Abstract
Background
We have developed a recombinant B cell epitope-based vaccine (BM32) for allergen-specific immunotherapy (AIT) of grass pollen allergy. The vaccine contains recombinant fusion proteins consisting of allergen-derived peptides and the hepatitis B surface protein domain preS as immunological carrier.
Methods
We conducted a randomized, double-blind, placebo-controlled AIT study to determine safety, clinical efficacy and immunological mechanism of three subcutaneous injections of three BM32 doses adsorbed to aluminum hydroxide versus aluminum hydroxide (placebo) applied monthly to grass pollen allergic patients (n = 70). Primary efficacy endpoint was the difference in total nasal symptom score (TNSS) through grass pollen chamber exposure before treatment and 4 weeks after the last injection. Secondary clinical endpoints were total ocular symptom score (TOSS) and allergen-specific skin response evaluated by titrated skin prick testing (SPT) at the same time points. Treatment-related side effects were evaluated as safety endpoints. Changes in allergen-specific antibody, cellular and cytokine responses were measured in patients before and after treatment.
Results
Sixty-eight patients completed the trial. TNSS significantly decreased with mean changes of − 1.41 (BM32/20 μg) (P = 0.03) and − 1.34 (BM32/40 μg) (P = 0.003) whereas mean changes in the BM32/10 μg and placebo group were not significant. TOSS and SPT reactions showed a dose-dependent decrease. No systemic immediate type side effects were observed. Only few grade 1 systemic late phase reactions occurred in BM32 treated patients. The number of local injection site reactions was similar in actively and placebo-treated patients. BM32 induced highly significant allergen-specific IgG responses (P < 0.0001) but no allergen-specific IgE. Allergen-induced basophil activation was reduced in BM32 treated patients and addition of therapy-induced IgG significantly suppressed T cell activation (P = 0.0063).
Conclusion
The B cell epitope-based recombinant grass pollen allergy vaccine BM32 is well tolerated and few doses are sufficient to suppress immediate allergic reactions as well as allergen-specific T cell responses via a selective induction of allergen-specific IgG antibodies. (ClinicalTrials.gov number, NCT01445002.)
Key words: Vaccine, Grass pollen, B cell-epitope, Challenge chamber, Immunotherapy
Highlights
-
•
BM32 is a recombinant allergy vaccine consisting of the preS domain of the large envelope protein of hepatitis B virus (HBV) and grass pollen allergen-derived peptides
-
•
Vaccination of allergic patients with BM32 is well tolerated and protects against allergic symptoms induced by grass pollen exposure in a challenge chamber by induction of allergen-specific IgG antibodies without boosting of allergic immune responses
-
•
BM32 may be useful as therapeutic and prophylactic vaccine for grass pollen allergy
Allergen-specific immunotherapy (AIT) is the only treatment for allergy which prevents the progression of disease to severe manifestations and has long-lasting effects even after discontinuation. However, current forms of AIT based on natural allergen extracts, induce side effects, require multiple administrations, inconvenient up-dosing protocols and thus patients compliance is poor. Our study is the first to investigate the effects of vaccination with a B cell epitope-based recombinant allergy vaccine in patients who were exposed to grass pollen in a pollen chamber. The vaccine contains recombinant fusion proteins consisting of allergen-derived peptides bound to a hepatitis B virus surface antigen as immunological carrier. It is demonstrated that three injections of the recombinant B cell epitope-based allergy vaccine are well tolerated and protect allergic patients against allergic symptoms by induction of allergen-specific IgG antibody responses without inducing allergic sensitization. Thus recombinant hypoallergenic B cell epitope-based vaccines have potential to improve safety and convenience of AIT when used as therapeutic vaccine and, due to low allergenicity, may be used even for prophylactic allergy vaccination.
1. Introduction
Allergen-specific immunotherapy (AIT) is the only disease-modifying treatment for allergy which prevents the progression of allergic rhinitis towards asthma (Larché et al., 2006, Jacobsen et al., 2007). Clinical and immunological effects of AIT are long-lasting, even after discontinuation (Durham et al., 1999). Furthermore, AIT has a cost-saving effect when compared to symptomatic drug treatment (Hankin and Cox, 2014). However, several factors limit the broad application of AIT. Current allergy vaccines are based on natural allergen extracts which are often of poor quality (e.g., varying allergen compositions, lack of important allergens, contaminations) and may induce severe immediate and late phase side effects (Focke et al., 2008, Casset et al., 2012, Winther et al., 2006, Focke et al., 2010). Accordingly current treatment schedules require inconvenient, multiple administrations of gradually increasing doses and, in sensitive patients, the therapeutically effective maintenance dose often cannot be reached due to side effects. As a result, the real-life adherence to AIT is low (Kiel et al., 2013).
Progress made regarding the molecular characterization of the disease-causing allergens has now opened the door for new forms of allergen-specific immunotherapy which allow reduced side effects and targeting of specific pathways of the allergic immune response (Niederberger et al., 2004, Creticos et al., 2006, Valenta et al., 2010). We have developed a strategy for AIT (i.e., B cell epitope-based allergy vaccines) which is based on fusion proteins consisting of non-allergenic peptides which are derived from the IgE binding sites of disease-causing allergens but do not bind IgE themselves and PreS, a surface protein from hepatitis B which serves as a non-allergenic carrier protein providing T cell help (Focke-Tejkl et al., 2015). The allergen-derived peptides used for the construction of the B cell epitope-based allergy vaccines lack IgE reactivity and therefore do not induce IgE-mediated side effects but focus blocking allergen-specific IgG towards the IgE binding sites of the allergen. In the vaccine allergen-specific T cell epitopes are reduced as much as possible (Focke-Tejkl et al., 2015, Niederberger et al., 2015) because, when present in previously engineered hypoallergenic allergen derivatives, they were found to cause late phase side effects (Campana et al., 2015). In the B cell epitope-based allergy vaccine concept the T cell help for the induction of allergen-specific IgG antibodies comes from T cell epitopes of the non-allergenic PreS carrier protein (Focke-Tejkl et al., 2015).
Since grass pollen is one of the most important allergen sources world-wide and causes severe respiratory manifestations in allergic patients (Suphioglu et al., 1992), we constructed a grass pollen vaccine consisting of four recombinant derivatives of the four major timothy grass pollen allergens (i.e., Phl p 1, Phl p 2, Phl p 5, Phl p 6) based on the B cell epitope-based strategy. It has been demonstrated that with a combination of these four timothy grass pollen allergens all grass pollen allergic patients can be diagnosed (Linhart et al., 2005, Hatzler et al., 2012). The hypoallergenic peptides incorporated in the grass pollen allergy vaccine have been identified in combined approaches using epitope mapping and structural analyses of the allergens (Focke et al., 2001, Focke-Tejkl et al., 2014, Focke-Tejkl et al., 2015). The in vitro immunological characterization, experimental animal data and a safety skin test study performed in grass pollen allergic patients indicated that the vaccine lacks allergenic activity and has the potential to induce allergen-specific IgG antibodies upon vaccination, which compete with allergic patients IgE antibodies for the binding sites on the natural allergens (Focke-Tejkl et al., 2015, Niederberger et al., 2015).
The current double-blind, placebo-controlled study was designed as a safety and dose-finding study carried out in the Vienna Challenge Chamber (VCC) to investigate the underlying immunological mechanisms and potential clinical effects of the B cell epitope-based grass pollen allergy vaccine in allergic patients for the first time.
2. Methods
2.1. Patients, inclusion and exclusion criteria, randomization
Adult male and female patients (18–60 years) suffering from grass pollen-induced allergic rhinoconjunctivitis for at least two years were recruited. Grass pollen-specific sensitization was confirmed by positive skin prick test to timothy grass pollen extract as well as by the presence in serum of grass pollen-specific IgE antibodies (at least 0.7kUA/l) as demonstrated by ImmunoCAP (Thermofisher, Uppsala, Sweden). Grass pollen-specific clinical reactivity with at least moderate symptoms of allergic rhinitis (itching, sneezing, rhinorrhea, nasal obstruction) documented by a TNSS of at least 6 within the first two hours of a 6 hour screening challenge in the Vienna Challenge Chamber (VCC) were required.
Patients with unstable asthma and other intercurrent diseases like perennial allergies or structural nasal abnormalities were not eligible. A detailed list of inclusion and exclusion criteria can be found in the study protocol (supplementary data). Excluded were pregnant women, subjects who received grass pollen specific AIT within 2 years prior to study start and patients under prohibited medications (i.e., depot corticosteroids for 12 weeks, oral corticosteroids for 8 weeks and inhaled corticosteroids for 4 weeks prior to study start and during entire study).
Block randomization with stratification for disease severity (moderate or severe) was conducted to ensure that patients in the four treatment arms had comparable disease severity as assessed by TNSS and titrated skin prick test during screening allergen challenge. The randomisation lists were generated by a CRO using a block size of 4 (Software RUNCODE Version 3.6, idv Gauting). Paper listings were used for performing the randomization and number allocation. Only the data manager generating the randomisation list at the CRO was unblinded. All other personnel at the CRO conducting data management and statistical analysis were blinded. Likewise, all personnel at the study site, in the laboratories performing analyses and at the sponsor were blinded.
One subject was excluded due to abnormal laboratory values before treatment. At the time of randomization there were no relevant differences between the subjects allocated to the four treatment groups regarding demographic data, TNSS in response to grass pollen exposure in the challenge chamber and grass pollen-specific immediate type skin responses (Table 1). Patients showed IgE reactivity to the recombinant timothy grass pollen allergens (Phl p 1, Phl p 2, Phl p 5, Phl p 6) but lacked relevant IgE reactivity to BM321, BM322, BM325 and BM326 (Supplemental Fig. 1). Patients with IgE reactivity against a clinically relevant minor allergen (i.e., Phl p 7) which was not included in BM32 were unevenly distributed in the four treatment groups (Table 1).
Table 1.
