Abstract
Until recently, adenomyosis has been associated with multiparity, not impaired fertility. Currently, adenomyosis is diagnosed with increasing frequency in infertile patients since women delay their first pregnancy until their late 30s or early 40s. Although an association between adenomyosis and infertility has not been fully established, based on the available information, recent studies suggested that adenomyosis has a negative impact on female fertility. Several uncontrolled studies with limited data also suggested that treatment of adenomyosis may improve fertility. This article discusses (i) the hypothesis and epidemiology of adenomyosis, (ii) diagnostic techniques, (iii) clinical evidence of correlation between adenomyosis and infertility, (iv) proposed mechanism of infertility in women with adenomyosis, (v) different treatment strategies and reproductive outcomes, and (vi) assisted reproductive technology outcome in women with adenomyosis.
Target Audience
Obstetricians and gynecologists, family physicians.
Learning Objectives
After completing this activity, the learner should be better able to: Recall the hypothesis and epidemiology of adenomyosis; Evaluate the important findings on improved imaging techniques to diagnose adenomyosis; Understand that the presence of adenomyosis may impair the reproductive outcomes in women with adenomyosis; Explain the proposed mechanism of infertility in women with adenomyosis; Give the most appropriate treatment for better reproductive outcomes in women with adenomyosis; and Advise patients that surgery could be effective in women with adenomyosis with a history of IVF failure although latter finding could be partly attributed to the higher rate of early miscarriage.
Chief Editor's Note: This article is part of a series of continuing education activities in this Journal through which a total of 36 AMA PRA Category 1 Credits™ can be earned in 2016. Instructions for how CME credits can be earned appear on the last page of the Table of Contents.
Adenomyosis is a benign uterine disorder characterized by the presence of heterotopic endometrial glands and stroma in the myometrium and reactive fibrosis of the surrounding smooth muscles cells of the myometrium. For the past 80 years, a number of theories have described how adenomyosis develops. Currently, the most widespread hypothesis is that adenomyosis originates from the invagination of the basalis of the endometrium into the myometrium. According to a second theory, this basalis invagination would proceed along the intramyometrial lymphatic system. A third theory suggests that a metaplastic process initiating from ectopic intramyometrial endometrial tissue is produced de novo.1–3
Reports show that approximately 20% of cases of adenomyosis involve women younger than 40 years, and 80% are 40 to 50 years old. The most severe symptoms are associated with the older group. Adenomyosis is completely asymptomatic in approximately one third of cases. The most frequent symptoms in the remaining two thirds are menorrhagia (50%), dysmenorrhea (30%), and metrorrhagia (20%). Dyspareunia may also be a complaint.4,5
From the epidemiologic data, a large number of births, spontaneous and induced abortions, and endometrial hyperplasia are related to increased risk of adenomyosis. Other risk factors associated with adenomyosis are endometriosis, smoking, and surgical trauma, such as cesarean section or curettage.6 Since adenomyosis has been reported in 60% of postmenopausal women taking tamoxifen therapy for a long time, it seems to reactivate lesions of preexisting adenomyosis,7 implying that this condition is estrogen dependent.
DIAGNOSIS OF ADENOMYOSIS
Until recently, adenomyosis was thought to be found only in parous women. With few physical findings, diagnosis of adenomyosis relied on surgical resection and pathological examination. With improved imaging techniques, however, it is frequently encountered in infertile patients. Hysterectomy has been advised for women with severe symptoms from adenomyosis, although alternative conservative treatment can preserve the uterus. Therefore, the role of preoperative diagnostic tools is important to avoid unnecessary surgery. In patients with uterine masses and infertility, adenomyosis needs to be excluded before other treatment options are given.8
Hysterosalpingography was the first imaging tool used to diagnose adenomyosis, but it is no longer used to evaluate patients because of its low overall accuracy.9
Uterine enlargement without any features of fibroids and asymmetric thickening of anterior and posterior myometrial walls are signs of adenomyosis that transabdominal ultrasonography (TAU) can reveal.10,11 In a retrospective review, Siedler et al.10 examined 80 patients using TAU and diagnosed adenomyosis with a sensitivity of 63%, a specificity of 97%, and a positive predictive value of 71%. However, because it is not possible to get sufficient image resolution to visualize the myometrium, TAU cannot reliably diagnose adenomyosis or differentiate it from leiomyoma.10,11
In clinically suspected adenomyosis cases, transvaginal sonography (TVS) should be considered as the primary diagnostic tool. Fundamental TVS signs for adenomyosis are heterogeneous and hypoechogenic poorly described areas in the myometrium, those areas with or without anechoic lacunae or cysts of varying size, linear striation radiating out from the endometrium into the myometrium, poor definition of the junctional zone (JZ), and pseudo-widening of the endometrium (enlargement of uterus with asymmetric thickening of the anterior or posterior walls). Adenomyosis is most often diagnosed in the presence of 3 or more sonographic criteria.8,12
On 3D TVS, features linked to adenomyosis were JZmax 8 mm or greater, myometrial asymmetry, and hypoechoic striations.13 When at least 2 of the described ultrasound features were present, the accuracy was 90% (sensitivity, 92%; specificity, 83%; positive predictive value, 99%; and negative predictive value, 71%).
According to Dueholm et al. (2001),12 magnetic resonance imaging (MRI) and TVS were equally good at identifying patients with adenomyosis, but MRI was superior to TVS to exclude the diagnosis of adenomyosis, with equal sensitivity but a higher specificity (sensitivity: MRI, 0.70 (0.46–0.87); and TVS, 0.68 (0.44–0.86) (P = 0.66); specificity: MRI, 0.86 (0.76–0.93); and TVS 0.65 (0.50–0.77) (P = 0.03). The combination of TVS and MRI had the highest sensitivity, but, surprisingly, it also had the lowest specificity. In addition, measuring the difference in junctional zone thickness may optimize the MRI diagnosis. Gordts et al.14 recently suggested the following adenomyosis classification: simple JZ hyperplasia (zone thickness >8 mm but <12 mm on T2-weighed images, in women aged 35 years or younger); partial or diffuse adenomyosis (thickness >12 mm; high signal intensity myometrial foci; involvement of the outer myometrium <1/3, <2/3, and >2/3), adenomyoma (myometrial mass with indistinct margins with primarily low signal intensity on all MRI sequences). However, this classification still remains to be proved. The consensus today is that adenomyosis can be strongly considered when JZ thickness is greater than 12 mm, although there is no definable JZ on MRI in approximately 20% of premenopausal women.15 A diagnosis can also be suspected when the thickness of the JZ is between 8 and 12 mm; if other signs, such as a relative thickening of JZ in a localized area, poor definition of the JZ borders, or high signal foci on T2- or T1-weighed sequences, are present.9
Uterine Junctional Zone (JZ)
In 1983, Hricak et al.16 first described the functional uterine zone, which is the junction between the endometrium and the inner myometrium. Today, in healthy women of reproductive age, through T2-weighted images, 3 distinct layers were noted: (i) a high signal intensity corresponding to the endometrial stripe, (ii) an inner low signal intensity that is adjacent to the basal endometrium, the JZ or subendometrial layer, and (iii) an outer medium signal intensity subserosal zone, or outer myometrium.17 However, diffuse thickening of the JZ should be carefully distinguished from normal physiological change, since the thickness of JZ varies considerably during the menstrual cycle.18 de Souza et al.19 reported an incidence of 54% myometrial JZ hyperplasia (a clear sign of adenomyosis) in subfertile patients complaining of menorrhagia or dysmenorrhea.
