Abstract
Background
Circulating biomarkers can predict clinical outcomes in colorectal cancer patients. The aim of the study was to evaluate the feasibility of our multigene biomarker chip for detecting circulating tumor cells for postoperative surveillance of stage I–III colorectal cancer patients.
Materials and Methods
In total, 298 stage I–III colorectal cancer patients were analyzed after curative resection between June 2010 and October 2014. During each follow-up, a postoperative surveillance strategy, including ESMO Guidelines Working Group recommendations and the biochip, was used.
Results
After a 28.4-month median follow-up, 48 (16.1%) patients had postoperative relapse. Univariate analysis revealed that the postoperative relapse risk factors were rectal tumor, perineural invasion, elevated preoperative and postoperative serum carcinoembryonic antigen levels, and positive biochip results (all P < 0.05). Multivariate analyses revealed that postoperative relapse correlated significantly with elevated postoperative serum carcinoembryonic antigen levels (odds ratio = 4.136, P = 0.008) and positive biochip results (odds ratio = 66.878, P < 0.001). However, the sensitivity (P = 0.003), specificity (P = 0.003), positive (P = 0.002) and negative (P = 0.006) predictive values, and accuracy (P < 0.001) of the biochip for predicting postoperative relapse were significantly higher than those of elevated postoperative serum carcinoembryonic antigen levels. Moreover, the median lead time between positive biochip result and postoperative relapse detection was significantly earlier than that between elevated postoperative serum carcinoembryonic antigen level and postoperative relapse detection (10.7 vs. 2.8 months, P < 0.001). Furthermore, positive biochip results correlated strongly with lower disease-free survival and overall survival of colorectal cancer patients (both P < 0.001).
Conclusion
Compared with conventional serum carcinoembryonic antigen detection, our multigene chip aided more accurate and earlier prediction of postoperative relapse during stage I–III colorectal cancer patient surveillance. In clinical practice, this biochip may facilitate early postoperative relapse diagnosis in colorectal cancer patients.
Introduction
According to the Ministry of Health and Welfare of Taiwan, since 2006, colorectal cancer (CRC) has become the most common cancer and the third cause of cancer-related death in Taiwan. In 2013, its incidence was 45.1 per 100,000 population, with more than 5,000 deaths per year and an average of 13.1 years of life lost (http://mohw.gov.tw/CHT/DOS/Index.aspx; accessed in March 2016). Although surgical resection is the primary treatment modality for CRC, 33%–50% of CRC patients relapse [1]. More than 90% of relapses occur during the first 5 years following surgery and at a particularly higher rate in the first 2 years. However, CRC-related deaths are majorly attributable to clinical relapse [1]. If the relapse is diagnosed earlier, it may be amenable to resection, leading to a higher rate of resectability and increasing the likelihood of long-term survival [2].
Several surveillance strategies for patients undergoing curative primary CRC resection have been reported. Carcinoembryonic antigen (CEA), an oncofetal antigen, is an extensively used disease relapse marker [3]; however, the utility of serial CEA testing remains uncertain: in 30%–40% of all CRC recurrences, the serum CEA shows unmeasurable elevations [4]. By contrast, transient elevations in CEA levels are observed in patients with resected CRC during adjuvant chemotherapy or immunotherapy. The false-positive rate for elevated serum CEA level detection during follow-up can be as high as 16% [5], unnecessarily increasing the difficulty in diagnosing recurrence and increasing patient anxiety [2]. Therefore, a more powerful tool for early detection of CRC relapse is required.
Studies for identifying novel panels of multiple molecular and biochemical markers usable for more precisely defining prognosis and predicting of adjuvant treatment benefits in CRC have been reported [6]. Reports have described the detection of circulating tumor cells (CTCs) in the peripheral blood of CRC patients; this method has major prognostic and therapeutic implications [7–10]. Our recently developed membrane array-based multigene biomarker assay can detect CTCs in the peripheral blood of CRC patients; this is a rational approach for the surveillance of postoperative CRC patients [6,11–15]. However, a detailed prospective comparative study regarding the diagnostic accuracy of the biomarker chip and serum CEA level detection is required. In the present study, we prospectively analyzed both the biomarker chip and serum CEA level detection periodically after curative resection in Union for International Cancer Control (UICC)/American Joint Committee on Cancer stage I–III CRC patients and identified whether the biochip was more efficient for their postoperative surveillance.
Materials and Methods
Patients
This prospective study was conducted by a surgical team in a single institution between June 2010 and February 2016. During June 2010 to October 2014, 331 patients were diagnosed with stage I–III CRC. Of these, 33 were excluded: 16 with a <1-year follow-up before death and 17 with other malignancies. Finally, 298 patients were enrolled after radical curative resection for primary CRC tumor: 82, 102, and 114 stage I, II, and III patients, respectively. The clinicopathological characteristics of these patients are listed in Table 1. The clinical stages and pathological features of the primary tumors were defined according to the seventh edition of the UICC tumor—node—metastasis (TNM) staging system [16].
Table 1. Clinicopathological features of 298 colorectal cancer patients.
| Gender (male/female) | 168/130 |
| Age (year) | |
| Median | 64.21 |
| Mean ± SD | 64.4±11.3 |
| Maximum tumor size≧5cm | 78 (26.2%) |
| Location (rectum/colon) | 77/221 |
| Depth of tumor invasion T (1/2/3/4) | 44/67/167/20 |
| Lymph node metastasis N (0/1/2) | 184/74/40 |
| Histology (WD/MD/PD) | 42/244/12 |
| TMN stage (I/II/III) | 82/102/114 |
| Vascular invasion | 86 (28.9%) |
| Perineural invasion | 61 (20.5%) |
| Abnormal preoperative CEA level | 98 (32.9%) |
| Abnormal postoperative CEA level | 71 (23.8%) |
| Postoperative relapse | 48 (16.1%) |
| Positive biomarker chip | 62 (20.8%) |
| Mortality | 26 (8.7%) |
| Follow up (month) | |
| Median, range | 28.4, 3.0–61.3 |
| Mean ± SD | 29.0±9.7 |
Ethics Statement
The study protocol was approved by the Institutional Review Board of Kaohsiung Medical University Hospital (KMUHIRB-950326 & KMUHIRB-2012-03-02(II)). Written informed consent was obtained from the patients, and all clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki. The patients also consented to the publication of the clinical details.