Patients characteristics at baseline
| Characteristic | Placebo N = 18 |
S1, 10 μg N = 17 |
S2, 20 μg N = 18 |
S3, 40 μg N = 17 |
|---|---|---|---|---|
| Age | ||||
| Mean | 29.4 | 28.9 | 28.6 | 29.8 |
| SD | 8.0 | 7.7 | 5.2 | 7.7 |
| Range | 21–52 | 19–48 | 23–47 | 19–52 |
| Gender | ||||
| Male (%) | 7 (39%) | 8 (47%) | 9 (50%) | 10 (59%) |
| Height (cm) | ||||
| Mean | 172.6 | 173.1 | 173.7 | 175.6 |
| SD | 9.0 | 8.4 | 9.4 | 9.2 |
| Range | 157–197 | 158–190 | 163–194 | 161–189 |
| Weight (kg) | ||||
| Mean | 69.4 | 69.1 | 72.0 | 74.4 |
| SD | 15.8 | 12.2 | 14.1 | 12.4 |
| Range | 51–114 | 52–90 | 53–98 | 58–98 |
| BMI (kg/m2) | ||||
| Mean | 23.0 | 22.9 | 23.8 | 24.1 |
| SD | 3.2 | 3.0 | 3.8 | 3.0 |
| Range | 18.4–29.4 | 19.0–28.0 | 18.7–33.3 | 19.5–30.1 |
| TNSS (baseline) | ||||
| Mean | 8.4 | 8.7 | 8.2 | 8.1 |
| SD | 0.2 | 0.4 | 0.2 | 0.3 |
| Range | 6.0–12.0 | 5.9–12.0 | 4.9–10.1 | 4.6–11.2 |
| Total IgE (kU/l) | ||||
| Mean | 156.4 | 268.8 | 437.6 | 260.2 |
| SD | 101,5 | 264,1 | 343,5 | 121,2 |
| Range | 20.9–485.0 | 26.3–921.0 | 30.4–1026.0 | 25.4–1442.0 |
| sIgE timothy grass (kU/l) | ||||
| Mean | 22.5 | 32.4 | 39.8 | 31.3 |
| SD | 25,4 | 27,3 | 39,3 | 24,2 |
| Range | 2.3–100.0 | 3.3–100.0 | 1.6–100.0 | 1.7–100.0 |
| Phl p 7 positive | ||||
| N | 0 | 5 | 2 | 1 |
| SPT grass pollen (mm2) | ||||
| Mean | 68.1 | 72.8 | 59.5 | 71.7 |
| SD | 33.9 | 37.8 | 31.3 | 45.8 |
| Range | 9.6–140.6 | 26.3–136.7 | 11.7–135.7 | 14.7–172.3 |
2.2. Sample size calculation
Sample size estimation for comparison of 3 treatments with a control was done according to (Horn and Vollandt, 1998). The difference between placebo and the highest dose group in the change of the TNSS score before and after treatment was assumed to be 2.65 (standard deviation 2.0). A sample size of 13 patients was derived, which finally led to a size of 15 patients per group with respect to a 15% drop-out rate (one-sided significance level 5%, power 90%).
2.3. Study design, study drug and concomitant medication
This study was a safety and dose finding phase II study investigating safety and dose-dependent effects of three subcutaneous injections of three different doses of an aluminum hydroxide-adsorbed, recombinant grass pollen vaccine (BM32) versus placebo (i.e., aluminum hydroxide alone). The grass pollen allergy vaccine BM32 included four recombinant fusion proteins consisting of non-allergenic peptides derived from the IgE-binding sites of the major grass pollen allergens (Phl p 1, Phl p 2, Phl p 5, Phl p 6) fused to hepatitis B virus-derived PreS which were adsorbed to aluminum hydroxide (Biomay AG, Polymun Scientific GmbH Vienna) (Fig. 1) (Focke-Tejkl et al., 2015). BM32/S1 contained 10 μg, BM32/S2 20 μg and BM32/S3 40μg of each of the four fusion proteins. Ready to use suspensions of BM32 of each of the three doses (S1, S2, S3) and placebo (aluminum hydroxide) were provided in single use 2ml glass vials. Four hundred μl were applied per s.c. injection. On demand use of inhaled short acting beta-agonists, antihistamines and decongestants with discontinuation at least 24 hours prior to next evaluation was allowed. Any concurrent medication had to be approved by the investigator and was recorded.
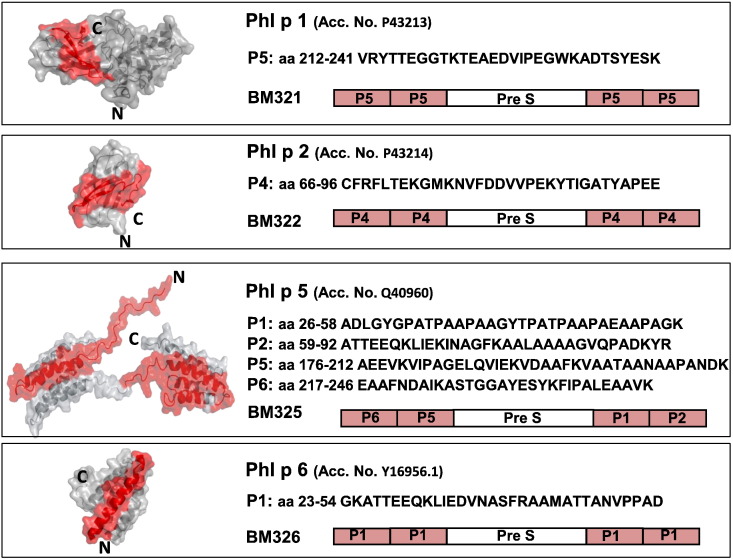
Fig. 1.
Characterization of the recombinant fusion proteins in BM32. Shown are the three-dimensional structures of the four timothy grass pollen allergens Phl p 1, Phl p 2, Phl p 5 and Phl p 6 (accession numbers for sequences in brackets) with the peptides (position, amino acid sequences) indicated in red which are part of the recombinant PreS fusion proteins BM321, BM322, BM325, BM326
The effects of vaccination on responses to grass pollen allergen challenge in the Vienna challenge chamber (VCC) and by skin testing were tested. In addition, effects of vaccination on grass pollen-specific humoral and cellular immune responses were studied in detail. The study has been registered at https://clinicaltrials.gov under the identifier: NCT01445002. It was conducted outside the grass pollen season between October 2011 (screening) and April 2012 (data base lock). The trial was approved by the Clinical Pharmacology Ethics Committee, Vienna, Austria and by the Austrian Regulatory Agency (AGES). Written informed consent was obtained from each patient ahead of any study specific procedure, and the study was conducted according to the principles of the declaration of Helsinki and the International Conference on Harmonization of Good Clinical Practice guidelines.
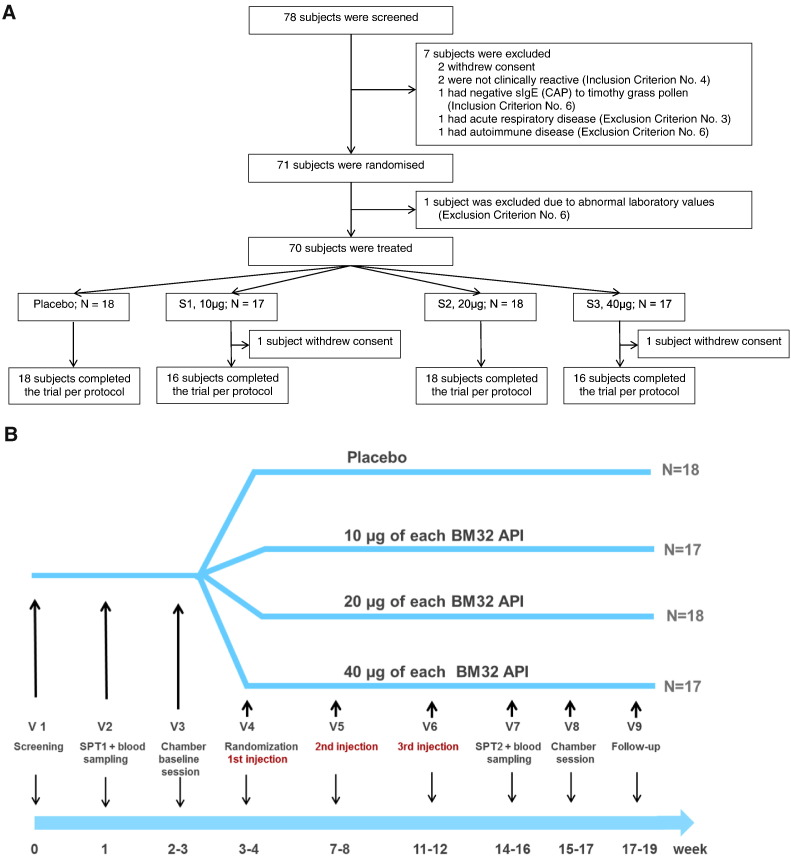
Seventy eight subjects were screened and 71 were randomized to the four treatment groups (S1, S2, S3, Placebo) (Fig. 2A) (Table 1). One to 4 weeks after skin testing and after the baseline session in the challenge chamber subjects received the first injection followed by two additional injections with approximately 1 month intervals (Fig. 2B). At these visits safety assessments including urine pregnancy testing in women were performed. During the entire study period, patients documented adverse events and concomitant medication in a personal diary. Approximately 4 weeks after the last injection subjects underwent skin testing and a second session in the challenge chamber. Blood samples for immunological analysis were obtained during screening and during the second challenge session. A follow up visit was done 1–2 weeks later (Fig. 2B).
Fig. 2.
Study enrolment, randomization (A) and study design (B). Patients screening, baseline SPT, blood sampling and baseline challenge chamber session (VCC) occurred in a period of 28 day before treatment was started. Then patients were randomized and received three subcutaneous injections in 3–4 week intervals. Three–4 weeks after the last injection SPT was performed, a blood sample was taken and patients underwent the second challenge chamber session (VCC). The concluding follow up visits were scheduled approximately 2 weeks after the last challenge chamber session.
2.4. Clinical endpoints and assessments
Primary efficacy endpoint was the difference in TNSS (itching, sneezing, rhinorrhea, nasal obstruction) before and after treatment. Clinical secondary endpoints were the change of TOSS (ocular itch, tearing, conjunctival injection) and changes in grass pollen-specific titrated skin prick test reactivity before and after treatment in terms of sum of wheal areas and threshold concentrations. Primary safety endpoint was the frequency and severity of treatment-related local and systemic adverse reactions.
For the assessment of TNSS grass pollen allergen challenges were conducted in the Vienna Challenge Chamber (VCC) before and approximately 4 weeks after treatment (Horak et al., 2009). During the 6 hour challenge, the chamber was charged with fresh air, which was cleaned, cooled, dried and then loaded with a qualitatively and quantitatively determined load of mixed grass pollen from Dactylis glomerata, Lolium perenne, Phleum pratense and Anthoxanthum odoratum. According to continuous monitoring patients were exposed to constant humidity (approximately 40%), temperature (approximately 24°C) and allergen loads (approximately 1500 pollen grains per cubic meter) throughout the exposure period. Patients (up to 20 per session) were under constant supervision by medical staff outside the chamber.
TNSS comprised the four components of nasal itching, sneezing, rhinorrhea and nasal congestion. Each symptom was scored on a four point scale from 0 to 3 (0 = absence of symptoms — 3 = severe symptoms) (Pfaar et al., 2014) giving a TNSS range from 0 to 12. Patients completed the TNSS assessment on a touch screen monitor immediately prior to entering the chamber, and at 15 minute intervals during the 6 h allergen challenge. TOSS was evaluated accordingly. Furthermore nasal air flow was measured using active anterior rhinomanometry immediately prior to entering the chamber and at 30 minute intervals during the challenge.
