Abstract
The aim of this study was to evaluate flexibly dosed brexpiprazole for early-episode schizophrenia through the assessment of efficacy, social functioning, and tolerability. This was an exploratory, 16-week, open-label, flexible-dose (1, 2, 3, or 4 mg/day; target dose 3 mg/day) study in outpatients with early-episode schizophrenia (18–35 years old, ≤5 years’ duration of illness). Efficacy was assessed by the Positive and Negative Syndrome Scale score (PANSS) and social functioning was assessed by changes from baseline in PANSS modified prosocial subscale, personal and social performance (PSP), and specific levels of functioning (SLOF) scales. Safety and tolerability were also evaluated. Overall, 25/49 patients completed the study. Symptoms of schizophrenia improved over the entire treatment period, as evidenced by reductions in PANSS total score from baseline (least squares mean change at week 16: −10.2). Improvements in social functioning were shown by least squares mean changes from baseline at week 16 in the PANSS prosocial subscale (−2.0), PSP (6.6), and SLOF (13.1). Brexpiprazole was generally well tolerated; the most common adverse events were insomnia (7/49 patients), somnolence (4/49), sedation, weight increase, and nausea (each 3/49). Brexpiprazole may represent a novel and effective treatment strategy for patients with early-episode schizophrenia and may be effective for improving social function.
Keywords: antipsychotic, brexpiprazole, early phase, schizophrenia, social functioning
Introduction
The first 5 years following a first psychotic episode are widely recognized as the critical period for optimal long-term outcomes (Birchwood et al., 1998; Crumlish et al., 2009). It has been proposed that longer durations of untreated psychosis are associated with reductions in the volume of gray matter within defined regions of the brain (Malla et al., 2011; Guo et al., 2013), leading to speculation that untreated psychosis may have a neurotoxic effect (Haijma et al., 2013). Without intervention, gray matter loss may continue throughout the course of illness (Guo et al., 2013) and such structural abnormalities have been associated with poor treatment response and unfavorable long-term outcomes (Crumlish et al., 2009; Owens et al., 2010; Penttilä et al., 2014), suggesting that early intervention is important for improving prognosis.
In addition to possible brain deterioration, the sequelae of unresolved psychosis include impaired functioning and poorer health-related quality of life in the long term (Kanahara et al., 2013; Cechnicki et al., 2014), and also predict short-term and long-term treatment success (Norman and Malla, 2001; Marshall et al., 2005; Carbon and Correll, 2014). Furthermore, decreased social function is a core attribute of schizophrenia and is present even at the onset of the illness (Grant et al., 2001).
Effective treatment of early episodes of schizophrenia may limit loss of social function, enabling patients to maintain social support networks with family and friends, thereby improving long-term outcomes (Norman et al., 2007, 2011). In addition, although high numbers of patients respond to treatment at first episode (>80%) (Malla et al., 2006), relapse rates are also high during the first few years following the first episode, reinforcing the need to maintain treatment and monitor medication adherence (Novick et al., 2010; Owens et al., 2010; Alvarez-Jimenez et al., 2012; Hui et al., 2013).
Atypical antipsychotics generally have a mode of action based on antagonism of dopamine D2 receptors and serotonin 5-HT2A receptors. These medications have shown efficacy in improving positive and, to a lesser extent, negative symptoms, with a reduced propensity for developing extrapyramidal symptoms (EPS) compared with typical antipsychotic agents, which generally show limited activity at 5-HT2A receptors (Reynolds and Kirk, 2010). However, despite improvements over first-generation treatments, some atypical antipsychotics are still associated with the development of EPS (Leucht et al., 2013), including akathisia (Kahn et al., 2008; Kane et al., 2009), and a range of other adverse events (AEs), such as metabolic abnormalities (De Hert et al., 2011), sedation, hyperprolactinemia, and QTc prolongation (Leucht et al., 2013). Consequently, there is a need for an antipsychotic treatment of schizophrenia that optimizes symptom control in patients during the early episodes of schizophrenia, without trade-offs in tolerability that can affect the patient’s ability to remain on treatment.
Brexpiprazole, which was approved by the FDA in July 2015 for the treatment of schizophrenia and as an adjunctive therapy to antidepressants for the treatment of major depressive disorder, has a unique pharmacological profile, acting as a serotonin–dopamine activity modulator that is a partial agonist with similar potency at serotonin 5-HT1A and dopamine D2 receptors, and a potent antagonist at serotonin 5-HT2A and noradrenaline α1B/2C receptors (Maeda et al., 2014). Brexpiprazole has a lower intrinsic activity at D2 receptors than the D2 partial agonist, aripiprazole, which may reduce the potential of brexpiprazole to induce AEs mediated by activity at this receptor, such as akathisia, agitation, insomnia, restlessness, and nausea (Fleischhacker, 2005; Maeda et al., 2014). Brexpiprazole also has a moderate affinity for histamine H1 receptors relative to its D2 receptor affinity (Maeda et al., 2014), which could potentially result in low levels of sedation. In two fixed-dose phase 3 studies, brexpiprazole treatment was efficacious in adult patients experiencing an acute exacerbation of schizophrenia, showed good tolerability, and was associated with a low incidence of sedation, EPS-related, and metabolic AEs (Correll et al., 2015; Kane et al., 2015).
The aim of this exploratory, open-label study was to investigate the effects of flexibly dosed brexpiprazole (1, 2, 3, or 4 mg/day; monotherapy target dose 3 mg/day) on efficacy, social functioning, and tolerability during treatment of early episodes of schizophrenia.
