Abstract
Currently, there is no available biomarker for lung cancer diagnosis. Here we recruited 844 lung cancer patients and 620 healthy participants from six hospitals. A total of four serum proteins was identified and subsequently assessed in the training and validation cohorts. The concentrations of four serum proteins were found to be significantly higher in lung cancer patients compared with healthy participants. The area under the curve (AUC) for the 4-biomarker were 0.86 in the training cohort, and 0.87 in the validation cohort. The classification improved to a corrected AUC of 0.90 and 0.89 respectively following addition of sex, age and smoking status. Similar results were observed for early-stage lung cancer. Remarkably, in a blinded test with a suspicious pulmonary nodule, the adjusted prediction model correctly discriminated the patients with 86.96% sensitivity and 98.25% specificity. These results demonstrated the 4-biomarker panel improved lung cancer prediction beyond that of known risk factors. Moreover, the biomarkers were valuable in differentiating benign nodules which will remain indolent from those that are likely to progress and therefore might serve as an adjuvant diagnosis tool for LDCT scanning.
Keywords: Lung cancer, Serum biomarkers, Risk factors, Early detection, LDCT
Highlights
-
•
A multicentric, case–control study was conducted to identify serum proteins for diagnosing lung cancer.
-
•
A 4-marker panel improved lung cancer prediction beyond that of known risk factors.
-
•
The biomarkers can detect lung cancer especially stage I–II from healthy controls or benign nodule patients.
Currently, low dose chest computed tomography (LDCT) leads to a high false-positive result. Here we identified a panel of four serum proteins, which provides the potential for accurate diagnosis of lung cancer in a multicentric study. More importantly, the biomarkers allowed accurate detection of lung cancer cases especially stage I-II cases from normal and benign nodule subjects. These results suggest that the 4-protein panel might be a useful biomarker for diagnosing lung cancer patients and help discriminate early stage patients, which may serve as an adjuvant diagnosis tool for LDCT scanning in the future.
1. Introduction
Lung cancer is continuously the leading cause of cancer-related deaths for both men and women worldwide (Cancer Facts & Figures 2015, American Cancer Society). The early detection of lung cancer presents an opportunity to dramatically reduce disease mortality. The overall 5-year relative survival rate was 17%; however, if the cancer is detected at stage Ia, the 5-year survival often exceeds 80% (Mahadevia et al., 2003). Currently, the National Lung Screening Trial (NLST) using low dose chest computed tomography (LDCT) in high-risk individuals demonstrates that a 20% reduction in lung cancer-specific mortality and a 6.7% reduction in all-cause mortality can be achieved (International Early Lung Cancer Action Program I et al., 2006, Bach et al., 2007, Henschke et al., 1999, Sone et al., 1998). However, there is a significant chance of a false-positive result for CT scan, which may require additional clinical testing, even an invasive procedure to specify an abnormality (Welch et al., 2007, Wilson et al., 2008, Swensen et al., 2005). Such finding greatly exacerbates the high cost of the technology and leads to unnecessary patient anxiety and surveillance. Therefore, a non-invasive test with a high specificity for distinguishing the indolent disease from lung cancer patients is highly demanded.
Serum biomarkers could be used as an invasive, cost-effective way to differentiate lung cancer patients. Several serum tumor markers have been studied extensively, such as carcinoembryonic antigen (CEA), serum cytokeratin 19 fragments, and pro-gastrin-releasing peptide; however, none has been demonstrated to provide clinical utility, mainly because of the poor reproducibility and lack of sufficient sensitivity and specificity (Buccheri et al., 2003, Pastor et al., 1997).
Recently, we described a non-invasive diagnostic system on Luminex xMAP platform to detect serum autoantibodies for diagnosis of lung cancer (Jia et al., 2014). Given the biological properties of cancer as a systemic disease, we predict that a combination of cancer associated serum proteins and autoantibody can be used to achieve superior levels of sensitivity and specificity. Here, we identified a diverse set of circulating proteins in the sera of patients with lung cancer and designed a large-scale, multicenter validation study to evaluate their utility in distinguishing lung cancer patients from matched healthy controls, with the goal of using these biomarkers to aid clinicians in making case management decisions.
2. Methods
2.1. Patients Population
The recruitment of patient with lung cancer was initiated from May 2009 and the discovery phase of the study included the patients collected until September 2009 from Hangzhou First People's Hospital, Zhejiang, China. The training cohort included the lung cancer patients collected between February 2011 and March 2012 from 3 hospitals, namely Hangzhou First People's Hospital, Hangzhou Cancer Hospital and Zhejiang Cancer Hospital, Zhejiang, China. The validation lung cancer patients were recruited from September 2012 to December 2013 from 6 hospitals, including the 3 hospitals mentioned in the training cohort, and the First Hospital of Jiaxing, the First Affiliated Hospital of Wenzhou Medical University, Shaoxing People's Hospital, Zhejiang, China.
Approval for the study was obtained from the institutional ethics review committee. All patients were provided written informed consent, according to the committees' regulations.
2.2. Patient and Serum Collection
The patients included in this study were all consecutive patients, and details regarding patient inclusion criteria and collection protocols have been previously reported (Jia et al., 2014). All cases used in this study were confirmed to be primary lung cancer by pathology review.
Healthy participants were recruited from the eligible blood donors with no evidence of pulmonary disorders of any type and were approximately age and sex-matched to the cancer cohorts presented. Patients who had a history of other solid tumors were excluded from the study.
2.3. Cancer Biomarker Screening and Detection
In discovery phase, samples were analyzed using the Bio-Plex Pro Biomarker Assays (Bio-Rad Laboratories), according to the manufacturer's instruction. CEA concentrations were measured with commercial ELISA (Roche), according to the manufacturer's recommendations. All measurements were done in duplicate.
2.4. Autoantigen Coupling to Luminex Microsphere and Detection of Autoantibody in Serum
The cDNA for NY-ESO-1 was cloned into the Flexi vector with a HQ-tag at N-terminus (Promega, USA) as previously described (Jia et al., 2014). The recombinant protein was expressed and purified using Ni-NTA agarose (Life Technologies) according to manufacturers' protocols. The purity was determined as > 95% by SDS-PAGE and Coomassie staining.
A total of 25 μg of purified protein was conjugated to microspheres by following the manufacture's protocol (Luminex, Austin). The microspheres conjugated with recombinant protein were aliquoted into a 96-well plate, and autoantibody in serum was detected by a Bio-Plex 200 System as previously described (Jia et al., 2014). All values reported are the raw median fluorescence intensities (MFI).
