Background/Aims:
Nonalcoholic fatty liver disease (NAFLD) has emerged as an important cause of chronic liver disease, cirrhosis, and hepatocellular carcinoma (HCC). The Barcelona Clinic Liver Cancer (BCLC) system is the preferred staging system to evaluate patients with HCC and links prognosis assessment with treatment recommendation. The aim of this retrospective study was to evaluate whether the BCLC staging system and its treatment algorithm are suitable for patients with HCC arising from NAFLD.
Methods:
Forty-two patients with HCC related to either to NAFLD or cryptogenic cirrhosis were retrieved retrospectively from 2 centers in Brazil. Patients were classified according to BCLC staging system. If the proposed HCC therapy could not be applied, the case was considered to represent deviations from the recommended BCLC guideline. Causes of treatment deviations were investigated.
Results:
There were 4 patients without evidence of cirrhosis according to liver biopsy and/or clinical evaluation. One (2%), 21 (50%), 10 (24%), 5 (12%), and 5 patients (12%) were classified initially to the very early (0), early (A), intermediate (B), advanced (C), and terminal (D) BCLC stages, respectively. Thirty-five patients (83%) were treated according to BCLC recommendations. There were 3 cases (of 5) of protocol deviation in BCLC C patients. The 1- and 2-year overall survival rates were 81% and 66%, respectively.
Conclusions:
The BCLC system is applied in most cases of NAFLD-related HCC cases. Deviation of BCLC is found more frequently in BCLC C stage patients.
Key Words: nonalcoholic fatty liver disease, hepatocellular carcinoma, BCLC staging system, liver cancer, management
Hepatocellular carcinoma (HCC), which accounts for >5% of cancers globally, is ranked as the sixth most common cancer and the third leading cause of cancer-related death worldwide.1 Cirrhosis is the main risk factor for HCC development. Most cases are associated to chronic hepatitis B and C infection as well as alcohol consumption.2 In recent years, nonalcoholic fatty liver disease (NAFLD) has appeared as an important cause of chronic liver disease, cirrhosis, and HCC. In the absence of effective surveillance strategies, the stage of presentation is often advanced.3,4
HCC is unique because in addition to cancer stage, the underlying liver function substantially affects prognosis. The Barcelona Clinic Liver Cancer (BCLC) staging system was proposed and has been validated by several groups in the United States and Europe and offers the best stage classification and guidance for HCC treatment allocation.5,6 BCLC staging system was developed mainly in European populations in which hepatitis C virus infection was dominant. Validation was also done by Asiatic group where hepatitis B virus is dominant.7
This system currently recommends radical therapies [liver transplantation, surgical resection, or percutaneous ablation, such as radiofrequency ablation (RFA) or percutaneous ethanol injection (PEI)] for very early (BCLC stage 0) or early-stage (BCLC stage A) HCC, transarterial chemoembolization (TACE) for intermediate-stage (BCLC stage B) HCC, sorafenib administration for advanced-stage (BCLC stage C) HCC, and supportive care for end-stage (BCLC stage D) HCC.8 However, although this staging system was developed using an evidence-based approach, its application can be problematic in NAFLD patients because of different clinical profile of these patients.9 There are few reports of BCLC application in NAFLD patients.
Evidence has emerged suggesting that the molecular pathogenesis, clinical features, and the prognosis of NAFLD-related HCC may differ from viral-induced HCC.4 Most of HCC cases are described in cirrhotic patients, but HCC is increasing also in the noncirrhotic setting.10 These findings suggest that hepatocarcinogenesis may be deregulated in early stages of this disease. Some comparative studies suggest that NAFLD-related HCC patients are older and present with comorbidities that may limit HCC therapy according to recommended guidelines.9,11
The main objective of this retrospective study was to evaluate whether the BCLC staging system and its treatment algorithm are suitable for patients with HCC arising from NAFLD. We also studied clinical characteristics of NAFLD-related HCC, and aspects related to diagnosis, overall survival, and predictors of survival.