Methods
Patients
Adult outpatients (18–35 years old) were recruited from 20 sites in the USA, with an axis-1 diagnosis of schizophrenia [Diagnostic and Statistical Manual of Mental Disorders, 4th ed., text revision (DSM-IV-TR)] of at least 6 months’ duration before screening. Diagnosis was confirmed by the Mini International Neuropsychiatric Interview for Schizophrenia and Psychotic Disorders (Sheehan et al., 2010) and a clinical psychiatric evaluation. Patients could be currently receiving antipsychotic medication and were eligible if their first schizophrenia episode occurred 5 years or less before the time of consent. In addition, eligible patients had a Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1999) total score of up to 80 at screening and baseline, and showed impaired social interaction with a score of at least 4 on at least one PANSS modified prosocial line item, including active social avoidance; emotional withdrawal; passive/apathetic social withdrawal; and difficulty in abstract thinking (Purnine et al., 2000; Docherty et al., 2010). Additional inclusion criteria were as follows: history of relapse and/or symptom exacerbation in the absence of antipsychotic treatment; good response in the last 12 months to outpatient antipsychotic treatment of at least 6 weeks’ duration; and potential to benefit from outpatient brexpiprazole treatment according to the investigator’s judgment.
Patients were excluded if they had received recent (≤60 days of screening) electroconvulsive therapy or transcranial magnetic stimulation, were hospitalized for psychotic symptoms in the last 6 months, had experienced acute depressive symptoms requiring treatment for up to 30 days before screening or had any other psychiatric diagnosis, had tardive dyskinesia or severe akathisia at enrollment, were receiving predefined concomitant medications, or were at serious risk of suicide.
Study design
This was an exploratory, open-label, flexible-dose study carried out between November 2013 and October 2014 (http://www.clinicaltrials.gov; NCT02013622).
The study comprised a 2–21-day screening phase, a 2–4-week conversion phase in which brexpiprazole was cross-titrated with previous antipsychotic medication, a 12–14-week, flexible-dose, open-label brexpiprazole monotherapy phase (1, 2, 3, or 4 mg/day; 3 mg/day target dose), and a 30-day (+2 day) follow-up phase (Fig. 1). Although brexpiprazole 3 mg/day was the target dose, study investigators used informed judgment to decrease dosage (brexpiprazole 1 or 2 mg/day) for enhanced tolerability or to increase dosage (brexpiprazole 4 mg/day) for enhanced efficacy.
Fig. 1.
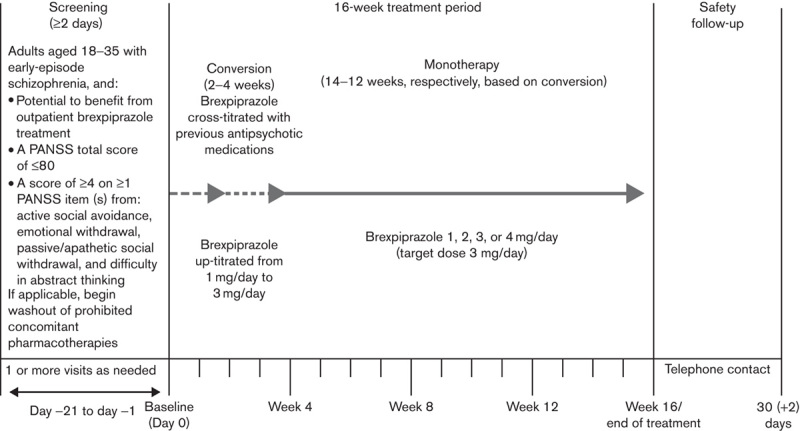
Study design. PANSS, Positive and Negative Syndrome Scale.
The final study protocol and informed consent forms were approved by the relevant institutional review board or independent ethics committee for each participating study center. The study was carried out in compliance with the Declaration of Helsinki, the International Conference on Harmonisation Good Clinical Practice Guideline, and applicable local laws and regulatory requirements. All patients provided written informed consent before study initiation.
Efficacy and safety assessments
The efficacy outcomes included changes from baseline to week 16 in PANSS total score (Kay et al., 1999), PANSS modified prosocial subscale scores (Purnine et al., 2000), Clinical Global Impressions – Severity of illness (CGI-S) score, Specific Levels of Functioning Scale (SLOF) (Schneider and Struening, 1983), Personal and Social Performance scale (PSP) (Morosini et al., 2000), Pittsburgh Sleep Quality Index (PSQI) (Buysse et al., 1989), and Treatment Satisfaction Questionnaire for Medication 14 items (TSQM-14) (Atkinson et al., 2004). CGI – Improvement (CGI-I) score at week 16 (Guy, 1976) was also assessed, as was the response rate [defined by CGI-I score of 1 (very much improved) or 2 (much improved)].
Safety assessments included AEs; physical examinations; vital signs; body weight; clinical laboratory tests; ECGs; Simpson Angus Scale (SAS) (Simpson and Angus, 1970); Abnormal Involuntary Movement Scale (AIMS) (Guy, 1976); Barnes Akathisia Rating Scale (BARS) (Barnes, 1989); and Columbia-Suicide Severity Rating Scale (C-SSRS) (Posner et al., 2011). AEs were coded by system organ class and preferred term using the Medical Dictionary for Regulatory Activities.
Statistical analysis
Analysis sets included safety analysis set (all patients who took at least one dose of study medication) and full analysis set (all treated patients who took at least one dose of study medication and who underwent a baseline assessment and at least one post-baseline efficacy assessment). Baseline measurement was defined as the last available measurement before the start of study medication, provided that the measurement took place at screening or day 0 visit.
A mixed-model repeated-measures (MMRM) analysis was used to investigate change in efficacy from baseline, with a statistical significance level of 0.05 (two sided). For the primary analysis, the model included fixed-class effect terms for visit, baseline PANSS total score, and baseline-by-visit interaction; imputation of missing data using MMRM was based on the observed cases dataset.
The MMRM model was also used to evaluate change from baseline to week 16 for the PANSS modified prosocial subscale scores, CGI-S score, PSP total score, SLOF total and subdomain scores, PSQI total score, and TSQM-14 total score. Last observation carried forward analyses were also carried out. Additional efficacy and safety variables were reported descriptively.
