Supplemental Digital Content is available in the text.
Keywords: brain attack, intracranial hemorrhage, seizure, stroke, transient ischemic attack
Background and Purpose—
Clinical identification of stroke in the pediatric emergency department is critical for improving access to hyperacute therapies. We identified key clinical features associated with childhood stroke or transient ischemic attack compared with mimics.
Methods—
Two hundred and eighty consecutive children presenting to the emergency department with mimics, prospectively recruited over 18 months from 2009 to 2010, were compared with 102 children with stroke or transient ischemic attack, prospectively/retrospectively recruited from 2003 to 2010.
Results—
Cerebrovascular diagnoses included arterial ischemic stroke (55), hemorrhagic stroke (37), and transient ischemic attack (10). Mimic diagnoses included migraine (84), seizures (46), Bell’s palsy (29), and conversion disorders (18). Being well in the week before presentation (odds ratio [OR] 5.76, 95% confidence interval [CI] 2.25–14.79), face weakness (OR 2.94, 95% CI 1.19–7.28), arm weakness (OR 8.66, 95% CI, 2.50–30.02), and inability to walk (OR 3.38, 95% CI 1.54–7.42) were independently associated with increased odds of stroke diagnosis. Other symptoms were independently associated with decreased odds of stroke diagnosis (OR 0.28, 95% CI 0.10–0.77). Associations were not observed between seizures or loss of consciousness. Factors associated with stroke differed by arterial and hemorrhagic subtypes.
Conclusions—
Being well in the week before presentation, inability to walk, face and arm weakness are associated with increased odds of stroke. The lack of positive or negative association between stroke and seizures or loss of consciousness is an important difference to adults. Pediatric stroke pathways and bedside tools need to factor in differences between children and adults and between stroke subtypes.
Long delays in diagnosis of childhood stroke limit access to hyperacute interventions that improve outcome in adults.1–3 However, a recent US study has demonstrated the benefits of developing a pediatric Code Stroke protocol for shortening time to diagnosis and increasing rates of thrombolysis and mechanical thrombectomy.4 Clinical differentiation of stroke from mimics by pediatric triage nurses and emergency physicians influences the decision-making process, in particular the rapidity of medical assessment, the need for emergent neuroimaging to confirm diagnosis, the initiation of antithrombotic treatment in ischemic stroke, and neurosurgical intervention in hemorrhagic stroke (HS). Correct clinical identification of stroke avoids unnecessary diagnostic or therapeutic procedures and allows early implementation of measures to maintain physiological homeostasis.
Three studies on adults have described clinical features that differentiate stroke from mimics. Focal neurological symptoms and signs were associated with increased odds of stroke, whereas loss of consciousness, altered mental state, dizziness, confusion, and seizures were associated with increased odds of mimic.5–7 It may not be appropriate to extrapolate data from adults because of different presenting features, such as frequent occurrence of seizures,8 and the different spectrum of disorders that mimic childhood stroke.9
Several prospective case series have reported the presenting features of childhood stroke,10–13 but most have focused on arterial ischemic stroke (AIS), and none have explored differences between stroke and other conditions that mimic stroke. Therefore, our primary aim was to identify key clinical features that were positively and negatively associated with pediatric stroke, compared with mimics, in children presenting with brain attack symptoms. We hypothesized that the clinical features associated with stroke are similar to those of adults, with the exception of seizures.
Materials and Methods
The study population comprised 2 groups of children presenting to the Royal Children’s Hospital Melbourne Emergency Department (ED). The first consisted of a consecutive group of children with stroke mimics, presenting between July 2009 and December 2010. The second consisted of a mixed prospective and retrospective group of children with radiologically confirmed AIS, transient ischemic attack (TIA), and HS, presenting between January 2003 and December 2010 (Figure).
Figure.
