Supplemental Digital Content is available in the text.
Keywords: antidepressant, apathy, depression, fatigue, serotonin and norepinephrine reuptake inhibitor
Abstract
The objective of this post-hoc analysis was to investigate the relationship between motivation/energy and functional impairment in patients with major depressive disorder (MDD). Data were taken from a phase 3 trial of levomilnacipran extended-release (ER) in adults with MDD (NCT01034462; N=429) that used the 18-item Motivation and Energy Inventory (MEI) to assess motivation/energy. Two subgroups with lower and higher motivation/energy were defined using baseline MEI total scores (≤28 and >28, respectively). Change from baseline in the Sheehan Disability Scale (SDS) total score was analyzed in the intent-to-treat (ITT) population and both subgroups. Path analyses were carried out in the ITT population and a lower MEI subgroup to assess the direct and indirect effects of levomilnacipran ER on SDS total score change. In the ITT population and the lower MEI subgroup, significant differences were found between levomilnacipran ER and placebo for changes in the SDS total score (−2.6 and −3.9, both P<0.01), but not in the higher MEI subgroup. The indirect effect of levomilnacipran ER on SDS total score improvement, as mediated by MEI total score change, was 79.9% in the lower MEI subgroup and 67.2% in the ITT population. Levomilnacipran ER was previously shown to improve motivation/energy in adults with MDD. The current analysis indicates that improvements in functional impairment were considerably mediated by improvements in motivation/energy, particularly in patients with lower motivation/energy at baseline.
Introduction
Decreased energy, fatigue, loss of interest or pleasure, and reduced drive or motivation are symptoms of major depressive disorder (MDD) that are associated with poor treatment outcomes (Uher et al., 2012; Calabrese et al., 2014). These symptoms might not be adequately improved with serotonergic agents, even in patients who otherwise respond to treatment. In the STAR*D trial (McClintock et al., 2011), for example, the majority of patients who had achieved at least 50% improvement in overall depression symptom severity after acute treatment with a selective serotonin reuptake inhibitor continued to experience decreased interest and energy (55.0 and 64.6%, respectively), whereas one-third continued to feel slowed down (35.6%). Such findings point to the ongoing need for more clinically relevant information on the causes and consequences of unresolved motivation and energy symptoms in MDD patients, such as the effects of decreased motivation/energy on treatment adherence in patients, the need for and/or consequences of medication switching by clinicians, the associated increases in healthcare utilization costs, and the impact of residual symptoms on patient functioning.
Restoration of functional ability remains a primary target in the management of MDD (APA, 2010), both in terms of improving individual patient well-being and reducing healthcare and disability costs (McKnight and Kashdan, 2009; Lam et al., 2011). As residual impairments in psychosocial functioning have been associated with an increased risk of episode recurrence (Hardeveld et al., 2010), further contributing toward the personal and social burden of MDD, identifying and alleviating symptoms associated with functional impairment are important clinical objectives. Fatigue, decreased energy, and loss of interest/pleasure, which are among most the common depressive symptoms encountered in clinical settings, have been shown to negatively affect patient functioning and workplace productivity (Stahl, 2002; Nutt et al., 2007). Improving these symptoms may therefore be an important component of effective MDD management. As fatigue, lack of energy, and loss of interest can be more difficult to treat than other depression symptoms (Nutt et al., 2007), there remains an ongoing need for treatments that effectively target these types of symptoms.
The psychopathological basis of reduced energy and motivation is not entirely known, but clinical experience with antidepressant medications suggests that these symptoms may be related to deficits in dopaminergic and noradrenergic activity (Stahl, 2002; Nutt et al., 2007). Decreased neurotransmission of dopamine in limbic regions and the prefrontal cortex is believed to be associated with reduced motivation and interest (Nutt et al., 2007). In the dorsolateral region of the prefrontal cortex, decreased dopaminergic and noradrenergic activity may result in mental fatigue and lack of energy, which contribute toward loss of interest and decreased motivation as well as indecisiveness, reduced attention, and other cognitive difficulties (Demyttenaere et al., 2005; Nutt et al., 2007). Given the data that support the role of noradrenaline in regulating these types of symptoms (El Mansari et al., 2010), drugs that increase both serotonin and noradrenaline levels may have favorable effects on motivation and energy in addition to other core depression symptoms.
Although studies have been carried out that evaluated the effects of antidepressants and other neuroactive drugs on energy or fatigue, research focusing on the relationship between energy and motivation, and the effect of these symptoms on other depressive outcomes such as functional impairment, is more limited, especially as they relate to the clinical management of MDD. One contribution to this field has been the introduction of the Motivation and Energy Inventory (MEI), which was developed specifically to evaluate the impact of both motivation and energy on daily functioning in depressed patients with the goal of detecting antidepressant treatment effects on these symptoms (Fehnel et al., 2004). Fatigue scales (e.g. Brief Fatigue Inventory) or items from depression scales (e.g. Hamilton Depression Rating Scale) or health status scales [e.g. Short Form-36 Health Survey (SF-36)] have been used to indirectly assess energy and/or motivation, but the MEI provides researchers with an instrument that recognizes the interrelationship between motivation and energy and evaluates how both of these symptom domains affect a patient’s ability to perform daily activities and willingness to engage in social interactions.