Results
Patient disposition and demographic characteristics
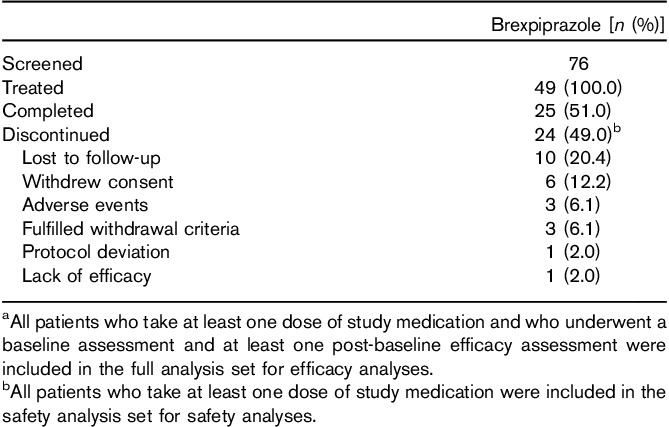
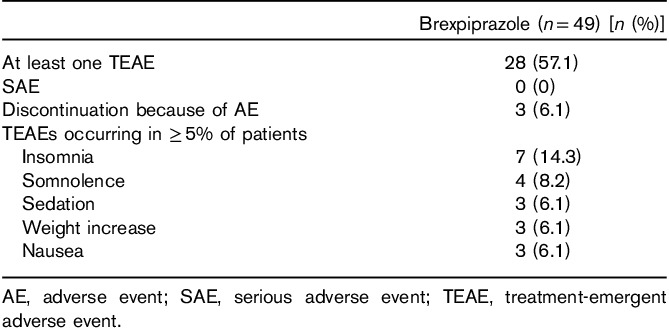
All 49 patients received at least one dose of study medication, had undergone baseline and at least one post-baseline efficacy assessments, and were included in the full analysis set. Overall, 25 (51.0%) patients completed the study (Table 1). The most common reason for discontinuation was loss to follow-up (20.4%, 10/49 patients), which represented more than 40% (10/24) of all discontinuations. Three patients (6.1%) were withdrawn because of AEs: one each because of psychotic disorder, insomnia/akathisia, and somnolence (Table 1).
Table 1.
Patient dispositiona,b
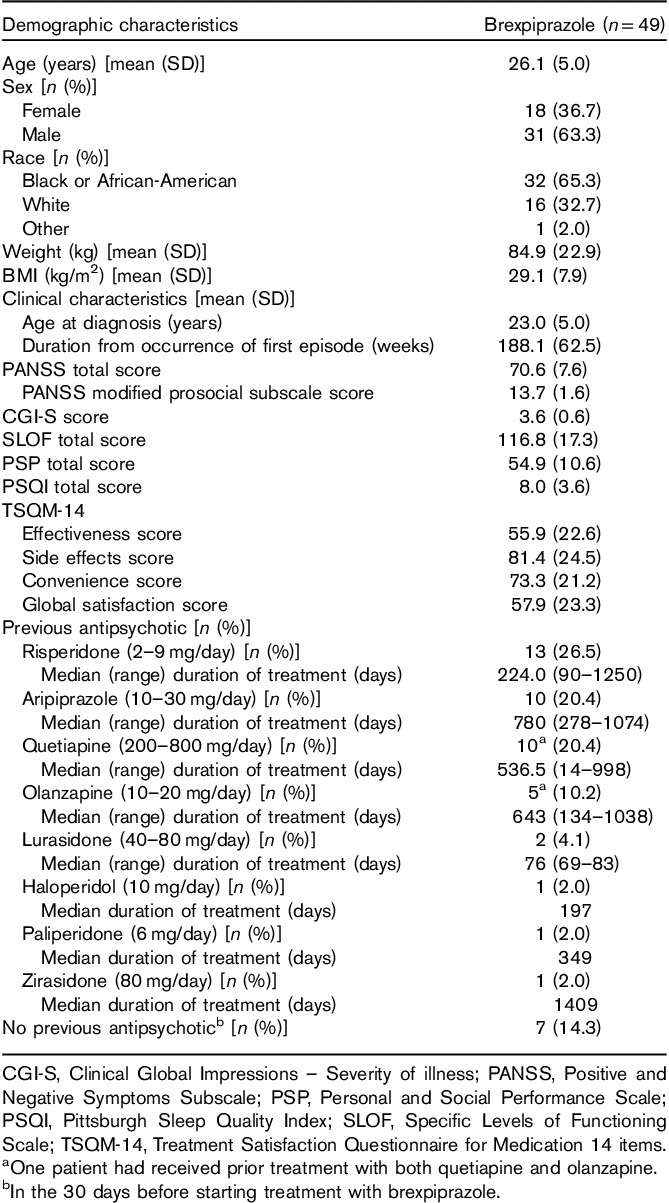
Patient demographics and baseline clinical characteristics are shown in Table 2. The study population was relatively young, with a mean (SD) age of 26.1 (5.0) years, mainly men (31/49, 63.3%), and predominantly Black or African-American (32/49, 65.3%) (Table 2). All patients had previously received antipsychotic treatment and 42 (85.7%) patients had received any antipsychotic therapy in the 30 days before starting brexpiprazole (Table 2).
Table 2.
Patient demographics and baseline clinical characteristics
Analysis of efficacy
Primary variable
Improvements in PANSS total score from baseline to week 16 were observed with brexpiprazole (Fig. 2); the least-squares (LS) mean improvement (SE) observed at week 16 was −10.2 (2.1) (P<0.0001 vs. baseline) (Fig. 2). Improvements in PANSS total score were observed as early as week 1 (P=0.0003) and continued throughout the entire treatment period.
Fig. 2.