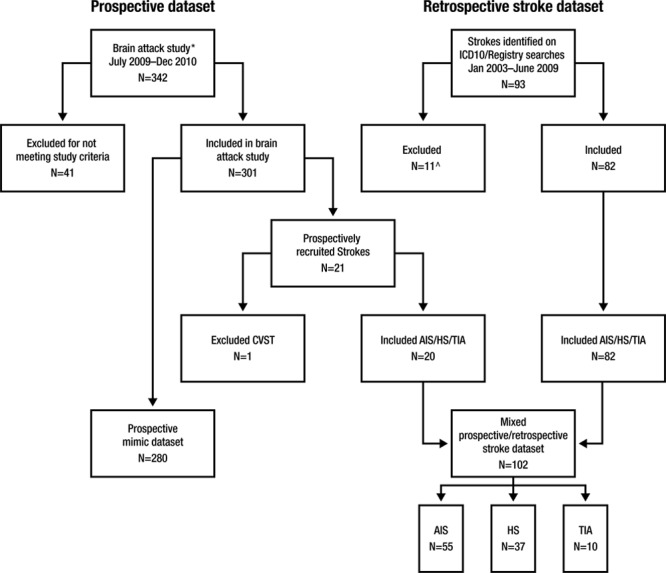
Case ascertainment for stroke and mimic data sets. Causes of ischemic strokes/TIAs: focal cerebral arteriopathy 19 (29%), bilateral cerebral arteriopathy 14 (22%), arterial dissection 5 (8%), cardioembolic 3 (5%), other determined etiology 5 (7%), multifactorial etiologies 2 (3%), and undetermined 17 (26%). Causes of hemorrhagic strokes: arteriovenous malformation 20 (54%), cavernous malformation 3 (8%), arterial dissection 3 (5%), subarachnoid, cause undetermined 2 (5%) and because of aneurysm 1 (3%), tumor-related bleed 1 (3%), focal cerebral arteriopathy with ruptured collaterals 1 (3%), and undetermined 7 (19%). AIS indicates arterial ischemic stroke; CVST, cerebral sinus venous thrombosis; HS, hemorrhagic stroke; N, number; and TIA, transient ischemic attack. *Brain attack study.7 ^Reasons for exclusion: CVST (1), direct admission to inpatient unit (5), and incomplete medical records (5).
Inclusion criteria, exclusion criteria, study procedure, and diagnostic definitions for the prospective cohort of patients with brain attack symptoms9 (defined as acute onset focal brain dysfunction) and retrospective stroke patients14 have been previously described. Children with stroke mimics (including those presenting with a first seizure) had persistent neurological symptoms (headache, subjective visual or sensory disturbance) on presentation to the ED or abnormal neurological signs on examination. TIA required a known history of cerebrovascular disease, a clinical history typical of a vascular brain event with full resolution of symptoms within 24 hours, and absence of acute infarction on magnetic resonance imaging.15 There was no time limit on duration of symptoms after arrival at ED in the mimic group. The following stroke subgroups were excluded: perinatal stroke, cerebral sinovenous thrombosis, admission directly to the ward, and incomplete medical records. Children with subdural or extradural hemorrhage were also excluded.
Variables collected included demographics and neurological symptoms and signs. Clinical variables were selected on the basis of being common presenting features of stroke or being discriminatory between stroke and mimics in the published studies on adults.5–7 Key demographic factors and neurological symptoms variables were compared between the prospective and retrospective cerebrovascular patients to account for threats to study validity by ensuring both statistical and substantive similarity of the 2 cohorts.
Analyses were performed for the following outcomes: (1) combined stroke (AIS and HS) or TIA versus mimics, (2) AIS subtype versus mimics, and (3) HS subtype versus mimics. For simplicity, stroke and TIA are referred to as stroke in the combined analysis section of the results. Categorical variables are descriptively presented as counts and percentages. Continuous variables are presented as mean and standard deviation for normally distributed data or median and interquartile range for non-normally distributed data.
Logistic regression modeling was used to investigate associations between independent variables and stroke diagnosis. Selection of variables for regression analyses was determined by a combination of computational analyses and clinical importance. Variables were initially individually examined by univariate logistic regression for associations with the outcome of interest. Odds ratios (ORs) and corresponding 95% confidence intervals (95%CIs) were estimated. Variables with P values of <0.05 were considered for entry into the multivariable model to determine the adjusted association with the outcomes of interest for combined AIS and HS diagnoses versus mimics. Multivariable modeling was not performed for AIS or HS stroke subtypes versus mimics because of the relatively small sample sizes. Unadjusted (produced by univariate models) and adjusted (produced by multivariable models) ORs are presented with corresponding 95% CIs and P values. CIs not including 1 are indicative of statistical significance, with the P value set at 0.05. Standard analyses of model fit and excessive collinearity diagnostics were performed, including (1) computation of pairwise correlation coefficients to assess interdependence of causal variables and (2) measures of multicollinearity, including variance inflation factors and condition number, to determine whether there was a linear association between ≥2 predictor variables. Statistical analyses were performed using STATA 13 (StataCorp, TX). This study was reviewed and approved by the Royal Children’s Hospital Human Research Ethics Committee (HREC30194A).