Association With Infertility
Diagnosing adenomyosis was difficult until recently, and in the past, it was associated with multiparous women not with infertility. Indeed, women often delay their first pregnancy, and adenomyosis is typically observed in their late 30s and 40s. When nonsurgical diagnosis, such as TVS and MRI, became possible, the role of adenomyosis in infertility and early miscarriage was recognized.20
CLINICAL EVIDENCE OF CORRELATION BETWEEN ADENOMYOSIS AND INFERTILITY
Dysfunctional Uterine JZ and Fertility Outcome
Chiang et al.21 suggested the link between spontaneous abortion rate and uterine JZ dysfunction in infertile patients undergoing in vitro fertilization (IVF) and found that the spontaneous abortion rate was higher in women with a diffusely enlarged uterus on ultrasound imaging without distinct uterine masses compared with those with a normal uterus. However, their pregnancy rates were not statistically significant. Piver et al.22 also proposed that MRI evaluation of JZ thickness is the best negative predictive factor of implantation failure, and an increase in JZ diameter is inversely correlated to implantation rate. Implantation failure was found to be high when the average junctional zone was greater than 7 mm.23
Achieving Pregnancy After Medical or Surgical Therapy
Infertile women reportedly achieve pregnancy after being treated for adenomyosis. Since 1993, published case reports or small series report adenomyosis treated with gonadotrophin-releasing hormone (GnRH) analog alone, conservative surgery, or combined therapy (Tables 1–3).24–42 A live birth rate after treatment with GnRH agonist (GnRH-a) for 5 months was first reported by Silva et al.25 In a case study by Nelson and Corson,24 the patient with histologically diagnosed adenomyosis who underwent a long-term course of GnRH-a conceived shortly after cessation of treatment. It was also reported that 2 cases of adenomyosis were conceived within 6 months after a short course of GnRH therapy with buserelin.26 A small Japanese study, in which 3 of 4 infertile patients successfully conceived after using a danazol IUD, is also additional evidence to link adenomyosis and infertility.42
TABLE 1.
Successful Pregnancies After GnRH-a in Women With Adenomyosis
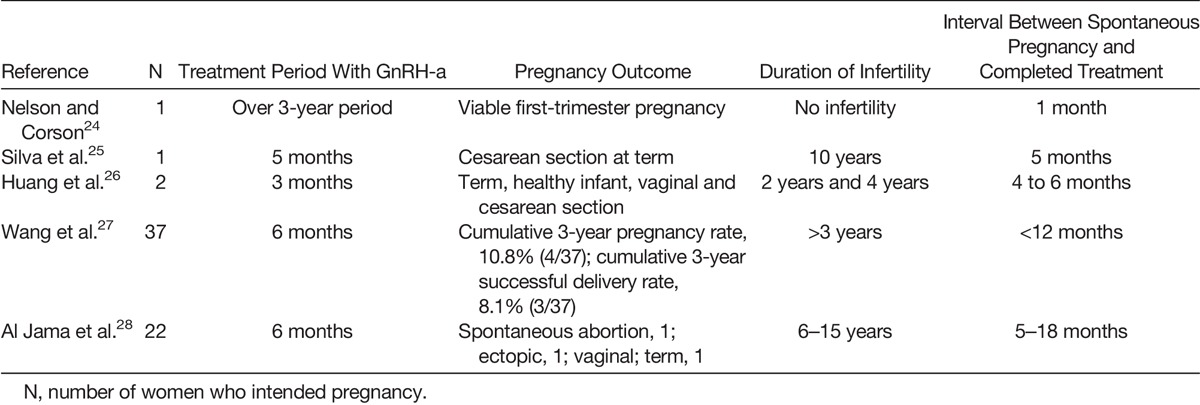
TABLE 3.
Successful Pregnancies After Combined Therapy in Women With Adenomyosis

Furthermore, Fujishita et al.29 described the modified reduction surgical technique for adenomyosis; and in this study, one patient conceived spontaneously 4 months after operation by H-incision technique. One prospective study used a triple-flap method for reconstructing the uterine wall for 104 patients with severe adenomyosis. Of these, 4 of 26 women who wished to conceive became pregnant after conservative surgery.34 Uterus-sparing surgery for adenomyosis-associated subfertile women were also demonstrated by Kishi et al.35 In the group aged younger than 39 years, 60.8% of women with a history of IVF failure achieved pregnancy after surgery, although there was no clear benefit of surgery on fertility outcomes for patients older than 40 years. Wang et al.30 also suggested that laparoscopic cytoreductive surgery might be suitable for women with localized adenomyosis who failed the usual infertility treatments and assisted reproductive technology (ART). They reported one spontaneous pregnancy that occurred 21 months after surgery.
Conservative surgery or combination treatment in subfertile women with adenomyosis also had significant benefits for not only controlling symptoms but also for increasing the pregnancy rate compared with GnRH-a alone.27,28 The cumulative 3-year clinical pregnancy rate and final successful delivery rate were higher in adenomyotic women who underwent conservative surgery with or without GnRH-a compared with those who received GnRH-a alone for 6 months.27
In one large prospective study, 55 of 165 patients with adenomyosis became pregnant after surgery followed by 6-course GnRH treatment or surgery alone, with a clinical pregnancy rate of 77.5%, and 49 women (69.0%) had a successful delivery by the end of the 2-year follow-up period.33 The combination of microsurgical cytoreduction and GnRH-a treatment could be appropriate for patients who failed GnRH-a alone or would not tolerate long-term GnRH-a treatment for presumed severe adenomyosis.40,41
Effect of Adenomyosis on Reproductive Outcome After Endometriosis Surgery
The impact of adenomyosis on pregnancy rates following surgery for both rectovaginal and colorectal endometriosis was reviewed in five articles from 2005 to 2010.32,43–46 Diagnosis of uterine adenomyosis based on TVS,44 MRI,32 and both TVS and MRI.43,45,46 Among the five selected studies, the criteria for the presence of adenomyosis were described in only one.32
Although fertility was not their primary study end point, in Landi et al.32 in 2008, a significantly higher pregnancy rate was recorded in women with endometriosis, but without adenomyosis, compared with those with concurrent endometriosis and adenomyosis after laparoscopic excision of deep, infiltrated endometriosis (Table 2). One prospective study evaluated the cumulative pregnancy rate after intracytoplasmic sperm injection (ICSI)/IVF in patients with colorectal endometriosis. Cumulative pregnancy rates in patients with associated adenomyosis was 19%, and in those who had endometriosis only, it was 82%, revealing that adenomyosis was associated with decreased cumulative pregnancy rate (Fig. 1).47
TABLE 2.
Successful Pregnancies After Conservative Surgery in Women With Adenomyosis
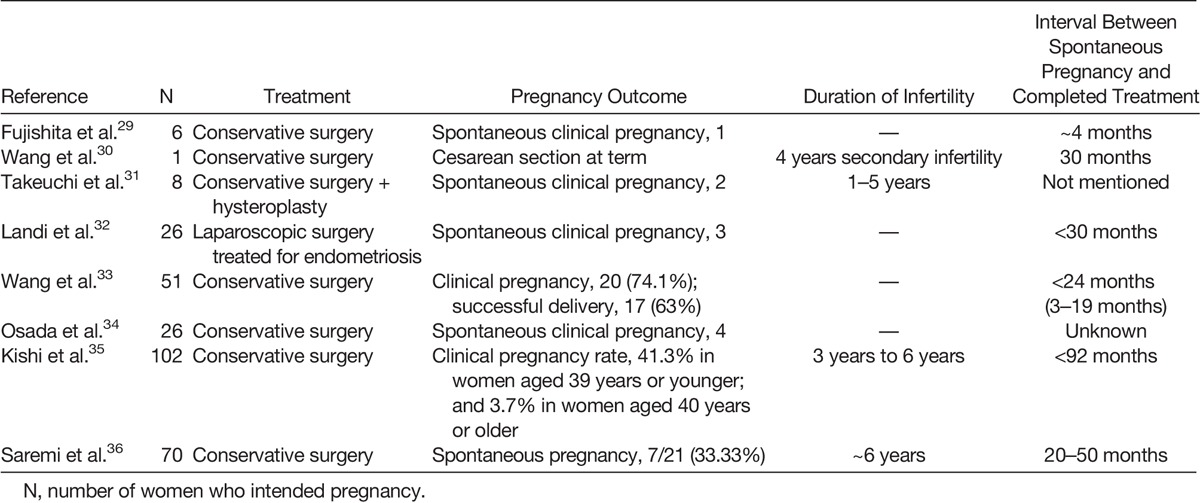
FIG. 1.