The MEI has been implemented in several depression studies, particularly with bupropion (Hewett et al., 2009, 2010a, 2010b; Soczynska et al., 2014), but continued efforts are needed to better understand the effects of different pharmacotherapies on the interrelated symptoms of motivation and energy and the extent to which improvements contribute toward overall depression symptoms and functional impairment. A validated short-form (18-item) version of the MEI (Fehnel and Mcleod, 2005) was used to evaluate motivation/energy in a phase 3 study of levomilnacipran extended-release (ER) (Sambunaris et al., 2014), which is now approved in the USA for the treatment of MDD in adults. A description of the short-form MEI is provided in Supplementary Table 1 (Supplemental digital content 1, http://links.lww.com/ICP/A19). Part of the rationale for implementing the MEI in this trial was the pharmacology of levomilnacipran ER, a serotonin and noradrenaline reuptake inhibitor that has shown preferential in-vitro activity at noradrenaline transporters (Auclair et al., 2013). As symptoms such as fatigue, loss of energy, diminished interest, and difficulties with concentration or attention are noradrenergic symptoms of MDD (Nutt et al., 2007), the MEI was included as an additional efficacy measure in this levomilnacipran ER study.
As presented in the primary report of this trial (Sambunaris et al., 2014), significant mean improvements with levomilnacipran ER relative to placebo were detected in the MEI total score and in both MEI subscale scores (i.e. social, cognitive). As reduced motivation and energy have been associated with poorer functional outcomes, the current post-hoc analysis of this trial was carried out to explore the effects of baseline motivation/energy on functional impairment and functional health as well as the relationship between improvements in motivation/energy and improvements in function-related measures.
Methods
Study design and treatment
Post-hoc analyses were carried out using data from a multicenter, randomized, double-blind, placebo-controlled trial of levomilnacipran ER in adults with MDD (Sambunaris et al., 2014). The study included a single-blind, placebo run-in period (1 week), a double-blind treatment period (8 weeks), and a double-blind down-taper period (2 weeks). Patients were randomized (1 : 1) to placebo or flexible-dose levomilnacipran ER (40–120 mg/day). Levomilnacipran ER treatment was initiated at 20 mg/day (days 1 and 2) and increased to 40 mg/day (day 3). An increase in dosage from 40 to 80 mg/day was allowed at end of week 1 or 2; an increase from 80 to 120 mg/day was allowed at the end of week 4 on the basis of inadequate patient response [<50% improvement in the Montgomery–Åsberg Depression Rating Scale (MADRS) total score from baseline] and tolerability. No dosage increase was allowed after week 4, but a decrease to the previous dose level was permitted if significant tolerability issues developed.
The study included male and female outpatients, 18–80 years of age, with a diagnosis of MDD per Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Text Revision (DSM-IV-TR) criteria, ongoing major depressive episode (duration ≥4 weeks), body mass index (BMI) greater than or equal to 18 to less than or equal to 40 kg/m2, and a MADRS total score of at least 30 at screening and baseline. Patients were excluded for any of the following reasons: Axis I disorder other than MDD (within the past 6 months); lifetime history of manic or hypomanic episode; depressive episode with psychotic features; substance abuse or dependence (within the past 6 months); other psychiatric disorders or cognitive disorders; history of nonresponse to greater than or equal to two antidepressants after adequate treatment (≥8 weeks at approved recommended dosages); suicide risk on the basis of investigator judgment, MADRS item 10 score of at least 5, and/or Columbia-Suicide Severity Rating Scale evaluation.
Efficacy analyses
Efficacy analyses were carried out in the intent-to-treat (ITT) population, defined as all patients who received at least one dose of double-blind treatment and had at least 1 postbaseline MADRS assessment. The primary efficacy endpoint was defined as the change from baseline to week 8 in MADRS total score. Change from baseline to week 8 in MEI total score was a predefined additional efficacy parameter in this study. The changes from baseline to week 8 in MEI subscale scores and the change from baseline by study visit in MEI total score were also analyzed. All MEI score changes were analyzed using a mixed-effects model for repeated measures with treatment group, pooled study center, visit, and treatment group-by-visit interaction as factors and corresponding baseline and baseline-by-visit interaction as covariates.
Post-hoc analyses were carried out in two subgroups of patients who were categorized using the median MEI total score at baseline: a lower motivation/energy subgroup (score ≤28) and a higher motivation/energy subgroup (score>28). No statistical analyses were carried out between these MEI subgroups.
