Abstract
Background
We have constructed and clinically evaluated a hypoallergenic vaccine for grass pollen allergy, BM32, which is based on fusion proteins consisting of peptides from the IgE binding sites of the major grass pollen allergens fused to preS (preS1 + preS2), a domain of the hepatitis B virus (HBV) large envelope protein which mediates the viral attachment and entry. Aim of this study was the characterization of the HBV-specific immune response induced by vaccination of allergic patients with BM32 and the investigation of the vaccines' potential to protect against infection with HBV.
Methods
Hepatitis B-specific antibody and T cell responses of patients vaccinated with BM32 were studied using recombinant preS and synthetic overlapping peptides spanning the preS sequence. The specificities of the antibody responses were compared with those of patients with chronic HBV infection. Furthermore, the capacity of BM32-induced antibodies, to inhibit HBV infection was investigated using HepG2-hNTCP cell-based in vitro virus neutralization assays.
Findings
IgG antibodies from BM32-vaccinated but not of HBV-infected individuals recognized the sequence motif implicated in NTCP (sodium-taurocholate co-transporting polypeptide)-receptor interaction of the hepatitis B virus and inhibited HBV infection.
Interpretation
Our study demonstrates that the recombinant hypoallergenic grass pollen allergy vaccine BM32 induces hepatitis B-specific immune responses which protect against hepatitis B virus infection in vitro.
Abbreviations: HBV, hepatitis B virus; HBcAg, hepatitis B virus core antigen; HBeAg, hepatitis B virus e antigen; HBsAg, hepatitis B virus surface antigen; NTCP, sodium-taurocholate co-transporting polypeptide; AIT, allergen-specific immunotherapy
Keywords: Allergy, B cell epitope-based allergy vaccine, Hepatitis B, Hepatitis B surface protein, preS, Virus neutralization
Highlights
-
•
BM32 is a recombinant allergy vaccine consisting of the preS domain of the large envelope protein of hepatitis B virus (HBV) and allergen-derived peptides.
-
•
Vaccination of allergic patients with BM32 induced preS-specific antibodies which inhibit hepatitis B infection in vitro.
-
•
BM32 may be useful as therapeutic vaccine in HBV-infected patients.
Infection with HBV remains a major cause of morbidity and mortality worldwide. Conventional HBV vaccines, consisting of SHBs particles solely, do not elicit adequate antibody production in 5–10% of vaccines and there is a need for therapeutic HBV vaccines. We have engineered an allergy vaccine which consists of allergen-derived peptides fused to the preS domain of the large envelope protein of HBV. Here we show that vaccination of allergic patients with this vaccine induces antibodies which protect against HBV infection in vitro. The preS-containing allergy vaccine may thus be also useful for therapeutic vaccination of HBV-infected patients.
1. Introduction
Allergy affects > 25% of the population. There are several forms of intervention to manage allergic disease. They include allergen avoidance, symptomatic pharmacotherapy, biologics and allergen-specific immunotherapy (AIT) but only AIT has disease-modifying and long-lasting effects (Durham et al., 1999, Larché et al., 2006, Jacobsen et al., 2007). In order to improve safety, efficacy, convenience and patients' compliance, hypoallergenic allergy vaccines based on recombinant allergen derivatives or synthetic allergen-derived peptides have been developed and advanced into clinical trials (Valenta, 2002, Jutel and Akdis, 2014, Marth et al., 2014, Sandrini et al., 2015).
The concept of B cell epitope-based allergy vaccines is based on recombinant fusion proteins which consist of per se non-allergenic peptides from the IgE binding sites of major allergens and an allergen unrelated carrier protein which provides T cell help without activating pro-inflammatory allergen-specific T cell responses (Focke et al., 2010, Focke-Tejkl and Valenta, 2012). As candidates for the allergen-unrelated carrier proteins, viral proteins from rhinovirus and hepatitis B virus have been considered (Edlmayr et al., 2011).
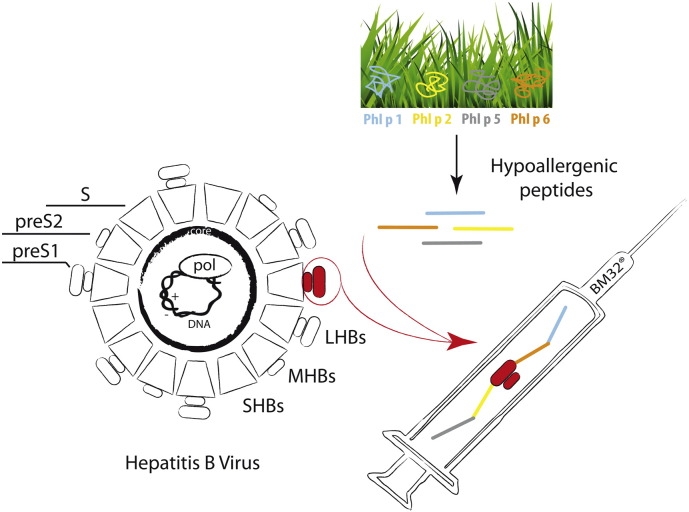
We have recently developed such a hypoallergenic vaccine for grass pollen allergy, BM32, which is based on fusion proteins consisting of non-allergenic peptides from the IgE binding sites of the four major grass pollen allergens, Phl p 1, Phl p 2, Phl p 5 and Phl p 6, fused to the hepatitis B virus-derived surface protein preS (preS1 + preS2), (Focke-Tejkl et al., 2015) (Fig. 1). The protein preS was selected as a carrier in BM32 because it has been used in HBV vaccines and was found to be safe (Rendi-Wagner et al., 2006) and preS-fusion proteins could be produced under Good Manufacturing Practice (GMP) conditions in a quality suitable for clinical trials. Due to lack of conformation the allergen-derived peptides are non-allergenic and the recombinant fusion proteins accordingly lacked IgE reactivity when tested with sera from grass pollen allergic patients and showed almost no induction of basophil activation. Furthermore, upon immunization of animals they induced IgG antibodies which recognized the natural grass pollen allergens and inhibited allergic patients' IgE binding and allergen-induced basophil activation (Focke-Tejkl et al., 2015). Skin testing of grass pollen allergic patients with the recombinant preS fusion proteins showed that they did not induce any relevant immediate or late phase skin reactions (Niederberger et al., 2015). Vaccination of grass pollen allergic patients with aluminium hydroxide-adsorbed preS-based fusion proteins revealed that the BM32 vaccine induced allergen-specific IgG responses and protected patients from grass pollen-induced rhinitis as determined in a pollen challenge chamber (ClinicalTrials.gov number: NCT01445002). Since it has been shown that preS1-specific antibodies neutralized HBV infectivity (Neurath et al., 1986, Glebe et al., 2003) we aimed to evaluate whether the preS-based grass pollen allergy vaccine BM32 could also induce HBV-specific immune responses. Using synthetic peptides spanning the preS-sequence, we mapped preS-specific antibody and T cell responses and compared the antibody responses of BM32-treated subjects with that of individuals, suffering from chronic HBV infection to explore the possible use of BM32 for therapeutic vaccination in the latter group. Furthermore we investigated the potential of BM32-induced antibodies to protect against HBV infection using an in vitro assay based on HBV receptor (sodium-taurocholate co-transporting polypeptide, NTCP) expressing HepG2 cell lines.
Fig. 1.
Scheme for the construction of the BM32 vaccine. BM32 contains fusion proteins consisting of the preS domain (i.e., preS1 and preS2) of the large hepatitis B virus envelope protein (LHB) fused with allergen-derived peptides. LHB: Large hepatitis B virus envelope protein; MHB: Middle hepatitis B virus envelope protein; SHB: Small hepatitis B virus envelope protein.