A titrated skin prick test was performed in duplicates before and 4 weeks after treatment. A registered standardized timothy grass pollen extract (Stallergenes, Antony, France) was diluted in 1:2 steps with a maximal dilution of 1:128 and the threshold concentration necessary to elicit a positive skin reaction was determined (Drachenberg et al., 2001).
Recordings of concomitant medication and adverse events (local and systemic reactions) were evaluated by keeping daily diaries. Treatment-related adverse events were recorded in the CRF and graded according to “Allergen immunotherapy — a position paper of the German Society for Allergology and Clinical Immunology” (Kleine-Tebbe et al., 2001). Patients were monitored in the study center for at least 30 minutes after injection of the investigational product.
2.5. Immunological analyses
Immunological endpoints were changes in allergen-specific IgG, IgG subclass (IgG1-IgG4) and IgE levels, in vitro allergen-induced cytokine responses and allergen-specific basophil activation as measured by CD203c expression before and four weeks after end of treatment. Allergen-induced T cell proliferation was assessed after end of treatment with allergens in the presence of pre- and post-treatment sera. Recombinant grass pollen allergens (rPhl p 1, rPhl p 2, rPhl p 5 and rPhl p 6) and recombinant BM32 proteins (BM321, BM322, BM325 and BM326) used in the immunological assays were produced by Biomay AG (Vienna, Austria). Recombinant PreS was expressed and purified as described (Niespodziana et al., 2011). Allergen- and PreS-specific IgG as well as IgG1-IgG4 subclass levels were measured by ELISA (Gallerano et al., 2015). Timothy grass pollen allergen extract-,rPhl p 1-, rPhl p 2-, rPhl p 5-, and rPhl p 6-specfic IgE antibody levels (kUA/l) before and after treatment were quantified with a Phadia 250 ImmunoCAP system (Thermo Fisher). A comparison of IgE reactivities to the purified recombinant grass pollen allergens, to the BM32 derivatives (BM321, BM322, BM325 and BM326) and PreS in pre-treatment sera was performed by ELISA (Niederberger et al., 2015).
Furthermore, allergen-specific IgE and IgG binding to micro-arrayed allergens was measured using ImmunoCAP ISAC technology (Thermo Fisher). This technology utilizes low amounts of micro-arrayed allergens and can be used to visualize the competition of allergen-specific IgG with allergen-specific IgE binding by a reduction of IgE binding (Lupinek et al., 2014, Wollmann et al., 2015).
Allergen-induced basophil activation was determined in heparinized blood samples obtained before and after treatment by measuring the up-regulation of CD203c expression on basophils upon exposure to a mix of increasing concentrations of the four major timothy grass pollen allergens (rPhl p 1,2,5,6) containing 1, 5, 25 and 125 pg/ml of each allergen compared to buffer control (phosphate-buffered saline = PBS)
Mean fluorescence intensities of stimulated (MFIstim) and unstimulated (MFIcontrol) cells are determined for triplicate cultures by flow cytometry and the up-regulation of CD203c expression is expressed as stimulation index (MFIstim : MFIcontrol) (SI) (Hauswirth et al., 2002).
Peripheral blood mononuclear cells (PBMC, 2 × 105/well) prepared from patients heparinized blood samples before and after treatment were cultured in triplicates in 96-well plates (Nunclone; Nalge Nunc International, Roskilde, Denmark) and stimulated with a mix containing 0.25 μg/well of each of the four major timothy grass pollen allergens allergens, rPhl p 1, 2, 5 and 6) as described (Niederberger et al., 2015). The levels of in vitro allergen-induced cytokine responses (IL-1b, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12, IL-13, IL-17, INF-γ, TNF-α, C-GSF, GM-CSF, MCP-1, and MIP-1b) were measured using Bio-Plex ProTM human cytokine 17-plex immunoassay (Bio-Rad Inc., Hercules, CA) using a Luminex 100 System (Luminex Corp. Austin, Tx). Allergen-induced T cell proliferation was determined in triplicate PBMC cultures obtained after treatment. This was done by measuring the T cell proliferation upon stimulation with the allergen mix in the presence of serum (50μl) obtained before or after treatment for each patient by thymidine up-take (Focke-Tejkl et al., 2015). Stimulation indices (SIs) were calculated as the quotient of the mean cpms obtained for cultures with allergen and medium alone. For patients with SIs higher than 1 the cpm differences between the allergen-induced proliferation with serum before and after treatment were calculated. For logistic reasons it was not possible to obtain and process samples for cellular experiments from all patients and therefore results are shown only for those patients where experiments could be performed.
2.6. Statistical analysis
The primary efficacy endpoint (difference in the TNSS2-6h before and after treatment) was analyzed using a stepwise, hierarchical procedure (Tamhane et al., 1996), which respects the overall α-level of 5%: The three BM32 dose groups were each compared to placebo using one-sided t-tests at α = 0.05. Further analyses on the primary efficacy endpoint were conducted using ANOVAs with fixed factors treatment and strata moderate and severe allergy. Changes in TNSS2-6h before and after treatment within each treatment group were analyzed using a paired t-test.
AUC2-6h of TNSS and TNNSS before and after treatment as well as the change in AUCs were analyzed with descriptive statistics and displayed graphically (mean and 95% CI) for each group. Absolute changes in AUC within each treatment group before and after treatment were looked at using paired t-tests. Comparisons between each verum and placebo group of absolute AUCs values at each visit and change from pre-treatment AUCs were done applying the same ANOVA model like for the primary efficacy parameter. The mean of TOSS (which is a component of TNNSS and thus a post hoc analysis) was calculated, the change between pre and post treatment was given in % of baseline value.
Changes in the skin reaction to grass pollen extract, determined by a titrated skin prick test before and after the treatment were evaluated via measuring wheal areas at screening and at visit 7. In addition, the change in the threshold concentration of grass pollen extract necessary to provoke a positive skin reaction was evaluated. Results were analyzed by descriptive statistics and Fisher´s exact t-test was applied to evaluate differences between the groups.
Antibody evaluations used a signed-rank Wilcoxon test for evaluating paired differences before and after treatment within each treatment group, and the Kruskal-Wallis test to check for a global difference between treatment groups.
All tests except the ones for the primary efficacy endpoint were done on an explorative two-sided 5% significance level. Details regarding the statistical analyses of secondary endpoints can be found in the Supplemental materials (Study protocol and Statistical analysis plan).
Confirmatory testing of the primary efficacy parameter was based on the full-analysis set (FAS). All secondary endpoints used the FAS as well. Further evaluations of the primary efficacy endpoint as well as selective secondary endpoints used the per-protocol population (PP) as indicated.
3. Results
3.1. Vaccination with BM32 reduces grass pollen-induced rhinitis and immediate skin responses
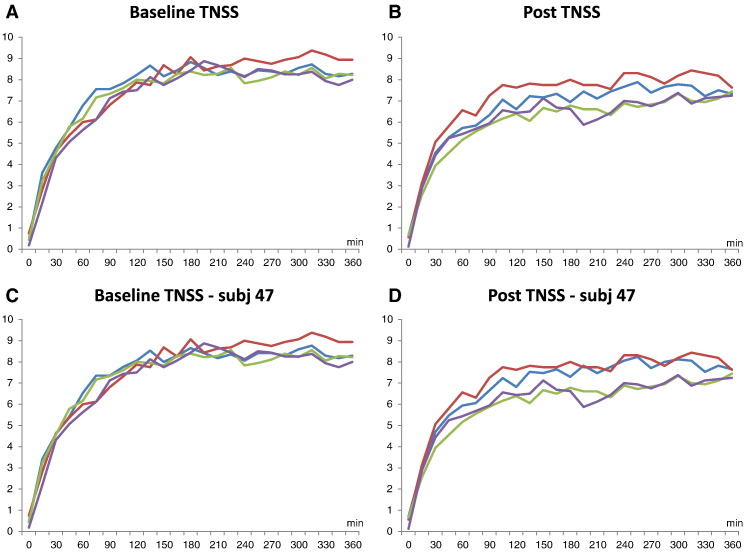
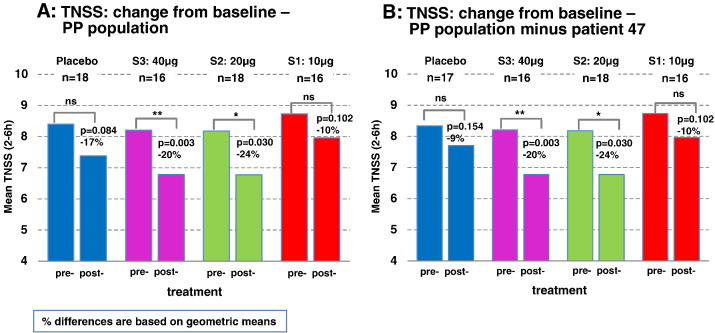
A significant decrease of the TNSS after treatment was found in patients of groups S2:20 μg and S3:40 μg with a mean reduction of 1.41 (p = 0.03) and 1.34 (p = 0.003), respectively (Fig. 3, Fig. 4). Mean changes of TNSS in the placebo and S1:10 μg groups were not significant (Fig. 4A). Fig. 3 shows no difference regarding the development of the TNSS in the four treatment groups over the 6 hours pollen exposure in the challenge chamber at baseline (Fig. 3A). After treatment patients from groups S2:20 μg and S3:40 μg showed lower TNSS during almost the entire chamber session (i.e., between hours 1 and 6) when compared to placebo and group S1:10 μg (Fig. 3B). Interestingly, patients from group S1:10 μg showed higher TNSS than placebo-treated patients. A post hoc analysis of the placebo-treated patients revealed that this difference was due to one placebo subject (i.e., subj. 47) who showed an unusual drop of TNSS of 10 points (i.e., 12 to 2) compared to the baseline session which was not compatible with objective parameters obtained by active anterior rhinomanometry (data not shown). The post hoc removal of subject 47 did not affect the TNSS results at baseline whereas TNSS results of the placebo and the S1:10 μg group became almost indistinguishable after treatment (Fig. 3C, D, Fig. 4B).
Fig. 3.
A–D Time course of the mean total nasal symptom scores recorded for the four treatment groups in the challenge chamber at baseline and after vaccination. Shown are the means of the total nasal symptom scores (y-axes) recorded for the four treatment groups over a period of 360 min (x-axes) before treatment at baseline and after the three injections (post) for all patients and after removal of subject 47 (A: baseline TNSS; B: TNSS post; C: baseline TNSS-subj 47; D: TNSS-subj 47 post). (S1: 10 μg — red lines; S2: 20μg — green lines; S3: 40 μg — purple lines; placebo — blue lines).