Functional impairment and functional health were evaluated using the Sheehan Disability Scale (SDS) and SF-36, respectively (Supplementary Table 1, Supplemental digital content 1, http://links.lww.com/ICP/A19). Changes from baseline to week 8 in SDS total and subscale scores were analyzed in the ITT population and in each MEI subgroup (lower and higher) using an mixed-effects model for repeated measures with treatment, pooled study center, visit, MEI subgroup, treatment-by-MEI subgroup, treatment-by-visit, visit-by-MEI subgroup, treatment-by-visit-by-MEI subgroup as fixed effects, and baseline score and baseline score-by-visit as covariates. Changes from baseline to end of treatment in SF-36 mental component summary (MCS), Physical Component Summary (PCS), and individual domain scores were analyzed in the ITT population and in each MEI subgroup using a last observation carried forward approach through an analysis of covariance model with treatment, pooled study center, MEI category, and treatment-by-MEI category interaction, and corresponding baseline as covariates. Treatment effect sizes for these score changes were estimated using Cohen’s d calculation.
Post-hoc path analyses were carried out using multiple regression models on the basis of available week 8 data from patients in the ITT population and the lower MEI subgroup who received levomilnacipran ER. The first model evaluated the direct effects of levomilnacipran ER (fixed effect) on SDS total score change (outcome), as well as the indirect effects of treatment on outcome through changes in MADRS and MEI total scores (explanatory variables). The second model included levomilnacipran ER as the fixed effect, change in SF-36 MCS total score as the outcome, and changes in MADRS and MEI total scores as explanatory variables. The direct and indirect treatment effects are presented as percentages of the overall effect of levomilnacipran on outcome (SDS total score change or SF-36 MCS score change); these effects do not account for measurement errors or other extraneous variables.
Results
Patients
At baseline, MEI total scores in the ITT population ranged from 0 to 90, representing almost the full gamut of possible scores (total score range, 0–108). The mean MEI total scores (±SD) in the lower and higher MEI subgroups did not overlap (Table 1), suggesting that these two subgroups had sufficiently different levels of motivation/energy at baseline.
Table 1.
Baseline characteristics in subgroups categorized by motivation/energy
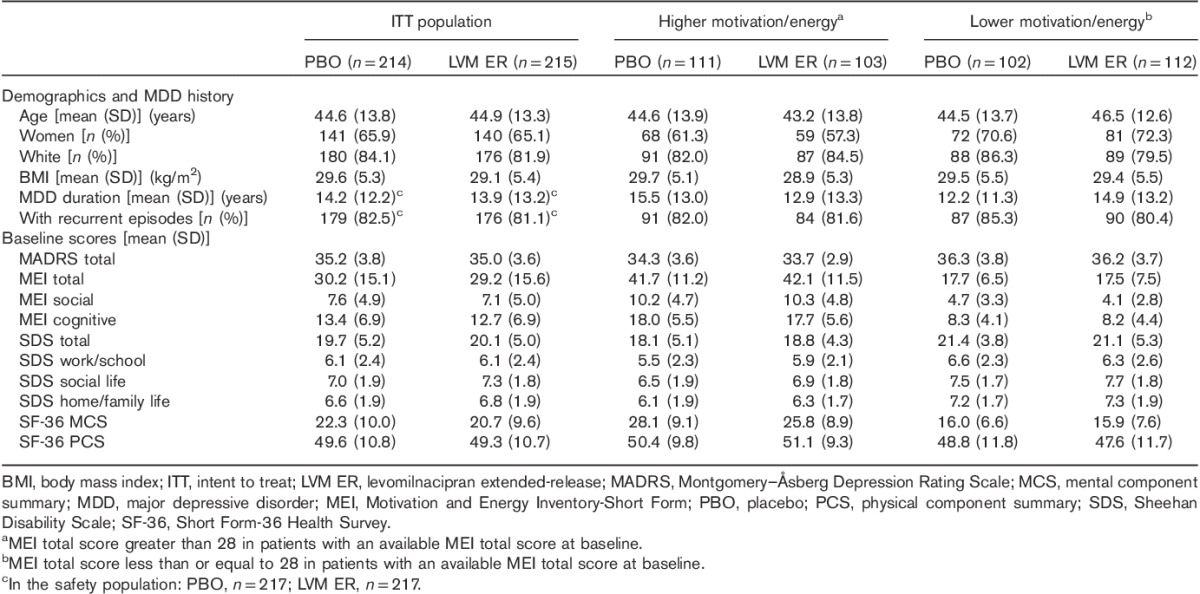
Demographics and MDD history were generally similar between the lower and the higher MEI subgroups, except for a larger percentage of women in the MEI up to 28 subgroup (Table 1). The mean baseline MADRS and SDS scores were similar between treatment groups in both the lower MEI subgroup and the higher MEI subgroup. In both MEI subgroups, the mean SF-36 MCS scores at baseline indicated clinically relevant decrements in mental functional health. The mean SF-36 PCS scores, however, indicated no or minimal decrements in physical functional health.
Effects of levomilnacipran ER on motivation/energy
As was reported previously for the ITT population (Sambunaris et al., 2014), the mean changes from baseline to week 8 in MEI total and subscale scores were significantly greater with levomilnacipran ER compared with placebo. The least squares mean difference (LSMD) between levomilnacipran ER and placebo for these outcomes were as follows: total score, 5.0; social subscale score, 1.5; and cognitive subscale score, 2.1; all P values less than 0.05. For MEI total score changes, significant differences between levomilnacipran ER and placebo were detected at all study visits (weeks 4, 6, and 8) (Fig. 1). Significant differences were detected at weeks 4 and 8 in the MEI social subscale and at week 8 in the cognitive subscale (Supplementary Fig. 1, Supplemental digital content 1, http://links.lww.com/ICP/A19).
