Abstract
Background
Metastatic breast cancer exhibits diverse and rapidly evolving intra- and inter-tumor heterogeneity. Patients with similar clinical presentations often display distinct tumor responses to standard of care (SOC) therapies. Genome landscape studies indicate that EGFR/HER2/RAS “pathway” activation is highly prevalent in malignant breast cancers. The identification of therapy-responsive and prognostic biomarkers is paramount important to stratify patients and guide therapies in clinical oncology and personalized medicine.
Methods
In this study, we analyzed matched pairs of tumor specimens collected from 182 patients who received neoadjuvant systemic therapies (NST). Statistical analyses were conducted to determine whether EGFR/HER2/RAS pathway biomarkers and clinicopathological predictors, alone and in combination, are prognostic in breast cancer.
Findings
SIAH and EGFR outperform ER, PR, HER2 and Ki67 as two logical, sensitive and prognostic biomarkers in metastatic breast cancer. We found that increased SIAH and EGFR expression correlated with advanced pathological stage and aggressive molecular subtypes. Both SIAH expression post-NST and NST-induced changes in EGFR expression in invasive mammary tumors are associated with tumor regression and increased survival, whereas ER, PR, and HER2 were not. These results suggest that SIAH and EGFR are two prognostic biomarkers in breast cancer with lymph node metastases.
Interpretation
The discovery of incorporating tumor heterogeneity-independent and growth-sensitive RAS pathway biomarkers, SIAH and EGFR, whose altered expression can be used to estimate therapeutic efficacy, detect emergence of resistant clones, forecast tumor regression, differentiate among partial responders, and predict patient survival in the neoadjuvant setting, has a clear clinical implication in personalizing breast cancer therapy.
Funding
This work was supported by the Dorothy G. Hoefer Foundation for Breast Cancer Research (A.H. Tang); Center for Innovative Technology (CIT)-Commonwealth Research Commercialization Fund (CRCF) (MF14S-009-LS to A.H. Tang), and National Cancer Institute (CA140550 to A.H. Tang).
Abbreviations: AUC, area under the curve; DRFS, distant recurrence-free survival; EGFR, epidermal growth factor receptor; ER, estrogen receptor; H&E, hematoxylin and eosin staining; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; LN, lymph node; MRI, magnetic resonance imaging; NRT, no residual tumor; NST, neoadjuvant systemic therapy; phospho-ERK, phosphorylated extracellular signal-regulated kinases; pCR, pathological complete response; pIR, pathological incomplete response; PH, proportional hazards; PR, progesterone receptor; ROC, receiver operating characteristic; SIAH, human homologues of Drosophila Seven-In-Absentia (SINA); SOC, standard of care; sROC, survival receiver operating characteristic (sROC); TNBC, triple-negative breast cancer
Keywords: Locally advanced and metastatic breast cancer, Neoadjuvant systemic therapies, Needle biopsies, The RAS pathway activation in breast cancer, SIAH E3 ligase, Clinicopathological predictors, And prognostic biomarkers
Graphical abstract
Highlights
-
•
EGFR/RAS pathway activation is prevalent in breast cancer with poor prognosis.
-
•
The two RAS pathway biomarkers, SIAH and EGFR, are prognostic in breast cancer.
-
•
The RAS pathway biomarkers can be used to stratify patients and guide therapies.
-
•
The RAS pathway biomarkers can be used to identify resistant tumor clones post NST.
-
•
SIAH and EGFR outperform ER, PR, HER2 and Ki67 in predicting patient survival.
The prognostic power of SIAH/EGFR is comparable to that of clinical predictors
Early stage breast cancer is amenable to standard of care therapies with excellent long-tern survival. Locally advanced and metastatic breast cancer has a much worse prognosis despite intense systemic and locoregional therapies. This disparity in prognosis underlines the acute need to identify resistant breast cancer cells, and administer and select effective therapies to eradicate resistant mammary tumors. We report that the activation of the EGFR/RAS/SIAH pathway can be used to identify resistant tumor clones and differentiate effective from ineffective therapies, and therefore predict tumor relapse and patient survival in human breast cancer during first-line neoadjuvant systemic therapy in clinic.
1. Introduction
Breast cancer is the second leading cause of cancer-related death among women in the United States (Siegel et al., 2016). An estimated 231,840 patients were diagnosed with breast cancer and 40,450 of them died of metastatic diseases in 2016 alone (Siegel et al., 2016, DeSantis et al., 2014). Increased screening, improved mammary imaging technologies, and targeted therapies have contributed to significant decreases in morbidity and mortality since the 1970s, and now more than 90-percent of patients with early-stage breast cancer survive longer than five years (Graham et al., 2014, DeSantis et al., 2015). Despite great scientific advancements and major clinical breakthroughs, patients diagnosed with invasive and malignant breast cancer still have a poor prognosis (Penault-Llorca and Radosevic-Robin, 2016, Rakha et al., 2008, Perou et al., 2000). The average survival for women diagnosed with metastatic disease is less than 2 years, despite aggressive therapies such as surgery, radiation, and chemo-, immuno- and targeted therapies (Graham et al., 2014, Swain et al., 2015, Zardavas et al., 2013, Baselga et al., 2012, Weigelt et al., 2005a). State-of-the-art treatment modalities, such as anti-HER2 therapy, anti-ER therapy, anti-PI3K and anti-mTOR therapy, tumor genome-guided combination therapies, stem cell therapy, and anti-CTLA-4/PD1 immunotherapy, alone or in combination, are not curative in eradicating metastatic breast cancer (El Saghir et al., 2011, McKeage and Perry, 2002, Romond et al., 2005, Piccart-Gebhart et al., 2005, Robert et al., 2006, Engelman, 2009). This is because invasive mammary tumors have tremendous inter- and intra-tumor heterogeneity that becomes more pronounced and diversified in metastatic diseases; therefore, effective therapies need to be personalized to shutdown the core tumor-driving pathways that promote resistant tumor clonal expansion in breast cancer.
Neoadjuvant systemic therapies (NST), such as cytotoxic, endocrine, targeted- and/or antibody-based agents, are routinely used to reduce tumor burden and control aggressive tumor growth in locally advanced, high-risk and malignant subtypes (King and Morrow, 2015). An increasing number of breast cancer patients with triple-negative breast cancer (TNBC), HER2-positive, inflammatory, invasive or basal-like breast cancer received NST prior to surgical resection (Arteaga et al., 2012, King and Morrow, 2015, Thompson and Moulder-Thompson, 2012, Nagayama et al., 2014). Preoperative NST provides a valuable window of opportunity for in vivo monitoring of tumor responses in real time, allowing evaluation of the antitumor therapeutic efficacy of a given therapy and, thus, offers invaluable prognostic information to predict outcome and survival. Patients who had “complete” responses to NST, with no residual tumor based on pathological analysis of breast tissues, survived longer than patients who had incomplete responses (Morrow, 2016, Wang-Lopez et al., 2015, Cortazar et al., 2014, Parinyanitikul et al., 2015, Petrarca et al., 2011). Therapy-induced tumor shrinkage measured by imaging technology in the clinicopathological settings can be used to distinguish effective versus ineffective NST and identify “complete” responders versus “non-responders” in clinical settings. However, determining therapeutic efficacy and quantifying tumor responses to a given therapeutic modality among “partial” responders—the largest percentage of breast cancer patients—remains a major challenge (Bulfoni et al., 2016, Falkson et al., 1992). Currently, there are no reliable clinical biomarkers that can be used to consistently predict survival and differentiate partial responders whose tumors regressed significantly versus the ones whose resistant tumor clones started emerging after tumor debulking post-NST.
In addition to imaging-guided technology to monitor the therapy efficacy, sensitive and reliable biomarker panels should be developed in the neoadjuvant setting to select, quantify, and optimize first-line therapies to treat locally advanced breast cancer. Breast tumor biomarkers such as ER, PR, and HER2 do not correlate with tumor response nor do they predict patient survival in high-grade, therapy-resistant, invasive and metastatic diseases (Tevaarwerk et al., 2013, Gown, 2008, Chen et al., 2012, Onitilo et al., 2009, Joensuu et al., 2013, Parise and Caggiano, 2014, Prat et al., 2015). Importantly, the clinical reality is that there is little to no flexibility in changing standard therapy once it is started. As a result, most patients with malignant tumors, especially those who only “partially” respond to a given therapy, have to endure the toxicities of the full regimens, often waiting months or years for tumor relapse, progressive diseases, and systematic metastasis before knowing whether the prescribed regimens were effective in eradicating their tumors. Patients who receive ineffective first-line therapies or experience therapy-induced resistant tumor clonal expansion will miss an important window if no effective follow-up therapy is added in a timely fashion to remedy the inadequacy. A majority of breast cancer patients fall under this category of “partial responders” and face uncertain futures. There is a pressing need to identify reliable and robust prognostic biomarkers to closely monitor tumor regression in real time, and importantly, to determine whether first-line therapies prescribed are effective against high-risk and invasive mammary tumors.
Although oncogenic K-RAS mutations are rare in mammary tumors (observed in about 5-percent of patients), genomic studies have indicated that the EGFR/HER2/K-RAS “pathway” is activated in a large proportion of aggressive and malignant breast cancers (Arteaga et al., 2012, Foulkes et al., 2010). EGFR/HER2/K-RAS activation has been correlated with shortened survival, resistance to therapy, and tumor relapse despite aggressive treatments in breast cancer (Tebbutt et al., 2013, Wright et al., 2015). As a major tumor-promoting signaling pathway, we investigated whether EGFR/HER2/RAS pathway biomarker expression can be added to evaluate therapy efficacy and predict patient survival in breast cancer. In this study, we report that activation or inactivation of the tumor-promoting RAS pathway biomarkers, SIAH and EGFR, is associated with tumor progression versus regression in mammary tumors post-NST. We find that NST-induced reduction of SIAH and EGFR expression can be used as surrogate prognostic biomarkers to quantify therapeutic efficacy, determine tumor responses, detect emerging resistant clones, and predict survival in invasive breast cancer, regardless of tumor heterogeneity, in the neoadjuvant setting.
2. Materials and Methods
2.1. Ethical Statement
With the proper approval by two Institutional Review Boards (IRB) at Eastern Virginia Medical School and Sentara Hospital Systems, this clinical study was conducted in full compliance of HIPAA regulations to protect patient privacy and confidentiality.
2.2. Patient Selection
This research project was designed and executed as per REMARK and RECIST criteria for tumor biomarker studies (McShane et al., 2005, Altman et al., 2012, Eisenhauer et al., 2009). This retrospective study was conducted using data from breast tumor tissue collected from all patients diagnosed with invasive and high-risk carcinoma of the breast between August 2007 and December 2010. A cohort of 182 women was identified who received NST treatment, and then surgical resection under the care of Sentara Hospital Systems. Clinicopathological and treatment course data were extracted and de-identified following extensive chart review of patients’ electronic medical records in Sentara’s EPIC database (Table 1). All patients received standard NST regimens as prescribed by their oncologists following NCI guideline (Supplemental Table S1). Patients typically received a combination of chemotherapies (anthracyclines, alkylating agents, taxanes, and/or metabolic inhibitors), plus hormone and/or anti-HER2 therapies in conjunction. Post-NST, all patients underwent surgical resections, performed by 24 local surgical oncologists, to excise their primary tumors (either total mastectomy, radical mastectomy, modified radical mastectomy, segmental mastectomy, or lumpectomy) (Supplemental Table S1).
Table 1.