Fig. 4.
Means of the TNSS recorded in the challenge chamber from hours 2 to 6. For each treatment group the mean TNSS at baseline (pre-) and after vaccination (post-) is shown with p-values and % differences for intra-group changes. Percentages (%) differences are based on geometric means (A: PP population, B: PP population without subject 47). (S1: 10 μg — red bars; S2: 20 μg — green bars; S3: 40 μg — purple bars; placebo — blue bars)
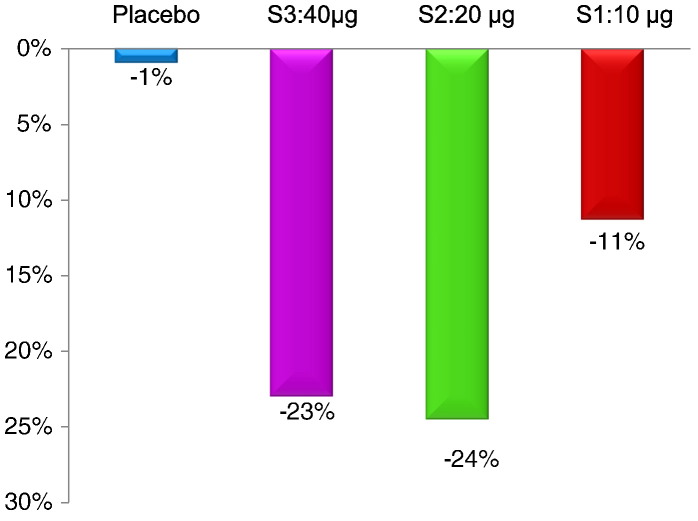
After treatment, a dose-dependent reduction of TOSS was observed (11% for S1:10μg; 23% for S2:20μg; 24% for S3:40μg) whereas the placebo group remained unchanged (Fig. 5).
Fig. 5.
TOSS in the treatment groups. Shown is the reduction of TOSS after treatment compared to baseline in the per protocol population (blue = placebo, purple = 40 μg dose, green = 20 μg dose, red = 10 μg dose). Percentage changes compared to baseline are indicated.
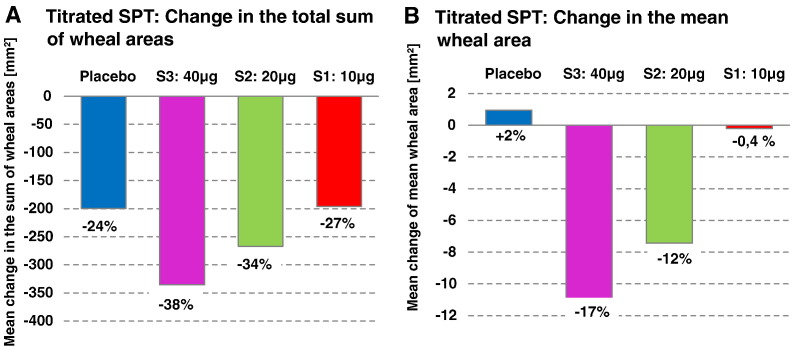
Quantitative skin prick test titration with grass pollen allergen extract before and after treatment showed a dose-dependent reduction of allergen-specific skin sensitivity after treatment which was most pronounced in the S2:20 μg and S3:40 μg group when compared to baseline (Fig. 6A, B). We found a dose-dependent reduction of the mean wheal areas in the patients of the S2:20 μg and S3:40 μg group when compared to baseline whereas the placebo and the S1:10 μg group showed no or a low reduction in wheal reactions (Fig. 6B). The mean reduction was largest for the S3:40 μg group (− 10.96 mm2; SD 24.64) followed by the S2:20 μg group (− 7.43 mm2; SD 18.89) whereas there were only very little changes for the S1:10 μg group (− 0.20 mm2; SD 16.41) and placebo group (+ 0.96 mm2; SD 16.04). (Fig. 6B). Likewise, we found a dose-dependent reduction of the sum of wheal areas of the S2:20 μg (− 266.8; SD 257.20) and S3:40 μg (−335.4; SD 371.06) group when compared to baseline and again the S1:10 μg group (− 195.8; SD 225.86) was similar to placebo (− 199.6; SD 284.71) (Fig. 6A).
Fig. 6.
Grass pollen allergen-specific skin reactions in the treatment groups. Shown are in (A) the changes of the sums of wheal areas after treatment compared to baseline and in (B) the changes in the mean wheal areas after treatment compared to baseline in the per protocol population (blue = placebo, purple = 40 μg dose, green = 20 μg dose, red = 10 μg dose). Percentage changes compared to baseline are indicated.
By SPT endpoint titration we found that 5 out of 18 patients from group S2:20μg and 5 out of 16 patients of group S3: 40μg shifted their sensitivities at least to 1:8 or 1:4 whereas such a shift was observed only for 2 out of 16 patients from group S1 and only for one out of 18 of the placebo group (data not shown).
3.2. High doses of BM32 are well-tolerated
All but two subjects concluded the study per protocol. Two subjects, one from the S1:10 μg and one from the S3:40 μg group, withdrew consent due to restriction in study visit availability (Fig. 2A). The majority of TAEs were local injection site late phase reactions. Local injection site reactions occurred in both actively- and placebo-treated patients. There was no significant difference regarding the frequency of occurrence of these reactions but a dose-dependent trend to higher intensity of the local reactions in the verum groups (Table 2). No systemic grade 2, 3 or 4 reactions were observed. All systemic TAEs were late phase reactions (i.e., occurred after several hours) of grade 1. They included allergic rhinitis in 7 actively treated patients and 1 conjunctivitis in the S3:40 μg group. Cutaneous reactions were one flush, one event of itching and palmar erythema and urticarial reactions in three patients (Table 2). No systemic reactions were observed in the placebo group.
