Abstract
Background
Transfusion of blood at the limits of approved storage time is associated with lower red blood cell (RBC) post-transfusion recovery and hemolysis, which increases plasma cell-free hemoglobin and iron, proposed to induce endothelial dysfunction and impair host defense. There is noted variability among donors in the intrinsic rate of storage changes and RBC post-transfusion recovery, yet genetic determinants that modulate this process are unclear.
Methods
We explore RBC storage stability and post-transfusion recovery in murine models of allogeneic and xenogeneic transfusion using blood from humanized transgenic sickle cell hemizygous mice (Hbatm1PazHbbtm1TowTg(HBA-HBBs)41Paz/J) and human donors with a common genetic mutation sickle cell trait (HbAS).
Findings
Human and transgenic HbAS RBCs demonstrate accelerated storage time-dependent hemolysis and reduced post-transfusion recovery in mice. The rapid post-transfusion clearance of stored HbAS RBC is unrelated to macrophage-mediated uptake or intravascular hemolysis, but by enhanced sequestration in the spleen, kidney and liver. HbAS RBCs are intrinsically different from HbAA RBCs, with reduced membrane deformability as cells age in cold storage, leading to accelerated clearance of transfused HbAS RBCs by entrapment in organ microcirculation.
Interpretation
The common genetic variant HbAS enhances RBC storage dysfunction and raises provocative questions about the use of HbAS RBCs at the limits of approved storage.
Keywords: Sickle cell trait, Red cell storage, Blood, Post-transfusion survival, Transfusion practice, RBC hemolysis
Highlights
-
•
Sickle cell trait (HbAS) RBC exhibit increased resistance to osmotic shock compared to normal (HbAA) RBCs.
-
•
HbAS RBC show accelerated storage-related aging and post-transfusion clearance after cold storage compared to HbAA RBC.
-
•
Reduced post-transfusion survival of stored HbAS RBCs is not due to intravascular hemolysis but due to tissue sequestration.
In allogeneic transfusions, red blood cells (RBCs) are collected and stored for up to 42 days. Historically, donor RBC genetic background is only considered in the context of major Rh and ABO blood groups. This study shows that donor-specific genetic factors such as sickle cell trait, the benign heterozygote state of sickle cell disease, accelerate storage-related hemolysis and reduces RBC post-transfusion survival in mice. Impaired post-transfusion recovery is due to enhanced sequestration in organ microcirculation. Further studies are warranted to determine an appropriate earlier outdate for HbAS RBC units, particularly in malaria-endemic regions where sickle cell trait prevalence is high.
1. Introduction
Sickle cell trait is the carrier status of sickle cell disease (SCD), a severe hemolytic disease that is caused by a point mutation in the gene encoding beta-hemoglobin (β6Glu → Val) that increases the hydrophobicity of this protein when deoxygenated. In SCD RBCs, this mutation causes Hb polymerization under small reductions in physiologic oxygen saturation leading to cell dehydration, increased membrane rigidity and hemolysis (Rees et al., 2010, Brittenham et al., 1985). These altered red cell properties promote vaso-occlusive events in microcirculation, causing severe pain and end-organ ischemia, infarction and progressive dysfunction. Red blood cells (RBCs) from individuals with sickle cell trait (SCT) contain 25–50% HbS that polymerizes only at low fractional oxygen saturations < 50% (Brittenham et al., 1985). Thus, under normal physiologic conditions, individuals with sickle cell trait are asymptomatic. However, under extreme conditions of hypoxia and dehydration, vaso-occlusive events can occur (Statius van Eps and De Jong, 1997).
Historically, donor RBC genetic background is considered benign if the donor lacks clinically relevant symptoms but prolonged storage exposes RBCs to non-physiologic stress conditions and may amplify the effects of occult mutations (Dern et al., 1967, Latham et al., 1982). Sickle cell trait has a high prevalence in malaria endemic regions, which increases the probability that patients requiring RBC transfusions in these regions will receive stored HbAS RBCs. Current transfusion practices supporting the use of sickle cell trait RBCs are based on limited studies performed decades ago, which reported no differences in post-transfusion survival or recovery of sickle trait RBCs compared with normal RBCs. However, storage duration was relatively short (< 21 days compared with present 42-day storage limits) and utilized less sensitive methods to evaluate RBC post-transfusion survival (Callender et al., 1949, Ray et al., 1959, Levin and Truax, 1960). Here, we show that sickle cell trait increases storage hemolysis and reduces red cell post-transfusion survival in mice, an effect that increases with increasing time in storage. Interestingly, transfused HbAS RBCs do not exhibit higher intravascular hemolysis compared to HbAA RBCs, but rather become entrapped in the systemic microcirculation. These findings raise concerns about the viability of stored sickle cell trait red blood cells after prolonged storage and suggest a need for further clinical evaluation of post-transfusion recovery of stored human HbAS containing RBCs.
2. Materials and Methods
2.1. Mice
8–12 week old wildtype (HbAA) mice (C57BL/6J), Berkeley hemizygous (HbAS) (Hbatm1PazHbbtm1TowTg(HBA-HBBs)41Paz/J) mice and transgenic mice expressing enhanced Green Fluorescent Protein in hematopoietic cells (C57BL/6-Tg(UBC-GFP)30Scha/J) were purchased from Jackson Laboratories. Berkeley hemizygous mice express human α-globin and sickle β-globin genes in addition to one copy of the murine β-globin, making them hemizygous for Sickle Cell Disease (HbAS). Our characterization studies and those of others suggest these mice express 20–30% HbS, which is similar to the 20–45% HbS distribution observed in sickle cell trait individuals (Noguchi et al., 2001, Steinberg and Embury, 1986). Procedures involving animals were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Both male and female mice were used as donors for RBC isolation. Recipient male mice were of C57Bl/6J strain and randomly assigned to experimental groups to receive either HbAA or HbAS blood. Experimenters performing the experiments was not blinded to group assignment but was blinded to outcome assessment. There was no exclusion of data from any animals.
2.2. Blood Collection and Storage
Leukoreduced human RBC units were stored under standard blood banking conditions from sickle cell trait donors and ABO blood type matched donors were obtained from Central Blood Bank (Pittsburgh, PA) and stored at 1–6 °C until specified times for testing. Whole blood (WB) was collected from mice via the inferior vena cava immediately following euthanasia using Citrate Phosphate Dextrose solution as an anti-coagulant (Sigma St. Louis, MO). Pooled WB from mice and human blood was leukoreduced using a Pall Purecell® NEO Neonatal High Efficiency Leukocyte Reduction Filter (Hod et al., 2010b). Leukoreduced blood was re-suspended in 14% CPDA-1 (Sigma St. Louis, MO), concentrated to a final hematocrit of 55%, and stored at 1–6 °C in glass vacutainers shielded from light for up to 11 days (Hod et al., 2010b).
2.3. In Vitro Hemolytic Assays
Storage hemolysis and stress-induced osmotic or mechanical hemolysis were measured by supernatant cell-free hemoglobin using Drabkin's assay (Moore et al., 1995). See Supplemental section.
2.4. Post-Transfusion Survival Studies
Fresh or 11-day stored murine RBCs (equivalent to 39–42 day stored human RBCs) (Mangalmurti et al., 2009, Gilson et al., 2009) for transfusion were labeled with lipophilic dyes 1,1′-dioctadecyl-3,3,3′3′-tetramethylindocarbocyanine perchlorate dyes DiI (D-383) or DiD (D-307) (Invitrogen, Carlsbad CA) prior to infusion in recipient mice by retro-orbital injection. RBC 24 h post-transfusion recovery was obtained by blood sampling via tail vein and enumerated by flow cytometry and analyzed using FlowJo (Ashland, OR). To confirm that fluorescent labeling dyes do not alter RBC post-transfusion survival, C57BL/6 mice expressing Green Fluorescent Protein (GFP) driven by the human ubiquitin C promoter (C57BL/6-Tg (UBC-GFP) 30Scha/J) were transfused with unlabeled fresh or stored HbAA and HbAS RBCs individually and analyzed using a negative FITC gate to quantify 24 h post-transfusion recovery.
