Abstract
In 1998, our group discovered a cDNA that encoded the precursor of two putative neuropeptides that we called hypocretins for their hypothalamic expression and their similarity to the secretin family of neuropeptides. In the past 15 years, numerous studies have placed the hypocretin system as an integrator of homeostatic functions with a crucial, nonredundant function as an arousal stabilizer. Here, we discuss some of the data that have accumulated over the years on the integrating capacity of these hypothalamic neurons and their role on sleep-to-wake transitions.
Keywords: lateral hypothalamus, sleep, arousal, narcolepsy, insomnia, addiction, reward
Introduction
In the past few years, the hypocretins (also known as orexins) have been shown to be critical components of the brain circuitry that modulates the states of vigilance (Mignot et al., 2002; Sutcliffe and de Lecea, 2002; Willie et al., 2001). Recent advances are yielding a clearer picture as to the mechanism of action of these peptides, and how they control multiple circuits to produce a coherent behavioral output. Here, I review the interactions of the hypocretinergic system with the major neurotransmitter networks and discuss the role of the neurons that contain hypocretin in integrating information that dictates the state of arousal.
Discovery and properties of the hypocretins
Analysis of the expression patterns of subtracted hypothalamus-enriched sequences (Gautvik et al., 1996) revealed that one of these was expressed exclusively by a bilaterally symmetric structure within the posterior hypothalamus (Fig. 1). Its nucleotide sequence (de Lecea et al., 1998) encoded a 130-residue putative secretory protein (preprohypocretin) with an apparent signal sequence and three additional sites for potential proteolytic maturation. Two of the 4 putative products of proteolysis had 14 amino acid identities across 20 residues. This region of one of the peptides contained a 7/7 match with secretin, suggesting that the prepropeptide gave rise to two peptide products that were structurally related both to each other and to secretin. Thus, these peptides were named hypocretin (Hcrt) 1 and 2 to reflect their hypothalamic origin and the similarity to secretin, which also extends to the secondary structure (Lee et al., 1999).
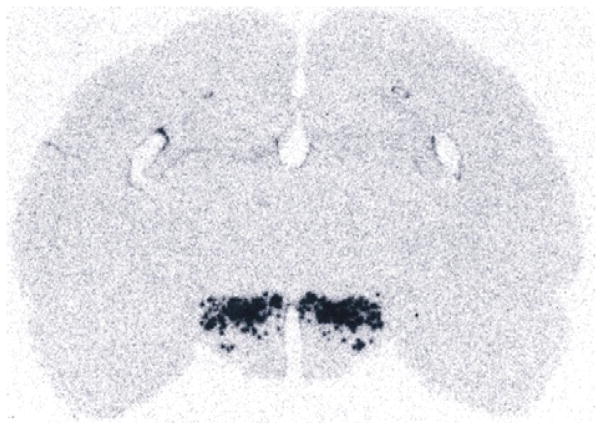
Fig. 1.
The first image of the hypocretin system, as reported by Gautvik et al. (1996). The picture shows an autoradiograph of an in situ hybridization of clone 1D4 corresponding to a gridded hypothalamic subtracted cDNA library.
Parallel work in another laboratory described the orexins as ligands of two G-protein-coupled receptors: HcrtR1 and HcrtR2 (Sakurai et al., 1998). Hcrt1 binds with equal affinity to both receptors, whereas Hcrt2 binds with preferential affinity to HcrtR2 (see Chapter 1). Immunocytochemical mapping using antisera against chemically synthesized hypocretin peptides has shown that hypocretin neurons project their terminals throughout the brain (Peyron et al., 1998). Within the synaptic terminals of these fibers, hypocretin immunoreactivity is associated with dense core secretory vesicles (de Lecea et al., 1998). Efferents of hypocretin neurons include an ascending pathway that projects to the basal forebrain, septum, and cerebral cortex; a very dense intra-hypothalamic network; and a descending pathway that connects the lateral hypothalamus with brainstem nuclei and the spinal cord (Peyron et al., 1998). Both hypocretin peptides (Hcrt1 and Hcrt2) are neuroexcitatory (de Lecea et al., 1998; van den Pol et al., 1998) and bind to postsynaptic Hcrt receptors (HcrtR1 and HcrtR2) with different selective affinities (Sakurai et al., 1998). The distribution of Hcrt fibers matches with that of the described hypocretin receptors (Marcus et al., 2001) and suggests that the hypocretins interact with multiple neurotransmitter networks involved in different functions.
