peripheral parasites every 2–3 d in 5 µl of blood diluted 1/5 in lysis buffer (0.75% NH4Cl, 0.2% Tris, pH 7.2) by direct microscopy examination in a Neubauer chamber. Mortality was recorded daily.
Immunotherapy of infected mice
Two different experimental approaches were used in this study. In one of them, animals infected with a lethal or sublethal dose of parasites were vaccinated during the acute phase of T. cruzi infection on days 0 and 10 post-infection (dpi). Another group of mice, infected with a sublethal dose of parasites received the treatment during the chronic phase of infection (90 and 100 dpi).
Immunotherapy was conducted using 2 different protocols. A naked DNA based therapeutic vaccine where T. cruzi infected mice were treated with naked (n) DNA by intramuscular injection of 2 doses of 100 µg of plasmid in the quadriceps, according to the following regimens: 1- Plasmid lacking an Insert- (nEmpty); 2- plasmid encoding GM-CSF (nGM-CSF); 3-plasmid encoding cruzipain (nCz); and 4- coadministration of plasmids encoding cruzipain and GM-CSF (nCz+ nGM-CSF).
The other protocol to treat the infected animals consisted in the administration of the same plasmids but employing Salmonella aro A7207 as DNA delivery system. Oral administration was achieved by feeding 20 µl of PBS containing 109 UFC to mice that were deprived of drinking water for 2 h. Groups were as follows: 1- Salmonella with a plasmid lacking an insert (SEmpty); 2- Salmonella carrying the plasmid encoding GM-CSF (SGM-CSF); 3- Salmonella with the plasmid encoding cruzipain (SCz); and 4- simultaneous administration of SCz and SGM-CSF (SCz+SGM−CSF).
Animals were killed by cervical dislocation at 100 dpi, for mice treated during the acute T. cruzi infection, and at 190 dpi for mice vaccinated during the chronic phase of Chagas disease. Blood and spleen cells were taken to analyze the immune response.
Antibody titers and IgG isotype determination
F105- or Cz-specific antibody titers were determined in serum samples by ELISA. Plates were sensitized with 1 µg/well of F105 or with 0.1 µg/well of rCz. Peroxidase-conjugated anti- mouse IgG (Sigma) or biotinylated anti- mouse IgG1 or IgG2a subclasses (PharMingen) were used as secondary antibody, followed by streptavidin-peroxidase. Plates were developed by adding OPD/H2O2, and the reaction was stopped using 4N H2SO4. End point titers were defined as the dilution that resulted in OD value greater than 0.1. Control negative samples included in the assay had values lower than 0.1.
SDS-PAGE and immunoblotting
Epimastigotes containing protease inhibitors were diluted in sample buffer for SDS-PAGE and electrophoretically separated on 3% stacking/ 10% resolving gel. Samples were electrophoresed and blotted onto nitrocellulose. After blocking in Tris buffer solution (TBS: 50mM Tris–HCl, 150 mM NaCl, pH 7.4) with 1% skim milk, nitrocellulose was cut into strips and incubated for 1 h at 37 °C with 1/100 dilution of mice sera. After three washes with TBS, strips were incubated with biotinylated rat monoclonal antibodies to mouse IgG1 or IgG2a subclasses, later on with streptavidin–peroxidase and developed with H2O2/4-Cl−1-naphtol[34].
Characterization of the cell-specific immune response
Delayed-type hypersensitivity (DTH) reactions were performed 3 months after treatment by intradermal administration of 5 µg of rCz or 50 µg F105. The thickness of hind footpads were measured before and 48 h after the antigen injection with a digital indicator. Results are expressed as the difference in footpad thickness after and before inoculation.