2.5. Calibrator for Autoantibody Measurement
Autoantibody in serum was detected by a Bio-Plex 200 System as previously described (Jia et al., 2014). Currently, there are no calibration standards for assays to measure cancer autoantibodies. Therefore, a modified calibration system was adopted based on a previous publication (Murray et al., 2010). Briefly, serum samples from lung cancer patients were screened for the autoantibody against NY-ESO-1 as described above. The positive sera were then further confirmed by western blot. For each serum sample, a calibration curve of background-corrected MFI versus log dilution was constructed to which a four-parameter logistic model plot was fitted. The MFI value for each unknown sample was then converted to a calibrated reference unit (RU) using the calibration curve. A calibration curve was prepared at the beginning of every assay run.
2.6. Statistical Analysis
In the discovery phase, the differences of the circulating concentrations of each biomarker between two groups were evaluated by the Mann-Whitney U test (continuous variables and nonparametric analyses). R version 3.0.1, 2-sided tests and a significance level of 0.05 were used. If not stated otherwise, we considered all patients with lung cancer as a single group regardless of stage.
For the training and validation cohorts, statistical analyses were carried out with MedCalc (version 15.8). Receiver operating characteristics (ROC) curves were used to quantify the biomarker performance by means of sensitivity, specificity, area under the curve (AUC) as well as corresponding 95% confidence intervals. To test the diagnostic accuracy of the panel of biomarkers, we estimated functions of the combined markers by logistic regression with or without adjustment for known risk factors for lung cancer (age, sex and smoking status), and the predictive probabilities were used as one marker and subjected to ROC analysis. We investigated the optimum cutoff value for diagnosis by maximizing the sum of sensitivity and specificity and minimizing the overall error (square root of the sum [1-sensitivity]2 + [1-specificity]2), and by minimizing the distance of the cutoff value to the top-left corner of the ROC curve. We also reported adjusted p-values corrected for multiple testing using the Benjamini-Holm method to control for the false positive error rate.
The correlation between the biomarkers in serum and clinicopathological characteristics was analyzed with Fisher's exact test. We took p < 0.05 (two sided) to be significant.
2.7. Prediction of Blinded Patients
The serum samples were collected pre-surgery from Hangzhou First People's Hospital, Zhejiang, China. The biomarkers were measured as described above, and the blinded patients were predicted as cancer or non-cancer by the BRB-Array Tools package (version 3.6) available at http://linus.nci.nih.gov/BRB-ArrayTools.html. Briefly, a predictor model was created using the 4-biomarker panel after adjustment for age, sex and smoking status in the training set, then subsequently tested in the validation cohort. A log base 2 transformation was applied to the raw data. Each sample's value was multiplied by the corresponding coefficients derived from univariate logistic regressions on the training set with cancer/non-cancer as a binary response variable, and then the values were totaled. The adjusted index scores were then assessed by the ROC curve, which provided a pure index of a test's accuracy by plotting the sensitivity against 1-specificity for each result value of the test. We computed the misclassification error of the models using leave-one-out cross-validation (LOOCV) method. For each LOOCV training set, the entire model-building process was repeated, including the biomarker selection process. The class labels were randomly permuted (100 permutations), and the entire LOOCV process was repeated. The significance level is the proportion of the random permutations that gave a cross-validated error rate no greater than the cross-validated error rate obtained with the real data. Each clinical sample was predicted to cancer or non-cancer group by the model. We computed a statistical significance level (p < 0.05) for each biomarker.
3. Results
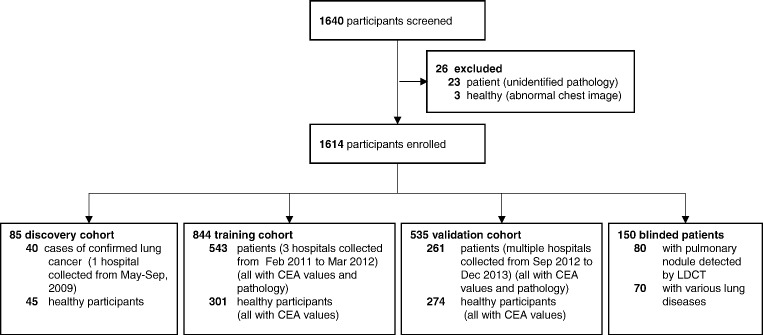
A total of 844 patients with lung cancer were included in this study, 40 in the discovery cohort, 543 in the training cohort and 261 in the validation cohort (Fig. 1). The healthy controls included 620 healthy participants. There is no significant difference in term of age and sex in both case and control groups, however, more current smokers in cases than controls. Clinicopathological characteristics of the study participants are summarized in Table 1. We also recruited 70 patients with various benign lung diseases, and 80 blinded patients with suspicious pulmonary nodule detected by LDCT. All these high-risk patients were either continuously followed for 2 years or underwent surgical resection if the CT image progressed.
Fig. 1.
Study design.
Table 1.
Demographics of patients and healthy participants in the discovery, training and validation cohorts.
| No. (%) of patients and healthy participants |
||||||||
|---|---|---|---|---|---|---|---|---|
| Discovery cohort (n = 85) |
Training cohort (n = 844) |
P | Validation cohort (n = 535) |
P | ||||
| Cases (n = 40) | Controls (n = 45) | Cases (n = 543) | Controls (n = 301) | Cases (n = 261) | Controls (n = 274) | |||
| Sex | 0.19 | 0.75 | ||||||
| Men | 22 (55) | 27 (60) | 380 (70) | 238 (79) | 188 (72) | 206 (75) | ||
| Women | 18 (45) | 18 (40) | 163 (30) | 63 (21) | 73 (28) | 68 (25) | ||
| Age at enrollment, years | 0.89 | 0.99 | ||||||
| < 60 | 16 (40) | 19 (42) | 221 (41) | 124 (42) | 96 (37) | 104 (38) | ||
| ≥ 60 | 24 (60) | 26 (58) | 322 (59) | 177 (58) | 165 (63) | 170 (62) | ||
| Smoking history | < 0.001 | 0.002 | ||||||
| Former | 1 (2) | 60 (11) | 82 (27) | 20 (8) | 52 (19) | |||
| Current | 24 (60) | 26 (58) | 271 (50) | 102 (34) | 113 (43) | 104 (38) | ||
| Never | 12 (30) | 14 (32) | 195 (36) | 108 (36) | 99 (38) | 93 (34) | ||
| Missing | 3 (8) | 5 (10) | 17 (3) | 9 (3) | 29 (11) | 25 (9) | ||
| Cancer stage | ||||||||
| I | 5 (13) | 54 (10) | 29 (11) | |||||
| II | 4 (10) | 61 (11) | 27 (10) | |||||
| III | 11 (27) | 129 (24) | 68 (26) | |||||
| IV | 16 (40) | 233 (43) | 113 (44) | |||||
| Unknown | 4 (10) | 66 (12) | 24 (9) | |||||
| Tumor type | ||||||||
| Adenocarcinoma | 15 (38) | 168 (31) | 86 (33) | |||||
| Squamous cell carcinoma | 16 (40) | 185 (34) | 104 (40) | |||||
| Large-cell carcinoma | 1 (2) | 27 (5) | 16 (6) | |||||
| Small-cell carcinoma | 3 (8) | 98 (18) | 37 (14) | |||||
| Non-small-cell carcinoma, unspecified | 5 (12) | 65 (12) | 18 (7) | |||||
P value calculated using χ2 test.