Loss of function
The studies showing that hypocretin mRNA is absent from narcoleptic brains (Peyron et al., 2000) and that Hcrt immunoreactivity is highly decreased in narcoleptic hypothalami (Thannickal et al., 2000) provide compelling evidence that the main function of the hypocretinergic system is the regulation of arousal circuits.
Narcoleptic patients with cataplexy have non- or barely detectable levels of Hcrt1 in the cerebrospinal fluid, (Nishino et al., 2000) in addition to the absence of preproHcrt gene transcripts in the hypothalamus (Peyron et al., 2000; Thannickal et al., 2000). Doberman narcoleptic dogs bear a mutation in HcrtR2, and all genetically engineered rodents with either a deletion of the Hcrt (Chemelli et al., 1999). HcrtR2 gene (Willie et al., 2003) or Hcrt cells present behavioral arrests that resemble cataplexy, the hallmark of narcolepsy. HcrtR1 KO mice do not show any overt sleep abnormality, and HcrtR2-deficient mice are less affected with cataplexy-like attacks of REM sleep compared to the mice deficient in peptide ligand that are more severely affected (Willie et al., 2003), suggesting that the altered REM sleep control in narcolepsy–cataplexy syndrome emerges from the loss of signaling through both HcrtR2-dependent and HcrtR2-independent pathways (Willie et al., 2003). These studies support a role for the Hcrt system in “lowering the arousal threshold” (Sutcliffe and de Lecea, 2002) resulting in a facilitation of wakefulness when animals are asleep.
Hypocretin neuronal activity
Recordings of Hcrt neuronal activity in freely moving (Mileykovskiy et al., 2005) and in head restraint (Lee et al., 2005) rats revealed that Hcrt neurons fire phasically in correlation with the locomotor activity and are mostly silent during NREM and REM sleep. Interestingly, the highest frequency of activity was found during the transitions of vigilance states and in anticipation of a reward signal. This phasic pattern of activity questioned the behavioral effects of the pharmacological experiments infusing large amounts of Hcrt peptide ligand in the brain, which would mimic, in the best possible conditions, an increase in tonic activity.
Recently, we and others have used optogenetic (Adamantidis et al., 2007) and pharmacogenetic (Sasaki et al., 2011) approaches to mimic phasic activity with millisecond resolution and determine the causal relationships between the activity of Hcrt neuronal circuit and arousal transitions. We found that direct, deep brain optical stimulation of hypocretin neurons in the hypothalamus increased the probability of transitions to wakefulness from either NREM or REM. Interestingly, photostimulation using 5–30 Hz light pulse trains reduced latency to wakefulness, whereas 1 Hz trains did not. We also asked whether Hcrt-mediated sleep-to-wake transitions are affected by light/dark period and sleep pressure. We found that stimulation of Hcrt neurons increased the probability of an awakening event throughout the entire light/dark period but that this effect was diminished with sleep pressure induced by 2 or 4 h of sleep deprivation (Carter et al., 2009). These results suggest that the Hcrt system promotes wakefulness throughout the light/dark period by activating multiple downstream targets, which themselves are inhibited with increased sleep pressure.
In contrast to the loss-of-function phenotype, overactivation of the Hcrt release has been associated with hyperarousal response associated with stress, panic disorder, and addictive behaviors (see below).
Arousal circuits modulated by the hypocretins
Hypothalamus
Hcrt neurons are localized in the lateral hypothalamus, an area long known as a key center for the regulation of energy homeostasis. Therefore, it was only logical that the first hypotheses about Hcrt function involved feeding and energy balance (Sakurai et al., 1998). Indeed, Hcrt neurons are connected with the main networks regulating feeding. The connectivity between NPY-positive neurons in the arcuate nucleus and Hcrt neurons has been demonstrated (Broberger et al., 1998; Elias et al., 1998). Hcrt neurons are also innervated by POMC-containing terminals. Hcrt neurons also appear to activate themselves through HcrtR2 (Yamanaka et al., 2010). Additional GABAergic input to Hcrt cells includes melanin-concentrating hormone neurons as well as neurons containing leptin receptor (Leinninger et al., 2009).
Further, hypocretin neurons are sensitive to glucose, ghrelin, triglycerides, and amino acids (Cai et al., 1999; Karnani et al., 2011; Lopez et al., 2000; Wortley et al., 2003; Yamanaka et al., 2003). In an elegant study, Hara et al. (2001) showed that genetic ablation of Hcrt neurons in transgenic mice results in obesity and hypophagia, suggesting that the balance between storage and expenditure is impaired in these mice. Together, the available data strongly suggest that the main function of the Hcrt peptides is not increasing food intake, but generating a coherent output that stabilizes brain states.