Follow-up
According to the clinical practice guidelines recommended by European Society for Medical Oncology Guidelines Working Group [1], postoperative surveillance during each follow-up comprised medical history-taking, physical examination, and laboratory studies including serum CEA levels. Abdominal ultrasonography or computed tomography (CT) was performed every 6 months, and chest plain radiography examinations and total colonofiberscopy were performed once a year. Furthermore, elevated CEA levels were defined when two consecutive CEA levels at a 3-month interval of regular follow-up were >5 ng/mL. In the case of elevated CEA or a positive multigene biomarker chip, high resolution MRI or contrast enhanced CT of the liver was performed before the annual follow-up. Patients were followed every 3 months in the first 3 years and at 6-month intervals thereafter. All patients were followed until death or February 2016. The development of recurrent or metastatic lesions was defined as postoperative relapse.
CTCs in the peripheral blood were detected using our previously constructed multigene biomarker chip with serial CEA assays at each follow-up [7, 12–15]. Additional 4 mL samples of peripheral blood were obtained for total RNA isolation. To prevent contamination by epithelial cells, peripheral blood samples were obtained through a catheter inserted into a peripheral vessel, and the first 5 mL of blood was discarded. Sample acquisition and subsequent use were approved by the institutional review board of the hospital.
Serum CEA Level Detection
Serum CEA levels were determined from additional 3 mL peripheral blood samples by using an enzyme immunoassay test kit (DPC Diagnostic Product Co., Los Angeles, CA, USA), with the upper limit of 5 ng/mL defined as normal, in accordance with the manufacturer instructions.
Gene Selection and Oligonucleotide Design
The authors used a method combining suppression subtractive hybridization and cDNA microarray chips to investigate changes in all genes involved in the carcinogenic pathway from colorectal adenomatous polyps to colorectal cancer [17]. 71 genes specific for colorectal cancer as diagnostic markers were successfully identified and the patents were obtained in Taiwan (No. I278519), the USA (No. US 7575928), and the European Union (No. 04 003 301.1). In order to implement the clinical application of specific gene groups, the oligonucleotide sequences of top 19 highly overexpressed target genes of the 71 genes were selected (Table 2), as described previously [18–20]; they were designed using the Oligo Explorer software program (Gene Link, Inc. New York, USA).
Table 2. Oligonucleotide sequences of 19 target genes.
| Gene | oligonucleotide sequences (5'→ 3') |
|---|---|
| PSG2 | CTCGGAAACTTTTGGTGGCTGGGCTTCAATCGTGACTTGGGCAGT |
| ELAVL4 | TTGCCCCTGTTTGCATGGGAGAAGGACAGTTTCTGTTGTTGCTGG |
| TK1 | CAGGGAGAACAGAAACTCAGCAGTGAAAGCCGCAGAGGGGAAGAA |
| UBE2C | AACTTCACTGTGGGCGCATTGTAAGGGTAGCCACTGGGGAACTCT |
| PDE6D | TGCCAGAGTATCTTCCCTGTCTCAGCATCCCGAAGGTTCATCCAA |
| PSAT1 | TTGACCTTGAATCAACAGCCGCTGAACCCAGGAGACCCCACAGAT |
| CHRNB1 | TAGGGTCCCAACGCTGGTGAAGATGATGAAAGTCCACAGGAAGAG |
| CEA | ATCCTGCATCGTTCCTTTTGACGCTGAGTAGAGTGAGGGTCATGT |
| BMI1 | CGAGGTCTATTGGCAAAAGAAGATTGGTGGTTACCGCTGGGGCTG |
| CAP2 | ACATGGCGGAGCCCTTTTGTAATTGCTTCTCCCTGGTTAAGTTGG |
| MMP13 | AAAGTGGCTTTTGCCGGTGTAGGTGTAGATAGGAAACATGAGTGC |
| OLFM4 | AGCAGGTGCCTCATCTACAGATCCTTCTGGGATTTATTTGCCATG |
| PTTG1 | TATCTATGTCACAGCAAACAGGTGGCAATTCAACATCCAGGGTCG |
| MYC | AGTGACTGTCCAGTTTTGAGAAGCGTCTAGCAAGTCCGAGCGTGTTCAAT |
| MET | CCCGAGTTCTTTCTATTGATGCGTTCATGCTCTTGACCCTGGTAG |
| MUC1 | CCTGGGGTAGAGCTTGCATGACCAGAACCTGTAACAACTGTAAGCACTGT |
| HMGB1 | ATTGCAGCCTATCACTAACCCTGCTGTTCGCTTGCATGTATCTTG |
| hTERT | AGGGGTGAACAATGGCGAATCTGGGGATGGACTATTCCTATGTGG |
| BIRC5 | CTCTAACCTGCCATTGGAACCTCACCCATAGCCCAGAAGCCTCAT |
| Oryza sativa | CTCGGTAACCTCTATTCCTCTACACCCTCGACCTCACCAACACCAGCCT |
| β-actin | ATGCTCGCTCCAACCGACTGCTGTCACCTTCACCGTTCCAGTTTT |
Multigene Biomarker Chip Preparation
The 19 synthesised oligonucleotides were dissolved in distilled water to a concentration of 100 mM and then applied to a BioDOT AD1500 nanoliter dispense system (BioDot, Inc., Irvine, CA, USA), which blotted each oligonucleotide solution sequentially on a Nytran SuperCharge nylon membrane (Schleicher and Schuell, Dassel, Germany) in triplicate. The oligonucleotides were then crosslinked to the membrane by using a UV Stratalinker 1800 (Stratagene, La Jolla, CA, USA). Each spot contained 20 ng of PCR-amplified DNA derived from sequence-verified cDNA clones. DMSO was also dispensed onto the membrane as a blank control.
