Abstract
In March of 2015, the United States Department of Health and Human Services identified 3 priority areas to reduce opioid use disorders and overdose, which are as follows: opioid-prescribing practices; expanded use and distribution of naloxone; and expansion of medication-assisted treatment. In this narrative review of overdose prevention and the role of prescribers and pharmacists in distributing naloxone, we address these priority areas and present a clinical scenario within the review involving a pharmacist, a patient with chronic pain and anxiety, and a primary care physician. We also discuss current laws related to naloxone prescribing and dispensing. This review was adapted from the Prescribe to Prevent online continuing medical education module created for prescribers and pharmacists (http://www.opioidprescribing.com/naloxone_module_1-landing).
Keywords: pain, prescription drug abuse, prevention, public health, substance abuse
Clinical scenario: Upon filling a prescription for oxycodone, a pharmacist reviews the patient's other medications: “In the Prescription Monitoring Program, I see that you’re also being prescribed clonazepam by another physician. I wanted to make sure that you understand that combining pain medications, like oxycodone, and anxiety medications, like clonazepam, can increase your risk of drowsiness, sedation, and even overdose.” The patient replies that she has been taking these medications for a long time and is not concerned. “I’m glad to hear that you’re aware of the risks,” the pharmacist says, “With your permission, I’m going to contact your prescribers, so we can monitor your risk and keep you safe.”
EPIDEMIOLOGY
Drug overdose, driven largely by use of prescription opioids and heroin, is now the leading cause of accidental injury death in the United States, surpassing those caused by motor vehicle crashes (U.S. Food and Drug Administration, 2014; U.S. Centers for Disease Control and Prevention, 2015a, 2015b). From 2005 to 2013, annual opioid-related overdose deaths almost doubled, from 12,937 to 24,492 (Jones et al., 2013; Wheeler et al., 2015). The economic costs to society have been projected to surpass $20 billion every year (Inocencio et al., 2013). This overdose crisis has been linked to the availability and potency of prescribed opioids (Okie, 2010; Modarai et al., 2013; Chen et al., 2014; Dart et al., 2015). Whereas 71% of those who are at risk to misuse pain relievers report obtaining the medications from family and friends, 79% of those family and friends report being prescribed those medicines by their doctor (Substance Abuse and Mental Health Services Administration, 2011). Both qualitative and epidemiologic studies have also demonstrated that heroin addiction is often preceded by prescription opioid misuse (Jones, 2013; Dasgupta et al., 2014; Mars et al., 2014; LaRochelle et al., 2015). Furthermore, fentanyl-related overdose fatalities have increased, involving fentanyl manufactured and distributed illicitly as part of the street drug market (U.S. Centers for Disease Control and Prevention, 2015c).
How Does an Opioid Overdose Occur?
Opioid receptors are found throughout the nervous system, including the sections of the brainstem that control breathing. Stimulation of the receptors by opioids can cause euphoria, pain relief, sedation, and decreased respiration. High doses of opioids cause reduced sensitivity to oxygen and carbon dioxide levels, reducing respiratory drive and allowing tidal volume and respiratory rate to decrease. The resulting hypoxia can cause a loss of consciousness, and eventually, death. Signs of an overdose include decreases in respiratory rate, abnormal breathing sounds (snoring, gurgling, choking, etc.), decreased consciousness, miosis, and a blue/gray tinge of the skin, especially the lips and nail beds (White and Irvine, 1999).
PREVENTION
What are Common Risks for Opioid Overdose?
Medical providers and pharmacists should understand, explain to patients, and take actions to reduce overdose risk. The following should be considered when assessing a patient's risk.
First, previous nonfatal overdose is associated with future overdose (Kinner et al., 2012). Second, higher opioid doses, such as daily doses higher than 50 morphine milli-equivalents (Dunn et al., 2010), and changes in dose or formulations increase overdose risk; people who use heroin are thus frequently at risk due to unpredictable changes in substance purity from, for example, adulteration with fentanyl (Rudd et al., 2015). Third, polypharmacy and mixing substances contribute to overdose risk, as opioid overdoses commonly involve other substances (Jones et al., 2013, 2014). Psychoactive medications of particular concern include barbiturates, stimulants, and benzodiazepines (Jann et al., 2014; Zedler et al., 2014; Jones and McAninch, 2015). Other medications that can have synergistically central nervous system depressive effects include clonidine (Beuger et al., 1998), promethazine (Shapiro et al., 2013; Lynch et al., 2015), and gabapentin (Smith et al., 2015). Fourth, socially isolated individuals who use prescription opioids or heroin alone have little chance of being rescued. Isolation is also associated with depression, which is itself associated with overdose (Cacioppo et al., 2006; Madadi et al., 2013). Fifth, chronic medical illnesses involving organs, such as the lung, liver, kidney, and brain, primarily responsible for metabolizing substances and respiration, can compromise the body's ability to handle opioids (Zedler et al., 2014). Sixth, periods of abstinence, such as periods of incarceration, hospitalization, and medical detoxification, cause reduced opioid tolerance and thus increased overdose risk. Accordingly, providers should educate and monitor patients restarting opioids or at risk of relapse (Wolff, 2002; Strang et al., 2003; Moller et al., 2010).
Assessing a Patient's Overdose Risk
Prescribers should assess overdose risk as part of a patient's history by the following means: reviewing medications and checking the Prescription Monitoring Program (PMP); reviewing medical and social history for above-mentioned risk factors; obtaining a focused substance use history; and obtaining an overdose history. The overdose history should determine if the patient has personally experienced an overdose (“Have you ever overdosed? What strategies do you use to prevent yourself from overdosing?”), witnessed an overdose (“Have you witnessed an overdose?”), or received training to prevent, recognize, and/or respond to one (“How would you respond to an overdose?”). Understanding the patient's experience and knowledge should guide the education provided.
Pharmacists should review medications, optimize medication safety, and provide patient education. This includes checking the PMP for psychoactive or sedating medications, addressing potential drug–drug interactions, ensuring that prescribers are aware of the patient's prescriptions, confirming understanding of the risks of opioids, providing overdose education, and filling naloxone prescriptions, or, when permitted, directly providing naloxone to the patient.