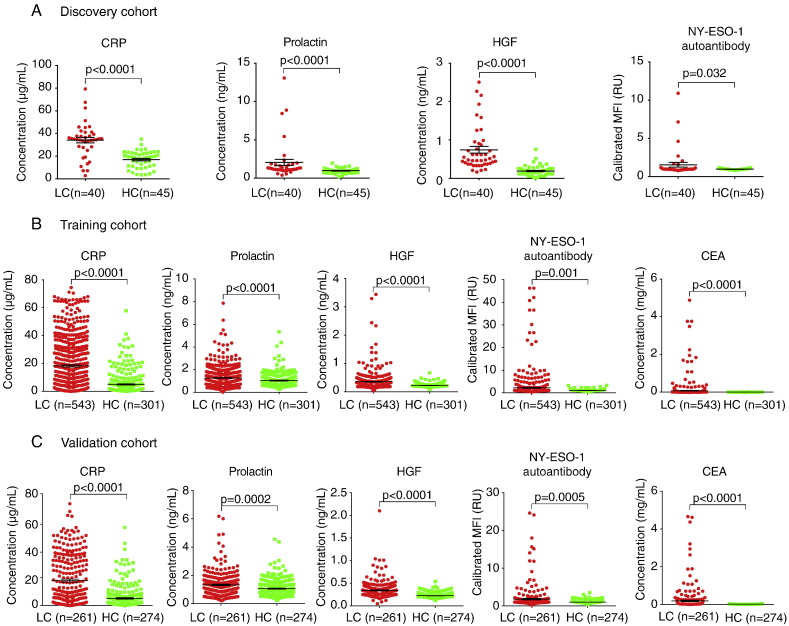
Our objective for the discovery phase was to identify a robust subset of biomarkers to discriminate the patients with lung cancer from the matched controls. Of the 20 circulating proteins evaluated in our study (Supplementary Fig. 1), three proteins, namely C-reactive protein (CRP), prolactin and hepatocyte growth factor (HGF), were found to differ significantly between the lung cancer and control group (p < 0.01) (Fig. 2A). Previously, we had demonstrated that circulating autoantibody against cancer-testis antigen NY-ESO-1 had the potential to separate patients with lung cancer from healthy participants in a multivariate statistical model (Jia et al., 2014). In this study, we further validated its performance and found that NY-ESO-1 autoantibody was significantly higher in patients with lung cancer than healthy controls (p < 0.05) (Fig. 2A), indicating its discriminatory utility for lung cancer detection.
Fig. 2.
Scatter dot blots of the biomarker concentration in serum in the discovery, training, and validation cohorts. (A) Discovery cohort. (B) Training cohort. (C) Validation cohort. The horizontal long line in each blot indicates the mean, while the top and bottom shorter lines mark the SEM. P values were calculated by Mann-Whitney test. LC = lung cancer, HC = healthy control.
The panel of 4 biomarkers consisted of three serum proteins and one autoantibody was further analyzed in the training and validation cohorts to determine their clinical utilities for non-invasive detection of lung cancer in a larger set of cases and controls. Importantly, the biomarkers were elevated in the cases with lung cancer in the training set than in the controls with a p value < 0.05. The scatter dot plots of these biomarkers appear in Fig. 2. The mean concentrations for CRP, Prolactin, HGF and NY-ESO-1 antibody in cancer group vs control group of the population are 18.20 μg/mL (95% CI 16.59–19.80) vs 5.03 μg/mL (95% CI 4.11–5.90), 1.27 ng/mL (95% CI 1.19–1.34) vs 1.03 ng/mL (95% CI 0.96–1.11), 0.36 ng/mL (95% CI 0.33–0.38) vs 0.22 ng/mL (95% CI 0.21–0.23) and 2.19 RU (95% CI 1.75–2.61) vs 1.06 RU (95% CI 1.02–1.10), respectively. As expected, the mean concentration of CEA in serum was higher in the cancer group compared with that in healthy controls (p < 0.001). There is no correlation between the 4 biomarkers and the clinical factors, such as gender, age and smoking status (Supplementary Table 1).
To assess if these biomarkers could predict subsequent diagnosis of lung cancer, we performed association study between the variables and lung cancer. In multivariable logistic regression analyses among all participants from both training and validation sets, the serum levels for the 4 biomarkers were statistically significantly associated with lung cancer (all p value < 0.05) (Table 2). We next examined if the associations between the 4 biomarkers and lung cancer were independent of factors that could potentially influence the associations. Results remained statistically significant after adjustment for age, gender, and smoking status (Table 2). These data suggest that the associations between the 4 biomarkers and lung cancer were independent of these tested potential confounding factors.
Table 2.
Association of the serum levels for 4 serum biomarkers with lung cancer with or without adjustment for the risk factors in both training and validation cohorts.a
| Variable | ORb (95% CI) | P valueb | ORc (95% CI) | P valuec |
|---|---|---|---|---|
| Sex | 1.03 (0.72–1.49) | 0.851 | ||
| Age | 1.02 (1.00–1.03) | 0.028 | ||
| Smoke | < 0.001 | |||
| Never | Reference | |||
| Former | 1.78 (1.09–2.91) | < 0.001 | ||
| Current | 3.12 (2.13–4.58) | 0.020 | ||
| Prolactin | 2.05 (1.56–2.68) | < 0.001 | 2.01 (1.48–2.75) | < 0.001 |
| CRP | 1.07 (1.05–1.08) | < 0.001 | 1.06 (1.04–1.08) | < 0.001 |
| NY-ESO-1 | 1.30 (1.08–1.56) | 0.005 | 1.32 (1.03–1.68) | 0.026 |
| HGF | 3.49 (2.06–5.88) | < 0.001 | 2.50 (2.04–4.79) | < 0.001 |
CI = confidence interval; OR = odds ratio.