METHODS
Patients
Between January 2010 and December 2012, 42 patients with HCC related either to NAFLD or cryptogenic cirrhosis were retrieved retrospectively from 2 centers in Brazil (Instituto do Câncer do Estado de São Paulo and Universidade Federal do Rio Grande do Sul). The Institutional Review Board of the University of São Paulo approved the study. Part of the included patients comes from an observatory of HCC in NAFLD of the Fatty Liver Inhibition of Progression (FLIP) consortium.12
Cirrhosis was diagnosed on histologic features and/or radiologic evidence (imaging studies suggesting chronic liver disease and portal hypertension), laboratory abnormalities such as thrombocytopenia, hypoalbuminemia, prolonged prothrombin time, or clinically decompensated disease (ascites, variceal bleeding, or hepatic encephalopathy). Other causes of chronic liver disease as hepatitis B, hepatitis C, autoimmune hepatitis, α1-antitrypsin deficiency, Wilson disease, and hemochromatosis were excluded by appropriate laboratory tests. Unless features of the metabolic syndrome were present, or NAFLD was evident from histology, cases were regarded as cryptogenic. Baseline information, including patient demographics; risk factors for NAFLD; serum biochemistries; liver biopsy when available, severity of cirrhosis (Child-Pugh classification and Model for End-Stage Liver Disease score); Eastern Cooperative Oncology Group performance status; cancer stage according to the BCLC staging system; α-fetoprotein level; and treatment modalities were collected at enrollment.
HCC was diagnosed according to noninvasive diagnostic criteria of the American Association for the Study of Liver Diseases updated in 2010 whenever cirrhosis was present.8 Liver histology confirmation was performed in all noncirrhotic patients and inconclusive cases by imaging examination.
Staging According to the BCLC
BCLC stages were determined based on tumor size and number, liver function tests, performance status, and cancer-related symptoms.8 All patients underwent abdominal dynamic computed tomography or magnetic resonance, chest computed tomography, and a bone scan for staging. Portal hypertension was defined by the presence of esophagogastric varices by upper endoscopy, ascites, or splenomegaly and a platelet count <100,000/mm3. Patients were classified as 0 (very early), A (early), B (intermediate), C (advanced), or D (terminal) using BCLC staging system.
Study Outcome
A local multidisciplinary team in each center composed of hepatobiliary surgeons, hepatologists, pathologists, oncologists, and interventional radiologists assessed all patients and decided on the best therapeutic option for each particular case, as performed routinely for each patient with HCC. For each patient the administered treatment strategy was compared with the 1 theoretically recommended by the BCLC stage. The main outcome of the study was to determine whether therapeutic options in agreement with the BCLC classification were applicable or not. In the latter case, we investigated the causes of treatment deviations from BCLC recommendations and analyzed the rate of deviation for each BCLC stage.
Treatment
Curative therapies included surgical resection, liver transplantation, and local ablative therapy such as RFA or PEI.8,13,14 Surgical resection was considered in patients with a single tumor, with well-preserved liver function, normal bilirubin, and absence of portal hypertension.15 This was also the first choice for noncirrhotic patients with a single nodule, regardless of tumor size. Liver transplantation was considered in patients fulfilling the Milan criteria and showed increased portal pressure or bilirubin. During the waiting time on the liver transplant list, patients were treated with percutaneous ablation or TACE as a bridge therapy to liver transplantation.8,13–15
If hepatectomy or liver transplantation were not indicated local ablative therapies such as RFA or PEI were performed depending on the size and number of tumor nodules. In multifocal tumors or large HCCs without extrahepatic spread or vascular invasion, TACE was the treatment of choice.8,13–15
Sorafenib was indicated for patients with advanced HCC. When treatment efficacy was considered limited or treatment-related risk was substantial due to extensive tumor burden, Child-Pugh class C status, or other medical comorbidities, supportive care was given.16,17
Statistical Analysis
Overall survival was measured from the date of diagnosis until death or last follow-up visit. Survival time was estimated using the Kaplan-Meier method and differences in survival between groups were assessed using the log-rank test.
RESULTS
Patients Characteristics
Baseline characteristics of the 42 patients are shown in Table 1. Median age was 66.5 years (range, 25 to 80 y) and male sex predominated (n=26; 62%).
TABLE 1.