Table 2.
Summary of treatment-associated adverse events.
| Organ specific reaction |
BM32/S1 (N = 17) |
BM32/S2 (N = 18) |
BM32/S3 (N = 17) |
Placebo (N = 18) |
Total (N = 70) |
|---|---|---|---|---|---|
| Number of subjects (%) event count | |||||
| Injection site reaction | 17 (100.0) 47 | 17 (94.4) 57 | 17 (100.0) 51 | 17 (94.4) 48 | 68 ( 97.1) 203 |
| Allergic rhinitis | 3 (17.6) 4 | 0 (0.0) 0 | 4 (23.5) 5 | 0 (0.0) 0 | 7 (10.0) 9 |
| Cutaneous reactions | 1 (5.9) 2 | 2 (11.1) 3 | 1 (5.9) 1 | 0 (0.0) 0 | 4 (5.7) 6 |
| Allergic conjunctivitis | 0 (0.0) 0 | 0 (0.0) 0 | 1 (5.9) 1 | 0 (0.0) 0 | 1 (1.4) 1 |
| Flushing | 1 (5.9) 1 | 0 (0.0) 0 | 0 (0.0) 0 | 0 (0.0) 0 | 1 (1.4) 1 |
3.3. Vaccination with BM32 induces grass pollen allergen-specific IgG without boosting allergen-specific IgE responses
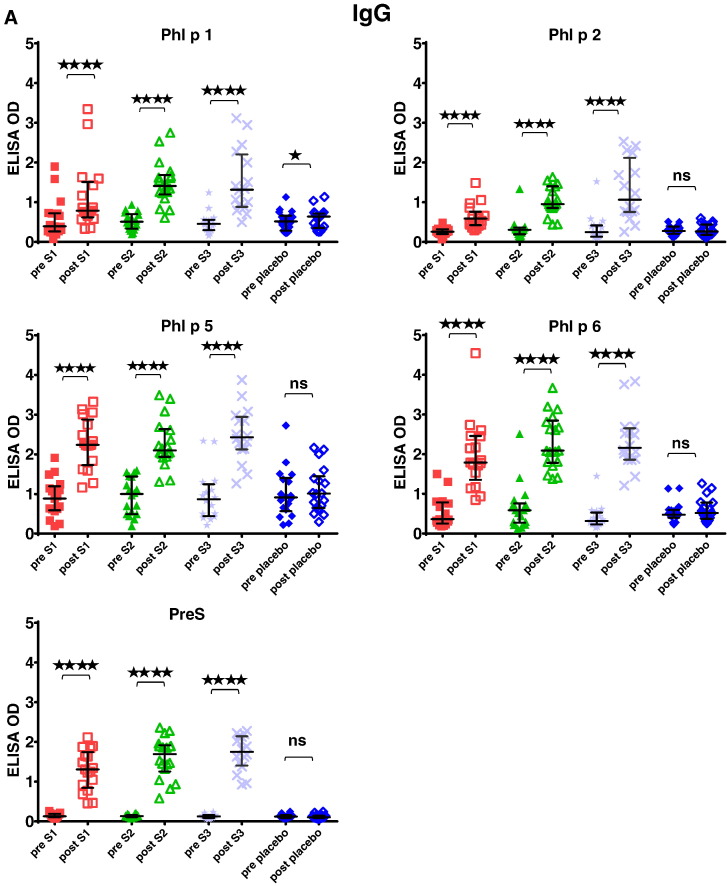
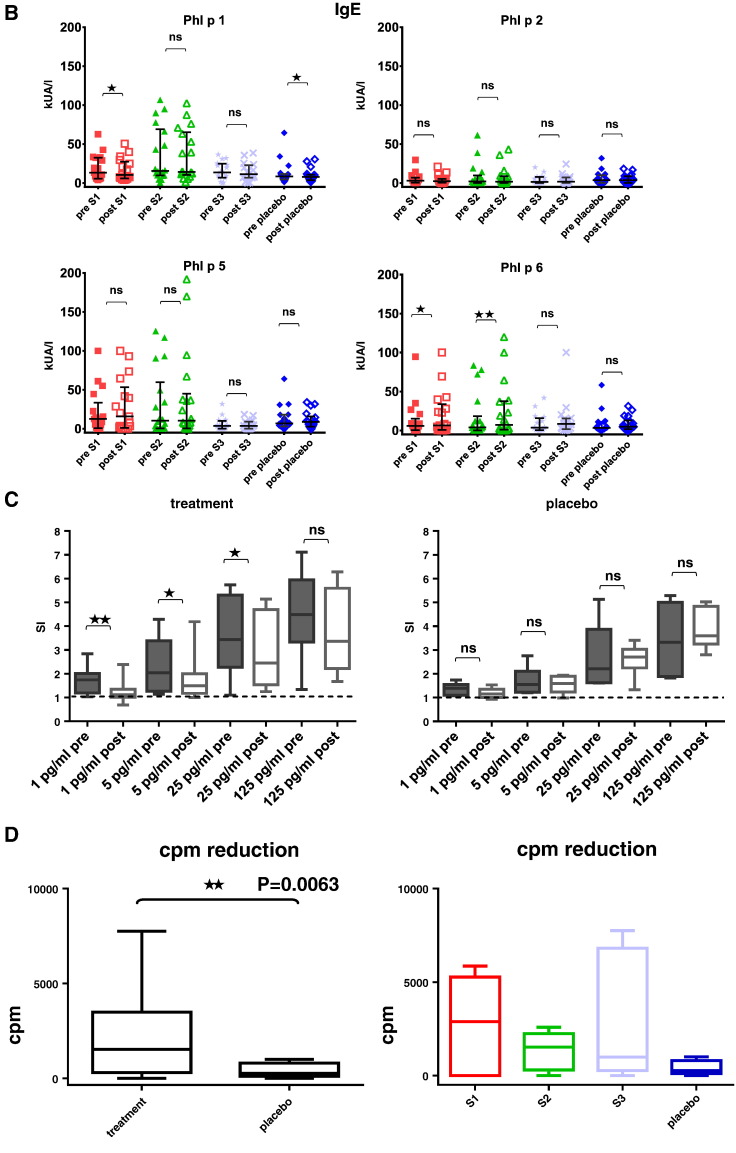
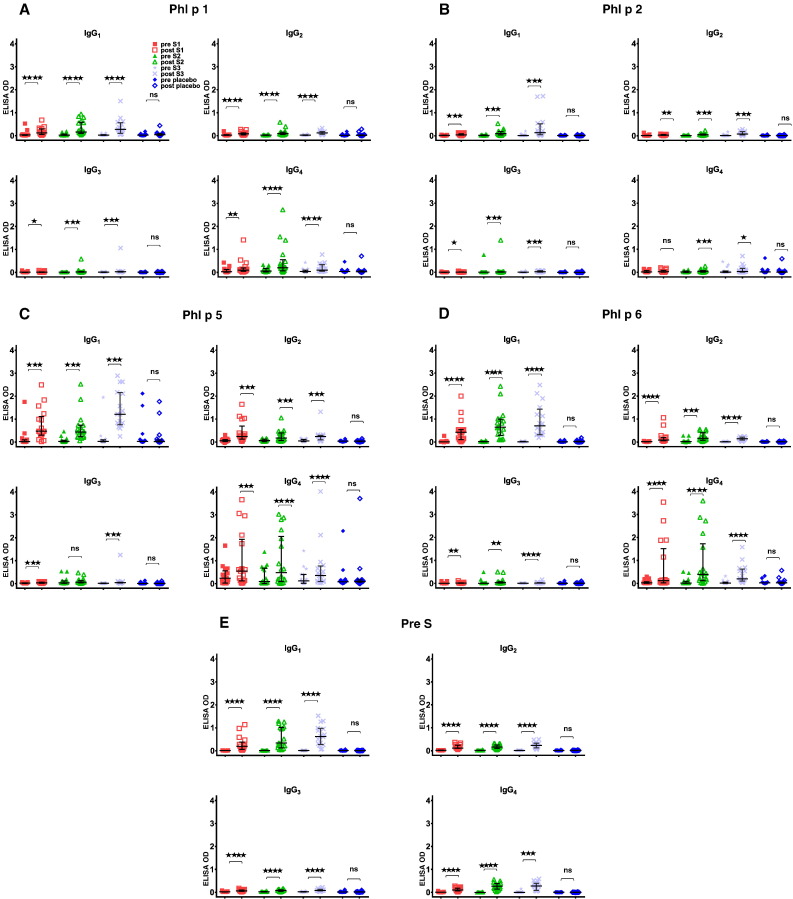
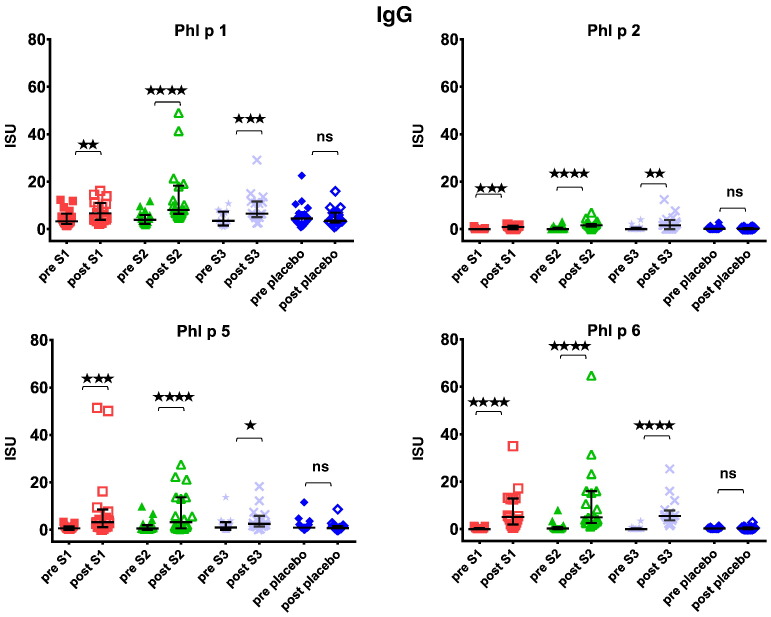
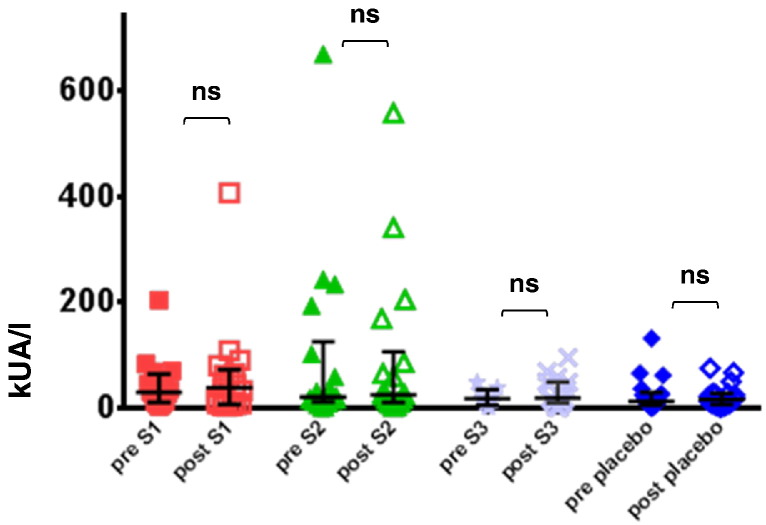
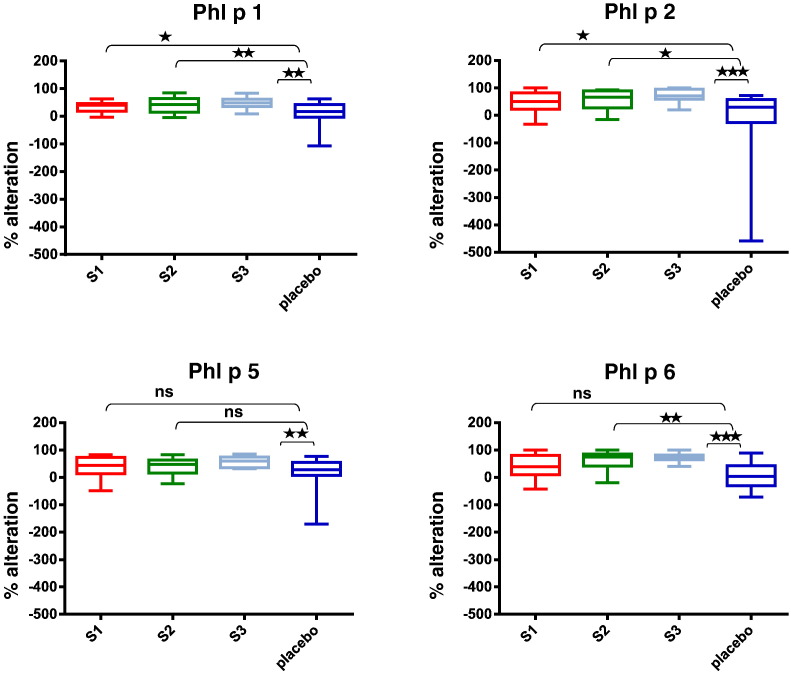
Fig. 7 shows that immunotherapy with each of the three doses of BM32 but not with placebo induced highly significant (p < 0.0001) increases of IgG antibodies specific for the four major grass pollen allergens as well as to the PreS carrier protein which influenced also cellular responses. The induced allergen-specific IgG antibodies are specific for the peptides included in BM32 but recognize also the peptides in the context of the complete allergens (data not shown; Focke-Tejkl et al., 2015). The BM32-induced IgG response was mainly composed of allergen- and PreS-specific IgG1 and IgG4 responses as well as some IgG2 responses whereas the IgG3 response was negligible (Fig. 8A-E). Significant BM32-induced allergen-specific IgG responses were also detected using micro-arrayed allergen molecules (Fig. 9). Immunotherapy with BM32 did not lead to any relevant increases of allergen-specific IgE levels (Fig. 7B). Only for the low doses (i.e., S1 and S2) a slight induction of Phl p 6-specific IgE was noted whereas no significant increases were found for the three doses for Phl p 1, Phl p 2, Phl p 5 and for the high dose (i.e., S3) for Phl p 6 (Fig. 7B). The quantification of timothy grass pollen allergen-specific IgE levels showed that there were no significant increases after treatments with BM32 (Fig. 10). When we assessed allergen-specific IgE reactivity to micro-arrayed allergens by chip testing, an assay where low amounts of allergens are immobilized to the solid phase, we found significant reductions of allergen-specific IgE binding for Phl p 1 and Phl p 2 when comparing the three BM32 doses with placebo and significant reductions of IgE binding for Phl p 5 (S3 versus placebo) and Phl p 6 (S2 and S3 versus placebo) (Fig. 11).
Fig. 7.
Grass pollen allergen-specific immunological changes in the treatment groups before and after vaccination. (A) IgG levels specific for Phl p 1, Phl p 2, Phl p 5, Phl p 6 and PreS (y-axes: OD levels, horizontal bars denote medians) in sera from the four patient groups (S1: 10 μg; S2: 20 μg; S3: 40 μg; placebo) before (pre) and after (post) treatment. (B) IgE levels specific for Phl p 1, Phl p 2, Phl p 5 and Phl p 6 (y-axes: OD levels, horizontal bars denote medians) in sera from the four patient groups (S1: 10 μg; S2: 20 μg; S3: 40 μg; placebo) before (pre) and after (post) treatment. Significant differences between pre and post samples are indicated (****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05).