2.5. Statistical Methods
To measure storage-related changes between HbAA and HbAS RBCs such as echinocyte formation, changes in hemolytic propensity and post-transfusion survival, two-way ANOVA with Bonferroni post-test for individual comparisons was used to perform statistical analysis. To determine differences in HbAA and HbAS RBC sequestration in tissues, Mann-Whitney U test was used for non-parametric analysis (GraphPad Prism 6, La Jolla, CA). Initial pilot studies examining RBC post-transfusion recovery provided the basis for sample size estimation of recipient mice. Details regarding statistics, technical and biological replicates performed are provided in the Figure Legends.
3. Results
3.1. HbAS Red Blood Cells Exhibit Higher Storage Hemolysis, Increased Resistance to Osmotic Stress
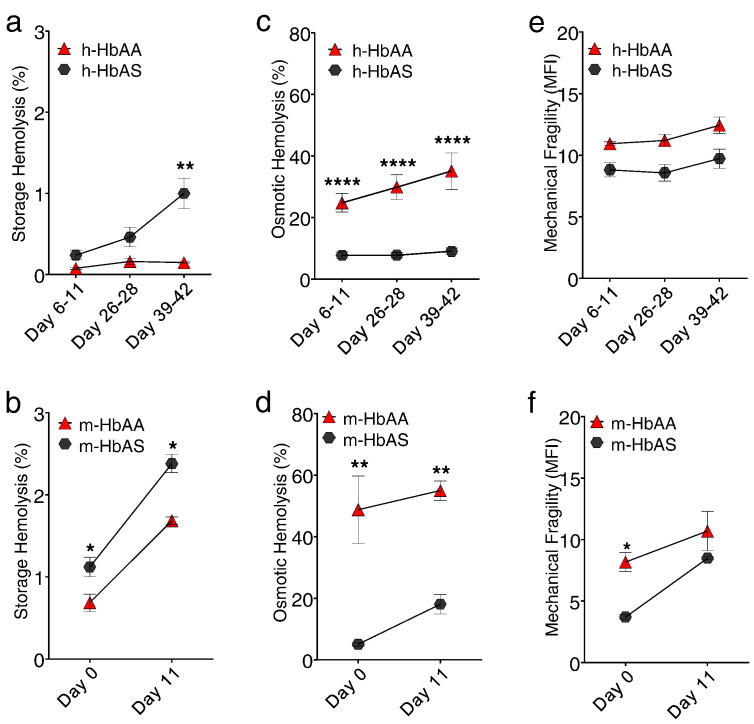
To assess the membrane properties of HbAA and HbAS RBCs, conventionally banked human and murine RBCs stored in standard preservative solution were assayed at the beginning and end of storage. Human RBCs under standard blood banking can be stored up to 42 days (Koch et al., 2008). Leukoreduced murine RBCs stored for up to 14 days show post-transfusion recovery that approximates what is observed with human RBCs at the limits of approved storage, suggesting that these shorter storage times represent a more appropriate and conservative model for day 39–42 human RBC storage (Hod et al., 2010b, Gilson et al., 2009). By 39–42 days of storage, conventionally banked human HbAS (h-HbAS) RBCs exhibited higher storage hemolysis compared to human HbAA (h-HbAA) RBC samples (1.0 ± 0.6% versus 0.15 ± 0.02%, p = 0.0035, Fig. 1a). RBCs from Berkeley hemizygous mice (m-HbAS) also exhibited higher storage hemolysis at the end of 11-day storage compared to murine HbAA (m-HbAA) RBCs (2.4 ± 0.16% versus 1.7 ± 0.1%, p = 0.0022, Fig. 1b). In both human and murine HbAS RBCs, free hemoglobin concentrations were higher at the beginning of storage compared to HbAA RBCs, a transient increase likely due to mechanical challenges suffered during leukoreduction (Fig. 1a–b) (Stroncek et al., 2002). The size distribution and hemoglobin content of human HbAA and HbAS RBCs showed no differences (Supplemental Fig. S1).
Fig. 1.
HbAS is associated with higher storage hemolysis in RBCs. Fresh leukoreduced human RBC units (n = 3 HbAA and n = 9 HbAS donors) processed and stored under standard blood banking conditions and murine RBC units (n = 3 HbAA RBCs and n = 3 HbAS RBCs pooled samples, where each pooled sample contained RBCs from n = 11 mice) were stored at 4 °C, sampled and tested at various times during a 42-day or 11-day storage period, respectively. (a,b) At the end of storage, there was a higher concentration of free hemoglobin in HbAS RBC samples compared to HbAA RBCs. (c,d) HbAS RBCs exhibited increased resilience under hypotonic-induced osmotic shock compared to HbAA RBCs throughout storage, as measured by the fraction of lysed RBCs in a hypotonic solution. (e,f) Human and murine RBCs showed no difference at the end of storage under mechanical stress induced by agitation with one metal bead (3/32″) for 180 min in a 96-well plate. HbAA RBCs are indicated as red triangle and HbAS RBCs are indicated as black circle. Human HbAA (h-HbAA), Human HbAS (h-HbAS), Mouse HbAA (m-HbAA), Mouse HbAS (m-HbAS). The results are presented as mean ± SEM. *p < 0.05; **p < 0.01; ****p < 0.0001 analyzed by 2Way ANOVA, GraphPad Prism 6.0.
To determine membrane resilience to osmotic shock, packed RBCs were re-suspended in a hypotonic buffer for 3 h. Similar to prior findings in HbSS RBCs,(Franco et al., 2000) human and murine HbAS RBCs showed reduced % osmotic hemolysis, or increased resilience to osmotic shock, compared with HbAA RBCs (h-HbAS and h-HbAA RBCs: 9.0 ± 3·1% vs. 35.1 ± 10.3% hemolysis; p < 0.0001, Fig. 1c; m-HbAS and m-HbAA RBCs: 18.1 ± 5.4% vs. 55 ± 5.4% hemolysis; p = 0.0001, Fig. 1d). The osmolarity at which RBCs exhibited maximum deformability (Osm Max) was lower for HbAS RBCs compared to HbAA RBCs at the beginning and end of storage (Day 42, h-HbAS: 217.7 ± 0.5 vs. h-HbAA: 249.7 ± 1.2 mOsm), indicating that HbAS RBCs are more dehydrated than HbAA RBCs and provides a possible explanation for HbAS RBC resistance to hypotonic-induced osmotic stress (Day 11, m-HbAS: 265.2 ± 6.0 vs. m-HbAA: 353.5 ± 5.6 mOsm) (Supplemental Fig. S2, Fig. 1c–d). Human and murine HbAS RBCs did not show increased hemolysis following mechanical stress compared with HbAA RBCs (Fig. 1e–f). Taken together, the Berkeley murine HbAS RBCs appear to show similar membrane properties to that of human HbAS RBCs during storage, suggesting that these mice serve as a suitable model of sickle cell trait for transfusion studies.