Spleen cells were aseptically removed and cell suspensions were prepared. Cell proliferation was assayed as previously reported by thymidine incorporation.35
Measurement of muscle damage
Muscle damage was evaluated through the determination of a panel of myopathy-linked enzyme markers. Serum levels of creatine kinase (CK), aspartate aminotransferase (AST) and lactate dehydrogenase (LDH) were measured at 35 and 100 dpi for the acute model and 190 dpi for the chronic model of T. cruzi infection. The assays were done by ultraviolet spectrophotometry following the manufacturer's specifications (Wiener Lab, Buenos Aires, Argentina).
The histological features of heart and skeletal muscles (quadriceps) from immunotherapy- treated mice were also investigated. A blind histological test was done, analyzing 10 microscopic fields in 10 sections of each organ.
Statistical analysis
Statistical analyses were carried out with the Prisma 3.0 Software (GraphPad) using one-way analysis of variance (ANOVA) for proliferation, antibody and enzyme assays, and parasitemia data. The log-rank test was used for survival curves. All comparisons were referred to control groups. Values of p < 0.05 were considered significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Financial support was received from: Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET PIP 0935 to SIC), University of Buenos Aires (20020100100603 to ELM), Fundación Bunge & Born to SIC, and Agencia Nacional de Investigaciones Científicas y Técnicas (PICT-2006–608 and PICT-2010-657 to ELM), Argentina.
References
- 1.Gascon J, Bern C, Pinazo MJ. Chagas disease in Spain, the United States and other non-endemic countries. Acta Trop 2010; 115:22-7; PMID:19646412; http://dx.doi.org/ 10.1016/j.actatropica.2009.07.019 [DOI] [PubMed] [Google Scholar]
- 2.Basile L, Jansà JM, Carlier Y, Salamanca DD, Angheben A, Bartoloni A, Seixas J, Van Gool T, Canavate C, Flores-Chavez M, et al.. Chagas disease in European countries: the challenge of a surveillance system. 1–10. Euro Surveill 2011; 16(37):pii=19968; PMID:21944556 [PubMed] [Google Scholar]
- 3.Pan American Health Organization, 2012. Enfermedad de Chagas (Trypanosomiasis Americana). Available at: http://new.paho.org/hq/index.php?option=com_content&view=category&layout=blog&id=3591&Itemid=3921&lang=es. Accessed March 1, 2015.
- 4.Kirchhoff LV. Epidemiology of American trypanosomiasis (Chagas disease). Adv Parasitol 2011; 75:1-18; PMID:21820549; http://dx.doi.org/ 10.1016/B978-0-12-385863-4.00001-0 [DOI] [PubMed] [Google Scholar]
- 5.Wilkinson SR, Kelly JM. Trypanocidal drugs: mechanisms, resistance and new targets. Expert Rev Mol Med 2009; October 29; 11:e31; PMID:19863838; http://dx.doi.org/ 10.1017/S1462399409001252 [DOI] [PubMed] [Google Scholar]
- 6.Garcia S, Ramos CO, Senra JF, Vilas-Boas F, Rodrigues MM, Campos-de-Carvalho AC, Ribeiro-Dos-Santos R, Soares MB. Treatment with benznidazole during the chronic phase of experimental Chagas' disease decreases cardiac alterations. Antimicrob Agents Chemother 2005. April; 49(4):1521-8; PMID:15793134; http://dx.doi.org/ 10.1128/AAC.49.4.1521-1528.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marin-Neto JA, Rassi A, Morillo CA, Avezum A, Connolly SJ, Sosa-Estani S, Rosas F, Yusuf S; BENEFIT Investigators . Rationale and design of a randomized placebo-controlled trial assessing the effects of etiologic treatment in Chagas' cardiomyopathy: the Benznidazole evaluation for interrupting trypanosomiasis (BENEFIT). Am Heart J 2008; 156(1):37-43; PMID:18585495; http://dx.doi.org/ 10.1016/j.ahj.2008.04.001 [DOI] [PubMed] [Google Scholar]
