Abstract
Background
Recently, the World Health Organization launched a campaign to eradicate the tropical disease yaws, caused by the bacterium Treponema pallidum subsp. pertenue; however, for decades researchers have questioned whether flies act as a vector for the pathogen that could facilitate transmission.
Methods
A total of 207 fly specimens were trapped in areas of Africa in which T. pallidum-induced skin ulcerations are common in wild baboons; 88 flies from Tarangire National Park and 119 from Lake Manyara National Park in Tanzania were analyzed by PCR for the presence of T. pallidum DNA.
Findings
We report that in the two study areas, T. pallidum DNA was found in 17–24% of wild-caught flies of the order Diptera. Treponemal DNA sequences obtained from many of the flies match sequences derived from nearby baboon T. pallidum strains, and one of the fly species with an especially high prevalence of T. pallidum DNA, Musca sorbens, has previously been shown to transmit yaws in an experimental setting.
Interpretation
Our results raise the possibility that flies play a role in yaws transmission; further research is warranted, given how important understanding transmission is for the eradication of this disfiguring disease.
Keywords: Treponema pallidum, Dipteria, Yaws, Nonhuman primates, Transmission
Highlights
-
•
Treponema pallidum DNA was found in 17–24% of wild-caught flies in the Manyara region of Tanzania.
-
•
Results further support the possibility that flies play a role in yaws transmission.
-
•
New theoretic route of inter-species transmission for Treponema
The discovery of Treponema pallidum DNA on necrophagous flies in Africa supports historical reports on possible transmission of the bacterium by flies as a mechanical vector. The bacterium (subsp. pertenue) causes human yaws, which is currently subject to eradication efforts. It has been shown that African nonhuman primates are also found to be infected with T. pallidum strains that are closely related to human yaws causing strains. The ecology of T. pallidum infection in primates is not yet fully understood and intra- and interspecies transmission pathways, apart from skin-to-skin contact in humans, are largely unknown.
1. Introduction
Human yaws, a neglected tropical disease caused by T. pallidum subsp. pertenue (TPE), has been targeted for global eradication by 2020, after previous attempts in the 1950’s (Mitja et al., 2013) did not eradicate the disease (Asiedu et al., 2014). Factors involved in the natural history of yaws, including transmission by flies and a possible nonhuman reservoir (Knauf et al., 2013), may have contributed to yaws re-emergence. In humans, Treponema pallidum subsp. pallidum (TPA) causes syphilis, while TPE causes yaws (Lukehart, 2008, Giacani and Lukehart, 2014). We reported previously high levels of treponemal infection in nonhuman primates (NHPs) with strains closely related to the human yaws-causing strains (Knauf et al., 2013). This was shown on the basis of DNA polymorphisms that are known to distinguish among the subspecies of T. pallidum (Harper et al., 2012, Knauf et al., 2012) as well as on the basis of the genome sequence of the Fribourg-Blanc strain (str. F-B) (Zobaníková et al., 2013), which was isolated from a baboon in Guinea in 1966 (Fribourg-Blanc and Mollaret, 1969). In addition, the subspecies of T. pallidum in humans (Sena et al., 2010) and those in NHPs (Knauf et al., 2015) are not distinguishable based on serology. The transmission of pathogenic treponemes by insects has been debated for over a century (Barnard, 1952a, Gudger, 1911, Gudger, 1910a, Gudger, 1910b, Barnard, 1952b, Lamborn, 1936), although there are no recent published studies.
The observation that flies function as carriers of human pathogens such as Chlamydia trachomatis (causing blinding trachoma) under natural conditions has already been reported (Emerson et al., 2000, Emerson et al., 1999). In contrast, the role of necrophagous flies in the transmission of yaws and other treponematoses, despite the early notes and models at the beginning of the last century (Lamborn, 1936, Castellani, 1907, Kumm, 1935a, Kumm et al., 1935, Kumm and Turner, 1936, Satchell and Harrison, 1953), is not clear. Treponema species have not yet been reported to be present on necrophagous flies caught in a natural ecosystem and, most importantly, molecular evidence of the presence of the different subspecies has not been presented in any reported studies to date. In addition, a better understanding of different modes of transmission of T. pallidum is important for the development of sustainable control strategies. Vector transmission of the yaws bacterium for example, would underline the importance of additional hygiene measures and wound coverage when treating infected individuals. Moreover, if the simian yaws-like treponemes were demonstrated to be infectious to humans (Knauf et al., 2013), it could add an additional route of inter-species transmission. The West African simian isolate (str. F-B) is reported to have caused a sustained infection after experimental inoculation into humans (Smith et al., 1971).
Based on previous reports, we hypothesized that flies function as a mechanical vector for the transmission of T. pallidum in areas of high prevalence of Treponema-induced skin ulceration in primates. To test this hypothesis, we performed a prospective cross-sectional study in areas that have populations of olive baboons (Papio anubis) with T. pallidum-associated genital ulcerations at Tarangire National Park (TNP; unpublished data) and Lake Manyara National Park (LMNP) (Knauf et al., 2012) in Tanzania.