Baseline Characteristics of the Study Population
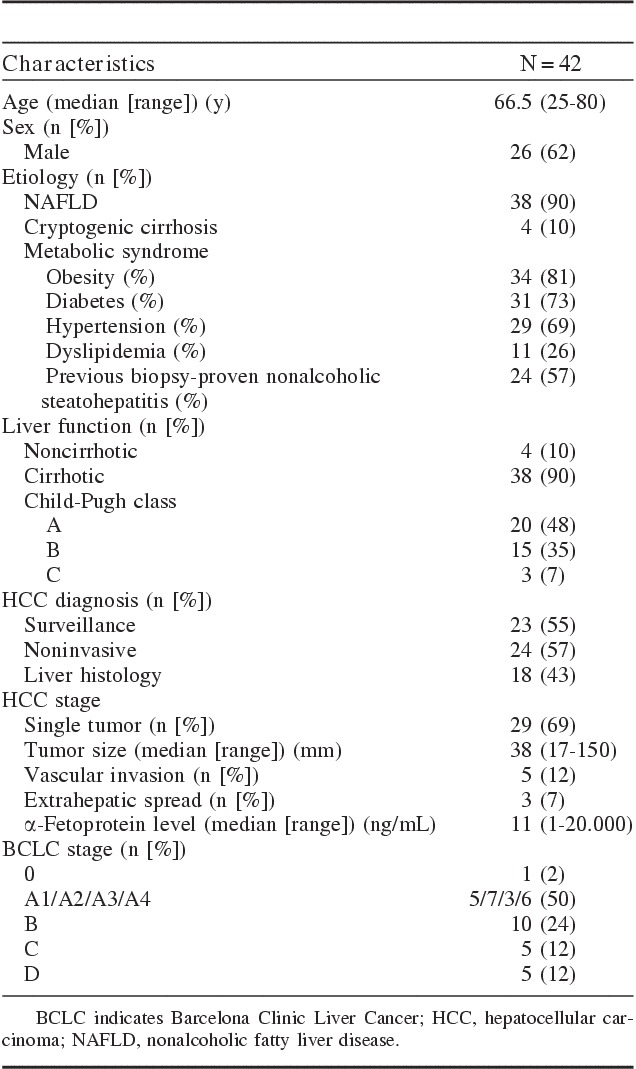
Four patients (10%) were classified as cryptogenic cirrhosis because no features of the metabolic syndrome were present, and there was no evidence of NAFLD in liver histology. NAFLD-related HCC were seen in 38 patients. In this group, there were 4 patients without evidence of cirrhosis according to liver biopsy and/or clinical evaluation. In NAFLD group, 24 patients had previous liver biopsy with macrovacuolar and microvacuolar fatty change, zonal distribution, foci of necrosis, portal and perivenular fibrosis, and inflammatory and fibrotic infiltrate with zonal distribution. In NAFLD group, 34 patients (81%) had obesity; 31 patients (73%) had type II diabetes, 29 patients (69%) had arterial hypertension, and 11 patients (26%) had dyslipidemia.
The numbers of patients in Child-Pugh classes A, B, and C were 20 (48%), 15 (35%), and 3 (7%), respectively. HCC was diagnosed based on noninvasive diagnostic criteria of the American Association for the Study of Liver Diseases in 24 patients (57%). Diagnosis of HCC was confirmed by histology in 18 patients (43%).
Twenty-nine patients (69%) had a single tumor and the median diameter of largest tumor was 38 mm (range, 17 to 150 mm). Five patients (12%) had macrovascular invasion and 3 (7%) extrahepatic spread. The median α-fetoprotein value was 11 ng/mL (range, 1 to 20,000 ng/mL).