Fig. 1.
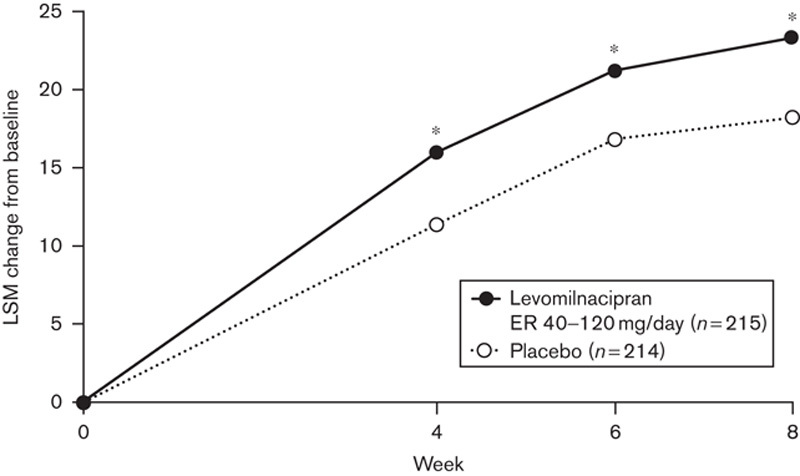
Change from baseline in MEI total score by study visit (ITT population, MMRM). Analysis includes all study visits at which MEI was evaluated. *P<0.05 versus placebo. ER, extended release; ITT, intent to treat; LSM, least squares mean; MEI, Motivation and Energy Inventory-Short Form; MMRM, mixed-effects model for repeated measures.
Effects of baseline motivation/energy on functional impairment (SDS) and functional health (SF-36)
In the ITT population and the lower MEI subgroup, statistically greater improvements were found with levomilnacipran ER versus placebo in the SDS total score and all three SDS subscale scores (Fig. 2). LSMDs between levomilnacipran ER and placebo for SDS total and subscale score changes were larger in the lower MEI subgroup than in the overall ITT population. Correspondingly, effect sizes in the lower MEI subgroup [range, 0.37 (work/school) to 0.51 (home/family life)] were larger than those in the ITT population or the higher MEI subgroup.
Fig. 2.
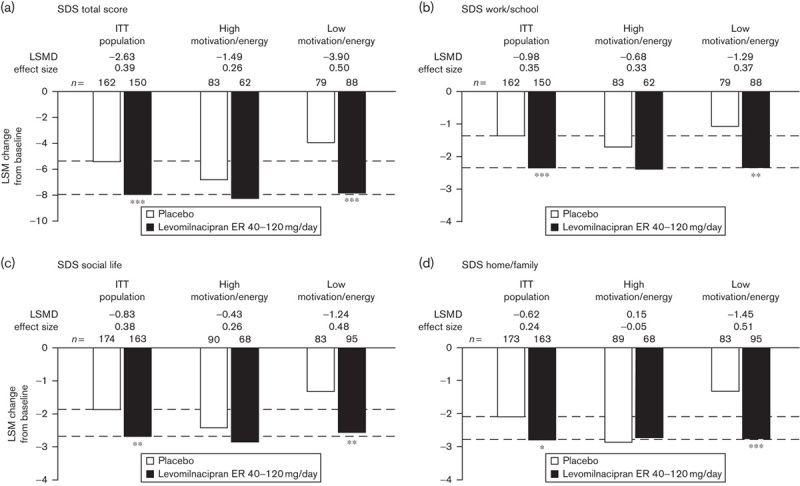
Mean changes from baseline in (a) SDS total score and in (b, c, d) SDS subscale scores; analyzed in the ITT population and MEI subgroups using an MMRM approach. Lower and higher motivation/energy subgroups were defined by baseline MEI total scores less than or equal to 28 and greater than 28, respectively; n-values represent the number of patients with nonmissing SDS values at baseline and at week 8. *P<0.05; **P<0.01; ***P≤0.001 versus placebo. ER, extended release; ITT, intent to treat; LSM, least squares mean; LSMD, least squares mean difference between treatment groups; MEI, Motivation and Energy Inventory-Short Form; MMRM, mixed-effects model for repeated measures; SDS, Sheehan Disability Scale.
In the ITT population, the levomilnacipran ER group showed a significantly greater mean improvement in the SF-36 MCS score relative to the placebo group (Fig. 3). Significant differences between treatment groups were also found in the SF-36 domains of general health, social functioning, and role emotional (Supplementary Table 2, Supplemental digital content 1, http://links.lww.com/ICP/A19). In the lower MEI subgroup, significant differences between levomilnacipran ER and placebo were found in the SF-36 MCS and all individual SF-36 domains, except for bodily pain, with treatment effect sizes ranging from 0.23 (bodily pain) to 0.41 (role emotional). SF-36 score improvements were greater in the lower MEI subgroup than in the ITT population.
Fig. 3.