2. Materials and Methods
2.1. Study Sites and Sample Size
TNP (latitude (lat.): − 3·83333, longitude (long.): 36·00000) and LMNP (lat.: − 3·58333, long.: 35·83333) are located in the north of Tanzania. The two parks are about 60 km apart and both belong to the same greater ecosystem (Fig. S1). TNP is mainly dominated by savanna grassland, while LMNP consists of a variety of different vegetation types: groundwater forest with freshwater sources, open grassland, and seasonal swamps in the north, savanna bush land in the center and acacia forest in the far south of the park. Though TNP is much greater in size (2600 qkm TNP vs. 330 qkm LMNP), both national parks face a substantial pressure of human activities along their borders (Kiffner et al., 2015). Wildlife corridors are narrowed down and are almost closed by human settlements. Baboons are often found crop-raiding in neighboring villages and thus are in very frequent conflict with humans.
Reports of T. pallidum infected NHPs at TNP were investigated in 2015 and were confirmed by clinical manifestations, serology, and PCR in 2016 (unpublished data; Chuma et al.). At LMNP, infection in olive baboons has been reported since 1994 and was extensively characterized by a study in 2007 (Harper et al., 2012, Knauf et al., 2012).
A total of 207 fly specimens were trapped in 2014 in areas inhabited by infected baboons: 88 flies from TNP and 119 from LMNP. Sampling was conducted along road transects within the national parks and only in areas where NHPs and in particular baboons were known to occur Areas with no history of NHPs were excluded from sampling.
Two different conservation methods (RNA Later Solution (Ambion, Cat# AM7020) and air dried) were used to store fly specimens until analysis in the laboratory. Further details can be found in the Supplementary material, including a detailed description of flytraps and sampling procedures.
2.2. DNA Extraction
In the laboratory, fly bodies in RNA Later Solution were separated from the liquid. Supernatant transport fluid was collected in a new reaction tube and immediately stored at 4 °C. All working steps were performed under a laminar flow workbench (BSL2). DNA extraction of fly bodies was performed using the First-DNA-All-Tissue-Kit (Gen-ial), following the manufacturer's instructions with some minor modifications. Briefly, fly bodies were smashed in their respective reaction tube using a stamp of a sterile 1-ml syringe. Tissue lysate was then incubated in a mixture of the Gen-ial Kit's lysis buffer #1 (1000 μl) and #2 (100 μl) as well as 20 μl of proteinase K and 10 μl 1 M dithiothreitol (DTT, Cleland's reagent, Sigma Aldrich, Cat# D9779). The lysate was continuously mixed at 600 rpm and incubated at 65 °C (Thermomixer comfort, Eppendorf) for 1 h. Next, temperature was reduced to 37 °C and samples were constantly shaken at 300 rpm overnight. The remaining protocol followed the manufacturer's instructions. DNA pellets were re-suspended in 20–50 μl molecular grade water. Total DNA content and purity were measured using a NanoDrop ND-1000 spectrophotometer (Thermo Scientific).
DNA from transport fluid was extracted using the (QIAamp DNA Mini Kit, Qiagen, Cat# 51306) as follows: fluids were centrifuged at 20,000 × g for 30 min at room temperature, followed by the removal of the supernatant and re-suspension of the cell pellet in 500 μl lysis buffer (10 mM Tris [pH 8·0], 0·1 M EDTA, and 0·5% SDS). After vigorous mixing, the sample was incubated with 30 μl proteinase K for 30 min at 56 °C. Next, 200 μl of the kit's AL buffer was added and incubation was prolonged for another 10 min at 70 °C. The subsequent steps followed the manufacturer's guidance. DNA was eluted from the columns with 200 μl molecular grade water.
DNA extraction from fly specimen supernatant was followed by DNA precipitation to further purify and concentrate the yielded DNA. Therefore, 2·5 volumes (500 μl) ethanol 100%, 2 μl glycogen (Thermo Fisher, Cat# R0561) and 20 μl sodium acetate (3 M, pH 4·8) were added to the DNA solution, mixed for 1 min and stored at − 20 °C overnight. On the next day, centrifugation followed for 45 min at 4 °C and 17,900 × g. Supernatant was discarded and the remaining DNA pellet was washed with 800 μl 70% ethanol. This last step was succeeded by immediate discard of the ethanol and drying of the DNA pellet by centrifugation in a SpeedVac (Concentrator plus, Eppendorf) for 5–10 min (until DNA was free of ethanol). DNA was re-suspended in 50 μl molecular grade water.
2.3. Amplification of Treponema DNA and Fly Species Identification
Fly specimens were analyzed by PCR for the presence of T. pallidum DNA, targeting three different loci: DNA polymerase I (polA) (Liu et al., 2001), tp0548 (Marra et al., 2010), and tp0574 (tp47) (Marra et al., 2016). Target regions were selected purposely to achieve high sensitivity and specificity (polA and tp0574) as well as to identify possible T. pallidum strain variation, using tp0548, a gene that is commonly used for multi-locus strain typing of T. pallidum (Marra et al., 2010).