Results
The study population comprised 280 children with mimics, prospectively recruited from July 2009 until December 2010 and 102 children with stroke, prospectively and retrospectively identified from January 2003 until December 2010. No statistically significant difference between the 2 stroke cohorts was observed, with sex (P=0.09) being the only variable approaching significance (Table I in the online-only Data Supplement). AIS was the most common stroke subtype in 55 children, followed by HS subtype in 37 and TIAs in 10 children. Twelve children with stroke were excluded: 2 had cerebral sinovenous thrombosis, 5 were direct admissions to the inpatient unit, and 5 had incomplete medical records (Figure). In children with neurological symptoms or signs on arrival at the ED, the most common mimic diagnoses included migraine in 84 children, febrile or afebrile seizures in 46, Bell’s palsy in 29, conversions disorders in 18, and syncope in 14 children. A full list of mimic diagnoses has been previously published.9
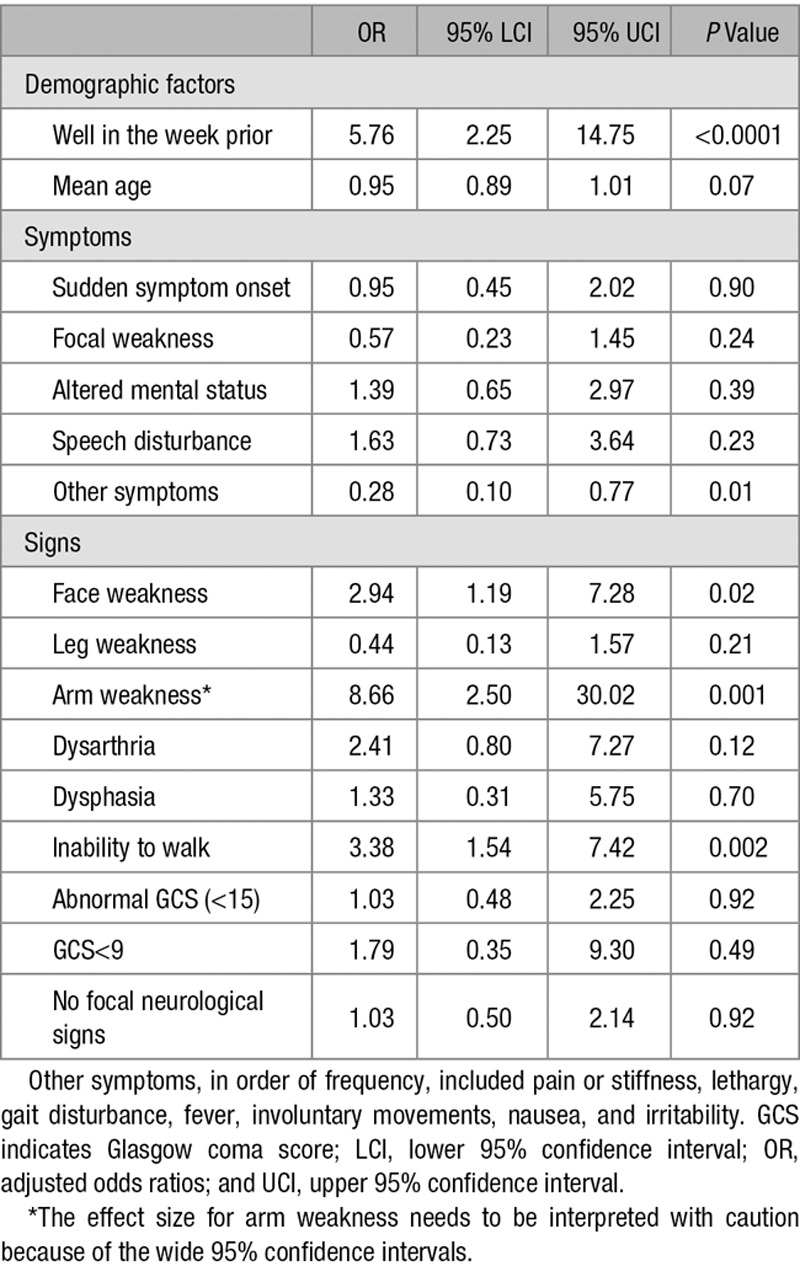
Fourteen factors associated with increased odds, and 2 factors with decreased odds of stroke diagnosis (Tables 1 and 2), were included in the multivariable logistic regression model. Four factors emerged as being significantly associated with stroke diagnosis in the adjusted analysis. Being well in the week before presentation (OR 5.76, 95% CI 2.25–14.79) was associated with increased odds of stroke. Arm weakness (OR 8.66, 95% CI 2.50–30.02) was the neurological sign most strongly associated with stroke, but the association was poorly estimated as indicated by the wide 95% confidence intervals. Face weakness (OR 2.94, 95% CI 1.19–7.28) and inability to walk (OR 3.38, 95% CI 1.54–7.42) were also associated with increased odds of stroke. In contrast, the presence of other symptoms (OR 0.28, 95% CI 0.10–0.77) was negatively associated with stroke diagnosis (Table 3).
Table 1.