3.2. Elevated Echinocyte Formation in HbAS RBCs Compared to HbAA RBCs During Storage
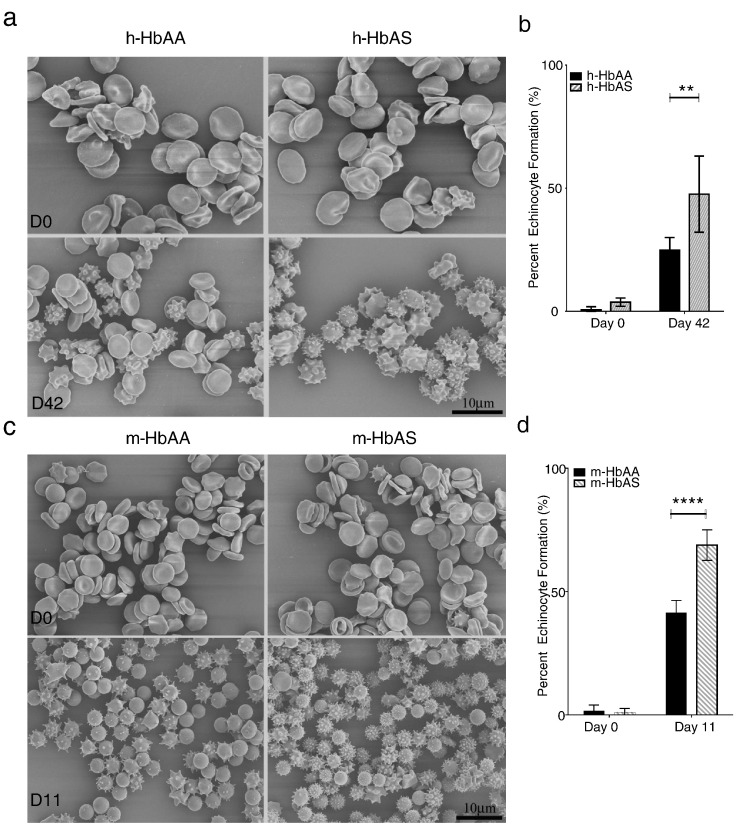
Echinocyte formation occurs due to various factors such as cell dehydration and ATP depletion, and has previously been used as a surrogate marker for RBC storage integrity (Flatt et al., 2014). Human and murine RBCs were examined by scanning electron microscopy at the beginning and end of 39–42 or 11-day storage, respectively. There were no differences in the percentage of echinocytes between h-HbAA and h-HbAS RBCs or m-HbAA and m-HbAS RBCs at the beginning of storage but h-HbAS RBCs showed higher percentage of echinocytes at the end of storage compared to h-HbAA RBCs (47.6% and 24.9%; p = 0.0048, Fig. 2a–b). A similar finding was observed in m-HbAS RBCs compared to m-HbAA RBCs (68.8% and 41.4%, p < 0.0001, Fig. 2c–d).
Fig. 2.
Echinocyte formation is increased in HbAS RBCs compared to HbAA RBCs during storage. RBC images were taken with a JEOL JSM- 6335F scanning electron microscope. (a) Upper panel shows representative fields from fresh and stored human HbAA and HbAS RBCs at the beginning and the end of 39–42 day storage. (b) Five fields per sample were counted and analyzed from one healthy and one SCT representative donors at the beginning and end of storage. Human and mouse RBC SEM analysis was performed twice. (c) Lower panel shows representative fields from fresh and 11-day stored murine RBCs obtained from WT C57BL/6 and Berkeley hemizygous mice (One pooled sample each where n = 11 mice donor RBCs/pooled sample). (d) Six fields were counted and analyzed for echinocyte formation from each pooled sample. The results are presented as mean ± SEM, where echinocyte formation represents % echinocyte per total number of cells in fields counted. **p < 0.01; ****p < 0.0001 analyzed by 2Way ANOVA, GraphPad Prism 6.0.
3.3. Stored HbAS RBCs Exhibit Accelerated 24 h Post-Transfusion Clearance Compared to Stored HbAA RBCs
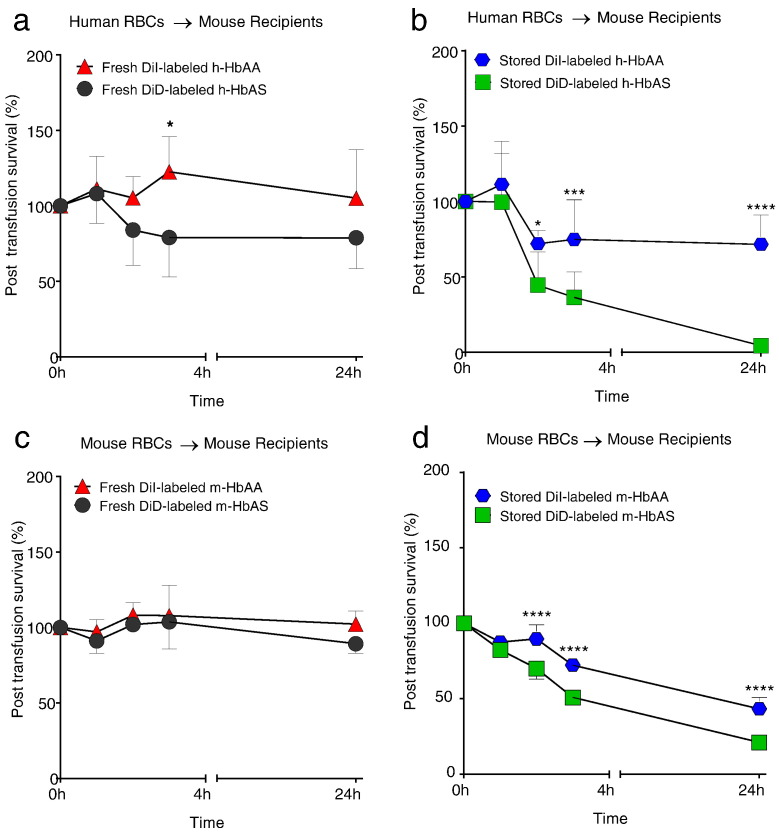
Next we sought to examine whether the increased storage hemolysis and increased echinocyte formation of human and mouse HbAS RBCs was indicative of accelerated aging that impacts post-transfusion survival. We established a murine model of allogeneic and xenogeneic transfusion, using transfusion of DiI-labeled and DiD-labeled donor RBCs (Hod et al., 2010b, Gilson et al., 2009, Hod et al., 2010a). There was no significant difference in the post-transfusion recovery of fresh DiI-labeled h-HbAA and fresh DiD-labeled h-HbAS RBCs in mice at 24 h (Fig. 3a). Fresh h-HbAA and h-HbAS showed 100% and 78.9% survival in circulation 24 h post-transfusion, respectively. The > 100% post-transfusion survival of fresh HbAA RBCs at earlier time points is due to incomplete distribution of transfused RBCs in the circulating blood volume at the time of the initial tail vein blood sampling (Ti = 5 min post-transfusion). As Ti is arbitrarily defined as 100% and all subsequent time points are referenced to Ti, fresh HbAA transfused RBCs, upon complete distribution, creates an artifact of > 100% survival in subsequent RBC recovery estimations given their negligible loss in circulation. This artifact has been previously reported in red cell survival post-transfusion studies (Franco et al., 2000, Mock et al., 1999). After 39–42 day storage, the mean 24 h post-transfusion survival was 71.6 ± 19.5% for stored h-HbAA and 4.3 ± 4.9% for stored h-HbAS RBCs (Fig. 3b). Consistent with human RBCs, stored m-HbAS RBCs showed accelerated clearance from circulation compared with stored m-HbAA RBCs, whereas no differences were noted in post-transfusion recovery of fresh m-HbAS and fresh m-HbAA RBCs (Fig. 3c–d).
Fig. 3.