In addition to the circuitry that modulates energy balance, hypocretin neurons contact several hypothalamic nuclei involved in sleep and wakefulness, including the ventrolateral preoptic nucleus (VLPO), the dorsomedial hypothalamus (DMH), and the tuberomammilary nucleus (TMN). Hypocretin neurons only account for 4% of the lateral hypothalamic input to the VLPO, which is mostly active during NREM sleep (Chou et al., 2002). The DMH is a key relay nucleus that receives input from the internal clock (Chou et al., 2003). Both hypocretin peptides excite histaminergic neurons of the TMN, probably acting through HcrtR2, and knockout mice deficient in histamine receptor 1 are impervious to hypocretin administration, suggesting that at least some of the effects of the Hcrts are caused by release of histamine and activation of postsynaptic H1 receptors (Huang et al., 2001). However, optogenetic stimulation of Hcrt neurons in histamine-deficient mice did not affect the ability of Hcrt to increase the probability of awakenings, suggesting that the Histamine is not an essential factor in this circuit.
Locus coeruleus
The densest projection of Hcrt fibers terminate in the locus coeruleus area, the main site of noradrenergic transmission. Thus, this system was one of the first targets of the hypocretinergic system to be analyzed (Bourgin et al., 2000; Hagan et al., 1999). Noradrenergic neurons of the locus coeruleus are active during wakefulness, display low activity during slow wave sleep, are silent during REM sleep, and are thought to be critical for the alternation of the REM–NREM sleep (Pace-Schott and Hobson, 2002). Most of the LC neurons express HcrtR1 but not Hcrt2. This is important because HcrtR1-deficient animals do not have overt sleep abnormalities or cataplexy (Willie et al., 2003). Local administration of Hcrt1 in the LC increases wakefulness and suppresses REM sleep in a dose-dependent manner, and this effect can be blocked by antisera that prevent binding of Hcrt to its receptors (Bourgin et al., 2000). Application of Hcrt1 peptide to slices of the locus coeruleus increased the firing rate of noradrenergic neurons, possibly by decreasing the afterhyperpolarization current (Horvath et al., 1999).
Recent optogenetic studies have shown that a brief train of pulses is sufficient to induce an awakening (Carter et al., 2010). In particular, combinations, frequencies, and durations that led to at least 20 pulses during 5 s were deterministic in inducing an awakening. Since noradrenergic neurons in the LC contain high concentrations of HcrtR1 (Bourgin et al., 2000), it is possible that a mild, phasic stimulation of Hcrt neurons facilitates awakenings directly by depolarizing LC neurons.
Brainstem cholinergic nuclei
The major cholinergic input to the thalamus is from the laterodorsal tegmental nucleus (LDT) and the adjacent pedunculopontine tegmental nucleus (PPT). These neurons act on the thalamocortical network to provoke the tonic activation subtending both sensory transmission and cortical activation during arousal (Steriade and Llinas, 1988). Considerable evidence has also indicated that mesopontine cholinergic nuclei also play a role in generating REM sleep, notably by stimulating the medial pontine reticular formation. Thus, cholinergic neurons in LDT and PPT, by promoting either EEG desynchronization and wakefulness or REM sleep, play a key role in regulating the vigilance state (Jones, 1991). The wide descending hypocretinergic projection includes the mesopontine cholinergic system (Peyron et al., 1998). Moreover, HcrtR1 mRNA has been detected in these mesopontine cholinergic nuclei (Greco and Shiromani, 2001; Marcus et al., 2001; Trivedi et al., 1998). Hcrt peptides excite cholinergic neurons in the LDT (Burlet et al., 2002; Takahashi et al., 2002), and injection of Hcrt1 into the rat LDT increases wakefulness at the expense of NREM sleep (Xi et al., 2001). It has been hypothesized that the hypocretin system may coordinate activation of the entire ascending reticular activating system (see below).
The basal forebrain
The majority of neurons in the magnocellular basal forebrain are wakefulness active with highest discharge activity during wakefulness and a marked reduction in activity just before and during the entry to NREM sleep. A variety of basal forebrain structures receive a moderate hypocretin innervation. Infusion of hypocretin peptides into the medial septal area significantly increases wakefulness (Espana et al., 2001). Infusion of Hcrt1 in slices shows a strong and direct excitatory effect on the cholinergic neurons of the basal forebrain. Interestingly, these effects are mediated through HcrtR2 which are those lacking in narcoleptic dogs. Interestingly, some studies have linked hypocretin secretion to the processing of beta amyloid and the progression of Alzheimer’s disease. Since cholinergic neurons in the basal forebrain are among the first to be affected in this disorder, it is possible that the hypocretins excite cholinergic neurons that release acetylcholine in the cerebral cortex and thereby contribute to cortical arousal.