Descriptive statistics of clinicopathological variables and molecular biomarker expression in this breast cancer patient cohort.
| Variables | Level | Median or Frequency | IQR or % | p-Value1 |
|---|---|---|---|---|
| A. Patient demographics | ||||
| Age (year) at diagnosis | 54.0 (27–88) | 17.8 | 0.00584 | |
| Body mass index (kg/m2) at diagnosis | 29.4 (16.8–60.6) | 9.0 | 0.608 | |
| Stage at diagnosis | 2.27 × 10− 8 | |||
| Stage I | 22 | 12.1% | ||
| Stage II | 101 | 55.5% | ||
| Stage III | 46 | 25.3% | ||
| Stage IV | 13 | 7.1% | ||
| Tumor size at diagnosis | 5.88 × 10− 10 | |||
| T1: ≤ 2-cm | 39 | 21.4% | ||
| T2: > 2-cm to ≤ 5-cm | 95 | 52.2% | ||
| T3: > 5-cm | 29 | 16.0% | ||
| T4: Inflammatory BC; chest wall infiltration | 19 | 10.4% | ||
| Tumor grade | 0.169 | |||
| I | 30 | 16.5% | ||
| II | 45 | 24.7% | ||
| III | 96 | 52.8% | ||
| Not determined | 11 | 6.0% | ||
| Tumor histology | 0.813 | |||
| Ductal | 159 | 87.4% | ||
| Lobular | 12 | 6.6% | ||
| Ductal + lobular | 10 | 5.5% | ||
| Mucinous | 1 | 0.5% | ||
| Lymph node status at diagnosis | 1.66 × 10− 7 | |||
| Negative | 79 | 43.4% | ||
| Positive | 103 | 56.6% | ||
| Molecular subtype | 0.0159 | |||
| Luminal A | 91 | 50.0% | ||
| Luminal B | 20 | 11.0% | ||
| HER2-type | 18 | 9.9% | ||
| Triple negative | 53 | 29.1% | ||
| B. Receptor status | ||||
| Variables | Level | Frequency | % | p-Value1 |
| Estrogen receptor (ER) status | 0.007 | |||
| Negative | 77 | 42.3% | ||
| Positive | 105 | 57.7% | ||
| Progesterone receptor (PR) status | 0.0237 | |||
| Negative | 88 | 48.4% | ||
| Positive | 94 | 51.6% | ||
| HER2 status | 0.644 | |||
| Negative | 144 | 79.1% | ||
| Positive | 38 | 20.9% | ||
| C. Treatment information | ||||
| Variables | Level | Frequency | % | p-Value1 |
| Neoadjuvant treatment | ||||
| Chemotherapy | Received | 153 | 84.1% | 0.245 |
| Did not receive | 29 | 15.9% | ||
| Hormone therapy | Received | 26 | 14.3% | 0.768 |
| Did not receive | 156 | 85.7% | ||
| Antibody therapy | Received | 32 | 17.6% | 0.652 |
| Did not receive | 150 | 82.4% | ||
| Pathological response after NAT | 0.0179 | |||
| Complete response (pCR) | No residual tumor | 33 | 18.1% | |
| Incomplete response (pIR) | Partial response | 117 | 64.3% | |
| No response | 15 | 8.2% | ||
| Unknown | 17 | 9.4% | ||
| Adjuvant treatment | ||||
| Chemotherapy | Received | 34 | 18.7% | 5.64 × 10− 6 |
| Did not receive | 148 | 81.3% | ||
| Hormone therapy | Received | 94 | 51.6% | 0.00692 |
| Did not receive | 88 | 48.4% | ||
| Antibody therapy | Received | 32 | 17.6% | 0.670 |
| Did not receive | 150 | 82.4% | ||
| Radiation therapy (XRT) | Received | 134 | 73.6% | 0.620 |
| Did not receive | 48 | 26.4% | ||
| D. RAS pathway biomarkers | ||||
| Variables | Level | Median or frequency |
IQR or % | p-Value1 |
| SIAH | ||||
| pre-NAT (needle biopsy) | Median expression, % | 40.0 (1.0–97.0) | 55.0 | |
| Low ≤ 30% | 83 | 45.6% | 0.0463 | |
| High > 30% | 95 | 52.2% | ||
| Not available | 4 | 2.2% | ||
| post-NAT (s/p resection) | Median expression, % | 3.0 (0.0–92.0) | 20.0 | |
| Low ≤ 30% | 150 | 82.4% | 0.00188 | |
| High > 30% | 30 | 16.5% | ||
| Not available | 2 | 1.1% | ||
| NAT-induced change | High > 30% ➔ Low ≤ 30% | 67 | 36.8% | 0.00144 |
| High > 30% ➔ High > 30% | 27 | 14.8% | ||
| Low ≤ 30% ➔ Low ≤ 30% | 81 | 44.5% | ||
| Not available | 7 | 3.9% | ||
| EGFR | ||||
| pre-NAT (needle biopsy) | Median expression, % | 0 (0-3 +) | 1 + | |
| Low ≤ + 1 | 131 | 72.0% | 0.00107 | |
| High > + 1 | 48 | 26.4% | ||
| Not available | 3 | 1.6% | ||
| post-NAT (s/p resection) | Median expression, % | 0 (0-3 +) | 1 + | |
| Low ≤ + 1 | 158 | 86.8% | 0.00513 | |
| High > + 1 | 23 | 12.6% | ||
| Not available | 1 | 0.6% | ||
| NAT-induced change | High > + 1 ➔ Low ≤ + 1 | 28 | 15.4% | 0.00146 |
| High > + 1 ➔ High > + 1 | 19 | 10.4% | ||
| Low ≤ + 1 ➔ Low ≤ + 1 | 127 | 69.8% | ||
| Not available | 8 | 4.4% | ||
| phospho-ERK | ||||
| pre-NAT | Median expression, % | 10.0 (0.0–95.0) | 27.5 | |
| Low < 1 | 39 | 21.4% | 0.429 | |
| High ≥ 1 | 138 | 75.8% | ||
| Not available | 5 | 2.8% | ||
| post-NAT | Median expression, % | 0.0 (0.0–95.0) | 0.0 | |
| Low < 1 | 150 | 82.4% | 0.141 | |
| High ≥ 1 | 29 | 15.9% | ||
| Not available | 3 | 1.7% | ||
| NAT-induced change | High ≥ 1 ➔ Low < 1 | 113 | 62.1% | 0.336 |
| High ≥ 1 ➔ High | 24 | 13.2% | ||
| Low < 1 ➔ Low < 1 | 35 | 19.2% | ||
| Not available | 10 | 5.5% | ||
| Ki67 | ||||
| pre-NAT | Median expression, % | 11.3 (0.0–92.5) | 21.9 | |
| Low ≤ 10% | 89 | 48.9% | 0.764 | |
| High > 10% | 89 | 48.9% | ||
| Not available | 4 | 2.2% | ||
| post-NAT | Median expression, % | 1.0 (0.0–94.5) | 5.5 | |
| Low ≤ 10% | 151 | 83.0% | 0.390 | |
| High > 10% | 30 | 16.5% | ||
| Not available | 1 | 0.5% | ||
| NAT-induced change | High > 10% ➔ Low ≤ 10% | 63 | 34.6% | 0.601 |
| High > 10% ➔ High > 10% | 25 | 13.7% | ||
| Low ≤ 10% ➔ Low ≤ 10% | 86 | 47.3% | ||
| Not available | 8 | 4.4% | ||
| E. Clinical outcomes of overall survival and vital status | ||||
| Variables | Level | Median or frequency | IQR or % | |
| Length of disease (days since diagnosis) | 1610 | 622 | ||
| (230-2438) | ||||
| Vital status at last follow-up | ||||
| Alive | 149 | 81.9% | ||
| without distant metastasis | 134 | 73.6% | ||
| with distant metastasis | 15 | 8.3% | ||
| Dead | 33 | 18.1% | ||
| without distant metastasis | 3 | 1.6% | ||
| with distant metastasis | 30 | 16.5% | ||
Log-rank test for categorical variables and Cox proportional hazards model (or Wilcoxen two-sample t-test) for continuous variable.
The date of diagnosis was defined as the date of initial fine-needle aspiration biopsy of the primary breast tumor that led to the initial diagnosis. The only selection criteria were the availability of the matched pre- and post-NST tumor biospecimens. Study endpoints were determined either by the date of patient death, or the last documented follow-up examination at time of chart review. Data on tumor stage at diagnosis (pathological and clinical), treatment courses including neoadjuvant and adjuvant therapies (chemotherapy, hormone therapy, and/or antibody therapy), patient outcome (cancer remission, metastasis, or recurrence), and vital status were obtained per review of patients’ records (see Table 1 and Supplemental Table S1). Data were collected on tumor histological type, size, pathological grade, hormone receptor (ER and PR) or HER2 expression, lymph node status, and response to NST from pathology reports in EPIC.
2.3. Pathology Samples
Following the dual IRB approval at EVMS and Sentara, we collected tumor samples and clinical data from a total of 182 patients with primary operable breast tumors, including 364 paraffin-embedded tumor blocks of invasive carcinoma collected pre- and post-NST from eight Sentara hospitals: Sentara Careplex Hospital (Hampton, VA), Sentara Leigh Hospital (Norfolk, VA), Sentara Norfolk General Hospital (Norfolk, VA), Sentara Obici Hospital (Suffolk, VA), Sentara Port Warwick Hospital (Newport News, VA), Sentara Princess Anne Hospital (Virginia Beach, VA), Sentara Virginia Beach General Hospital (Virginia Beach, VA), and Sentara Williamsburg Regional Medical Center (Williamsburg, VA).
Histology and pathology analyses were performed by two board-certified clinical pathologists, JSW and CFO. Pathology and immunohistochemistry protocols have been optimized to detect the ER, PR, HER2, EGFR, phosphorylated ERK (phospho-ERK), Ki67, and SIAH expression from the matched pairs of tumor blocks pre- and post-NST. All staining and pathological analyses were conducted at the Pathology Sciences Medical Group and Sentara Norfolk General Hospital’s Pathology Unit under the supervision of J.S. Winston, M.D., a breast disease expert and clinical pathologist who identified representative tumor paraffin blocks, coordinated and guided the pathological study, controlled the quality of the IHC staining, and scored the slides independently along with C.F. O’Connor in a double blind fashion. The diagnostic tumor needle biopsy pre-NST and surgically resected tumor tissues post-NST were selected for serial immunohistochemistry (IHC) staining and clinicopathological studies.
2.4. Immunohistochemistry
Four-micrometer tissue sections were cut from tumor paraffin blocks and slides were stained using Ventana Benchmark Ultra, according to the manufacturer’s protocol. The IHC experiments were carried out using monoclonal anti-EGFR (Ventana clone 5B7, pre-diluted, Ventana Medical Systems Cat# 790-4347, Roche, USA, RRID:AB_2617183), monoclonal anti-phospho-ERK (1:750 dilution, Cell Signaling, Danvers, MA, RRID:AB_331646), monoclonal anti-SIAH 24E6H3 (1:40 dilution, Novus, CA, RRID:AB_1217916), monoclonal anti-Ki67 (1:100 dilution, Dako, Denmark, RRID:AB_2142367), monoclonal anti-ER (Ventana clone SP1, pre-diluted, Roche, USA, RRID:AB_2335977), monoclonal anti-PR (Ventana clone 1E2, pre-diluted, Roche, USA, RRID:AB_2335976), and monoclonal anti-HER2 (Ventana clone 4B5, pre-diluted, Roche, USA, RRID:AB_2335975) antibodies. The IHC was performed on a BenchMark ULTRA fully automated IHC/ISH staining instrument (Ventana Medical Systems, Inc., Tucson, AZ).
2.5. Tumor Block Review and the Immunohistochemical Quantification
Original hematoxylin and eosin (H&E) and immunohistochemistry (IHC) slides used for clinical diagnosis collected pre- and post-NST for each patient (1-3 slides per patient) were independently reviewed and scored in a double blind manner by JSW and CFO. Slides stained for ER, PR, Ki67, or SIAH, were scored using the percentage of positive IHC staining within the tumor. Slides with a score discrepancy of greater than 10-percent were then dually re-evaluated by two pathologists together to reach a final consensus score. Slides stained for HER2 or EGFR were scored as 0 (no membrane staining), 1 + (partial membrane staining), 2 + (complete membrane staining in less than 30% of the tumor cells), or 3 + (complete membrane staining in more than 30% of the tumor cells). Slides stained for phospho-ERK were scored as the percentage of tumor cells with cytoplasmic staining. All histology images presented were captured at 400-fold magnification using a Leica compound microscope and Leica DC500 digital camera.
2.6. Statistical Analyses
Overall survival times ranged from 230 days (0.6 years) to 2438 days (6.7 years), with a median survival of 1610 days (4.4 years). For patients initially diagnosed without distant metastasis, time of distant recurrence-free survival was calculated either as the number of days from the date of initial diagnosis until the date of first documented metastasis per imaging studies, or censored at the date of last documented follow-up evaluation listed in the EPIC database (as of August 31, 2014).