Univariate Analyses of Demographic Factors, Time Course, and Symptoms Associated With Stroke and Mimic Diagnoses
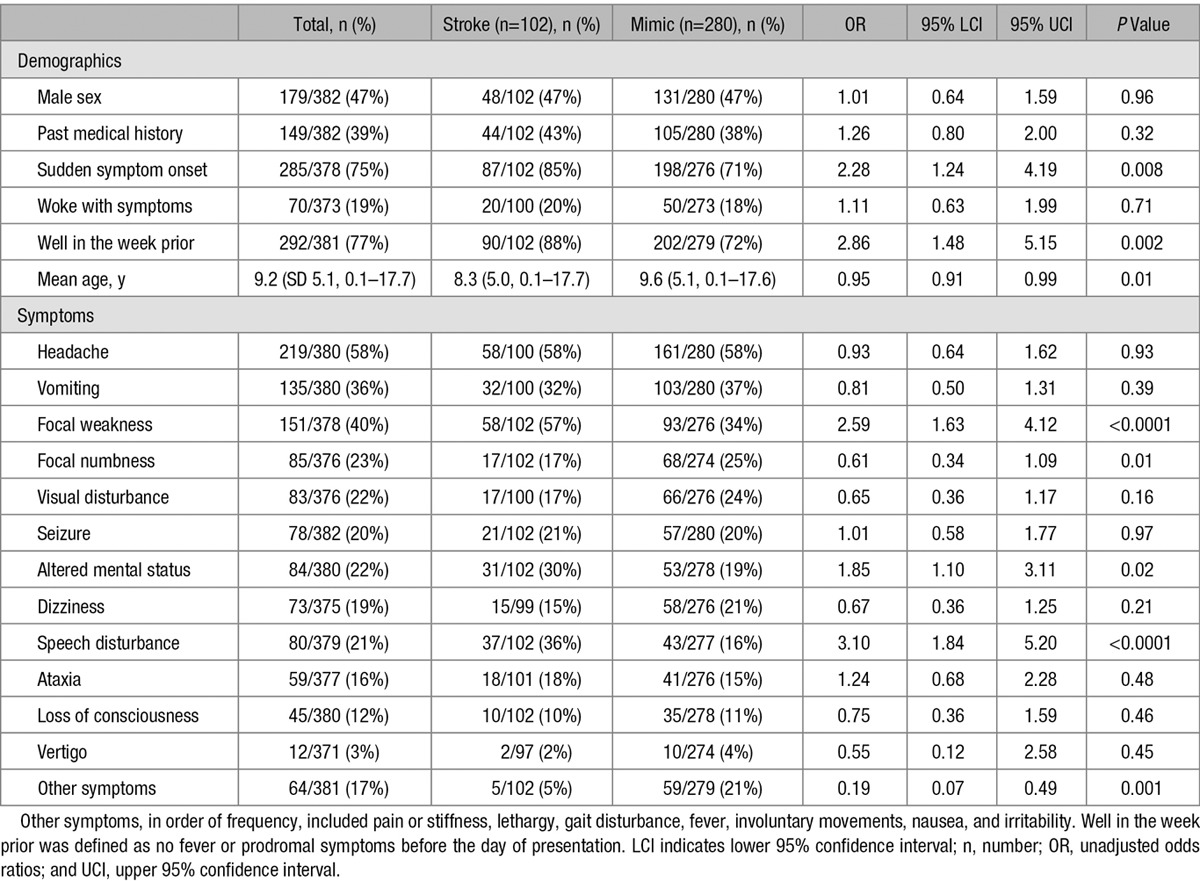
Table 2.
Univariate Analyses of Signs Associated With Stroke and Mimic Diagnoses
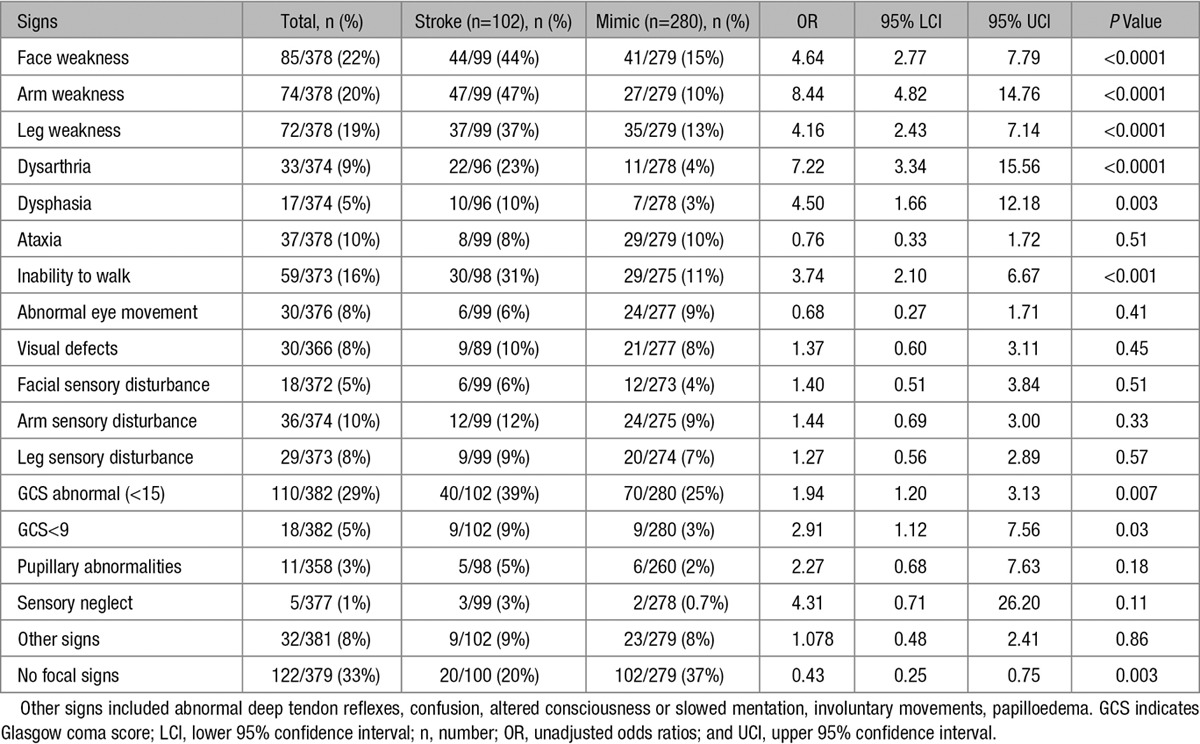
Table 3.