Detection of Multigene Expression on the Biomarker Chip
A GeneCling Enzymatic Gene Chip Detection Kit (CaryGene Biotechnology Co., Ltd., Kaohsiung, Taiwan) was used as follows: Beads first were added for RNA extraction; RT buffer and RT enzyme were then added to synthesize cDNA. Next, Biotin Mix Label was added to the cDNA for biotin-labelling probe synthesis (probe synthesis). The labelled cDNA was then hybridised with the prepared biomarker chip at 42°C for 6–17 h. Wash buffer was then used to wash off the nonhybridized probes, followed by the addition of Streptavidin-AP—NBT/BCIP mixture and incubation at 37°C for a color reaction. Finally, after coloration, the biochips were completely dried, and the software was used to analyze the colorimetric values of the individual genes; these values were translated to indicate the relative intensities of the various gene expressions.
Biomarker Chip Intervention
Subsequent quantification analysis of the intensity of each spot was carried out using AlphaEase® FC software (Alpha Innotech Corp., San Leandro, CA, USA). Spots consistently carrying a factor of two or more were considered to be differentially expressed. A deformable template extracted the gene spots and quantified their expression levels by determining the integrated intensity of each spot after background subtraction (Fig 1). The fold ratio of each gene was normalized on the basis of the reference gene (β-actin) density, as follows: spot intensity ratio = mean intensity of the target gene/mean intensity of β-actin. Fig 1 provides the schematic representation of the membrane array with 19 candidate genes, one positive control (β-actin), one negative control (Oryza sativa sequence), and the blank control (dd water). If the gene presented a color density >2-fold higher than the positive control (β-actin) did, the result was defined as positive, whereas if the density was <2-fold higher than that of the positive control, the result was defined as negative. Each overexpressed spot was then multiplied by the respective weighted values ranging from 1 to 4, according to the principle described previously [18–22].
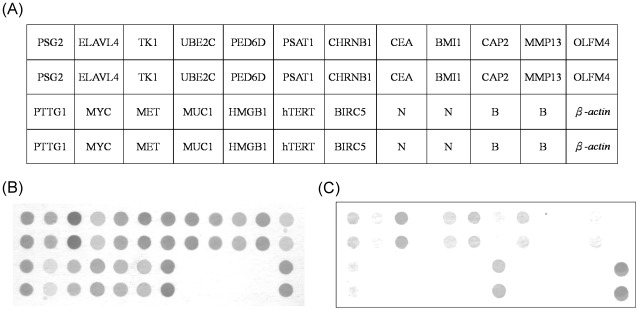
Fig 1.
(A) Schematic representation of the colorectal cancer biomarker chip evacuated using the weighted enzymatic chip array method with the 19 candidate genes, a positive control (β-actin), a negative control (Oryza sativa), and a blank control (double distilled water). Oligonucleotide fragments were blotted on the membranes in triplicate, and the expression levels of each gene spot were quantified and then normalized on the basis of the color density of a reference gene (β-actin). (B) Positive biochip result. (C) Negative biochip result.
ROC Curve and Determination of Cutoff Levels of the Multigene Biomarker Chip
The optimal cutoff value of the multigene biomarker chip was determined according to a prior study [18]. Receiver operating characteristic (ROC) curve analysis was performed on 557 participants, including 298 CRC patients (not the enrolled patients in the present study) and 259 normal individuals. Based on the calculated cutoff value, the expression of the biomarker chip was defined as either positive or negative. The optimal cutoff value and AUC were 23.5 and 0.980 (95% CI, 0.971–0.989), respectively. When the total score was ≥ 24 by two consecutive analysis, the biomarker chip results were defined as positive.
Data Collection and Statistical Analysis
Student t and chi-squared tests were used to compare continuous and categorical descriptive variables, respectively, between relapsed and non-relapsed patients. Univariate and multivariate logistic regression analyses were also used to examine the factors influencing the postoperative relapse. The cumulative disease-free survival (DFS) and overall survival (OS) rates were calculated using the Kaplan—Meier method, and the differences in the rates were analyzed using the log-rank test. Results are expressed as the mean with standard deviation or effect and 95% CI where appropriate. A P value of <0.05 denoted statistical significance. All data were analyzed using SPSS (version 19.0; SPSS, Inc., Chicago, IL, USA).
Results
Descriptive Data
The median follow-up time was 28.4 months (range, 3.0–61.3 months). Of the 298 patients, 168 were men (56.4%). The average age was 64.4 ± 11.3 years (range, 20–91 years). Regarding tumor histology, 42 (14.1%) were well-differentiated carcinomas, 244 (81.9%) were moderately differentiated, and 12 (4.0%) were poorly differentiated. Forty-eight (16.1%) patients had postoperative relapse and 26 (8.7%) died. Of the 298 CRC patients, 62 (20.8%) had a total biomarker chip score higher than the cutoff value. Of 48 relapsed patients, 42 (87.5%) showed positive biochip results prior to relapse. The positive biochip results were significantly associated with postoperative relapse (P < 0.001; Table 3). The clinicopathological characteristics of all 298 CRC patients are listed in Table 1. The raw data from the multigene biomarker chip may compromise the privacy of study participants and may not be shared publicly. Data are available upon request to the authors.