The VTA/NAcc reward circuit
Ventral tegmental area (VTA) contains cell bodies of dopaminergic neurons projecting to the nucleus accumbens, amygdala, hippocampus, and prefrontal cortex. Defined as the mesocorticolimbic dopamine system (Albanese and Minciacchi, 1983), these neurons are critically implicated in brain mechanisms of reward, reinforcement, and emotional arousal (Wise and Rompre, 1989). Their activity has been closely correlated to the availability of primary rewards such as food, water, and sexual behavior (Schultz, 1998). The mesolimbic dopamine system, which is an established component of the reward system, receives glutamatergic input from cortical structures including the medial and occipital prefrontal cortex and amygdala, GABAergic inputs from striatal sources, and cholinergic input from the brainstem (Wise, 2002). Hypocretin activity may mimic lateral hypothalamic self stimulation, activating the LDT/PPTg nuclei and subsequently increase the activity of dopaminergic neurons in the VTA (Wise, 2002). In addition of the hypothetical indirect activation of VTA dopaminergic neurons by Hcrt via the LDT/PPT brainstem nuclei, Hcrt neurons directly excite dopamine fibers in the VTA (Fadel and Deutch, 2002; Korotkova et al., 2003; Uramura et al., 2001) and that the VTA dopaminergic system is critically involved in hypocretin-induced hyperlocomotion and stereotypy (Nakamura et al., 2000). Hcrt appears to increase glutamatergic excitability in VTA synapses (Borgland et al., 2006, 2009). Lastly, hypocretin-immunoreactive fibers and receptors are present in the nucleus accumbens (Peyron et al., 1998), and Hcrt peptides modify the response to glutamate and GABA in this nucleus (Martin et al., 2002). We and others (Boutrel et al., 2005; Harris et al., 2005) demonstrated a functional association between Hcrt activation and relapse of drug-seeking behavior, suggesting that Hcrt activation increases the allostatic load that may develop into pathological hyperarousal associated with compulsivity and addictive behaviors.
The HPA axis
Hcrt peptides interact with autonomic, neuroendocrine, and neuroregulatory systems (Date et al., 2000; Hagan et al., 1999) and have recently been shown to be mediators of the stress response (Ida et al., 2000). Thus, the hypocretinergic system has been associated with increased sympathetic tone (Samson et al., 1999). Immunocytochemical studies have shown long descending Hcrt-containing axonal projections from the lateral hypothalamus to the spinal cord (van den Pol, 1999). Innervation of the intermediolateral column and lamina 10 suggests that the Hcrt may participate in the sympathetic and parasympathetic components of the autonomic nervous system. Indeed, injection of an agonist for the Hcrt1 receptor increases heart rate, blood pressure, cerebral blood flow, and renal sympathetic activity in awake animals (Samson et al., 1999; Shirasaka et al., 2002), as well as gastric secretion (Takahashi et al., 1999).
Hcrt neurons are modulated by adrenergic input (Hajszan et al., 2002). Moreover, centrally administered orexin/Hcrt activates HPA axis in rats (Kuru et al., 2000; Nakamura et al., 2000), induces plasma ACTH and corticosterone (Ida et al., 2000; Kuru et al., 2000; Malendowicz et al., 1999) and c-fos mRNA in the parvocellular division of the PVN. In addition, glucocorticoids modulate hypothalamic hypocretin mRNA expression (Stricker-Krongrad et al., 2002), suggesting that this system could constitute a sensitive key relay for mediating stress behavior. Interestingly, hypocretin receptors have been detected in adrenal gland: HcrtR1 is expressed in the cortex of the normal human adrenal gland (glomerulosa, fasciculata, and reticular zones) and HcrtR2 is located in the medulla (epinephrine and norepinephrine cells) (Blanco et al., 2002; Lopez et al., 1999), and addition of Hcrt to adrenocortical cultures stimulates norepinephrine release (Nanmoku et al., 2002). However, the origin of the ligand that would bind to Hcrt receptors in the periphery is unclear.