Factors Independently Associated With Stroke and Mimic Diagnoses on Multivariable Regression Analyses
Factors significantly associated with AIS diagnosis on univariate analyses included symptoms of focal weakness and speech disturbance and signs of face, arm, or leg weakness, dysarthria, dysphasia, and inability to walk. No child with AIS presented with loss of consciousness or coma (GCS [Glasgow coma score] of <9). In contrast, factors significantly associated with mimic diagnosis on univariate analyses included presence of other symptoms and absence of neurological signs on examination (Table II in the online-only Data Supplement). Factors associated with HS diagnosis on univariate analyses included sudden onset of symptoms, vomiting, altered mental state, inability to walk, abnormal GCS, and coma. All children with HS were well in the week before presentation, whereas none presented with vertigo (Table III in the online-only Data Supplement).
Discussion
Significant delays to diagnosis of pediatric stroke, well beyond those reported in adults,1–3,16,17 mean that children are unlikely to access time-critical treatments that have been shown to improve outcome in adults.18 Prehospital factors contribute more to delayed diagnosis in adults,19 but recent studies suggest that in-hospital factors are more important contributors to delayed diagnosis in children.1–3 It is likely that poor recognition of stroke among pediatric physicians2 or limited knowledge of neurologically relevant brain attack symptoms are contributing factors.
Triage nurses and emergency physicians play a critical role in the diagnosis of stroke because they are the first point of contact in ≈50% of adults with stroke.19 Clinical differentiation of stroke from mimics is a crucial first step that influences decisions about type and urgency of investigations and selection of the most appropriate treatments. Assessment of patients with brain attack symptoms is challenging for non-neurologists, with accuracy of emergency physician diagnosis, before completion of investigations, ranging from 51% to 81%.5–7,20–22 The low a priori probability of childhood stroke makes diagnosis even more challenging for pediatric ED staff.9
The key findings of this study are as follows: (1) there are differences in the clinical presenting features of childhood stroke and mimics and (2) factors that discriminate stroke from mimics differ by stroke subtype. Few studies have investigated the clinical features that distinguish stroke from mimics in adults. Exact time of onset,7 abnormal vascular findings (such as hypertension, atrial fibrillation, and valvular heart disease),7 and sudden-onset symptoms of face, arm, or leg weakness and speech6 are associated with increased odds of stroke. Lateralized signs,7 face, arm, or leg weakness, eye movement abnormalities, visuospatial neglect, hemiparetic or ataxic gait disturbance, sensory disturbance of the arm or leg,6 and abnormal visual fields6 are also associated with increased odds of stroke. In contrast, altered consciousness,5,6 cognitive impairment,7 dizziness, confusion, loss of consciousness, and seizures5,6 are associated with increased odds of mimic diagnosis.
We found that 16 variables were associated with combined stroke or mimic on univariate analysis but only 5 remained significant on multivariable analysis. Being well in the week before arrival, inability to walk, and examination findings of focal face or arm weakness were associated with stroke diagnosis, consistent with those in the literature on adults. Visual symptoms were not independently associated with stroke diagnosis, which may be explained by the different spectrum of disorders mimicking pediatric stroke. For example, visual disturbance is the most frequent type of aura in migraine, and migraine is the most common pediatric stroke mimic.9 Sudden symptom onset was not independently associated with stroke. The lack of independent association between sudden symptom onset and stroke may be because of selection bias, because brain attack symptoms were defined as acute-onset focal brain dysfunction, or variable interpretation of the term’s meaning among ED physicians.