Stored human and murine HbAS RBCs show accelerated post-transfusion clearance compared to stored HbAA RBCs. (a) WT C57BL/6 recipient mice (n = 6) were transfused with a 50:50 mixture of fresh DiI-labeled h-HbAA RBCs (indicated as red triangle) and fresh DiD-labeled h-HbAS RBCs (indicated as black circle). (b) N = 9 WT C57BL/6 recipients were transfused with a 50:50 mixture of 39-day stored DiI-labeled h-HbAA RBCs (indicated as blue circle) and stored DiD-labeled h-HbAS RBCs (indicated as green square) both re-suspended to a 55% hematocrit following labeling. (c) WT C57BL/6 mice recipients (n = 6) were transfused with a 50:50 mixture of fresh DiI-labeled m-HbAA RBCs (indicated as red triangle) and fresh DiD-labeled m-HbAS RBCs (indicated as black circle). (d) WT recipient mice (n = 6) were transfused with 11-day stored DiI-labeled m-HbAA RBCs (indicated as blue circle) and DiD-labeled stored m-HbAS RBCs (indicated as green square). All mice were 8–12 weeks of age and received a total volume of 200 μl of leukoreduced HbAA and HbAS RBCs (100 μl each). To mimic similar conditions, human and murine RBCs were stored in glass. Post-transfusion recovery was measured by dual-label cell tracking by flow cytometry unless stated otherwise. The results are presented as mean ± SD. *p < 0.05; ***p < 0.001; ****p < 0.0001 analyzed by 2Way ANOVA, GraphPad Prism 6.0.
The high 24 h post-transfusion survival of fresh human and murine HbAA or HbAS in recipient mice underscore the lack of an immune-mediated clearance of transfused RBCs. The findings confirm previous reports indicating the absence of anti-major histocompatibility complex antibodies responsible for the clearance of incompatible red cells in mice, and suggest that the rapid clearance of human and murine HbAS RBCs is due to storage-dependent changes and not alloantibody-mediated RBC clearance (Stimpfling et al., 1976, Hod et al., 2010a, Ong and Mattes, 1989) (Rice and Crowson, 1950). To ensure that labeling dyes did not significantly impact RBC clearance, C57BL/B6 mice expressing GFP were transfused with unlabeled m-HbAA or m-HbAS RBCs (Supplemental Fig. S3). No differences were observed in the post-transfusion survival when fresh unlabeled m-HbAA and m-HbAS RBCs were transfused into separate groups of mice expressing GFP (Supplemental Figs. S3C, S4), while we again observed that the transfusion of unlabeled stored and aged m-HbAS RBCs showed accelerated clearance compared to unlabeled stored and aged m-HbAA RBCs (Supplemental Fig. S3D). Furthermore, there was no difference in the post-transfusion survival of stored human RBCs following 39–41 day storage in standard plasticizer bags or in glass (Supplemental Fig. S5) suggesting that HbAS RBC in vivo behavior following storage was not due to specific storage conditions. The post-transfusion clearance of stored human and murine RBCs show a bi-phasic curve with rapid disappearance of both HbAA and HbAS RBCs within 2 h post-transfusion, followed by slower rate of disappearance where HbAS RBCs exhibited two-fold rate of disappearance (rate of disappearance per hour, h-HbAS: 31.8% and h-HbAA: 12.6%; p = 0.0043) (Fig. 3b) and (m-HbAS: 24.5% versus m-HbAA RBC 13.8% p = 0.0022) at 2 h (Fig. 3d).
3.4. Clodronate Treatment or Splenectomy Does Not Alter Stored HbAS RBC 24 h Post-Transfusion Survival
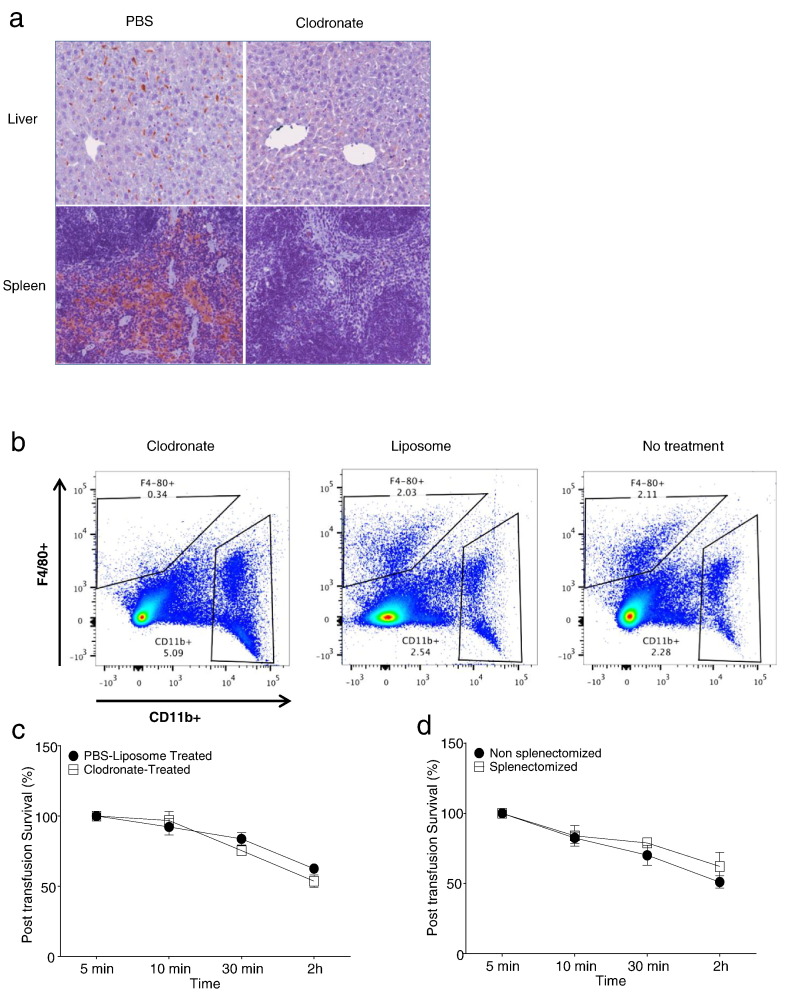
To determine whether stored HbAS RBCs are preferentially cleared by the mononuclear phagocyte system, we depleted F4/80+ macrophages in the liver and spleen using liposomal clodronate prior to transfusion. Intraperitoneal administration of liposomal clodronate effectively depleted F4/80+ macrophages in both the liver and spleen, with > 85% depletion of splenic F4/80+ macrophages 24 h post-treatment (Fig. 4a, b). We show clodronate depletion of macrophages enhances early post-transfusion survival of stored m-HbAA RBCs as previously shown but not stored m-HbAS RBCs (Fig. 4c–d, S6A–B)(Hod et al., 2010b). We next performed surgical splenectomies and survived mice for 5 days until stable for transfusion studies, we found that consistent with the RBC post-transfusion survival studies in splenectomized mice (Fig. 4d), splenectomy did not alter tissue sequestration of stored m-HbAS RBCs within the kidneys and livers following transfusion (Fig. 5a–b). Taken together, these findings suggest that the mechanism of reduced post-transfusion survival of stored m-HbAS RBCs is different from that of stored m-HbAA RBCs where macrophage- and spleen-mediated clearance mechanisms may play a predominant role.
Fig. 4.