2. Material & Methods
2.1. Expression and Purification of Recombinant PreS, Synthesis of PreS Overlapping Peptides, Sequence Alignments
The procedure of the expression and purification of a hexahistidine-tagged recombinant preS protein (preS1 + preS2; genotype A; subtype adw2, GenBank: AAT28735.1) in Escherichia coli BL21 (DE3, Stratagene, La Jolla, CA) is described elsewhere (Niespodziana et al., 2011).
Eight peptides at a length of approximately 30 amino acids and an overlap of 10 amino acids spanning the complete sequence of preS (genotype A, subtype adw2; Supplemental Fig. 1) were synthesized by a Fmoc (9-fluorenylmethoxycarbonyl) - strategy with HBTU [2-(1H-Benzotriazol-1-yl)1,1,3,3 tetramethyluronium hexafluorophosphat] activation (Liberty Microwave Peptide Synthesis, CEM Corporation, Matthews, NC) as described previously (Focke et al., 2001). Peptides were purified by preparative HPLC and their identity was confirmed by mass spectrometry (Microflex MALDI-TOF, Bruker, Billerica, MA).
An alignment of the preS genotype A, serotype adw2 sequence and peptide sequences thereof with HBV genotypes B–H was performed with CLUSTAL Ω using reference sequences from the HBV data base (HBVdb: https://hbvdb.ibcp.fr/HBVdb/HBVdbIndex),(Hayer et al., 2012).
2.2. Immunization of Rabbits and Mice
Specific rabbit antibodies against recombinant preS were raised by immunization of a New Zealand white rabbit with purified preS (200 μg per injection) using Freund's complete adjuvant (CFA) for the first and incomplete Freund's adjuvant (IFA) for the second and third injection (Charles River, Kisslegg, Germany). In addition, New Zealand white rabbits were immunized three times with a mix containing 20 μg (n = 2) or 40 μg (n = 2) of each of the four BM32 components (BM32–20/BM32–40) using Al(OH)3 as adjuvant (Focke-Tejkl et al., 2015).
Furthermore, rabbit antibodies specific for the registered HBV vaccine ENGERIX-B were obtained by immunizing New Zealand white rabbits (n = 2) three-times with commercially available ready-to-use pre-filled syringes (20 μg HBsAg/ml) at an interval of one month.
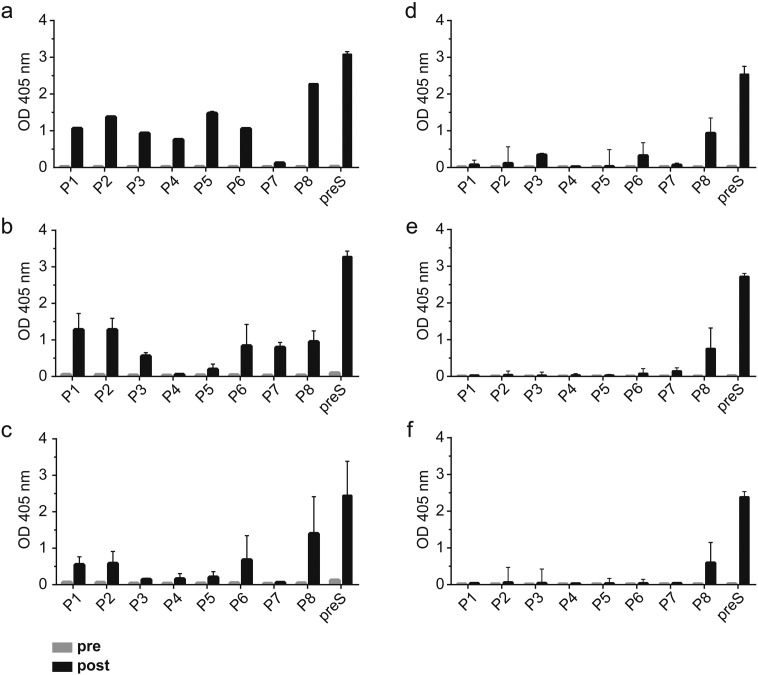
With approval of the animal ethics committee, six-week-old BALB/c mice (Charles River) (six animals/group) were immunized three times in a four weeks interval subcutaneously with Al(OH)3-adsorbed mixes containing 10, 20 and 30 μg of each of the BM32 components. Animals were kept in the animal care unit of the Department of Pathophysiology and Allergy Research, Medical University of Vienna according to the local guideline for animal care. Serum samples were obtained before immunization and approximately four weeks after the third immunization and stored at − 20 °C until analysis for preS-specific antibodies (Fig. 2).
Fig. 2.
IgG responses towards preS and synthetic preS-derived overlapping peptides. Optical density values (y-axes: OD values at 405 nm) corresponding to IgG levels of rabbits immunized with (a) preS (n = 1), (b) 20 μg of BM32 (n = 2), (c) 40 μg of BM32 (n = 2) or to IgG1 levels of groups of mice (n = 6) immunized with (d) 10 μg, (e) 20 μg or (f) 30 μg of BM32 prior to (grey bars) and after (black bars) immunization, towards preS and synthetic preS overlapping peptides P1–P8 (x-axes). Results represent medians ± interquartile ranges from triplicate determinations.
2.3. Human Subjects
Serum samples from BM32 immunized subjects were obtained in the course of two clinical studies. One set of serum samples was obtained in the course of a safety and dose-finding phase IIa study (ClinicalTrials.gov number: NCT01445002) during which patients received three injections of Al(OH)3-adsorbed BM32 (i.e., mixes of 10, 20 or 40 μg of each BM32 component or placebo, i.e., Al(OH)3). Sera were collected before and four weeks after the third immunization and stored at − 20 °C until usage. This study was approved by the Clinical Pharmacology Ethics Committee of the city of Vienna. A second set of serum samples was obtained in the course of a phase IIb study (ClinicalTrials.gov number: NCT01538979) during which patients were treated over a period of two years with seven subcutaneous injections of Al(OH)3-adsorbed BM32 (mixes of 20 or 40 μg of each BM32 component) or Al(OH)3 as placebo. An overview of the latter study is provided in Fig. 3. Supplemental Table 1 summarizes the characteristics of the patients (n = 30). This study was approved by the Ethics committee of the Medical University of Vienna, Austria (EK2092/2012) and all procedures were performed in accordance with the Declaration of Helsinki. Informed consent was obtained from all subjects included in the study.
Fig. 3.
Overview of the treatment period of the BM32 trial. (a) Subjects received seven injections of placebo or BM32 over two years as depicted in the time line. Visits during which blood samples were obtained are indicated. (b) IgG responses of subjects vaccinated with BM32 or placebo towards preS and synthetic preS-derived overlapping peptides. Shown are optical density values (y-axes: OD values, means of triplicate determination) corresponding to IgG levels towards preS and peptides P1–P8 measured in subjects with (red symbols) or without (black symbols) prior HBV vaccination who had been immunized with BM32 or placebo before (V5) and at different time points after immunization (V8 and V15) (x-axes). Medians (horizontal bars) and significant differences are indicated: *P < 0.05, **P < 0.01, ***P < 0.001.
In addition, serum samples were obtained from individuals suffering from chronic HBV infection which was diagnosed based on clinical data, liver function testing and HBV serum markers (Supplemental Table 2) at the Division of Gastroenterology and Hepatology of the Medical University of Vienna.
All sera analyzed, were screened for serological markers for HBV (i.e., HBV surface antigen [HBsAg]; antibodies to the HBV surface antigen [anti-HBs] as well as antibodies to the HBV core antigen [anti-HBc] at the Department of Virology, Medical University of Vienna).