Further supporting the role of the hypocretins in the activation of the HPA axis, Ida et al. (2000) have shown that icv administration of the alpha helical CRF antagonist blocks Hcrt-induced grooming behavior. Also recently, Espana et al. (2002) have shown that mild stress increases c-fos immunoreactivity in hypocretin positive neurons in the perifornical area. Interestingly, the effect of Hcrt on the stress response appears to be specific and finely regulated, since in vitro, Hcrt1 inhibits CRF-induced ACTH release via a pertussis toxin-sensitive mechanism, but does not affect baseline levels of ACTH, or release of LH, PRL, or FSH from the pituitary (Samson and Taylor, 2001). These data, and our own preliminary evidence showing that hypocretin neurons are activated by CRF, suggest that the hypocretinergic system is an important component of the neural circuitry modulating the stress response. Along this line, the hypocretin system may also be involved in hyperarousal associated with panic and posttraumatic stress disorder, as silencing Hcrt neurons prevent panic disorder in rats, and some human patients with panic disorder show elevated levels of Hcrt in CSF (Johnson et al., 2010),
The hypocretins as an integrator circuit in arousal
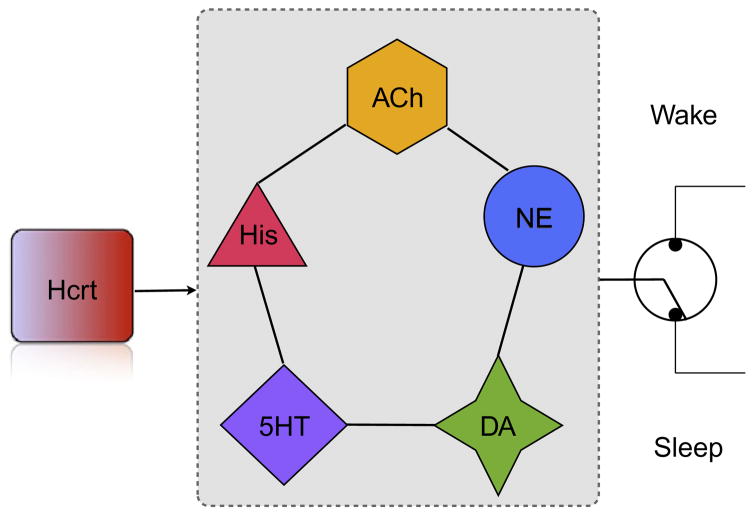
The anatomical localization and functional connectivity of Hcrt neurons reveals a prominent role in homeostatic control of physiological switches. Data from multiple laboratories have shown a very diverse set of classical and peptide transmitters as well as metabolites that modulate Hcrt activity. Recent data showing phasic activity of Hcrt in correlation with goal oriented behaviors, locomotor activity, and behavioral state transitions also suggest that these neurons provide physiological signals in a changing environment and prepare other neuronal circuits to adapt to new situations. Following Cannon’s original concept of homeostasis, Hcrt neurons act as integrators that convey possibly conflicting physiological signals into a coherent output to other effector systems, which include norepinephrine neurons for arousal transitions and dopaminergic neurons of the mesocorticolimbic system to engage in rewarding activities (Fig. 2). The deconstruction of hypothalamic circuits using opto and pharmacogenetic methods will undoubtedly reveal new ways of integration that underlie complex behaviors.
Fig. 2.
Multiple lines of evidence suggest that the Hcrt system integrates multiple variables including metabolite concentration and limbic tone to produce a coherent output to arousal systems. Each of these arousal systems contribute in different ways to sleep-to-wake transitions. For instance, optogenetic experiments suggest that noradrenergic neurons in the locus coeruleus (NE) are strong effectors of arousal systems, as only a few action potentials are sufficient to induce an awakening (Carter et al., 2010). In contrast, histamineric neurons in the tuberomammilary region of the posterior hypothalamus (His) do not appear essential to elicit transitions (Carter et al., 2009) but contribute to the length of the wake bout. Serotoninergic neurons have been proposed as gates to REM sleep, whereas dopaminergic and cholinergic systems have different effects on cortical excitability and affect different frequency bands. Coordination of these systems by Hcrt neurons is essential for arousal stability, and narcolepsy with cataplexy may be the result of chaotic signaling during transitions.