Clodronate treatment or splenectomy does not alter stored m-HbAS RBC post-transfusion survival. (a) WT recipient mice were treated with clodronate or PBS liposomes 24 h prior to liver and spleen harvest for immunohistochemistry. Representative images are shown indicating staining for F4/80 + macrophages. (b) WT mice received either clodronate, PBS liposomes or no treatment. Mice were euthanized after 24 h. Spleens were homogenized and stained with F4/80 and CD11b antibodies. Samples were analyzed using flow cytometry to quantify the F4/80 + population. (c) WT recipient mice were transfused with 11-day stored m-HbAS RBCs 24 h following clodronate (n = 3) or PBS liposome (n = 3) injection (2 mg i.p.). (d) WT recipient mice were transfused with 11-day stored m-HbAS RBCs 5 d following splenectomy (n = 4) or sham procedure (n = 6) Results presented are mean ± SD.
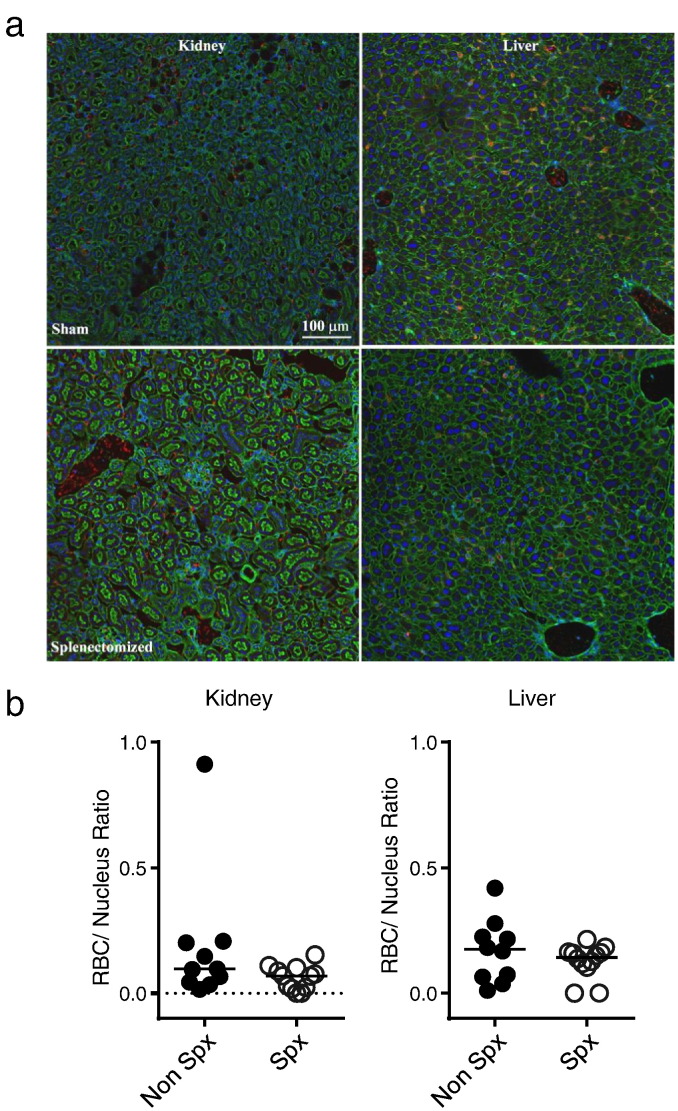
Fig. 5.
Splenectomy does not increase kidney and liver sequestration of stored m-HbAS RBCs following transfusion. Splenectomized WT C57BL/6 recipient mice (n = 7) or sham operated (n = 5) were transfused with stored m-HbAS RBCs. Kidney and liver organs were harvested 2 h post-transfusion, fixed in 2% PFA and processed for confocal imaging. (a) Confocal images showing sequestered m-HbAS RBCs in recipient mouse tissues (Magnification 20 ×, Green: phalloidin, Blue: DAPI, Red: Cy3-labeled m-HbAS RBCs). (b) Individual points represent sequestered m-HbAS RBCs normalized to nuclei number per section quantified by fluorescence intensity using NIS Elements. Two sections from each recipient mouse organ were analyzed.
We further investigated whether intravascular hemolysis contributes to the reduced post-transfusion recovery of stored m-HbAS RBCs. Transfused mice recipients were assayed for plasma and urinary free hemoglobin at various time intervals. Recipient mice transfused with stored m-HbAS RBCs did not show higher levels of plasma or urinary free hemoglobin levels at 5 min and 4 h post-transfusion compared with mouse recipients transfused with m-HbAA RBCs (Supplemental Fig. S7), indicating that intravascular hemolysis did not contribute significantly to the reduced post-transfusion recovery of m-HbAS RBCs.
3.5. Increased Sequestration of Stored HbAS RBCs Within Kidney, Liver and Spleen Following Transfusion
To evaluate whether stored HbAS and HbAA RBC show differences in tissue entrapment following transfusion and provide an explanation for the rapid clearance of stored m-HbAS RBCs in intact WT recipient mice, we transfused cy3-labeled stored m-HbAA or m-HbAS RBCs and harvested kidney, liver and spleen of recipient mice 2 h post-transfusion for confocal imaging and quantitative analysis (Fig. 6, Fig. 7). There was higher sequestration of stored m-HbAS RBCs within the recipient kidney, liver and spleen when compared to recipients transfused with m-HbAA RBCs (Fig. 6a and b). When stored h-HbAS and h-HbAA RBCs were transfused into murine recipients, h-HbAS RBCs were entrapped in the spleen to a greater extent than h-HbAA RBCs (Fig. 7a–b), presumably due to the narrower internal diameter of splenic sinusoidal vessels (Schroit et al., 1984, Mebius and Kraal, 2005, Connor et al., 1994). Collectively, these findings indicate that enhanced mechanical entrapment in tissue accounts for the rapid clearance of transfused HbAS RBCs from circulation of intact mice. It remains unclear why splenectomy did not improve RBC recovery in the circulation since this appears to be a major site of RBC entrapment. This may reflect the propensity of HbAS RBCs to entrap in the liver, kidney and other organs (Fig. 6a–b).
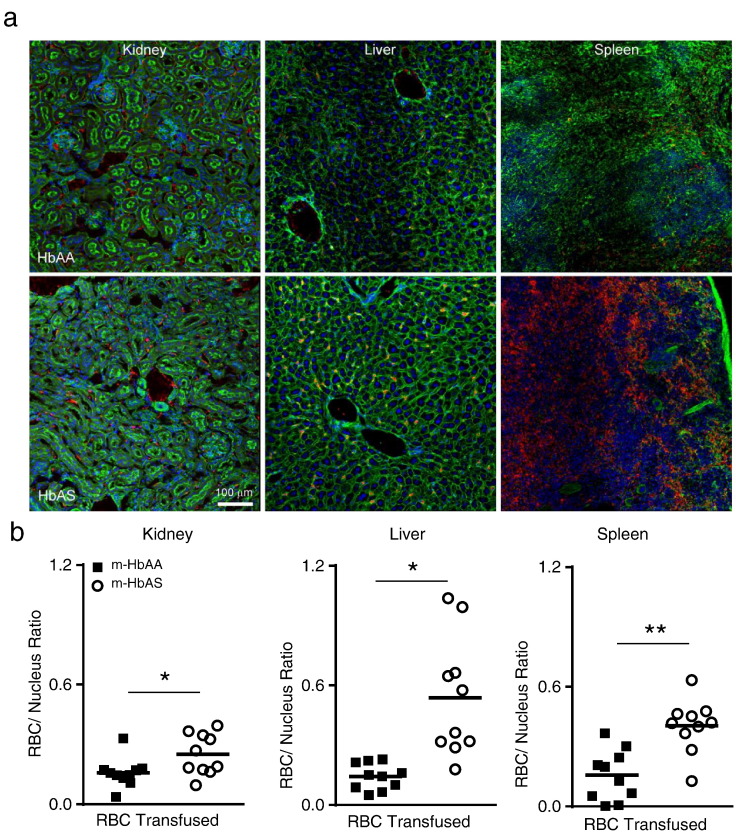
Fig. 6.