Classification of BCLC Stage
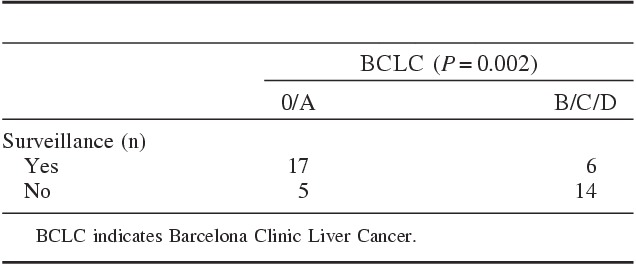
Noncirrhotic patients were incorporated to BCLC staging system because, in the real-life, they are practically treated with BCLC algorithm. One (2%), 21 (50%), 10 (24%), 5 (12%), and 5 patients (12%) were classified initially to the BCLC 0, A, B, C, and D stages, respectively. Among 42 patients, HCC was diagnosed in a screening program in 55% (there was 1 noncirrhotic patient). Patients with HCC diagnosed outside of a surveillance program (n=19) were mostly not candidates to curative therapies (73%) (P=0.002) (Table 2).
TABLE 2.
BCLC Stage According to Surveillance Program
Allocation of Treatment According BCLC Stages
Curative treatments, which included resection, local ablative therapy, and liver transplantation, were performed in 23 (55%) patients, whereas palliative treatments such as TACE or sorafenib were given in 11 (26%) patients. Eight (19%) patients received only the best supportive care. Table 3 summarizes the detailed treatment modalities used for patients classified according to the BCLC staging system.
TABLE 3.
Allocation of Treatment Modalities According BCLC Staging Systems
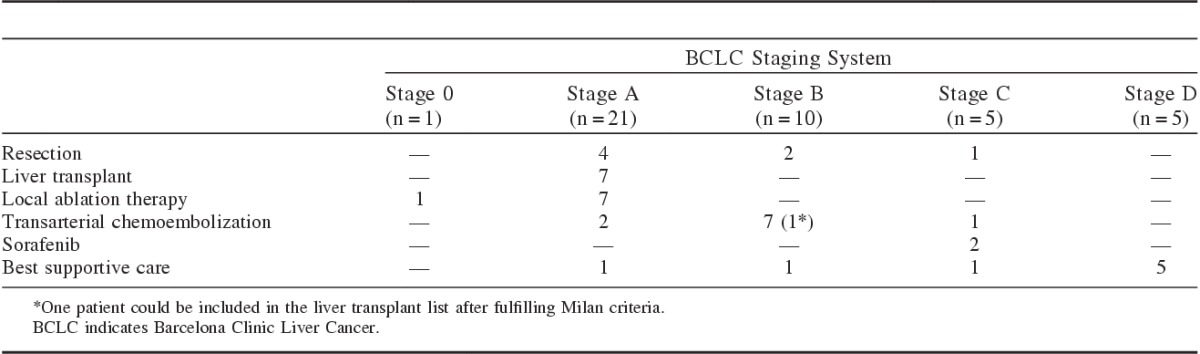
Liver resection was the first choice therapy in all noncirrhotic patients, based on the decision of the multidisciplinary team as the best therapeutic option in each case. The first patient had 1 nodule of 37 mm. The second patient had 1 nodule of 109 mm. The other 2 patients had >2 nodules, but in the same liver segment. All noncirrhotic patients survived >2 years after treatment.
Most patients (85%) in BCLC stage A were treated according to BCLC recommendations. Liver resection was performed in 4 patients, liver transplant in 7 patients, and local ablation therapy in 7 patients. There were 3 protocol deviations in this group. Two patients were treated with TACE and 1 received best supportive care.
Most patients at the BCLC stage B were treated with TACE. In this group of 7 patients, 1 could be included in the liver transplant list after fulfilling Milan criteria. Only 1 patient presented a BCLC protocol deviation (ie, could not be administered TACE) because of significant comorbidity (heart failure) and received only best supportive care. Two patients in this group were treated with resection, because they were noncirrhotic.
Most cases of protocol deviation were found in BCLC C patients (3 of the 5 BCLC C patients). Two patients was initially treated with sorafenib (one of them was included in a clinical trial with brivanib plus sorafenib). One patient was treated with liver resection and 1 received TACE. These 2 cases of protocol deviation had preserved liver function (Child-Pugh A) and were classified as BCLC C because of macrovascular invasion at a segmental branch level. This was an attempt to a more curative therapy in these 2 cases. The third patient presented with rapid deterioration of general status and liver decompensation and received best supportive care.