The pharmacist calls the primary care doctor: “I saw your patient has prescriptions for both oxycodone and clonazepam and informed her of the risks of combining these medications” The doctor replies, “I was not aware of the clonazepam prescription. Who is the prescriber?” The pharmacist offers the other prescriber's name, which the doctor recognizes. “That makes sense. He's a psychiatrist that I refer my patients to. I’ll discuss the risks with the patient at our appointment coming up.”
Strategies to Address Overdose
There are several existing strategies that address the Department of Health and Humans Services’ priority areas of improving opioid-prescribing safety and access to medication-assisted treatment (U.S. Department of Health and Human Services, 2015). Safe opioid-prescribing education has been supported by the Federal Drug Administration (FDA) and is mandated in some states like Massachusetts for prescribers to be relicensed (U.S. Food and Drug Administration, 2013). PMPs allow for prescribers and pharmacists to monitor what controlled substances a patient is filling at the pharmacy (Paulozzi et al., 2011; Davis et al., 2014a). Evidence of their effectiveness as overdose prevention tools is still mixed (Li et al., 2014; Green et al., 2015a). Prescription drug disposal in many communities occurs at kiosks, often hosted at local police stations and other locations, with support from Drug Enforcement Administration (DEA) which holds nationwide “take-back” events (Gray and Hagemeier, 2012; U.S. Drug Enforcement Administration, 2015). The DEA now also permits manufacturers, distributors, treatment programs, pharmacies, and healthcare facilities to become authorized collectors of prescription medications (U.S. Drug Enforcement Administration, 2014). Finally, medication for opioid use disorders, specifically methadone, buprenorphine, and naltrexone, is supported by evidence for increased abstinence and decreased opioid use (Schwartz et al., 2006; Mattick et al., 2009; Krupitsky et al., 2011; Mattick et al., 2014). Methadone and buprenorphine treatment is also associated with decreased criminal activity (Bell et al., 1997; Dolan et al., 2005; Lobmann and Verthein, 2009; Bukten et al., 2012; Soyka et al., 2012), improved birth outcomes (Johnson et al., 2001; Fajemirokun-Odudeyi et al., 2006; Meyer et al., 2012), and less overdose (Langendam et al., 2001; Clausen et al., 2009; Schwartz et al., 2013).
“Your pharmacist contacted me about the risks of taking both clonazepam and oxycodone,” the doctor informs the patient at their appointment. The patient does not understand the concern. The doctor explains that these medications have interactions that increase the chance of drowsiness and overdose, especially if she uses substances like alcohol or other drugs. “Do you ever feel drowsy or sedated from your medications?” The patient acknowledges one event when she took an extra oxycodone and clonazepam, after which her husband had trouble waking her up. The patient says that she now never takes anything more than what she is prescribed, and knows to call the doctor if she is struggling with pain, and her psychiatrist if she is struggling with her anxiety.
When the doctor asks how she stores her medication, she reports that she has started hiding pills from her son, “He got his wisdom teeth out and was given pain medication, and then he sprained his ankle and was given even more. I’m worried he has a drug problem and has switched over to heroin.” According to the patient, on previous occasions after leaving treatment, he immediately relapsed, and she is now worried about him overdosing. The doctor reiterates the risk of overdose following a period of abstinence, and asks if she has a plan if her son should overdose. The patient says that she knows to call an ambulance and start rescue breathing, “But what else can I do?”
RESPONSE
What are Naloxone Rescue Kits?
Naloxone is an opioid antagonist that displaces opioids from brain receptors and restores breathing and consciousness. Naloxone is a prescription medication, but not a controlled substance. It usually takes 2 to 5 minutes to take effect and wears off after 30 to 90 minutes, so patients on long-acting opioids like methadone and extended-release oxycodone may have recurrent reduced respirations and overdose (Chamberlain and Klein, 1994). Overdose responders should be encouraged to stay with the person overdosing after administration of naloxone. In cases of polysubstance or high potency fentanyl-related overdose, standard doses of naloxone may be insufficient, and thus rescue breathing and help from the emergency medical providers may be necessary to reverse the overdose. Administering naloxone may precipitate withdrawal symptoms in the victim.
There are multiple reasons to equip people in the community with a naloxone rescue kit. First, most opioid users do not use alone, and so have people around them that can intervene should an overdose occur (Baca and Grant, 2007; Powis et al., 1999). Second, risk factors for overdose have been identified, as discussed above. Third, the extent of hypoxic brain injury is time-dependent, so the sooner hypoxia is reversed, the better (Michiels, 2004). Fourth, bystanders can be trained to recognize and respond effectively to overdoses with naloxone (Green et al., 2008; Clark et al., 2014). Finally, fear of being arrested sometimes discourages bystanders from calling for medical assistance, and thus makes naloxone an important tool for people hesitant to call for help (Davis et al., 2013).
Naloxone rescue kits have been endorsed by numerous organizations, including the American Medical Association (American Medical Association, 2015), American Society of Addiction Medicine (American Society of Addiction Medicine, 2014), American Pharmacists Association (American Pharmacists Association, 2014), Substance Abuse and Mental Health Services Administration (Substance Abuse and Mental Health Services Administration, 2014), World Health Organization (World Health Organization, 2014), and United Nations Office on Drugs and Crime (United Nations Office on Drugs and Crime, 2014). In 2015, the American Heart Association published guidelines on opioid overdose response with naloxone in an update to their guidelines for cardiopulmonary resuscitation and emergency cardiovascular care (Lavonas et al., 2015, Figure 1).
FIGURE 1.