2.3.1. DNA Polymerase I (polA)
The PCR targeting the T. pallidum polymerase I gene (polA) was performed as described elsewhere (Liu et al., 2001). Briefly, PCR was performed in a reaction volume of 30 μl, which contained 1 U Biotherm Taq 5000 DNA polymerase (Ares Bioscience, Cat# GC-002-1000), 1 × reaction buffer including MgCl2, 0·64 mM dNTPs, 0·33 μM for each primer, 0·6 mg/ml BSA, 0·7% TritonX-100 and 2 μl template DNA. PCR conditions comprised a pre-denaturation step at 94 °C for 2 min, followed by 50 cycles of denaturation at 94 °C for 1 min, annealing at 60 °C for 1 min, and elongation at 72 °C for another min. Amplification was completed with a final extension step at 72 °C for 5 min.
2.3.2. tp0548
Amplification of tp0548 was performed in a reaction volume of 25 μl using primers published elsewhere (Marra et al., 2010). Briefly, the reaction mix consisted of 12·5 μl Universe Hot Start High-Fidelity 2 × PCR Master Mix (Biotool, Cat# 22,101) containing Universal Hot Start High-Fidelity DNA polymerase. The mix was completed with 1 μl of each 10 μM primer and 4 μl DNA extract, independent of the overall genomic DNA content. PCR conditions comprised a pre-denaturation step at 95 °C for 3 min, followed by 50 cycles of denaturation at 95 °C for 15 s, annealing at 62 °C for 15 s, and elongation at 72 °C for 30 s. Amplification was completed with a final extension step at 72 °C for 5 min.
2.3.3. tp0574 (tp47)
Amplification of a 132 bp-long fragment of the tp47 gene was performed in a reaction volume of 25 μl. Primers were used as published elsewhere (Marra et al., 2016). The reaction mix was identical to the one described for tp0548. PCR conditions comprised a pre-denaturation step at 94 °C for 3 min, followed by 45 cycles of denaturation at 94 °C for 15 s, annealing at 55 °C for 15 s, and elongation at 72 °C for 15 s. Amplification was completed with a final extension step at 72 °C for 5 min.
2.3.4. rDNA Internal Transcribed Spacer 2 (ITS-2) for Diptera Species Identification
Fly species identification was performed utilizing a PCR targeting a 500 bp-long fragment of the ITS-2 gene as described elsewhere (Song et al., 2008). PCR conditions were equal to the ones described for amplification of polA, with the exception of the annealing temperature (52 °C).
2.4. Gel Electrophoresis, Purification and DNA Sequencing
All PCR products were run on 1% agarose gels to check for PCR performance and correct amplicon size. PCR products of polA and tp0548 of the correct size were excised from the gel and purified with the Qiagen Gel Extraction Kit (Qiagen, Cat# 28,706) according to the manufacturer's protocol. Unspecific PCR products were occasionally observed, but ignored for further analysis. No sequence variation was expected for tp0574 (tp47). Therefore, only PCR products amplified from two independent fly samples were Sanger sequenced to receive final proof of the correct PCR product (data not shown). Sanger sequencing was performed utilizing the BigDye Terminator Cycle Sequencing kit (Applied Biosystems, Cat# 4,337,455) and the respective forward and reverse amplification primers. Sequencing was conducted on an ABI 3130xl sequencer.
2.5. Data Analysis
Sequences were analyzed and edited using 4Peaks 1.8 (Griekspoor and Groothuis, nucleobytes.com) and SeaView 4.5.4 software (Gouy et al., 2010). Sequences were compared to respective orthologous sequences available in GenBank using the standard nucleotide (nt) BLAST search option (http://blast.ncbi.nlm.nih.gov/Blast.cgi) at the NCBI homepage.
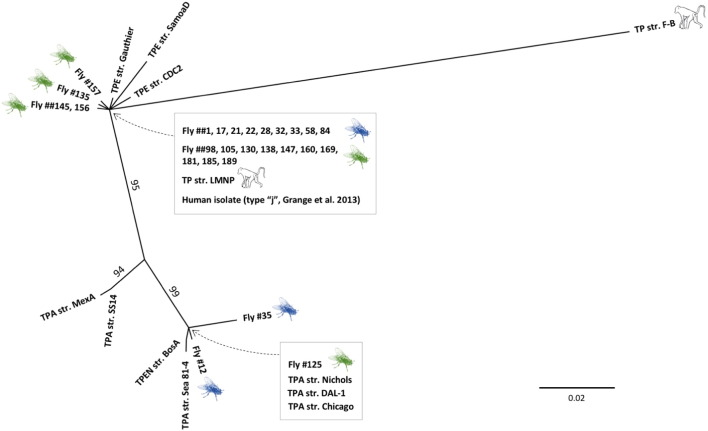
Phylogenetic tree reconstruction was conducted using 26 tp0548 sequences obtained from fly samples (accession numbers can be found under 2.6. or in Table S2) and one of 28 identical sequences from LMNP baboon skin tissue isolates as well as TPA and TPE reference strain sequence data obtained from GenBank. Accession numbers of reference strain sequences are as follows: TPE str.: Gauthier [CP002376], and CDC-2 [CP002375]; TP str. F-B [CP003902]; TPA str.: Nichols [AE000520], Dallas 1 [CP003115], Chicago [CP001752], and Seattle 81-4 [CP003679]); T. p. subsp. endemicum str. Bosnia A [CP007548]. The accession number of a sequence of tp0548 of type “j”, which was obtained from a human, was not available. Data were copied from the respective publication (Grange et al., 2013) and the extension of these data to cover the full sequence (nt 40 to 344) that was obtained from the flies was added from unpublished data (DS). A maximum parsimony (MP) tree with gaps as fifth character and 1000 bootstrap replicates was constructed in SeaView. Tree visualization and editing was performed in FigTree 1.4.2 (http://tree.bio.ed.ac.uk/software/figtree/). Alignment data of sequences used in this study can be found in Fig. S3.