To describe the data, medians and interquartile ranges (IQR) were used for continuous variables while frequencies and percentages were used for categorical variables. Spearman’s correlation coefficients were calculated to evaluate correlations among clinicopathological and molecular biomarker variables (Table 2). The Kaplan-Meier method was used for all survival curve analyses. In univariate analysis, the log-rank test was used to calculate the difference in survival for categorical variables. The Cox proportional hazards model was used for continuous variables; this model was also used for multivariate survival analyses of the association of survival with patient age, tumor size, molecular subtypes, stage, presence of lymph node metastasis, and the level of ER, PR, HER2, phospho-ERK, Ki67, SIAH, or EGFR (Table 3). The proportional hazards model assumptions were verified using the log-transformation plot; goodness-of-fit of the models were checked by martingale and deviance residual plots. The time-dependent receiver operating characteristic (survival ROC) was applied to display and compare the sensitivity and specificity of the predictive models based on the multivariate survival analysis (Heagerty and Zheng, 2005). The survival ROC curves were used to accommodate the time-dependent nature and censoring in the survival data. All P-values calculated were two-sided. A P-value of less than 0.05 was considered statistically significant. Statistical plots were generated using R software Version 3.2.1 (R Core Team, 2015).
Table 2.
Spearman's correlation coefficients between clinical variables, ER, PR, HER2 and RAS pathway biomarkers.
| Clinical variables or Biomarkers | Stage | Tumor Size | Tumor grade | Lymph node status | Molecular subtype | ER | PR | HER2 |
pre-NAT |
post-NAT |
Relative change |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SIAH | EGFR | SIAH | EGFR | SIAH | EGFR | ||||||||||
| Stage | 1 | ||||||||||||||
| Tumor size | 0.66 | 1 | |||||||||||||
| Tumor grade | 1 | ||||||||||||||
| Lymph node status | 0.53 | 0.38 | 1 | ||||||||||||
| Molecular subtype | 0.50 | 1 | |||||||||||||
| ER | 0.91 | 1 | |||||||||||||
| PR | -0.48 | 0.79 | 0.75 | 1 | |||||||||||
| HER2 | 1 | ||||||||||||||
| pre-NAT | SIAH | 0.48 | 0.45 | − 0.47 | -0.41 | 1 | |||||||||
| EGFR | 0.31 | 0.32 | − 0.37 | -0.34 | 0.35 | 1 | |||||||||
| post-NAT | SIAH | 0.49 | 0.47 | − 0.53 | -0.43 | 0.80 | 0.71 | 1 | |||||||
| EGFR | 0.47 | 0.50 | − 0.57 | -0.47 | 0.51 | 0.37 | 0.53 | 1 | |||||||
| Relative change | SIAH | 0.32 | 0.30 | − 0.36 | -0.31 | 0.35 | 0.55 | 0.52 | 0.57 | 1 | |||||
| EGFR | 0.43 | 0.36 | − 0.44 | -0.36 | 0.48 | 0.42 | 0.79 | 0.54 | 1 | ||||||
Note: Only correlations that are statistically significant (p ≤ 0.05), with coefficients greater than | 0.30 | are shown.
Table 3.
Cox proportional hazards regression models with clinical variables and RAS pathway biomarkers
| Predictors | Level | Hazard ratio | 95% Confidence interval |
p-Value | |
|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||
| A. Pre-neoadjuvant therapy | |||||
| Age | – | 1.0409 | 1.0079 | 1.0749 | 0.01459* |
| Molecular subtype | Luminal A | 0.2437 | 0.09739 | 0.6097 | 0.002549** |
| Luminal B | |||||
| HER-2 type | |||||
| TNBC | |||||
| Histology | Ductal | 2.1953 | 1.0874 | 4.4318 | 0.02825* |
| Lobular | |||||
| Mixed | |||||
| Tumor size | T1 | 2.1612 | 1.3838 | 3.3754 | 0.0007050*** |
| T2 | |||||
| T3 | |||||
| T4 | |||||
| ER | Negative | 15.0042 | 1.4257 | 157.9058 | 0.02411* |
| Positive | |||||
| pre-Ki67 | Low ≤ 10% | 0.3518 | 0.1497 | 0.8267 | 0.01655* |
| High > 10% | |||||
| pre-SIAH | Low ≤ 30% | 1.2926 | 0.5117 | 3.2652 | 0.5872 |
| High > 30% | |||||
| pre-EGFR | Low ≤ + 1 | 2.1204 | 0.8789 | 5.1150 | 0.09435 |
| High > + 1 | |||||
| B. Post-neoadjuvant therapy | |||||
| Age | – | 1.0283 | 0.9951 | 1.0636 | 0.09590 |
| Molecular subtype | Luminal A | 0.3467 | 0.1412 | 0.8513 | 0.0208* |
| Luminal B | |||||
| HER-2 type | |||||
| TNBC | |||||
| Histology | Ductal | 2.6777 | 1.2805 | 5.5994 | 0.008876** |
| Lobular | |||||
| Mixed | |||||
| Tumor size | T1 | 2.07757 | 1.3575 | 3.1795 | 0.000758*** |
| T2 | |||||
| T3 | |||||
| T4 | |||||
| ER | Negative | 7.8666 | 0.7989 | 77.4615 | 0.07714 |
| Positive | |||||
| post-Ki67 | Low ≤ 10% | 0.2680 | 0.07119 | 1.0087 | 0.05152 |
| High > 10% | |||||
| post-SIAH | Low ≤ 30% | 4.4579 | 1.3383 | 14.8494 | 0.01491* |
| High > 30% | |||||
| post-EGFR | Low ≤ + 1 | 2.4207 | 0.8759 | 6.6906 | 0.088304 |
| High > + 1 | |||||
| C. Relative change after neoadjuvant therapy | |||||
| Age | – | 1.0532 | 1.0176 | 1.0901 | 0.00316** |
| Molecular subtype | Luminal A | 0.2199 | 0.08659 | 0.5586 | 0.00145** |
| Luminal B | |||||
| HER-2 type | |||||
| TNBC | |||||
| Histology | Ductal | 2.3088 | 1.1511 | 4.6308 | 0.01846* |
| Lobular | |||||
| Mixed | |||||
| Tumor size | T1 | 1.8953 | 1.2286 | 2.9239 | 0.00384** |
| T2 | |||||
| T3 | |||||
| T4 | |||||
| ER | Negative | 24.2180 | 2.1442 | 273.5388 | 0.00998** |
| Positive | |||||
| ΔKi67 | Low ➔ Low | 0.4504 | 0.2086 | 0.9728 | 0.04234* |
| High ➔ Low | |||||
| High ➔ High | |||||
| ΔSIAH | Low ➔ Low | 1.8881 | 0.8338 | 4.2757 | 0.1275 |
| High ➔ Low | |||||
| High ➔ High | |||||
| ΔEGFR | Low ➔ Low | 2.5837 | 1.3507 | 4.9426 | 0.00413** |
| High ➔ Low | |||||
| High ➔ High | |||||
P < 0.05
P < 0.01
P < 0.001
3. Results
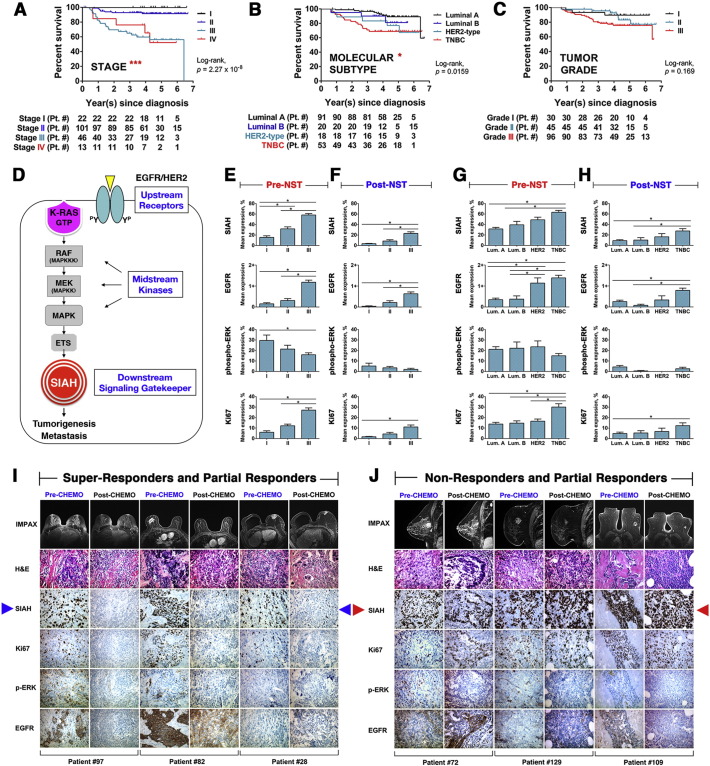
3.1. Increased Expression of SIAH and EGFR, Two RAS Pathway Biomarkers, are Correlated With Advanced Tumor Grade and Aggressive Molecular Subtypes
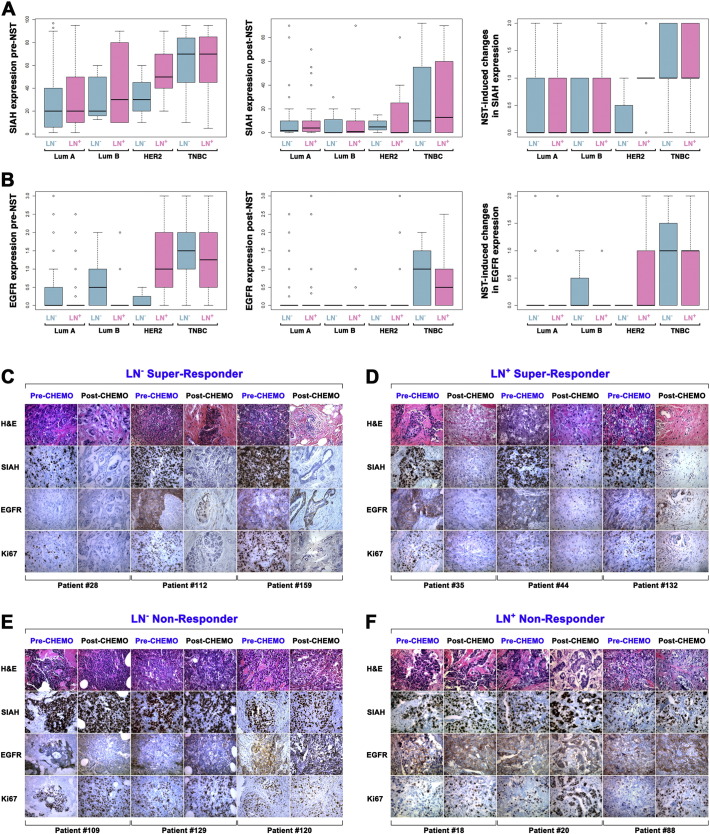
It is challenging to predict accurately which individual breast cancer patients will benefit from standard therapies and which ones will not, based on the existing biomarker panels and advanced imaging technology. Consistent with the published literature, the increased pathological stage and aggressive molecular subtypes were correlated with poor clinical outcome and reduced patient survival (Fig. 1A and B), while increased tumor grades were not associated with survival at 7-years (Fig. 1C). To demonstrate the prognostic values of the tumor-driving RAS pathway biomarkers in high-risk breast cancer patients, we compared altered expression levels of 4 biomarkers in the RAS pathway in the paired tumor biospecimens collected in pre- and post-NST (Fig. 1D). That includes two upstream receptors (HER2 and EGFR), a mid-stream kinase (phospho-ERK) and the most downstream E3 ligase (SIAH) in paired tumor biospecimens collected from 182 breast cancer patients who received NST at Sentara Hospitals. The 4 mammary tumor biomarkers, HER2, ER, PR and Ki67, were utilized as internal controls for this study. Among all the 182 treatment-naive needle biopsy tumor biospecimens, SIAH expression was detected in 97.8% of all the SIAH IHC slides – 52.2% of them displayed a high SIAH expression level that marked more than 30% of tumor cells, whereas 45.6% of them displayed a low SIAH expression that marked equal or less than 30% of tumor cells; Similarly, EGFR expression was detected in 26.4% of all the EGFR IHC slides, with an EGFR score that was higher than 1; and phospho-ERK expression was detected in 75.8% of the phospho-ERK IHC slides (Table 1). As controls, HER2 was detected in 20.9% of treatment-naïve tumor biospecimens, ER in 57.7% and PR in 51.6% (Table 1). SIAH expression was associated with active tumor cell proliferation, and it was robust, tumor-specific, and therapy-responsive (Fig. 1 and Supplemental Fig. S1). Mean levels of SIAH, EGFR, and Ki67 increased with tumor grade and aggressive molecular subtypes at treatment-naïve tumors (Fig. 1E and G). Increased tumor grade and aggressive molecular subtype exhibited a much higher level of endogenous SIAH or EGFR expression pre- and post-NST in breast cancer (Fig. 1F and H).