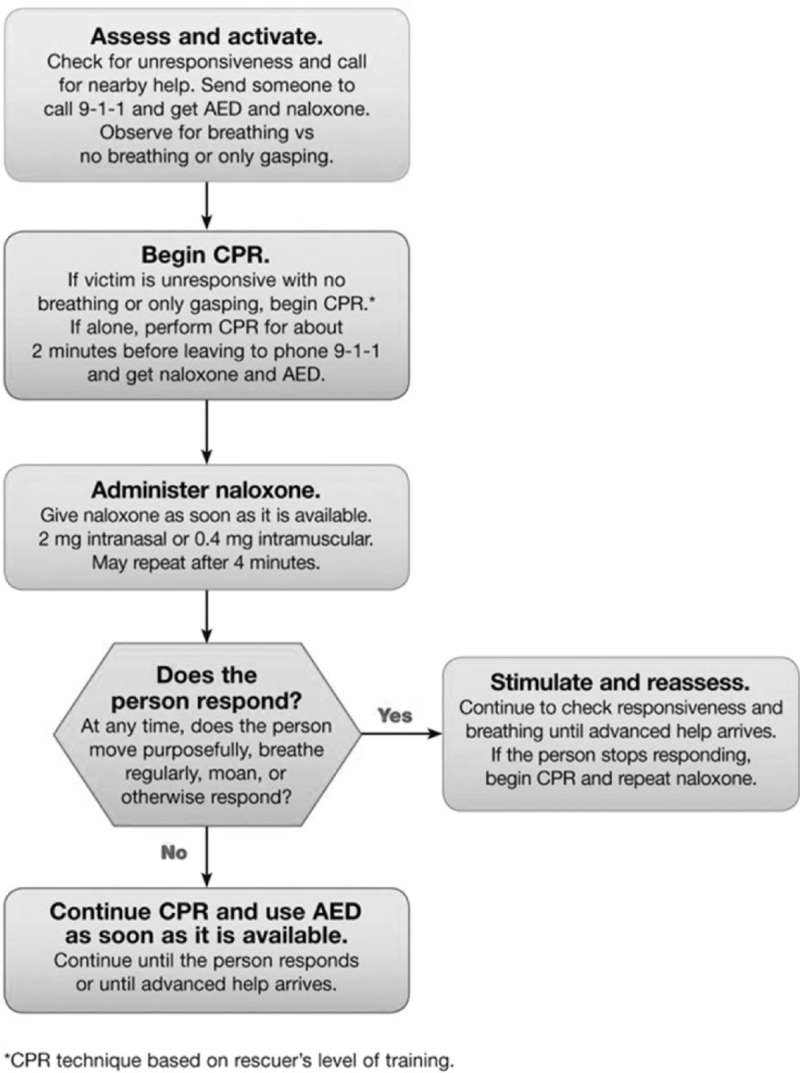
American Heart Association Opioid-Associated Life-Threatening Emergency (Adult) Algorithm. Reprinted with permission. Circulation. 2015;132:S501-S518. © 2015 American Heart Association, Inc. (http://circ.ahajournals.org/content/132/18_suppl_2/S501.long).
What Evidence Exists to Support Overdose Prevention Education and Naloxone Rescue Kits?
Overdose education and naloxone distribution programs have existed since the late 1990s as community-based initiatives based out of harm-reduction programs (Maxwell et al., 2006; Sporer and Kral, 2007; Wheeler et al., 2012). Between 1996 and 2014, community organizations reported providing naloxone rescue kits to 152,283 laypersons and received reports of 26,463 overdose reversals (Wheeler et al., 2015).
Studies have shown feasibility of naloxone rescue kits in several different populations (Piper et al., 2008; Doe-Simkins et al., 2009; Enteen et al., 2010; Bennett et al., 2011; Walley et al., 2013a). Nonmedical bystanders can be effectively trained to respond to overdose (Green et al., 2008; Tobin et al., 2009; Wagner et al., 2010). Some have expressed concern that naloxone availability would result in increased opioid use, but this has not been observed. Drug treatment rates have either stayed constant or increased (Seal et al., 2005; Doe-Simkins et al., 2014). In several communities in which programs were implemented, overdose rates have decreased (Maxwell et al., 2006). In Massachusetts, where there was heterogeneous rollout of overdose prevention programs, communities with naloxone distributed to 1 to 100 people per 100,000 population saw opioid overdose death rates decrease by 27%, whereas in communities with naloxone distributed to over 100 people per 100,000 population, opioid overdose death rates declined by 46% (Walley et al., 2013b). In a 2013 study, a best-case scenario determined a cost of $438 per quality-adjusted life year (QALY) gained, whereas in the worst-case scenario, the cost was $14,000 per QALY gained (Coffin and Sullivan, 2013).
Talking with Patients About Overdose Prevention and Response with Naloxone
Messages that providers can convey to patients:
Only take opioids prescribed to you and as directed;
If you are worried about your use of opioids, you can talk to me about it;
If you are not taking opioids safely, I can help you find treatment;
Make sure your prescribers and pharmacist are aware of all of your medications;
Don’t mix opioids with other drugs or alcohol;
Store medication in a safe and secure place, and dispose of unused medication;
Abstinence can change tolerance, so if you stop taking opioids, a lower dose may be needed upon restart;
Teach friends and family how to respond to an overdose, including how to use naloxone and where you store it;
Know how to recognize and respond to an overdose when you witness one.
The doctor provides guidance for the patient and her son. “First, I’d like to monitor your medications more closely. I’m going to call your psychiatrist to discuss whether we should adjust your medications to something safer. I also recommend that you use a lock box, which you can get at the pharmacy, to secure your medications.” The doctor then devises a plan for her son. “Ask him to consider a treatment program that includes a medication for opioid addiction, like methadone or buprenorphine—here is a card to contact the treatment hotline. Lastly, for both you and your son, I’m going to prescribe a naloxone rescue kit. The kit includes a medication called naloxone, and comes with instructions on how to recognize and respond to an overdose.”
Naloxone Rescue Kits at the Pharmacy: Prescribing, Stocking, Filling, and Billing
Prescribing naloxone rescue kits should be a collaborative effort between prescribers and pharmacists. Both have the duty to recognize and mitigate overdose risk.
Most major wholesalers carry naloxone formulations (Table 1). Outside of the inpatient setting, the most common methods of naloxone administration are intranasal and intramuscular. Evzio is an intramuscular formulation of naloxone in the form of an auto-injector (Beletsky, 2015).
TABLE 1.