Table 3. Comparison between non-relapsed and relapsed colorectal cancer patients.
| Non-relapse N = 250 | Relapse N = 48 | P | |
|---|---|---|---|
| Gender (Male/Female) | 144/106 | 24/24 | 0.331 |
| Age (year) | 64.2±11.0 | 65.3±12.8 | 0.546 |
| Maximum tumor size≧5cm | 66 (26.4%) | 12 (25%) | 0.840 |
| Location (rectum/colon) | 58/192 | 19/29 | 0.018 |
| Depth of tumor invasion | 96/154 | 15/33 | 0.348 |
| T (1+2/3+4) | |||
| Lymph node metastasis | 157/93 | 27/21 | 0.392 |
| N (0/1+2) | |||
| Histology (WD+MD/PD) | 214/9 | 45/3 | 0.392 |
| TNM stage (I-II/III) | 157/93 | 27/21 | 0.392 |
| Vascular invasion | 68 (27.2%) | 18 (37.5%) | 0.149 |
| Perineural invasion | 45 (18.0%) | 16 (33.3%) | 0.016 |
| Abnormal preoperative CEA level | 72 (28.8%) | 26 (54.2%) | 0.001 |
| Abnormal postoperative CEA level | 42 (16.8%) | 29 (60.4%) | <0.001 |
| Positive biomarker chip | 20 (8.0%) | 42 (87.5%) | <0.001 |
| Mortality | 6 (1.6%) | 20 (29.5%) | <0.001 |
| Follow up (month) | |||
| Median, range | 29.6, 7.3–59.8 | 24.1, 3.0–61.3 | 0.058 |
| Mean ± SD | 29.5±9.5 | 26.6±10.6 | |
Univariate and Multivariate Analyses
During the follow-up period, 29 of 221 (13.1%) colon cancer patients and 19 of 77 (24.7%) rectal cancer patients showed postoperative relapse. In comparison with the patients without postoperative relapse, rectal neoplasms (P = 0.018), perineural invasion (P = 0.016), elevated preoperative serum CEA levels (P = 0.001), elevated postoperative serum CEA levels (P < 0.001), and positive biochip results (P < 0.001) were more frequently noted in the patients with relapse (Table 4). However, sex, age, tumor size, tumor invasion depth, lymph node metastasis, histology, TNM stage, vascular invasion, and follow-up duration did not differ significantly between the studied groups (all P > 0.05).
Table 4. Factors influencing the relapse estimated by univariate and multivariate logistic regression analyses.
| Univariate regression | Multivariate regression | |||
|---|---|---|---|---|
| Odds ratio (95% CI) | P | Odds ratio (95% CI) | P | |
| Maximum tumor size≧5cm | 0.929 (0.456, 1.893) | 0.840 | - | - |
| Location (rectum/colon) | 2.169 (1.134, 4.149) | 0.019 | 1.566 (0.564, 4.348) | 0.389 |
| Depth of tumor invasion | 1.371 (0.708, 2.657) | 0.349 | - | - |
| T (1+2/3+4) | ||||
| Lymph node metastasis | 1.313 (0.703, 2.454) | 0.393 | - | - |
| N (0/1+2) | ||||
| Histology (WD+MD/PD) | 1.785 (0.465, 6.851) | 0.398 | - | - |
| TNM stage (I+II/III) | 1.313 (0.703, 2.454) | 0.393 | - | - |
| Vascular invasion | 1.606 (0.841, 3.068) | 0.152 | - | - |
| Perineural invasion | 2.278 (1.152, 4.502) | 0.018 | 2.181 (0.716, 6.644) | 0.170 |
| Abnormal preoperative CEA level | 2.922 (1.556, 5.488) | 0.001 | 2.538 (0.885, 7.277) | 0.083 |
| Abnormal postoperative CEA level | 7.559 (3.880, 14.724) | <0.001 | 4.136 (1.455, 11.755) | 0.008 |
| Positive biomarker chip | 80.500 (30.523, 212.309) | <0.001 | 66.878 (23.229, 192.548) | <0.001 |
95% CI: 95% confidence interval
In the multivariate logistic regression analysis, elevated postoperative serum CEA levels (OR = 4.136, 95% CI: 1.455–11.755; P = 0.008) and positive biochip results (OR = 66.878, 95% CI: 23.229–192.548; P < 0.001) were revealed to be independent predictors for postoperative relapse (Table 5). However, tumor location (rectum or colon), perineural invasion, and preoperative CEA elevation did not differ significantly between the studied groups (all P > 0.05).
Table 5. Sensitivity, specificity, postitive predictive value, negative predictive value, and accuracy of postoperative serum CEA level and biomarker chip.
| Abnormal postoperative CEA level (95% CI) | Positive biomarker chip (95% CI) | P | |
|---|---|---|---|
| Sensitivity | 60.4% | 87.5% | 0.003 |
| (45.3%-74.2%) | (74.8%-95.3%) | ||
| Specificity | 83.2% | 92.0% | 0.003 |
| (78.0%-87.6%) | (87.9%-95.1%) | ||
| Positive predictive value | 40.8% | 67.7% | 0.002 |
| (29.3%-53.2%) | (54.7%-79.1%) | ||
| Negative predictive value | 91.6% | 97.5% | 0.006 |
| (87.2%-94.9%) | (94.6%-99.1%) | ||
| Accuracy | 79.5% | 91.3% | <0.001 |
| (74.9%-84.1%) | (88.1%-94.5%) |
95% CI: 95% confidence interval
Sensitivities, Specificities, and Accuracies of Postoperative Serum CEA levels and Biomarker Chip for Predicting Postoperative Relapse
Positive biochip results with elevated postoperative serum CEA levels for predicting postoperative relapse were thoroughly compared (Table 5). The biomarker chip demonstrated higher sensitivity (biochip: 87.5%, CEA: 60.4%; P = 0.003), specificity (biochip: 92.0%, CEA, 83.2%; P = 0.003), positive predictive value (biochip: 67.7%, CEA: 40.8%; P = 0.002), negative predictive value (biochip: 97.5%, CEA: 91.6%; P = 0.006), and accuracy (biochip: 91.3%, CEA: 79.5%; P < 0.001) than postoperative serum CEA levels did. Therefore, our multigene biomarker chip would be a more accurate tool for predicting postoperative relapse than postoperative serum CEA is.