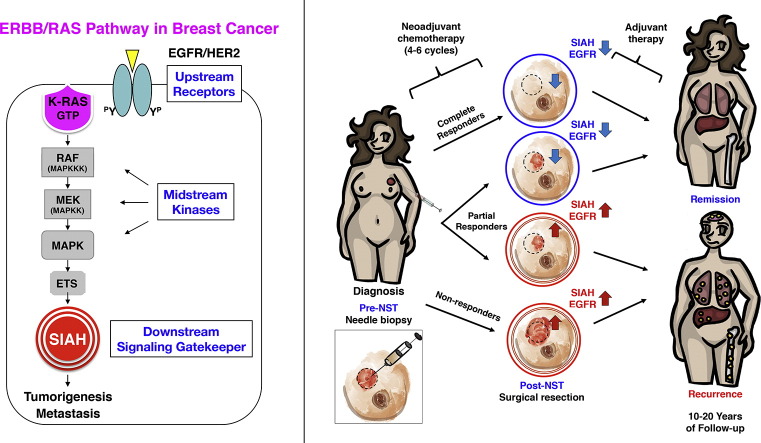
Fig. 1.
Two RAS pathway biomarkers, SIAH and EGFR, whose expression in breast cancer correlated with increased tumor grades and aggressive molecular subtypes pre- and post-NST.
(A) Pathological stages are statistically associated with patient survival with P value of 2.27 × 10− 8. (B) Molecular subtypes are statistically associated with patient survival with P value of 0.016. (C) Tumor grade are not associated with patient survival. (D) A schematic illustration of the EGFR/HER2/RAS signaling pathway is shown. The expression of the two upstream receptors, EGFR/HER2, the midstream kinases, phospho-ERK, and the most downstream signaling gatekeeper, SIAH E3 ligase, were examined. (E–F) Levels of SIAH, EGFR, and Ki67 expression in breast cancer increased with tumor grades, pre- and post-NST. NST significantly reduced levels of EGFR, SIAH, phospho-ERK, and Ki67. (G–H) Levels of SIAH, EGFR, and Ki67 expression increased in aggressive molecular subtypes of mammary tumors, pre- and post-NST. Levels of phospho-ERK expression were not correlated with molecular subtypes. NST significantly reduced levels of EGFR, SIAH, phospho-ERK, and Ki67, in a majority of resected primary mammary tumors. (I–J) Radiographic (MRI) images of human breasts and breast cancer pre- and post-NST were shown. Treatment-naïve tumor cells in high-grade and high-risk mammary tumors expressed high SIAH. (I) Fewer tumor cells expressed high SIAH post-NST. Based on diminished SIAH expression, we categorized patients as super-responders, whose tumor regressed completely post-NST. Three patients had dramatic reductions in SIAH expression (marked by blue arrows); more than 95% of tumor cells expressed SIAH pre-NST, compared with less than 1% tumor cells expressed SIAH post-NST. These patients had no evidence of tumor recurrence 7 years post-NST. (J) Three patients whose tumors had a high SIAH expression post-NST (marked by red arrows) were categorized as non-responders, whose tumors regress less than 20-30% post-NST, and who had shortened survival time due to progressive diseases and LN metastases.
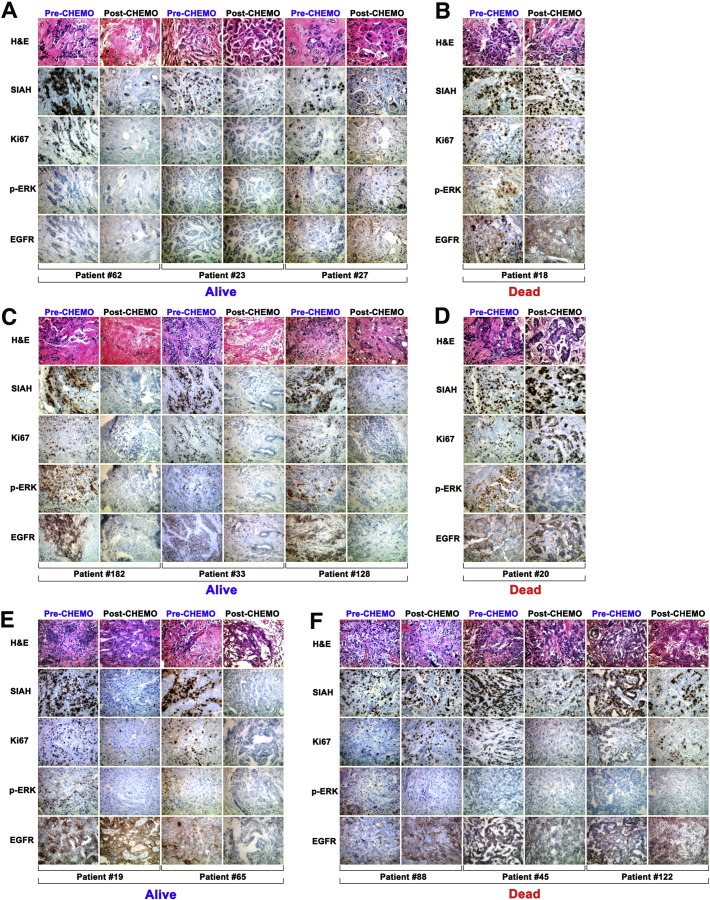
Supplementary Fig. S1.
Low or high SIAH and/or EGFR expression post-NST is associated with increased or reduced patient survival in breast cancer at 5 years
Representative results from immunohistochemical analyses of patients who were alive at 5-year post-NST (A, C, and E), or who were dead at 5-year post-NST (B, D, and F). Patients with luminal-type breast tumors (A and B), HER2-positive breast tumors (C and D), or triple negative breast cancer (E and F). (A, C and E) Loss of SIAH expression post NST correlates with increased patient survival. Persistent high SIAH or high EGFR expression post-NST is associated with decreased patient survival. For all mammary tumor subtypes, patients who survived for longer than 5 years had low or markedly reduced level of SIAH expression post-NST, than patients who succumbed in less than 5 years.
3.2. A marked Reduction of SIAH and EGFR Expression Post-NST is Correlated With Tumor Regression, as Reflected in the NST Efficacy and Increased Patient Survival
We hypothesized that effective NST, which has been shown to slow tumor growth and reduce tumor volume, would inactivate or decrease the RAS pathway signal that drives mammary tumor progression and metastasis. Ineffective therapy would therefore neither impede the active RAS pathway signal nor inhibit tumor growth. Importantly, therapy-resistant tumor clones can be easily identified by SIAH-positive staining in the surgically resected tumors post-NST. Thus, loss of and/or decrease in SIAH expression, which reflects the inactivation of this tumor-promoting RAS pathway, may serve as a reliable indicator of an effective NST modality. In contrast, persistent SIAH expression, reflecting RAS pathway activation, may serve as a reliable predictor of an ineffective NST modality and a sensitive readout of emerging resistant clones in invasive breast cancer.
Compared to the mean expression levels of SIAH, EGFR, and Ki67 in treatment-naïve tumors (Fig. 1E and G), SIAH, EGFR, and Ki67 expression decreased significantly in a majority of resected tumors post-NST, suggesting that current standard NST is effective in suppressing mammary tumor growth (Fig. 1F and H). The pattern of the mean level of SIAH, EGFR and Ki67 expression remained similar and statistically significant pre- and post-NST, correlated with grades, stages and molecular subtypes (Fig. 1E–H). In contrast, mean levels of phospho-ERK expression decreased with increased tumor grades (Fig. 1E). The NST-induced loss of SIAH expression could be used to identify super-responders and partial responders with good outcome and best survival (Fig. 1I and Supplemental Fig. S1A, S1C and S1E), whereas the persistent high SIAH expression post NST seemed to identify non-responders and partial responders with poor outcome and reduced survival (Fig. 1J and Supplemental Fig. S1B, S1D and S1F).
3.3. The Prognostic Value of SIAH and EGFR Expression in Breast Cancer Pre- and Post-NST
To determine whether the therapy-induced reduction in SIAH, EGFR and Ki67 pre- and post-NST have added prognostic values in breast cancer, we conducted statistical analyses to examine the clinical outcome of the breast cancer patients whose tumors retained high SIAH/EGFR/Ki67 expression post-NST, versus that of the patients whose tumors lost SIAH/EGFR/Ki67 expression completely or partially post-NST when compared to their treatment-naïve tumors. We segregated treatment-naïve tumors (182 of them) into two roughly equal groups: “high” SIAH expression (> 30% positive tumor cells) and “low” SIAH expression (≤ 30% positive tumor cells). At the time of diagnosis prior to NST, about 50% patients had tumors with high SIAH expression and another 50% patients had low SIAH expression (Table 1). The same criteria of high (> 30%) or low (≤ 30%) SIAH expression was applied to the surgically resected tumors post-NST. Following clinical classification, high EGFR expression is defined as > 1+ (score of 2+ or 3+); while negative or low EGFR expression is defined as ≤ 1+ (score of 1+ or 0) in tumor cells. About 50% patients had tumors with high Ki67 expression (> 10% positive tumor cells) and another 50% patients had low Ki67 expression (≤ 10% positive tumor cells) pre-NST. The same criteria of high (> 10%) or low (≤ 10%) Ki67 biomarker expression was applied to the surgically resected mammary tumors post-NST (Table 1).
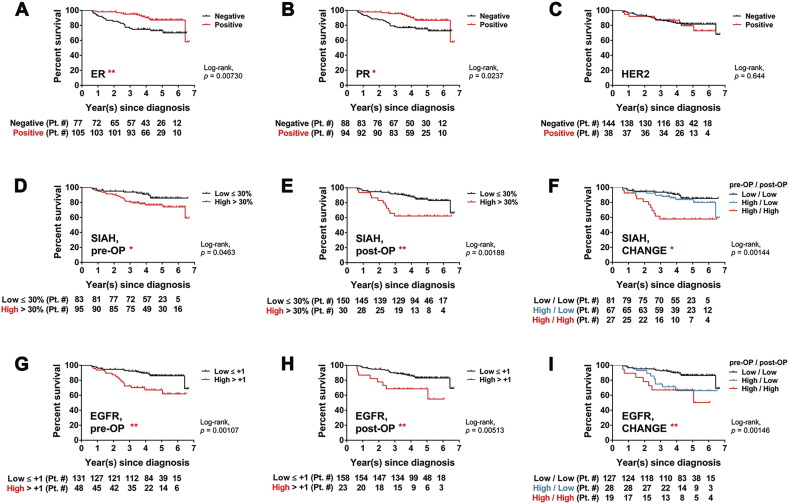
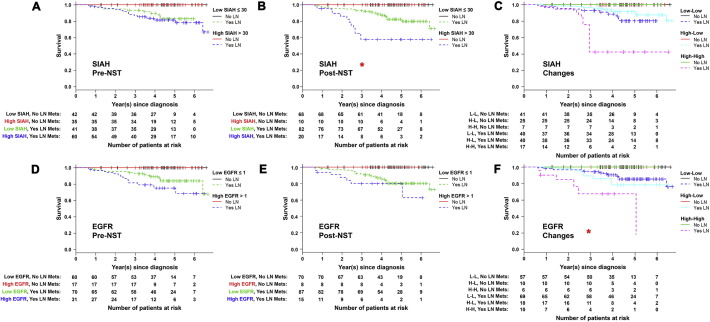
By performing the univariate analysis over a 7-year period, we found that ER, PR, SIAH, and EGFR expression was statistically associated with survival (log-rank P < 0.05) (Fig. 2). The ER and PR positivity in luminal breast tumors was a biomarker of extended survival (Fig. 2A and B). In contrast, the level of HER2, phospho-ERK or Ki67 expression was not associated with tumor response or patient survival (Fig. 2C and data not shown). Activation of the EGFR/SIAH pathway is indicative of tumor progression, while its inactivation is indicative of mammary tumor regression post-NST. Hence, low levels of SIAH and/or EGFR expression post-NST correlated with tumor regression and increased survival, whereas high levels of SIAH and/or EGFR expression in tumor biospecimens pre- and post-NST were associated with progressive diseases, therapy-induced resistance, metastasis to sentinel lymph node and beyond, and decreased survival (Fig. 1, Fig. 2). Univariate analysis showed that the endogenous SIAH/EGFR expression in mammary tumors pre- and post-NST, and therapy-induced reduction in SIAH or EGFR expression post-NST, were prognostic of patient survival (Fig. 2D–I).
Fig. 2.
Kaplan-Meier survival curves of SIAH and EGFR expression in univariate analyses.