All patients in BCLC D stage received best supportive care exclusively. There were 3 patients that received only best supportive care and were included in the group of protocol deviation because they were classified as BCLC A, B, and C staging system. The reason for not treating these patients is the association of poor liver function, compromised general status and advanced age. On the other side, the others patients who did not follow BCLC algorithm presented a very compensated liver function and a more aggressive therapy was attempted in each case. Two patients were classified as BCLC B, but without signs of liver cirrhosis and resection was indicated by the multidisciplinary group. The other 2 patients were those classified as BCLC C because of macrovascular invasion, but the multidisciplinary team decision was for resection in one case and TACE in the other case before indicating sorafenib.
Survival
During the follow-up period (median, 11 mo; range, 0 to 85 mo), 12 patients died of tumor progression or cirrhosis-related complications. The 1- and 2-year overall survival rates were 81% and 66%, respectively (Fig. 1). All noncirrhotic patients were alive after 2 years of follow-up. Child-Pugh A cirrhotic patients presented a better overall survival rate (P=0.02) than Child-Pugh B and C patients. BCLC stage 0 and A patients had a better survival compared with BCLC B, C, and D patients, but it was not statistically significant.
FIGURE 1.
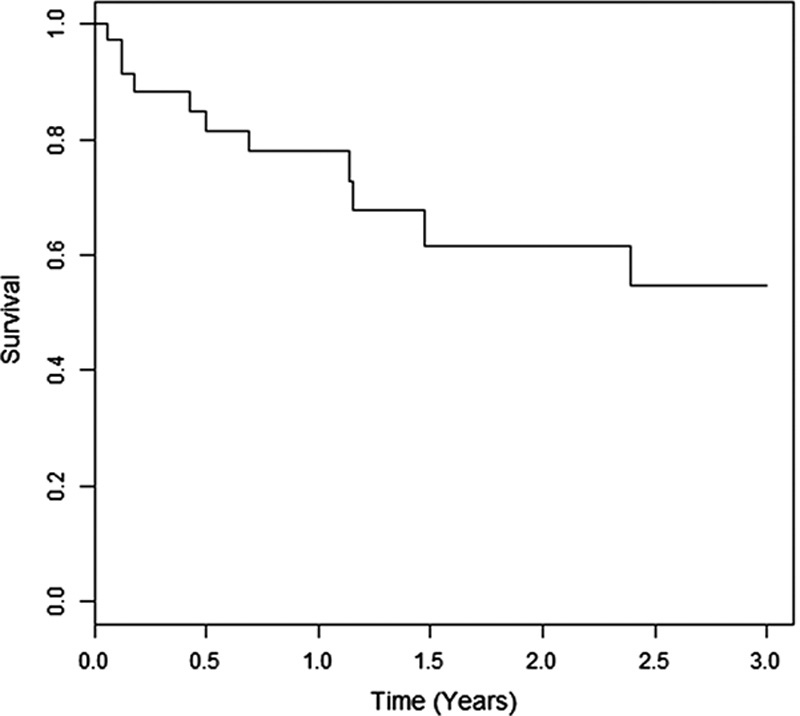
Overall survival of 42 patients.
DISCUSSION
HCC is the main cause of death in patients with cirrhosis. The BCLC system is the preferred staging system to evaluate patients with HCC as it takes into account the characteristics of the tumor, the degree of liver impairment, and the physical performance.5,7 Moreover, it is the only one that links prognosis assessment with treatment recommendation. NAFLD is a well-recognized etiology of HCC. Although clinical profile and hepatocarcinogenesis of these patients may be different from viral hepatitis infected patients, the BCLC could be applied in most cases of HCC arising in the context of NAFLD.