- 8.Urbina JA. Specific chemotherapy of Chagas disease: relevance, current limitations and new approaches. Acta Trop 2010; 115(1–2):55-68; PMID:19900395; http://dx.doi.org/ 10.1016/j.actatropica.2009.10.023 [DOI] [PubMed] [Google Scholar]
- 9.Castro J a, de Mecca MM, Bartel LC. Toxic side effects of drugs used to treat Chagas' disease (American trypanosomiasis). Hum Exp Toxicol 2006; 25(8):471-9; PMID:16937919; http://dx.doi.org/ 10.1191/0960327106het653oa [DOI] [PubMed] [Google Scholar]
- 10.Parussini F, Duschak VG, Cazzulo JJ. Membrane-bound cysteine proteinase isoforms in different developmental stages of Trypanosoma cruzi. Cell Mol Biol (Noisy-le-grand) 1998. May; 44(3):513-9; PMID:9620448 [PubMed] [Google Scholar]
- 11.Berasain P, Carmona C, Frangione B, Cazzulo JJ, Goñi F. Specific cleavage sites on human IgG subclasses by cruzipain, the major cysteine proteinase from Trypanosoma cruzi. Mol Biochem Parasitol 2003; 130(1):23-9; PMID:14550893; http://dx.doi.org/ 10.1016/S0166-6851(03)00139-7 [DOI] [PubMed] [Google Scholar]
- 12.Souto-padron T, Campetella OE, Cazzulo JJ. Cysteine proteinase in Trypanosoma cruzi: immunocytochemical localization and involvement in parasite-host cell interaction. J Cell Sci 1990. July; 96 (Pt 3):485-90; PMID:2229199 [DOI] [PubMed] [Google Scholar]
- 13.Malchiodi EL, Chiaramonte MG, Taranto NJ, Zwirner NW, Margni RA. Cross-reactivity studies and differential serodiagnosis of human infections caused by Trypanosoma cruzi and Leishmania spp; use of immunoblotting and ELISA with a purified antigen (Ag163B6). Clin Exp Immunol 1994; September; 97(3):417-23; PMID:8082296; http://dx.doi.org/ 10.1111/j.1365-2249.1994.tb06104.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fissolo N, Montalban X, Comabella M. DNA vaccination techniques. In: Weissert R, ed. Multiple Sclerosis: New York: Springer, 2016:39–50. [DOI] [PubMed] [Google Scholar]
- 15.Peng S, Song L, Knoff J, Wang JW, Chang YN, Hannaman D, Wu TC, Alvarez RD, Roden RB, Hung CF. Control of HPV-associated tumors by innovative therapeutic HPV DNA vaccine in the absence of CD4+ T cells. Cell Biosci 2014. March 4; 4(1):11; PMID:24594273; http://dx.doi.org/ 10.1186/2045-3701-4-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diniz MO, Cariri FA, Aps LR and Ferreira LC. Enhanced therapeutic effects conferred by an experimental DNA vaccine targeting human papillomavirus-induced tumors. Hum Gene Ther 2013. October; 24(10):861-70; PMID:24007495; http://dx.doi.org/ 10.1089/hum.2013.102 [DOI] [PubMed] [Google Scholar]