2.4. Assessment of PreS- and PreS Peptide-specific Humoral Immune Responses
ELISA plates (Nunc, Maxisorb, Roskilde, Denmark) were coated with the antigens (recombinant preS, synthetic preS-overlapping peptides: P1–P8) or human serum albumin (negative control) (Behring, King of Prussia, PA), blocked and washed as described elsewhere (Gallerano et al., 2015). Incubation was performed with rabbit sera in a dilution of 1:10,000 (CFA) or 1:500 (BM32–20/BM32–40), with mouse sera in a dilution of 1:1000 and with human sera diluted differently for the isotypes and IgG subclasses. For the detection of human total IgG, sera were diluted 1:100, for IgA, IgG1, IgG2, IgG3, IgG4 as well as IgM, sera were diluted 1:20 and for detection of IgE antibodies sera were diluted 1:10.
Rabbit IgG was detected by donkey anti-rabbit horse radish peroxidase-conjugated IgG antibodies, diluted 1:2,500 (GE Healthcare Cat# NA934-1 ml RRID:AB_772206). Bound mouse IgG1 was detected by monoclonal rat anti-mouse IgG1 (BD Biosciences Cat# 553440 RRID:AB_394860) diluted 1:1000, followed by horse radish peroxidase-conjugated goat anti-rat IgG antibodies (GE Healthcare Cat# NA935 RRID:AB_772207), diluted 1:2,500.
Human IgG was detected by rabbit anti-human IgG Fc-specific antibody (Jackson ImmunoResearch Labs Cat# 309-005-008 RRID:AB_2339626) diluted 1:10,000, followed by peroxidase-linked donkey anti-rabbit IgG (GE Healthcare Cat# NA934-1 ml RRID:AB_772206) at a dilution of 1:2500. Human IgA, IgG subclasses IgG1, IgG2 and IgG4 as well as human IgM were detected by purified mouse anti-human IgA1/IgA2 (BD Biosciences Cat# 555886 RRID:AB_396198), IgG1 (BD Biosciences Cat# 555868 RRID:AB_396186), IgG2 (BD Biosciences Cat# 555873 RRID:AB_396189), IgG4 (BD Biosciences Cat# 555878 RRID:AB_396191) and IgM (BD Biosciences Cat# 555856 RRID:AB_396178) antibodies, diluted 1:1000 respectively, followed by peroxidase-linked sheep anti mouse IgG (GE Healthcare Cat# NA931-1 ml RRID:AB_772210) at a dilution of 1:2500. Monoclonal anti-human IgG3 (Sigma-Aldrich Cat# B3523 RRID:AB_258549) was diluted 1:5000. Human IgE was detected by goat anti-human horse radish peroxidase-conjugated IgE antibodies (KPL Cat# 074-1004 RRID:AB_2616558).
2.5. Assessment of T Cell Responses
Peripheral blood mononuclear cells (PBMC) were obtained from heparinized blood samples of patients from the phase IIb study (NCT01538979) through density gradient centrifugation using Ficoll (Amersham Biosciences, Uppsala, Sweden). PreS-specific PBMC proliferation was determined for BM32-vaccinated subjects when blood samples could be obtained (n = 19) at visits 5, 8, M1 and M2 (Fig. 3) by 3H-thymidine incorporation (Focke-Tejkl et al., 2014).
For certain BM32-immunized patients (n = 11), CD4 and CD8 T cell responses could be assessed at visit M2 by carboxyfluorescein succinimidyl ester (CFSE) labelling (Quah et al., 2007).
Fluorescent dye-labeled cells were seeded at 200,000 cells/well in Ultra culture™ serum-free medium (Lonza, Verviers, Belgium) supplemented with 2 mmol/L l-glutamine (Sigma Aldrich, St. Louis, MO), 50 mmol/L β-mercaptoethanol (Sigma Aldrich), and 0.02 mg of gentamicin per milliliter (Sigma Aldrich), in a total volume of 200 μl in 96 well microplates with U shaped bottom (Thermo Fisher, Waltham, MA). Cells were either left unstimulated (negative control) or were stimulated with Dynabeads® Human T-Activator CD3/CD28 (3 μg/well (Invitrogen, Carlsbad, CA)) as positive control or with preS (0.15 μg/well), equimolar quantities of preS-overlapping peptides (0.03 μg/well) or with a mixture of the preS-derived overlapping peptides containing 0.03 μg/well of each peptide and cultured at 37 °C in 5% CO2 for seven days before antibody staining and FACS analysis was conducted.
For flow cytometry the following reagents were used: PerCP/Cy5.5 anti-human CD3 antibody (Clone HIT3a; BioLegend Cat# 300328 RRID:AB_1575008), Brilliant Violet 421™ anti-human CD4 antibody (Clone RPA-T4; BioLegend Cat# 300532 RRID:AB_10965645), APC anti-human CD8a antibody (Clone HIT8a; BioLegend Cat# 300911 RRID:AB_314115) as well as isotype controls: PerCP/Cy5.5 mouse IgG2a (BioLegend Cat# 400251 RRID:AB_893682), Brilliant Violet 421™ mouse IgG1 (BioLegend Cat# 400158 RRID:AB_11150232), APC mouse IgG1 (BioLegend Cat# 400119 RRID:AB_326441) and Fixable Viability Dye eFluor® 780 (eBioscience, San Diego, CA).
Flow Cytometry was performed on a BD FACS Canto II (Becton, Dickinson and Company, Franklin Lakes, NJ). Twenty thousand events were acquired per sample and analysis was performed via FlowJo Software, Version 10. Lymphocytes were gated according to morphological criteria on a forward and sideward scatter dot blot, dead cells were excluded by staining of viability dye and gating was focused on CD3CD4 and CD3CD8-positive T cells. Those cells that proliferated in response to antigen stimulation were identified by their reduction in CFSE fluorescence intensity. Results represent means of triplicate cultures and median percentages stimulation of CD3+ CD4+ and CD3+ CD8+ above background are shown for the different antigens and the analyzed patients.
2.6. Hepatitis B Virus Neutralization Assays
The HBV inoculum for infection was prepared from supernatants of HepAd38 cells using a heparin column (GE Healthcare) to isolate viral particles.
HepG2-hNTCP cells (Ni et al., 2014) were seeded at a density of 3 × 105 cells/well in a 24 well plate. At day two after seeding, the infection medium (DMEM, Invitrogen, Carlsbad, CA) was supplemented with 2.5% Dimethyl sulfoxide (DMSO) (Merck, Darmstadt, Germany) and at day three cells were infected with HBV. For the neutralization of HBV particles, patients' sera (10 μl) were pre-incubated with the HBV inoculum (i.e., 5 μl) (6.9 × 107 genome equivalents/well) for 30 min at 37 °C, followed by co-incubation of cells with the patient's sera and virus in presence of 4% polyethylene glycol 8000 (Sigma Aldrich, St. Louis, MO) for 16 h at 37 °C. The HBV-neutralizing monoclonal antibody Ma18/7 (14 μg/ml), (Glebe et al., 2003) was used as positive control.
Following inoculation, cells were washed extensively with PBS and fresh differentiation medium, supplemented with 2.5% DMSO (Invitrogen, Carlsbad, CA) was added. Additional medium changes were performed at day three and day five post infection (p.i.).
Quantification of HBV infection was conducted by the measurement of secreted hepatitis B e antigen (HBeAg) in the supernatant from cells collected from day five to seven p.i. HBeAg was determined by ADVIA Centaur XPT automated chemiluminescence system (Siemens, Munich, Germany). Samples were considered as positive at a signal above an index of 1. For the quantification of the percentage of inhibition the given index of a treated sample was divided by the given index of the untreated sample and normalized to 100%.