(C) Grass pollen allergen-induced up-regulation of CD203c expression (y-axes: SIs as box plots with indicated medians, whiskers = minimum and maximum; boxes = 25th to 75th percentiles) in tested blood samples from patients of the active treatment groups (S1: 10 μg, n = 4; S2: 20 μg, n = 4; S3: 40 μg, n = 2) or the placebo group (n = 6) for different allergen concentrations (x-axes) before (pre) and after (post) treatment. Significant differences between pre and post samples are indicated (**p < 0.01; *p < 0.05).
(D) Reduction of allergen-induced T cell proliferation in PBMCs (y-axes: counts per minute, cpm reductions as box plots with indicated medians, whiskers = minimum and maximum; boxes = 25th to 75th percentiles) after pre-incubation of allergens with post-treatment sera versus pre-incubation with pre-treatment sera for placebo (n = 6) and actively treated patients (n = 19) and for the individual treatment groups (S1: 10 μg, n = 6; S2: 20 μg, n = 7; S3: 40 μg, n = 6; placebo, n = 6). Significant differences in the cpm reductions between actively and placebo treated patients are indicated.
Fig. 8.
IgG subclass responses to grass pollen allergens and PreS in the treatment groups. Shown are IgG1, IgG2, IgG3 and IgG4 subclass levels specific for Phl p 1 (panel A), Phl p 2 (panel B), Phl p 5 (panel C), Phl p 6 (panel D) and PreS (panel E) (y-axes: OD levels, horizontal bars denote medians) in sera from the four patient groups (S1: 10 μg; S2: 20 μg; S3: 40 μg; placebo) before (pre) and after (post) treatment. Significant differences between pre and post samples are indicated (****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05).
Fig. 9.
Allergen-specific IgG responses to micro-arrayed grass pollen allergens. Shown are IgG levels (y-axes: ISU; horizontal bars denote medians) for Phl p 1, Phl p 2, Phl p 5, Phl p 6 in sera from the four patient groups (S1: 10 μg; S2: 20 μg; S3: 40 μg; placebo) before (pre) and after (post) treatment. Significant differences between pre and post samples are indicated (****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05).
Fig. 10.
Quantification of grass pollen allergen-specific IgE levels in the treatment groups before and after vaccination. Timothy grass pollen allergen extract-specific IgE levels determined by ImmunoCAP (y-axes: kUA/l, horizontal bars denote medians) in sera from the four patient groups (S1: 10 μg; S2: 20 μg; S3: 40 μg; placebo) before (pre) and after (post) treatment.
Fig. 11.
Changes of IgE-binding to micro-arrayed allergens in the treatment groups. Shown are the percentages reduction of IgE binding (box plot representations of the 25th to 75th percentiles, horizontal bars denote medians, whiskers = minimum and maximum) to Phl p 1, Phl p 2, Phl p 5 and Phl p 6 in the four patient groups (S1: 10 μg; S2: 20 μg; S3: 40 μg; placebo) after treatment. Significant differences between actively and placebo-treated patients are indicated (***p < 0.001; **p < 0.01; *p < 0.05).
3.4. IgG antibodies induced by vaccination with BM32 inhibit allergen-induced basophil degranulation and T cell proliferation
We found a significant reduction of allergen-induced up-regulation of CD203c expression for the BM32-treated patients in the increasing part of the curve of the dose-dependent basophil activation whereas no reduction of basophil activation was observed for placebo-treated patients after treatment (Fig. 7C).
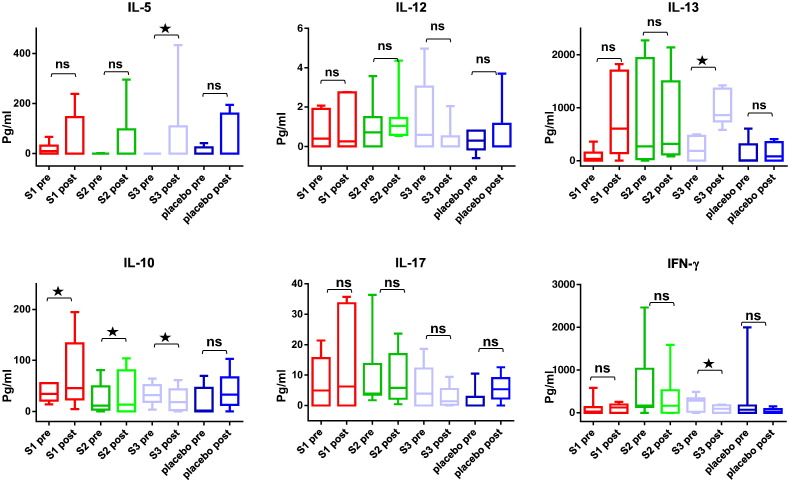
Next we assessed whether BM32-induced allergen-specific IgG antibodies influence allergen-induced T cell proliferation (Fig. 7D). Pre-incubation of allergens with post-treatment sera containing treatment-induced allergen-specific IgG antibodies obtained from BM32-treated patients inhibited PBMC proliferation more than pre-incubation with post-treatment sera from placebo-treated patients without treatment-induced allergen-specific IgG antibodies for each of the three BM32 doses (Fig. 7D). This effect (i.e., reduction of T cell proliferation as measured by counts per minute reduction) was statistically significant when the results from the three BM32 doses were compared to placebo (p = 0.0063). Fig. 12 shows the levels of allergen-induced cytokines (IL-5, IL-12, IL-13, IL-10, IL-17 and interferon-gamma) detected before and after treatment in patients PBMC culture supernatants. We noted a significant (p < 0.05) induction of IL-10 for the lower doses S1 and S2 whereas for S3 a significant decrease (p < 0.05) of IL-10 production which was associated with significant increases of IL-5 and IL-13 (p < 0.05) and a significant decrease of interferon gamma (p < 0.05) was noted. The analysis of the secretion of additional 11 cytokines (see methods) in PBMC cultures obtained before and after treatment showed no significant changes (data not shown).
Fig. 12.
Changes of allergen-induced cytokine secretion in cultured PBMC obtained from the patients before and after treatment. Shown are the levels of cytokines (IL-5, IL-12, IL-13, IL-10, IL-17, IFN-gamma) (y-axes: pg/ml; box plot representations of the 25th to 75th percentiles, horizontal bars denote medians, whiskers = minimum and maximum) measured in cultured PBMCs stimulated with grass pollen allergens before and after treatment in the four patient groups (S1: 10 μg; S2: 20 μg; S3: 40 μg; placebo). Significant differences between before and after cultures are indicated (*p < 0.05).
4. Discussion
This AIT study is the first to investigate safety as well as clinical and immunological effects of a new strategy for AIT which is based on a B cell epitope-based recombinant allergy vaccine. It shows that three monthly injections of a vaccine consisting of four recombinant fusion proteins combining non-allergenic peptides derived from the four major timothy grass pollen allergens, Phl p 1, 2, 5 and 6 with the hepatitis B preS protein are effective in reducing allergen-induced immediate allergic reactions as demonstrated by the reduction of TNSS, TOSS and immediate skin responses in grass pollen allergic patients. We consider the reduction of the TNSS observed after vaccination with the 20 and 40 μg dose of BM32 as relevant because its magnitude is comparable to that observed after administration of systemic antihistamines in the pollen chamber model (Horak et al., 2010). Furthermore, it has been demonstrated that a one point difference in the rhinoconjunctivitis total symptom score is clinically relevant in grass pollen-induced allergic rhinoconjunctivitis (Devillier et al., 2014).
The study further shows that the new B cell epitope based vaccine was very well tolerated although high doses of up to 160 μg of the four recombinant proteins per injection were administered without any up-dosing. In fact, the previous in vitro assessment of BM32 had shown that the vaccine exhibited almost no IgE reactivity and allergenic activity when exposed to basophils of allergic patients (Focke-Tejkl et al., 2015). It was found that allergic patients failed to exhibit any relevant immediate type skin reactions even when tested at the height of the grass pollen season (Niederberger et al., 2015). We also had reduced the presence of grass allergen-specific T cell epitopes in BM32 but a complete elimination of all T cell epitopes is not possible due to the diversity of allergic patients T cell epitope recognition. However, we found that the reduction of allergen-specific T cell epitopes in the vaccine resulted in a strong reduction of T cell and pro-inflammatory cytokine responses when BM32 was tested in PBMC cultures from grass pollen allergic patients (Focke-Tejkl et al., 2015). Accordingly, no late skin responses were found in grass pollen allergic patients when BM32 was applied by atopy patch testing (Niederberger et al., 2015).The latter study indeed suggests that BM32 has a strongly reduced potential to induce late phase, T cell-mediated side effects in patients.
Here, we treated 52 patients with doses of 40–160 μg of the four recombinant proteins and thus administered 155 injections. Given that very high doses were injected, the vaccine was very well tolerated because very few (i.e., 9 events of late phase rhinitis, 6 events of late phase cutaneous reactions, 1 late phase conjunctivitis and one late phase flushing) and mild (i.e., grade 1) late phase systemic reactions were observed. In contrast, previous “hypoallergenic vaccines” which included allergen-specific T cell epitopes frequently induced late phase reactions (Niederberger et al., 2004, Purohit et al., 2008, Spertini et al., 2014).The analyses of recombinant hypoallergenic T cell epitope-containing derivatives of the major birch pollen allergen, Bet v 1 (Purohit et al., 2008) by atopy patch testing indicated that these reactions most likely originated from T cell activation (Campana et al., 2015). Likewise, it was found that a T cell epitope-containing vaccine for birch pollen allergy (AllerT) based on peptides of the major birch pollen allergen Bet v 1 frequently caused late phase reactions (Spertini et al., 2014). During the administration of 75 injections of T cell epitope-containing peptides of Bet v 1, 31 events of late phase dyspnoe and 36 late phase cutaneous reactions were observed with AllerT (Spertini et al., 2014). We therefore think that the reduction of allergen-specific T cell epitopes in BM32 and their replacement by the allergen-unrelated carrier protein PreS has contributed to the tolerability of BM32. We have already constructed several hypoallergenic derivatives for allergens from different allergen sources (e.g., cat, birch, house dust mites) using PreS as carrier protein (Niespodziana et al., 2011, Marth 2012, Banerjee et al., 2014).The in vitro and experimental animal data collected for all these derivatives suggest a similar mode of action of these vaccines. Upon injection they induce allergen-specific IgG responses towards the IgE binding sites of the corresponding allergens with T cell help derived from the PreS carrier protein. In fact our study demonstrates for the first time in humans that BM32 induces strong allergen-specific IgG responses which appear to suppress immediate type allergic reactions as shown by the reduction of TNSS upon pollen exposure and by the reduction of immediate type skin responses. The reduction of allergen-induced basophil responses after treatment indeed suggests that the treatment induced IgG antibodies which prevent the allergens from interacting with IgE on the surface of mast cells and basophils and that this may be the major underlying mechanism. This was also mirrored by results from an in vitro assay in which we measured the reduction of IgE binding by therapy-induced IgG in an IgE binding assay in which we can visualize the IgE-blocking effect of IgG using small amounts of solid phase bound allergens using allergen micro-arrays (Fig. 11). AIT with BM32 such as subcutaneous AIT with natural allergens reduces IgE binding to micro-arrayed allergens through induction of IgG competing with IgE for allergen binding (Wollmann et al., 2015, Schmid et al., 2016, Lupinek et al., 2016). The analysis of the allergen-specific isotype and subclass responses showed that BM32 mainly induced allergen-specific IgG responses (IgG1=IgG4 > IgG2) which is similar to allergen-extract based vaccines which are in clinical use and are formulated with Aluminum hydroxide (Gadermaier et al., 2010, Gadermaier et al., 2011) or with Th1-driving adjuvants such as monophosphoryl lipid-A (Mothes et al., 2003).