Increased tissue sequestration of stored m-HbAS RBCs within kidney, liver and spleen organs following transfusion when compared with stored m-HbAA RBCs. WT C57BL/6 recipient mice (n = 5 per group) were transfused with stored cy3 labeled m-HbAA or m-HbAS RBCs. Kidney, liver and spleen organs were harvested 2 h post-transfusion for confocal imaging. (a) Confocal images showing sequestered m-HbAA and m-HbAS RBCs in recipient mouse tissues (Magnification 20 ×, Green: phalloidin, Blue: DAPI, Red: cy3 RBCs). (b) Individual points represent sequestered cy3-labeled RBCs normalized to nuclei number per section quantified by fluorescence intensity using NIS Elements. Two sections from each recipient mouse organ were analyzed (n = 10 sections per group). Statistical Analysis by two-tailed Mann Whitney U, *p < 0.05, **p < 0.01, lines indicate the median.
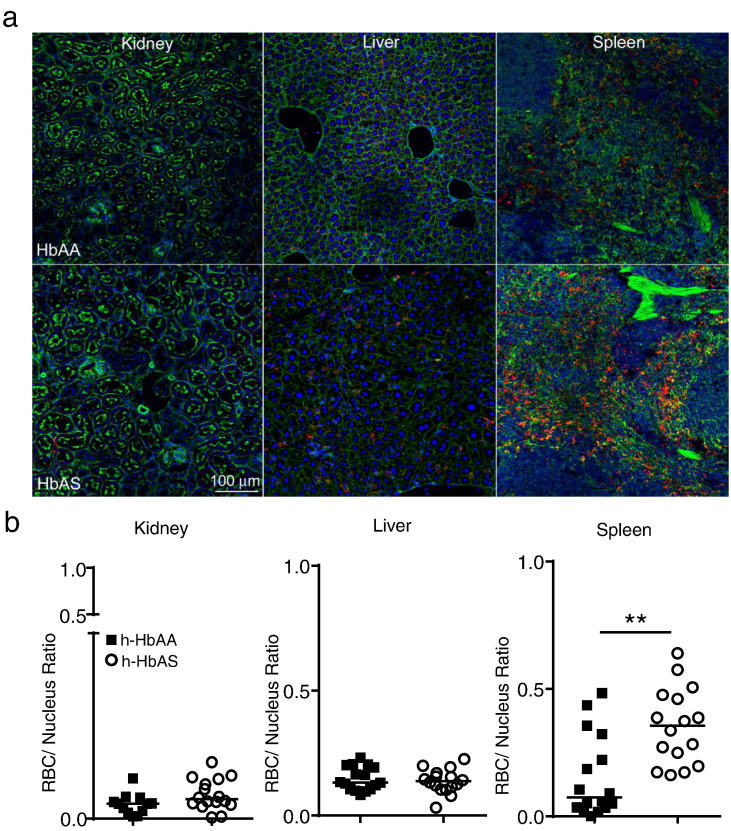
Fig. 7.
Increased sequestration of stored h-HbAS RBCs in the spleen following transfusion when compared with stored h-HbAA RBCs. WT C57BL/6 recipient mice (n = 8 per group) were transfused with conventionally stored h-HbAA or h-HbAS RBCs. Kidney, liver and spleen organs were harvested 2 h post-transfusion for confocal imaging. (a) Confocal images showing sequestered h-HbAA and h-HbAS RBCs in recipient mouse tissues (Magnification 20 ×, Green: phalloidin, Blue: DAPI, Red: Glycophorin A: FITC antibody for human RBCs). (b) Individual points represent sequestered h-RBCs normalized to nuclei number per section quantified by fluorescence intensity using NIS Elements. Two sections from each recipient mouse organ were analyzed (n = 16 sections per group). Statistical Analysis by two-tailed Mann Whitney U, **p < 0.01, lines indicate the median.
4. Discussion
The main findings of this study are that both human and murine HbAS RBCs show accelerated storage-related aging and reduced post-transfusion survival after prolonged cold storage compared to HbAA RBCs. There was no difference in the post-transfusion recovery of fresh murine and human HbAA and HbAS RBCs when transfused into wild type C57Bl/6 mouse recipients, suggesting accelerated storage-induced aging of HbAS RBCs. Our findings indicate that enhanced mechanical entrapment in the kidney, liver and spleen account for the rapid clearance of stored m-HbAS RBCs following transfusion, an observation possibly consistent with rare clinical observations of organ injury in sickle cell trait individuals (Rice and Crowson, 1950).
To date, over 1000 mutations in the human genome relate to the evolved response to endemic malaria infection, with sickle cell trait being one of the most common mutations that confers protection against this endemic parasite (Hedrick, 2011, Jallow et al., 2009). It still remains to be determined whether other hemoglobinopathies, carrier status of alpha thalassemia, and glucose-6-phospate dehydrogenase (G6PD) deficiency that occur with high frequency in African Americans and populations originally from malaria endemic regions, exhibit similar behavior as sickle cell trait during storage and following transfusion. This is particularly important because these populations represent a donor pool from which sickle cell disease patients are likely to be transfused to reduce the risk of RBC allo-immunization. Carriers of these mutations usually do not present with clinical symptoms, however, as shown in this study, prolonged exposure of RBCs to storage-induced stress may unmask subtle perturbations of the heterozygous state.
Our data support previous studies showing no difference in the storage hemolysis of h-HbAA and h-HbAS RBCs at earlier time-points (up to 21 days) (Levin and Truax, 1960, Ray et al., 1959). However, after 28 days, this accumulation intensifies in h-HbAS RBC units. These studies suggest a need to re-evaluate the suitability of HbAS RBCs for transfusion after prolonged storage since prior studies only examined RBCs that were stored for shorter periods (< 21 days) of time in ACD anticoagulant and utilized less precise methods to assess RBC post-transfusion survival. In this controlled study, we utilize highly sensitive tracking methods in examining the storage integrity and post-transfusion survival of h-HbAS RBCs at the limits of the current approved storage time of 42 days. The relevance of this study is underscored by the observation that fresh h-HbAA and h-HbAS RBCs exhibit a high post-transfusion survival and only show decreased post-transfusion survival in wild type C57Bl/6 mice following storage, which cannot be explained by mechanical restraints due to the larger diameter of human RBCs compared to murine RBCs, since even stored h-HbAA RBCs still exhibit high post-transfusion survival.
There are no comprehensive studies evaluating clearance of human RBCs in immunocompetent mice. Prior studies utilized NOD-scid mice for xenotransfusion of human RBCs based upon an assumption that xenotransfusion in immunocompetent mice would induce rapid immune-mediated clearance of human RBCs (Bazin et al., 2002, Moore et al., 1995). However, even in NOD-scid mice, fresh human RBCs showed significant clearance over the first 4 h, arguing against clearance by cross-reactive anti-hRBC antibodies as NOD-scid mice lack such antibodies (Moore et al., 1995, Bazin et al., 2002). It is also unlikely that cross-species differences in RBCs induce rapid alternative complement-mediated lysis of h-RBC in mice, as mouse complement fails to lyse human RBCs (Ish et al., 1993). In contrast, we observe near 100% post-transfusion recovery of fresh human AA RBCs transfused into immunocompetent C57Bl/6 mice. This discrepancy may be related to differences in the labeling method of transfused RBCs, as prior studies utilized chromium-labeling as an in vivo tracking method (Bazin et al., 2002). Chromium binds to hemoglobin but it is possible that chromium may elute from transfused human hemoglobin and result in the artefactual appearance of rapid RBC clearance (Awwad et al., 1966, Gimlette, 1978). We utilized a lipophilic fluorescent dye that directly intercalates with the RBC membrane.