References
- Adamantidis A, Zhang F, Aravanis A, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albanese A, Minciacchi D. Organization of the ascending projections from the ventral tegmental area: A multiple fluorescent retrograde tracer study in the rat. The Journal of Comparative Neurology. 1983;216:406–420. doi: 10.1002/cne.902160406. [DOI] [PubMed] [Google Scholar]
- Blanco M, Garcia-Caballero T, Fraga M, Gallego R, Cuevas J, Forteza J, et al. Cellular localization of orexin receptors in human adrenal gland, adrenocortical adenomas and pheochromocytomas. Regulatory Peptides. 2002;104:161–165. doi: 10.1016/s0167-0115(01)00359-7. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, et al. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2009;29:11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Bourgin P, Huitron-Resendiz S, Spier AD, Fabre V, Morte B, Criado JR, et al. Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2000;20:7760–7765. doi: 10.1523/JNEUROSCI.20-20-07760.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, et al. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberger C, de Lecea L, Sutcliffe JG, Hökfelt T. Hypocretin/orexin- and melanin-concentrating hormone expressing cells form distinct populations in the rodent lateral hypothalamus: Relationship to neuropeptide Y innervation. The Journal of Comparative Neurology. 1998;402:460–474. [PubMed] [Google Scholar]
- Burlet S, Tyler CJ, Leonard CS. Direct and indirect excitation of laterodorsal tegmental neurons by hypocretin/orexin peptides: Implications for wakefulness and narcolepsy. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2002;22:2862–2872. doi: 10.1523/JNEUROSCI.22-07-02862.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai XJ, Widdowson PS, Harrold J, Wilson S, Buckingham RE, Arch JR, et al. Hypothalamic orexin expression: Modulation by blood glucose and feeding. Diabetes. 1999;48:2132–2137. doi: 10.2337/diabetes.48.11.2132. [DOI] [PubMed] [Google Scholar]
- Carter ME, Adamantidis A, Ohtsu H, Deisseroth K, de Lecea L. Sleep homeostasis modulates hypocretin-mediated sleep-to-wake transitions. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2009;29:10939–10949. doi: 10.1523/JNEUROSCI.1205-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nature Neuroscience. 2010;13:1526–1533. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, et al. Narcolepsy in orexin knockout mice: Molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Chou TC, Bjorkum AA, Gaus SE, Lu J, Scammell TE, Saper CB. Afferents to the ventrolateral preoptic nucleus. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2002;22:977–990. doi: 10.1523/JNEUROSCI.22-03-00977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TC, Scammell TE, Gooley JJ, Gaus SE, Saper CB, Lu J. Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2003;23:10691–10702. doi: 10.1523/JNEUROSCI.23-33-10691.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date Y, Mondal MS, Matsukura S, Ueta Y, Yamashita H, Kaiya H, et al. Distribution of orexin/hypocretin in the rat median eminence and pituitary. Brain Research. Molecular Brain Research. 2000;76:1–6. doi: 10.1016/s0169-328x(99)00317-4. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias CF, Saper CB, Maratos-Flier E, Tritos NA, Lee C, Kelly J, et al. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. The Journal of Comparative Neurology. 1998;402:442–459. [PubMed] [Google Scholar]
- Espana RA, Baldo BA, Kelley AE, Berridge CW. Wake-promoting and sleep-suppressing actions of hypocretin (orexin): Basal forebrain sites of action. Neuroscience. 2001;106:699–715. doi: 10.1016/s0306-4522(01)00319-0. [DOI] [PubMed] [Google Scholar]
- Espana RA, Valentino RJ, Berridge CW. Abstract Viewer/Itinerary Planner. Society for Neuroscience; 2002. Fos expression in hypocretin-1 receptor-bearing and hypocretin-synthesizing neurons: Effects of diurnal and nocturnal waking, stress and Hcrt-1 administration. Program No. 776.775. [DOI] [PubMed] [Google Scholar]
- Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: Lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Gautvik KM, de Lecea L, Gautvik VT, Danielson PE, Tranque P, Dopazo A, et al. Overview of the most prevalent hypothalamus-specific mRNAs, as identified by directional tag PCR subtraction. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:8733–8738. doi: 10.1073/pnas.93.16.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco MA, Shiromani PJ. Hypocretin receptor protein and mRNA expression in the dorsolateral pons of rats. Brain Research. Molecular Brain Research. 2001;88:176–182. doi: 10.1016/s0169-328x(01)00039-0. [DOI] [PubMed] [Google Scholar]
- Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, et al. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajszan T, Liposits Z, Zaborszky L. Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2002. Adrenergic input to the perifornical orexin-containing neurons in the rat. 2002 Online, Program No. 735.739. [Google Scholar]
- Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Kilduff TS, et al. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. The Journal of Comparative Neurology. 1999;415:145–159. [PubMed] [Google Scholar]
- Huang ZL, Qu WM, Li WD, Mochizuki T, Eguchi N, Watanabe T, et al. Arousal effect of orexin A depends on activation of the histaminergic system. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:9965–9970. doi: 10.1073/pnas.181330998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ida T, Nakahara K, Murakami T, Hanada R, Nakazato M, Murakami N. Possible involvement of orexin in the stress reaction in rats. Biochemical and Biophysical Research Communications. 2000;270:318–323. doi: 10.1006/bbrc.2000.2412. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, et al. A key role for orexin in panic anxiety. Nature Medicine. 2010;16:111–115. doi: 10.1038/nm.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BE. The role of noradrenergic locus coeruleus neurons and neighboring cholinergic neurons of the pontomesencephalic tegmentum in sleep–wake states. Progress in Brain Research. 1991;88:533–543. doi: 10.1016/s0079-6123(08)63832-7. [DOI] [PubMed] [Google Scholar]
- Karnani MM, Apergis-Schoute J, Adamantidis A, Jensen LT, de Lecea L, Fugger L, et al. Activation of central orexin/hypocretin neurons by dietary amino acids. Neuron. 2011;72:616–629. doi: 10.1016/j.neuron.2011.08.027. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuru M, Ueta Y, Serino R, Nakazato M, Yamamoto Y, Shibuya I, et al. Centrally administered orexin/hypocretin activates HPA axis in rats [In Process Citation] Neuroreport. 2000;11:1977–1980. doi: 10.1097/00001756-200006260-00034. [DOI] [PubMed] [Google Scholar]
- Lee JH, Bang E, Chae KJ, Kim JY, Lee DW, Lee W. Solution structure of a new hypothalamic neuropeptide, human hypocretin-2/orexin-B. European Journal of Biochemistry. 1999;266:831–839. doi: 10.1046/j.1432-1327.1999.00911.x. [DOI] [PubMed] [Google Scholar]
- Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinninger GM, Jo YH, Leshan RL, Louis GW, Yang H, Barrera JG, et al. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metabolism. 2009;10:89–98. doi: 10.1016/j.cmet.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez M, Senaris R, Gallego R, Garcia-Caballero T, Lago F, Seoane L, et al. Orexin receptors are expressed in the adrenal medulla of the rat. Endocrinology. 1999;140:5991–5994. doi: 10.1210/endo.140.12.7287. [DOI] [PubMed] [Google Scholar]
- Lopez M, Seoane L, Garcia MC, Lago F, Casanueva FF, Senaris R, et al. Leptin regulation of prepro-orexin and orexin receptor mRNA levels in the hypothalamus. Biochemical and Biophysical Research Communications. 2000;269:41–45. doi: 10.1006/bbrc.2000.2245. [DOI] [PubMed] [Google Scholar]
- Malendowicz LK, Tortorella C, Nussdorfer GG. Orexins stimulate corticosterone secretion of rat adrenocortical cells, through the activation of the adenylate cyclase-dependent signaling cascade. The Journal of Steroid Biochemistry and Molecular Biology. 1999;70:185–188. doi: 10.1016/s0960-0760(99)00110-7. [DOI] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. The Journal of Comparative Neurology. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- Martin G, Fabre V, Siggins GR, de Lecea L. Interaction of the hypocretins with neurotransmitters in the nucleus accumbens. Regulatory Peptides. 2002;104:111–117. doi: 10.1016/s0167-0115(01)00354-8. [DOI] [PubMed] [Google Scholar]
- Mignot E, Taheri S, Nishino S. Sleeping with the hypothalamus: Emerging therapeutic targets for sleep disorders. Nature Neuroscience. 2002;5(Suppl):1071–1075. doi: 10.1038/nn944. [DOI] [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Uramura K, Nambu T, Yada T, Goto K, Yanagisawa M, et al. Orexin-induced hyperlocomotion and stereotypy are mediated by the dopaminergic system. Brain Research. 2000;873:181–187. doi: 10.1016/s0006-8993(00)02555-5. [DOI] [PubMed] [Google Scholar]
- Nanmoku T, Isobe K, Sakurai T, Yamanaka A, Takekoshi K, Kawakami Y, et al. Effects of orexin on cultured porcine adrenal medullary and cortex cells. Regulatory Peptides. 2002;104:125–130. doi: 10.1016/s0167-0115(01)00356-1. [DOI] [PubMed] [Google Scholar]
- Nishino S, Okura M, Mignot E. Narcolepsy: Genetic predisposition and neuropharmacological mechanisms. Sleep Medicine Reviews. 2000;4:57–99. doi: 10.1053/smrv.1999.0069. [DOI] [PubMed] [Google Scholar]