Equally important were the findings that seizures and loss of consciousness were not independently associated with mimic diagnosis, and therefore, in contrast to adults, they are not useful discriminators for mimic diagnosis in children. The relatively frequent occurrence of seizures in childhood stroke is well described in the pediatric literature, with reported rates ranging from 11% to 52% for ischemic stroke1,10–13,16,17,23 and from 37% to 41% for HS.24,25 This contrasts with the low rates of seizures in adults, ranging from 0.5% to 11%.6,7 Increased rates of seizures may reflect age-related cortical hyperexcitability or decreased inhibitory influences in the developing brain. Loss of consciousness, which has been noted in 18% to 41% of adults with mimics,6,7 was also nondiscriminatory, occurring in low numbers of children in both groups. Differences in discriminatory factors between adults and children may also be related to the higher proportion of HS in children because presenting features differ by stroke subtype.3,14 Thirty-six percent of children in our stroke cohort had HS, consistent with the 37% to 46% rates reported in population-based studies.26–29
Exploratory analyses suggest that factors that discriminate AIS from mimics are different from those that discriminate HS from mimics. In contrast to the analyses comparing all strokes to mimics, being well in the week before presentation was not associated with AIS, and face or arm weakness was not associated with HS. Understanding factors that discriminate ischemic or HS subtypes from mimics may assist the clinician in selecting the most appropriate neuroimaging modality to minimize delays and maximize diagnostic yield. However, magnetic resonance imaging has similar sensitivity to computed tomography for detection of hemorrhage, and recent studies have demonstrated feasibility of rapid magnetic resonance imaging screening protocols that avoid the need for anesthesia in young children.4
Our study has limitations. The wide 95% CIs in the multivariable analyses for some signs suggest that the effect sizes need to be interpreted with caution. We did not have data on exact time of onset, assess for presence of positive signs in other systems, or use standardized stroke severity scales. Therefore, we were unable to assess the value of other factors that have been shown to be discriminatory in adults.7 The majority of our stroke group was identified retrospectively but we did not find significant differences in the presenting features when compared with the smaller prospective group. Relatively small numbers of children limited our ability to determine adjusted associations for the arterial ischemic and HS subgroups. Children with sinovenous thrombosis were excluded from the analyses and, therefore, they require further study. Accuracy of the clinical examination findings may have been influenced by level of experience of emergency physicians.7 The discriminatory values of conventional stroke risk factors were not assessed because the causes of stroke differ from adults.11,13 We did not assess the influence of discriminatory factors in AIS for anterior and posterior circulation. Finally, the study findings may not apply to children with in-hospital strokes, such as those with cardiac disease, because of differences in demographic factors, comorbid problems, such as infection, and presenting clinical features.
Development of ED brain attack protocols4 and decision support tools to improve accuracy of stroke diagnosis by frontline emergency staff need to take into account differences between children and adults30 and differences between ischemic stroke and HS subtypes. Further work is required to assess discriminatory factors for stroke compared with more common mimic diagnoses, such as migraine, and with less common but more serious diagnoses requiring rapid intervention, such as encephalitis, acute disseminated encephalomyelitis, and central nervous system tumors.
Acknowledgments
We acknowledge the assistance of Zhi Kai Chua and Michelle Lee with data entry.
Sources of Funding
Grant support received from the National Stroke Foundation, Melbourne, Australia; the Collier Charitable Fund, Melbourne, Australia; and the Murdoch Childrens Research Institute, Melbourne, Australia, to use research assistances to recruit subjects and enter data to the CRF.
Disclosures
None.
Supplementary Material
Footnotes
The online-only Data Supplement is available with this article at http://stroke.ahajournals.org/lookup/suppl/doi:10.1161/STROKEAHA.116.014179/-/DC1.