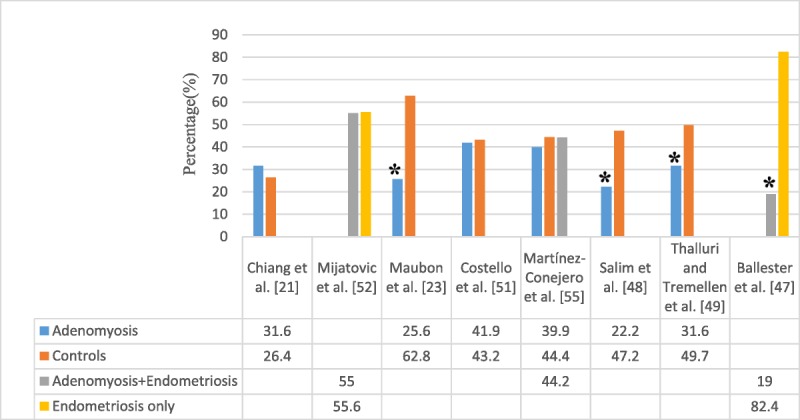
Clinical pregnancies in women with adenomyosis.
Presurgical hormone treatment (GnRH agonists for 3 months) was used in 2 studies.45,46 Surgery was performed in 3 studies with laparoscopy32,44,45 and with laparotomy or laparoscopy in 2 studies.44,46
In 3 of 5 studies,43–45 no pregnancy occurred in women older than 35 years, and in the study by Darai et al.,46 only 1 of 24 women who conceived was older than 35 years. Moreover, the percentage of women with adenomyosis seeking pregnancy was 4 of 18 in Darai et al.,45 8 of 17 in Ferrero et al.,43 11 of 20 in Darai et al.,46 and 10 of 20 in Stepniewska et al.44
ART (IVF/ICSI) Outcome in Patients With Adenomyosis
Many studies reported reproductive outcome after ART in women with adenomyosis.21,48,49 Most concluded that adenomyosis causes infertility, but more prospective studies with a large population should be performed to further evaluate this causal interaction and unravel the mechanisms responsible for this negative effect. One of the first studies that connect the thickness of JZ in women with adenomyosis with embryo implantation failure came from Maubon et al.23 Two recent large population studies confirmed lower pregnancy rates in women with adenomyosis who underwent IVF.48,49 There are significantly lower clinical pregnancy rates in women with adenomyosis diagnosed by ultrasound (23.6%) compared with the nonadenomyosis group (44.6%) after stimulation with a GnRH antagonist protocol for IVF. Possibly, the age factor in older women with adenomyosis may be related to a lower pregnancy rate. This difference was still significant (P = 0.031) even after normalization of groups for age using regression analysis.49 In a study by Tremellen et al.,50 4 patients with adenomyosis who previously had multiple unsuccessful IVF cycles promptly resulted in successful pregnancy with ART after prolonged down-regulation with GnRH-a.
However, Costello et al.51 investigated the effect of TVS-diagnosed adenomyosis on subsequent IVF/ICSI outcome. After a single cycle of IVF/ICSI, reproductive outcome was compared in women with and women without adenomyosis excluding patients with severe endometriosis. There was no difference in live birth rate per patient (cycle) between the 2 groups. Mijatovic et al.52 also showed reproductive outcome in infertile patients with surgically proven endometriosis who were pretreated with more than 3 months of long-term pituitary down-regulation (GnRH-a) before IVF/ICSI. No significant differences in IVF/ICSI outcome were observed between women with and women without adenomyosis. However, in this study, the authors point out that endometriosis was an important confounder, and 90.4% of the patients with endometriosis were surgically staged with moderate to severe endometriosis (revised American Society of Reproductive Medicine stages III–IV; Figs. 1, 2). Having a study population of asymptomatic women with adenomyosis undergoing IVF attempts, Benaglia et al.53 failed to show a detrimental effect of the disease in the pregnancy rate of these women. The authors proposed that GnRH-a pretreatment might have a therapeutic effect on adenomyosis.
FIG. 2.
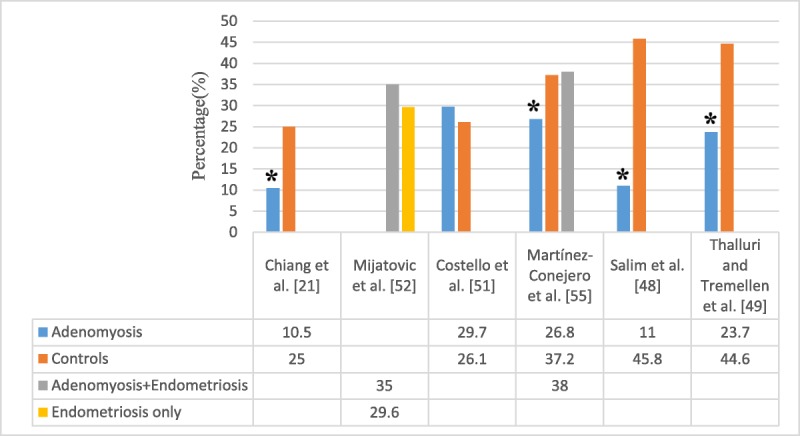
Ongoing pregnancies in women with adenomyosis.
Association With Early Miscarriage
Two recent prospective studies concluded that adenomyosis reduces implantation and number of embryos transferred, clinical pregnancy rate, and ongoing pregnancy rate in women undergoing IVF. The first trimester miscarriage rate was also higher in women with adenomyosis compared with the control group.48,54 Martínez-Conejero et al.55 examined the endometrium of women with adenomyosis undergoing oocyte donation and found a similar endometrial gene expression pattern in both the adenomyosis and the control group. The implantation and clinical pregnancy rates were not different in all groups, but the miscarriage rate was significantly higher in the adenomyosis group (13.1%) than adenomyosis + endometriosis group (6.1%) and the controls (7.2%). The term pregnancy rate was also lower in the adenomyosis group (26.8%) than in the adenomyosis + endometriosis (38%) and the control group (37.1%), showing that implantation is not affected by adenomyosis. However, the higher rate of miscarriages associated with this condition led to lower term pregnancy rates, indicating a negative effect on the final outcome of oocyte donation.