Naloxone Formulations and Features
| Injectable (and Nasal) Generic | Intranasal Branded | Injectable Generic | Auto-injector Branded | |
 |
||||
| FDA approval | X (for IV, IM, SC) | X | X | X |
| Layperson experience | X | X | X | |
| Fragile; requires assembly | X | X | ||
| Can titrate dose | X | X | ||
| Strength | 1 mg/mL | 4 mg/0.1 mL | 0.4 mg/mL or 4 mg/10 mL | 0.4 mg/0.4 mL |
| Cost per kit | $$ | $$ | $ | $$$ |
| Rx and quantity | #2, 2 mL Luer-Jet Luer-Lock needleless syringe plus #2 mucosal atomizer devices (MAD-300) | #1 two-pack of two 4-mg/0.1 mL intranasal devices | #2 single-use 1-mL vials or #1 10-mL multidose fliptop vial PLUS #2 3 mL syringe with 23 to 25 gauge 1–1.5 inch IM needles | #1 two-pack of two 0.4 mg/0.4 mL prefilled auto-injector devices |
| Sig. (for suspected opioid overdose) | Spray 1 mL (1/2 of syringe) into each nostril. Repeat after 2 to 3 minutes if no or minimal response | Spray 0.1 mL into one nostril. Repeat with second device into other nostril after 2 to 3 minutes if no or minimal response | Inject 1 mL in shoulder or thigh. Repeat after 2 to 3 minutes if no or minimal response | Inject into outer thigh as directed by English voice-prompt system. Place black side firmly on outer thigh and depress and hold for 5 seconds. Repeat with second device in 2 to 3 minutes if no or minimal response |
Some portions developed with funding from NIDA R01 DA034634.
To maintain its shelf life of 12 to 18 months, the product should only be drawn (for the intramuscular injection) or screwed (for the intranasal spray) into the syringe immediately before use. To maintain the shelf life, store at room temperature, avoid environments with fluctuating extreme temperatures like inside unattended automobiles, and away from direct light (International Medications Systems Limited, 2015). Previously, the only option for intranasal naloxone was with the use of the intramuscular formulation with a luer-lock mucosal atomizer device (MAD), which must be stocked separately and does not have a national drug code. However, in November of 2015, the FDA approved a nasal naloxone spray, which requires no assembly (U.S. Food and Drug Administration, 2015). It is important to consider the patient's individual preferences and needs when choosing between naloxone formulations.
A growing number of public and private insurers are covering naloxone formulations. Pharmacists can determine coverage and prior authorization requirements. The cost of naloxone varies by formulation and distributor, but is around $40 to $150 for an intranasal regimen—less for the intramuscular regimen, more for the auto-injector—which may place them out of reach for many uninsured patients (Massachusetts Department of Public Health, 2015). Kaléo, the manufacturer of the auto-injector (Evzio), has a patient assistance program that limits the out-of-pocket cost for eligible patients (Evzio, 2015). The MAD has an out-of-pocket cost of around $5 each.
Three models exist for accessing naloxone rescue kits through a pharmacy (Green et al., 2015b). First, a prescriber can write a prescription for naloxone and the patient can fill it at the pharmacy. There, pharmacists can determine if naloxone is covered by the patient's insurance. Pharmacy staff should demonstrate the assembly and use of naloxone, and also provide further information on how to prevent, recognize, and respond to overdoses. Second, prescribers can write a prescription and, if permitted by state law, dispense the naloxone onsite. Third, pharmacies can provide naloxone directly to the patient via a pharmacist prescriber, or under a standing order, protocol, or collaborative practice agreement (CPA), explained in more detail below. In states that do not permit standing orders, protocols, or CPAs, pharmacists can contact the prescriber to have him or her call in or electronically send a prescription.
Variability exists across the country regarding availability and regulation of naloxone. Providers are encouraged to identify pharmacies in their areas that stock naloxone, and be aware of their state-specific laws surrounding naloxone prescribing.
The patient visits the pharmacy to pick up her naloxone. The patient asks for some help to determine which is best for her. “I’d be happy to demonstrate,” the pharmacist replies.
The Legal Environment for Naloxone Prescribing and Dispensing
The legal environment for naloxone prescribing is in general no different than that for any other prescription medication. Prescribers must ensure that prescriptions are issued in good faith, in the usual course of professional practice, and for a legitimate medical purpose (Abood, 2010). The prescription of naloxone for a patient at risk of overdose satisfies all 3 criteria (Burris et al., 2009).
Whereas intranasal use with a MAD is technically considered an off-label use of the intramuscular formulation, prescription of the medication for intranasal administration does not change liability risk. Depending on the setting, 20% to 80% of all prescriptions are written off-label, and the use of naloxone intranasally is a well-established practice (O’ Reilly and Dalal, 2003; Kerr et al., 2009; Wittich et al., 2012). A recent review of relevant law discovered no instances in which a prescriber or dispenser was sued for prescribing or providing naloxone for community use (Davis et al., 2015a). As with any medication, both prescriber and pharmacist should ensure that the patient understands the indications of the medication, possible side effects, and how to administer it in the event of an overdose.
As legislators have realized the need to increase naloxone access to laypersons, the majority of states have implemented laws and regulations to expand opportunities for naloxone prescription and distribution. Additionally, a number of states have passed laws that limit civil and criminal liability for prescribers, dispensers, and administrators, and also “Good Samaritans” who call for help in an overdose emergency, and the overdose victim (Davis et al., 2013; Davis and Carr, 2015).
Third Party Prescribing
In recognition of the fact that the person who is at risk of overdose is often not the person that the clinician encounters (ie, often a friend or family member expresses concern to the prescriber about another person at risk), a number of state legislatures have taken action to permit prescribers to prescribe naloxone to people with whom they do not have a prescriber–patient relationship. This is termed “third party prescribing.” In a state that allows for third party prescribing, the legal risk in doing so is no different than that if the prescriber were prescribing directly to the person at risk. Typically, the actual prescription will be written in the name of the person who has directly contacted the prescriber. As of September 2015, 37 states had passed laws permitting third party prescribing of naloxone. The question of whose insurance gets billed for third party prescriptions is an unresolved issue and policies currently differ from place to place.
Enhanced pharmacy access
Some pharmacists practicing in federal agencies such as the Indian Health Service and Veterans Administration have prescribing authority, and several states have recently passed laws that permit some pharmacists to prescribe naloxone (U.S. Department of Health and Human Services, 1996; Clause et al., 2001; Davis and Carr, 2015). Where the pharmacist is the prescriber, he or she is generally bound by the same requirements that apply to physicians and other prescribers.