Model containing the 4-marker panel (continuous).
Model containing sex (Male, Female), age at enrollment (continuous), smoking status (Never, Former, Current) and the 4-marker panel (continuous).
The performance of these biomarkers, in combination, in distinguishing lung cancer patients from healthy controls was further evaluated by receiver operating characteristic (ROC) analysis in both training and validation sets. We estimated functions of the combined marker by logistic regression, and used the parameters in the training set to further evaluate in the validation set. With an optimal Youden index score of 0.6145, the AUC of the 4-marker panel in the training set was 0.86 (95% CI: 0.83–0.88). The optimal sensitivity and specificity were 69.98% and 87.04%, respectively (Table 3). On the basis of the cutoff index score, we evaluated both training and validation samples in this study. The optimal cutoff value for CEA was 3.72 μg/mL in the training set (AUC 0.768, 0.738–0.796, sensitivity 57.83%, specificity of 86.38%). Notably, the biomarkers appeared to have similar diagnostic accuracies for SCLC or NSCLC (Supplementary Figs. 2 and 3). The detail predictive values and likelihood ratios for the biomarkers in the diagnosis of lung cancer and subtypes of the patients are shown in Table 3.
Table 3.
Performance of the 4-biomarker panel (with or without adjustment) on detection of lung cancer in both training and validation cohorts.
| Training |
Validation |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC (95% CI) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Positive LR | Negative LR | AUC (95% CI) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Positive LR | Negative LR | |
| All LC vs HC | ||||||||||||||
| 4-marker | 0.86 (0.83–0.88) | 69.98 | 87.04 | 90.7 | 61.6 | 5.40 | 0.34 | 0.87 (0.83–0.89) | 69.35 | 87.23 | 83.8 | 74.9 | 5.43 | 0.35 |
| Adj 4-markera | 0.90 (0.87–0.93) | 88.16 | 80.43 | 93.7 | 67.3 | 4.51 | 0.15 | 0.89 (0.86–0.93) | 85.12 | 78.91 | 87.1 | 75.9 | 4.04 | 0.19 |
| CEA | 0.76 (0.73–0.79) | 57.83 | 86.38 | 88.5 | 53.2 | 4.25 | 0.49 | 0.80 (0.76–0.83) | 55.17 | 94.16 | 90.0 | 68.8 | 9.45 | 0.48 |
| 4-marker + CEA | 0.91 (0.89–0.93) | 81.95 | 83.72 | 90.1 | 72.0 | 5.03 | 0.22 | 0.93 (0.91–0.95) | 80.84 | 90.51 | 89.0 | 83.2 | 8.52 | 0.21 |
| SCLC vs HC | ||||||||||||||
| 4-marker | 0.86 (0.82–0.89) | 69.44 | 87.04 | 39.1 | 96.0 | 5.36 | 0.35 | 0.85 (0.80–0.89) | 73.68 | 87.23 | 28.6 | 98.0 | 5.77 | 0.30 |
| Adj 4-markera | 0.92 (0.87–0.97) | 87.50 | 85.21 | 52.8 | 97.3 | 5.92 | 0.15 | 0.88 (0.79–0.96) | 86.67 | 72.67 | 22.8 | 98.3 | 3.17 | 0.18 |
| CEA | 0.74 (0.69–0.79) | 58.33 | 86.38 | 33.9 | 94.5 | 4.28 | 0.48 | 0.67 (0.61–0.72) | 42.11 | 94.16 | 33.3 | 95.9 | 7.21 | 0.61 |
| 4-marker + CEA | 0.88 (0.84–0.91) | 80.56 | 83.72 | 37.2 | 97.3 | 4.95 | 0.23 | 0.90 (0.86–0.93) | 63.16 | 90.51 | 31.6 | 97.3 | 6.66 | 0.41 |
| NSCLC vs HC | ||||||||||||||
| 4-marker | 0.85 (0.82–0.88) | 68.60 | 87.04 | 85.2 | 71.8 | 5.29 | 0.36 | 0.86 (0.83–0.90) | 71.43 | 87.23 | 74.1 | 85.7 | 5.59 | 0.33 |
| Adj 4-markera | 0.90 (0.87–0.93) | 87.54 | 82.84 | 89.7 | 79.5 | 5.10 | 0.15 | 0.90 (0.86–0.94) | 88.33 | 78.26 | 75.2 | 90.0 | 4.06 | 0.15 |
| CEA | 0.77 (0.73–0.80) | 57.62 | 86.38 | 82.2 | 65.2 | 4.23 | 0.49 | 0.83 (0.79–0.87) | 59.29 | 94.16 | 83.8 | 81.9 | 10.15 | 0.43 |
| 4-marker + CEA | 0.91 (0.88–0.93) | 81.40 | 83.72 | 84.5 | 80.5 | 5.00 | 0.22 | 0.94 (0.91–0.96) | 84.29 | 90.51 | 81.9 | 91.9 | 8.88 | 0.17 |
| Stage I & II LC vs HC | ||||||||||||||
| 4-marker | 0.84 (0.81–0.88) | 62.61 | 87.04 | 64.9 | 85.9 | 4.83 | 0.43 | 0.84 (0.80–0.88) | 64.29 | 87.23 | 50.7 | 92.3 | 5.03 | 0.41 |
| Adj 4-markera | 0.91 (0.87–0.94) | 83.17 | 86.98 | 79.2 | 89.6 | 6.39 | 0.19 | 0.88 (0.83–0.94) | 76.6 | 86.3 | 62.1 | 92.7 | 5.61 | 0.27 |
| CEA | 0.69 (0.64–0.73) | 42.61 | 86.38 | 54.4 | 79.8 | 3.13 | 0.66 | 0.76 (0.71–0.81) | 44.64 | 94.16 | 61.0 | 89.3 | 7.65 | 0.59 |
| 4-marker + CEA | 0.87 (0.84–0.90) | 73.04 | 83.72 | 63.2 | 89.0 | 4.49 | 0.32 | 0.93 (0.90–0.95) | 75.00 | 90.51 | 61.8 | 94.7 | 7.90 | 0.28 |
| Stage III & IV LC vs HC | ||||||||||||||
| 4-marker | 0.87 (0.84–0.89) | 72.88 | 87.04 | 87.2 | 72.5 | 5.62 | 0.31 | 0.87 (0.84–0.90) | 69.66 | 87.23 | 78.0 | 81.6 | 5.45 | 0.35 |
| Adj 4-markera | 0.91 (0.88–0.94) | 84.82 | 86.98 | 92.1 | 76.2 | 6.52 | 0.17 | 0.91 (0.88–0.94) | 86.99 | 80.75 | 80.4 | 87.2 | 4.52 | 0.16 |
| CEA | 0.78 (0.74–0.81) | 60.55 | 86.38 | 84.4 | 64.4 | 4.45 | 0.46 | 0.80 (0.76–0.84) | 55.62 | 94.16 | 86.1 | 76.6 | 9.52 | 0.47 |
| 4-marker + CEA | 0.92 (0.90–0.94) | 85.21 | 83.72 | 86.4 | 82.4 | 5.23 | 0.18 | 0.93 (0.90–0.95) | 81.46 | 90.51 | 84.8 | 88.3 | 8.58 | 0.20 |
HC = healthy control. LC = lung cancer. SCLC = small cell lung cancer. NSCLC = non-small cell lung cancer.