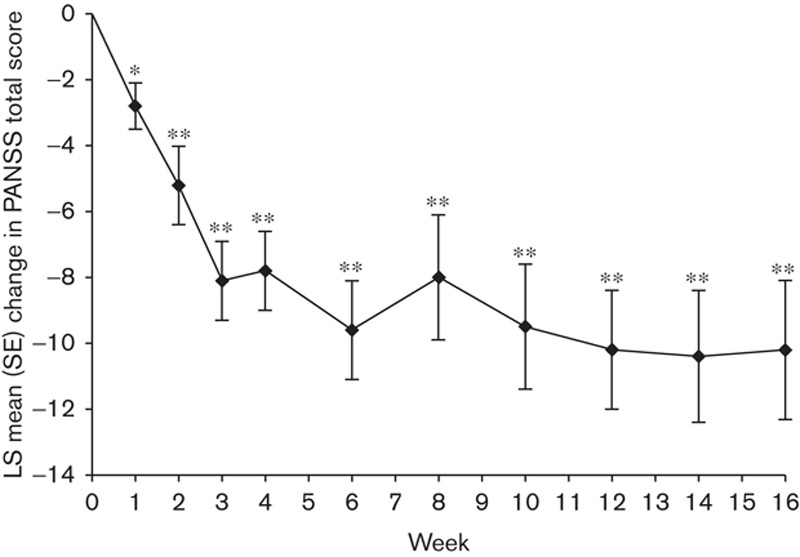
Mean change from baseline in PANSS total score during the brexpiprazole treatment period. Efficacy population, MMRM analysis; n=49. PANSS total mean score at baseline: 70.6. *P=0.0003; **P<0.0001. P-values are relative to baseline (week 0). LS, least squares; MMRM, mixed-model repeated-measures; PANSS, Positive and Negative Syndrome Scale.
Other variables
Improvements in PANSS total score were reflected in improvements in CGI-S (Table 3) and reductions in CGI-I scores compared with baseline. Improvements in CGI-S scores over baseline were observed as early as week 1 (P=0.0066) and were maintained at week 16 (P<0.0001; Table 3). The mean CGI-I scores supported the observed changes in PANSS and CGI-S scores, and the mean CGI-I (SD) score at week 16 was 2.8 (1.3). Increases in the proportion of patients who were responders [CGI-I score of 1 (very much improved) or 2 (much improved)] were noted. By weeks 2, 4, and 16, 25.0, 39.6, and 41.7% of patients, respectively, were classified as responders (Fig. 3).
Table 3.
LS mean (SE) change from baseline to week 16 in other efficacy variables
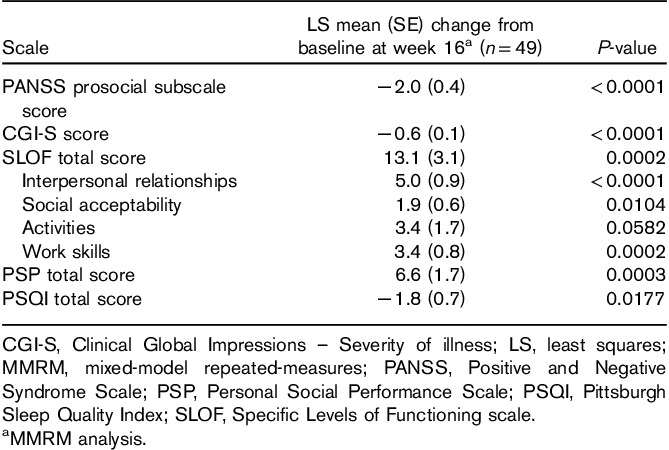
Fig. 3.
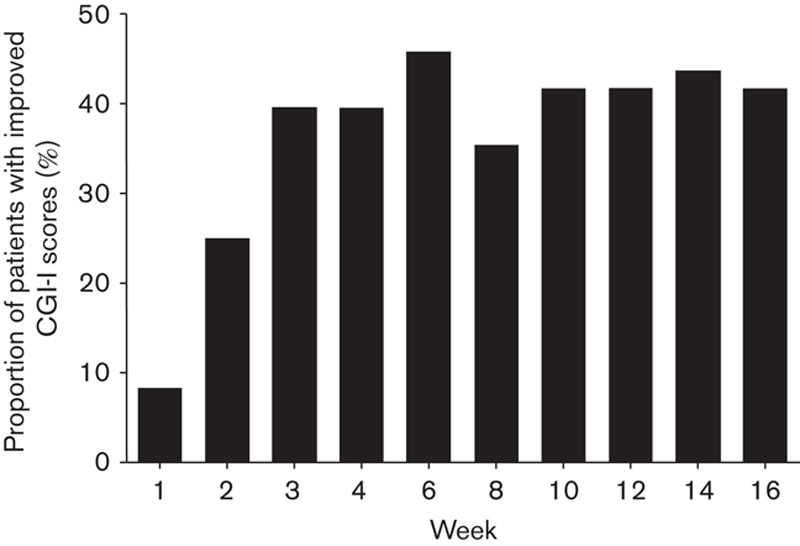
Proportion of patients with improved CGI-I scoresa,b during the brexpiprazole treatment period. aLOCF; bpercentage of patients fulfilling criteria for CGI-I response defined as a CGI-I score of 1 (very much improved) or 2 (much improved). CGI-I, Clinical Global Impressions – Improvement; LOCF, last observation carried forward.
Improvements from baseline were observed at week 16 in measures of patient function (SLOF and PSP scores) that included social function (Table 3). Improvements were also supported by results on the PANSS modified prosocial subscale score, for which the LS mean improvement observed at week 16 was −2.0 (P<0.0001 vs. baseline) (Table 3). In addition, improvements were observed in sleep quality on the basis of change in PSQI score (P=0.0177 vs. baseline) (Table 3).