A majority of states have passed laws that permit prescribers and pharmacists to establish CPAs or standing or protocol orders for naloxone. In these states, a pharmacist can dispense naloxone to any individual who meets criteria specified in the agreement or order. Rules and requirements are state-specific, though all states require a written agreement between prescriber and the pharmacy or pharmacist that sets out the terms. As of September 2015, 27 states permit pharmacy standing orders and 12 also permit naloxone to be distributed by laypersons acting under the authority of a prescriber at locations such as community-based organizations and drug treatment centers (Davis et al., 2015b; Wheeler et al., 2015). In many states, standing orders are also used to authorize nonmedical first responders such as firefighters and police officers to possess and administer naloxone (Davis et al., 2014b, 2015a).
Immunity for Prescribers and Dispensers
Fear of legal consequences may still discourage some medical professionals from issuing naloxone prescriptions (Beletsky et al., 2007). To address this concern, 32 states have changed their laws to provide full or partial civil immunity to medical professionals who prescribe naloxone as permitted by law, whereas 30 provide such immunity to dispensers and 36 to the person who administers the medication in the event of an overdose. Most of these laws require that the medical professional not act negligently or recklessly in prescribing or dispensing. Because of these laws, prescribing or dispensing naloxone entails less risk in most states than prescribing or dispensing other medications.
Good Samaritan Laws
Many people who use illicit drugs or medications other than prescribed may be wary of calling 911 in an overdose for fear of criminal sanctions. Overdose Good Samaritan laws, sometimes known as medical amnesty laws, are intended to encourage bystanders to call for help by providing limited criminal immunity to the person that makes the call, and also to the victim. This immunity is typically limited to relatively minor crimes, although in some states it extends to violations of probation or parole. For prescribers and pharmacists, it is important to let patients prescribed naloxone know of these laws, and reinforce the importance of not only administering naloxone, but also calling emergency medical response (Tobin et al., 2005; Lagu et al., 2006; Pollini et al., 2006).
State-specific naloxone laws can be found at the Public Health Law Research Program's Law Atlas (www.lawatlas.org).
CONCLUSIONS
Prescribers and pharmacists should acknowledge and embrace their role in helping patients to prevent and respond to opioid overdoses. Clinical and legal environments have become more conducive to providing overdose prevention education and naloxone rescue kits. Engaging prescribers and pharmacists is crucial to addressing the overdose epidemic.
Acknowledgments
The Web site www.prescribetoprevent.org is a free online resource to help health care providers educate their patients to reduce overdose risk and provide naloxone rescue kits to patients. We would like to thank the contributors to the Web site.
Footnotes
Funding: This program is supported by an unrestricted educational grant from the Substance Abuse and Mental Health Services Administration of the U.S. Department of Health and Human Services. The online CME activity is hosted by the Boston University School of Medicine Continuing Medical Education Office.
Conflicts of interest: The authors declare no conflicts of interest.
REFERENCES
- Abood R. Pharmacy Practice and the Law, 6th ed. Accessed December 19, 2015 Jones & Bartlett; 2010: 223. [Google Scholar]
- American Medical Association. It's about saving lives: increasing access to naloxone. Available at: http://www.ama-assn.org/ama/ama-wire/post/its-saving-lives-increasing-access-naloxone. Published June 29, 2015. Accessed July 12, 2015. [Google Scholar]
- American Pharmacists’ Association. APhA Policy: controlled substances and other medications with the potential for abuse and use of opioid reversal agents. Available at: http://www.pharmacist.com/policy/controlled-substances-and-other-medications-potential-abuse-and-use-opioid-reversal-agents-2. Published 2014. Accessed July 12, 2015. [Google Scholar]
- American Society of Addiction Medicine. Public policy statement on the use of naloxone for the prevention of drug overdose deaths. Chevy Chase, MD: Available at: http://www.asam.org/docs/default-source/publicy-policy-statements/1naloxone-rev-8-14.pdf. Revised 2014. Accessed July 12, 2015. [Google Scholar]
- Baca CT, Grant KJ. What heroin users tell us about overdose. J Addict Dis 2007; 26:63–68. [DOI] [PubMed] [Google Scholar]
- Beletsky L, Ruthazer R, Macalino GE, et al. Physicians’ knowledge of and willingness to prescribe naloxone to reverse accidental opiate overdose: challenges and opportunities. J Urban Health 2007; 84:126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beletsky L. The benefits and potential drawbacks in the approval of EVZIO for lay reversal of opioid overdose. Am J Prev Med 2015; 48:357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J, Mattick R, Hay A, et al. Methadone maintenance and drug-related crime. J Subst Abuse 1997; 9:15–25. [DOI] [PubMed] [Google Scholar]
- Bennett AS, Bell A, Tomedi L, et al. Characteristics of an overdose prevention, response, and naloxone distribution program in Pittsburgh and Allegheny County, Pennsylvania. J Urban Health 2011; 88:1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuger M, Tommasello A, Schwartz R, et al. Clonidine use and abuse among methadone program applicants and patients. J Subst Abuse Treat 1998; 15:589–593. [DOI] [PubMed] [Google Scholar]
- Bukten A, Skurtveit S, Gossop M, et al. Engagement with opioid maintenance treatment and reductions in crime: a longitudinal national cohort study. Addiction 2012; 107:393–399. [DOI] [PubMed] [Google Scholar]
- Burris S, Beletsky L, Castagna C, et al. Stopping an invisible epidemic: legal issues in the provision of naloxone to prevent opioid overdose. Drexel Law Rev 2009; 1:273–339. [Google Scholar]
- Cacioppo JT, Hughts ME, Waite LJ, et al. Loneliness as a specific risk factor for depressive symptoms: cross-sectional and longitudinal analyses. Psychol Aging 2006; 21:140–151. [DOI] [PubMed] [Google Scholar]