To prevent the negative impact of hemolysis, the U.S. Food and Drug Administration set the upper threshold of hemolysis in a RBC unit to be transfused at 1% but some RBC units stored under standard blood banking conditions fail to meet this requirement due to factors that may be donor specific (Mishler et al., 1979, Dumont and Aubuchon, 2008). The human HbAS RBCs purchased from a commercial blood bank for this study would not have met the regulatory requirement near the end of the FDA accepted shelf-life of 42 days, as these units exhibited a hemolysis level of > 1%. Other occult genetic mutations such as sickle cell trait that increase storage hemolysis may explain why some RBCs age faster during storage.
The enhanced tissue sequestration of HbAS RBCs that we observed may be due to reduced deformability of HbAS RBC membrane that worsens during storage and increases the propensity for microvascular obstruction. However, splenectomy of recipient mice did not alter post-transfusion recovery of stored m-HbAS RBC. This observation is likely due to the fact that the liver is significantly larger and about ten times the spleen weight in mice, so that increases in circulating RBCs after splenectomy are not measurable (Sisto et al., 2003). The limitation in our technique to fully quantify the capacity of the liver to entrap and clear large numbers of stored RBCs in real time may account for the lack of observable differences.
One limitation of this study and previous studies utilizing murine RBCs to study the effect of storage lesion on RBC post-transfusion survival is the number of mice that are needed to obtain sufficient volumes (~ 75 ml) for conventional storage in pediatric transfer bags with diethylhexyl phthalate (DEHP). It has also been shown that storage in pediatric transfer bags increases RBC hemolysis and alters osmotic fragility (Kanias et al., 2013). These considerations demonstrate the practical challenges of an acceptable storage model to mimic RBC behavior in standard-sized blood bags. Of note, studies performed decades ago to determine the transfusion suitability of stored h-HbAS RBCs were not under current approved conditions (Levin and Truax, 1960, Ray et al., 1959). Nonetheless, murine RBCs stored under similar conditions exhibited progressive reduction in 24 h post-transfusion survival with 64% post-transfusion survival following 14-day storage as well as phosphatidylserine externalization and reduced CD47 expression similar to trends observed in human RBCs at the end of 42-day storage (Gilson et al., 2009).
In conclusion, HbAS RBCs harbor subtle differences in membrane resistance to osmotic stress, show accelerated degradation during prolonged storage, and reduced survival in circulation following transfusion. It remains unknown whether our findings of impaired transfusion recovery of both murine and human HbAS in mice reflect that of stored human HbAS red cells in humans. Nevertheless, these findings suggest that further investigation should be performed to determine a shorter length of allowable storage to improve the efficacy of stored HbAS RBCs and post-transfusion recovery in humans.
Funding Sources
MTG is supported by NIH grants 2R01HL098032, 1R01HL125886-01, and P01HL103455, T32HL110849, T32HL007563, ITxM and Hemophilia Center of Western Pennsylvania, DOH is funded by NHLBI Diversity Supplementary grant under 2R01 HL098032. JSL is supported by NIH grants 5R01HL086884 and 1R21AI119042. DKS and work conducted at Wake Forest is supported by NIH grants HL058091 and HL098032.
Conflict of Interest Statement
The authors have declared that no conflict of interest exists.
Authorship Contribution
D.O.H, J.S.L, and M.T.G. conceived the study, designed the experiments, executed the experimental plan, interpreted the data, and wrote the manuscript. D.O.H., T.K., C.S., M.J., Z.X, J.F., Q.X., E.N., J.T.S., K.P., R.B., S.B., M.B., D.T., D.K.S. were involved in data acquisition, analysis, and interpretation of those data. All authors reviewed and edited the manuscript.
Acknowledgements
This work was also partially supported by NIH grant HL058091 (DKS). Center for Biological Imaging is supported by NIH grant P01HL114453 and 1S10 OD019973. We would like to thank Christina Morse and Dr. Prabir Ray for their assistance with immunohistochemical staining of F4/80+ cells.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2016.08.006.
Contributor Information
Janet S. Lee, Email: jsl26@pitt.edu.
Mark T. Gladwin, Email: gladwinmt@upmc.edu.
Appendix A. Supplementary data
Supplementary material
References
- Awwad H.K., Moussa L., Sheraki A.S. The effect of red cell aging on chromium-51 binding and in vitro elution. J. Nucl. Med. 1966;7:687–695. [PubMed] [Google Scholar]
- Bazin R., Aubin E., Boyer L., St-AMOUR I., Roberge C., Lemieux R. Functional in vivo characterization of human monoclonal anti-D in NOD-scid mice. Blood. 2002;99:1267–1272. doi: 10.1182/blood.v99.4.1267. [DOI] [PubMed] [Google Scholar]
- Brittenham G.M., Schechter A.N., Noguchi C.T. Hemoglobin S polymerization: primary determinant of the hemolytic and clinical severity of the sickling syndromes. Blood. 1985;65:183–189. [PubMed] [Google Scholar]
- Callender S.T., Nickel J.F. Sickle cell disease; studied by measuring the survival of transfused red blood cells. J. Lab. Clin. Med. 1949;34:90–104. [PubMed] [Google Scholar]
- Connor J., Pak C.C., Schroit A.J. Exposure of phosphatidylserine in the outer leaflet of human red blood cells. Relationship to cell density, cell age, and clearance by mononuclear cells. J. Biol. Chem. 1994;269:2399–2404. [PubMed] [Google Scholar]
- Dern R.J., Brewer G.J., Wiorkowski J.J. Studies on the preservation of human blood. II. The relationship of erythrocyte adenosine triphosphate levels and other in vitro measures to red cell storageability. J. Lab. Clin. Med. 1967;69:968–978. [PubMed] [Google Scholar]
- Dumont L.J., Aubuchon J.P. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion. 2008;48:1053–1060. doi: 10.1111/j.1537-2995.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- Flatt J.F., Bawazir W.M., Bruce L.J. The involvement of cation leaks in the storage lesion of red blood cells. Front. Physiol. 2014;5:214. doi: 10.3389/fphys.2014.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco R.S., Yasin Z., Lohmann J.M., Palascak M.B., Nemeth T.A., Weiner M., Joiner C.H., Rucknagel D.L. The survival characteristics of dense sickle cells. Blood. 2000;96:3610–3617. [PubMed] [Google Scholar]
- Gilson C.R., Kraus T.S., Hod E.A., Hendrickson J.E., Spitalnik S.L., Hillyer C.D., Shaz B.H., Zimring J.C. A novel mouse model of red blood cell storage and posttransfusion in vivo survival. Transfusion. 2009;49:1546–1553. doi: 10.1111/j.1537-2995.2009.02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimlette T.M.D. Transfusion of autologous and aliogeneic chromium-51 labelled red cells in ponies. J. R. Soc. Med. 1978;71:576–581. [Google Scholar]