Model corrected by sex (Male, Female), age at enrollment (continuous), smoking status (Never, Former, Current) and the 4-marker panel (continuous).
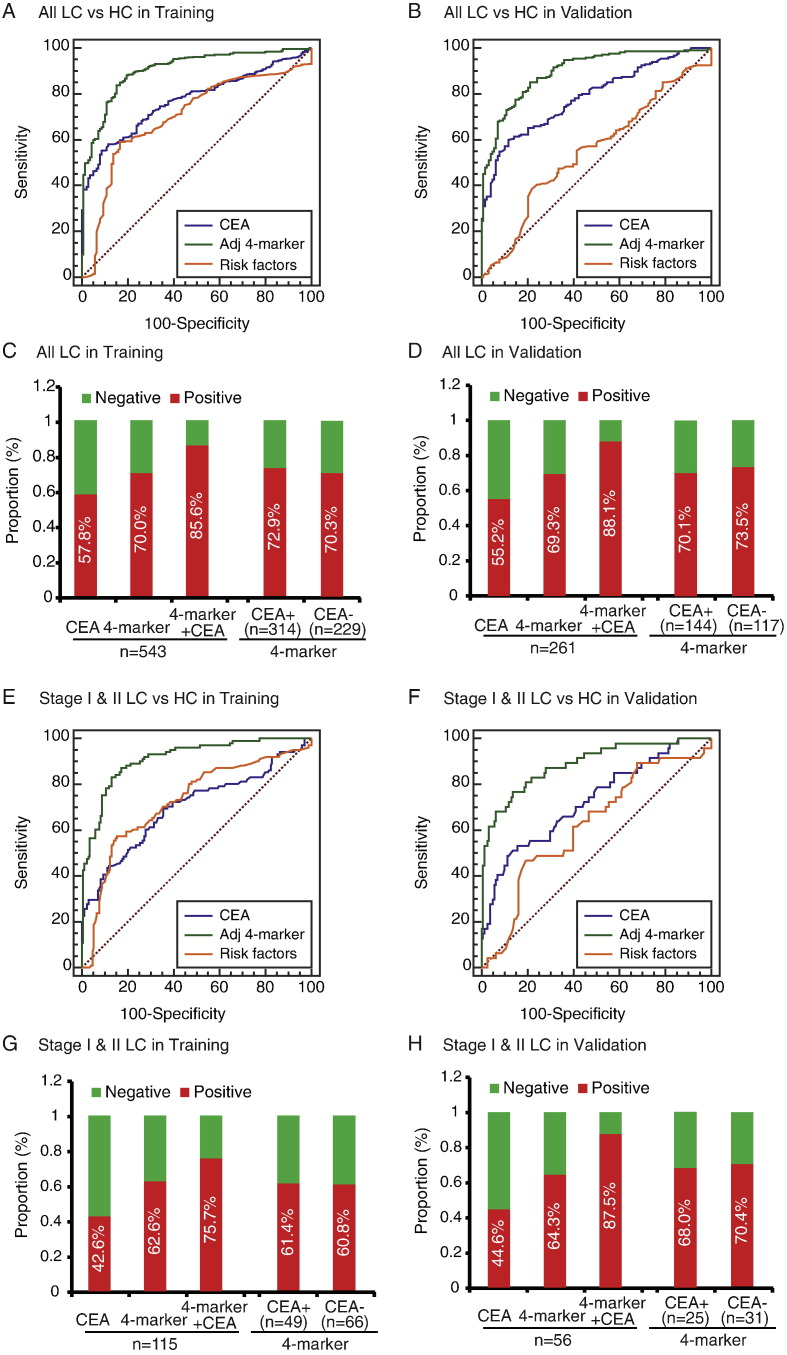
To assess the extent to which the 4-biomarker panel improves over current risk factor models, we fit the logistic regression model with the 4-marker panel and adjusted for main lung cancer risk factors, namely sex, age at enrollment, and smoking status. We found the discriminatory power attributable to the 4-marker panel is substantial compared with that of the main lung cancer risk factors (Fig. 3). The AUCs for the full logistic models with and without the 4-marker panel in the training cohort were 0.90 (95%CI: 0.87–0.93) and 0.70 (95%CI: 0.65–0.75), respectively. The predictive value was of similar magnitude (AUC: 0.90; 95% CI: 0.87–0.93) when considering the current smokers only. Notably, even though derived from both histological types, the adjusted 4-marker panel performed equally well in both SCLC (AUC: 0.92) and NSCLC (AUC: 0.90). To assess the differential ability of the biomarkers on early-stage of lung cancers, we focused on 115 (21%) of 543 patients in the training cohort with stage I & II disease. The adjusted predictor performed well in differential diagnosis of early-stage lung cancer from all controls, with an AUC of 0.91 (Fig. 3 and Table 3).
Fig. 3.
Performance of the adjusted 4-biomarker panel in the diagnosis of lung cancer.
ROC curve for the adjusted 4-marker panel, CEA, and the risk factors for all patients with lung cancer versus all controls in the training cohort (A) and validation cohort (B). The proportion of positive samples for the 4-marker panel, CEA, or their combination in all lung cancer patients, and for the 4-marker panel by CEA status, in the training cohort (C) and validation cohort (D). ROC curve for the adjusted 4-marker panel, CEA, and the risk factors for the early stage lung cancer patients versus all controls in the training cohort (E) and validation cohort (F). The proportion of positive samples for the 4-marker panel, CEA, or their combination in the early stage lung cancer patients, and for the 4-marker panel by CEA status, in the training cohort (G) and validation cohort (H). ROC = receiver operating characteristics. LC = lung cancer. HC = healthy control.