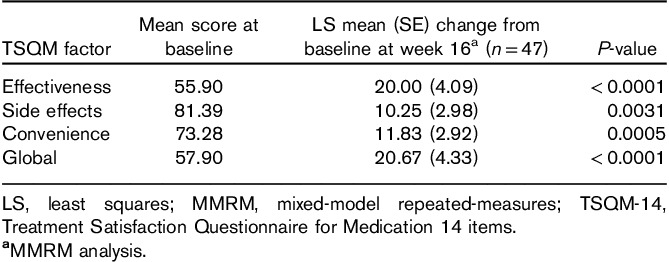
Patient satisfaction with treatment, on the basis of TSQM-14 scores, showed improvements throughout the treatment period, with effectiveness, side effects, convenience, and global factors all showing improvement at week 16 (Table 4).
Table 4.
LS mean (SE) change from baseline in TSQM-14 scores
Safety and tolerability
A total of 25 patients were exposed to brexpiprazole for between 105 and 111 days, with a mean daily dose of 3.4 mg (range 2.0–4.0 mg). Approximately 70% of patients completed cross-titration in 2 weeks, with the remaining patients completing cross-titration within the 4-week period. Brexpiprazole was well tolerated; 3/49 (6.1%) patients discontinued because of AEs (psychotic disorder, insomnia/akathisia, and somnolence, each in one patient) and no patient experienced a serious AE (Table 5). Treatment-emergent AEs (TEAEs) were all mild or moderate in severity; the most common AEs were insomnia, somnolence, sedation, weight increase, and nausea (Table 5).
Table 5.
Treatment-emergent adverse events
Clinical laboratory, body weight, and other safety parameters
The mean (SD) weight increase from baseline at week 16 was 2.24 (2.71) kg (n=25) and 1.50 (3.03) kg at last visit (n=39). Weight increase was reported as an AE by three patients (6.1%); however, no patient experienced clinically relevant weight gain (≥7% increase from baseline of body weight). One patient experienced clinically relevant weight loss (≥7% decrease from the baseline body weight) at week 8 and at last visit. No clinically relevant mean changes in metabolic parameters were observed from baseline to week 16, although some potentially clinically relevant laboratory values, for individual patients, were reported.
Overall, there were no clinically relevant changes in physical examinations, vital signs (excluding ≥7% decrease in body weight and one incidence of orthostatic hypotension), clinical laboratory tests, or ECGs.
The mean changes from baseline at week 16 for all ECG parameters were minimal and none were considered to be clinically relevant; no patients experienced a new-onset QT more than 450 ms, five patients experienced an increase of 30–60 ms in the QTcB interval, and two patients experienced an increase of 30–60 ms in the QTcF interval.
A total of four patients (8.2%) experienced EPS-related AEs during the study, one each of dystonia (2.0%) and tremor (2.0%), and two of akathisia (4.1%). LS mean (SD) changes in EPS scales at week 16 were not clinically relevant [SAS, 0.0 (0.2); AIMS, 0.0 (0.0); BARS, 0.0 (0.0)].
No suicidal behavior was reported on the C-SSRS. At week 16, a total of five patients (10.2%) had experienced at least one occurrence of suicidal ideation on the C-SSRS compared with one patient at baseline. All occurrences were identified as a ‘wish to be dead’ and none of these events were reported as TEAEs. Of the four patients who had emergence of suicide ideation during the study, none were considered to be serious.
Discussion
A number of antipsychotic treatments have been evaluated in the treatment of early episodes of schizophrenia and appear to reduce schizophrenia symptoms in this patient population (Kahn et al., 2008; Docherty et al., 2010). Aripiprazole seems to be better tolerated than quetiapine (Melnik et al., 2010; Belgamwar and El-Sayeh, 2011; Citrome, 2013) and may help to improve functioning in patients with schizophrenia and achieve better control of positive symptoms compared with other antipsychotics (Docherty et al., 2010; Crespo-Facorro et al., 2013). Importantly, use of low-dose atypical antipsychotics in cases of first-episode psychosis in the context of a comprehensive early intervention service has been shown to achieve very high rates of symptom resolution, particularly positive symptoms (Malla et al., 2006, 2016).
In this open-label trial of patients with relatively stable schizophrenia in its early stage (mean baseline PANSS total score, 70.6) who were responsive to outpatient antipsychotic treatment in the last 12 months, symptoms of schizophrenia improved over the entire treatment period in patients treated with brexpiprazole, as shown by reductions in PANSS total score across the 16-week study (LS mean reduction at week 16: −10.2 points). Considerable improvements were also indicated in CGI-I scores (CGI-I results presented here were indicative of additional response to brexpiprazole over previous antipsychotic) and corresponding response rates, whereas the withdrawal rate for lack of efficacy was 2.0% (1/49). Our findings related to overall efficacy are broadly consistent with results of the short-term phase 3 trials, which showed that brexpiprazole is efficacious in adult patients with acute schizophrenia (Correll et al., 2015; Kane et al., 2015).
Importantly, our results in early-episode schizophrenia patients also suggest an improvement in social engagement and functioning with brexpiprazole, as measured by PANSS modified prosocial subscale score, SLOF total and subdomain scores, and PSP score (Schneider and Struening, 1983; Morosini et al., 2000; Docherty et al., 2010). The PANSS modified prosocial subscale evaluates social interaction, scoring patients according to active social avoidance, emotional withdrawal, passive/apathetic social withdrawal, and difficulty in abstract thinking. The magnitude of improvement observed with brexpiprazole on the modified prosocial subscale was similar to that reported for aripiprazole at week 18 in a post-hoc analysis of social functioning in patients with early episodes of schizophrenia (LS mean change from baseline –3.16) (Docherty et al., 2010). The PSP scale assesses social functioning, and shows good reliability and validity in patients with acute and stabilized schizophrenia (Morosini et al., 2000; Patrick et al., 2006). On the basis of these statistical considerations and clinical reasoning, the minimal clinically important difference for PSP has been reported previously to be 10 points (Patrick et al., 2006). Although a difference of this magnitude was not reported in the present study, it is noteworthy that improvements were observed in PSP at week 16, despite the study cohort having a mean PSP score at baseline of 54.9, indicative of a relatively high level of personal and social functioning. In addition, SLOF represents another reliable and valid instrument for the assessment of social functioning, measuring practical aspects of everyday function within four domains: interpersonal relationships, social acceptability, activities, and work skills (Schneider and Struening, 1983; Mucci et al., 2014). Improvements in all four domains were observed with brexpiprazole treatment. Again, the clinical relevance of the improvements in SLOF observed in the current study is currently unclear as a minimal clinically important difference in the SLOF score has not been determined. Although the improvement in social functioning was clearly observed, we are not able to test whether this was mediated by improvement in symptoms or independent of such change in symptoms.