(A) Tumor expression of ER is associated with increased patient survival with P value of 0.007. (B) Tumor expression of PR is associated with increased patient survival with P value of 0.024. (C) Tumor expression of HER2 is not associated with patient survival. (D) Tumor expression of high SIAH expression (> 30% of tumor cells expressing SIAH) pre-NST is associated with decreased patient survival with P value of 0.046. (E) Tumor expression of high SIAH level (< 30% of tumor cells expressing SIAH) post-NST is associated with decreased patient survival with P value of 0.002. (F) Therapy-induced reduction in SIAH expression post-NST is associated with increased patient survival with P value of 0.001. (G) Tumor expression of high EGFR level (> 1) pre-NST is associated with decreased patient survival with P value of 0.001. (H) Tumor expression of high EGFR level (> 1) post-NST is associated with decreased patient survival with P value of 0.005. (I) Therapy-induced reduction in EGFR expression post-NST is associated with increased survival time with P value of 0.001.
3.4. The Prognostic Value of SIAH and EGFR in Metastatic Breast Cancer
Univariate analyses showed an added prognostic value of SIAH and EGFR, to predict tumor response, therapy efficacy and patient survival in breast cancer (Fig. 2). Clinically, the detection of sentinel lymph node (LN) metastases at diagnosis is clearly associated with a reduced survival in breast cancer (Penault-Llorca and Radosevic-Robin, 2016, Jatoi et al., 1999, Weigelt et al., 2005a, Ozmen et al., 2015, Gipponi et al., 2004, Rakha et al., 2012). Cancer-related deaths occurred only in patients whose tumors had spread to the sentinel lymph nodes (LN) and beyond. Increased SIAH, EGFR and Ki67 expression were detected in metastatic breast cancer, including malignant tumors that had invaded sentinel LN, and/or metastasized to distant organs, as compared to mammary tumors with no detectable LN metastases (Fig. 1, Fig. 3, and Table 1). Here, our survival analyses were further stratified by LN status. All patients without LN metastases survived for the 7-year duration of this study, and thus their 5–7 years survivals are independent of tumor grades, pathological stages, molecular subtypes, and biomarker expression (Fig. 4). Importantly, we found that SIAH and EGFR have additional prognostic values to predict patient survival in metastatic breast cancer (Fig. 3, Fig. 4).
Fig. 3.
SIAH and EGFR expression in node-negative and node-positive breast cancer pre- and post-NST.
(A–B) The box-and-whisker plots were used to graphically illustrate the population distribution of SIAH (A) and EGFR (B) expression levels in both node-positive (as marked by purple color bar graphs) and node-negative (as marked by teal color bar graphs) mammary tumors. The SIAH/EGFR expression patterns in the 4 molecular subtypes, Luminal A (Lum A) or Luminal B (Lum B), HER2+ and TNBC, were shown pre- and post-NST treatment. The error bars or whiskers in the histogram and bar charts represent the 95% CI, and in the box plots, they represent the upper (top) and lower quartiles (bottom) data distribution – with points beyond representing outliers. (C–F). Serial micro-sections were cut from each tumor paraffin blocks and the tissue slides were stained with H&E, SIAH, EGFR or Ki67 monoclonal antibody. Representative images of SIAH, EGFR, Ki67 staining in node-negative super-responders (C), node-positive super-responders (D), and node-negative non-responders (E) and node-positive non-responders (F), were shown. SIAH marked proliferating tumor cells, independent of nodal status in breast cancer. Note the lack of SIAH expression in super-responders post-NST correlated with good outcome, whereas persisted SIAH expression post-NST in non-responders correlated with poor survival.
Fig. 4.
Among patients with LN metastases, decreased SIAH expression post-NST and therapy-induced reduction in EGFR expression, are correlated with improved patient survival.
Kaplan-Meier analysis was used to generate survival curves to compare survival time among patients with and without LN metastases, whose tumors have high versus low SIAH/EGFR expression pre- and post-NST. Death occurred only in patients with LN metastases: all patients without sentinel LN metastases survived for 7-years, independent of biomarker expression. (A) SIAH expression in treatment-naïve tumors did not correlate with survival. (B) SIAH expression post-NST statistically correlated with survival. (C) Therapy-induced changes in SIAH expression did not correlate with survival. (D) EGFR expression in treatment-naïve tumors did not correlate with survival. (E) EGFR expression post-NST did not correlate with survival. (F) Therapy-induced changes in EGFR statistically correlated with patient survival. Numbers of patients at risk are listed under each K-M curve.
Among patients with LN metastases, the therapy-induced reduction in SIAH expression seemed to correlate with increased survival; i.e., the patients with low SIAH expression in both pre- and post-NST settings have the best prognosis and the greatest survival benefit despite their invasion phenotypes, whereas patients with high SIAH expression in both pre- and post-NST settings have the worst prognosis, suggesting that either ineffective therapy was administered or therapy-induced resistant tumor clones were emerging (Figs. 3A, C–F, 4A, B and C). The patients with high SIAH expression at diagnosis and low SIAH expression post-NST have intermediate survival, suggesting that an effective NST modality was administered to inhibit tumorigenesis (Figs. 3A, 4A, B and C). Among patients with LN metastases, the therapy-induced reduction in EGFR expression is statistically significant to correlate with increased survival (Figs. 3B, C–F, 4D, E and F). Patients with low EGFR expression in both pre- and post-NST settings have the best prognosis despite their invasive phenotypes and LN positivity, whereas patients with high EGFR expression in both pre- and post-NST settings have the worst prognosis, and patients with high EGFR expression at diagnosis and low EGFR expression post-NST have intermediate survival (Figs. 3B, 4D, E and F). In the cases of persistent high EGFR expression post-NST, additional anti-EGFR therapy should be added to control these therapy-refractory and EGFR-positive mammary tumors that have metastasized.
3.5. Multivariate Survival Analysis
To demonstrate the clinical prognostic values of SIAH and EGFR in breast cancer, we performed multivariate analyses of 7 molecular biomarkers (SIAH, EGFR, phospho-ERK, ER, PR, HER2 and Ki67) and compared them to 4 clinicopathological predictors (pathological stages, molecular subtypes, tumor size and LN positivity). ER, PR, HER2 and Ki67 were used as biomarker controls. Variables that were significantly associated (P < 0.05) with patient survival included molecular subtype, histology, tumor size, Ki67 expression at diagnosis, SIAH expression post-NST, and a NST-induced reduction in EGFR and Ki67 expression in patients with metastatic diseases (Fig. 3, Table 2 and Table 3). Older age pre-NST and high SIAH expression in tumors post-NST were significantly associated with reduced survival (Table 3). Survival curves, stratified by LN status, showed that a significantly smaller proportion of patients whose invasive tumors had high levels of SIAH and EGFR pre- and post-NST survived for 7 years post-NST, compared to patients whose invasive tumors had low levels of SIAH and EGFR expression pre- and post-NST (Fig. 4 and Table 3).
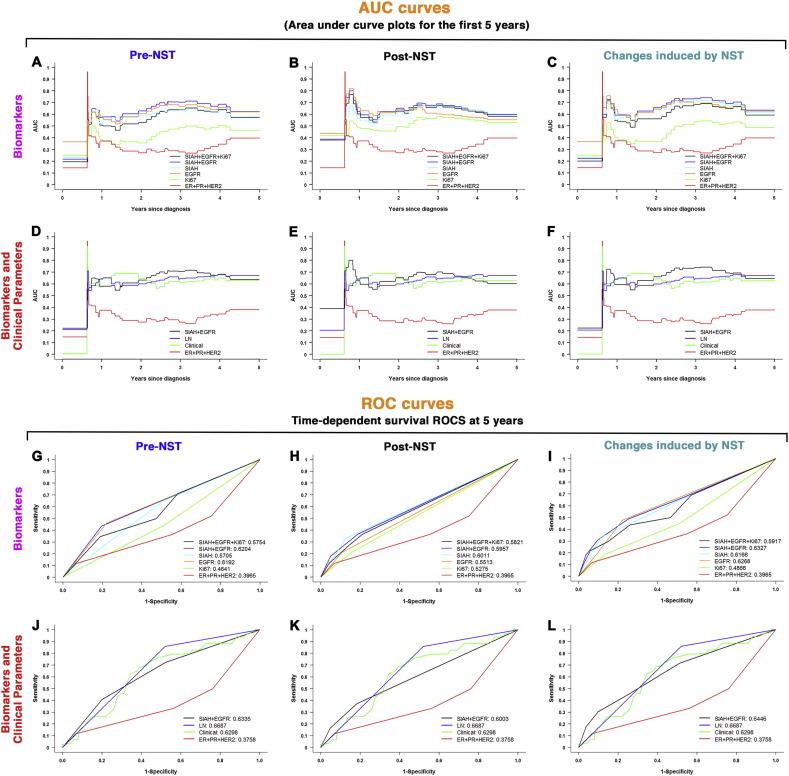
Multivariate time-dependent survival receiver operating characteristic (survival ROC) and area under the curve (AUC) plots were generated to determine the ability of various combinations of 7 molecular biomarkers and 4 clinicopathological predictors to predict patient survival (Fig. 5). Of all biomarker combinations analyzed, SIAH, EGFR and SIAH/EGFR combination had the highest AUC and ROC values in predicting patient survival (Fig. 5A, B and C, and Fig. 5G, H and I). Notably, these two RAS pathway biomarkers alone have demonstrated an impressive prognostic power that is comparable to that of the clinical gold standards, i.e., LN metastases and/or 4 clinical predictors in combination (Fig. 5D, E, and F, and Fig. 5J, K and L). The prognostic values of SIAH and EGFR are far superior to that of Ki67, and/or ER, PR, and HER2 — the universally used biomarker standards in breast cancer clinics (Fig. 5A, B and C, and Fig. 5J, K and L). The ROC and AUC analyses demonstrated that clinicopathological predictors are still a reliable predictor of patient survival at 5–7 years, and we discovered that SIAH and EGFR are highly prognostic in breast cancer (Fig. 5). It is important to note that the prognostic values of SIAH and EGFR are comparable to the best clinical prognostic tools available in metastatic breast cancer (Fig. 5).
Fig. 5.
Multivariate analyses of six biomarkers, LN metastases, and four clinicopathological predictors in predicting patient survival in breast cancer.
(A–C) Area under curve (AUC) plots for the first 5-years based on SIAH, EGFR, Ki67 and/or ER/PR/HER2 expression, alone or in combination, are shown, pre-NST (A), post-NST (B), and NST-induced reduction of these six biomarkers (C). The AUC plots include ER, PR and HER2 in combination (red line), Ki67 (neon green line), EGFR (orange line), SIAH (light teal line), SIAH and EGFR (blue line), SIAH, EGFR and Ki67 in combination (black line). The data showed that SIAH and EGFR, alone or in combination, outperform ER, PR, HER2 and/or Ki67 in predicting patient survival. (D–F) AUC plots for the first 5-years based on SIAH and EGFR, ER/PR/HER2 expression, LN metastases and/or four clinicopathological predictors, alone or in combination, in pre-NST (D), post-NST (E), and NST-induced reduction of these six biomarkers and four clinicopathological predictors (F). The AUC plots include ER, PR and HER2 in combination (red line), 4 clinicopathological predictors (neon green line), LN metastases (blue line), SIAH and EGFR in combination (black line). The data showed that the prognostic values of SIAH and EGFR, alone or in combination, are comparable to the four clinicopathological predictors, in predicting survival. (G–I) The time-dependent survival ROC curves for the first 5 years based on SIAH, EGFR, Ki67 and/or ER/PR/HER2 expression, alone or in combination, are shown, in pre-NST (G), post-NST (H), and NST-induced reduction of these six biomarkers (I) in predicting survival. The survival ROC curves include ER, PR and HER2 in combination (red line), Ki67 (neon green line), EGFR (orange line), SIAH (light teal line), SIAH and EGFR (blue line), SIAH, EGFR and Ki67 in combination (black line). SIAH and EGFR, two RAS pathway biomarkers alone or in combination, outperform ER, PR, HER2 and Ki67 in predicting patient survival. (J–L) Time-dependent survival ROC curves for the first 5-years based on SIAH and EGFR, and/or ER/PR/HER2 in combination with LN metastases, and the four clinicopathological predictors, alone or in combination, are shown, in pre-NST (J), post-NST settings (K), and NST-induced reduction of these six biomarkers (L). The survival ROC curves include ER, PR and HER2 in combination (red line), SIAH and EGFR in combination (black line), LN metastases (blue line), and 4 clinical predictors together (neon green line). The data showed that the prognostic values of SIAH and EGFR, alone or in combination, are comparable to the four clinicopathological predictors, in predicting survival in breast cancer.