We then compared the performance of the 4-marker panel with CEA. The 4-biomarker panel had greater AUC, sensitivity, and specificity than did CEA in patients with lung cancer compared with healthy controls (Fig. 3, Table 3). A greater proportion of patients in the training cohort were positive for the 4-biomarker than for CEA only (380 [70.0%] vs 314 [57.8%] of 543 patients; Fig. 3). In addition, 72.9% (229 out of 314) of CEA-positive patients with lung cancer and 70.3% (161 out of 229) of CEA-negative patients had positive 4-biomarker results, indicating the diagnostic ability of the 4-biomarker irrespective of CEA status. Multiplexing the 4-marker panel and CEA remarkably increased the diagnostic accuracy for lung cancer compared with either biomarker alone (AUC 0.913, 95% CI 0.892–0.931, sensitivity 81.9% and specificity 83.7%; 4-marker + CEA vs 4-marker alone, p < 0.0001; 4-marker + CEA vs CEA alone, p < 0.0001; Table 3). Likewise, a much higher proportion of patients with early-stage lung cancer had positive results for the 4-biomarker than for CEA, and similar proportion of patients had positive 4-biomarker results for both CEA-negative patients (38 [60.8%] of 66) and CEA-positive patients (34 [61.4%] of 49) (Fig. 3). Diagnostic accuracy using 4-marker + CEA remained improved in the detection of early-stage lung cancer as well as other lung cancer subgroups (Fig. 3, Table 3).
In order to further assess the specificity of the 4-biomarker panel in detecting lung cancer patients, we collected a group of patients with benign lung diseases, including granuloma, pulmonary inflammatory pseudotumor, tuberculosis, pulmonary nodule and sequestration. Of note, these patients were diagnosed based on the clinical examination with 2 years of following up. Supplementary Table 2 demonstrated the clinical characteristics of these patients. Interestingly, of the 70 patients with benign lung diseases, 56 patients (4/6 granuloma, 4/4 pseudotumor, 8/10 pulmonary nodule, 1/2 sequestration, and 39/48 tuberculosis) were classified as benign by the adjusted model, resulting in a specificity of 80% (Supplementary Table 3), indicating the robustness of the biomarkers in detecting the patients with benign lung diseases.
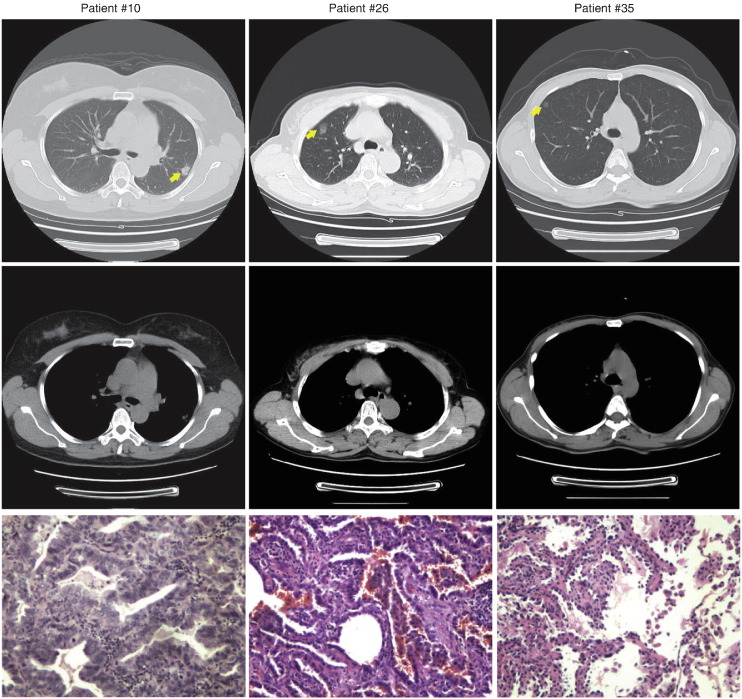
We next investigated the potential clinical application of the 4-biomarker panel in predicting the blinded patient samples. The clinical characteristics of the 80 patients are demonstrated in Table 4 and the median sizes of nodule are 1.7 cm and 0.54 cm for cases and controls respectively. All patients received blood drawn before any invasive clinical intervention. The measurement of the 4 biomarkers and the generation of the adjusted prediction model were as described above. Table 5 showed the prediction of the patients as well as the predictive values and likelihood ratios. Importantly, of the 23 lung cancer patients validated by pathology, 20 individuals were classified as cancers, while 56 non-cancer patients as followed for 2 years were predicted as benign, which resulted in 86.96% sensitivity and 98.25% specificity. Fig. 4 showed the representative CT images from three patients as well as hematoxylin and eosin stained tissue sections after surgery. These results are very intriguing as the high specificity indicates that the 4-biomarker panel has robust discriminatory power in distinguishing indolent diseases from early-stage patients with lung cancer and therefore might serve as an adjuvant non-invasive diagnosis tool for unspecified pulmonary abnormalities by CT screening.
Table 4.
Clinical characteristics of the blinded patients with pulmonary nodule detected by LDCT.
| No. of lung cancer patients (as confirmed by surgery) |
No. of none-cancer patients (as followed for 2 years) |
|
|---|---|---|
| N = 23 | N = 57 | |
| Sex | ||
| Men | 13 | 23 |
| Women | 10 | 34 |
| Age, median (range), y | 53.5 (46–66) | 53 (40–78) |
| Smoking history | ||
| Former | 0 | 1 |
| Current | 8 | 14 |
| Never | 6 | 30 |
| Missing | 9 | 12 |
| Nodule size, median (range), cm | 1.7 (0.5–4.12) | 0.54 (0.23–2.5) |
| < 1 | 7 | 44 |
| 1–2 | 10 | 11 |
| 2–3 | 5 | 2 |
| > 3 | 1 | |
| Cancer stage | ||
| I | 12 | |
| II | 10 | |
| Unknown | 1 |
Table 5.
Performance of the adjusted 4-biomarker panel on diagnosis of the blinded patients. The prediction model was corrected by sex (Male, Female), age at enrollment (continuous), smoking status (Never, Former, Current) and the 4-marker panel (continuous).
| Patients with pulmonary nodule |
Sensitivity (%) | Specificity (%) | PPV (%) | PPV (%) | Positive LR | Negative LR | ||
|---|---|---|---|---|---|---|---|---|
| Cancer (as confirmed by surgery) | Non-cancer (as followed for 2 years) | |||||||
| Classified as cancer | 20 | 1 | 86.96 | 98.25 | 95.24 | 94.92 | 49.56 | 0.13 |
| Classified as non-cancer | 3 | 56 | ||||||
Fig. 4.