- Chamberlain JM, Klein BL. A comprehensive review of naloxone for the emergency physician. Am J Emerg Med 1994; 12:650–660. [DOI] [PubMed] [Google Scholar]
- Chen LH, Hedegaard H, Warner M. Drug-poisoning deaths involving opioid analgesics: United States, 1999–2011. NCHS Data Brief 2014; 1–8. [PubMed] [Google Scholar]
- Clark AK, Wilder CM, Winstanley EL. A systematic review of community opioid overdose prevention and naloxone distribution programs. J Addict Med 2014; 8:153–163. [DOI] [PubMed] [Google Scholar]
- Clause S, Fudin J, Mergner A, et al. Prescribing privileges among pharmacists in veterans affairs medical centers. Am J Health Syst Pharm 2001; 58:1143–1145. [DOI] [PubMed] [Google Scholar]
- Clausen T, Waal H, Thoresen M, et al. Mortality among opiate users: opioid maintenance therapy, age and causes of death. Addiction 2009; 104:1356–1362. [DOI] [PubMed] [Google Scholar]
- Coffin PO, Sullivan SD. Cost-effectiveness of distributing naloxone to heroin users for lay overdose reversal. Ann Intern Med 2013; 158:1–9. [DOI] [PubMed] [Google Scholar]
- Dart RC, Surratt HL, Cicero JF, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med 2015; 372:241–248. [DOI] [PubMed] [Google Scholar]
- Dasgupta N, Creppage K, Austin A, et al. Observed transition from opioid analgesic deaths toward heroin. Drug Alcohol Depend 2014; 145:238–241. [DOI] [PubMed] [Google Scholar]
- Davis CS, Carr D. Legal changes to increase access to naloxone for opioid overdose reversal in the United States. Drug Alcohol Depend 2015; 157:112–120. [DOI] [PubMed] [Google Scholar]
- Davis CS, Webb D, Burris S. Changing law from barrier to facilitator of opioid overdose prevention. J Law Med Ethics 2013; 41 Suppl 1:33–36. [DOI] [PubMed] [Google Scholar]
- Davis CS, Pierce M, Dasgupta N. Evolution and convergence of state laws governing controlled substance prescription monitoring programs, 1998–2011. Am J Public Health 2014a; 104:1389–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CS, Southwell JK, Niehaus VR, et al. Emergency medical services naloxone access: a national systematic legal review. Acad Emerg Med 2014b; 21:1173–1177. [DOI] [PubMed] [Google Scholar]
- Davis CS, Carr D, Southwell JK, et al. Engaging law enforcement in overdose reversal initiatives: authorization and liability for naloxone administration. Am J Public Health 2015a; 105:1530–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CS, Walley AY, Bridger CM. Lessons learned from the expansion of naloxone access in Massachusetts and North Carolina. J Law Med Ethics 2015b; 43 Suppl 1:19–22. [DOI] [PubMed] [Google Scholar]
- Doe-Simkins M, Walley AY, Epstein A, et al. Saved by the nose: bystander-administered intranasal naloxone hydrochloride for opioid overdose. Am J Public Health 2009; 99:788–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe-Simkins M, Quinn E, Xuan Z, et al. Overdose rescues by trained and untrained participants and change in opioid use among substance-using participants in overdose education and naloxone distribution programs: a retrospective cohort study. BMC Public Health 2014; 14:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan KA, Shearer J, White B, Zhou J, et al. Four-year follow-up of imprisoned male heroin users and methadone treatment: mortality, re-incarceration and hepatitis C infection. Addiction 2005; 100:820–828. [DOI] [PubMed] [Google Scholar]
- Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med 2010; 152:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enteen L, Bauer J, McLean R, et al. Overdose prevention and naloxone prescription for opioid users in San Francisco. J Urban Health 2010; 87:931–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evzio. Kaléo Cares Patient Assistance Program. Available at: http://evzio.com/hcp/patient-savings/kaleo-cares-patient-assistance.php. Accessed July 9, 2015. [Google Scholar]
- Fajemirokun-Odudeyi O, Sinha C, Tutty S, et al. Pregnancy outcome in women who use opiates. Eur J Obstet Gynecol Reprod Biol 2006; 126:170–175. [DOI] [PubMed] [Google Scholar]
- Gray JA, Hagemeier NE. Prescription drug abuse and DEA-sanctioned drug take-back events: characteristics and outcomes in rural appalachia. Arch Intern Med 2012; 172:1186–1187. [DOI] [PubMed] [Google Scholar]
- Green TC, Heimer R, Grau LE. Distinguishing signs of opioid overdose and indication for naloxone: an evaluation of six overdose training and naloxone distribution programs in the United States. Addiction 2008; 103:979–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TC, Bowman S, Davis C, et al. Discrepancies in addressing overdose prevention through prescription monitoring programs. Drug Alcohol Depend 2015a; 153:355–358. [DOI] [PubMed] [Google Scholar]
- Green TC, Dauria EF, Bratberg J, et al. Orienting patients to greater opioid safety: models of community pharmacy-based naloxone. Harm Reduct J 2015b; 12:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inocencio TJ, Carroll NV, Read EJ, et al. The economic burden of opioid-related poisoning in the United States. Pain Med 2013; 14:1534–1547. [DOI] [PubMed] [Google Scholar]
- International Medications Systems Limited; 2015. Naloxone hydrochloride [package insert]. El Monte, CA. [Google Scholar]
- Jann M, Kennedy WK, Lopez G. Benzodiazepines: a major component in unintentional prescription drug overdoses with opioid analgesics. J Pharm Pract 2014; 27:5–16. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Jones HE, Jasinski DR, et al. Buprenorphine treatment of pregnant opioid-dependent women: maternal and neonatal outcomes. Drug Alcohol Depend 2001; 63:97–103. [DOI] [PubMed] [Google Scholar]
- Jones CM. Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers: United States, 2002–2004 and 2008–2010. Drug Alcohol Depend 2013; 132:95–100. [DOI] [PubMed] [Google Scholar]
- Jones CM, McAninch JK. Emergency department visits and overdose deaths from combined use of opioids and benzodiazepines. Am J Prev Med 2015;S0749-3797(15):00163–4. [DOI] [PubMed] [Google Scholar]
- Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA 2013; 309:657–659. [DOI] [PubMed] [Google Scholar]
- Jones CM, Paulozzi LJ, Mack KA. Alcohol involvement in opioid pain reliever and benzodiazepine drug abuse-related emergency department visits and drug-related deaths. MMWR Morb Mortal Wkly Rep 2014; 63:881–885. [PMC free article] [PubMed] [Google Scholar]