Lifelong Primary Infertility in Baboons
After assessing their necropsy records, 37 baboons diagnosed with adenomyosis and 38 baboons with normal uteri histology were compared in one case-control study. The authors analyzed the association between adenomyosis, primary infertility, and the presence of coexisting endometriosis. They found that adenomyosis is strongly associated with lifelong infertility (P < 0.001) and was maintained even after excluding coexisting endometriosis cases (N = 17). The weakness in that study lies in the selection of the negative controls. Unless uteri are exhaustively sectioned, adenomyosis cannot be excluded. However, this study showed that adenomyosis is strongly associated with the presence of endometriosis and lifelong infertility.56
PROPOSED MECHANISM OF INFERTILITY IN PATIENTS WITH ADENOMYOSIS
Abnormal Uterotubal Transport
Intrauterine Abnormalities
Anatomical distortion of the uterine cavity may be one important factor leading to infertility, although the mechanism by which uterine adenomyoma has a detrimental effect on reproductive function remains unknown. Adenomyoma that distorts the uterine cavity may obstruct the tubal ostia and interfere with sperm migration and embryo transport. Several studies have demonstrated that submucosal and intramural fibroids in the presence of endometrial cavity distortion are associated with reduced implantation and pregnancy rates.57,58
Disturbed Uterine Peristalsis and Sperm Transport
Using transabdominal ultrasound, the presence of distinct contraction waves in the myometrium can be seen. This peristaltic activity originates solely from the JZ, whereas the outer myometrium remains inactive.59 Directed sperm transport toward the peritoneal opening of the tubes on the side of dominant follicle by uterine peristalsis is fundamental to the early reproductive process, and it depends on the architecture of the myometrial wall with circular muscular fibers.60 In women with adenomyosis, normal architecture of the “archimyometrium” (JZ myometrium) was destroyed owing to invagination of the endometrial glands and stroma. This gives rise to the development of hyperplastic muscular tissue that causes dysfunctional uterine hyperperistalsis with increased intrauterine pressure.61 It seems reasonable to suppose that those changes may affect fertility in patients with adenomyosis. Increased uterine JZ activity just before embryo transfer in IVF is also associated with a reduced pregnancy rate and increased frequency of ectopic pregnancy.62
Destruction of Normal Myometrial Architecture and Function
Mehasseb et al.63 studied the ultrastructural features of myometrium in the presence or absence of adenomyosis. Myocytes of adenomyosis uteri are ultrastructurally different from those of normal uteri. In the presence of adenomyosis, JZ showed cellular and nuclear hypertrophy, abnormal nuclear and mitochondrial shape, abundant myelin bodies, and other abnormalities. This suggests that those ultrastructural abnormalities may cause a disturbance in normal calcium cycling in affected myocytes, with subsequent loss of normal rhythmic contraction, eventually affecting uterotubal transport.
Altered Endometrial Function and Receptivity
Aberrant Endometrial Metabolism
Altered Endometrial Steroid Metabolism
Kitawaki et al.64 demonstrated the expression of aromatase cytochrome P450 (P450arom) protein and mRNA only in adenomyotic tissues and the eutopic endometrium of patients with adenomyosis, but not in the normal endometrium of women without adenomyosis. P450arom is an enzyme that catalyzes the conversion of androgens to estrogens. Takahashi et al.65 found menstrual blood estradiol levels were highest in women with adenomyosis, whereas they were within normal levels in peripheral blood, suggesting that local estrogen production may increase estrogen concentration in the menstrual blood of women with adenomyosis. Lessey et al.66 also showed that overexpression of P450 aromatase increases local estrogen production within the endometrium. Clinical pregnancy rates were statistically lower in women with high endometrial P450arom mRNA levels,67 and they suggest that P450arom mRNA expression can identify women at increased risk of IVF failure. Moreover, treatment with GnRH agonist or danazol decreased expression of P450arom in the eutopic endometrium of women with adenomyosis.68
Abnormal Inflammatory Response
Macrophages have the capacity to produce not only proinflammatory cytokines, such as TNF-α and IL-1, but also reactive oxygen species that can be toxic to embryos.69,70 Tremellan et al.70 reported women with severe adenomyosis who, after a failed implantation, have significantly greater stromal macrophage density than the nonadenomyosis controls. Additionally, IL-6 mRNA expression was increased in macrophage-cocultured endometriotic stromal cells in adenomyosis.71 Wang et al.72 have shown that IL-10 staining intensity in women with adenomyosis was higher in epithelial cells of both eutopic and ectopic endometrium than controls, suggesting that an abnormal inflammatory response may impair fertility (Fig. 3).
FIG. 3.
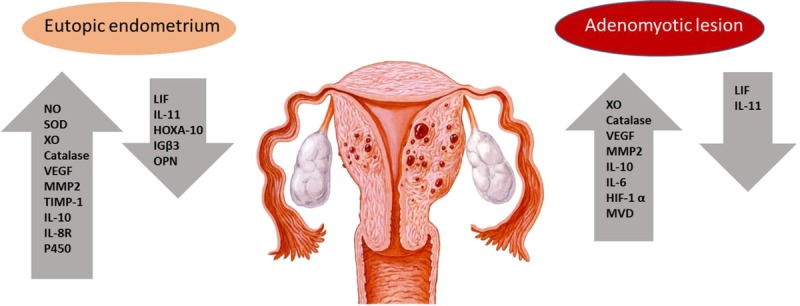
Implantation factors showing altered concentration in adenomyosis-associated infertility.
Altered Expression of Estrogen and Progesterone Receptors
Since IL-6 can activate the estrogen receptor in breast cancer cells,73 overexpressed IL-6 could lead to an increased estrogen receptor expression in adenomyosis women. Progesterone has antiproliferative activity through its receptors. Within the adenomyotic tissue, there was reduction in the expression of progesterone receptors (A and B) in all layers,74,75 resulting in up-regulation of ER-α, since down-regulation of estrogen receptor-α is one of the primary functions of progesterone. Overexpression of ER-α reduces β-3 integrin secretion and alters uterine receptivity.76
Altered Uterine Oxidative Stress Environment
A low oxygen environment in the uterus needs to be maintained for implantation of fertilized eggs because an excessive free radical environment may damage fertilized eggs and interfere with embryo development.77 In a normal woman, concentrations of nitric oxide synthase, xanthine oxidase, superoxide dismutase are low during the proliferative phase and increase during the early and midsecretory phases.78,79 In women with adenomyosis, levels do not fluctuate during the menstrual cycle and are overexpressed.80 Abnormal levels of intrauterine free radicals seem to cause infertility in women with adenomyosis.
Some studies with animal models demonstrated that excess or deficient levels of free radicals mediated by inflammatory factors in reproductive tissue may inhibit both embryo development in vitro and implantation in vivo, resulting in a low pregnancy rate (Fig. 3).81–83
Impaired Implantation
Lack of Expression of Adhesion Molecules
Several cell adhesion molecules (integrin, selectin, and cadherin) expressed by the endometrium are necessary for embryo and endometrium interaction. Integrins are the best studied markers of endometrial receptivity.84 Abnormal endometrial expression of integrin subtype α-5 and β-3 takes place in patients with IVF failure despite good embryo quality.85 Osteopontin (OPN) is a small integrin-binding ligand, N-linked glycoprotein in the endometrium. It binds to integrin 3, giving rise to speculation that it may mediate trophoblast endometrial interaction during implantation.86 Integrin β-3 and OPN levels were significantly lower in patients with adenomyosis than in controls. Dysregulation of both integrin β-3 and OPN mRNA and protein in the endometrium during the implantation window suggests that adenomyosis is associated with impaired implantation.87
Reduced Expression of Implantation Markers
Another factor associated with endometrial receptivity, leukemia inhibitory factor (LIF),88 is present during the implantation window. Leukemia inhibitory factor is an essential cytokine for successful egg implantation during human reproduction.89 In addition, adenomyotic endometrium shows abnormalities in the production of LIF, which may contribute to altering uterine receptivity.90 Leukemia inhibitory factor concentrations in uterine flushing are lower in women with infertility than in controls. Additionally, LIF expression is lower in the endometrium of women with adenomyosis during the midsecretory phase.89 Leukemia inhibitory factor and IL-6 expression is controlled in endometrial cells by nuclear factor kappa B (NF-kB) activation. Nuclear factor kappa B is a transcription factor and critical regulator of innate immune response and inflammation. Ponce et al.91 reported that NF-kB binding, phosphorylated NF-kB, and IL-6 expression were down-regulated in the late secretory phase in the eutopic endometrium of women with endometriosis. Nuclear factor kappa B activities in the eutopic endometrium of patients with adenomyosis are an intriguing target for future investigation.