In clinical practice, the two independent tests can be combined to be more confident of the diagnosis. The combined specificity becomes 1-(1–0.832)×(1–0.92) = 1–0.001344 = 0.998656 = 99.8656%. The combined sensitivity becomes = 0.604×0.875 = 0.5285 = 52.85%. The combined specificity of 99.87% allowed us to rule in the diagnosis: until proved otherwise, this patient had postoperative relapse.
The diagnostic/prognostic values of the biochip and postoperative CEA were evaluated according to different clinical features and shown in Table 6. The positive biochip results showed prominent association with rectal tumor (P = 0.009), perineural invasion (P = 0.010), postoperative relapse (P < 0.001) and mortality (P < 0.001).
Table 6. Clinical features associated with diagnostic/prognostic values of postoperative CEA and the biochip.
| Postoperative CEA (+/-) | Biochip (+/-) | P | |
|---|---|---|---|
| Maximum tumor size | 0.690 | ||
| ≧5cm | 20/58 | 15/63 | |
| <5cm | 51/169 | 47/173 | |
| Location | 0.009 | ||
| Rectum | 22/55 | 24/53 | |
| Colon | 49/172 | 38/183 | |
| Depth of tumor invasion | 0.361 | ||
| T (1+2) | 23/88 | 20/91 | |
| T (3+4) | 48/139 | 42/145 | |
| Lymph node metastasis | 0.934 | ||
| N (0) | 42/142 | 38/146 | |
| N (1+2) | 29/85 | 24/90 | |
| Histology | 0.069 | ||
| WD+MD | 68/218 | 57/229 | |
| PD | 3/9 | 5/7 | |
| TNM stage | 0.934 | ||
| I+II | 42/142 | 38/146 | |
| III | 29/85 | 24/90 | |
| Vascular invasion | 0.328 | ||
| Positive | 24/62 | 21/65 | |
| Negative | 47/165 | 41/171 | |
| Perineural invasion | 0.010 | ||
| Positive | 12/49 | 20/41 | |
| Negative | 59/178 | 42/195 | |
| Postoperative relapse | <0.001 | ||
| Yes | 29/19 | 42/6 | |
| No | 42/208 | 20/230 | |
| Mortality | <0.001 | ||
| Yes | 15/11 | 19/7 | |
| No | 56/216 | 43/229 |
Multigene Biomarker Chip versus Postoperative Serum CEA Levels for Predicting Postoperative Relapse and Clinical Outcomes
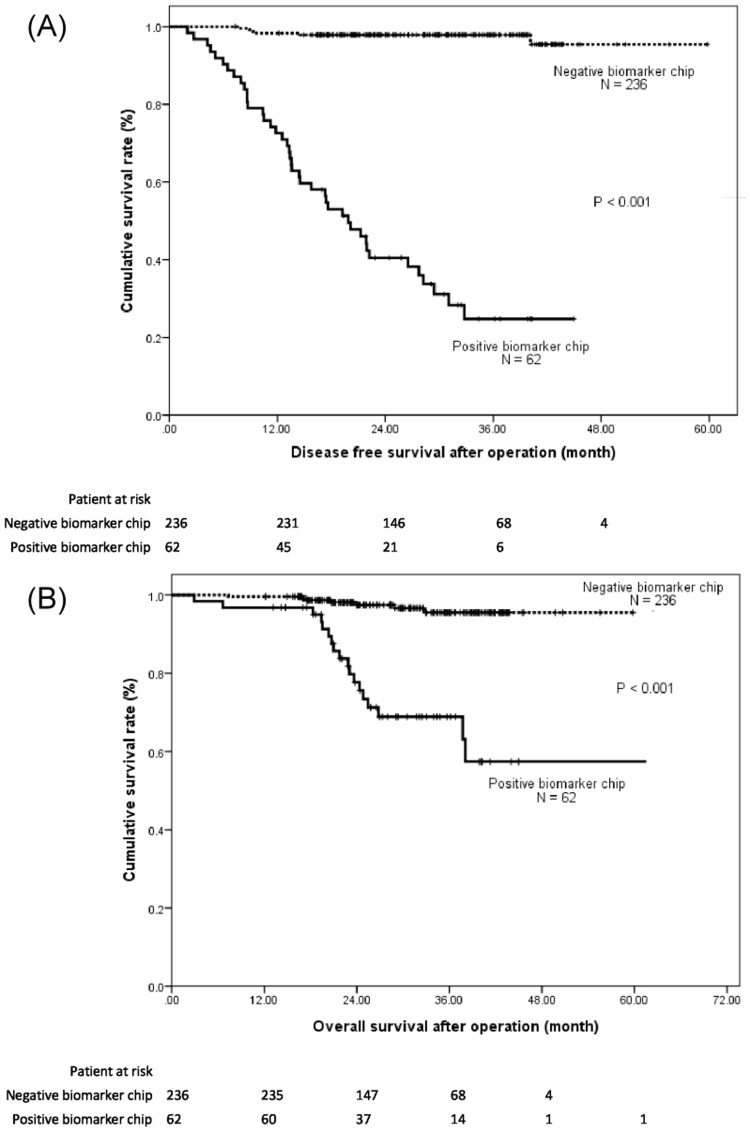
In postoperative surveillance, both multigene biomarker chip analysis and CEA assays were performed at each follow-up. Of the 48 relapsed patients, 42 (87.5%) showed positive biochip results and a median lead time from detection of 10.7 months (11.0 ± 7.3 months; Table 7). However, only 29 (60.4%) relapsed patients had elevated postoperative serum CEA levels, and the median lead time was 2.8 months (3.4 ± 2.8 months). The median lead time between the positive biochip results and subsequent postoperative relapse detection was considerably earlier than that between the elevated postoperative serum CEA levels and postoperative relapse detection (P < 0.001). The median DFS rate was significantly lower among patients with positive biochip results than among patients with negative biochip results (20.4 vs. 48.0 months, P < 0.001; Fig 2A). The cumulative DFS rate at the end of the study was 95% and 26% for the patients with negative and positive biochip results, respectively. Similarly, the median OS rate was significantly lower among the patients with positive biochip results than among those with negative biochip results (P < 0.001; Fig 2B). The cumulative proportion OS rate at 48 months was 96% and 51% for those with negative and positive biochip results, respectively.