On the basis of our results from a relatively brief study of 16 weeks, further controlled and longer-term studies are warranted to confirm the improvements observed in this open-label study, and to evaluate the clinical relevance of the improvements in social functioning observed in early-episode patients with schizophrenia treated with brexpiprazole. Improved social functioning is particularly important in early schizophrenia as more engaged patients are likely to take advantage of psychosocial and recovery-oriented interventions, with these interventions also being able to influence prevention of relapses (Malla et al., 2008; Horan et al., 2009, 2011). Given that deficits in social functioning are present in the early stages of schizophrenia (Grant et al., 2001), the improvements in social functioning observed with brexpiprazole treatment may facilitate a greater degree of overall functional recovery in patients in this stage of the disease.
Consistent with the pivotal trials (Correll et al., 2015; Kane et al., 2015), brexpiprazole was well tolerated in this study in patients with early-episode schizophrenia, with low numbers of TEAEs and discontinuations because of TEAEs. The most common AE was insomnia, which should be monitored in all patients with schizophrenia, especially in patients with early-episode schizophrenia. Loss to follow-up accounted for more than 40% of total study discontinuations, likely reflecting the propensity for patients to disengage with care systems, as opposed to a direct effect of the medication. Improvements from baseline in TSQM scores, including subscales of convenience, effectiveness, and side effects, were noted at week 16, suggesting that these patients, who had already experienced other antipsychotic treatment, were satisfied with brexpiprazole and did not find the process of switching therapy difficult. Although no method is universally accepted for switching from one antipsychotic to another, the cross-titration schedule used in this study appeared to work well, with ∼70% of patients completing within 2 weeks. Overall, the brexpiprazole target dosage of 3 mg/day was achieved, with a mean daily dose of 3.4 mg.
Results from the C-SSRS showed that five patients had at least one occurrence of suicide ideation, all of which were identified as a ‘wish to be dead’. None of these events were reported as TEAEs. Of the four patients who had emergence of suicide ideation during the study, none were considered to be serious.
A moderate increase in body weight was observed in patients over 16 weeks of treatment. The magnitude of mean body weight change (2.24 kg) was marginally greater than that reported in previous phase 3 trials of brexpiprazole (1.23–1.89 kg), which could be because of differences in patient demographics or duration of studies. A greater proportion of patients in the present study were Black or African-American (65.3%), compared with patients receiving brexpiprazole during the phase 3 studies (23.2–23.9%), and the patient population was younger, with an entry criterion of up to 35 years [mean (SD) age, 26.1 (5.0) years]. It is not clear whether these demographic differences could have contributed to the greater mean weight changes and, given the relatively brief nature of this study, we cannot speculate as to whether weight gain would continue or is likely to reach a plateau.
We acknowledge the limitations associated with evaluating multiple outcome measures (which may not be independent) when using a small sample size. This may also restrict the applicability of the findings to a wider, more diverse population of patients with schizophrenia in this relatively early phase. However, the study was exploratory in nature and the promising results suggest that further investigation is warranted. Other limitations included the lack of a placebo or an active comparator to contextualize the improvements observed with brexpiprazole. Nevertheless, these patients, who had all been treated previously with antipsychotics and were enrolled because their physicians believed that they may benefit from therapy, showed improvement following the initiation of brexpiprazole.
In conclusion, the results of this exploratory study suggest that brexpiprazole may be efficacious in the treatment of patients with schizophrenia in its early phase. The findings extend those from the pivotal studies, in which patients within 3 years of first diagnosis were excluded, where the efficacy of brexpiprazole in the treatment of patients with acute schizophrenia was shown (Correll et al., 2015; Kane et al., 2015). More specifically, the data suggest that brexpiprazole may improve functioning and treatment satisfaction in young, early-episode outpatients (18–35 years) with schizophrenia, potentially improving long-term outcomes. These results warrant further studies of the effectiveness of brexpiprazole in patients still in their early phase of schizophrenia who may benefit from a change in treatment as well as in patients who have had limited exposure to antipsychotic medications.
Acknowledgements
The authors would like to thank the patients who participated in this study. Kris Holmes (QXV Communications, an Ashfield business, part of UDG Healthcare plc, Macclesfield, UK) provided medical writing support, which was funded by Otsuka Pharmaceutical Commercialization and Development Inc. (Princeton, New Jersey, USA) and H. Lundbeck A/S (Valby, Denmark).
Conflicts of interest
Ashok Malla is a consultant to Otsuka Pharmaceutical Development & Commercialization Inc. and H. Lundbeck A/S, and has received honoraria for advisory boards and lecture honoraria from BMS, Otsuka, Lundbeck, Janssen, and Pfizer. Ai Ota and Kazuhiro Nagamizu are full-time employees of Otsuka Pharmaceutical Co. Ltd, Tokyo, Japan. Pamela Perry and Ross A. Baker are full-time employees of Otsuka Pharmaceutical Development & Commercialization Inc. Emmanuelle Weiller is a full-time employee of H. Lundbeck A/S.