- 17.Godon O, Fontaine H, Kahi S, Meritet J, Scott-Algara D, Pol S, Michel M, Bourgine M. Immunological and antiviral responses after therapeutic DNA immunization in chronic hepatitis B patients efficiently treated by analogues. Mol Ther 2014. March; 22(3):675-84; PMID:24394187; http://dx.doi.org/ 10.1038/mt.2013.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson CC, Newman MJ, Livingston BD, MaWhinney S, Forster JE, Scott J, Schooley RT, Benson CA. Clinical phase 1 testing of the safety and immunogenicity of an epitope-based DNA vaccine in human immunodeficiency virus type 1-infected subjects receiving highly active antiretroviral therapy. Clin Vaccine Immunol 2008. June; 15(6):986-94; PMID:18400976; http://dx.doi.org/ 10.1128/CVI.00492-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palma P, Romiti ML, Montesano C, Santilli V, Mora N, Aquilani A, Dispinseri S, Tchidjou HK, Montano M, Eriksson LE, et al.. Therapeutic DNA vaccination of vertically HIV-infected children: report of the first pediatric randomised trial (PEDVAC). PLoS One 2013; 8(11):e79957; PMID:24312194; http://dx.doi.org/ 10.1371/journal.pone.0079957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zur Megede J, Sanders-Beer B, Silvera P, Golightly D, Bowlsbey A, Hebblewaite D, Sites D, Nieves-Duran L, Srivastava R, Otten GR, et al.. A therapeutic SIV DNA vaccine elicits T-cell immune responses, but no sustained control of viremia in SIVmac239-infected rhesus macaques. AIDS Res Hum Retroviruses 2008. August; 24(8):1103-16; PMID:18620495; http://dx.doi.org/ 10.1089/aid.2008.0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skinner AM, Buddle BM, Wedlock DN, Keen D, de Lisle GW, Tascon RE, Ferraz JC, Lowrie DB, Cockle PJ, Vordermeier HM, et al.. A DNA Prime-Mycobacterium bovis BCG boost vaccination strategy for cattle induces protection against bovine tuberculosis. Infect Immun 2003. September; 71(9):4901-7; PMID:12933831; http://dx.doi.org/ 10.1128/IAI.71.9.4901-4907.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maroof A, Brown N, Smith B, Hodgkinson MR, Maxwell A, Losch FO, Fritz U, Walden P, Lacey CN, Smith DF, et al.. Therapeutic vaccination with recombinant adenovirus reduces splenic parasite burden in experimental visceral leishmaniasis. J Infect Dis 2012. March 1; 205(5):853-63; PMID:22301630; http://dx.doi.org/ 10.1093/infdis/jir842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Handman E, Noormohammadi AH, Curtis JM, Baldwin T, Sjölander A. Therapy of murine cutaneous leishmaniasis by DNA vaccination. Vaccine 2000; 18(26):3011-7; PMID:10825604; http://dx.doi.org/ 10.1016/S0264-410X(00)00109-2 [DOI] [PubMed] [Google Scholar]
- 24.Dupré , Herv M, Schacht AM, Capron A, Riveau G. Control of schistosomiasis pathology by combination of Sm28GST DNA immunization and praziquantel treatment. J Infect Dis 1999. August; 180(2):454-63; PMID:10395862; http://dx.doi.org/ 10.1086/314875 [DOI] [PubMed] [Google Scholar]
- 25.Dumonteil E, Escobedo-ortegon J, Reyes-rodriguez N, Arjona-Torres A, Ramirez-Sierra MJ. Immunotherapy of Trypanosoma cruzi infection with DNA vaccines in mice. Infect Immun 2004. January; 72(1):46-53; PMID:14688079; http://dx.doi.org/ 10.1128/IAI.72.1.46-53.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quijano-Hernández I, Castro-Barcena A, Vázquez-Chagoyán JC, Bolio-González ME, Ortega-López J, Dumonteil E. Preventive and therapeutic DNA vaccination partially protect dogs against an infectious challenge with Trypanosoma cruzi. Vaccine 2013. April 26; 31(18):2246-52; PMID:23499599; http://dx.doi.org/ 10.1016/j.vaccine.2013.03.005 [DOI] [PubMed] [Google Scholar]