Table 7. Comparison of the expression prior to the diagnosis in 48 relapsed patients.
| Postoperative CEA level | Biomarker chip | P | |
|---|---|---|---|
| Positive result N (%) | 29 (60.4) | 42 (87.5) | 0.003 |
| Lead-time (month) | <0.001 | ||
| Median (Range) | 2.8 (0.5–11.0) | 10.7 (0.5–30.7) | |
| Mean ± SD | 3.4 ± 2.8 | 11.0 ± 7.3 |
Fig 2. (A) Cumulative disease-free survival (DFS) and (B) overall survival (OS) rates of the 298 colorectal cancer patients calculated using the Kaplan—Meier method.
Positive biochip results correlated strongly with lower DFS and OS rates of colorectal cancer patients (both P < 0.001).
Discussion
Postoperative surveillance of CRC patients facilitates early diagnosis of disease relapse, which may then be surgically or medically treated. Of the numerous reported follow-up strategies, serial CEA monitoring is the most sensitive for detecting recurrence compared with history-taking, physical examination, liver function tests, abdominal sonography, chest radiography, and colonofiberoscopy; it remains one of the most crucial postoperative work-ups [23–25]. In the literature, the estimated sensitivity of serum CEA for detecting relapsed disease in patients with completely resected CRC is 58%–89%, with a 1.5–6.0-month lead time between serum CEA level elevation and recurrence detection [26–29]. In the present study, the sensitivity and specificity of elevated serum CEA level detection were 60.4% and 83.2%, with a median lead time of 2.8 months between serum CEA level and relapse detection, consistent with the previous reports. However, the sensitivity and specificity of relapse detection depends largely on the definition of elevated serum CEA levels (cutoff value). The higher the cutoff value for the elevated serum CEA levels is, the higher (lower) the specificity (sensitivity) would be [27, 30].
Elevated preoperative serum CEA levels indicate a poor prognosis and are correlated with a reduced OS after surgical resection [27, 31–32]. In the present study, the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of elevated preoperative serum CEA levels for predicting postoperative relapse were 54.1%, 71.2%, 26.5%, 89.0%, and 68.5%, respectively. In addition, the elevated preoperative serum CEA levels were more frequent in patients with relapse than in those without relapse (P = 0.001). However, in the multivariate analysis, the postoperative relapse was not associated with elevated preoperative serum CEA levels (P = 0.083). Of all clinicopathological features, well known factors, such as depth of invasion and lymph node metastasis, did not affect the recurrence rate in univariate analysis, and the relatively short follow-up period may in part be responsible for it. The elevated postoperative serum CEA levels and positive biochip results were the only two independent predictors of postoperative relapse (P = 0.008 and P < 0.001, respectively). Of the two independent predictors of relapse, our multigene biomarker biochip was more accurate than postoperative serum CEA levels (91.3% vs. 79.5%, P < 0.001). Moreover, the median lead time between positive biochip results and relapse detection was 10.7 months, considerably earlier than that between elevated postoperative serum CEA levels and relapse detection (2.8 months, P < 0.001). The detection of overexpressed molecular biomarkers on the biochip may facilitate earlier detection of relapsed disease and enable physicians to select early therapeutic strategies.
A weighted enzymatic chip array (WEnCA) platform is a sensitive technique for detecting activated KRAS from the peripheral blood in patients with various malignancies [18,21,22]. The selection of the target gene and modification of the weighted values for the corresponding genes contribute the most to the accuracy in clinical applications. To reduce the false-negative detection CTCs when predicting postoperative relapse, cDNA of multiple biomarkers from the peripheral blood were analyzed at each follow-up; the biomarker chips of the CRC patients with subsequent relapse showed that most of the gene spots expressed more prominently with time.
In the present study, the specificity (92.0%) and accuracy (91.3%), but not the sensitivity (87.5%), of the biomarker chip were similar to those reported in previous studies that used the WEnCA platform [18–22]. The false-positive rate of the biomarker chip in early prediction of postoperative relapse was 32.3%; nevertheless, postoperative serum CEA levels showed a higher false-positive rate (59.2%). These rates may have been low because of using unadjusted weighted value and limited follow-up period. Therefore, to obtain an acceptable low false-positive rate, individual weighted values of each gene should be further investigated on the WEnCA platform.
Of the 242 patients with negative biochip results, 1 and 4 stage II and III patients relapsed during the follow-up period, respectively. In the biomarker chip of all 5 patients, some gene spots indicating metastatic potential lacked overexpression. Such negative biochip results may be attributable to the histological dedifferentiation or heterogeneity of relapsed tumor or the relapse caused by provocative agents in the environment [33].
In the present study, 9, 18, and 21 stage I, II, and III patients demonstrated postoperative relapse (P = 0.328) and a median DFS of 21.3, 12.8, and 9.3 months (P = 0.081), respectively. The positive predictive value of the biomarker biochip was more favourable than that of postoperative serum CEA levels (67.7% vs. 40.8%, P = 0.002). However, of the 62 patients with a positive biomarker biochip result, 5, 7, and 8 stage I, II, and III patients remained relapse-free until the end of the follow-up period, respectively.