The expression of HBV core protein (HBcAg) was detected by immunofluorescence at day seven p.i. Cells were washed with PBS prior to the fixation with 4% paraformaldehyde (Sigma Aldrich, St. Louis, MO) for 30 min at room temperature (RT). Next, cells were washed with PBS followed by permeabilization in 0.25% Triton X-100 (AppliChem GmbH, Darmstadt, Germany) in PBS for 30 min at RT. Subsequently, cells were incubated overnight at 4 °C with the primary antibody (anti-HBV core, rabbit polyclonal AK; Dako Cat# B0586 RRID:AB_2335704) diluted in 2% w/v BSA, PBS. On the next day, cells were washed with PBS and incubated with the secondary antibody (goat anti rabbit Alexa 488; Molecular Probes Cat# A11008 RRID:AB_143165) and 4′, 6-Diamidin-2-phenylindo/Hoechst 33342 (Roche Applied Science, Penzberg, Germany) in the dark. For the detection of HBcAg-positive cells, the secondary antibody was incubated for 2 h at RT, protected from light. Cells were examined under an epifluorescence microscope at 480 nm for Alexa-488-labeled secondary antibodies (Invitrogen, Carlsbad, CA) and 360 nm for the nuclear staining.
2.7. Allergen Microarray
The assessment of specific IgE towards > 170 allergen molecules in sera of HBV-infected individuals was conducted using micro-arrayed allergens by chip technology (Lupinek et al., 2014). Levels of allergen-specific IgE antibodies were reported in ISAC Standardized Units (ISU) with a cut-off of 0.3 ISU.
2.8. Statistical Analysis
Statistics were performed using Prism 6.0 software (GraphPad, San Diego, CA). Statistical significances were determined by 2-tailed paired Student's t-test in case of normal distribution of data and alternatively by Wilcoxon rank-sum test when population was not assumed to be distributed normally. In all figures with multiple n, data are presented as median (± interquartile ranges). A P value of < 0.05 was considered significant.
3. Results
3.1. Immunization With BM32 Induces IgG Antibodies With Specificity for Sequential PreS Epitopes
Fig. 2 shows a comparison of the IgG antibody responses towards preS and synthetic preS-derived peptides induced in rabbits with CFA-formulated preS or aluminium hydroxide-adsorbed BM32 (Fig. 2b, c) as well as IgG1 responses of BALB/c mice immunized with aluminium hydroxide-adsorbed BM32 (Fig. 2d–f). Rabbit antibodies induced with CFA-formulated preS recognized preS as well as each of the preS-derived peptides except for P7 (Fig. 2a). Both doses of aluminium-hydroxide adsorbed BM32 (20 and 40 μg) induced preS-specific IgG antibodies and IgG antibodies directed mainly to the N-terminal peptides P1, P2, peptide P6 and towards the C-terminal peptide P8 (Fig. 2a). Interestingly, the 20 μg dose appeared to induce stronger IgG responses than the 40 μg dose but the difference was statistically not significant. IgG1 responses of BALB/c mice immunized with three different doses of BM32 (10, 20, 30 μg) were directed against preS and mainly against the C-terminal peptide P8 (Fig. 2d–f). No preS or peptide-specific IgG responses were detected in rabbits or mice before immunization (Fig. 2, grey bars).
3.2. PreS-specific Antibody Responses of BM32 Immunized Subjects Are Not Influenced by Already Existing HBV Immunity
Serum samples from grass pollen allergic patients who had received immunotherapy with the hypoallergenic grass pollen vaccine BM32 or with placebo were tested for IgG reactivity to preS and synthetic preS peptides (Fig. 3). These patients (n = 30) had been screened for HBV serum markers (HBsAg, anti-HBs and anti-HBc antibodies) before treatment and were found to be negative for HBsAg and anti-HBc antibodies. Due to previous vaccination with a conventional HBV vaccine, twenty-two of the subjects contained anti-HBs antibodies, indicated as red symbols whereas non-HB-vaccinated subjects are shown in black symbols (Fig. 3). In contrast to placebo-treated patients, we found that each of the patients who had received immunotherapy with BM32, developed robust preS-specific IgG responses when sera were tested after the third (V8) as well as after the seventh (V15) injection (Fig. 3). Since conventional HBV vaccines do not contain preS it was not unexpected that there was no difference regarding the development of preS-specific antibody responses in subject who had been or were not HBV vaccinated before. The preS-specific IgG responses increased significantly from baseline before immunotherapy (i.e., visit 5 versus visit 8) and further increased significantly between visit 8 and visit 15 (i.e., after the seventh injection) (Fig. 3). The preS-specific IgG responses in these patients were directed mainly towards the N-terminal peptides P1, P2 and P3 (Supplemental Fig. 1) and again P1- and P2-specific IgG responses showed significant increases from baseline (visit 5) to visit 8 and from visit 8 to visit 15 (Fig. 3). We also found increases of IgG responses against the other preS-derived peptides P4, P5, P6, P7 and P8 in sera from patients who had received immunotherapy with BM32 but not in placebo-treated patients (Fig. 3).
3.3. PreS-specific Antibody Responses of BM32 Immunized Subjects Are Directed Against Neutralizing Epitopes and Differ From Those of Hepatitis B Virus-infected Individuals
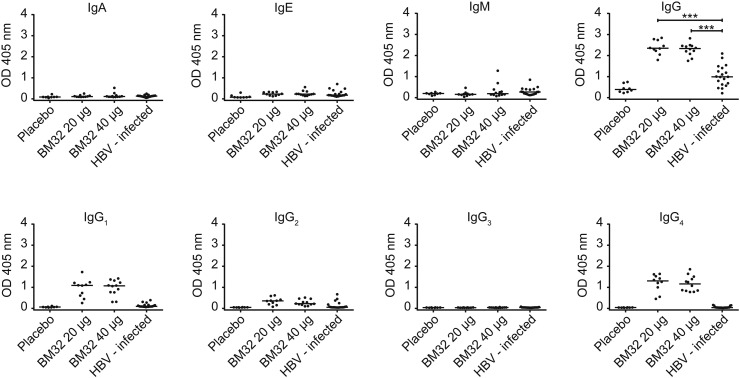
Fig. 4 depicts a comparison of the preS-specific isotype and IgG subclass responses of allergic patients after immunotherapy with BM32 or placebo with that of chronically HBV-infected individuals. Immunotherapy with both doses of BM32 induced a robust preS-specific IgG response in each of the treated patients which was significantly higher than the IgG response in HBV-infected individuals (Fig. 4). No relevant preS-specific IgA, IgE or IgM responses were detected in sera of BM32- or placebo treated patients as well as in HBV-infected individuals (Fig. 4). Moreover, the preS-specific IgG subclass response differed between BM32-treated subjects and HBV-infected individuals. BM32-treated subjects showed a preferential IgG1 and IgG4 subclass response to preS whereas HBV-infected individuals mounted some IgG1 and IgG2 responses towards preS (Fig. 4). The preferential IgG1 and IgG4 subclass response in BM32-treated patients which is indicative of a Th2-like immune response cannot solely be attributed to the fact that they are allergic as six out of the nineteen HBV-infected individuals showed IgE sensitizations but lacked preS-specific IgG4 responses (Supplemental Table 2, Fig. 4).
Fig. 4.
PreS-specific antibody responses of subjects vaccinated with BM32 or placebo and of HBV-infected individuals. Shown are optical density values (y-axes: OD values) of IgA, IgE, IgM, IgG and IgG subclass (IgG1–IgG4) levels specific for preS of subjects immunized with placebo (n = 8), 20 μg (n = 10) or 40 μg of BM32 (n = 12) at visit 15 as well as of HBV-infected individuals (n = 19) (x-axes). Medians are indicated by horizontal lines, significant differences are indicated: ***P < 0.001.