References
- Alvarez-Jimenez M, Priede A, Hetrick SE, Bendall S, Killackey E, Parker AG, et al. (2012). Risk factors for relapse following treatment for first episode psychosis: a systematic review and meta-analysis of longitudinal studies. Schizophr Res 139:116–128. [DOI] [PubMed] [Google Scholar]
- Atkinson MJ, Sinha A, Hass SL, Colman SS, Kumar RN, Brod M, Rowland CR. (2004). Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes 2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes TR. (1989). A rating scale for drug-induced akathisia. Br J Psychiatry 154:672–676. [DOI] [PubMed] [Google Scholar]
- Belgamwar RB, El-Sayeh HG. (2011). Aripiprazole versus placebo for schizophrenia. Cochrane Database Syst Rev 8:CD006622. [DOI] [PubMed] [Google Scholar]
- Birchwood M, Todd P, Jackson C. (1998). Early intervention in psychosis. The critical period hypothesis. Br J Psychiatry Suppl 172:53–59. [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. (1989). The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28:193–213. [DOI] [PubMed] [Google Scholar]
- Carbon M, Correll CU. (2014). Clinical predictors of therapeutic response to antipsychotics in schizophrenia. Dialogues Clin Neurosci 16:505–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cechnicki A, Cichocki Ł, Kalisz A, Błądziński P, Adamczyk P, Franczyk-Glita J. (2014). Duration of untreated psychosis (DUP) and the course of schizophrenia in a 20-year follow-up study. Psychiatry Res 219:420–425. [DOI] [PubMed] [Google Scholar]
- Citrome L. (2013). A review of the pharmacology, efficacy and tolerability of recently approved and upcoming oral antipsychotics: an evidence-based medicine approach. CNS Drugs 27:879–911. [DOI] [PubMed] [Google Scholar]
- Correll CU, Skuban A, Ouyang J, Hobart M, Pfister S, McQuade RD, et al. (2015). Efficacy and safety of brexpiprazole for the treatment of acute schizophrenia: a 6-week randomized, double-blind, placebo-controlled trial. Am J Psychiatry 172:870–880. [DOI] [PubMed] [Google Scholar]
- Crespo-Facorro B, Pérez-Iglesias R, Mata I, Ortiz-Garcia de la Foz V, Martínez-Garcia O, Valdizan EM, Vazquez-Barquero JL. (2013). Aripiprazole, ziprasidone, and quetiapine in the treatment of first-episode nonaffective psychosis: results of a 6-week, randomized, flexible-dose, open-label comparison. J Clin Psychopharmacol 33:215–220. [DOI] [PubMed] [Google Scholar]
- Crumlish N, Whitty P, Clarke M, Browne S, Kamali M, Gervin M, et al. (2009). Beyond the critical period: longitudinal study of 8-year outcome in first-episode non-affective psychosis. Br J Psychiatry 194:18–24. [DOI] [PubMed] [Google Scholar]
- De Hert M, Detraux J, van Winkel R, Yu W, Correll CU. (2011). Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol 8:114–126. [DOI] [PubMed] [Google Scholar]
- Docherty JP, Baker RA, Eudicone J, Mathew S, Marcus RN, McQuade RD, Mankoski R. (2010). Effect of aripiprazole versus haloperidol on PANSS Prosocial items in early-episode patients with schizophrenia. Schizophr Res 120:199–203. [DOI] [PubMed] [Google Scholar]
- Fleischhacker WW. (2005). Aripiprazole. Expert Opin Pharmacother 6:2091–2101. [DOI] [PubMed] [Google Scholar]
- Grant C, Addington J, Addington D, Konnert C. (2001). Social functioning in first- and multiepisode schizophrenia. Can J Psychiatry 46:746–749. [DOI] [PubMed] [Google Scholar]
- Guo X, Li J, Wei Q, Fan X, Kennedy DN, Shen Y, et al. (2013). Duration of untreated psychosis is associated with temporal and occipitotemporal gray matter volume decrease in treatment naive schizophrenia. PLoS One 8:e83679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy W. (1976). ECDEU assessment manual for psychopharmacology. Rockville, MD, USA: US Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs. [Google Scholar]
- Haijma SV, Van Haren N, Cahn W, Koolschijn PC, Hulshoff Pol HE, Kahn RS. (2013). Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull 39:1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Kern RS, Shokat-Fadai K, Sergi MJ, Wynn JK, Green MF. (2009). Social cognitive skills training in schizophrenia: an initial efficacy study of stabilized outpatients. Schizophr Res 107:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Kern RS, Tripp C, Hellemann G, Wynn JK, Bell M, et al. (2011). Efficacy and specificity of social cognitive skills training for outpatients with psychotic disorders. J Psychiatr Res 45:1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui CL, Wong GH, Tang JY, Chang WC, Chan SK, Lee EH, et al. (2013). Predicting 1-year risk for relapse in patients who have discontinued or continued quetiapine after remission from first-episode psychosis. Schizophr Res 150:297–302. [DOI] [PubMed] [Google Scholar]
- Kahn RS, Fleischhacker WW, Boter H, Davidson M, Vergouwe Y, Keet IP, et al. (2008). Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet 371:1085–1097. [DOI] [PubMed] [Google Scholar]
- Kanahara N, Yoshida T, Oda Y, Yamanaka H, Moriyama T, Hayashi H, et al. (2013). Onset pattern and long-term prognosis in schizophrenia: 10-year longitudinal follow-up study. PLoS One 8:e67273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane JM, Fleischhacker WW, Hansen L, Perlis R, Pikalov A, 3rd, Assunção-Talbott S. (2009). Akathisia: an updated review focusing on second-generation antipsychotics. J Clin Psychiatry 70:627–643. [DOI] [PubMed] [Google Scholar]
- Kane JM, Skuban A, Ouyang J, Hobart M, Pfister S, McQuade RD, et al. (2015). A multicenter, randomized, double-blind, controlled phase 3 trial of fixed-dose brexpiprazole for the treatment of adults with acute schizophrenia. Schizophr Res 164:127–135. [DOI] [PubMed] [Google Scholar]