In this study, we characterized the outcome of patients with HCC arising on NAFLD or cryptogenic liver disease. Patients enrolled in a regular surveillance program were more often diagnosed at an early stage. Surveillance in patients with established cirrhosis and nonalcoholic steatohepatitis (NASH) is part of international recommendations. Recently, a Japanese group observed that in 28% of NAFLD patients, HCC developed in less advanced stages of fibrosis.18 In the present study, we observed HCC in 4 noncirrhotic patients that were treated with liver resection. However, recommendation of surveillance in this subgroup of patients can not be generalized and must be made with caution because it is estimated that in the United States, NAFLD can affect 30% of the general population.19
Recently, 166 new cases were collected over 22 months in the FLIP consortium and the demographic aspects found in our study were similar to this larger database.12 However, in the FLIP study, 59% patients were symptomatic and consequently BCLC stage C or D, whereas in the present study, 47% were BCLC stage 0 and A. Besides, applicability of the BCLC system was not evaluated in FLIP study. Another difference was the larger proportion of noncirrhotic patients in the FLIP study (43%) compared with 10% in our study. Preliminary data show that almost all noncirrhotic cases occurred in steatohepatitis rather than steatosis alone in both studies.12
The majority of studies describing HCC in the context of NAFLD usually focus on risk factors for liver carcinogenesis. Very little information is available on tumor stage and treatment of HCC after diagnosis. Besides, several factors affect the choice of treatment options and as the BCLC staging system is based on clinical and radiologic findings, it is not possible to determine exact stage in some patients.7
In our study, there were 4 noncirrhotic patients who were treated with liver resection. Most cases of protocol deviation were found in BCLC C stage (advanced stage) patients that sorafenib was the treatment of choice in these cases.8 One patient was treated with liver resection despite the presence of vascular invasion because it affected a segmental branch. The other patient who was classified as BCLC C was not evaluated initially by multidisciplinar team approach and received TACE in a private setting. This patient showed a very good tumor control response with TACE sessions besides the presence of segmental vascular invasion. These facts are problematic for the BCLC staging system not only in NAFLD patients, but in all types of HCC because its recommended treatment options are dependent on tumor stage. In another Korean study, applicability of the BCLC staging system to patients with HCC demonstrated that donor shortage, financial problems, the relatively limited efficacy of molecular targeting agents, and the presence of an indeterminate nodule were the main causes of deviation from BCLC recommendations.20
In the other side, there are many concerns about HCC treatment in NAFLD patients. Comparative studies demonstrated that these patients are older, have higher body mass index, and are more likely to be diabetic, obese, or hypertensive.9,21 All these factors may difficult treatment schedule according to BCLC guidelines. They may be older to be candidate to liver transplant or have more renal dysfunction to TACE or have too many comorbidities to surgical resection. In our study, only 1 patient from BCLC A did not receive HCC therapy due to his comorbidities. Another patient did not follow BCLC guideline and received TACE because of technical contraindication to others procedures. In general, protocol deviation was not associated to age or presence of comorbidities. Deviations were associated to the presence of a good liver function allowing a more aggressive oncologic treatment.
Liver transplant was performed in 8 patients. United Network for Organ Sharing database showed that the frequency of liver transplant in NASH patients is increasing in the last decades and it is projected that NASH would likely overtake hepatitis C virus as an indication of LT by the year 2020. Besides age and risk factors, NASH patients had an excellent outcome (5-y survival of 80% to 85%) after liver transplantation.21 Survival after resection was also comparable with viral hepatitis patients (P=0.391), but patients with NAFLD showed better disease-free survival on univariate (P=0.048) and multivariate (P=0.020) analyses.9
NAFLD is recognized as a risk factor for the development of HCC. Most cases are believed to develop in a background of cirrhosis. However, an increasing number of NAFLD-related HCC arising from a noncirrhotic liver has been reported.10 In the present study, 4 of 42 (10%) patients who developed HCC in the context of NAFLD or cryptogenic liver disease had a noncirrhotic liver. In all the cases, HCC diagnosis was confirmed by liver histology. All patients in this subgroup were treated with surgical resection even with tumors >50 mm (50%) or >1 nodule (50%). In this context, a more aggressive HCC therapy was possible because of absence of liver dysfunction and portal hypertension. All patients were alive after 2 years of follow-up. However, in the literature, the BCLC guideline is recommended for cirrhotic patients. In noncirrhotic patients, a more aggressive oncology treatment should be performed.8
The main limitation of this study is the small sample size and the short period of follow-up. A better overall survival and progression-free survival of patients who were treated aggressively than those in the same stage that followed the BCLC algorithm could not be performed due to these limitations.