- 27.Cazorla SI, Becker PD, Frank FM, Ebensen T, Sartori MJ, Corral RS, Malchiodi EL, Guzmán CA. Oral vaccination with Salmonella enterica as a cruzipain-DNA delivery system confers protective immunity against Trypanosoma cruzi. Infect Immun 2008. January; 76(1):324-33; PMID:17967857; http://dx.doi.org/ 10.1128/IAI.01163-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cazorla SI, Matos MN, Cerny N, Ramirez C, Alberti AS, Bivona AE, Morales C, Guzmán CA, Malchiodi EL. Oral multicomponent DNA vaccine delivered by attenuated Salmonella elicited immunoprotection against American trypanosomiasis. J Inf Dis 2015; 211(5):698-707; PMID:25160983; http://dx.doi.org/ 10.1093/infdis/jiu480 [DOI] [PubMed] [Google Scholar]
- 29.Chiari E, Camargo EP. Culturing and cloning of Trypanosoma cruzi In: Morel CM, editor. Genes and antigens of parasites. Rio de Janeiro: Fundacao Oswaldo Cruz; 1984. p. 23-6 [Google Scholar]
- 30.Braka K, Doyleb PS, McKerrowb JH, Ellman JA. Identification of a new class of nonpeptidic inhibitors of cruzain. J Am Chem Soc 2008. May 21; 130(20):6404-10; PMID:18435536; http://dx.doi.org/ 10.1021/ja710254m [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen YT, Liraa R, Hansell E, McKerrow JH, Roush WR. Synthesis of macrocyclic trypanosomal cysteine protease inhibitors. Bioorg Med Chem Lett 2008. November 15; 18(22):5860-3; PMID:18585034; http://dx.doi.org/ 10.1016/j.bmcl.2008.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bellera CL, Balcazar E, Alberca L, Labriola CA, Talevi A, Carrillo C. Application of computer-aided drug repurposing in the search of new cruzipain inhibitors: discovery of amiodarone and bromocriptine inhibitory effects. J Chem Inf Model 2013. September 23; 53(9):2402-8; PMID:23906322; http://dx.doi.org/ 10.1021/ci400284v [DOI] [PubMed] [Google Scholar]
- 33.Ndao M, Beaulieu C, Black WC, Isabel E, Vasquez-Camargo F, Nath-Chowdhury M, Massé F, Mellon C, Methot N, Nicoll-Griffith DA. Reversible cysteine protease inhibitors show promise for a Chagas disease cure. Antimicrob Agents Chemother 2014. February; 58(2):1167-78; PMID:24323474; http://dx.doi.org/ 10.1128/AAC.01855-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frank FM, Petray PB, Cazorla SI, Muñoz MC, Corral RS, Malchiodi EL. Use of a purified Trypanosoma cruzi antigen and CpG oligodeoxynucleotides for immunoprotection against a lethal challenge with trypomastigotes. Vaccine 2003; 22(1):77-86; PMID:14604574; http://dx.doi.org/ 10.1016/S0264-410X(03)00541-3 [DOI] [PubMed] [Google Scholar]
- 35.Cazorla SI, Frank FM, Becker PD, Corral RS, Guzmán CA, Malchiodi EL. Prime-boost immunization with cruzipain co-administered with MALP-2 triggers a protective immune response able to decrease parasite burden and tissue injury in an experimental Trypanosoma cruzi infection model. J Infect Dis 2010. July 1; 202(1):136-44; PMID:20497050; http://dx.doi.org/ 10.1086/652872 [DOI] [PubMed] [Google Scholar]
- 36.Cazorla SI, Frank FM, Becker PD, Arnaiz M, Mirkin GA, Corral RS, Guzmán CA, Malchiodi EL. Redirection of the immune response to the functional catalitic domain of the cystein proteinasa cruzipain improves protective immunity against Trypanosoma cruzi infection. J Infect Dis 2010. July 1; 202(1):136-44; PMID:20497050; http://dx.doi.org/ 10.1086/652872 [DOI] [PubMed] [Google Scholar]