We furthermore observed previously that allergen-specific IgG responses induced by PreS-based vaccines can inhibit IgE-facilitated allergen presentation to T cells in in vitro studies (Marth et al., 2013). In this study we were able to demonstrate that this mechanism, which was earlier described for AIT with natural allergens (van Neerven et al., 1999), may be also operative in BM32-treated patients. In fact, the addition of post-treatment sera containing therapy-induced allergen-specific IgG to allergens significantly suppressed allergen-induced T cell proliferation suggesting that one can obtain “T cell tolerance” with a B cell epitope-based vaccine.
Another potential advantage is that BM32 induced selectively allergen-specific IgG responses but did not induce boosts of allergen-specific IgE production. In fact, allergen extract-based but also peptide-based vaccines have been shown to induce strong boosts of allergen-specific IgE production. For example, sublingual treatment with grass pollen extract induced a five-fold increase of allergen-specific IgE production (Durham et al., 2006) and a 3-fold increase of allergen-specific IgE was found upon AIT with hypoallergenic Bet v 1-peptides (Spertini et al., 2014). While this may not be a problem for therapeutic vaccination when much larger amounts of allergen-specific IgG are induced at the same time, it may be a prohibitive feature if one considers to use a vaccine strategy for prophylactic intervention in only sensitized but not yet symptomatic children or perhaps even for the prevention of sensitization (Valenta et al., 2012). It is therefore quite possible that BM32 and B cell epitope-based vaccines constructed according to a similar principle may be especially suitable for prophylactic intervention because our data suggest that they have low IgE sensitization capacity. In fact data from experimental animal models indeed indicate that preventive vaccination with carrier-bound B cell epitope peptides can prevent allergic sensitization (Linhart et al., 2014). A general limitation in the field of AIT is that different forms of AIT (e.g., SCIT, SLIT) have not been compared in controlled clinical studies and this also applies for the present study conducted with BM32.
We also found that BM32 induced PreS-specific IgG antibodies (Figs. 7A, 8E). The mapping of the PreS-specific antibody responses using synthetic PreS-derived overlapping peptides revealed that the BM32 induced IgG antibodies reacted with peptides important for the infection of hepatocytes by hepatitis B virus and also suppressed in vitro the infection of hepatocytes by hepatitis B (Cornelius et al., 2016). When we analyzed sera from the present study and another AIT study conducted in grass pollen allergic patients during two years of natural grass pollen exposure (ClinicalTrials.gov number, NCT01538979) we found no difference regarding persons who have received previous HBV vaccination and persons who had not been HBV vaccinated regarding the development of HBV-specific antibody responses or adverse events. This finding together with data from hepatitis B vaccination studies conducted with PreS-containing vaccines (Rendi-Wagner et al., 2006) would even indicate that BM32 may not only protect against grass pollen allergy, but eventually also against hepatitis B infections which would offer an additional advantage (Gerlich and Glebe, 2016). Persons with HBV-infections have not yet been treated with BM32 but it is planned to study if BM32 or one of its components can be used for therapeutic and eventually prophylactic vaccination against HBV infections.
Taken together our results encourage to further evaluate BM32 in larger clinical studies for its efficacy for treating grass pollen allergic patients. Data from a phase IIb study in which more than 150 patients have been treated with BM32 for two years indeed indicate that BM32 is clinically effective for the treatment of grass pollen allergy induced by natural grass pollen exposure (ClinicalTrials.gov number, NCT01538979). The present study provides evidence for clinical efficacy after a short course of treatment in a pollen chamber setting. A limitation of our study is that we do not have data if prolonged treatment with BM32 has long-term beneficial effects on grass pollen allergy. Therefore, future studies are necessary to investigate if vaccination with BM32 has such long-term and disease-modifying effects. Furthermore, work is in progress to move B cell epitope-based vaccines for house dust mite, cat and birch pollen allergy based on the PreS-fusion protein into clinical evaluation.
The following are the supplementary data related to this article.
IgE reactivity of study subjects to grass pollen allergens, recombinant BM fusion proteins and PreS. Box plot representation of IgE levels (y-axis: OD values, whiskers = minimum and maximum; horizontal bars denote medians; boxes = 25th to 75th percentiles) specific for the grass pollen allergens (Phl p 1, Phl p 2, Phl p 5, Phl p 6), the PreS fusion proteins (BM321, BM322, BM325, BM326) and PreS of the study population at baseline. Significant differences between allergens and BM fusion proteins are indicated (****p < 0.0001; **p < 0.01).
Supplementary material 1
Supplementary material 2
Contributors
PZ, PL, RZ, AN, FH, RH, and RV contributed to the study design. PZ, MFT, PL, RZ, MW, RK, KB, FS, HH contributed to data collection. RS, PL, RZ, FH, AK did the statistical analysis of the data. PZ, PV, FS, AN, FH, RH, RV did the initial data interpretation. PZ and RV wrote the first draft of the manuscript. All authors contributed to final data interpretation and contributed to and approved the final draft of the manuscript.
Declaration of interests
Rudolf Valenta has received research grants from BIOMAY AG, Vienna, Austria and from Thermofisher, Uppsala, Sweden and serves as a consultant for these companies and for Fresenius Medical Care, Bad Homburg, Germany. Frank Stolz, Hans Huber, Angela Neubauer and Rainer Henning are employees of BIOMAY AG, Vienna, Austria. Petra Zieglmayer, Rene Schmutz, Patrick Lemell, Rene Zieglmayer and Friedrich Horak are employees of Research Consult GesmbH, Vienna, Austria and have received payment for the conductance of the study. Margarete Focke-Tejkl, Milena Weber, Renata Kiss, Katharina Blatt, Anette Knoll and Peter Valent declare no competing interests.
Acknowledgements
We thank Prof. Walter Keller from the University of Graz and Dr. Christoph Göbl from the Technical University of Munich for preparing the surface representation figures of the grass pollen allergens. This study was supported by grants F4605 and F4611 of the Austrian Science Fund (FWF), grants 830432, 835415 of the Austrian Research Promotion Agency (FFG) and by BIOMAY AG (Vienna, Austria). We thank Thermofisher, Uppsala, Sweden for having provided allergen micro-arrays for the assessment of allergen-specific IgE and IgG responses.
References
- Banerjee S., Weber M., Blatt K. Conversion of Der p 23, a new major house dust mite allergen, into a hypoallergenic vaccine. J. Immunol. 2014;192:4867–4875. doi: 10.4049/jimmunol.1400064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campana R., Moritz K., Marth K. Frequent occurrence of T cell-mediated late reactions revealed by atopy patch testing with hypoallergenic rBet v 1 fragments. J. Allergy Clin. Immunol. 2015;137(2):601–609. doi: 10.1016/j.jaci.2015.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casset A., Mari A., Purohit A. Varying allergen composition and content affects the in vivo allergenic activity of commercial Dermatophagoides pteronyssinus extracts. Int. Arch. Allergy Immunol. 2012;159(3):253–262. doi: 10.1159/000337654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius C., Schöneweis K., Georgi F., Weber M., Niederberger V., Zieglmayer P., Niespodziana K., Trauner M., Hofer H., Urban S., Valenta R. Immunotherapy With the PreS-based Grass Pollen Allergy Vaccine BM32 Induces Antibody Responses Protecting Against Hepatitis B Infection. EBioMedicine. 2016;11:58–67. doi: 10.1016/j.ebiom.2016.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creticos P.S., Schroeder J.T., Hamilton R.G. Immune tolerance network group. Immunotherapy with a ragweed-toll-like receptor 9 agonist vaccine for allergic rhinitis. N. Engl. J. Med. 2006;355:445–455. doi: 10.1056/NEJMoa052916. [DOI] [PubMed] [Google Scholar]
- Devillier P., Chassany O., Vicaut E. The minimally important difference in the Rhinoconjunctivitis Total Symptom Score in grass-pollen-induced allergic rhinoconjunctivitis. Allergy. 2014;69(12):1689–1695. doi: 10.1111/all.12518. [DOI] [PubMed] [Google Scholar]
- Drachenberg K.J., Wheeler A.W., Stuebner P., Horak F. A well tolerated grass pollen-specific allergy vaccine containing a novel adjuvant, monophosphoryl lipid A, reduces allergic symptoms after only four preseasonal injections. Allergy. 2001;56:498–505. doi: 10.1034/j.1398-9995.2001.056006498.x. [DOI] [PubMed] [Google Scholar]
- Durham S.R., Walker S.M., Varga E.M. Long-term clinical efficacy of grass-pollen immunotherapy. N. Engl. J. Med. 1999;341:468–475. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- Durham S.R., Yang W.H., Pedersen M.R. Sublingual immunotherapy with once-daily grass allergen tablets: a randomized controlled trial in seasonal allergic rhinoconjunctivitis. J. Allergy Clin. Immunol. 2006;117:802–809. doi: 10.1016/j.jaci.2005.12.1358. [DOI] [PubMed] [Google Scholar]
- Focke M., Mahler V., Ball T. Nonanaphylactic synthetic peptides derived from B cell epitopes of the major grass pollen allergen, Phl p 1, for allergy vaccination. FASEB J. 2001;15(11):2042–2044. doi: 10.1096/fj.01-0016fje. [DOI] [PubMed] [Google Scholar]
- Focke M., Marth K., Flicker S., Valenta R. Heterogeneity of commercial timothy grass pollen extracts. Clin. Exp. Allergy. 2008;38:1400–1408. doi: 10.1111/j.1365-2222.2008.03031.x. [DOI] [PubMed] [Google Scholar]
- Focke M., Swoboda I., Marth K., Valenta R. Developments in allergen-specific immunotherapy: from allergen extracts to allergy vaccines bypassing allergen-specific immunoglobulin E and T cell reactivity. Clin. Exp. Allergy. 2010;40:385–389. doi: 10.1111/j.1365-2222.2009.03443.x. [DOI] [PubMed] [Google Scholar]