CONCLUSIONS
The BCLC system is applicable in most cases of NAFLD-related HCC cases. Deviation from BCLC-based therapeutic recommendations is found more frequently in BCLC C stage patients. In noncirrhotic patients, a more aggressive oncology treatment should be performed, preferably with liver resection.
ACKNOWLEDGMENTS
The authors thank Alves de Queiroz Family Fund for Research for financial support and Laboratory of Epidemiology and Statistics of Department of Gastroenterology, School of Medicine, University of São Paulo, SP, Brazil.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. [DOI] [PubMed] [Google Scholar]
- 2.Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47SupplS2–S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27–S34. [DOI] [PubMed] [Google Scholar]
- 4.McCullough AJ. The clinical features, diagnosis and natural history of nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8:521–533viii. [DOI] [PubMed] [Google Scholar]
- 5.Marrero JA, Fontana RJ, Barrat A, et al. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology. 2005;41:707–716. [DOI] [PubMed] [Google Scholar]
- 6.Cillo U, Vitale A, Grigoletto F, et al. Prospective validation of the Barcelona Clinic Liver Cancer staging system. J Hepatol. 2006;44:723–731. [DOI] [PubMed] [Google Scholar]
- 7.Kim BK, Kim SU, Park JY, et al. Applicability of BCLC stage for prognostic stratification in comparison with other staging systems: single centre experience from long-term clinical outcomes of 1717 treatment-naive patients with hepatocellular carcinoma. Liver Int. 2012;32:1120–1127. [DOI] [PubMed] [Google Scholar]
- 8.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wakai T, Shirai Y, Sakata J, et al. Surgical outcomes for hepatocellular carcinoma in nonalcoholic fatty liver disease. J Gastrointest Surg. 2011;15:1450–1458. [DOI] [PubMed] [Google Scholar]
- 10.Kawada N, Imanaka K, Kawaguchi T, et al. Hepatocellular carcinoma arising from non-cirrhotic nonalcoholic steatohepatitis. J Gastroenterol. 2009;44:1190–1194. [DOI] [PubMed] [Google Scholar]
- 11.Duan XY, Qiao L, Fan JG. Clinical features of nonalcoholic fatty liver disease-associated hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2012;11:18–27. [DOI] [PubMed] [Google Scholar]
- 12.Reeves H, Villa E, Bellentani S, et al. The emerging impact of hepatocellular carcinoma arising on a background of NAFLD. J Hepatol. 2012;56:S3. [Google Scholar]
- 13.Villanueva A, Hernandez-Gea V, Llovet JM. Medical therapies for hepatocellular carcinoma: a critical view of the evidence. Nat Rev Gastroenterol Hepatol. 2013;10:34–42. [DOI] [PubMed] [Google Scholar]
- 14.Ferenci P, Fried M, Labrecque D, et al. World Gastroenterology Organisation Guideline. Hepatocellular carcinoma (HCC): a global perspective. J Gastroint Liver Dis. 2010;19:311–317. [PubMed] [Google Scholar]
- 15.Bruix J, Castells A, Bosch J, et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology. 1996;111:1018–1022. [DOI] [PubMed] [Google Scholar]
- 16.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. [DOI] [PubMed] [Google Scholar]
- 17.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. [DOI] [PubMed] [Google Scholar]
- 18.Yasui K, Hashimoto E, Tokushige K, et al. Clinical and pathological progression of non-alcoholic steatohepatitis to hepatocellular carcinoma. Hepatol Res. 2012;42:767–773. [DOI] [PubMed] [Google Scholar]
- 19.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–1832. [DOI] [PubMed] [Google Scholar]
- 20.Chen CH, Hu FC, Huang GT, et al. Applicability of staging systems for patients with hepatocellular carcinoma is dependent on treatment method—analysis of 2010 Taiwanese patients. Eur J Cancer. 2009;45:1630–1639. [DOI] [PubMed] [Google Scholar]
- 21.Singal AK, Guturu P, Hmoud B, et al. Evolving frequency and outcomes of liver transplantation based on etiology of liver disease. Transplantation. 2013;95:755–760. [DOI] [PubMed] [Google Scholar]