Altered Function of the Gene for Embryonic Development
HOXA 10 gene, essential for embryonic uterine development and proper adult endometrial growth during the menstrual cycle, may be involved in creating an impairment of implantation in women with adenomyosis.92 Its expression is necessary for endometrial receptivity, and it is significantly lower in the endometrial stroma of women with adenomyosis compared with fertile controls.93
PHARMACOLOGICAL AND SURGICAL TREATMENT OF ADENOMYOSIS
Hormonal Treatments
GnRH Agonists
A lack of randomized, controlled trials exploring the impact of GnRH-a treatment on fertility hinders our understanding of adenomyosis. One case study reported that treatment of severe adenomyosis with GnRH-a for 16 weeks resulted in the live birth of a healthy male infant.39
Progestogens
Women with adenomyosis are characterized by a lower expression of progesterone receptors (A and B) in endometrial lesions, but also in the outer and inner layers of myometrium. The treatment of adenomyosis with progesterone may be restricted owing to the abnormal expression of progesterone receptors.94
Dienogest
Dienogest (progestin) has been used to treat adenomyosis pharmacologically. Dienogest directly inhibited cellular proliferation and induced apoptosis in human adenomyotic stromal cells.95 Two nonrandomized studies on a small number of patients have been published, but neither refers to the patients' fertility. The first study compared 2 groups of approximately 20 subjects treated with danazol and dienogest for adenomyosis.96 That study did not clearly describe the effectiveness of the therapeutic protocols. Adenomyosis patients treated with dienogest are at higher risk of discontinuation owing to uterine bleeding. The second study presented a correlation of 3 factors: age younger than 38 years, lower hemoglobin levels before the starting point of the therapeutic protocol, and estradiol levels after 3 months of dienogest therapy.97
Levonorgestrel Intrauterine System
Levonorgestrel intrauterine system (LNG-IUS) is approved for treating women with adenomyosis who have completed their childbearing. Levonorgestrel intrauterine system treatment is accompanied by decreased pain and heavy uterine bleeding, which could be explained by the following mechanisms: (i) a progestogenic influence on adenomyosis foci; (ii) atrophy of the eutopic endometrium; and (iii) controlling of endometrial factors that changed during adenomyosis. Choi et al.98 described decreased expression of growth factor and the related receptors in women with heavy bleeding and adenomyosis after LNG-IUS treatment. In another randomized study, Maia et al.99 described a positive effect of LNG-IUS in approximately 100 women with adenomyosis experiencing heavy menstrual bleeding.
Exploring Surgery Treatments and Pregnancy Rates
Conservative Surgery Alone
The results of conservative surgery, which consists of laparoscopy or laparotomy, are based on 3 studies in women with a diagnosis with adenomyosis. Two studies reported birth rate,31,100 and one reported pregnancy rate.38 The surgical techniques presented in all the studies consisted of excision of the adenomyoma and hysteroplasty using laparoscopy or laparotomy. The overall average birth rate reached 36.2% (21 of 58) in women who had this surgery.
Comparison of 2 Surgical Techniques
A retrospective study of 104 patients undergoing conservative surgery compared the classical adenomyomectomy with a new technique, the H-incision surgery. The classical technique was performed in 5 women with adenomyosis who were selected retrospectively among 104 patients. The newer technique was used in 6 of 83 patients who desired to preserve fertility. The classical technique involves incision of the uterine wall and a stepwise resection of adenomyomatic tissue. The newer technique consists of an H-shape incision and excision of the adenomyomatic tissue. In this study, the newer technique resulted in one spontaneous pregnancy 4 months after operation compared with no pregnancy in women undergoing the classical technique. (Classical technique pregnancy rate, 0.14 [95% confidence interval].) Time between surgery and pregnancy was 4 to 6 months followed by a live birth, with one continuing pregnancy at the time the study finished.29
Adenomyomectomy
When women experience the severe symptoms of advanced adenomyosis, hysterectomy has been advised. However, the more conservative adenomyomectomy preserves the uterus. One study reported that from a pool of 103 patients, 55.34% presented with infertility, and of those, 16.50% had IVF failure, 8.74% had recurrent miscarriages, and 19.42% had abnormal uterine bleeding. Of the 103 patients, 70 attempted pregnancy, 21 naturally through intercourse, and 49 through IVF. Pregnancy outcomes were 30% pregnancy, with 23% live births. The symptoms of dysmenorrhea/hypermenorrhea lessened after surgery. Only one patient had a recurrence of adenomyosis.36
CONCLUSIONS
In summary, adenomyosis is a common gynecological disorder with unclear etiology. Several studies have demonstrated that the presence of adenomyosis may impair the fertility by affecting the uterotubal transport and altering endometrial function and receptivity. Some indirect proofs have shown that women with adenomyosis have poor reproductive outcomes compared with those without adenomyosis. Based on limited available evidence, it has been reported that infertile women who experience adenomyosis achieved pregnancy after being treated with different strategies, indirectly revealing poor reproductive outcomes in women with adenomyosis. Furthermore, surgery could be effective in women with adenomyosis with a history of IVF failure, although latter finding could be partly attributed to the higher rate of early miscarriage.
However, in the clinical situation, it is still difficult to determine whether adenomyosis is the cause of the infertility or not because, as previous studies have made us aware, unknown or as yet undiagnosed cases of endometriosis may be present in both cases and controls. Additionally, studies on treatment are limited to case series and a retrospective data without control groups. Better studies are needed to determine the molecular mechanism of implantation failure in women with adenomyosis and the impact of adenomyosis on infertile women with or without endometriosis.
Footnotes
All authors and staff in a position to control the content of this CME activity and their spouses/life partners (if any) have disclosed that they have no financial relationships with, or financial interests in, any commercial organizations pertaining to this educational activity.