In the present study, we confirmed that our constructed multigene biomarker chip is feasible for the accurate early prediction of postoperative relapse in stage I–III CRC patients. The biomarker chip may be used periodically in clinical practice for postoperative surveillance to improve early detection. However, a multicenter trial for CRC patients with a longer follow-up duration is required in order to confirm the long-term effectiveness.
Acknowledgments
Conflicts of Interest: All authors have read the journal’s policy on disclosure of potential conflicts of interest and have none to declare.
Data Availability
Data may compromise the privacy of study participants and may not be shared publicly. Data are available upon request to the authors.
Funding Statement
This work was supported by Ministry of Science and Technology, Taiwan MOST1042325B037001 Prof. JawYuan Wang, Ministry of Health and Welfare MOHW105TDUB212134007 Prof. JawYuan Wang, Kaohsiung Medical University Hospital KMUH102-2M26, KMUH104-4R19, KMUHS10522, KMUHS10505 Prof. JawYuan Wang, Kaohsiung Medical University KMU-TP105C00, KMU-TP105C01, KMU-TP105C02, KMU-TP106005, D08-00005-10401, KMU-TP105A12 Prof. JawYuan Wang.
References
- 1.Labianca R, Nordlinger B, Beretta GD, Brouquet A, Cervantes A, On behalf of the ESMO Guidelines Working Group. Primary colon cancer: ESMO Clinical Practice Guidelines for diagnosis, adjuvant treatment and follow-up. Ann Oncol 2010;21 Suppl 5:v70–v77. 10.1093/annonc/mdq168 [DOI] [PubMed] [Google Scholar]
- 2.Scheer A, Auer RA. Surveillance after Curative Resection of Colorectal Cancer. Clin Colon Rectal Surg 2009;22:242–250. 10.1055/s-0029-1242464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gold P, Freedman SO. Specific carcinoembryonic antigens of the human digestive system. J Exp Med 1965;122:467–481. 10.1084/jem.122.3.467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desch CE, Benson AB 3rd, Somerfield MR, Flynn PJ, Krause C, Loprinzi CL, et al. Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol 2005;23:8512–8519. 10.1200/JCO.2005.04.0063 [DOI] [PubMed] [Google Scholar]
- 5.Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Tangen C. An evaluation of the carcinoembryonic antigen (CEA) test for monitoring patients with resected colon cancer. JAMA 1993;270:943–947. 10.1001/jama.1993.03510080047030 [DOI] [PubMed] [Google Scholar]
- 6.Uen YH, Lin SR, Wu DC, Su YC, Wu JY, Cheng TL, et al. Prognostic significance of multiple molecular markers for patients with stage II colorectal cancer undergoing curative resection. Ann Surg 2007;246:1040–1046. 10.1097/SLA.0b013e318142d918 [DOI] [PubMed] [Google Scholar]
- 7.Weitz J, Kienle P, Lacroix J, Willeke F, Benner A, Lehnert T, et al. Dissemination of tumor cells in patients undergoing surgery for colorectal cancer. Clin Cancer Res 1998;4:343–348. [PubMed] [Google Scholar]
- 8.Wharton RQ, Jonas SK, Glover C, Khan ZA, Klokouzas A, Quinn H, et al. Increased detection of circulating tumor cells in the blood of colorectal carcinoma patients using two reverse transcription-PCR assays and multiple blood samples. Clin Cancer Res 1999;5:4158–4163. [PubMed] [Google Scholar]
- 9.Wong IH, Yeo W, Chan AT, Johnson PJ. Quantitative relationship of the circulating tumor burden assessed by reverse transcription-polymerase chain reaction for cytokeratin 19 mRNA in peripheral blood of colorectal cancer patients with Dukes' stage, serum carcinoembryonic antigen level and tumor progression. Cancer Lett 2001;162:65–73. 10.1016/S0304-3835(00)00630-3 [DOI] [PubMed] [Google Scholar]
- 10.Wang JY, Wu CH, Lu CY, Hsieh JS, Wu DC, Huang SY, et al. Molecular detection of circulating tumor cells in the peripheral blood of patients with colorectal cancer using RT-PCR: significance in the prediction of postoperative metastasis. World J Surg 2006;30:1007–1013. 10.1007/s00268-005-0485-z [DOI] [PubMed] [Google Scholar]
- 11.Wang JY, Lin SR, Wu DC, Lu CY, Yu FJ, Hsieh JS, et al. Multiple molecular markers as predictors of colorectal cancer in patients with normal perioperative serum carcinoembryonic antigen levels. Clin Cancer Res 2007;13:2406–2413. 10.1158/1078-0432.CCR-06-2054 [DOI] [PubMed] [Google Scholar]
- 12.Lu CY, Tsai HL, Uen YH, Hu HM, Chen CW, Cheng TL, et al. Circulating tumor cells as a surrogate marker for determining clinical outcome to mFOLFOX chemotherapy in patients with stage III colon cancer. Br J Cancer 2013;108:791–797. 10.1038/bjc.2012.595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen YF, Wang JY, Wu CH, Chen FM, Cheng TL, Lin SR. Detecting circulating cancer cells with K-ras oncogene using membrane array. Cancer Lett 2005;229:115–122. 10.1016/j.canlet.2004.12.026 [DOI] [PubMed] [Google Scholar]
- 14.Wang JY, Yeh CS, Chen YF, Wu CH, Hsieh JS, Huang TJ, et al. Development and evaluation of a colorimetric membrane-array method for the detection of circulating tumor cells in the peripheral blood of Taiwanese patients with colorectal cancer. Int J Mol Med 2006;17:737–747. 10.3892/ijmm.17.5.737 [DOI] [PubMed] [Google Scholar]