The representative axial CT images from three patients at the level of the heart displayed in bone settings reveal the pulmonary nodules in the upper lobe (yellow arrows). The bottom panels show hematoxylin and eosin stain of the corresponding tumor section.
Using the same index scores for all the variables in logistic regression model as in the training set, we observed similar results in the validation cohort to those in the training cohort. The 4-marker panel had significantly higher diagnostic accuracy for all lung cancer, early-stage lung cancer as well as other cancer subtypes. In addition, the 4-marker was able to detect lung cancer in CEA-negative patients from the validation cohort, especially those with early-stage disease. The improvement in diagnostic accuracy for lung cancer by 4-marker + CEA was also proven in the validation cohort (Table 3, Fig. 3).
4. Discussion
Identification of blood-based biomarkers has been previously reported. A pre-specified 22 miRNA signature has significant diagnostic and prognostic performance, and could reduce the false-positive rate of LDCT (Sozzi et al., 2014). A multiplexed serum protein was able to discriminate clinical lung cancer patients from high-risk individuals, thus having the potential to aid in the early detection of lung cancer (Bigbee et al., 2012). A panel of six autoantibodies has been tested and validated in case–control settings and may be a detection tool for early lung cancer patients (Lam et al., 2011, Jett et al., 2014). These results further ensure serum biomarkers could be a noninvasive approach to differentiate early lung cancers.
In this study we identified a highly performing panel of protein biomarkers in serum, and measurement of the panel had diagnostic value for lung cancer better than that of CEA, especially for patients with small unidentified pulmonary nodules. The significance of the discovery was further validated by a large-scale population (> 1000) collected from multiple hospitals/medical centers across 5 years, and the multiplexed biomarkers have the clinical diagnostic relevance for lung cancer, particularly for the high-risk population with suspicious pulmonary nodules detected by CT screening (size < 2 cm). The high specificity indicate that the panel of biomarkers could be suitable for detecting lung cancer patients, especially early stage disease, thus assisting the physician and the patients to make decision for immediate medical intervention. More importantly, this panel could be useful to guide CT scanning to clarify the pulmonary abnormalities. In this setting, the biomarkers would dramatically reduce the patient anxiety and the cost of additional testing, further benefiting the patients as well as the public health in all.
The protein biomarkers described in this study have all been associated with lung cancer, and characterized previously for cancer diagnostic potential. The elevated CRP preceded lung cancer diagnosis by several years (Chaturvedi et al., 2010), and were associated with increasing lung cancer risk (Xu et al., 2013). Elevation of serum prolactin could be served as diagnostic biomarkers for lung cancer (Bigbee et al., 2012, Nolen et al., 2011). Sera HGF were significantly elevated in lung cancer patients (Tanaka et al., 2011), and a high level of blood HGF exhibited a poor prognosis of metastatic disease in primary lung cancer patients (Hosoda et al., 2012). NY-ESO-1 autoantibody frequencies in lung cancer have been reported to range from 4 to 23% (Chapman et al., 2008, Tureci et al., 2006, Stockert et al., 1998). More importantly, NY-ESO-1 autoantibody was detected in patients with small primary tumors and more frequently before distant metastasis occurred, suggesting that NY-ESO-1 antibody is an early event (Tureci et al., 2006).
Although the biomarker panel we described here could not detect every lung cancer patient, it offers a high specificity in detecting healthy controls as well as the patients with benign lung diseases. After the appropriate confirmatory clinical trials, one could envision that the most immediate scenario in which this panel could be used is to improve interpretation of CT images in the setting of a suspicious pulmonary nodule. In this context, a diagnostic biomarker panel must perform at a maximal level of specificity in order to reduce the number of false positive results rendered by CT. Actually, of the 80 prospective patients, the 4-biomarker predictor produced 82.6% sensitivity and 98.2% specificity as determined by post-surgery histologic results or 2 years clinical follow-up. Therefore, as a supplementary diagnostic approach to CT scan, such a strategy could significantly reduce the number of futile invasive procedures. Upon further validation and optimization, these serum biomarkers could provide an effective means to further assess the malignant patients designated as having a high risk for lung cancer by LDCT scanning.
5. Conclusions
This study identified 4 serum biomarkers that had the ability to distinguish patients with lung cancer from benign controls. These biomarkers have potential to aid in detecting early stage of lung cancer and more accurate interpretation of the pulmonary abnormalities, thus serving as an adjuvant diagnosis tool for LDCT screening. Although we validated the panel, our findings are preliminary. Further study is necessary to validate whether the 4-biomarker panel alone has clinical implications as a screening test for early diagnosis of lung cancer.
Authors Contributions
Conception and design: SM, XW; Experiment: WW, XZ; Analysis and interpretation: SM, WW, BX, SZ, HY; Samples collection: SM, HJ, WM; Manuscript writing and editing: SM, BX, SZ, XW.
Funding
The study was supported by grants from the Major Science and Technology Innovation Project of Hangzhou (20112312A01), the National Natural Science Foundation (81172072), the Science Foundation for Distinguished Young Scholars of Zhejiang (R2101405), the International Science and Technology Cooperation Project of Zhejiang (2012C24008), and the Collaborative Innovation Projects of Science and Technology Department of Zhejiang (2014F50014), China. The funders had no role in study design, data collection, data analysis, interpretation and writing of the report.
Conflict of Interest
All authors declare that there is no conflict of interest in relation to this article.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2016.08.018.