References
- 1.Rafay MF, Pontigon AM, Chiang J, Adams M, Jarvis DA, Silver F, et al. Delay to diagnosis in acute pediatric arterial ischemic stroke. Stroke. 2009;40:58–64. doi: 10.1161/STROKEAHA.108.519066. doi: 10.1161/STROKEAHA.108.519066. [DOI] [PubMed] [Google Scholar]
- 2.Srinivasan J, Miller SP, Phan TG, Mackay MT. Delayed recognition of initial stroke in children: need for increased awareness. Pediatrics. 2009;124:e227–e234. doi: 10.1542/peds.2008-3544. doi: 10.1542/peds.2008-3544. [DOI] [PubMed] [Google Scholar]
- 3.Mallick AA, Ganesan V, Kirkham FJ, Fallon P, Hedderly T, McShane T, et al. Diagnostic delays in paediatric stroke. J Neurol Neurosurg Psychiatry. 2015;86:917–921. doi: 10.1136/jnnp-2014-309188. doi: 10.1136/jnnp-2014-309188. [DOI] [PubMed] [Google Scholar]
- 4.Ladner TR, Mahdi J, Gindville MC, Gordon A, Harris ZL, Crossman K, et al. Pediatric acute stroke protocol activation in a children’s hospital emergency department. Stroke. 2015;46:2328–2331. doi: 10.1161/STROKEAHA.115.009961. doi: 10.1161/STROKEAHA.115.009961. [DOI] [PubMed] [Google Scholar]
- 5.Libman RB, Wirkowski E, Alvir J, Rao TH. Conditions that mimic stroke in the emergency department. Implications for acute stroke trials. Arch Neurol. 1995;52:1119–1122. doi: 10.1001/archneur.1995.00540350113023. [DOI] [PubMed] [Google Scholar]
- 6.Nor AM, Davis J, Sen B, Shipsey D, Louw SJ, Dyker AG, et al. The Recognition of Stroke in the Emergency Room (ROSIER) scale: development and validation of a stroke recognition instrument. Lancet Neurol. 2005;4:727–734. doi: 10.1016/S1474-4422(05)70201-5. doi: 10.1016/S1474-4422(05)70201-5. [DOI] [PubMed] [Google Scholar]
- 7.Hand PJ, Kwan J, Lindley RI, Dennis MS, Wardlaw JM. Distinguishing between stroke and mimic at the bedside: the brain attack study. Stroke. 2006;37:769–775. doi: 10.1161/01.STR.0000204041.13466.4c. doi: 10.1161/01.STR.0000204041.13466.4c. [DOI] [PubMed] [Google Scholar]
- 8.Chadehumbe MA, Khatri P, Khoury JC, Alwell K, Szaflarski JP, Broderick JP, et al. Seizures are common in the acute setting of childhood stroke: a population-based study. J Child Neurol. 2009;24:9–12. doi: 10.1177/0883073808320756. doi: 10.1177/0883073808320756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mackay MT, Chua ZK, Lee M, Yock-Corrales A, Churilov L, Monagle P, et al. Stroke and nonstroke brain attacks in children. Neurology. 2014;82:1434–1440. doi: 10.1212/WNL.0000000000000343. doi: 10.1212/WNL.0000000000000343. [DOI] [PubMed] [Google Scholar]
- 10.Mallick AA, Ganesan V, Kirkham FJ, Fallon P, Hedderly T, McShane T, et al. Childhood arterial ischaemic stroke incidence, presenting features, and risk factors: a prospective population-based study. Lancet Neurol. 2014;13:35–43. doi: 10.1016/S1474-4422(13)70290-4. doi: 10.1016/S1474-4422(13)70290-4. [DOI] [PubMed] [Google Scholar]
- 11.Mackay MT, Wiznitzer M, Benedict SL, Lee KJ, Deveber GA, Ganesan V International Pediatric Stroke Study Group. Arterial ischemic stroke risk factors: the International Pediatric Stroke Study. Ann Neurol. 2011;69:130–140. doi: 10.1002/ana.22224. doi: 10.1002/ana.22224. [DOI] [PubMed] [Google Scholar]
- 12.Zimmer JA, Garg BP, Williams LS, Golomb MR. Age-related variation in presenting signs of childhood arterial ischemic stroke. Pediatr Neurol. 2007;37:171–175. doi: 10.1016/j.pediatrneurol.2007.05.010. doi: 10.1016/j.pediatrneurol.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Steinlin M, Pfister I, Pavlovic J, Everts R, Boltshauser E, Capone Mori A, et al. Swiss Societies of Paediatric Neurology and Neonatology. The first three years of the Swiss Neuropaediatric Stroke Registry (SNPSR): a population-based study of incidence, symptoms and risk factors. Neuropediatrics. 2005;36:90–97. doi: 10.1055/s-2005-837658. doi: 10.1055/s-2005-837658. [DOI] [PubMed] [Google Scholar]
- 14.Yock-Corrales A, Mackay MT, Mosley I, Maixner W, Babl FE. Acute childhood arterial ischemic and hemorrhagic stroke in the emergency department. Ann Emerg Med. 2011;58:156–163. doi: 10.1016/j.annemergmed.2010.10.013. doi: 10.1016/j.annemergmed.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 15.Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, et al. American Heart Association Stroke Council, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Interdisciplinary Council on Quality of Care and Outcomes Research. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:227–276. doi: 10.1161/STR.0b013e3181f7d043. doi: 10.1161/STR.0b013e3181f7d043. [DOI] [PubMed] [Google Scholar]