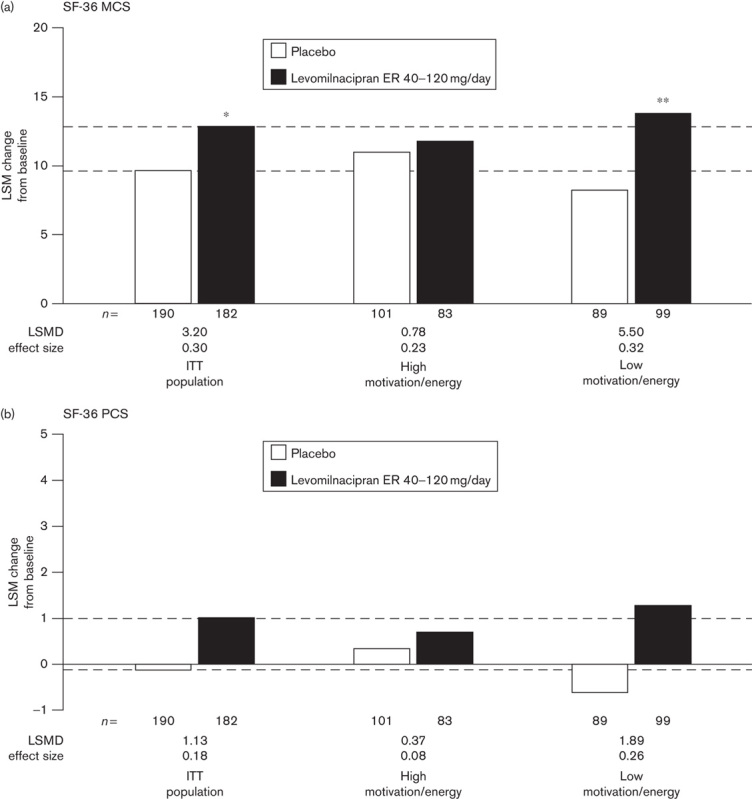
Mean changes from baseline in (a) SF-36 MCS scores and (b) SF-36 PCS scores; analyzed in the ITT population and MEI subgroups using an LOCF approach. Lower and higher motivation/energy subgroups were defined by baseline MEI total scores less than or equal to 28 and greater than 28, respectively; n-values represent the number of patients with nonmissing SF-36 values at baseline and at the end of treatment. *P<0.05; **P<0.01 versus placebo. ER, extended release; ITT, intent to treat; LOCF, last observation carried forward; LSM, least squares mean; LSMD, least-squares mean difference between treatment groups; MCS, mental component summary; MEI, Motivation and Energy Inventory-Short Form; SF-36, Short Form-36 Health Survey; PCS, physical component summary.
In the higher MEI subgroup, no significant LSMDs between levomilnacipran ER and placebo were detected for any SDS or SF-36 outcome, although a relatively strong treatment effect was found in the SF-36 domain of social functioning (d=0.43). In the ITT population and in both MEI subgroups, changes in SF-36 PCS scores were minimal.
Path analyses
In the ITT population and the lower MEI subgroup, the direct effect of levomilnacipran ER on SDS total score change was negligible (Table 2). The direct effect of treatment on SF-36 MCS score change was 6–7%.
Table 2.
Path analyses
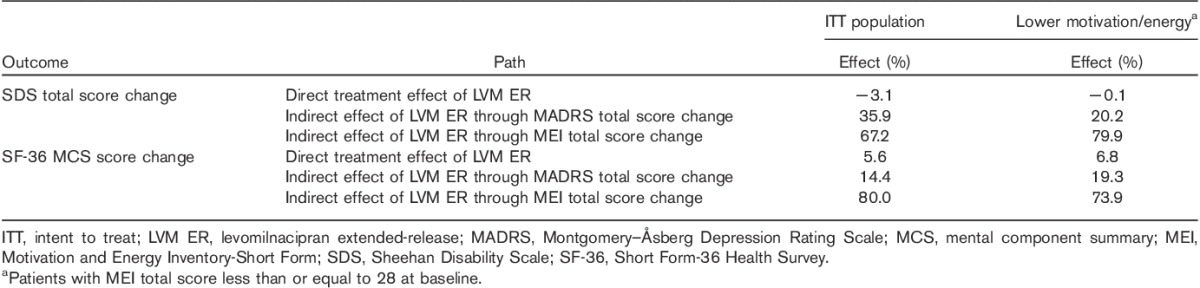
For both function-related outcomes (SDS total score, SF-36 MCS score), the indirect effects of levomilnacipran ER through MADRS and MEI total score changes were statistically significant in the ITT population and in the lower MEI subgroup (all P<0.05). However, the mediating effects of MEI total score on these outcomes were greater than the mediating effects of MADRS total score (Table 2). The indirect effects of levomilnacipran ER on SDS total score change through MEI total score improvement were 67.2 and 79.9% in the ITT population and the lower MEI subgroup, respectively. The indirect effects on SF-36 MCS through MEI total score were 80.0 and 73.9% in the ITT population and the lower MEI subgroup, respectively.