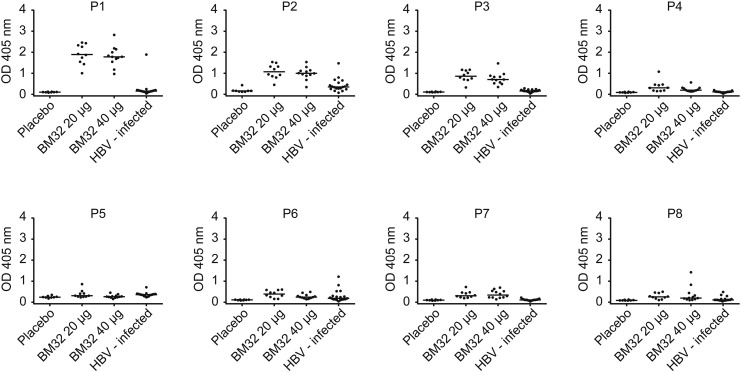
We also found striking differences regarding the epitope specificity of preS-specific antibodies in BM32-treated patients versus HBV-infected individuals (Fig. 5). BM32-immunized patients but not HBV-infected individuals showed strong IgG responses towards P1, P2 and P3 (Fig. 5). This finding is interesting as the region defined by P1 and P2 corresponds to a motif within preS1 (Supplemental Fig. 1, red box) that has been described as an essential domain regarding HBV infectivity (Glebe et al., 2005, Gripon et al., 2005). Moreover, P2 contains a motif (Supplemental Fig. 1, blue box) which has previously been shown as an essential domain for inhibition of infection with HBV (Neurath et al., 1986, Bremer et al., 2011). Furthermore, P7 was recognized exclusively by BM32-treated subjects whereas IgG responses towards P2 and P6 were found in both groups (Fig. 5).
Fig. 5.
IgG responses of subjects vaccinated with BM32 or placebo and of hepatitis B virus-infected individuals specific for preS peptides. Shown are optical density values (y-axes: OD values) of IgG levels specific for preS-derived peptides (P1–P8) of subjects immunized with placebo (n = 8), 20 μg (n = 10) or 40 μg of BM32 (n = 12) at visit 15 as well as of HBV-infected individuals (n = 19) (x-axes). Medians are indicated by horizontal lines.
P1 (aa 2–31); P2 (aa 22–51); P3 (aa 42–71); P4 (aa 62–91); P5 (aa 82–111); P6 (aa 102–131); P7 (aa 122–151); P8 (aa 142–174).
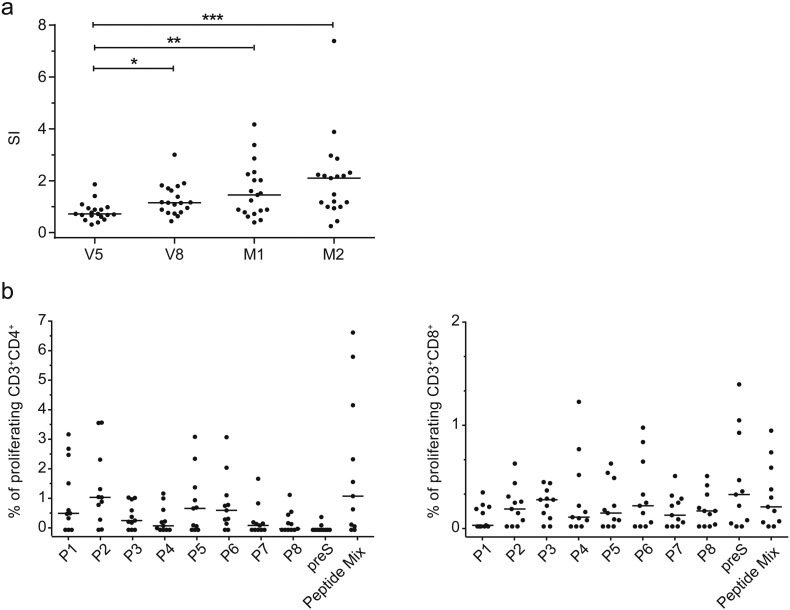
3.4. Characterization of the PreS-specific T Cell Responses of BM32 Immunized Subjects
The development of preS-specific T cell responses in patients who had received immunotherapy with BM32 is depicted in Fig. 6a. We found a gradually increasing preS-specific T cell response which was significantly higher at visits V8, M1 and M2 as compared to baseline at visit 5 (Fig. 6a). Analysis of the epitope specificity of the preS-specific CD4 cell responses by CFSE staining revealed that P1, P2, P5 and P6 induced the strongest CD4 cell proliferation whereas CD4 responses towards P3, P4 and P7 generated intermediate proliferation (Fig. 6b). Interestingly, the peptides as well as the peptide mix induced stronger CD4 cell proliferation than the preS protein itself (Fig. 6b). Albeit at low frequency, preS and preS peptide-specific CD8 cell responses were detected, which were mainly directed towards P2, P3, P6 and P8 and complete preS (Fig. 6b).
Fig. 6.
PreS- and peptide-specific T cell responses. (a) PreS-specific PBMC proliferations (y-axis: stimulation index SI) assessed by [3H] thymidine incorporation in subjects immunized with BM32 (n = 19) at different time points (x-axis). Medians (horizontal bars) and significant differences are indicated: *P < 0.05, **P < 0.001, ***P < 0.001. (b) Percentages of proliferated CD4 (left panel) and CD8 (right panel) T cells (y-axes) after stimulation with preS peptides (P1–P8), preS or an equimolar peptide mix (x-axes) in blood samples of BM32-immunized subjects (n = 11) at time point M2. Results are means of triplicates in each patient, medians for all tested subjects are denoted by the horizontal lines.
P1 (aa 2–31); P2 (aa 22–51); P3 (aa 42–71); P4 (aa 62–91); P5 (aa 82–111); P6 (aa 102–131); P7 (aa 122–151); P8 (aa 142–174).