4. Discussion
The confounding clinical reality is that cancer patients with similar clinical diagnoses often exhibit diverse tumor responses and varied survival following standard-of-care therapies, emphasizing the clinical need and intellectual challenges of personalized medicine in breast cancer (Tevaarwerk et al., 2013, Gyorffy et al., 2015, Zardavas et al., 2015, Vogelstein et al., 2013). There is a pressing need to develop and incorporate reliable prognostic tools that can determine which invasive mammary tumors are most likely to benefit from the prescribed neoadjuvant and adjuvant modalities, given intrinsic tumor heterogeneity and the emergence of resistant clones post systemic therapy (Zardavas et al., 2013, King and Morrow, 2015, Hutchinson, 2010, Coley, 2008, Holohan et al., 2013, Haddad and Goetz, 2015). Molecular assessment tools can be combined with clinicopathological predictors to identify unique tumor vulnerability, optimize therapies, forecast tumor response, estimate risk of recurrence and predict survival, include Oncotype DX and MammaPrint (Gyorffy et al., 2015, Sparano et al., 2015, Albain et al., 2010). Despite great promises, these multigene prognostic tools require clinical refinements to increase prognostic accuracy (Gyorffy et al., 2015, Oakman et al., 2010, Goncalves and Bose, 2013). Therapy-responsive and growth-dependent biomarkers are needed to identify which patients’ tumors are responding to the given therapies, independent of mammary tumor heterogeneity, so that effective therapy can be tailored and enhanced in response to the emergence of resistant tumor clones in real time. Hence, new techniques are needed to select the most effective first-line therapies for breast cancer patients with metastatic diseases to extend their survival (Weigelt et al., 2005b, Yap et al., 2012, Gerlinger et al., 2012).
The administration of NST has become a standard therapy for breast cancer patients with invasive, inflammatory and high-risk disease (Swain et al., 2015, Zardavas et al., 2013, King and Morrow, 2015, Tevaarwerk et al., 2013). NST reduces tumor burden before surgical resection, and it provides a valuable opportunity to assess therapy efficacy using pre-operative and post-operative tumor biospecimens (Graham et al., 2014). Patients have achieved complete tumor remission based on clinicopathological analysis post-NST, have increased disease-free survival (Thompson and Moulder-Thompson, 2012, Morrow, 2016, Schott and Hayes, 2012). Identifying patients as “super-responders”, “partial responders” and “non-responders” using an improved panel of logical, integrated and robust molecular biomarkers could help oncologists identify effective first-line therapies for patients with high-risk and malignant breast cancer. Therapies for invasive and high-risk breast cancer (luminal, basal-like, HER2-positive and TNBC) are often selected based on tumor ER, PR and HER2 status as well as clinicopathological predictors such as age, tumor size, stage, lymph node status, local invasion and systemic metastasis (Baselga et al., 2012, Bevers et al., 2009, Redden and Fuhrman, 2013, Tolaney et al., 2015). However, ER, PR, and HER2 expression in high-grade, therapy-resistant, invasive and metastatic mammary tumors does not correlate with progression-free or overall survival, nor predict tumor response to NST (Tevaarwerk et al., 2013, Gown, 2008, Chen et al., 2012, Onitilo et al., 2009, Parise and Caggiano, 2014, Prat et al., 2015).
As a major tumor-promoting pathway, RAS pathway activation/inactivation is ideally positioned to serve as a therapy-responsive, tumor heterogeneity-independent, and growth-dependent prognostic biomarker in breast cancer. Here, we conducted a retrospective study to determine whether the RAS pathway biomarkers can be added to evaluate tumor response and therapy efficacy, forecast patient survival, and predict which patients with invasive breast cancer are likely to benefit from standard NST regimens, or which patients will benefit from additional regimens after ineffective first-line therapies are identified. SIAH function is required for proper HER2/EGFR/K-RAS signal transduction, cancer cell proliferation and survival (Schmidt et al., 2007, Ahmed et al., 2008, Qin et al., 2015, Tang et al., 1997, Adam et al., 2015). SIAH is expressed in proliferating tumor cells. We have previously associated increased SIAH expression with the progression of ductal carcinoma in situ (DCIS) to invasive carcinoma (Behling et al., 2010). We showed that combining five molecular biomarkers (EGFR, SIAH, phospho-ERK, Ki67 and HIF1α) in the oncogenic K-RAS/Ki67/HIF1α pathways, with four clinicopathological predictors can be used to predict patient survival post surgery in human pancreatic cancer (Qin et al., 2015). Other groups have shown that SIAH expressed in breast cancer (Wright et al., 2015, Palmieri et al., 2009, Bruzzoni-Giovanelli et al., 2010, Confalonieri et al., 2009, Chan et al., 2011). Importantly, we were the first group to demonstrate the efficacy of an anti-SIAH-based anti-K-RAS and anticancer strategy to shutdown the “undruggable” oncogenic K-RAS activation and successfully block oncogenic K-RAS-driven tumorigenesis in several animal models of human cancer (Schmidt et al., 2007, Ahmed et al., 2008, Van Sciver et al., 2016, Wong and Moller, 2013).
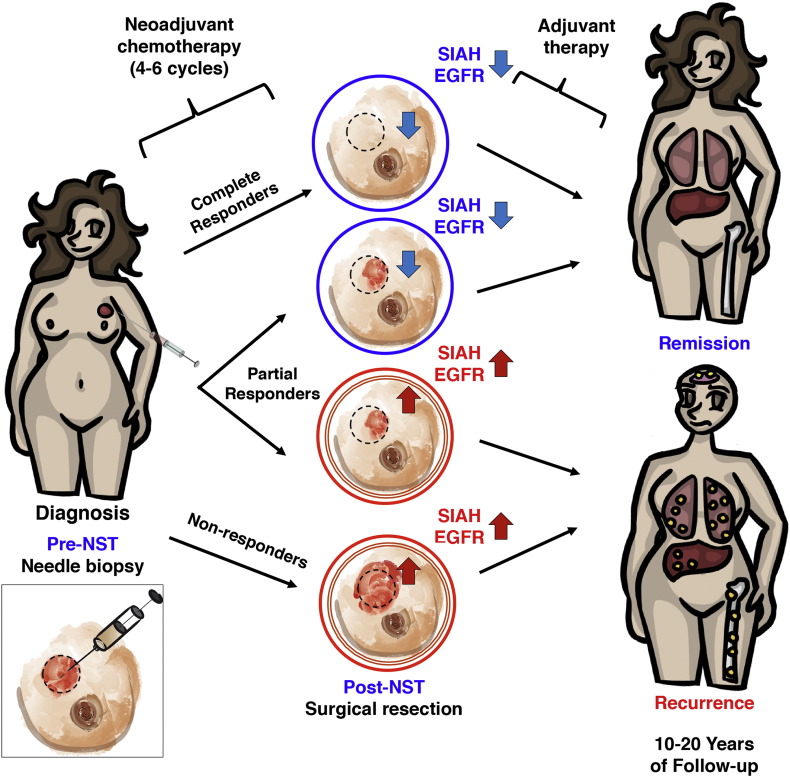
SIAH expression reflects RAS pathway activation, cell proliferation, and tumor growth (Schmidt et al., 2007, Ahmed et al., 2008, Tang et al., 1997). As a therapy-responsive and tumor heterogeneity-independent RAS pathway biomarker, SIAH is uniquely positioned to identify super-responders, partial-responders, and non-responders in the neoadjuvant setting to allow real-time monitoring, augmentation and quantification of therapy efficacy, to improve clinical outcome and patient survival (Fig. 6, a schematic illustration). We found that SIAH is a robust, tumor-specific and therapy-responsive tumor biomarker that can be used to identify which patients are most likely to benefit from a given NST regimen as a first-line therapy, and which patients should receive additional augmented and combinational therapies in the case of ineffective NST (Fig. 2, Fig. 3, Fig. 4, Fig. 5). Independent of therapy-induced tumor debulking, SIAH expression post-NST appears to have important prognostic value to detect the emergence of resistant tumor clones, differentiate among partial responders, forecast tumor relapse or remission, and predict survival in malignant breast cancer (Fig. 6).
Fig. 6.
A schematic illustration of adding two RAS pathway biomarkers, SIAH and EGFR, to evaluate therapy efficacy, tumor response, and predict patient survival in breast cancer.
SIAH and/or EGFR expression can be used to monitor tumor responses and identify resistant tumor clones post-NST and stratify patients. SIAH and EGFR outperform ER, PR, HER2 and Ki67 as two robust, sensitive and prognostic biomarkers to predict survival in breast cancer patients with lymph node metastases. The prognostic power of SIAH and EGFR, alone or in combination, is comparable to the clinical gold standards of clinical predictors (LN positivity, mammary tumor size, grade, stage and molecular subtypes in combination), and imaging-guided technology. A marked reduction in SIAH/EGFR expression post-NST would indicate effective therapy and increased survival, while persistent high SIAH/EGFR expression post-NST would indicate ineffective therapy and decreased survival. We found that the reduction of SIAH and/or the changes in EGFR expression post-NST are prognostic in predicting patient survival, especially among partial responders. The therapy-induced changes in SIAH and EGFR expression are highly prognostic in identifying effective/ineffective therapies, differentiating partial responders, identifying resistant tumor clones and predicting remission/relapse in breast cancer patients with lymph node (LN) metastases in neoadjuvant settings. The identification of therapy-responsive and prognostic biomarkers is of paramount importance to stratify patients and guide therapies in clinical oncology and personalized medicine. By developing the two RAS pathway-centered prognostic biomarkers, we hope to identify, personalize, and synergize effective therapies, improve survival for breast cancer patients with metastatic diseases in the future.
Despite showing promising prognostic potential, there are several limitations to consider when interpreting our results. It is retrospective in design and included a limited number of 182 breast cancer patients. Up to 7 years post initial diagnosis, survival was shortest among patients whose tumors spread to the lymph nodes and beyond, whereas patients with node-negative mammary tumors all survived for 7-years (Fig. 3, Fig. 4, Fig. 5). A longer study (10–15 years) will be followed up to determine the prognostic power of SIAH and EGFR in noninvasive mammary tumors. Although the survival curves indicate that reduced levels of SIAH and EGFR post-NST are good prognostic biomarkers that predict effective treatment, and tumor remission (Fig. 2, Fig. 3, Fig. 4, Fig. 5), the ultimate clinical incorporation of SIAH and EGFR in metastatic breast cancer will require large-scale and independent validations at multiple NCI-designated comprehensive cancer centers.
Our study identified that roughly 20% of locally advanced and metastatic mammary tumors have upregulated EGFR, and that EGFR expression levels decrease post-NST (Fig. 1, Fig. 3, and Table 1). EGFR expression is associated with aggressive molecular subtypes such as TNBC subtype independent of LN status, and HER2+ subtype with LN metastases. Post NST treatment, EGFR expression persisted in TNBC subtype independent of LN status, suggesting that anti-EGFR therapy is likely to offer additional therapeutic benefits to the patients with therapy-refractory and EGFR-positive TNBC tumors (Fig. 3B). Our findings may be important because they indicate that FDA-approved anti-EGFR therapeutics should be added to treat TNBC patients whose tumors are therapy-refractory and EGFR-positive. By comparing the percentage reduction in SIAH or EGFR expression pre- and post-NST, we will be able to identify and quantify super-, partial- and non-responders among breast cancer patients who received standard therapies. Successful validation of the prognosis of these RAS pathway biomarkers among “partial responders” with invasive breast cancer may have clear clinical impact (Fig. 6). As a control, Ki67 has showed limited or contradictory prognostic values (Loehberg et al., 2013). SIAH seems to be a better, more sensitive and therapy-responsive biomarker than Ki67 in breast cancer (Fig. 1, Fig. 5, and Supplemental Fig. S1). The prognostic value of SIAH and EGFR expression in locally advanced and metastatic breast cancer is superior and/or comparable to LN status and established clinicopathological predictors (AUC and ROC curves, Fig. 5). This SIAH/EGFR-centered biomarker panel may be used to determine tumor response to NST, tailor and optimize NST, and improve patient survival in the future. Importantly, our results show that therapy-induced reduction in SIAH and/or EGFR expression reflects RAS pathway activation/inactivation, and outperforms ER, PR, HER2 and Ki67 as a promising and prognostic biomarker in invasive and metastatic breast cancer. SIAH is expressed in 97.8% treatment-naïve breast cancer whereas EGFR is expressed in 20% of untreated population. Thus, the prognostic value of SIAH outperforms EGFR in malignant breast cancer (Fig. 2, Fig. 3, Fig. 4, Fig. 5). Conceptually, SIAH and EGFR offers an accurate readout of therapeutic efficacy independent of tumor heterogeneity, thus providing a valuable window of opportunity to quantify tumor responses, to reduce drug toxicity and to improve therapy efficacy against invasive breast cancer in real time. A prospective study will be performed to validate the prognostic value and therapy-responsiveness of SIAH and EGFR, to forecast and monitor therapy-induced tumor regression, estimate NST efficacy, differentiate the “partial responders” and identify therapy-induced resistant clonal expansion, and to ultimately improve patient survival in the future.