- 37.Schnapp AR, Eickhoff CS, Sizemore D, Curtiss R 3rd, Hoft DF. Cruzipain induces both mucosal and systemic protection against Trypanosoma cruzi in mice. Infect Immun 2002. September; 70(9):5065-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matos MN, Cazorla SI, Bivona AE, Morales C, Guzmán CA, Malchiodi EL. Tc52 amino-terminal-domain DNA carried by attenuated Salmonella enterica serovar Typhimurium induces protection against a Trypanosoma cruzi lethal challenge. Infect Immun 2014. October; 82(10):4265-75; PMID:25069980; http://dx.doi.org/ 10.1128/IAI.02190-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chianese-Bullock K, Pressley J, Garbee C, Hibbitts S, Murphy C, Yamshchikov G, Petroni GR, Bissonette EA, Neese PY, Grosh WW, et al.. MAGE-A1-, MAGE-A10-, and gp100-derived peptides are immunogenic when combined with granulocyte-macrophage colony-stimulating factor and montanide ISA-51 adjuvant and administered as part of a multipeptide vaccine for melanoma. J Immunol 2005. March 1; 174(5):3080-6; PMID:15728523; http://dx.doi.org/ 10.4049/jimmunol.174.5.3080 [DOI] [PubMed] [Google Scholar]
- 40.Kaufman HL, Ruby CE, Hughes T, Slingluff CL. Current status of granulocyte-macrophage colony-stimulating factor in the immunotherapy of melanoma. J Immunother Cancer 2014; 2:11; PMID:24971166; http://dx.doi.org/ 10.1186/2051-1426-2-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Machado AV, Cardoso JE, Claser C, Rodrigues MM, Gazzinelli RT, Bruna-Romero O. Long-term protective immunity induced against Trypanosoma cruzi infection after vaccination with recombinant adenoviruses encoding amastigote surface protein-2 and trans-sialidase. Hum Gene Ther 2006. September; 17(9):898-908; PMID:16972758; http://dx.doi.org/ 10.1089/hum.2006.17.898 [DOI] [PubMed] [Google Scholar]
- 42.Frank FM, Cazorla SI, Sartori MJ, Corral RS. Elicitation of specific, Th1-biased immune response precludes skeletal muscle damage in cruzipain-vaccinated mice. Exp Mol Pathol 2008. February; 84(1):64-70; PMID:18054912; http://dx.doi.org/ 10.1016/j.yexmp.2007.10.004 [DOI] [PubMed] [Google Scholar]
- 43.Sullivan NL, Eickhoff CS, Sagartz J, Hoft DF. Deficiency of antigen-specific B cells results in decreased Trypanosoma cruzi systemic but not mucosal immunity due to CD8 T cell exhaustion. J Immunol 2015. February 15; 194(4):1806-18; PMID:25595788; http://dx.doi.org/ 10.4049/jimmunol.1303163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pereira IR, Vilar-Pereira G, Marques V, da Silva AA, Caetano B, Moreira OC, Machado AV, Bruna-Romero O, Rodrigues MM, Gazzinelli RT, et al.. A human type 5 adenovirus-based Trypanosoma cruzi therapeutic vaccine re-programs immune response and reverses chronic cardiomyopathy. PLoS Pathog 2015. January 24; 11(1):e1004594; PMID:25617628; http://dx.doi.org/ 10.1371/journal.ppat.1004594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Espuelas S, Plano D, Nguewa P, Font M, Palop JA, Irache JM, Sanmartín C. Innovative lead compounds and formulation strategies as newer kinetoplastid therapies. Curr Med Chem 2012; 19(25):4259-88; PMID:22834813; http://dx.doi.org/ 10.2174/092986712802884222 [DOI] [PubMed] [Google Scholar]
- 46.González MN, Dey N, Garg NJ, Postan M. G ranulocyte colony-stimulating factor partially repairs the damage provoked by Trypanosoma cruzi in murine myocardium. Int J Cardiol 2013. October 3; 168(3):2567-74; PMID:23597573; http://dx.doi.org/ 10.1016/j.ijcard.2013.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]