Discussion
After 8 weeks of double-blind treatment, adult MDD patients who received levomilnacipran ER showed significantly greater improvements in MEI total and subscale scores than patients who received placebo. The difference between treatment groups for MEI total score change in this study (LSMD=5.0; P<0.05) was similar to outcomes found with venlafaxine extended-release (LSMD=5.5; P<0.05) and larger than the effect found with bupropion extended-release (LSMD=2.5; P=0.245) in a previously published study of MDD patients (Hewett et al., 2010b). Significant between-group differences in MEI total score were detected at all study visits, indicating that patients began experiencing the effects of levomilnacipran ER on motivation/energy within 4 weeks of initiating treatment.
To explore the effects of motivation/energy on function-related outcomes, two subgroups (lower and higher MEI) were defined using the median baseline MEI total score (≤28 and >28, respectively). This cut-off seems to have adequately segregated the subgroups as the mean baseline MEI total scores and standard deviations in the higher and lower subgroups (18±7 and 42±11, respectively) did not overlap. Although the MEI subgroups were sufficiently disparate in terms of motivation/energy levels, they had similar mean SDS total scores at baseline. This finding was not entirely surprising as functional impairment is likely associated with various core symptoms of depression (Lam et al., 2011).
However, baseline levels of motivation/energy did appear to influence the magnitude of levomilnacipran ER’s effects on functional impairment. After 8 weeks of double-blind treatment, the difference between levomilnacipran ER and placebo for SDS total score change, as well as the Cohen’s treatment effect size, was almost twice greater in the lower MEI subgroup (LSMD=−2.90; d=0.50; P<0.001) than in the higher MEI subgroup (LSMD=−1.49; d=0.26; P>0.05). Similar treatment effect sizes (∼0.50) were found in the lower MEI subgroup for the SDS social life and home/family life subscales. In all of the SDS analyses (total and subscale scores), the mean score changes with levomilnacipran ER (from baseline to week 8) were similar in the lower and higher MEI subgroups; however, score changes with placebo were markedly smaller in the lower MEI subgroup than in the higher MEI subgroup. In addition to accounting for the larger LSMDs and effect sizes, this relatively small placebo effect in the lower MEI subgroup suggests that active pharmacologic treatment may be particularly important in restoring function in MDD patients who present with reduced motivation and energy. This conjecture is supported by results from the path analysis that was carried out in levomilnacipran ER-treated patients, which showed the indirect effect of treatment on SDS total score change through improvement in MEI total score to be somewhat larger in the lower MEI subgroup (79.9%) than in the ITT population (67.2%). This path analysis result suggests that in patients with reduced motivation/energy at baseline, improving these symptoms may be an important driver of functional improvement.
The mean SF-36 PCS scores at baseline indicated normal levels of physical functional health in the overall study population and both MEI subgroups, which did not leave much room for improvement. As expected, no significant differences between treatment groups on this measure were found in the ITT population or in either MEI subgroup, although the lower MEI subgroup did experience a slight worsening with placebo. Consequently, the treatment difference and effect size for SF-36 PCS score change were larger in the lower MEI subgroup (LSMD=1.89; d=0.26; P>0.05) than in the higher MEI subgroup (LSMD=0.37; d=0.08; P>0.05).
In contrast to baseline SF-36 PCS scores, baseline SF-36 MCS indicated decrements in mental functional health. As was found with SDS total and subscale score changes, the treatment difference and Cohen’s effect size for the SF-36 MCS score change were greater in the lower MEI subgroup (LSMD=5.50; d=0.32; P<0.01) than in the higher MEI subgroup (LSMD=0.78; d=0.23; P>0.05). In the path analysis, comparable indirect effects of levomilnacipran on SF-36 MCS score change through MEI total score were found in the ITT population and the lower MEI subgroup (80.0 and 73.9%, respectively). These results suggest that treatment with levomilnacipran ER may lead to improvements in mental functional health that are considerably mediated through improvements in motivation/energy, irrespective of baseline motivation/energy levels.
Teasing out the impact of reduced motivation and energy on treatment outcomes in MDD patients is difficult because of the complex and heterogeneous symptomatology of this disorder. Studies have shown, however, that reduced motivation/energy is related to behavioral, cognitive, and emotional deficits (Rothschild et al., 2014) as well as decreased levels of activity, interest, enjoyment, and ability to feel (Uher et al., 2012). More importantly, from a treatment perspective, patients with decreased motivation/energy were found to have lower rates of symptom remission, higher recurrence of MDD episodes, and greater functional impairment (Stahl, 2002; Nutt et al., 2007) Hardeveld et al., 2010; Uher et al., 2012. The results of this post-hoc analysis indicate that if recovery of psychosocial functioning remains a primary goal of MDD treatment (APA, 2010), recognizing patients with decreased motivation/energy and choosing therapies that alleviate these symptoms are important aspects of a comprehensive clinical strategy.