The following are the supplementary data related to this article.
Neoadjuvant systemic therapies (NST) used to treat breast cancer patients at Sentara Hospitals.
Conflict of Interests Disclosure Statement
The authors describe no conflict of interests.
Funding Source Statement
The Dorothy G. Hoefer Foundation for Breast Cancer Research to AHT provides the support to the histology, pathology and the IHC study. The EVMS startup, developmental and incentive funds to AHT provide support for data collection and salary support. The Center for Innovative Technology (CIT)-Commonwealth Research Commercialization Fund (CRCF) (MF14S-009-LS to AHT) provides funding support for us to complete this study. The National Cancer Institute (CA140550 to AHT) provided additional research training opportunity in support of this study. None of the authors have been paid to write this article by a pharmaceutical company or other agency. As the corresponding author, AHT has the full access to all the data in the study and had final responsibility for the decision to submit for publication.
Authors' Contributions
LLSvR, VZ, JSW, share co-1st authorship with equal contribution. AHT was the principal investigator of this study. AHT designed, supervised, prepared the manuscript with the help and input from all team members. VZ, LLSvR, JSW, CFO, AJI, MB, REVS, AMTT, PTB, VJH, KAD, BWK and AHT are involved in data acquisition. AMTT and LSSVR generated the artistic schematic used in Fig. 5. JSW and CFO are responsible for IHC staining and scoring. RJJ, RQ, LLSvR and AHT are responsible for data management, analysis, statistical analysis, and interpretation. RAH, RRP, CAA, EAH and DZC provide clinical guidance and administrative support.
Acknowledgements
Correspondence should be addressed to A.H.T. The authors thank the breast cancer patients who generously donated tissues to this study. The authors thank the top leadership at EVMS and Sentara, Richard V. Homan, M.D., Mr. Harry T. Lester, Ms. Claudia Keenan, Mrs. Mary L. Blunt, Paul Chidester, M.D., Mr. Mark Szalwinski, Barbara H. Amaker, M.D., Mrs. Linda S. McKee and Ms. Meredith B. Strand, for supporting this work at its inception since 2012. The authors thank Mrs. Jennie Capps and Mrs. Linda Church at the Chesapeake Bay Wine Classic Foundation, and Dr. Judith Salerno, Mrs. Sharon Laderberg and Mrs. Miki Donovan at the Susan G. Komen Foundation for their staunch support. The authors thank Drs. Edward B. Leof, Stephen I. Deutsch, Jeffrey L. Platt, Christine Novak, and Rebecca L. Schmidt for their critical pre-submission manuscript review.
References
- Adam M.G., Matt S., Christian S., Hess-Stumpp H., Haegebarth A., Hofmann T.G., Algire C. SIAH ubiquitin ligases regulate breast cancer cell migration and invasion independent of the oxygen status. Cell Cycle. 2015;14:3734–3747. doi: 10.1080/15384101.2015.1104441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A.U., Schmidt R.L., Park C.H., Reed N.R., Hesse S.E., Thomas C.F., Molina J.R., Deschamps C., Yang P., Aubry M.C. Effect of disrupting seven-in-absentia homolog 2 function on lung cancer cell growth. J. Natl. Cancer Inst. 2008;100:1606–1629. doi: 10.1093/jnci/djn365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albain K.S., Barlow W.E., Shak S., Hortobagyi G.N., Livingston R.B., Yeh I.T., Ravdin P., Bugarini R., Baehner F.L., Davidson N.E. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman D.G., McShane L.M., Sauerbrei W., Taube S.E. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): explanation and elaboration. PLoS Med. 2012;9 doi: 10.1371/journal.pmed.1001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arteaga C.L., Sliwkowski M.X., Osborne C.K., Perez E.A., Puglisi F., Gianni L. Treatment of HER2-positive breast cancer: current status and future perspectives. Nat. Rev. Clin. Oncol. 2012;9:16–32. doi: 10.1038/nrclinonc.2011.177. [DOI] [PubMed] [Google Scholar]
- Baselga J., Cortes J., Kim S.B., Im S.A., Hegg R., Im Y.H., Roman L., Pedrini J.L., Pienkowski T., Knott A. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N. Engl. J. Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behling K.C., Tang A., Freydin B., Chervoneva I., Kadakia S., Schwartz G.F., Rui H., Witkiewicz A.K. Increased SIAH expression predicts ductal carcinoma in situ (DCIS) progression to invasive carcinoma. Breast Cancer Res. Treat. 2010 doi: 10.1007/s10549-010-1254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevers T.B., Anderson B.O., Bonaccio E., Buys S., Daly M.B., Dempsey P.J., Farrar W.B., Fleming I., Garber J.E., Harris R.E. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. J. Natl. Compr. Cancer Netw. 2009;7:1060–1096. doi: 10.6004/jnccn.2009.0070. [DOI] [PubMed] [Google Scholar]
- Bruzzoni-Giovanelli H., Fernandez P., Veiga L., Podgorniak M.P., Powell D.J., Candeias M.M., Mourah S., Calvo F., Marin M. Distinct expression patterns of the E3 ligase SIAH-1 and its partner Kid/KIF22 in normal tissues and in the breast tumoral processes. J. Exp. Clin. Cancer Res. 2010;29:10. doi: 10.1186/1756-9966-29-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulfoni M., Gerratana L., Del Ben F., Marzinotto S., Sorrentino M., Turetta M., Scoles G., Toffoletto B., Isola M., Beltrami C.A. In patients with metastatic breast cancer the identification of circulating tumor cells in epithelial-to-mesenchymal transition is associated with a poor prognosis. Breast Cancer Res. 2016;18:30. doi: 10.1186/s13058-016-0687-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P., Moller A., Liu M.C., Sceneay J.E., Wong C.S., Waddell N., Huang K.T., Dobrovic A., Millar E.K., O'Toole S.A. The expression of the ubiquitin ligase SIAH2 (seven in absentia homolog 2) is mediated through gene copy number in breast cancer and is associated with a basal-like phenotype and p53 expression. Breast Cancer Res. 2011;13:R19. doi: 10.1186/bcr2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Chen C.M., Yu K.D., Zhou R.J., Shao Z.M. Prognostic value of a positive-to-negative change in hormone receptor status after neoadjuvant chemotherapy in patients with hormone receptor-positive breast cancer. Ann. Surg. Oncol. 2012;19:3002–3011. doi: 10.1245/s10434-012-2318-2. [DOI] [PubMed] [Google Scholar]
- Coley H.M. Mechanisms and strategies to overcome chemotherapy resistance in metastatic breast cancer. Cancer Treat. Rev. 2008;34:378–390. doi: 10.1016/j.ctrv.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Confalonieri S., Quarto M., Goisis G., Nuciforo P., Donzelli M., Jodice G., Pelosi G., Viale G., Pece S., Di Fiore P.P. Alterations of ubiquitin ligases in human cancer and their association with the natural history of the tumor. Oncogene. 2009;28:2959–2968. doi: 10.1038/onc.2009.156. [DOI] [PubMed] [Google Scholar]
- Cortazar P., Zhang L., Untch M., Mehta K., Costantino J.P., Wolmark N., Bonnefoi H., Cameron D., Gianni L., Valagussa P. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- DeSantis C., Ma J., Bryan L., Jemal A. Breast cancer statistics, 2013. CA Cancer J. Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- DeSantis C.E., Fedewa S.A., Goding Sauer A., Kramer J.L., Smith R.A., Jemal A. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA Cancer J. Clin. 2015 doi: 10.3322/caac.21320. [DOI] [PubMed] [Google Scholar]
- Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R., Dancey J., Arbuck S., Gwyther S., Mooney M. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- El Saghir N.S., Tfayli A., Hatoum H.A., Nachef Z., Dinh P., Awada A. Treatment of metastatic breast cancer: state-of-the-art, subtypes and perspectives. Crit. Rev. Oncol. Hematol. 2011;80:433–449. doi: 10.1016/j.critrevonc.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Engelman J.A. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat. Rev. Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- Falkson G., Gelman R.S., Glick J., Falkson C.I., Harris J. Metastatic breast cancer: higher versus low dose maintenance treatment when only a partial response or a no change status is obtained following doxorubicin induction treatment. An Eastern Cooperative Oncology Group study. Ann. Oncol. 1992;3:768–770. doi: 10.1093/oxfordjournals.annonc.a058337. [DOI] [PubMed] [Google Scholar]
- Foulkes W.D., Smith I.E., Reis-Filho J.S. Triple-negative breast cancer. N. Engl. J. Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- Gerlinger M., Rowan A.J., Horswell S., Larkin J., Endesfelder D., Gronroos E., Martinez P., Matthews N., Stewart A., Tarpey P. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipponi M., Bassetti C., Canavese G., Catturich A., Di Somma C., Vecchio C., Nicolo G., Schenone F., Tomei D., Cafiero F. Sentinel lymph node as a new marker for therapeutic planning in breast cancer patients. J. Surg. Oncol. 2004;85:102–111. doi: 10.1002/jso.20022. [DOI] [PubMed] [Google Scholar]
- Goncalves R., Bose R. Using multigene tests to select treatment for early-stage breast cancer. J. Natl. Compr. Cancer Netw. 2013;11:174–182. doi: 10.6004/jnccn.2013.0025. quiz 182. [DOI] [PubMed] [Google Scholar]
- Gown A.M. Current issues in ER and HER2 testing by IHC in breast cancer. Mod. Pathol. 2008;21(Suppl 2):S8–S15. doi: 10.1038/modpathol.2008.34. [DOI] [PubMed] [Google Scholar]
- Graham L.J., Shupe M.P., Schneble E.J., Flynt F.L., Clemenshaw M.N., Kirkpatrick A.D., Gallagher C., Nissan A., Henry L., Stojadinovic A. Current approaches and challenges in monitoring treatment responses in breast cancer. J. Cancer. 2014;5:58–68. doi: 10.7150/jca.7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorffy B., Hatzis C., Sanft T., Hofstatter E., Aktas B., Pusztai L. Multigene prognostic tests in breast cancer: past, present, future. Breast Cancer Res. 2015;17:11. doi: 10.1186/s13058-015-0514-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad T.C., Goetz M.P. Landscape of neoadjuvant therapy for breast cancer. Ann. Surg. Oncol. 2015;22:1408–1415. doi: 10.1245/s10434-015-4405-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heagerty P.J., Zheng Y. Survival model predictive accuracy and ROC curves. Biometrics. 2005;61:92–105. doi: 10.1111/j.0006-341X.2005.030814.x. [DOI] [PubMed] [Google Scholar]
- Holohan C., Van Schaeybroeck S., Longley D.B., Johnston P.G. Cancer drug resistance: an evolving paradigm. Nat. Rev. Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- Hutchinson L. Breast cancer: challenges, controversies, breakthroughs. Nat. Rev. Clin. Oncol. 2010;7:669–670. doi: 10.1038/nrclinonc.2010.192. [DOI] [PubMed] [Google Scholar]
- Jatoi I., Hilsenbeck S.G., Clark G.M., Osborne C.K. Significance of axillary lymph node metastasis in primary breast cancer. J. Clin. Oncol. 1999;17:2334–2340. doi: 10.1200/JCO.1999.17.8.2334. [DOI] [PubMed] [Google Scholar]
- Joensuu K., Leidenius M., Kero M., Andersson L.C., Horwitz K.B., Heikkila P. ER, PR, HER2, Ki-67 and CK5 in Early and Late Relapsing Breast Cancer-Reduced CK5 Expression in Metastases. Breast Cancer (Auckl.) 2013;7:23–34. doi: 10.4137/BCBCR.S10701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King T.A., Morrow M. Surgical issues in patients with breast cancer receiving neoadjuvant chemotherapy. Nat. Rev. Clin. Oncol. 2015;12:335–343. doi: 10.1038/nrclinonc.2015.63. [DOI] [PubMed] [Google Scholar]