REFERENCEs
- 1.Moll R, Levy R, Czernobilsky B, et al. Cytokeratins of normal epithelia and some neoplasms of the female genital tract. Lab Invest. 1983;49:599–610. [PubMed] [Google Scholar]
- 2.Sahin AA, Silva EG, Landon G, et al. Endometrial tissue in myometrial vessels not associated with menstruation. Int J Gynecol Pathol. 1989;8:139–146. [DOI] [PubMed] [Google Scholar]
- 3.Ferenczy A. Pathophysiology of adenomyosis. Hum Reprod Update. 1998;4:312–322. [DOI] [PubMed] [Google Scholar]
- 4.Benson RC, Sneeden VD. Adenomyosis: a reappraisal of symptomatology. Am J Obstet GynecoI. 1958;76:1044–1057; discussion 1057–61. [DOI] [PubMed] [Google Scholar]
- 5.Nishida M. Relationship between the onset of dysmenorrhea and histologic findings in adenomyosis. Am J Obstet Gynecol. 1991;165:229–231. [DOI] [PubMed] [Google Scholar]
- 6.Vercellini P, Viganò P, Somigliana E, et al. Adenomyosis: epidemiological factors. Best Pract Res Clin Obstet Gynaecol. 2006;20:465–477. [DOI] [PubMed] [Google Scholar]
- 7.Bergeron C, Amant F, Ferenczy A. Pathology and physiopathology of adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2006;20:511–521. [DOI] [PubMed] [Google Scholar]
- 8.Dueholm M. Transvaginal ultrasound for diagnosis of adenomyosis: a review. Best Pract Res Clin Obstet Gynaecol. 2006;20:569–582. [DOI] [PubMed] [Google Scholar]
- 9.Reinhold C, Tafazoli F, Wang L. Imaging features of adenomyosis. Hum Reprod Update. 1998;4:337–349. [DOI] [PubMed] [Google Scholar]
- 10.Siedler D, Laing FC, Jeffrey RB, Jr, et al. Uterine adenomyosis. A difficult sonographic diagnosis. J Ultrasound Med. 1987;6:34–39. [DOI] [PubMed] [Google Scholar]
- 11.Arnold LL, Ascher SM, Schruefer JJ, et al. The nonsurgical diagnosis of adenomyosis. Obstet Gynecol. 1995;86:461–465. [DOI] [PubMed] [Google Scholar]
- 12.Dueholm M, Lundorf E, Hansen ES, et al. Magnetic resonance imaging and transvaginal ultrasonography for the diagnosis of adenomyosis. Fertil Steril. 2001;76:588–594. [DOI] [PubMed] [Google Scholar]
- 13.Luciano DE, Exacoustos C, Albrecht L, et al. Three-dimensional ultrasound in diagnosis of adenomyosis: histologic correlation with ultrasound targeted biopsies of the uterus. J Minim Invasive Gynecol. 2013;20:803–810. [DOI] [PubMed] [Google Scholar]
- 14.Gordts S, Brosens JJ, Fusi L, et al. Uterine adenomyosis: a need for uniform terminology and consensus classification. Reprod Biomed Online. 2008;17:244–248. [DOI] [PubMed] [Google Scholar]
- 15.Novellas S, Chassang M, Delotte J, et al. MRI characteristics of the uterine junctional zone: from normal to the diagnosis of adenomyosis. AJR Am J Roentgenol. 2011;196:1206–1213. [DOI] [PubMed] [Google Scholar]
- 16.Hricak H, Alpers C, Crooks LE, et al. Magnetic resonance imaging of the female pelvis: initial experience. AJR Am J Roentgenol. 1983;141:1119–1128. [DOI] [PubMed] [Google Scholar]
- 17.Tamai K, Koyama T, Umeoka S, et al. Spectrum of MR features in adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2006;20:583–602. [DOI] [PubMed] [Google Scholar]
- 18.Masui T, Katayama M, Kobayashi S, et al. Changes in myometrial and junctional zone thickness and signal intensity: demonstration with kinematic T2-weighted MR imaging. Radiology. 2001;221:75–85. [DOI] [PubMed] [Google Scholar]
- 19.de Souza NM, Brosens JJ, Schwieso JE, et al. The potential value of magnetic resonance imaging in infertility. Clin Radiol. 1995;50:75–79. [DOI] [PubMed] [Google Scholar]
- 20.Devlieger R, D'Hooghe T, Timmerman D. Uterine adenomyosis in the infertility clinic. Hum Reprod Update. 2003;9:139–147. [DOI] [PubMed] [Google Scholar]
- 21.Chiang CH, Chang MY, Shiau CS, et al. Effect of a sonographically diffusely enlarged uterus without distinct uterine masses on the outcome of in vitro fertilization-embryo transfer. J Assist Reprod Genet. 1999;16:369–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piver P. Uterine factors limiting ART coverage. J Gynecol Obstet Biol Reprod (Paris). 2005;34:5S30–5S33. [PubMed] [Google Scholar]
- 23.Maubon A, Faury A, Kapella M, et al. Uterine junctional zone at magnetic resonance imaging: a predictor of in vitro fertilization implantation failure. J Obstet Gynaecol Res. 2010;36:611–618. [DOI] [PubMed] [Google Scholar]
- 24.Nelson JR, Corson SL. Long-term management of adenomyosis with a gonadotropin-releasing hormone agonist. Fertil Steril. 1993;59:441–443. [DOI] [PubMed] [Google Scholar]
- 25.Silva PD, Perkins HE, Schauberger CW. Live birth after treatment of severe adenomyosis with a gonadotropin-releasing hormone agonist. Fertil Steril. 1994;61:171–172. [DOI] [PubMed] [Google Scholar]
- 26.Huang FJ, Kung FT, Chang SY, et al. Effects of short-course buserelin therapy on adenomyosis. A report of two cases. J Reprod Med. 1999;44:741–744. [PubMed] [Google Scholar]
- 27.Wang PH, Fuh JL, Chao HT, et al. Is the surgical approach beneficial to subfertile women with symptomatic extensive adenomyosis? J Obstet Gynaecol Res. 2009;35:495–502. [DOI] [PubMed] [Google Scholar]
- 28.Al Jama FE. Management of Adenomyosis in subfertile women and pregnancy outcome. Oman Med J. 2011;26:178–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujishita A, Masuzaki H, Khan KN, et al. Modified reduction surgery for adenomyosis. A preliminary report of the transverse H incision technique. Gynecol Obstet Invest. 2004;57:132–138. [DOI] [PubMed] [Google Scholar]
- 30.Wang CJ, Yuen LT, Chang SD, et al. Use of laparoscopic cytoreductive surgery to treat infertile women with localized adenomyosis. Fertil Steril. 2006;86:462.e5–462.e8. [DOI] [PubMed] [Google Scholar]
- 31.Takeuchi H, Kitade M, Kikuchi I, et al. Laparoscopic adenomyomectomy and hysteroplasty: a novel method. J Minim Invasive Gynecol. 2006;13:150–154. [DOI] [PubMed] [Google Scholar]
- 32.Landi S, Mereu L, Pontrelli G, et al. The influence of adenomyosis in patients laparoscopically treated for deep endometriosis. J Minim Invasive Gynecol. 2008;15:566–570. [DOI] [PubMed] [Google Scholar]
- 33.Wang PH, Liu WM, Fuh JL, et al. Comparison of surgery alone and combined surgical-medical treatment in the management of symptomatic uterine adenomyoma. Fertil Steril. 2009;92:876–885. [DOI] [PubMed] [Google Scholar]
- 34.Osada H, Silber S, Kakinuma T, et al. Surgical procedure to conserve the uterus for future pregnancy in patients suffering from massive adenomyosis. Reprod Biomed Online. 2011;22:94–99. [DOI] [PubMed] [Google Scholar]
- 35.Kishi Y, Yabuta M, Taniguchi F. Who will benefit from uterus-sparing surgery in adenomyosis-associated subfertility? Fertil Steril. 2014;102:802.e1–807.e1. [DOI] [PubMed] [Google Scholar]
- 36.Saremi A, Bahrami H, Salehian P, et al. Treatment of adenomyomectomy in women with severe uterine adenomyosis using a novel technique. Reprod Biomed Online. 2014;28:753–760. [DOI] [PubMed] [Google Scholar]