2.6. GenBank Accession Numbers
Sequences obtained from fly specimens are deposited in GenBank under the following accession numbers: KX301264-7 and KX301269-71. The baboon skin tissue isolate from LMNP is deposited under accession number KX301268. Details can be found in Table S2.
3. Results
Overall, 17·0% of the TNP and 23·5% of the LMNP fly specimens were positive for T. pallidum DNA (Table 1). Not all samples were positive or were tested for all three loci (Table 1, Table 2, and Table S2). The polA PCR reaction was positive less frequently than the detection rates obtained from the PCRs targeting tp0548 or tp0574.
Table 1.
Summary of genetic analysis in 207 fly specimens. Qualitative PCR results obtained from DNA extracted from individual fly bodies as well as transport fluids. “Overall” refers to the number of specimens tested positive in at least one of the gene targets. For details, see Supplementary material. Body = Fly body, TF = Transport fluid (RNA-Later Solution).
|
polA |
tp0548 |
tp0574 (tp47) |
Overall |
||||
|---|---|---|---|---|---|---|---|
| Body | Body | TF | Body | TF | Body + TF | ||
| TNP | n tested | 88 | 88 | 8 | 40 | 5 | 88 |
| n positive (%) | 0 (0%) | 10 (11·4%) | 4 (50·0%) | 6 (15·0%) | 4 (80·0%) | 15 (17·0%) | |
| LMNP | n tested | 118 | 118 | 11 | 46 | 6 | 119 |
| n positive (%) | 6 (5·1%) | 17 (14·4%) | 6 (54·5%) | 10 (21·7%) | 4 (66·7%) | 28 (23·5%) | |
Table 2.
Number of loci tested for T. pallidum per fly sample. TNP and LMNP samples are combined. Details can be found in Table 1 and Table S2.
Number of loci tested for T. pallidum per fly sample. TNP and LMNP samples are combined. Details can be found in Table 1 and Table S2.
| 3 loci tested | 2 loci tested (polA and tp0548) | |
|---|---|---|
| n samples tested (total n = 207) | 86 | 121 |
| 3 loci positive/sample | 3 | n/a |
| 2 loci positive/sample | 6 | 1 |
| 1 locus positive/sample | 16 | 17 |
In 17 fly specimens, DNA extracts from both the body and the corresponding transport fluid were tested (Table S2). Two of the specimens were positive for tp0548 in the body extract and transport fluid and seven specimens became positive only when the transport fluid was PCR analyzed. Sequences derived from both extracts of the same fly specimen were identical (fly# 105, Table S2). For the tp0547 gene extracts from eight fly bodies were positive, of which seven were also positive in the transport fluid.
The seven polA and two tp0574 sequences were identical to published orthologs from other T. pallidum subspecies (reference sequences in GenBank: U57757 [polA], M88769 [tp0574]). There was no variation at these two loci in all tested fly specimens.
In contrast, in the tp0548 gene (nt 40 to 347, GenBank: CP000805; Fig. S3) variation was found among various T. pallidum subspecies and strains, which were included as reference, as well as among sequence data obtained from the fly samples. Among the 308 investigated sites, 66 were variable, of which 24 were parsimony-informative (including gaps; Fig. S3). Phylogenetic tree reconstructions based on tp0548 sequence data (Fig. 1) show that the T. pallidum sequences obtained from fly specimens were diverse. This does not change even when a neighbor-joining tree is constructed (Fig. S4). Most sequences obtained from flies cluster with human reference TPE strains (str. Gauthier and str. CDC2), as well as str. F-B, and the sequences derived from T. pallidum-infected baboons at LMNP (n = 28, sample material originates from a study published elsewhere (Knauf et al., 2012)). Interestingly, 19 of the fly specimens (TNP n = 9, LMNP n = 10) exhibit a tp0548 sequence that is identical to that obtained from samples of infected baboons as well as the type “j” sequence that was obtained from a human patient with genital ulceration and infected with a strain closely related to T. pallidum subsp. endemicum (Grange et al., 2013) (unpublished data; Smajs et al.). Four additional sequences (three haplotypes) obtained from fly specimens, all from LMNP, also cluster within the TPE-containing clade, each differing from the LMNP baboon strain sequence in just a single nucleotide.
Fig. 1.