3.5. Antibodies Induced by Immunization with BM32 Inhibit Hepatitis B Virus Infections In Vitro
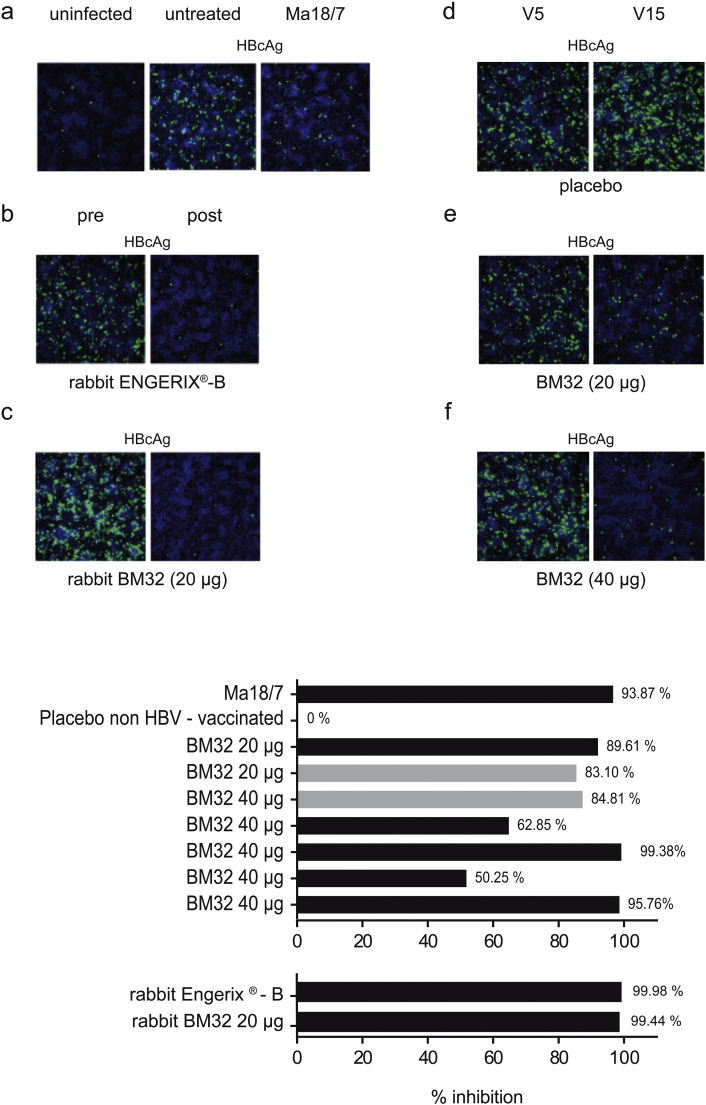
Next, we investigated whether BM32-induced antibodies can inhibit HBV infection using in vitro virus neutralization assay based on HepG2-hNTCP cells (Fig. 7). In the first type of assay the expression of HBcAg after infection of cells is detected by specific immunofluorescence. Fig. 7a shows that no HBcAg is detected in uninfected cells whereas it is stained green in infected but untreated cells (Fig. 7a, middle image). Further on, expression can be prevented by pre-incubation of virus with the neutralizing monoclonal antibody Ma18/7 (Glebe et al., 2003) which is directed against the preS1 domain of the large HBV surface protein (Fig. 7a, right image: positive control). Likewise we found that pre-incubation of HBV with rabbit antibodies induced by the commercial vaccine Engerix-B or with rabbit anti-BM32 (20 μg dose) antibodies inhibited infection of HepG2-hNTCP cells as visualized by a lack of green HBcAg-staining (Fig. 7b,c). A similar set of experiments was performed with sera from BM32- or placebo-treated patients and three representative examples are depicted in Fig. 7d–f. Sera obtained from a patient before and after immunization with placebo did not inhibit infection of HepG2-hNTCP cells (Fig. 7d), whereas sera obtained from a patient after immunization with 20 μg (Fig. 7e) or from a patient after immunization with 40 μg (Fig. 7f) strongly inhibited infection of HepG2-hNTCP cells.
Fig. 7.
Inhibition of hepatitis B virus infection of in vitro infected HepG2-hNTCP cell lines (Ni et al., 2014) by BM32-induced antibodies. Intracellular staining of HBV core antigen (HBcAg: green) in cultured HepG2-hNTCP cells seven days after addition of (a) buffer (uninfected), HBV without or with the neutralizing antibody (Ma 18/7), (b) serum from rabbits before (pre) or after (post) immunization with Engerix-B or (c) BM32 (20 μg) or sera obtained at visits V5 and V15 from subjects immunized with (d) placebo, (e) 20 μg or (f) 40 μg of BM32.
Bottom: Percentages of the inhibition (HBeAg secretion) of hepatitis B virus infection of cultured HepG2-hNTCP (x-axis) achieved by pre-incubation of virus with, Ma18/7, serum from a placebo-treated subject without prior HBV vaccination, sera from subjects after three (grey bars) or seven (black bars) immunizations with BM32 and sera from rabbits immunized with Engerix-B or BM32.
In addition to the staining of the HBcAg we used an assay based on the measurement of secreted hepatitis B e antigen (HBeAg) by HepG2-hNTCP cells seven days post infection with HBV as another surrogate marker to quantify the inhibition of HBV infection (Fig. 7 bottom). Supplemental Table 3 provides the mean of the absolute HbeAg values measured in the supernatant of HBV-infected HepG2-hNTCP cells showing the reduction of HBeAg secretion upon incubation of the viral inoculum with the patient sera. We found that sera from BM32-treated individuals inhibited HBV infection between 50 and 99% (Fig. 7 bottom). Furthermore, no relevant difference was found depending on the dose and number of BM32 injections, as a similar inhibition was observed for sera from patients who had received three injections (Fig. 7 bottom, grey bars) as well as for sera from patients who had received seven injections (Fig. 7 bottom, black bars). Also, there was no obvious difference regarding the degree of inhibition between patients who had received the 20 μg or 40 μg dose of BM32 (Fig. 7 bottom). In relation with HBcAg expression, no inhibition of secreted HBeAg was observed for serum from a placebo-treated patient. Further on, we observed a > 90% reduction in secreted HBeAg after the treatment with the monoclonal antibody Ma 18/7 (Fig. 7 bottom). Rabbit anti-Engerix and rabbit anti-BM32 antibodies induced a > 99% inhibition of HBV infection (Fig. 7 bottom).
4. Discussion
The preS domain of the hepatitis B virus large surface protein has recently been used as a carrier molecule for the construction of recombinant hypoallergenic vaccines for the treatment of allergy to cat, birch and grass pollen (Niespodziana et al., 2011, Marth et al., 2013, Focke-Tejkl et al., 2015). In these vaccines, preS serves as a carrier protein providing T cell help for the production of allergen-specific IgG antibodies which by this technology can be focused towards the IgE binding sites of the allergens (Marth et al., 2013). The induction of allergen-specific IgG antibodies directed against the IgE binding sites on allergens is achieved by fusing per se non-allergenic peptides from the IgE binding sites to preS in the form of recombinant fusion proteins. The grass pollen allergy vaccine BM32 is the so far most advanced version of the hypoallergenic B cell epitope-based vaccines as it has been evaluated not only in vitro and in experimental animal models (Focke-Tejkl et al., 2015) but also in vivo in allergic patients (Niederberger et al., 2015) (ClinicalTrials.gov numbers: NCT01445002, NCT01538979). In fact, its hypoallergenic nature has been confirmed in vitro by basophil activation testing and in vivo by skin testing. Furthermore, it has been shown that it induces allergen-specific IgG antibodies which inhibit patients' IgE binding to the natural allergens and allergen-induced effector cell activation. However, no information has been available about the ability of BM32 to induce preS-specific immune responses, the epitope-specificity of such a response and the potential of those antibodies to neutralize HBV infection. Our results show that immunization of animals (i.e., mice, rabbits) as well as of allergic patients with BM32 induces robust preS-specific IgG antibody responses. There was no difference in the development of preS-specific antibody responses in subjects who had previously received HBV vaccines and/or contained pre-existing HBV-specific antibody responses as compared to subjects without pre-existing HBV immune responses indicating that a pre-existing HBV immunity has no influence on the immunogenicity of BM32. Since conventional HBV vaccines do not contain preS this result was not unexpected. Our peptide epitope mapping studies showed that the preS-specific antibody responses are directed against several sequential epitopes, among them N-terminal peptides (i.e., P1, P2) which are supposed to contain inhibitory potential in regard to HBV infection (Neurath et al., 1986, Bremer et al., 2011) Interestingly, patients with chronic HBV infections failed to generate IgG antibodies towards these peptides but recognized different other sequential preS epitopes which are so far not found to be involved in protection against infection. In fact it has been previously reported that patients with chronic HBV infections contain preS1-specific non-protective antibodies (Alberti et al., 1990). In accordance with our results, another study found that the C-terminal peptides of preS1 induced antibodies but these did not protect (Bremer et al., 2011). A limitation of our study is that we could not distinguish between so-called immunotolerant HBeAg-positive HBV carriers with very high viremia and those without HBeAg or high viremia as well as HBV convalescents who reportedly contain preS1-specific antibodies (Deepen et al., 1990) but we did not have access to sera from such subjects.
The quality of the IgG antibody response was different between BM32-immunized subjects (IgG4 = IgG1 > IgG2) as compared to patients with chronic HBV infections (IgG1 > IgG2). We think that the predominance of IgG4 responses in the BM32-immunized subjects is not due to their allergic condition but rather due to features of the BM32 vaccine, as also allergic patients with chronic HBV infections failed to mount preS-specific IgG4 responses.