- 16.Gabis LV, Yangala R, Lenn NJ. Time lag to diagnosis of stroke in children. Pediatrics. 2002;110:924–928. doi: 10.1542/peds.110.5.924. [DOI] [PubMed] [Google Scholar]
- 17.McGlennan C, Ganesan V. Delays in investigation and management of acute arterial ischaemic stroke in children. Dev Med Child Neurol. 2008;50:537–540. doi: 10.1111/j.1469-8749.2008.03012.x. doi: 10.1111/j.1469-8749.2008.03012.x. [DOI] [PubMed] [Google Scholar]
- 18.Wardlaw JM, Murray V, Berge E, del Zoppo G, Sandercock P, Lindley RL, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet. 2012;379:2364–2372. doi: 10.1016/S0140-6736(12)60738-7. doi: 10.1016/S0140-6736(12)60738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tong D, Reeves MJ, Hernandez AF, Zhao X, Olson DM, Fonarow GC, et al. Times from symptom onset to hospital arrival in the Get with the Guidelines–Stroke Program 2002 to 2009: temporal trends and implications. Stroke. 2012;43:1912–1917. doi: 10.1161/STROKEAHA.111.644963. doi: 10.1161/STROKEAHA.111.644963. [DOI] [PubMed] [Google Scholar]
- 20.Whiteley WN, Wardlaw JM, Dennis MS, Sandercock PA. Clinical scores for the identification of stroke and transient ischaemic attack in the emergency department: a cross-sectional study. J Neurol Neurosurg Psychiatry. 2011;82:1006–1010. doi: 10.1136/jnnp.2010.235010. doi: 10.1136/jnnp.2010.235010. [DOI] [PubMed] [Google Scholar]
- 21.Rizos T, Ringleb PA, Huttner HB, Kohrmann M, Juttler E. Evolution of stroke diagnosis in the emergency room–a prospective observational study. Cerebrovasc Dis. 2009;28:448–453. doi: 10.1159/000235989. doi: 10.1159/000235989. [DOI] [PubMed] [Google Scholar]
- 22.Harbison J, Hossain O, Jenkinson D, Davis J, Louw SJ, Ford GA. Diagnostic accuracy of stroke referrals from primary care, emergency room physicians, and ambulance staff using the face arm speech test. Stroke. 2003;34:71–76. doi: 10.1161/01.str.0000044170.46643.5e. [DOI] [PubMed] [Google Scholar]
- 23.Braun KP, Rafay MF, Uiterwaal CS, Pontigon AM, DeVeber G. Mode of onset predicts etiological diagnosis of arterial ischemic stroke in children. Stroke. 2007;38:298–302. doi: 10.1161/01.STR.0000254484.10680.c6. doi: 10.1161/01.STR.0000254484.10680.c6. [DOI] [PubMed] [Google Scholar]
- 24.Al-Jarallah A, Al-Rifai MT, Riela AR, Roach ES. Nontraumatic brain hemorrhage in children: etiology and presentation. J Child Neurol. 2000;15:284–289. doi: 10.1177/088307380001500503. [DOI] [PubMed] [Google Scholar]
- 25.Beslow LA, Licht DJ, Smith SE, Storm PB, Heuer GG, Zimmerman RA, et al. Predictors of outcome in childhood intracerebral hemorrhage: a prospective consecutive cohort study. Stroke. 2010;41:313–318. doi: 10.1161/STROKEAHA.109.568071. doi: 10.1161/STROKEAHA.109.568071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Broderick J, Talbot GT, Prenger E, Leach A, Brott T. Stroke in children within a major metropolitan area: the surprising importance of intracerebral hemorrhage. J Child Neurol. 1993;8:250–255. doi: 10.1177/088307389300800308. [DOI] [PubMed] [Google Scholar]
- 27.Giroud M, Lemesle M, Gouyon JB, Nivelon JL, Milan C, Dumas R. Cerebrovascular disease in children under 16 years of age in the city of Dijon, France: a study of incidence and clinical features from 1985 to 1993. J Clin Epidemiol. 1995;48:1343–1348. doi: 10.1016/0895-4356(95)00039-9. [DOI] [PubMed] [Google Scholar]
- 28.Fullerton HJ, Wu YW, Zhao S, Johnston SC. Risk of stroke in children: ethnic and gender disparities. Neurology. 2003;61:189–194. doi: 10.1212/01.wnl.0000078894.79866.95. [DOI] [PubMed] [Google Scholar]
- 29.Jordan LC, Kleinman JT, Hillis AE. Intracerebral hemorrhage volume predicts poor neurologic outcome in children. Stroke. 2009;40:1666–1671. doi: 10.1161/STROKEAHA.108.541383. doi: 10.1161/STROKEAHA.108.541383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mackay MT, Churilov L, Donnan GA, Babl FE, Monagle P. Performance of bedside stroke recognition tools in discriminating childhood stroke from mimics. Neurology. 2016;86:2154–2161. doi: 10.1212/WNL.0000000000002736. doi: 10.1212/WNL.0000000000002736. [DOI] [PubMed] [Google Scholar]