Unrooted maximum parsimony tree based on tp0548 sequence data of human TPA, TPE, and TPEN strains as well as orthologs of T. pallidum from LMNP baboons and fly isolates from TNP and LMNP. The tree includes tp0548 sequences from 26 flies and 28 baboons plus TPA , TPE, and TPEN reference strains as indicated in the Materials and Methods. The type “j” sequence (Grange et al., 2013), which is identical to the baboon derived sequence, has been re-amplified and sequenced to cover a greater part of the previously published tp0548 sequence. It should be noted, however, that despite the tp0548 identity, other gene sequences differ from the TP str. LMNP sequence (unpublished data). The tree is based on 308 sites of which 24 are parsimony-informative. Gaps were coded as fifth character and 1000 bootstrap replicates were performed. Bootstrap values greater than 90% are displayed at respective nodes. Genbank accession numbers are provided in the Materials and Methods and Table S2, as well as the corresponding sequence data alignment, which can be seen in Fig. S3. TPA = T. p. pallidum, TPE = T. p. pertenue, TPEN = T. p. endemicum, str. = strain, F-B = Fribourg-Blanc, TNP = Tarangire National Park; LMNP = Lake Manyara National Park; green symbol: LMNP, blue: TNP. The bar refers to substitutions per site. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Unrooted maximum parsimony tree based on tp0548 sequence data of human TPA, TPE, and TPEN strains as well as orthologs of T. pallidum from LMNP baboons and fly isolates from TNP and LMNP. The tree includes tp0548 sequences from 26 flies and 28 baboons plus TPA, TPE, and TPEN reference strains as indicated in the Materials and Methods. The type “j” sequence (Grange et al., 2013), which is identical to the baboon derived sequence, has been re-amplified and sequenced to cover a greater part of the previously published tp0548 sequence. It should be noted, however, that despite the tp0548 identity, other gene sequences differ from the TP str. LMNP sequence (unpublished data). The tree is based on 308 sites of which 24 are parsimony-informative. Gaps were coded as fifth character and 1000 bootstrap replicates were performed. Bootstrap values greater than 90% are displayed at respective nodes. Genbank accession numbers are provided in the Materials and Methods and Table S2, as well as the corresponding sequence data alignment, which can be seen in Fig. S3. TPA = T. p. pallidum, TPE = T. p. pertenue, TPEN = T. p. endemicum, str. = strain, F-B = Fribourg-Blanc, TNP = Tarangire National Park; LMNP = Lake Manyara National Park; green symbol: LMNP, blue: TNP. The bar refers to substitutions per site. Fly image source: http://www.oldskoolman.de/bilder/plog-content/images/freigestellte-bilder/natur-tiere/fliege-mit-ruessel.jpg (modified).
tp0548 sequence data were also evaluated on the basis of the enhanced typing system (Marra et al., 2010), which uses only the sequence region between nt 130 and 215 (with reference to GenBank: CP000805) (Marra et al., 2010). As a result and with regard to the currently described subtypes (Read et al., 2016), the subtype distribution within the fly samples encompasses 23 sequences of dominating type “j”, two sequences of subtype “a” and a yet undescribed subtype “o” (n = 1; Fig. S5, Table S2).
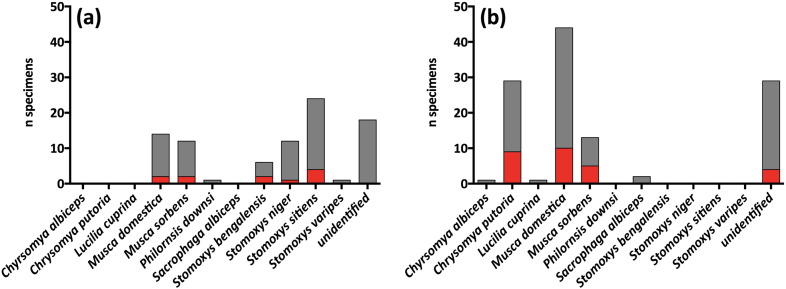
Fly species varied between the national parks (Fig. 2). Two fly species, Chrysomya putoria and Musca sorbens, seem to be disproportionally involved in the acquisition of T. pallidum. A full dataset of all fly specimens is presented in the Supplementary material.
Fig. 2.
Fly species composition and occurrence of T. pallidum DNA on flies caught at (a) TNP (n = 88) and (b) LMNP (n = 119). Fly species identification is based on the output from BLAST search using ITS-2 sequence data. Fly specimens that did not generate identifiable sequence data are classified as “unidentified”. Red = T. pallidum positive, grey = T. pallidum negative.
4. Discussion
Our results indicate that T. pallidum DNA can be frequently detected on wild caught flies, at least in areas of high prevalence of T. pallidum infection in primates. The molecular confirmation of T. pallidum DNA isolated from flies in this study, together with the recent detection of treponemal DNA on fly specimens from a yaws-endemic area of Papua New Guinea (unpublished data; Mitja et al.), supports the hypothesis of vector carriage of treponematoses, including yaws.