- 15.Yeh CS, Wang JY, Wu CH, Chong IW, Chung FY, Wang YH, et al. Molecular detection of circulating cancer cells in the peripheral blood of patients with colorectal cancer by using membrane array with a multiple mRNA marker panel. Int J Oncol 2006;28:411–420. 10.3892/ijo.28.2.411 [DOI] [PubMed] [Google Scholar]
- 16.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual, 7th ed New York: Springer; 2010. [Google Scholar]
- 17.Wang JY, Yeh CS, Tzou WS, Hsieh JS, Chen FM, Lu CY, et al. Analysis of progressively overexpressed genes in tumorigenesis of colorectal cancers using cDNA microarray. Oncol Rep. 2005;14:65–72. [PubMed] [Google Scholar]
- 18.Huang MY, Liu HC, Yen LC, Chang JY, Huang JJ, Wang JY, et al. Detection of activated KRAS from cancer patient peripheral blood using a weighted enzymatic chip array. J Transl Med 2014;12:147 10.1186/1479-5876-12-147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsao DA, Yang MJ, Chang HJ, Yen LC, Chiu HH, Hsueh EJ, et al. A fast and convenient new technique to detect the therapeutic target, K-ras mutant, from peripheral blood in non-small cell lung cancer patients. Lung Cancer 2010;68:51–57. 10.1016/j.lungcan.2009.05.023 [DOI] [PubMed] [Google Scholar]
- 20.Yang MJ, Chiu HH, Wang HM, Yen LC, Tsao DA, Hsiao CP, et al. Enhancing detection of circulating tumor cells with activating KRAS oncogene in patients with colorectal cancer by weighted chemiluminescent membrane array method. Ann Surg Oncol 2010;17:624–633. 10.1245/s10434-009-0831-8 [DOI] [PubMed] [Google Scholar]
- 21.Hsiung SK, Chang HJ, Yang MJ, Chang MS, Tsao DA, Chiu HH, et al. A novel technique for detecting the therapeutic target, KRAS mutant, from peripheral blood using the automatic chipball device with weighted enzymatic chip array. Fooyin J Health Sci 2009;1:72–80. 10.1016/S1877-8607(10)60003-3 [DOI] [Google Scholar]
- 22.Hsiung SK, Lin SR, Chang HJ, Chen YF, Huang MY. Clinical application of automatic gene chip analyzer wenca chipball for mutant Kras detection in peripheral circulating cancer cells of cancer patients In: Komorowska MA, Olsztynska-Janus S, editors. Biomedical Engineering, Trends, Research and Technologies. Croatia: InTech; 2011. pp. 151–168 10.5772/12940 [DOI] [Google Scholar]
- 23.Graham RA, Wang S, Catalano PJ, Haller DG. Postsurgical surveillance of colon cancer: preliminary cost analysis of physician examination, carcinoembryonic antigen testing, chest x-ray, and colonoscopy. Ann Surg 1998;228:59–63. 10.1097/00000658-199807000-00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rocklin MS, Senagore AJ, Talbott TM. Role of carcinoembryonic antigen and liver function tests in the detection of recurrent colorectal carcinoma. Dis Colon Rectum 1991;34:794–797. 10.1007/BF02051073 [DOI] [PubMed] [Google Scholar]
- 25.De Salvo L, Razzetta F, Arezzo A, Tassone U, Bogliolo G, Bruzzone D, et al. Surveillance after colorectal cancer surgery. Eur J Surg Oncol 1997;23:522–525. 10.1016/S0748-7983(97)93045-6 [DOI] [PubMed] [Google Scholar]
- 26.Minton JP, Hoehn JL, Gerber DM, Horsley JS, Connolly DP, Salwan F, et al. Results of a 400-patient carcinoembryonic antigen second-look colorectal cancer study. Cancer 1985;55:1284–1290. [DOI] [PubMed] [Google Scholar]
- 27.McCall JL, Black RB, Rich CA, Harvey JR, Baker RA, Watts JM, et al. The value of serum carcinoembryonic antigen in predicting recurrent disease following curative resection of colorectal cancer. Dis Colon Rectum 1994;37:875–881. 10.1007/BF02052591 [DOI] [PubMed] [Google Scholar]
- 28.Hine KR, Dykes PW. Serum CEA testing in the post-operative surveillance of colorectal carcinoma. Br J Cancer 1984;49:689–693. 10.1038/bjc.1984.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin EW Jr, Cooperman M, Carey LC, Minton JP. Sixty second-look procedures indicated primarily by rise in serial carcinoembryonic antigen. J Surg Res 1980;28:389–394. 10.1016/0022-4804(80)90100-6 [DOI] [PubMed] [Google Scholar]
- 30.Wanebo HJ, Llaneras M, Martin T, Kaiser D. Prospective monitoring trial for carcinoma of colon and rectum after surgical resection. Surg Gynecol Obstet 1989;169:479–487. [PubMed] [Google Scholar]
- 31.Slentz K, Senagore A, Hibbert J, Mazier WP, Talbott TM. Can preoperative and postoperative CEA predict survival after colon cancer resection? Am Surg 1994;60:528–531. [PubMed] [Google Scholar]
- 32.Wiratkapun S, Kraemer M, Seow-Choen F, Ho YH, Eu KW. High preoperative serum carcinoembryonic antigen predicts metastatic recurrence in potentially curative colonic cancer: results of a five-year study. Dis Colon Rectum 2001;44:231–235. 10.1007/BF02234298 [DOI] [PubMed] [Google Scholar]
- 33.Seyfried TN, Shelton LM. Cancer as a metabolic disease. Nutri Metab 2010;7:7 10.1186/1743-7075-7-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data may compromise the privacy of study participants and may not be shared publicly. Data are available upon request to the authors.