- Pace-Schott EF, Hobson JA. The neurobiology of sleep: Genetics, cellular physiology and subcortical networks. Nature Reviews. Neuroscience. 2002;3:591–605. doi: 10.1038/nrn895. [DOI] [PubMed] [Google Scholar]
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nature Medicine. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Samson WK, Gosnell B, Chang JK, Resch ZT, Murphy TC. Cardiovascular regulatory actions of the hypocretins in brain. Brain Research. 1999;831:248–253. doi: 10.1016/s0006-8993(99)01457-2. [DOI] [PubMed] [Google Scholar]
- Samson WK, Taylor MM. Hypocretin/orexin suppresses corticotroph responsiveness in vitro. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2001;281:R1140–R1145. doi: 10.1152/ajpregu.2001.281.4.R1140. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Suzuki M, Mieda M, Tsujino N, Roth B, Sakurai T. Pharmacogenetic modulation of orexin neurons alters sleep/wakefulness states in mice. PLoS One. 2011;6:e20360. doi: 10.1371/journal.pone.0020360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. Journal of Neurophysiology. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Shirasaka T, Kunitake T, Takasaki M, Kannan H. Neuronal effects of orexins: Relevant to sympathetic and cardiovascular functions. Regulatory Peptides. 2002;104:91–95. doi: 10.1016/s0167-0115(01)00352-4. [DOI] [PubMed] [Google Scholar]
- Steriade M, Llinas RR. The functional states of the thalamus and the associated neuronal interplay. Physiological Reviews. 1988;68:649–742. doi: 10.1152/physrev.1988.68.3.649. [DOI] [PubMed] [Google Scholar]
- Stricker-Krongrad A, Richy S, Beck B. Orexins/hypocretins in the ob/ob mouse: Hypothalamic gene expression, peptide content and metabolic effects. Regulatory Peptides. 2002;104:11–20. doi: 10.1016/s0167-0115(01)00344-5. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JG, de Lecea L. The hypocretins: Setting the arousal threshold. Nature Reviews. Neuroscience. 2002;3:339–349. doi: 10.1038/nrn808. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Koyama Y, Kayama Y, Yamamoto M. Effects of orexin on the laterodorsal tegmental neurones. Psychiatry and Clinical Neurosciences. 2002;56:335–336. doi: 10.1046/j.1440-1819.2002.00967.x. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Okumura T, Yamada H, Kohgo Y. Stimulation of gastric acid secretion by centrally administered orexin-A in conscious rats. Biochemical and Biophysical Research Communications. 1999;254:623–627. doi: 10.1006/bbrc.1998.9994. [DOI] [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Letters. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- Uramura K, Funahashi H, Muroya S, Shioda S, Takigawa M, Yada T. Orexin-a activates phospholipase C- and protein kinase C-mediated Ca2+ signaling in dopamine neurons of the ventral tegmental area. Neuroreport. 2001;12:1885–1889. doi: 10.1097/00001756-200107030-00024. [DOI] [PubMed] [Google Scholar]
- van den Pol AN. Hypothalamic hypocretin (orexin): Robust innervation of the spinal cord. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1999;19:3171–3182. doi: 10.1523/JNEUROSCI.19-08-03171.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Gao XB, Obrietan K, Kilduff TS, Belousov AB. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1998;18:7962–7971. doi: 10.1523/JNEUROSCI.18-19-07962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, Tokita S, Williams SC, Kisanuki YY, et al. Distinct narcolepsy syndromes in Orexin receptor-2 and Orexin null mice: Molecular genetic dissection of non-REM and REM sleep regulatory processes. Neuron. 2003;38:715–730. doi: 10.1016/s0896-6273(03)00330-1. [DOI] [PubMed] [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annual Review of Neuroscience. 2001;24:429–458. doi: 10.1146/annurev.neuro.24.1.429. [DOI] [PubMed] [Google Scholar]
- Wise RA. Brain reward circuitry: Insights from unsensed incentives. Neuron. 2002;36:229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Annual Review of Psychology. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Wortley KE, Chang GQ, Davydova Z, Leibowitz SF. Orexin gene expression is increased during states of hypertriglyceridemia. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2003;284:R1454–R1465. doi: 10.1152/ajpregu.00286.2002. [DOI] [PubMed] [Google Scholar]
- Xi M, Morales FR, Chase MH. Effects on sleep and wakefulness of the injection of hypocretin-1 (orexin-A) into the laterodorsal tegmental nucleus of the cat. Brain Research. 2001;901:259–264. doi: 10.1016/s0006-8993(01)02317-4. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Tabuchi S, Tsunematsu T, Fukazawa Y, Tominaga M. Orexin directly excites orexin neurons through orexin 2 receptor. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2010;30:12642–12652. doi: 10.1523/JNEUROSCI.2120-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]