Variation in frequency of loci tested positive in fly specimens (Table 2) is most likely associated with a low copy number of treponemes in fly specimens as well as differences of sensitivity of the different PCR assays. However, it must be noted that also the amount of template DNA might have influenced the results since we have used 2 instead of 4 μl template DNA in the polA PCR. All PCRs applied in this study have been published elsewhere and are frequently used for the detection of T. pallidum in humans. The identity of the tp0548 sequence isolated from olive baboons and the recovery of the same sequence from multiple fly specimens from Tanzania strongly suggests that flies often come into contact with the spirochete on NHPs. Our data are limited in that we did not attempt to isolate viable T. pallidum from the flies, which would be necessary to investigate the contagiousness of insects that carry the bacterium or the organism's ubiquity in the environment. T. pallidum is still not cultivable by standard microbiological techniques. Furthermore, based on the three loci that were amplified, it is not possible to identify the sources of contact with the bacteria, although it is most likely that, within the national park, baboon lesions are the major target for necrophagous flies near baboon groups. Our findings are consistent with earlier studies that demonstrated the presence (Kumm et al., 1935, Kumm, 1935b), and fly-associated transmission of treponemes (Lamborn, 1936, Castellani, 1907, Kumm and Turner, 1936, Satchell and Harrison, 1953, Thomson and Lamborn, 1934).
The finding that four additional tp0548 sequences (three haplotypes) obtained from fly specimens, all from LMNP and which cluster within the TPE-containing clade, differ from the LMNP baboon strain sequence in just a single nucleotide, is likely caused by intra-strain variation and therefore does not argue for significant separation from the LMNP simian strain isolate. In contrast, three other fly-derived sequences (two from TNP and one from LMNP) cluster with human TPA reference strains (str. Nichols, str. Dallas 1, str. Chicago, and str. Seattle 81-4) as well as a TPEN str. Bosnia A. These findings may be explained either by the fact that different simian strains are present within the two baboon populations or that some flies had contact with human TPA strains circulating in villages surrounding the national parks. Laboratory contamination with TPA DNA is highly unlikely (Supplementary material).
Geographically, fly specimens with sequences that differ from the dominating tp0548 sequence (Fig. 1), were collected in the northeastern zone of TNP (Fig. S1: A. TNP sampling sites 2 and 4) and in the north (Fig. S1: B. LMNP sampling sites 2, 6, and 7) and south (Fig. S1: B. LMNP sampling site 12) of LMNP. The finding that two of the three tp0548 subtypes (“j” and “a”) were recovered multiple times as well as at least two independent PCRs generated the same sequence of the yet undescribed subtype “o” provides evidence that these subtypes are not associated with sequencing error. Technically, tp0548 sequencing errors were minimized by using proof-reading polymerases and by repeating PCR amplification and sequencing.
A substantial number of samples with negative test results for the fly's body DNA extract became positive for T. pallidum DNA when the corresponding transport fluid was analyzed. This may be explained by two different mechanisms. First, the biology of necrophagous flies forces the insects to land directly on infectious lesions and treponemes may stick to the fly's exoskeleton. Second, Treponema-containing lesion exudate from the fly's esophagous may have been vomited into the transport medium when numbed fly specimens were transferred from the trapping chamber into the reaction tubes. Presumably a combination of both happened. However, since many samples were positive only when the transport fluid was analyzed, this strongly suggests that the treponemes are located mainly on the outside of the exoskeleton. If the esophagous were the major source of contamination of the transport fluid, one would expect that more DNA samples extracted from the fly bodies would be positive.
The variation of fly species composition between the national parks (Fig. 2) reflects the different ecologies of the two sampling areas. While TNP is dominated by dry grassland and is heavily influenced by the Masai people herding their livestock in the surrounding area (Kiffner et al., 2015), LMNP represents a more isolated area, bordered on one side by Lake Manyara and on the other side by the Great East African Rift. At TNP, more livestock-associated flies were found (e.g., Stomoxys spp.), whereas LMNP seems to harbor a more primate/wildlife/wetland-dominated species composition (e.g., Musca spp.) (Fig. 2). In past studies, M. sorbens and M. domestica were demonstrated to transmit treponemes under experimental conditions (Lamborn, 1936, Castellani, 1907, Satchell and Harrison, 1953, Thomson and Lamborn, 1934, Robertson, 1908), although the molecular identification of the spirochetes in these old studies was not possible. Interestingly, M. sorbens was also one of the two fly species that were disproportionally often PCR positive for T. pallidum DNA in the two investigated study areas in Tanzania.
Though the risk for primates to acquire treponemal infection through fly transmission was not examined here, necrophagous flies may play a role as a potential vector for transmission. This is particularly relevant in areas where NHPs have a high prevalence of moist lesions that contain T. pallidum (Knauf et al., 2012). It has been shown that motile spirochetes are demonstrable in the esophagus of flies for up to 7 h following consumption of infectious lesion material (Kumm et al., 1935). Although the spirochetes in those studies were not definitively shown to be T. pallidum, this would provide a reasonable window of time for transmission in a setting where host abundance is high. While there are no recent reports of human yaws cases in East Africa, the area is known to have been endemic for yaws in the past and the current level of surveillance for yaws is unknown. Despite this uncertainty, if fly carriage of viable T. pallidum bacteria is confirmed, in combination with a possible nonhuman reservoir, flies could possibly facilitate the (inter-species) transmission of T. pallidum, which may result in new index cases even if yaws is eradicated in humans. This underlines the importance of continued intense surveillance after mass drug administration to eradicate human yaws, particularly in areas where NHPs are infected with treponemes, e.g. Cote d'Ivoire, Cameroon, Republic of Congo (Knauf et al., 2013).