Many contributing factors certainly need to be considered when treating patients with reduced motivation/energy, such as their beliefs about the potential rewards associated with a task, activity, or social interaction, and the effects of these beliefs on motivation levels (Pulcu and Elliott, 2015). Attending to such factors may require education or cognitive-based therapies. However, there are also symptoms such as physical and mental fatigue – which can contribute toward decreased motivation/energy and vice versa – that may be responsive to pharmacologic therapies. Results from the lower MEI subgroup in this post-hoc analysis showed diminished placebo effects for functional improvements, which suggests that reduced motivation and energy may encompass a cluster of endogenous MDD symptoms (e.g. fatigue, cognition) that could be effectively targeted by noradrenergic medications such as levomilnacipran ER. Further research, such as path analyses that compare selective serotonin reuptake inhibitor with serotonin and noradrenaline reuptake inhibitor, could help to clarify the different ways in which noradrenergic and serotonergic medications improve functional impairment by direct treatment effects and indirectly through improvements in motivation/energy or other core depression symptoms.
Whether achieved through psychosocial therapy, pharmacologic treatment, or both, improving motivation/energy may be vital for recovery in patients with MDD. In the current post-hoc analyses, the effects of baseline motivation/energy levels on functional outcomes, along with path analysis results showing improved motivation/energy to be an important driver of functional improvement, highlight the need to identify MDD patients with low/motivation energy and to effectively alleviate their symptoms. From a clinical perspective, this may require asking specific questions about patients’ motivation/energy levels and continuing to monitor the effects of medication on those symptoms. Especially as adequate levels of motivation/energy may be needed for patients to continue adhering to their treatments (both pharmacologic and nonpharmacologic), identifying and successfully managing these symptoms are important components in developing a comprehensive therapeutic strategy.
Limitations
The primary limitation of these analyses is that they were carried out post hoc. Thus, although the numbers of patients in the predefined ITT population were balanced between treatment groups, the numbers of patients were less evenly distributed in the MEI subgroups. In addition, no patients were entered into or excluded from the study on the basis of their baseline motivation/energy levels. Other potential limitations include the short treatment duration, which does not allow for any conclusions to be drawn on the long-term effects of levomilnacipran ER on motivation and energy, and the eligibility criteria. Although this study population was similar to those in many other large MDD trials, the inclusion and exclusion criteria may limit the generalizability of these findings to a broader clinical population.
Conclusion
In adults with MDD, treatment with levomilnacipran ER versus placebo significantly improved motivation and energy overall (MEI total score) and in the domains related to cognitive and social difficulties (MEI subscale scores). The treatment effects of levomilnacipran ER on functional impairment (SDS total and subscale scores) and mental functional health (SF-36 MCS score) were more pronounced in patients with lower levels of motivation/energy at baseline, in large part because of the smaller placebo effect found in these patients relative to patients with higher motivation/energy levels at baseline. Path analysis results indicate that the indirect effects of levomilnacipran ER on these functional outcomes were mediated by improvements in motivation/energy (MEI total score), and to a lesser degree, by improvements in core depression symptoms (MADRS total score). As reduced motivation and energy may be associated with poorer treatment outcomes, including diminished psychosocial functioning, choosing a pharmacologic agent that targets the neurotransmitters associated with these symptoms may be an important clinical consideration.
Supplementary Material
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website (www.intclinpsychopharm.com).
Acknowledgements
The authors would like to thank Jeff Lai of Allergan plc (Irvine, California, USA) for assistance with the path analyses. Writing and editorial assistance was provided by Mildred Bahn at Prescott Medical Communications Group (Chicago, Illinois, USA) with support from Forest Research Institute, an Allergan affiliate.
Conflicts of interest
Dr Thase has received grants from the Agency for Healthcare Research and Quality, Alkermes, Forest Laboratories (an Allergan affiliate), National Institute of Mental Health, Otsuka, PharmaNeuroboost, and Roche; has acted as an advisor or a consultant for Alkermes, AstraZeneca, Bristol-Myers Squibb, Cerecor, Eli Lilly, Forest Laboratories, Gerson Lehman Group, GlaxoSmithKline, Guidepoint Global, Lundbeck, MedAvante, Merck, Neuronetics, Novartis, Ortho-McNeil Pharmaceuticals, Otsuka, Pamlab, Pfizer, Shire, Sunovion, and Takeda; has received royalties from American Psychiatric Association, Guilford Publications, Herald House, and W. W. Norton & Company; and holds equity in MedAvante Inc. Mr Gommoll, Dr Chen, and Dr Kramer are full-time employees of Allergan, Inc. Dr Sambunaris has received grants from Forest Laboratories (an Allergan affiliate) and consultant fees from Forest Research Institute. He has also received clinical research grants from Alkermes, Cerecor, Indivior, Lundbeck, Otsuka, Palatin, Pfizer, Sunovion, and Tal Medical. For the remaining authors there are no conflicts of interest.