- 37.Hirata JD, Moghissi KS, Ginsburg KA. Pregnancy after medical therapy of adenomyosis with a gonadotropin-releasing hormone agonist. Fertil Steril. 1993;59:444–445. [DOI] [PubMed] [Google Scholar]
- 38.Strizhakov AN, Davydov AI. Myometrectomy—a method of choice for the therapy of adenomyosis patients in the reproductive period [In Russian]. Akush Ginekol (Mosk). 1995;5:31–33. [PubMed] [Google Scholar]
- 39.Ozaki T, Takahashi K, Okada M, et al. Live birth after conservative surgery for severe adenomyosis following magnetic resonance imaging and gonadotropin-releasing hormone agonist therapy. Int J Fertil Womens Med. 1999;44:260–264. [PubMed] [Google Scholar]
- 40.Wang PH, Yang TS, Lee WL, et al. Treatment of infertile women with adenomyosis with a conservative microsurgical technique and a gonadotropin-releasing hormone agonist. Fertil Steril. 2000;73:1061–1062. [DOI] [PubMed] [Google Scholar]
- 41.Huang BS, Seow KM, Tsui KH, et al. Fertility outcome of infertile women with adenomyosis treated with the combination of a conservative microsurgical technique and GnRH agonist: long-term follow-up in a series of nine patients. Taiwan J Obstet Gynecol. 2012;51:212–216. [DOI] [PubMed] [Google Scholar]
- 42.Igarashi M, Abe Y, Fukuda M, et al. Novel conservative medical therapy for uterine adenomyosis with a danazol loaded intrauterine device. Fertil Steril. 2000;74:412–413. [DOI] [PubMed] [Google Scholar]
- 43.Ferrero S, Anserini P, Abbamonte LH, et al. Fertility after bowel resection for endometriosis. Fertil Steril. 2009;92:41–46. [DOI] [PubMed] [Google Scholar]
- 44.Stepniewska A, Pomini P, Scioscia M, et al. Fertility and clinical outcome after bowel resection in infertile women with endometriosis. Reprod Biomed Online. 2010;20:602–609. [DOI] [PubMed] [Google Scholar]
- 45.Daraï E, Marpeau O, Thomassin I, et al. Fertility after laparoscopic colorectal resection for endometriosis: preliminary results. Fertil Steril. 2005;84:945–950. [DOI] [PubMed] [Google Scholar]
- 46.Daraï E, Carbonnel M, Dubernard G, et al. Determinant factors of fertility outcomes after laparoscopic colorectal resection for endometriosis. Eur J Obstet Gynecol Reprod Biol. 2010b;149:210–214. [DOI] [PubMed] [Google Scholar]
- 47.Ballester M, d'Argent EM, Morcel K, et al. Cumulative pregnancy rate after ICSI-IVF in patients with colorectal endometriosis: results of a multicentre study. Hum Reprod. 2012;27:1043–1049. [DOI] [PubMed] [Google Scholar]
- 48.Salim R, Riris S, Saab W, et al. Adenomyosis reduces pregnancy rates in infertile women undergoing IVF. Reprod BioMed Online. 2012;25:273–277. [DOI] [PubMed] [Google Scholar]
- 49.Thalluri V, Tremellen KP. Ultrasound diagnosed adenomyosis has a negative impact on successful implantation following GnRH antagonist IVF treatment. Hum Reprod. 2012;27:3487–3492. [DOI] [PubMed] [Google Scholar]
- 50.Tremellen K, Russell P. Adenomyosis is a potential cause of recurrent implantation failure during IVF treatment. Aust N Z J Obstet Gynaecol. 2011;51:280–283. [DOI] [PubMed] [Google Scholar]
- 51.Costello MF, Lindsay K, McNally G. The effect of adenomyosis on in vitro fertilisation and intra-cytoplasmic sperm injection treatment outcome. Eur J Obstet Gynecol Reprod Biol. 2011;158:229–234. [DOI] [PubMed] [Google Scholar]
- 52.Mijatovic V, Florijn E, Halim N, et al. Adenomyosis has no adverse effects on IVF/ICSI outcomes in women with endometriosis treated with long-term pituitary down-regulation before IVF/ICSI. Eur J Obstet Gynecol Reprod Biol. 2010;151:62–65. [DOI] [PubMed] [Google Scholar]
- 53.Benaglia L, Cardellicchio L, Leonardi M, et al. Asymptomatic adenomyosis and embryo implantation in IVF cycles. Reprod Biomed Online. 2014;29:606–611. [DOI] [PubMed] [Google Scholar]
- 54.Vercellini P, Consonni D, Dridi D, et al. Uterine adenomyosis and in vitro fertilization outcome: a systematic review and meta-analysis. Hum Reprod. 2014a;29:964–977. [DOI] [PubMed] [Google Scholar]
- 55.Martínez-Conejero JA, Morgan M, Montesinos M, et al. Adenomyosis does not affect implantation, but is associated with miscarriage in patients undergoing oocyte donation. Fertil Steril. 2011;96:943–950. [DOI] [PubMed] [Google Scholar]
- 56.Barrier BF, Malinowski MJ, Dick EJ, Jr, et al. Adenomyosis in the baboon is associated with primary infertility. Fertil Steril. 2004;82(Suppl 3):1091–1094. [DOI] [PubMed] [Google Scholar]
- 57.Farhi J, Ashkenazi J, Feldberg D. Effect of uterine leiomyomata on the results of in-vitro fertilization treatment. Hum Reprod. 1995;10:2576–2578. [DOI] [PubMed] [Google Scholar]
- 58.Stovall DW, Parrish SB, Van Voorhis BJ. Uterine leiomyomas reduce the efficacy of assisted reproduction cycles: results of a matched follow-up study. Hum Reprod. 1998;13:192–197. [DOI] [PubMed] [Google Scholar]
- 59.Birnholz JC. Ultrasonic visualization of endometrial movements. Fertil Steril. 1984;41:157–158. [DOI] [PubMed] [Google Scholar]
- 60.Kunz G, Beil D, Deininger H, et al. The dynamics of rapid sperm transport through the female genital tract: evidence from vaginal sonography of uterine peristalsis and hysterosalpingoscintigraphy. Hum Reprod. 1996;11:627–632. [DOI] [PubMed] [Google Scholar]
- 61.Kunz G, Beil D, Huppert P, et al. Structural abnormalities of the uterine wall in women with endometriosis and infertility visualized by vaginal sonography and magnetic resonance imaging. Hum Reprod. 2000;15:76–82. [DOI] [PubMed] [Google Scholar]
- 62.Lesny P, Killick SR. The junctional zone of the uterus and its contractions. BJOG. 2004;111:1182–1189. [DOI] [PubMed] [Google Scholar]
- 63.Mehasseb MK, Bell SC, Pringle JH, et al. Uterine adenomyosis is associated with ultrastructural features of altered contractility in the inner myometrium. Fertil Steril. 2010;93:2130–2136. [DOI] [PubMed] [Google Scholar]
- 64.Kitawaki J, Noguchi T, Amatsu T, et al. Expression of aromatase cytochrome P450 protein and messenger ribonucleic acid in human endometriotic and adenomyotic tissues but not in normal endometrium. Biol Reprod. 1997;57:514–519. [DOI] [PubMed] [Google Scholar]
- 65.Takahashi K, Nagata H, Kitao M. Clinical usefulness of determination of estradiol level in the menstrual blood for patients with endometriosis. Nihon Sanka Fujinka Gakkai Zasshi. 1989;41:1849–1850. [PubMed] [Google Scholar]
- 66.Lessey BA, Palomino WA, Apparao KB, et al. Estrogen receptor-alpha (ER-alpha) and defects in uterine receptivity in women. Reprod Biol Endocrinol. 2006;4(Suppl 1):S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brosens J, Verhoeven H, Campo R, et al. High endometrial aromatase P450 mRNA expression is associated with poor IVF outcome. Hum Reprod. 2004;19:352–356. [DOI] [PubMed] [Google Scholar]