- Focke-Tejkl M., Campana R., Reininger R. Dissection of the IgE and T-cell recognition of the major group 5 grass pollen allergen Phl p 5. J. Allergy Clin. Immunol. 2014;133(3):836–845. doi: 10.1016/j.jaci.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focke-Tejkl M., Weber M., Niespodziana K. Development and characterization of a recombinant, hypoallergenic, peptide-based vaccine for grass pollen allergy. J. Allergy Clin. Immunol. 2015;135:1207. doi: 10.1016/j.jaci.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadermaier E., Flicker S., Aberer W. Analysis of the antibody responses induced by subcutaneous injection immunotherapy with birch and Fagales pollen extracts adsorbed onto aluminum hydroxide. Int. Arch. Allergy Immunol. 2010;151 doi: 10.1159/000232567. (17-2) [DOI] [PubMed] [Google Scholar]
- Gadermaier E., Staikuniene J., Scheiblhofer S. Recombinant allergen-based monitoring of antibody responses during injection grass pollen immunotherapy and after 5 years of discontinuation. Allergy. 2011;66:1174–1182. doi: 10.1111/j.1398-9995.2011.02592.x. [DOI] [PubMed] [Google Scholar]
- Gallerano D., Ndlovu P., Makupe I. Comparison of the specificities of IgG, IgG-Subclass, IgA and IgM reactivities in African and European HIV-infected individuals with an HIV-1 Clade C proteome-based array. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0117204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlich W.H., Glebe D. Development of an Allergy Immunotherapy Leads to a New Type of Hepatitis B Vaccine. EBioMedicine. 2016;11:5–6. doi: 10.1016/j.ebiom.2016.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin C.S., Cox L. Allergy immunotherapy: what is the evidence for cost saving? Curr. Opin. Allergy Clin. Immunol. 2014;14:363–370. doi: 10.1097/ACI.0000000000000084. [DOI] [PubMed] [Google Scholar]
- Hatzler L., Panetta V., Lau S. Molecular spreading and predictive value of preclinical IgE response to Phleum pratense in children with hay fever. J. Allergy Clin. Immunol. 2012;130(4):894–901. doi: 10.1016/j.jaci.2012.05.053. [DOI] [PubMed] [Google Scholar]
- Hauswirth A.W., Natter S., Ghannadan M. Recombinant allergens promote expression of CD203c on basophils in sensitized individuals. J. Allergy Clin. Immunol. 2002;110:102–109. doi: 10.1067/mai.2002.125257. [DOI] [PubMed] [Google Scholar]
- Horak F., Zieglmayer P., Zieglmayer R. Early onset of action of a 5-grass-pollen 300-IR sublingual immunotherapy tablet evaluated in an allergen challenge chamber. J. Allergy Clin. Immunol. 2009;124:471–477. doi: 10.1016/j.jaci.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Horak F., Zieglmayer P., Zieglmayer R. The effects of bilastine compared with cetirizine, fexofenadine, and placebo on allergen-induced nasal and ocular symptoms in patients exposed to aeroallergen in the Vienna Challenge Chamber. Inflamm. Res. 2010;59:391–398. doi: 10.1007/s00011-009-0117-4. [DOI] [PubMed] [Google Scholar]
- Horn M., Vollandt R. Sample sizes for comparisons of k treatments with a control based on different definitions on power. Biom. J. 1998;40:589–612. [Google Scholar]
- Jacobsen L., Niggemann B., Dreborg S. (The PAT investigator group). Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007;62:943–948. doi: 10.1111/j.1398-9995.2007.01451.x. [DOI] [PubMed] [Google Scholar]
- Kiel M.A., Röder E., Gerth van Wijk R. Real-life compliance and persistence among users of subcutaneous and sublingual allergen immunotherapy. J. Allergy Clin. Immunol. 2013;132:353–360. doi: 10.1016/j.jaci.2013.03.013. [DOI] [PubMed] [Google Scholar]
- Kleine-Tebbe J., Fuchs T., Klimek L. Allergen immunotherapy — a position paper of the German society for allergology and clinical immunology. Pneumologie. 2001;55:438–444. doi: 10.1055/s-2001-16954. [DOI] [PubMed] [Google Scholar]
- Larché M., Akdis C.A., Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat. Rev. Immunol. 2006;6:761–771. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- Linhart B., Hartl A., Jahn-Schmid B. A hybrid molecule resembling the epitope spectrum of grass pollen for allergy vaccination. J. Allergy Clin. Immunol. 2005;115(5):1010–1016. doi: 10.1016/j.jaci.2004.12.1142. [DOI] [PubMed] [Google Scholar]
- Linhart B., Narayanan M., Focke-Tejkl M. Prophylactic and therapeutic vaccination with carrier-bound Bet v 1 peptides lacking allergen-specific T cell epitopes reduces Bet v 1-specific T cell responses via blocking antibodies in a murine model for birch pollen allergy. Clin. Exp. Allergy. 2014;44:278–287. doi: 10.1111/cea.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupinek C., Wollmann E., Baar A. Advances in allergen-microarray technology for diagnosis and monitoring of allergy: the MeDALL allergen-chip. Methods. 2014;66:106–119. doi: 10.1016/j.ymeth.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupinek C., Wollmann E., Valenta R. Monitoring allergen immunotherapy effects by microarray. Curr. Treat. Options Allergy. 2016;3:189–203. doi: 10.1007/s40521-016-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth K., Breyer I., Focke-Tejkl M. A nonallergenic birch pollen allergy vaccine consisting of hepatitis PreS-fused Bet v 1 peptides focuses blocking IgG toward IgE epitopes and shifts immune responses to a tolerogenic and Th1 phenotype. J. Immunol. 2013;190:3068–3078. doi: 10.4049/jimmunol.1202441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothes N., Heinzkill M., Drachenberg K.J. Allergen-specific immunotherapy with a monophosphoryl lipid A-adjuvanted vaccine: reduced seasonally boosted immunoglobulin E production and inhibition of basophil histamine release by therapy-induced blocking antibodies. Clin. Exp. Allergy. 2003;33:1198–1208. doi: 10.1046/j.1365-2222.2003.01699.x. [DOI] [PubMed] [Google Scholar]
- Niederberger V., Horak F., Vrtala S. Vaccination with genetically engineered allergens prevents progression of allergic disease. Proc. Natl. Acad. Sci. U. S. A. 2004;101(Suppl. 2):14677–14682. doi: 10.1073/pnas.0404735101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederberger V., Marth K., Eckl-Dorna J. Skin test evaluation of a novel peptide carrier-based vaccine, BM32, in grass pollen-allergic patients. J. Allergy Clin. Immunol. 2015;136:1101–1103. doi: 10.1016/j.jaci.2015.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niespodziana K., Focke-Tejkl M., Linhart B. A hypoallergenic cat vaccine based on Fel d 1-derived peptides fused to hepatitis B PreS. J. Allergy Clin. Immunol. 2011;127 doi: 10.1016/j.jaci.2011.02.004. (1562-70.e6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaar O., Demoly P., van Wijk R G. European Academy of Allergy and Clinical Immunology. Recommendations for the standardization of clinical outcomes used in allergen immunotherapy trials for allergic rhinoconjunctivitis: an EAACI Position Paper. Allergy. 2014;69:854–867. doi: 10.1111/all.12383. [DOI] [PubMed] [Google Scholar]
- Purohit A., Niederberger V., Kronqvist M. Clinical effects of immunotherapy with genetically modified recombinant birch pollen Bet v 1 derivatives. Clin. Exp. Allergy. 2008;38:1514–1525. doi: 10.1111/j.1365-2222.2008.03042.x. [DOI] [PubMed] [Google Scholar]
- Rendi-Wagner P., Shouval D., Genton B. Comparative immunogenicity of a PreS/S hepatitis B vaccine in non- and low responders to conventional vaccine. Vaccine. 2006;24:2781–2789. doi: 10.1016/j.vaccine.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Schmid J.M., Würtzen P.A., Dahl R., Hoffmann H.J. Pretreatment IgE sensitization patterns determine the molecular profile of the IgG4 response during updosing of subcutaneous immunotherapy with timothy grass pollen extract. J. Allergy Clin. Immunol. 2016;137(2):562–570. doi: 10.1016/j.jaci.2015.05.023. [DOI] [PubMed] [Google Scholar]
- Spertini F., Perrin Y., Audran R. Safety and immunogenicity of immunotherapy with Bet v 1-derived contiguous overlapping peptides. J. Allergy Clin. Immunol. 2014;34:239–240. doi: 10.1016/j.jaci.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Suphioglu C., Singh M.B., Taylor P. Mechanism of grass-pollen-induced asthma. Lancet. 1992;7:569–572. doi: 10.1016/0140-6736(92)90864-y. [DOI] [PubMed] [Google Scholar]
- Tamhane A.C., Hochberg Y., Dunnett C.W. Multiple test procedures for dose finding. Biometrics. 1996;52:21–37. [PubMed] [Google Scholar]
- Valenta R., Ferreira F., Focke-Tejkl M. From allergen genes to allergy vaccines. Annu. Rev. Immunol. 2010;28:211–214. doi: 10.1146/annurev-immunol-030409-101218. [DOI] [PubMed] [Google Scholar]
- Valenta R., Campana R., Marth K., van Hage M. Allergen-specific immunotherapy: from therapeutic vaccines to prophylactic approaches. J. Intern. Med. 2012;272:144–157. doi: 10.1111/j.1365-2796.2012.02556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Neerven R.J., Wikborg T., Lund G. Blocking antibodies induced by specific allergy vaccination prevent the activation of CD4 + T cells by inhibiting serum-IgE-facilitated allergen presentation. J. Immunol. 1999;163(5):2944–2952. [PubMed] [Google Scholar]
- Winther L., Arnved J., Malling H.J. Side-effects of allergen-specific immunotherapy: a prospective multi-centre study. Clin. Exp. Allergy. 2006;36:254–260. doi: 10.1111/j.1365-2222.2006.02340.x. [DOI] [PubMed] [Google Scholar]
- Wollmann E., Lupinek C., Kundi M. Reduction in allergen-specific IgE binding as measured by microarray: a possible surrogate marker for effects of specific immunotherapy. J. Allergy Clin. Immunol. 2015;136:806–809. doi: 10.1016/j.jaci.2015.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
IgE reactivity of study subjects to grass pollen allergens, recombinant BM fusion proteins and PreS. Box plot representation of IgE levels (y-axis: OD values, whiskers = minimum and maximum; horizontal bars denote medians; boxes = 25th to 75th percentiles) specific for the grass pollen allergens (Phl p 1, Phl p 2, Phl p 5, Phl p 6), the PreS fusion proteins (BM321, BM322, BM325, BM326) and PreS of the study population at baseline. Significant differences between allergens and BM fusion proteins are indicated (****p < 0.0001; **p < 0.01).
Supplementary material 1
Supplementary material 2