The following are the supplementary data related to this article.
Supplementary material.
1 = positive, 0 = negative, ex = excluded due to laboratory error, ns = no sequence of suitable quality obtained.
Funding Sources
This work was supported, in part, by R01AI42143 from the National Institutes of Health (to SAL) as well as partly by grants of the German Research Foundation (DFG): KN1097/3-1 and KN1097/4-1 (to SK), RO3055/2-1 (to CR), as well as ZI548/5-1 (to DZ).
The findings and conclusion in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Disclosure of Potential Conflict of Interest
The authors have nothing to declare.
Author Contributions
The study was designed by SK, JR, ISC, HL, CR, and SAL. DNA extraction and PCRs (polA, tp47, tp0548, and ITS-2) were performed at the laboratory of the German Primate Center (SK, SL, CS, CR). Additional PCR testing in a sample subset was run at University of Washington (CG, SAL). Data analyses and manuscript preparation was done by SK, JR, OM, IAVL, ISC, EKB, JDK, RF, HL, DS, PG, DZ, CR, and SAL.
Acknowledgments
We thank Tanzania National Parks and Tanzania Wildlife Research Institute for continuous support during the study. LMNP and TNP Headquarters are acknowledged for their hospitality and field assistance. Franziska Aron is thanked for her assistance in molecular analysis of sample material. SK and HL thank Kristin Harper for fruitful scientific discussions.
References
- Asiedu K., Fitzpatrick C., Jannin J. Eradication of yaws: historical efforts and achieving WHO's 2020 target. PLoS Negl. Trop. Dis. 2014;8(9) doi: 10.1371/journal.pntd.0003016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard C.C. Yaws and flies; past and present opinions on the role of flies in the transmission of framboesia tropica. J. Trop. Med. Hyg. 1952;55(5):100–114. (contd) [PubMed] [Google Scholar]
- Barnard C.C. Yaws and flies; past and present opinions on the role of flies in the transmission of framboesia tropica. II. J. Trop. Med. Hyg. 1952;55(6):135–141. [PubMed] [Google Scholar]
- Castellani A. Experimental investigations on framboesia tropica (yaws) J. Hyg. 1907;7(4):558–569. doi: 10.1017/s0022172400033520. (Cambridge) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson P.M., Bailey R.L., Mahdi O.S., Walraven G.E., Lindsay S.W. Transmission ecology of the fly Musca sorbens, a putative vector of trachoma. Trans. R. Soc. Trop. Med. Hyg. 2000;94(1):28–32. doi: 10.1016/s0035-9203(00)90427-9. [DOI] [PubMed] [Google Scholar]
- Emerson P.M., Lindsay S.W., Walraven G.E. Effect of fly control on trachoma and diarrhoea. Lancet. 1999;353(9162):1401–1403. doi: 10.1016/S0140-6736(98)09158-2. [DOI] [PubMed] [Google Scholar]
- Fribourg-Blanc A., Mollaret H.H. Natural treponematosis of the African primate. Primates Med. 1969;3(0):113–121. [PubMed] [Google Scholar]
- Giacani L., Lukehart S.A. The endemic treponematoses. Clin. Microbiol. Rev. 2014;27(1):89–115. doi: 10.1128/CMR.00070-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouy M., Guindon S., Gascuel O. SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 2010;27(2):221–224. doi: 10.1093/molbev/msp259. [DOI] [PubMed] [Google Scholar]
- Grange P.A., Allix-Beguec C., Chanal J. Molecular subtyping of Treponema pallidum in Paris, France. Sex. Transm. Dis. 2013;40(8):641–644. doi: 10.1097/OLQ.0000000000000006. [DOI] [PubMed] [Google Scholar]
- Gudger E.W. A second early note on the transmission of yaws by flies. Science. 1910;32(827):632–633. doi: 10.1126/science.32.827.632. [DOI] [PubMed] [Google Scholar]
- Gudger E.W. An early note on flies as transmitters of disease. Science. 1910;31(784):31–32. doi: 10.1126/science.31.784.31. [DOI] [PubMed] [Google Scholar]
- Gudger E.W. Further early notes on the transmission by flies of the disease called yaws. Science. 1911;33(846):427–428. doi: 10.1126/science.33.846.427. [DOI] [PubMed] [Google Scholar]
- Harper K.N., Fyumagwa R.D., Hoare R. Treponema pallidum infection in the wild baboons of East Africa: distribution and genetic characterization of the strains responsible. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiffner C., Wenner C., LaViolet A., Yeh K., Kioko J. From savannah to farmland: effects of land-use on mammal communities in the Tarangire-Manyara ecosystem, Tanzania. Afr. J. Ecol. 2015;53(2):156–166. [Google Scholar]
- Knauf S., Batamuzi E.K., Mlengeya T. Treponema infection associated with genital ulceration in wild baboons. Vet. Pathol. 2012;49(2):292–303. doi: 10.1177/0300985811402839. [DOI] [PubMed] [Google Scholar]