- Loehberg C.R., Almstedt K., Jud S.M., Haeberle L., Fasching P.A., Hack C.C., Lux M.P., Thiel F.C., Schrauder M.G., Brunner M. Prognostic relevance of Ki-67 in the primary tumor for survival after a diagnosis of distant metastasis. Breast Cancer Res. Treat. 2013;138:899–908. doi: 10.1007/s10549-013-2460-y. [DOI] [PubMed] [Google Scholar]
- McKeage K., Perry C.M. Trastuzumab: a review of its use in the treatment of metastatic breast cancer overexpressing HER2. Drugs. 2002;62:209–243. doi: 10.2165/00003495-200262010-00008. [DOI] [PubMed] [Google Scholar]
- McShane L.M., Altman D.G., Sauerbrei W., Taube S.E., Gion M., Clark G.M. REporting recommendations for tumor MARKer prognostic studies (REMARK) Nat. Clin. Pract. Urol. 2005;2:416–422. [PubMed] [Google Scholar]
- Morrow M. Parsing Pathologic Complete Response in Patients Receiving Neoadjuvant Chemotherapy for Breast Cancer. JAMA Oncol. 2016;2:516–517. doi: 10.1001/jamaoncol.2015.4919. [DOI] [PubMed] [Google Scholar]
- Nagayama A., Hayashida T., Jinno H., Takahashi M., Seki T., Matsumoto A., Murata T., Ashrafian H., Athanasiou T., Okabayashi K. Comparative effectiveness of neoadjuvant therapy for HER2-positive breast cancer: a network meta-analysis. J. Natl. Cancer Inst. 2014;106 doi: 10.1093/jnci/dju203. [DOI] [PubMed] [Google Scholar]
- Oakman C., Santarpia L., Di Leo A. Breast cancer assessment tools and optimizing adjuvant therapy. Nat. Rev. Clin. Oncol. 2010;7:725–732. doi: 10.1038/nrclinonc.2010.170. [DOI] [PubMed] [Google Scholar]
- Onitilo A.A., Engel J.M., Greenlee R.T., Mukesh B.N. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin. Med. Res. 2009;7:4–13. doi: 10.3121/cmr.2009.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozmen V., Ozcinar B., Bozdogan A., Eralp Y., Yavuz E., Dincer M. The effect of internal mammary lymph node biopsy on the therapeutic decision and survival of patients with breast cancer. Eur. J. Surg. Oncol. 2015;41:1368–1372. doi: 10.1016/j.ejso.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Palmieri D., Fitzgerald D., Shreeve S.M., Hua E., Bronder J.L., Weil R.J., Davis S., Stark A.M., Merino M.J., Kurek R. Analyses of resected human brain metastases of breast cancer reveal the association between up-regulation of hexokinase 2 and poor prognosis. Mol. Cancer Res. 2009;7:1438–1445. doi: 10.1158/1541-7786.MCR-09-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parinyanitikul N., Lei X., Chavez-MacGregor M., Liu S., Mittendorf E.A., Litton J.K., Woodward W., Zhang A.H., Hortobagyi G.N., Valero V. Receptor status change from primary to residual breast cancer after neoadjuvant chemotherapy and analysis of survival outcomes. Clin. Breast Cancer. 2015;15:153–160. doi: 10.1016/j.clbc.2014.09.006. [DOI] [PubMed] [Google Scholar]
- Parise C.A., Caggiano V. Breast Cancer Survival Defined by the ER/PR/HER2 Subtypes and a Surrogate Classification according to Tumor Grade and Immunohistochemical Biomarkers. J. Cancer Epidemiol. 2014;2014:469251. doi: 10.1155/2014/469251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penault-Llorca F., Radosevic-Robin N. Biomarkers of residual disease after neoadjuvant therapy for breast cancer. Nat. Rev. Clin. Oncol. 2016;13:487–503. doi: 10.1038/nrclinonc.2016.1. [DOI] [PubMed] [Google Scholar]
- Perou C.M., Sorlie T., Eisen M.B., van de Rijn M., Jeffrey S.S., Rees C.A., Pollack J.R., Ross D.T., Johnsen H., Akslen L.A. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- Petrarca C.R., Brunetto A.T., Duval V., Brondani A., Carvalho G.P., Garicochea B. Survivin as a predictive biomarker of complete pathologic response to neoadjuvant chemotherapy in patients with stage II and stage III breast cancer. Clin. Breast Cancer. 2011;11:129–134. doi: 10.1016/j.clbc.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Piccart-Gebhart M.J., Procter M., Leyland-Jones B., Goldhirsch A., Untch M., Smith I., Gianni L., Baselga J., Bell R., Jackisch C. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N. Engl. J. Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- Prat A., Fan C., Fernandez A., Hoadley K.A., Martinello R., Vidal M., Viladot M., Pineda E., Arance A., Munoz M. Response and survival of breast cancer intrinsic subtypes following multi-agent neoadjuvant chemotherapy. BMC Med. 2015;13:303. doi: 10.1186/s12916-015-0540-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin R., Smyrk T.C., Reed N.R., Schmidt R.L., Schnelldorfer T., Chari S.T., Petersen G.M., Tang A.H. Combining clinicopathological predictors and molecular biomarkers in the oncogenic K-RAS/Ki67/HIF-1alpha pathway to predict survival in resectable pancreatic cancer. Br. J. Cancer. 2015;112:514–522. doi: 10.1038/bjc.2014.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2015. R: A language and environment for statistical computing. [Google Scholar]
- Rakha E.A., Reis-Filho J.S., Ellis I.O. Basal-like breast cancer: a critical review. J. Clin. Oncol. 2008;26:2568–2581. doi: 10.1200/JCO.2007.13.1748. [DOI] [PubMed] [Google Scholar]
- Rakha E.A., Morgan D., Macmillan D. The prognostic significance of early stage lymph node positivity in operable invasive breast carcinoma: number or stage. J. Clin. Pathol. 2012;65:624–630. doi: 10.1136/jclinpath-2012-200755. [DOI] [PubMed] [Google Scholar]
- Redden M.H., Fuhrman G.M. Neoadjuvant chemotherapy in the treatment of breast cancer. Surg. Clin. North Am. 2013;93:493–499. doi: 10.1016/j.suc.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Robert N., Leyland-Jones B., Asmar L., Belt R., Ilegbodu D., Loesch D., Raju R., Valentine E., Sayre R., Cobleigh M. Randomized phase III study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2-overexpressing metastatic breast cancer. J. Clin. Oncol. 2006;24:2786–2792. doi: 10.1200/JCO.2005.04.1764. [DOI] [PubMed] [Google Scholar]
- Romond E.H., Perez E.A., Bryant J., Suman V.J., Geyer C.E., Jr., Davidson N.E., Tan-Chiu E., Martino S., Paik S., Kaufman P.A. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N. Engl. J. Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- Schmidt R.L., Park C.H., Ahmed A.U., Gundelach J.H., Reed N.R., Cheng S., Knudsen B.E., Tang A.H. Inhibition of RAS-mediated transformation and tumorigenesis by targeting the downstream E3 ubiquitin ligase seven in absentia homologue. Cancer Res. 2007;67:11798–11810. doi: 10.1158/0008-5472.CAN-06-4471. [DOI] [PubMed] [Google Scholar]
- Schott A.F., Hayes D.F. Defining the benefits of neoadjuvant chemotherapy for breast cancer. J. Clin. Oncol. 2012;30:1747–1749. doi: 10.1200/JCO.2011.41.3161. [DOI] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J. Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Sparano J.A., Gray R.J., Makower D.F., Pritchard K.I., Albain K.S., Hayes D.F., Geyer C.E., Jr., Dees E.C., Perez E.A., Olson J.A., Jr. Prospective Validation of a 21-Gene Expression Assay in Breast Cancer. N. Engl. J. Med. 2015 doi: 10.1056/NEJMoa1510764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S.M., Clark E., Baselga J. Treatment of HER2-positive metastatic breast cancer. N. Engl. J. Med. 2015;372:1964–1965. doi: 10.1056/NEJMc1503446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang A.H., Neufeld T.P., Kwan E., Rubin G.M. PHYL acts to down-regulate TTK88, a transcriptional repressor of neuronal cell fates, by a SINA-dependent mechanism. Cell. 1997;90:459–467. doi: 10.1016/s0092-8674(00)80506-1. [DOI] [PubMed] [Google Scholar]
- Tebbutt N., Pedersen M.W., Johns T.G. Targeting the ERBB family in cancer: couples therapy. Nat. Rev. Cancer. 2013;13:663–673. doi: 10.1038/nrc3559. [DOI] [PubMed] [Google Scholar]
- Tevaarwerk A.J., Gray R.J., Schneider B.P., Smith M.L., Wagner L.I., Fetting J.H., Davidson N., Goldstein L.J., Miller K.D., Sparano J.A. Survival in patients with metastatic recurrent breast cancer after adjuvant chemotherapy: little evidence of improvement over the past 30 years. Cancer. 2013;119:1140–1148. doi: 10.1002/cncr.27819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A.M., Moulder-Thompson S.L. Neoadjuvant treatment of breast cancer. Ann. Oncol. 2012;23(Suppl 10):x231–x236. doi: 10.1093/annonc/mds324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolaney S.M., Barry W.T., Dang C.T., Yardley D.A., Moy B., Marcom P.K., Albain K.S., Rugo H.S., Ellis M., Shapira I. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N. Engl. J. Med. 2015;372:134–141. doi: 10.1056/NEJMoa1406281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sciver R.E., Njogu M.M., Isbell A.J., Odanga J.J., Bian M., Svyatova E., van Reesema L.L.S., Zheleva V., Eisner J.L., Bruflat J.K. Blocking SIAH proteolysis, an important K-RAS vulnerability, to control and eradicate K-RAS-driven metastatic cancer. In: Azmi A., editor. Conquering RAS. Elsevier, Inc.; 2016. [Google Scholar]
- Vogelstein B., Papadopoulos N., Velculescu V.E., Zhou S., Diaz L.A., Jr., Kinzler K.W. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang-Lopez Q., Chalabi N., Abrial C., Radosevic-Robin N., Durando X., Mouret-Reynier M.A., Benmammar K.E., Kullab S., Bahadoor M., Chollet P. Can pathologic complete response (pCR) be used as a surrogate marker of survival after neoadjuvant therapy for breast cancer? Crit. Rev. Oncol. Hematol. 2015;95:88–104. doi: 10.1016/j.critrevonc.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Weigelt B., Hu Z., He X., Livasy C., Carey L.A., Ewend M.G., Glas A.M., Perou C.M., Van't Veer L.J. Molecular portraits and 70-gene prognosis signature are preserved throughout the metastatic process of breast cancer. Cancer Res. 2005;65:9155–9158. doi: 10.1158/0008-5472.CAN-05-2553. [DOI] [PubMed] [Google Scholar]
- Weigelt B., Peterse J.L., van 't Veer L.J. Breast cancer metastasis: markers and models. Nat. Rev. Cancer. 2005;5:591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- Wong C.S., Moller A. Siah: a promising anticancer target. Cancer Res. 2013;73:2400–2406. doi: 10.1158/0008-5472.CAN-12-4348. [DOI] [PubMed] [Google Scholar]
- Wright K.L., Adams J.R., Liu J.C., Loch A.J., Wong R.G., Jo C.E., Beck L.A., Santhanam D.R., Weiss L., Mei X. Ras Signaling Is a Key Determinant for Metastatic Dissemination and Poor Survival of Luminal Breast Cancer Patients. Cancer Res. 2015;75:4960–4972. doi: 10.1158/0008-5472.CAN-14-2992. [DOI] [PubMed] [Google Scholar]
- Yap T.A., Gerlinger M., Futreal P.A., Pusztai L., Swanton C. Intratumor heterogeneity: seeing the wood for the trees. Sci. Transl. Med. 2012;4 doi: 10.1126/scitranslmed.3003854. (127ps110) [DOI] [PubMed] [Google Scholar]
- Zardavas D., Baselga J., Piccart M. Emerging targeted agents in metastatic breast cancer. Nat. Rev. Clin. Oncol. 2013;10:191–210. doi: 10.1038/nrclinonc.2013.29. [DOI] [PubMed] [Google Scholar]
- Zardavas D., Irrthum A., Swanton C., Piccart M. Clinical management of breast cancer heterogeneity. Nat. Rev. Clin. Oncol. 2015;12:381–394. doi: 10.1038/nrclinonc.2015.73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Neoadjuvant systemic therapies (NST) used to treat breast cancer patients at Sentara Hospitals.