Appendix A. Supplementary Data
Supplementary material
References
- Bach P.B., Jett J.R., Pastorino U., Tockman M.S., Swensen S.J., Begg C.B. Computed tomography screening and lung cancer outcomes. JAMA. 2007;297(9):953–961. doi: 10.1001/jama.297.9.953. [DOI] [PubMed] [Google Scholar]
- Bigbee W.L., Gopalakrishnan V., Weissfeld J.L., Wilson D.O., Dacic S., Lokshin A.E. A multiplexed serum biomarker immunoassay panel discriminates clinical lung cancer patients from high-risk individuals found to be cancer-free by CT screening. J. Thorac. Oncol. 2012;7(4):698–708. doi: 10.1097/JTO.0b013e31824ab6b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccheri G., Torchio P., Ferrigno D. Clinical equivalence of two cytokeratin markers in mon-small cell lung cancer: a study of tissue polypeptide antigen and cytokeratin 19 fragments. Chest. 2003;124(2):622–632. doi: 10.1378/chest.124.2.622. [DOI] [PubMed] [Google Scholar]
- Chapman C.J., Murray A., McElveen J.E., Sahin U., Luxemburger U., Tureci O. Autoantibodies in lung cancer: possibilities for early detection and subsequent cure. Thorax. 2008;63(3):228–233. doi: 10.1136/thx.2007.083592. [DOI] [PubMed] [Google Scholar]
- Chaturvedi A.K., Caporaso N.E., Katki H.A., Wong H.L., Chatterjee N., Pine S.R. C-reactive protein and risk of lung cancer. J. Clin. Oncol. 2010;28(16):2719–2726. doi: 10.1200/JCO.2009.27.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henschke C.I., McCauley D.I., Yankelevitz D.F., Naidich D.P., McGuinness G., Miettinen O.S. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet. 1999;354(9173):99–105. doi: 10.1016/S0140-6736(99)06093-6. [DOI] [PubMed] [Google Scholar]
- Hosoda H., Izumi H., Tukada Y., Takagiwa J., Chiaki T., Yano M. Plasma hepatocyte growth factor elevation may be associated with early metastatic disease in primary lung cancer patients. Ann. Thorac. Cardiovasc. Surg. 2012;18(1):1–7. doi: 10.5761/atcs.oa.09.01522. [DOI] [PubMed] [Google Scholar]
- International Early Lung Cancer Action Program I, Henschke C.I., Yankelevitz D.F., Libby D.M., Pasmantier M.W., Smith J.P. Survival of patients with stage I lung cancer detected on CT screening. N. Engl. J. Med. 2006;355(17):1763–1771. doi: 10.1056/NEJMoa060476. [DOI] [PubMed] [Google Scholar]
- Jett J.R., Peek L.J., Fredericks L., Jewell W., Pingleton W.W., Robertson J.F. Audit of the autoantibody test, EarlyCDT(R)-lung, in 1600 patients: an evaluation of its performance in routine clinical practice. Lung Cancer. 2014;83(1):51–55. doi: 10.1016/j.lungcan.2013.10.008. [DOI] [PubMed] [Google Scholar]
- Jia J., Wang W., Meng W., Ding M., Ma S., Wang X. Development of a multiplex autoantibody test for detection of lung cancer. PLoS One. 2014;9(4):e95444. doi: 10.1371/journal.pone.0095444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam S., Boyle P., Healey G.F., Maddison P., Peek L., Murray A. EarlyCDT-Lung: an immunobiomarker test as an aid to early detection of lung cancer. Cancer Prev. Res. 2011;4(7):1126–1134. doi: 10.1158/1940-6207.CAPR-10-0328. [DOI] [PubMed] [Google Scholar]
- Mahadevia P.J., Fleisher L.A., Frick K.D., Eng J., Goodman S.N., Powe N.R. Lung cancer screening with helical computed tomography in older adult smokers: a decision and cost-effectiveness analysis. JAMA. 2003;289(3):313–322. doi: 10.1001/jama.289.3.313. [DOI] [PubMed] [Google Scholar]
- Murray A., Chapman C.J., Healey G., Peek L.J., Parsons G., Baldwin D. Technical validation of an autoantibody test for lung cancer. Ann. Oncol. 2010;21(8):1687–1693. doi: 10.1093/annonc/mdp606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen B.M., Langmead C.J., Choi S., Lomakin A., Marrangoni A., Bigbee W.L. Serum biomarker profiles as diagnostic tools in lung cancer. Cancer Biomark. 2011;10(1):3–12. doi: 10.3233/CBM-2012-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor A., Menendez R., Cremades M.J., Pastor V., Llopis R., Aznar J. Diagnostic value of SCC, CEA and CYFRA 21.1 in lung cancer: a Bayesian analysis. Eur. Respir. J. 1997;10(3):603–609. [PubMed] [Google Scholar]
- Sone S., Takashima S., Li F., Yang Z., Honda T., Maruyama Y. Mass screening for lung cancer with mobile spiral computed tomography scanner. Lancet. 1998;351(9111):1242–1245. doi: 10.1016/S0140-6736(97)08229-9. [DOI] [PubMed] [Google Scholar]
- Sozzi G., Boeri M., Rossi M., Verri C., Suatoni P., Bravi F. Clinical utility of a plasma-based miRNA signature classifier within computed tomography lung cancer screening: a correlative MILD trial study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014;32(8):768–773. doi: 10.1200/JCO.2013.50.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockert E., Jager E., Chen Y.T., Scanlan M.J., Gout I., Karbach J. A survey of the humoral immune response of cancer patients to a panel of human tumor antigens. J. Exp. Med. 1998;187(8):1349–1354. doi: 10.1084/jem.187.8.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swensen S.J., Jett J.R., Hartman T.E., Midthun D.E., Mandrekar S.J., Hillman S.L. CT screening for lung cancer: five-year prospective experience. Radiology. 2005;235(1):259–265. doi: 10.1148/radiol.2351041662. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Kimura T., Kudoh S., Mitsuoka S., Watanabe T., Suzumura T. Reaction of plasma hepatocyte growth factor levels in non-small cell lung cancer patients treated with EGFR-TKIs. Int. J. Cancer. 2011;129(6):1410–1416. doi: 10.1002/ijc.25799. [DOI] [PubMed] [Google Scholar]
- Tureci O., Mack U., Luxemburger U., Heinen H., Krummenauer F., Sester M. Humoral immune responses of lung cancer patients against tumor antigen NY-ESO-1. Cancer Lett. 2006;236(1):64–71. doi: 10.1016/j.canlet.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Welch H.G., Woloshin S., Schwartz L.M., Gordis L., Gotzsche P.C., Harris R. Overstating the evidence for lung cancer screening: the International Early Lung Cancer Action Program (I-ELCAP) study. Arch. Intern. Med. 2007;167(21):2289–2295. doi: 10.1001/archinte.167.21.2289. [DOI] [PubMed] [Google Scholar]
- Wilson D.O., Weissfeld J.L., Fuhrman C.R., Fisher S.N., Balogh P., Landreneau R.J. The Pittsburgh Lung Screening Study (PLuSS): outcomes within 3 years of a first computed tomography scan. Am. J. Respir. Crit. Care Med. 2008;178(9):956–961. doi: 10.1164/rccm.200802-336OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M., Zhu M., Du Y., Yan B., Wang Q., Wang C. Serum C-reactive protein and risk of lung cancer: a case–control study. Med. Oncol. 2013;30(1):319. doi: 10.1007/s12032-012-0319-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material