- Knauf S., Dahlmann F., Batamuzi E.K., Frischmann S., Liu H. Validation of serological tests for the detection of antibodies against Treponema pallidum in nonhuman primates. PLoS Negl. Trop. Dis. 2015;9(3) doi: 10.1371/journal.pntd.0003637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf S., Liu H., Harper K.N. Treponemal infection in nonhuman primates as possible reservoir for human yaws. Emerg. Infect. Dis. 2013;19(12):2058–2060. doi: 10.3201/eid1912.130863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumm H.W. The digestive mechanism of one of the west Indian ‘eye gnats’: Hippelates pallipes Loew. Ann. Trop. Med. Parasitol. 1935;29(3):283–303. [Google Scholar]
- Kumm H.W. The natural infection of Hippelates pallipes Loew with the spirochaetes of yaws. Trans. R. Soc. Trop. Med. Hyg. 1935;29(3):265–272. [Google Scholar]
- Kumm H.W., Turner T.B. The transmission of yaws from man to rabbits by an insect vector, Hippelates pallipes Loew. Am.J.Trop. Med. Hyg. 1936;s1–16(3):245–271. [Google Scholar]
- Kumm H.W., Turner T.B., Peat A.A. The duration of motility of the spirochaetes of yaws in a small West Indian fly—Hippelates pallipes Loew. Am.J.Trop. Med. Hyg. 1935;s1–15(2):209–223. [Google Scholar]
- Lamborn W.A. The experimental transmission to man of Treponema pertenue by the fly Musca sorbens, WD. J. Trop. Med. Hyg. 1936;15(39):235–239. [Google Scholar]
- Liu H., Rodes B., Chen C.Y., Steiner B. New tests for syphilis: rational design of a PCR method for detection of Treponema pallidum in clinical specimens using unique regions of the DNA polymerase I gene. J. Clin. Microbiol. 2001;39(5):1941–1946. doi: 10.1128/JCM.39.5.1941-1946.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukehart S. Biology of Treponemes. In: Holmes K.K., Sparling P.F., Stamm W.E., editors. Sexually Transmitted Diseases. 4 ed. Mc Graw Hill Medical; New York: 2008. pp. 647–659. [Google Scholar]
- Marra C., Sahi S., Tantalo L. Enhanced molecular typing of Treponema pallidum: geographical distribution of strain types and association with neurosyphilis. J. Infect. Dis. 2010;202(9):1380–1388. doi: 10.1086/656533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra C.M., Tantalo L.C., Sahi S.K., Dunaway S.B., Lukehart S.A. Reduced Treponema pallidum-specific opsonic antibody activity in HIV-infected patients with syphilis. J. Infect. Dis. 2016;213(8):1348–1354. doi: 10.1093/infdis/jiv591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitja O., Asiedu K., Mabey D. Yaws. Lancet. 2013;381(9868):763–773. doi: 10.1016/S0140-6736(12)62130-8. [DOI] [PubMed] [Google Scholar]
- Read P., Tagg K.A., Jeoffreys N., Guy R.J., Gilbert G.L., Donovan B. Treponema pallidum strain-types and association with macrolide resistance in Sydney, Australia: new tp0548 types identified. J. Clin. Microbiol. 2016 doi: 10.1128/JCM.00959-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson A. Flies as carriers of contagion in yaws (framboesia tropica) J. Trop. Med. 1908;213 [Google Scholar]
- Satchell G.H., Harrison R.A. Experimental observations on the possibility of transmission of yaws by wound-feeding Diptera, in Western Samoa. Trans. R. Soc. Trop. Med. Hyg. 1953;47(2):148–153. doi: 10.1016/0035-9203(53)90068-6. [DOI] [PubMed] [Google Scholar]
- Sena A.C., White B.L., Sparling P.F. Novel Treponema pallidum serologic tests: a paradigm shift in syphilis screening for the 21st century. Clin. Infect. Dis. 2010;51(6):700–708. doi: 10.1086/655832. [DOI] [PubMed] [Google Scholar]
- Smith J.L., David N.J., Indgin S. Neuro-ophthalmological study of late yaws and pinta. II. The Caracas project. Br. J. Vener. Dis. 1971;47(4):226–251. doi: 10.1136/sti.47.4.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z., Wang X., Liang G. Species identification of some common necrophagous flies in Guangdong province, southern China based on the rDNA internal transcribed spacer 2 (ITS2) Forensic Sci. Int. 2008;175(1):17–22. doi: 10.1016/j.forsciint.2007.04.227. [DOI] [PubMed] [Google Scholar]
- Thomson J.G., Lamborn W.A. Mechanical transmission of trypanosomiasis, leishmaniasis, and yaws through the agency of non-biting Haematophagous flies: (preliminary note on experiments) Br. Med. J. 1934;2(3845):506–509. doi: 10.1136/bmj.2.3845.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobaníková M., Strouhal M., Mikalova L. Whole genome sequence of the Treponema Fribourg-Blanc: unspecified simian isolate is highly similar to the yaws subspecies. PLoS Negl. Trop. Dis. 2013;7(4) doi: 10.1371/journal.pntd.0002172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.
1 = positive, 0 = negative, ex = excluded due to laboratory error, ns = no sequence of suitable quality obtained.