- Hedrick P.W. Population genetics of malaria resistance in humans. Heredity (Edinb) 2011;107:283–304. doi: 10.1038/hdy.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hod E.A., Arinsburg S.A., Francis R.O., Hendrickson J.E., Zimring J.C., Spitalnik S.L. Use of mouse models to study the mechanisms and consequences of RBC clearance. Vox Sang. 2010;99:99–111. doi: 10.1111/j.1423-0410.2010.01327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hod E.A., Zhang N., Sokol S.A., Wojczyk B.S., Francis R.O., Ansaldi D., Francis K.P., Della-LATTA P., Whittier S., Sheth S., Hendrickson J.E., Zimring J.C., Brittenham G.M., Spitalnik S.L. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115:4284–4292. doi: 10.1182/blood-2009-10-245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish C., Ong G.L., Desai N., Mattes M.J. The specificity of alternative complement pathway-mediated lysis of erythrocytes: a survey of complement and target cells from 25 species. Scand. J. Immunol. 1993;38:113–122. doi: 10.1111/j.1365-3083.1993.tb01701.x. [DOI] [PubMed] [Google Scholar]
- Jallow M., Teo Y.Y., Small K.S., Rockett K.A., Deloukas P., Clark T.G., Kivinen K., Bojang K.A., Conway D.J., Pinder M., Sirugo G., Sisay-Joof F., Usen S., Auburn S., Bumpstead S.J., Campino S., Coffey A., Dunham A., Fry A.E., Green A., Gwilliam R., Hunt S.E., Inouye M., Jeffreys A.E., Mendy A., Palotie A., Potter S., Ragoussis J., Rogers J., Rowlands K., Somaskantharajah E., Whittaker P., Widden C., Donnelly P., Howie B., Marchini J., Morris A., Sanjoaquin M., Achidi E.A., Agbenyega T., Allen A., Amodu O., Corran P., Djimde A., Dolo A., Doumbo O.K., Drakeley C., Dunstan S., Evans J., Farrar J., Fernando D., Hien T.T., Horstmann R.D., Ibrahim M., Karunaweera N., Kokwaro G., Koram K.A., Lemnge M., Makani J., Marsh K., Michon P., Modiano D., Molyneux M.E., Mueller I., Parker M., Peshu N., Plowe C.V., Puijalon O., Reeder J., Reyburn H., Riley E.M., Sakuntabhai A., Singhasivanon P., Sirima S., Tall A., Taylor T.E., Thera M., Troye-Blomberg M., Williams T.N., Wilson M., Kwiatkowski D.P., Wellcome Trust Case Control, C., Malaria Genomic Epidemiology, N. Genome-wide and fine-resolution association analysis of malaria in west Africa. Nat. Genet. 2009;41:657–665. doi: 10.1038/ng.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanias T., Lanteri M., Sinchar D., Busch M., Gladwin M.T. 55th Annual Meeting (2013) for American Society of Hematology. 2013. Red blood cell storage in pediatric transfer bags is correlated with increased levels of hemolysis and altered osmotic fragility. (New Orleans) [Google Scholar]
- Koch C.G., Li L., Sessler D.I., Figueroa P., Hoeltge G.A., Mihaljevic T., Blackstone E.H. Duration of red-cell storage and complications after cardiac surgery. N. Engl. J. Med. 2008;358:1229–1239. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- Latham J.T., Jr., Bove J.R., Weirich F.L. Chemical and hematologic changes in stored CPDA-1 blood. Transfusion. 1982;22:158–159. doi: 10.1046/j.1537-2995.1982.22282177126.x. [DOI] [PubMed] [Google Scholar]
- Levin W.C., Truax W.E. The influence of storage on erythrocyte survival in blood obtained from donors with sickle cell trait. J. Lab. Clin. Med. 1960;55:94–97. [PubMed] [Google Scholar]
- Mangalmurti N.S., Xiong Z., Hulver M., Ranganathan M., Liu X.H., Oriss T., Fitzpatrick M., Rubin M., Triulzi D., Choi A., Lee J.S. Loss of red cell chemokine scavenging promotes transfusion-related lung inflammation. Blood. 2009;113:1158–1166. doi: 10.1182/blood-2008-07-166264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebius R.E., Kraal G. Structure and function of the spleen. Nat. Rev. Immunol. 2005;5:606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- Mishler J.M., Darley J.H., Haworth C., Mollison P.L. Viability of red cells stored in diminished concentration of citrate. Br. J. Haematol. 1979;43:63–67. doi: 10.1111/j.1365-2141.1979.tb03720.x. [DOI] [PubMed] [Google Scholar]
- Mock D.M., Lankford G.L., Widness J.A., Burmeister L.F., Kahn D., Strauss R.G. Measurement of red cell survival using biotin-labeled red cells: validation against 51Cr-labeled red cells. Transfusion. 1999;39:156–162. doi: 10.1046/j.1537-2995.1999.39299154729.x. [DOI] [PubMed] [Google Scholar]
- Moore J.M., Kumar N., Shultz L.D., Rajan T.V. Maintenance of the human malarial parasite, Plasmodium falciparum, in scid mice and transmission of gametocytes to mosquitoes. J. Exp. Med. 1995;181:2265–2270. doi: 10.1084/jem.181.6.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi C.T., Gladwin M., Diwan B., Merciris P., Smith R., Yu X., Buzard G., Fitzhugh A., Keefer L.K., Schechter A.N., Mohandas N. Pathophysiology of a sickle cell trait mouse model: human alpha(beta)(S) transgenes with one mouse beta-globin allele. Blood Cells Mol. Dis. 2001;27:971–977. doi: 10.1006/bcmd.2001.0469. [DOI] [PubMed] [Google Scholar]
- Ong G.L., Mattes M.J. Mouse strains with typical mammalian levels of complement activity. J. Immunol. Methods. 1989;125:147–158. doi: 10.1016/0022-1759(89)90088-4. [DOI] [PubMed] [Google Scholar]
- Ray R.N., Cassell M., Chaplin H., Jr. In vitro and in vivo observations on stored sickle trait red blood cells. Am. J. Clin. Pathol. 1959;32:430–435. doi: 10.1093/ajcp/32.5.430. [DOI] [PubMed] [Google Scholar]
- Rees D.C., Williams T.N., Gladwin M.T. Sickle-cell disease. Lancet. 2010;376:2018–2031. doi: 10.1016/S0140-6736(10)61029-X. [DOI] [PubMed] [Google Scholar]
- Rice C.E., Crowson C.N. The interchangeability of the complement components of different animal species in the hemolysis of sheep erythrocytes sensitized with rabbit amboceptor. J. Immunol. 1950;65:201–210. [PubMed] [Google Scholar]
- Schroit A.J., Tanaka Y., Madsen J., Fidler I.J. The recognition of red blood cells by macrophages: role of phosphatidylserine and possible implications of membrane phospholipid asymmetry. Biol. Cell. 1984;51:227–238. doi: 10.1111/j.1768-322x.1984.tb00303.x. [DOI] [PubMed] [Google Scholar]
- Sisto F., Miluzio A., Leopardi O., Mirra M., Boelaert J.R., Taramelli D. Differential cytokine pattern in the spleens and livers of BALB/c mice infected with Penicillium marneffei: protective role of gamma interferon. Infect. Immun. 2003;71:465–473. doi: 10.1128/IAI.71.1.465-473.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statius van Eps L., De Jong P.E. Disease of the Kidney. sixth ed. Vol. 1. 1997. Sickle cell disease; pp. 2201–2219. [Google Scholar]
- Steinberg M.H., Embury S.H. Alpha-thalassemia in blacks: genetic and clinical aspects and interactions with the sickle hemoglobin gene. Blood. 1986;68:985–990. [PubMed] [Google Scholar]
- Stimpfling J.H., Reichert A.E., Blanchard S. Genetic control of the immune response to the Ea-2 alloantigen system of the mouse. I. The humoral antibody response. J. Immunol. 1976;116:1096–1098. [PubMed] [Google Scholar]
- Stroncek D.F., Rainer T., Sharon V., Byrne K.M., Noguchi C.T., Klein H.G., Schechter A.N., Leitman S.F. Sickle Hb polymerization in RBC components from donors with sickle cell trait prevents effective WBC reduction by filtration. Transfusion. 2002;42:1466–1472. doi: 10.1046/j.1537-2995.2002.00206.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material