- Kay SR, Opler LA, Fiszbein A. (1999). Positive and negative syndrome scale (PANSS) rating criteria. North Tonawanda, NY: Multi-Health Systems. [Google Scholar]
- Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, et al. (2013). Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 382:951–962. [DOI] [PubMed] [Google Scholar]
- Maeda K, Sugino H, Akazawa H, Amada N, Shimada J, Futamura T, et al. (2014). Brexpiprazole I: in vitro and in vivo characterization of a novel serotonin-dopamine activity modulator. J Pharmacol Exp Ther 350:589–604. [DOI] [PubMed] [Google Scholar]
- Malla A, Norman R, Schmitz N, Manchanda R, Béchard-Evans L, Takhar J, Haricharan R. (2006). Predictors of rate and time to remission in first-episode psychosis: a two-year outcome study. Psychol Med 36:649–658. [DOI] [PubMed] [Google Scholar]
- Malla A, Norman R, Bechard-Evans L, Schmitz N, Manchanda R, Cassidy C. (2008). Factors influencing relapse during a 2-year follow-up of first-episode psychosis in a specialised early intervention service. Psychol Med 38:1585–1593. [DOI] [PubMed] [Google Scholar]
- Malla AK, Bodnar M, Joober R, Lepage M. (2011). Duration of untreated psychosis is associated with orbital-frontal grey matter volume reductions in first episode psychosis. Schizophr Res 125:13–20. [DOI] [PubMed] [Google Scholar]
- Malla A, Mustafa S, Rho A, Abadi S, Lepage M, Joober R. (2016). Therapeutic effectiveness and tolerability of aripiprazole as initial choice of treatment in first episode psychosis in an early intervention service: a one-year outcome study. Schizophr Res 174:120–125. [DOI] [PubMed] [Google Scholar]
- Marshall M, Lewis S, Lockwood A, Drake R, Jones P, Croudace T. (2005). Association between duration of untreated psychosis and outcome in cohorts of first-episode patients: a systematic review. Arch Gen Psychiatry 62:975–983. [DOI] [PubMed] [Google Scholar]
- Melnik T, Soares BG, Puga ME, Atallah AN. (2010). Efficacy and safety of atypical antipsychotic drugs (quetiapine, risperidone, aripiprazole and paliperidone) compared with placebo or typical antipsychotic drugs for treating refractory schizophrenia: overview of systematic reviews. Sao Paulo Med J 128:141–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morosini PL, Magliano L, Brambilla L, Ugolini S, Pioli R. (2000). Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand 101:323–329. [PubMed] [Google Scholar]
- Mucci A, Rucci P, Rocca P, Bucci P, Gibertoni D, Merlotti E, et al. (2014). The Specific Level of Functioning Scale: construct validity, internal consistency and factor structure in a large Italian sample of people with schizophrenia living in the community. Schizophr Res 159:144–150. [DOI] [PubMed] [Google Scholar]
- Norman RM, Malla AK. (2001). Duration of untreated psychosis: a critical examination of the concept and its importance. Psychol Med 31:381–400. [DOI] [PubMed] [Google Scholar]
- Norman RM, Mallal AK, Manchanda R, Windell D, Harricharan R, Takhar J, Northcott S. (2007). Does treatment delay predict occupational functioning in first-episode psychosis? Schizophr Res 91:259–262. [DOI] [PubMed] [Google Scholar]
- Norman RM, Manchanda R, Malla AK, Windell D, Harricharan R, Northcott S. (2011). Symptom and functional outcomes for a 5 year early intervention program for psychoses. Schizophr Res 129:111–115. [DOI] [PubMed] [Google Scholar]
- Novick D, Haro JM, Suarez D, Perez V, Dittmann RW, Haddad PM. (2010). Predictors and clinical consequences of non-adherence with antipsychotic medication in the outpatient treatment of schizophrenia. Psychiatry Res 176:109–113. [DOI] [PubMed] [Google Scholar]
- Owens DC, Johnstone EC, Miller P, Macmillan JF, Crow TJ. (2010). Duration of untreated illness and outcome in schizophrenia: test of predictions in relation to relapse risk. Br J Psychiatry 196:296–301. [DOI] [PubMed] [Google Scholar]
- Patrick D, Adriaenssen I, Morosini PL, Rothman M. (2006). Reliability, validity and sensitivity to change of the personal and social performance scale in patients with acute schizophrenia. Int J Neuropsychopharmacol 9:S287–S288. [Google Scholar]
- Penttilä M, Jääskeläinen E, Hirvonen N, Isohanni M, Miettunen J. (2014). Duration of untreated psychosis as predictor of long-term outcome in schizophrenia: systematic review and meta-analysis. Br J Psychiatry 205:88–94. [DOI] [PubMed] [Google Scholar]
- Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, et al. (2011). The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 168:1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnine DM, Carey KB, Maisto SA, Carey MP. (2000). Assessing positive and negative symptoms in outpatients with schizophrenia and mood disorders. J Nerv Ment Dis 188:653–661. [DOI] [PubMed] [Google Scholar]
- Reynolds GP, Kirk SL. (2010). Metabolic side effects of antipsychotic drug treatment--pharmacological mechanisms. Pharmacol Ther 125:169–179. [DOI] [PubMed] [Google Scholar]
- Schneider LC, Struening EL. (1983). SLOF: a behavioral rating scale for assessing the mentally ill. Soc Work Res Abstr 19:9–21. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Janavs J, Harnett-Sheehan K, Sheehan M, Gray C, Lecrubier Y. (2010). Mini International Neuropsychiatric Interview for schizophrenia and psychotic disorders studies, version 6.0.0. Available at: http://www.medical-outcomes.com. [Accessed 24 April 2015].
- Simpson GM, Angus JW. (1970). A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl 212:11–19. [DOI] [PubMed] [Google Scholar]