The development of preS-specific antibody responses in the BM32-immunized subjects was accompanied by a preS-specific CD4 T cell response which increased continuously in the course of the immunization. Several clinical trials have shown that preS-containing HBV vaccines are effective and even superior to HBV vaccines containing only the S protein (Madaliński et al., 2004, Rendi-Wagner et al., 2006, Schumann et al., 2007, Krawczyk et al., 2014, Shouval et al., 2015). This might be attributed to the induction of preS-specific antibody responses by these vaccines but the role of anti-preS antibodies was not analyzed in these studies. It is also possible that the better immunogenicity may have been caused by the conformational epitopes of the mammalian cell culture derived HBsAg and possibly by T helper epitopes from preS (Gerlich, 2015). Furthermore, our study showed that the preS-containing grass pollen allergy vaccine BM32 induced IgG antibodies against the important epitopes involved in attachment of HBV to hepatocytes. We therefore investigated whether BM32-induced antibodies may protect against HBV-infections. For this purpose, we immunized rabbits with the currently registered HBV vaccine Engerix-B as recommended by the manufacturer or with three injections of BM32 as it is used in the building-up phase of BM32 allergen-specific immunotherapy. Rabbit antibodies, induced with both types of vaccines inhibited HBV infection of hepatocytes completely and equally well. We also found that sera from grass pollen allergic patients immunized with BM32 but not sera from patients immunized with placebo inhibited HBV infection of hepatocytes in vitro.
The latter results indicate, that BM32 not only induces grass pollen allergen-specific IgG antibodies but also HBV-neutralizing antibodies and it is therefore tempting to speculate that BM32 may not only have anti-allergic but also HBV-neutralizing effects. In summary, our study reveals a so far unknown feature (i.e., potential HBV-neutralizing activity) of the grass pollen allergy vaccine BM32 and warrants further studies designed to investigate the usefulness of BM32 also for HBV vaccination as there is evidence that a considerable proportion of individuals show poor response to conventional HBV vaccines, lacking preS.
Author Contribution
Study conception and design: Carolin Cornelius, Stephan Urban, Rudolf Valenta.
Collection of data: Carolin Cornelius, Katrin Schöneweis, Fanny Georgi, Milena Weber.
Data analysis: Carolin Cornelius, Katrin Schöneweis, Fanny Georgi, Milena Weber, Verena Niederberger, Petra Zieglmayer, Katarzyna Niespodziana, Michael Trauner, Harald Hofer, Stephan Urban, Rudolf Valenta.
Contribution of reagents/materials/analytical tools: Verena Niederberger, Petra Zieglmayer, Katarzyna Niespodziana, Michael Trauner, Harald Hofer, Stephan Urban, Rudolf Valenta.
Manuscript preparation: Carolin Cornelius, Katrin Schöneweis, Fanny Georgi, Milena Weber, Verena Niederberger, Petra Zieglmayer, Katarzyna Niespodziana, Michael Trauner, Harald Hofer, Stephan Urban, Rudolf Valenta.
Conflict of Interest
Rudolf Valenta has received research grants from Biomay AG, Vienna, Austria and serves as a consultant for this company. The other authors have no conflict of interest to report.
Grant Support
This work was supported by the Austrian Science Fund (FWF) SFBF46, project F4605 and F4613, by a research grant from BIOMAY AG and Viravaxx Gmbh, Vienna, Austria and by the German Centre for Infection Research (DZIF), TTU Hepatitis, Project 5.807 and 5.704.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2016.07.023.
Appendix A. Supplementary data
Supplementary materials
References
- Alberti A., Cavalletto D., Chemello L., Belussi F., Fattovich G., Pontisso P., Milanesi G., Ruol A. Fine specificity of human antibody response to the PreS1 domain of hepatitis B virus. Hepatol. Baltim. Md. 1990;12:199–203. doi: 10.1002/hep.1840120204. [DOI] [PubMed] [Google Scholar]
- Bremer C.M., Sominskaya I., Skrastina D., Pumpens P., El Wahed A.A., Beutling U., Frank R., Fritz H.-J., Hunsmann G., Gerlich W.H., Glebe D. N-terminal myristoylation-dependent masking of neutralizing epitopes in the preS1 attachment site of hepatitis B virus. J. Hepatol. 2011;55:29–37. doi: 10.1016/j.jhep.2010.10.019. [DOI] [PubMed] [Google Scholar]
- Deepen R., Heermann K.H., Uy A., Thomssen R., Gerlich W.H. Assay of preS epitopes and preS1 antibody in hepatitis B virus carriers and immune persons. Med. Microbiol. Immunol. (Berl.) 1990;179:49–60. doi: 10.1007/BF00190150. [DOI] [PubMed] [Google Scholar]
- Durham S.R., Walker S.M., Varga E.M., Jacobson M.R., O'Brien F., Noble W., Till S.J., Hamid Q.A., Nouri-Aria K.T. Long-term clinical efficacy of grass-pollen immunotherapy. N. Engl. J. Med. 1999;341:468–475. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- Edlmayr J., Niespodziana K., Focke-Tejkl M., Linhart B., Valenta R. Allergen-specific immunotherapy: towards combination vaccines for allergic and infectious diseases. In: Valenta R., Coffman R.L., editors. Vaccines against Allergies, Current Topics in Microbiology and Immunology. Springer; Berlin Heidelberg: 2011. pp. 121–140. [DOI] [PubMed] [Google Scholar]
- Focke M., Mahler V., Ball T., Sperr W.R., Majlesi Y., Valent P., Kraft D., Valenta R. Nonanaphylactic synthetic peptides derived from B cell epitopes of the major grass pollen allergen, Phl p 1, for allergy vaccination. FASEB J. 2001 doi: 10.1096/fj.01-0016fje. [DOI] [PubMed] [Google Scholar]
- Focke M., Swoboda I., Marth K., Valenta R. Developments in allergen-specific immunotherapy: from allergen extracts to allergy vaccines bypassing allergen-specific immunoglobulin E and T cell reactivity. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2010;40:385–397. doi: 10.1111/j.1365-2222.2009.03443.x. [DOI] [PubMed] [Google Scholar]
- Focke-Tejkl M., Valenta R. Safety of engineered allergen-specific immunotherapy vaccines. Curr. Opin. Allergy Clin. Immunol. 2012;12:555–563. doi: 10.1097/ACI.0b013e328357ca53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focke-Tejkl M., Campana R., Reininger R., Lupinek C., Blatt K., Valent P., Pavkov-Keller T., Keller W., Valenta R. Dissection of the IgE and T-cell recognition of the major group 5 grass pollen allergen Phl p 5. J. Allergy Clin. Immunol. 2014;133:836–845. doi: 10.1016/j.jaci.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focke-Tejkl M., Weber M., Niespodziana K., Neubauer A., Huber H., Henning R., Stegfellner G., Maderegger B., Hauer M., Stolz F., Niederberger V., Marth K., Eckl-Dorna J., Weiss R., Thalhamer J., Blatt K., Valent P., Valenta R. Development and characterization of a recombinant, hypoallergenic, peptide-based vaccine for grass pollen allergy. J. Allergy Clin. Immunol. 2015;135:1207–1217. doi: 10.1016/j.jaci.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallerano D., Ndlovu P., Makupe I., Focke-Tejkl M., Fauland K., Wollmann E., Puchhammer-Stöckl E., Keller W., Sibanda E., Valenta R. Comparison of the specificities of IgG, IgG-subclass, IgA and IgM reactivities in African and European HIV-infected individuals with an HIV-1 clade C proteome-based array. PLoS One. 2015;10 doi: 10.1371/journal.pone.0117204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlich W.H. Prophylactic vaccination against hepatitis B: achievements, challenges and perspectives. Med. Microbiol. Immunol. (Berl.) 2015;204:39–55. doi: 10.1007/s00430-014-0373-y. [DOI] [PubMed] [Google Scholar]