- Kerr D, Kelly AM, Dietze P, et al. Randomized controlled trial comparing the effectiveness and safety of intranasal and intramuscular naloxone for the treatment of suspected heroin overdose. Addiction 2009; 104:2067–2074. [DOI] [PubMed] [Google Scholar]
- Kinner SA, Milloy M-J, Wood E, et al. Incidence and risk factors for non-fatal overdose among a cohort of recently incarcerated illicit drug users. Addict Behav 2012; 37:691–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupitsky E, Nunes EV, Ling W, et al. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicenter randomised trial. Lancet 2011; 377:1506–1513. [DOI] [PubMed] [Google Scholar]
- Lagu T, Anderson BJ, Stein M. Overdoses among friends: drug users are willing to administer naloxone to others. J Subst Abuse Treat 2006; 30:129–133. [DOI] [PubMed] [Google Scholar]
- Langendam MW, van Brussel GH, Coutinho RA, et al. The impact of harm-reduction-based methadone treatment on mortality among heroin users. Am J Public Health 2001; 91:774–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRochelle MR, Zhang F, Ross-Degnan D, et al. Rates of opioid dispensing and overdose after introduction of abuse-deterrent extended-release oxycodone and withdrawal of propoxyphene. JAMA Intern Med 2015; 175:978–987. [DOI] [PubMed] [Google Scholar]
- Lavonas EJ, Drennan IR, Gabrielli A, et al. Part 10: Special Circumstances of Resuscitation: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015; 132 (18 Suppl 2):S501–S518. [DOI] [PubMed] [Google Scholar]
- Li G, Brady JE, Lang BH, et al. Prescription drug monitoring and drug overdose mortality. Injury Epidemiol 2014; 1 9: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobmann R, Verthein U. Explaining the effectiveness of heroin-assisted treatment on crime reductions. Law Hum Behav 2009; 33:83–95. [DOI] [PubMed] [Google Scholar]
- Lynch KL, Shapiro BJ, Coffa D, et al. Promethazine use among chronic pain patients. Drug Alcohol Depend 2015; 150:92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madadi P, Hildebrandt D, Lauwers AE, et al. Characteristics of opioid-users whose death was related to opioid-toxicity: a population-based study in Ontario, Canada. PLoS One 2013; 8:e60600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars SG, Bourgois P, Karandinos G, et al. Every ‘never’ I ever said same true”: transitions from opioid pills to heroin injecting. Int J Drug Policy 2014; 25:257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massachusetts Department of Public Health Bureau of Substance Abuse Services. Overdose Education and Naloxone Distribution (OEND) Pilot Expansion. Boston, MA: Available at: http://www.mass.gov/eohhs/docs/dph/substance-abuse/opioid/fy-15-oend-pilot-expansion-report.pdf. Published 2015. Accessed July 13, 2015. [Google Scholar]
- Mattick RP, Breen C, Kimber J, et al. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev 2009; 8:CD002209. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kinber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev 2014; 2:CD002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell S, Bigg D, Stanczykiewicz K, et al. Prescribing naloxone to actively injecting heroin users: a program to reduce heroin overdose deaths. J Addict Dis 2006; 25:89–96. [DOI] [PubMed] [Google Scholar]
- Meyer M, Benvenuto A, Howard D, et al. Development of a substance abuse program for opioid-dependent nonurban pregnant women improves outcome. J Addict Med 2012; 6:124–130. [DOI] [PubMed] [Google Scholar]
- Michiels C. Physiological and pathological responses to hypoxia. Am J Pathol 2004; 164:1875–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modarai F, Mack K, Hicks P, et al. Relationship of opioid prescription sales and overdoses, North Carolina. Drug Alcohol Depend 2013; 132:81–86. [DOI] [PubMed] [Google Scholar]
- Moller LF, Matic S, van den Bergh BJ, et al. Acute drug-related mortality of people recently released from prisons. Public Health 2010; 124:637–639. [DOI] [PubMed] [Google Scholar]
- Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med 2010; 363:1981–1985. [DOI] [PubMed] [Google Scholar]
- O’Reilly J, Dalal A. Off-label or out of bounds? Prescriber and marketer liability for unapproved uses of FDA-approved drugs. Ann Health Law 2003; 12:295–324. [PubMed] [Google Scholar]
- Paulozzi LJ, Kilbourne EM, Desai HA. Prescription drug monitoring programs and death rates from drug overdose. Pain Med 2011; 12:747–754. [DOI] [PubMed] [Google Scholar]
- Piper TM, Stancliff S, Rudenstine S, et al. Evaluation of a naloxone distribution and administration program in New York City. Subst Use and Misuse 2008; 43:858–870. [DOI] [PubMed] [Google Scholar]
- Pollini RA, McCall L, Mehta SH, et al. Response to overdose among injection drug users. Am J Prev Med 2006; 31:261–264. [DOI] [PubMed] [Google Scholar]
- Powis B, Strang J, Griffiths P, et al. Self-reported overdose among injecting drug users in London: extent and nature of the problem. Addiction 1999; 94:471–478. [DOI] [PubMed] [Google Scholar]
- Rudd RA, Aleshire N, Zibbell JE, et al. Increases in drug and opioid overdose deaths: United States, 2000–2014. MMWR Morb Mortal Wkly Rep 2015; 64:1–5. [DOI] [PubMed] [Google Scholar]
- Schwartz RP, Highfield DA, Jaffe JH, et al. A randomized controlled trial of interim methadone maintenance. Arch Gen Psychiatry 2006; 63:102–109. [DOI] [PubMed] [Google Scholar]
- Schwartz RP, Gryczynski J, O’Grady, et al. Opioid agonist treatments and heroin overdose deaths in Baltimore, Maryland, 1995–2009. Am J Public Health 2013; 103:917–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal KH, Thawley R, Gee L, et al. Naloxone distribution and cardiopulmonary resuscitation training for injection drug users to prevent heroin overdose death: a pilot intervention study. J Urban Health 2005; 82:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro BJ, Lynch KL, Tochinda T, et al. Promethazine misuse among methadone maintenance patients and community-based injection drug users. J Addict Med 2013; 7:96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RV, Lofwall MR, Havens JR. Abuse and diversion of gabapentin among nonmedical prescription opioid users in Appalachian Kentucky. Am J Psychiatry 2015; 172:487–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyka M, Trader A, Klotsche J, et al. Criminal behavior in opioid-dependent patients before and during maintenance therapy: 6-year follow-up of a nationally representative cohort sample. J Forensic Sci 2012; 57:1524–1530. [DOI] [PubMed] [Google Scholar]