- 68.Ishihara H, Kitawaki J, Kado N, et al. Gonadotropin-releasing hormone agonist and danazol normalize aromatase cytochrome P450 expression in eutopic endometrium from women with endometriosis, adenomyosis, or leiomyomas. Fertil Steril. 2003;79(Suppl 1):735–742. [DOI] [PubMed] [Google Scholar]
- 69.Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2005;3:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tremellen KP, Russell P. The distribution of immune cells and macrophages in the endometrium of women with recurrent reproductive failure. II: adenomyosis and macrophages. J Reprod Immunol. 2012;93:58–63. [DOI] [PubMed] [Google Scholar]
- 71.Yang JH, Wu MY, Chang DY, et al. Increased interleukin-6 messenger RNA expression in macrophage-cocultured endometrial stromal cells in adenomyosis. Am J Reprod Immunol. 2006;55:181–187. [DOI] [PubMed] [Google Scholar]
- 72.Wang F, Li H, Yang Z, et al. Expression of Interleukin-10 in patients with adenomyosis. Fertil Steril. 2009;91:1681–1685. [DOI] [PubMed] [Google Scholar]
- 73.Fontanini G, Campani D, Roncella M, et al. Expression of interleukin 6 (IL-6) correlates with oestrogen receptor in human breast carcinoma. Br J Cancer. 1990;80:579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fung HY, Wong YL, Wong FW, et al. Study of oestrogen and progesterone receptors in normal human endometrium during the menstrual cycle by immunocytochemical analysis. Gynecol Obstet Invest. 1994;38:186–190. [DOI] [PubMed] [Google Scholar]
- 75.Fang Z, Yang S, Lydon JP, et al. Intact progesterone receptors are essential to counteract the proliferative effect of estradiol in a genetically engineered mouse model of endometriosis. Fertil Steril. 2004;82:673–678. [DOI] [PubMed] [Google Scholar]
- 76.Franco HL, Jeong JW, Tsai SY, et al. In vivo analysis of progesterone receptor action in the uterus during embryo implantation. Semin Cell Dev Biol. 2008;19:178–186. [DOI] [PubMed] [Google Scholar]
- 77.Noda Y, Matsumoto H, Umaoka Y, et al. Involvement of superoxide radicals in the mouse two-cell block. Mol Reprod Dev. 1991;28:356–360. [DOI] [PubMed] [Google Scholar]
- 78.Narimoto K, Noda Y, Shiotani M, et al. Immunohistochemical assessment of super oxide dismutase expression in human endometrium throughout the menstrual cycle. Acta Histochem Cytochem. 1990;23:487–497. [Google Scholar]
- 79.Telfer JF, Lyall F, Norman JE, et al. Identification of nitric oxide synthase in human uterus. Hum Reprod. 1995;10:19–23. [DOI] [PubMed] [Google Scholar]
- 80.Ota H, Igarashi S, Hatazawa J, et al. Immunohistochemical assessment of superoxide dismutase expression in the endometrium in endometriosis and adenomyosis. Fertil Steril. 1999;72:129–134. [DOI] [PubMed] [Google Scholar]
- 81.Barroso RP, Osuamkpe C, Nagamani M, et al. Nitric oxide inhibits development of embryos and implantation in mice. Mol Hum Reprod. 1998;4:503–507. [DOI] [PubMed] [Google Scholar]
- 82.Biswas S, Kabir SN, Pal AK. The role of nitric oxide in the process of implantation in rats. J Reprod Fertil. 1998;114:157–161. [DOI] [PubMed] [Google Scholar]
- 83.Ota H, Igarashi S, Oyama N, et al. Optimal levels of nitric oxide are crucial for implantation in mice. Reprod Fertil Dev. 1999;11:183–188. [DOI] [PubMed] [Google Scholar]
- 84.Lessey BA, Castelbaum AJ, Buck CA, et al. Further characterization of endometrial integrins during the menstrual cycle and in pregnancy. Fertil Steril. 1994a;62:497–506. [PubMed] [Google Scholar]
- 85.Surrey ES, Minjarez DA, Schoolcraft WB. The incidence of aberrant endometrial α-β 3 vironectin expression in high risk infertility population: could prolonged GnRH agonist therapy play a role? J Assist Reprod Genet. 2001;24:553–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wei Q, St Clair JB, Fu T, et al. Reduced expression of biomarkers associated with the implantation window in women with endometriosis. Fertil Steril. 2009;91:1686–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xiao Y, Li T, Xia E, et al. Expression of integrin β3 and osteopontin in the eutopic endometrium of adenomyosis during the implantation window. Eur J Obstet Gynecol Reprod Biol. 2013;170:419–422. [DOI] [PubMed] [Google Scholar]
- 88.Mikolajczyk M, Wirstlein P, Skrzypczak J. Leukaemia inhibitory factor and interleukin 11 levels in uterine flushings of infertile patients with endometriosis. Hum Reprod. 2006;21:3054–3058. [DOI] [PubMed] [Google Scholar]
- 89.Takahashi Y, Takahashi M, Carpino N, et al. Leukemia inhibitory factor regulates trophoblast giant cell differentiation via Janus kinase 1-signal transducer and activator of transcription 3-suppressor of cytokine signaling 3 pathway. Mol Endocrinol. 2008;22:1673–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xiao Y, Sun X, Yang X, et al. Leukemia inhibitory factor is dysregulated in the endometrium and uterine flushing fluids of patients with adenomyosis during implantation window. Fertil Steril. 2010;94:85–89. [DOI] [PubMed] [Google Scholar]
- 91.Ponce C, Torres M, Galleguillos C, et al. Nuclear factor kappaB pathway and interleukin-6 are affected in eutopic endometrium of women with endometriosis. Reproduction. 2009;13:727–737. [DOI] [PubMed] [Google Scholar]
- 92.Taylor HS. The role of HOX genes in human implantation. Hum Reprod Update. 2000;6:75–79. [DOI] [PubMed] [Google Scholar]
- 93.Fischer CP, Kayisili U, Taylor HS. HOXA10 expression is decreased in endometrium of women with adenomyosis. Fertil Steril. 2011;95:1133–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mehasseb MK, Panchal R, Taylor AH, et al. Estrogen and progesterone receptor isoform distribution through the menstrual cycle in uteri with and without adenomyosis. Fertil Steril. 2011;95:2228–2235. [DOI] [PubMed] [Google Scholar]
- 95.Yamanaka A, Kimura F, Kishi Y, et al. Progesterone and synthetic progestin, dienogest, induce apoptosis of human primary cultures of adenomyotic stromal cells. Eur J Obstet Gynecol Reprod Biol. 2014;179:170–174. [DOI] [PubMed] [Google Scholar]
- 96.Sasa H, Imai K, Suzuki A, et al. Comparison of low-dose dienogest with low-dose danazol for long-term treatment of adenomyosis. Obstet Gynecol. 2014;123:97S–98S. [Google Scholar]
- 97.Nagata C, Yanagida S, Okamoto A, et al. Risk factors of treatment discontinuation due to uterine bleeding in adenomyosis patients treated with dienogest. J Obstet Gynaecol Res. 2012;38:639–644. [DOI] [PubMed] [Google Scholar]
- 98.Choi YS, Cho S, Lim KJ. Effects of LNG-IUS on nerve growth factor and its receptors expression in patients with adenomyosis. Growth Factors. 2010;28:452–460. [DOI] [PubMed] [Google Scholar]
- 99.Maia H, Jr, Maltez A, Coelho G, et al. Insertion of mirena after endometrial resection in patients with adenomyosis. J Am Assoc Gynecol Laparosc. 2003;10:512–516. [DOI] [PubMed] [Google Scholar]
- 100.Tadjerouni A, Henry-Suchet J, Loysel T, et al. Adenomyosis and infertility surgical treatment. Gynecol Rev Gynecol. 1995;3:380–386. [Google Scholar]