References
- APA (2010). Practice guideline for the treatment of patients with major depressive disorder, 3rd ed Arlignton, VA: American Psychiatric Association. [Google Scholar]
- Auclair AL, Martel JC, Assié MB, Bardin L, Heusler P, Cussac D, et al. (2013). Levomilnacipran (F2695), a norepinephrine-preferring SNRI: profile in vitro and in models of depression and anxiety. Neuropharmacology 70:338–347. [DOI] [PubMed] [Google Scholar]
- Calabrese SR, Fava M, Garibaldi G, Grunze H, Krystal AD, Laughren T, et al. (2014). Methodological approaches and magnitude of the clinical unmet need associated with amotivation in mood disorders. J Affect Disord 168:439–451. [DOI] [PubMed] [Google Scholar]
- Demyttenaere K, de Fruyt J, Stahl SM. (2005). The many faces of fatigue in major depressive disorder. Int J Neuropsychopharmacol 8:93–105. [DOI] [PubMed] [Google Scholar]
- El Mansari M, Guiard BP, Chernoloz O, Ghanbari R, Katz N, Blier P. (2010). Relevance of norepinephrine-dopamine interactions in the treatment of major depressive disorder. CNS Neurosci Ther 16:e1–e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehnel SE, Mcleod LD. (2005). Psychometric evaulation of the motivation and energy inventory – short form (MEI-SF). Presented at the International Society for Quality of Life Research (ISOQOL) Annual Meeting; San Francisco, CA: 19–22 October 2005.
- Fehnel SE, Bann CM, Hogue SL, Kwong WJ, Mahajan SS. (2004). The development and psychometric evaluation of the motivation and energy inventory (MEI). Qual Life Res 13:1321–1336. [DOI] [PubMed] [Google Scholar]
- Hardeveld F, Spijker J, de Graaf R, Nolen WA, Beekman AT. (2010). Prevalence and predictors of recurrence of major depressive disorder in the adult population. Acta Psychiatr Scand 122:184–191. [DOI] [PubMed] [Google Scholar]
- Hewett K, Chrzanowski W, Schmitz M, Savela A, Milanova V, Gee M, et al. (2009). Eight-week, placebo-controlled, double-blind comparison of the antidepressant efficacy and tolerability of bupropion XR and venlafaxine XR. J Psychopharmacol 23:531–538. [DOI] [PubMed] [Google Scholar]
- Hewett K, Chrzanowski W, Jokinen R, Felgentreff R, Shrivastava RK, Gee MD, et al. (2010a). Double-blind, placebo-controlled evaluation of extended-release bupropion in elderly patients with major depressive disorder. J Psychopharmacol 24:521–529. [DOI] [PubMed] [Google Scholar]
- Hewett K, Gee MD, Krishen A, Wunderlich HP, le Clus A, et al. (2010b). Double-blind, placebo-controlled comparison of the antidepressant efficacy and tolerability of bupropion XR and venlafaxine XR. J Psychopharmacol 24:1209–1216. [DOI] [PubMed] [Google Scholar]
- Lam RW, Filteau MJ, Milev R. (2011). Clinical effectiveness: the importance of psychosocial functioning outcomes. J Affect Disord 132 (Suppl 1):S9–S13. [DOI] [PubMed] [Google Scholar]
- McClintock SM, Husain MM, Wisniewski SR, Nierenberg AA, Stewart JW, et al. (2011). Residual symptoms in depressed outpatients who respond by 50% but do not remit to antidepressant medication. J Clin Psychopharmacol 31:180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight PE, Kashdan TB. (2009). The importance of functional impairment to mental health outcomes: a case for reassessing our goals in depression treatment research. Clin Psychol Rev 29:243–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt D, Demyttenaere K, Janka Z, Aarre T, Bourin M, Canonico PL, et al. (2007). The other face of depression, reduced positive affect: the role of catecholamines in causation and cure. J Psychopharmacol 21:461–471. [DOI] [PubMed] [Google Scholar]
- Pulcu E, Elliott R. (2015). Neural origins of psychosocial functioning impairments in major depression. Lancet Psychiatry 2:835–843. [DOI] [PubMed] [Google Scholar]
- Rothschild AJ, Raskin J, Wang CN, Marangell LB, Fava M. (2014). The relationship between change in apathy and changes in cognition and functional outcomes in currently non-depressed SSRI-treated patients with major depressive disorder. Compr Psychiatry 55:1–10. [DOI] [PubMed] [Google Scholar]
- Sambunaris A, Bose A, Gommoll CP, Chen C, Greenberg WM, Sheehan DV. (2014). A phase III, double-blind, placebo-controlled, flexible-dose study of levomilnacipran extended-release in patients with major depressive disorder. J Clin Psychopharmacol 34:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soczynska JK, Ravindran LN, Styra R, McIntyre RS, Cyriac A, Manierka MS, Kennedy SH. (2014). The effect of bupropion XL and escitalopram on memory and functional outcomes in adults with major depressive disorder: results from a randomized controlled trial. Psychiatry Res 220:245–250. [DOI] [PubMed] [Google Scholar]
- Stahl SM. (2002). The psychopharmacology of energy and fatigue. J Clin Psychiatry 63:7–8. [DOI] [PubMed] [Google Scholar]
- Uher R, Perlis RH, Henigsberg N, Zobel A, Rietschel M, Mors O, et al. (2012). Depression symptom dimensions as predictors of antidepressant treatment outcome: replicable evidence for interest-activity symptoms. Psychol Med 42:967–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website (www.intclinpsychopharm.com).