- Sporer KA, Kral AH. Prescription naloxone: a novel approach to heroin overdose prevention. Ann Emerg Med 2007; 49:172–177. [DOI] [PubMed] [Google Scholar]
- Strang J, McCambridge J, Best D, et al. Loss of tolerance and overdose mortality after inpatient opiate detoxification: follow up study. BMJ 2003; 326:959–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2010 Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Center for Behavioral Health Statistics and Quality; Available at: http://www.samhsa.gov/data/sites/default/files/NSDUHNationalFindingsResults2010-web/2k10ResultsRev/NSDUHresultsRev2010.pdf. Published 2011. Accessed June 22, 2015. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. “Opioid Overdose Prevention Toolkit.” Rockville, MD: Available at: http://store.samhsa.gov/shin/content//SMA14-4742/Overdose_Toolkit.pdf. Revised 2014. Accessed July 12, 2015. [Google Scholar]
- Tobin KE, Davey MA, Latkin CA. Calling emergency medical services during drug overdose: an examination of individual, social and setting correlates. Addiction 2005; 100:397–404. [DOI] [PubMed] [Google Scholar]
- Tobin KE, Sherman SG, Beilenson P, et al. Evaluation of the Staying Alive programme: training injection drug users to properly administer naloxone and save lives. Intl J Drug Policy 2009; 20:131–136. [DOI] [PubMed] [Google Scholar]
- U.S. Centers for Disease Control and Prevention. Multiple causes of death, 1993–2013 query. Available at: http://wonder.cdc.gov/mcd-icd10.html. Accessed June 22, 2015a. [Google Scholar]
- U.S. Centers for Disease Control and Prevention. Deaths from Prescription Overdose. Available at: http://www.cdc.gov/drugoverdose/data/overdose.html. Published April 30, 2015b. Accessed July 6, 2015. [Google Scholar]
- U.S. Centers for Disease Control and Prevention. Increases in Fentanyl Drug Confiscation and Fentanyl-related Overdose Fatalities. Available at: http://emergency.cdc.gov/han/han00384.asp. Published October 26, 2015c. Accessed December 11, 2015. [Google Scholar]
- U.S. Department of Health and Human Services. Special General Memorandum 96-2: Designation of Pharmacists as Primary Care Providers with Prescriptive Authority. Available at: http://www.ihs.gov/IHM/index.cfm?module=dsp_ihm_sgm_main&sgm=ihm_sgm_9602. Published October 18, 1996. Accessed July 27, 2015. [Google Scholar]
- U.S. Department of Health and Human Services. Opioid Abuse in the U.S. and HHS Actions to Address Opioid-Drug Related Overdoses and Deaths. Available at: http://aspe.hhs.gov/sp/reports/2015/OpioidInitiative/ib_OpioidInitiative.cfm. Published March 26, 2015. Accessed July 10, 2015 [DOI] [PubMed] [Google Scholar]
- U.S. Drug Enforcement Administration. Final Rule on Disposal of Controlled Substances. Available at: https://www.federalregister.gov/articles/2014/09/09/2014-20926/disposal-of-controlled-substances. Published Septebmer 9, 2014. Accessed June 22, 2015. [Google Scholar]
- U.S. Drug Enforcement Administration. National Take-Back Initiative. Available at: http://www.deadiversion.usdoj.gov/drug_disposal/takeback/. Published 2015. Accessed June 22, 2015. [Google Scholar]
- U.S. Food and Drug Administration. FDA Commissioner Margaret A. Hamburg Statement on Prescription Opioid Abuse. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm391590.htm. Published April 3, 2014. Accessed July 6, 2015. [Google Scholar]
- U.S. Food and Drug Administration. FDA moves quickly to approve easy-to-use nasal spray to treat opioid overdose. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm473505.htm. Published November 18, 2015. Accessed December 12, 2015. [Google Scholar]
- U.S. Food and Drug Administration. FDA's Efforts to Address the Misuse and Abuse of Opioids. Available at: http://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm337852.htm. Published February 6, 2013. Accessed August 13, 2015. [Google Scholar]
- United Nations Office on Drugs and Crime. World Drug Report 2014. Vienna, Austria: Available at: http://www.unodc.org/documents/wdr2014/World_Drug_Report_2014_web.pdf. Published 2014. Accessed July 12, 2015. [Google Scholar]
- Wagner KD, Valente TW, Casanova M, et al. Evaluation of an overdose prevention and response training programme for injection drug users in the Skid Row area of Los Angeles, CA. Intl J Drug Policy 2010; 21:186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walley AY, Doe-Simkins M, Quinn E, et al. Opioid overdose prevention with intranasal naloxone among people who take methadone. J Subst Abuse Treat 2013a; 44:241–247. [DOI] [PubMed] [Google Scholar]
- Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ 2013b; 346:f174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler E, Davidson PJ, Jones TS, et al. Community-based opioid overdose prevention programs providing naloxone: United States, 2010. MMWR Morb Mortal Wkly Rep 2012; 61:101–105. [PMC free article] [PubMed] [Google Scholar]
- Wheeler E, Jones TS, Gilbert M, et al. Opioid overdose prevention programs providing naloxone to laypersons. MMWR Morb Mortal Wkly Rep 2015; 64:631–635. [PMC free article] [PubMed] [Google Scholar]
- White JM, Irvine RJ. Mechanism of fatal opioid overdose. Addiction 1999; 94:961–972. [PubMed] [Google Scholar]
- Wittich CM, Burkle CM, Lanier WL. Ten common questions (and their answers) about off-label drug use. Mayo Clin Proc 2012; 87:982–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff K. Characterization of methadone overdose: clinical considerations and the scientific evidence. Drug Monit 2002; 24:457–470. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Community management of opioid overdose; Geneva, Switzerland: Available at: http://apps.who.int/iris/bitstream/10665/137462/1/9789241548816_eng.pdf?ua=1&ua=1. Published 2014. Accessed July 12, 2015. [Google Scholar]
- Zedler B, Xie L, Wang L, et al. Risk factors for serious prescription opioid-related toxicity or overdose among Veterans Health Administration patients. Pain Med 2014; 15:1911–1929. [DOI] [PubMed] [Google Scholar]