- Glebe D., Aliakbari M., Krass P., Knoop E.V., Valerius K.P., Gerlich W.H. Pre-S1 antigen-dependent infection of tupaia hepatocyte cultures with human hepatitis B virus. J. Virol. 2003;77:9511–9521. doi: 10.1128/JVI.77.17.9511-9521.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glebe D., Urban S., Knoop E.V., Cag N., Krass P., Grün S., Bulavaite A., Sasnauskas K., Gerlich W.H. Mapping of the hepatitis B virus attachment site by use of infection-inhibiting preS1 lipopeptides and tupaia hepatocytes. Gastroenterology. 2005;129:234–245. doi: 10.1053/j.gastro.2005.03.090. [DOI] [PubMed] [Google Scholar]
- Gripon P., Cannie I., Urban S. Efficient inhibition of hepatitis B virus infection by acylated peptides derived from the large viral surface protein. J. Virol. 2005;79:1613–1622. doi: 10.1128/JVI.79.3.1613-1622.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayer J., Jadeau F., Deléage G., Kay A., Zoulim F., Combet C. HBVdb: a knowledge database for hepatitis B virus. Nucleic Acids Res. 2012 doi: 10.1093/nar/gks1022. (gks1022. doi:10.1093/nar/gks1022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen L., Niggemann B., Dreborg S., Ferdousi H.A., Halken S., Høst A., Koivikko A., Norberg L.A., Valovirta E., Wahn U., Möller C., The PAT investigator group Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007;62:943–948. doi: 10.1111/j.1398-9995.2007.01451.x. [DOI] [PubMed] [Google Scholar]
- Jutel M., Akdis C.A. Novel immunotherapy vaccine development. Curr. Opin. Allergy Clin. Immunol. 2014;14:557–563. doi: 10.1097/ACI.0000000000000121. [DOI] [PubMed] [Google Scholar]
- Krawczyk A., Ludwig C., Jochum C., Fiedler M., Heinemann F.M., Shouval D., Roggendorf M., Roggendorf H., Lindemann M. Induction of a robust T- and B-cell immune response in non- and low-responders to conventional vaccination against hepatitis B by using a third generation PreS/S vaccine. Vaccine. 2014;32:5077–5082. doi: 10.1016/j.vaccine.2014.06.076. [DOI] [PubMed] [Google Scholar]
- Larché M., Akdis C.A., Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat. Rev. Immunol. 2006;6:761–771. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- Lupinek C., Wollmann E., Baar A., Banerjee S., Breiteneder H., Broecker B.M., Bublin M., Curin M., Flicker S., Garmatiuk T., Hochwallner H., Mittermann I., Pahr S., Resch Y., Roux K.H., Srinivasan B., Stentzel S., Vrtala S., Willison L.N., Wickman M., Lødrup-Carlsen K.C., Antó J.M., Bousquet J., Bachert C., Ebner D., Schlederer T., Harwanegg C., Valenta R. Advances in allergen-microarray technology for diagnosis and monitoring of allergy: the MeDALL allergen-chip. Methods San Diego Calif. 2014;66:106–119. doi: 10.1016/j.ymeth.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madaliński K., Sylvan S.P., Hellstrom U., Mikołajewicz J., Dzierzanowska-Fangrat K. Presence of anti-preS1, anti-preS2, and anti-HBs antibodies in newborns immunized with bio-Hep-B vaccine. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2004;10:PI10–PI17. [PubMed] [Google Scholar]
- Marth K., Breyer I., Focke-Tejkl M., Blatt K., Shamji M.H., Layhadi J., Gieras A., Swoboda I., Zafred D., Keller W., Valent P., Durham S.R., Valenta R. A nonallergenic birch pollen allergy vaccine consisting of hepatitis PreS-fused bet v 1 peptides focuses blocking IgG toward IgE epitopes and shifts immune responses to a tolerogenic and Th1 phenotype. J. Immunol. Baltim. Md. 2013;1950(190):3068–3078. doi: 10.4049/jimmunol.1202441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth K., Focke-Tejkl M., Lupinek C., Valenta R., Niederberger V. Allergen peptides, recombinant allergens and hypoallergens for allergen-specific immunotherapy. Curr. Treat. Options Allergy. 2014;1:91–106. doi: 10.1007/s40521-013-0006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath A.R., Kent S.B., Parker K., Prince A.M., Strick N., Brotman B., Sproul P. Antibodies to a synthetic peptide from the preS 120–145 region of the hepatitis B virus envelope are virus neutralizing. Vaccine. 1986;4:35–37. doi: 10.1016/s0264-410x(86)80001-9. [DOI] [PubMed] [Google Scholar]
- Ni Y., Lempp F.A., Mehrle S., Nkongolo S., Kaufman C., Fälth M., Stindt J., Königer C., Nassal M., Kubitz R., Sültmann H., Urban S. Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes. Gastroenterology. 2014;146:1070–1083. doi: 10.1053/j.gastro.2013.12.024. [DOI] [PubMed] [Google Scholar]
- Niederberger V., Marth K., Eckl-Dorna J., Focke-Tejkl M., Weber M., Hemmer W., Berger U., Neubauer A., Stolz F., Henning R., Valenta R. Skin test evaluation of a novel peptide carrier-based vaccine, BM32, in grass pollen-allergic patients. J. Allergy Clin. Immunol. 2015;136:1101–1103. doi: 10.1016/j.jaci.2015.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niespodziana K., Focke-Tejkl M., Linhart B., Civaj V., Blatt K., Valent P., van Hage M., Grönlund H., Valenta R. A hypoallergenic cat vaccine based on Fel d 1-derived peptides fused to hepatitis B PreS. J. Allergy Clin. Immunol. 2011;127:1562–1570. doi: 10.1016/j.jaci.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quah B.J.C., Warren H.S., Parish C.R. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester. Nat. Protoc. 2007;2:2049–2056. doi: 10.1038/nprot.2007.296. [DOI] [PubMed] [Google Scholar]
- Rendi-Wagner P., Shouval D., Genton B., Lurie Y., Rümke H., Boland G., Cerny A., Heim M., Bach D., Schroeder M., Kollaritsch H. Comparative immunogenicity of a PreS/S hepatitis B vaccine in non- and low-responders to conventional vaccine. Vaccine. 2006;24:2781–2789. doi: 10.1016/j.vaccine.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Sandrini A., Rolland J.M., O'Hehir R.E. Current developments for improving efficacy of allergy vaccines. Expert Rev. Vaccines. 2015;14:1073–1087. doi: 10.1586/14760584.2015.1050385. [DOI] [PubMed] [Google Scholar]
- Schumann A., Fiedler M., Dahmen U., Grosse-Wilde H., Roggendorf M., Lindemann M. Cellular and humoral immune response to a third generation hepatitis B vaccine. J. Viral Hepat. 2007;14:592–598. doi: 10.1111/j.1365-2893.2007.00848.x. [DOI] [PubMed] [Google Scholar]
- Shouval D., Roggendorf H., Roggendorf M. Enhanced immune response to hepatitis B vaccination through immunization with a Pre-S1/Pre-S2/S vaccine. Med. Microbiol. Immunol. (Berl.) 2015;204:57–68. doi: 10.1007/s00430-014-0374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenta R. The future of antigen-specific immunotherapy of allergy. Nat. Rev. Immunol. 2002;2:446–453. doi: 10.1038/nri824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials