Abstract
Background
Providing renal support for small children is very challenging using the machinery currently available in the United States. As the extra-corporeal volume (ECV) relative to blood volume increases, and the state of critical illness worsens, the chance for instability during continuous renal replacement therapy (CRRT) initiation rises. CRRT machines with smaller ECV could reduce the risks and improve outcomes.
Methods
1) Case series of small children (n = 12) who received continuous veno-venous hemofiltration (CVVH) via Aquadex™ machine (ECV = 33 mL) with 30 mL/kg/h of pre-replacement fluids at Children’s of Alabama between December 2013 and April 2015. 2) In vitro assessment of fluid precision using the adapted CVVH system.
Results
We utilized 101 circuits over 261 days to provide CVVH for 12 children (median age = 30 days; median weight 3.4 kg). Median CVVH duration = 14.5 days (IQR = 10, 22.8 days). Most circuits were routinely stopped for change after 72 h. Only 5/101 (5 %) initiations were associated with mild transient change in vital signs. Complications were infrequent (three transient hypothermia; three puncture-site bleeding, one systemic bleeding, and one right atrial thrombus). Most patients (7/12, 58 %) were discharged from ICU, and 6/12 (50 %) were discharged home.
Conclusions
CRRT machines with low ECV can enable clinicians to provide adequate, timely, safe and efficient renal support to small critically ill infants.
Keywords: Renal Support Therapy, continuous veno-venous hemofiltration, Aquadex™ Machine, Pediatric Nephrology, children
Introduction
Despite rates of acute kidney injury (AKI) higher than most critically ill populations [1], neonates receive renal support infrequently. The technical challenges of traditional machines make continuous renal replacement therapy (CRRT) initiation very difficult, even at experienced tertiary children’s hospitals. In the United States, the smallest CRRT circuit is around 100 mL (i.e., Prismaflex M60), and no circuits are FDA approved for use in children <20 kg. Some centers have eliminated this circuit from use at their institutions due to the bradykinin release response that has been seen in filters using an AN-69 membrane, and instead, they have chosen to only provide therapy with a circuit with an extra-corporeal volume (ECV) of 165 mL. Some centers do not offer CRRT to any infant, let alone for critically ill neonates or premature infants, because of the inherent risk of the initiation. When used, these larger machines necessitate that small children receive CRRT with proportionally larger filters, higher blood flows, massive clearance rates, and big vascular catheters compared to bigger children [2]. Priming the CRRT circuit with blood reduces morbidity; yet blood primes are not without risk, as they can cause acidosis, hypocalcemia, hyperkalemia, thrombocytopenia, hypotension and coagulopathy, especially as the percentage ECV to patient blood volume increases. These complications and the need to alter the CRRT prescription stem from the markedly large ECV inherent to available machinery.
Despite protocols to reduce the risk of CRRT in small patients — using fresh packed red blood cells (pRBCs), diluting pRBC to more physiologic hematocrit, normalizing pH with sodium bicarbonate, and giving intravenous calcium to counteract the low ionized calcium — the initiation of CRRT therapy has historically been fraught with anxiety and hemodynamic compromise in small infants. In our experience, the higher the % ECV and the more critically ill the patient is before initiation, the more likely problems will arise as a consequence of CRRT initiation. Using the Pediatric Prospective Continuous Renal Replacement Therapy (ppCRRT) registry, which included 13 centers and over 350 children on CRRT, we showed that children <10 kg have a significantly lower survival rate than children who are >10kg (33 % vs. 67 %; p <0.05) [2]. The lower rates of survival are perhaps due to patient selection, initiation of CRRT only as “last resort”, the added risks of circuit initiation, or a combination of these factors. We and others have suggested that CRRT machines with smaller ECV could reduce the risks associated with the therapy and improve outcomes.
Several groups have begun to develop dialysis and convective clearance devices that use a smaller size circuit. Two machines have been designed specifically for small infants, the Newcastle Infant Dialysis Ultrafiltration System (NIDUS) that has an ECV of 6.5 mL for filters of 0.045 m2, and the Cardiac And Renal Pediatric Dialysis Emergency (CARPEDIEM™) that has available circuits of 27, 34 and 45 mL for filters of 0.075, 0.15 and 0.25 m2 [3, 4]. Unfortunately, because these machines are not available in the United States, pediatric nephrologists who are faced with a small child in need of renal support therapy must either use CRRT machines with high ECV, or deny their patients proper renal support therapy.
To mitigate concerns posed by CRRT machines with large ECV in relation to blood volume size, we adapted the Aquadex™ machine to provide pre-filter replacement fluids to allow for continuous veno-venous hemofiltration (CVVH). In 2007, the FDA approved the use of the Aquadex™ Machine for ultrafiltration of plasma volume in adults with heart failure who did not respond to diuretic therapy. We adapted this machine to be used for CVVH to help improve the ability to initiate CRRT in small children who required renal support at our Institution. We designed policies and procedures, trained physicians, dialysis nurses and bedside nurses on its use, and began this therapy in December 2013. As for any other renal support therapy procedure, we designed the procedure with the goals of maintaining electrolyte and water homeostasis as well as clearing waste products, while minimizing risks, particularly during CRRT initiation. In this report, we describe how we adapted the machine and our policies, report in vitro data documenting fluid precision of the system, and describe the first 12 infants who received CVVH at our Institution using the Aquadex™ machine.
Methods
Procedure
The Aquadex™ circuit (Baxter Corporation, Minneapolis, Minnesota) has a 33 ml volume when using the ultrafiltration (UF) 500 filter set with the hematrocrit (HCT) monitor. A roller pump provides negative pressure through the withdraw (access) line to bring blood from the patient to the hemofilter. A second pump provides negative pressure on the hemofilter to remove plasma volume. The filter is 0.12 m2 and is made of polysulfone membranes. The sieving coefficients for urea, creatinine, vitamin B12, and albumin are 0.98, 0.98, 0.98 and <0.02, respectively. Blood is returned via an inlet (return) line to the patient. The circuit has an in-line continuous HCT sensor, and there is software in place to automatically stop removing fluid if the HCT hits the prescribed HCT limit. The tubing set has pigtails before and after the filter. The blood pump can run at 10 to 40 ml/min (at intervals of 5), and has an accuracy of +15 − 20 % of setting for withdraw pressure of 250 mmHg. The UF pump has a range of 0 to 500 mL/h in 10 mL/h increments, and an accuracy of ±10 % of setting. The machine has an air detector and a blood leak detector. The operational range for the access (withdraw), return (infusion) and ultrafiltration (transmembrane) pressures is −350 to 500 mmHg. Ultrafiltration rates of up to 500 mL/h are ample for clearance of waste products in small children in CVVH mode; no port is present to provide countercurrent dialysis (Aquadex™ Flex Flow user guide).
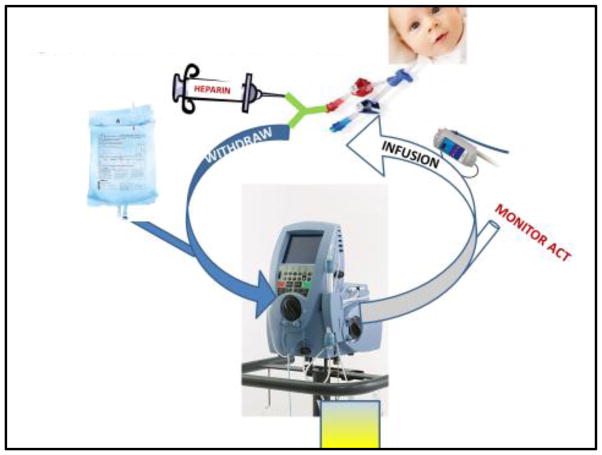
The schematic for the machine in CVVH mode is shown in Fig. 1. Venous access is placed by the surgical or intensive care team in consultation with the pediatric nephrologist. Blood flow rate is titrated to 40 mL/min. A y-connector is placed on the withdraw (access) line to provide continuous heparin for anticoagulation. Pre-filter replacement fluid is infused with an Alaris™ (head unit model #8015; large volume pump module #8100) fluid infusion system via the proximal pigtail of the circuit. Anticoagulation monitoring is obtained from the distal pigtail of the circuit. Heparin infusion rate is titrated to activated clotting time (ACT) goals specified by the team. We initially set the target ACT for 180 – 220 sec, and afterwards reduced it to 160 – 180 sec. Ultrafiltration rates are adjusted to match the intake of the previous hour, in addition to the prescribed amount to “leave on” or “take off”. A blood warmer (AstoFlo Plus™ Stihler Electronic, Stuttgart, Germany) is placed on the infusion (return) line of the circuit. The hematocrit limit is set to 50 %. Circuits run continuously and must be changed out every 72 h.
FIGURE 1. Pre-filter Continuous Veno-Venous Hemofiltration.
Continuous veno-venous hemofiltration (CVVH) using the Aquadex™ machine. Heparin is infused through a y-connector attached to the withdraw (access) line of the patient’s catheter. Using an in-line medication infusion machine, pre-replacement fluid is infused via the proximal pigtail of the circuit. There are two pumps on the machine, the blood pump (which can pump blood to a maximum of 40 mL/min), and the ultrafiltration pump (which can perform ultrafiltration to a maximum of 500 mL/h when the blood flow is 40 mL/min). Blood for anticoagulation monitoring is obtained through the distal pigtail of the circuit. A blood warmer is attached to the tubing on the infusion (return) line before it is returned to the patient.
Circuits were primed with pRBCs or saline according to our Institution’s guidelines. Therefore, in accordance with Institutional protocols, if a patient had <10 % of ECV and was clinically stable, a saline prime was used, while if a patient had an ECV ≥10 % the circuit was primed with pRBCs. To get the pRBC close to physiologic, the blood prime is diluted 1:1 with sodium bicarbonate (to correct low pH and decrease HCT), and 1 mL of 10 % calcium gluconate is infused to the patient (to compensate for the low ionized calcium level of banked blood) as we previously described [5]. If criteria for blood prime is met, and the circuit runs for 72 h, or a planned circuit change occurs, we cross-primed the new circuit with the blood of the expiring circuit, according to Institutional protocols.
In vitro assessment of fluid accuracy
We performed in vitro experiments to determine the fluid precision using the adapted CVVH system. We tested three different access pressure conditions (−50, −125, and −200 mmHg) and three different replacement fluid flow rates (100, 300 and 500 mL/h). Each set of conditions was performed in triplicate for a total of 27 individual runs. We calculated percentage of error as follows: % error = (delivered − prescribed)/delivered; where the amount delivered was calculated by subtracting the weight of the bag before infusion from the weight of the bag after the infusion. We examined differences in fluid precision by pressure differences and by fluid flow differences. Because data was non-normally distributed and we were comparing three groups, we used Kruskal-Wallis test to compare differences among groups. We then compared differences between groups using Tukey’s test. For all analysis, a p value <0.05 was considered statistically significant.
Results
Case series
We performed a case series on all children (12 subjects) who received CVVH using the adapted Aquadex™ system between December 2013 and April 2015, at Children’s of Alabama, Birmingham, Alabama. Informed consent for CRRT was obtained after the benefits and risks were discussed with the family in accordance with policies for all acute renal replacement therapy procedures (hemodialysis, peritoneal dialysis or CRRT) at our Institution. The University of Alabama at Birmingham Institutional Review Board approved this retrospective report of the case series with waiver of informed research consent. Data was extracted from our electronic medical record and the CRRT clinical quality improvement database.
The decision to start CRRT, as well as the machine type and access, were determined by the pediatric nephrologist in consultation with the intensive care team. In general, Aquadex™ was chosen if the child was <15 kg, peritoneal dialysis (PD) was contraindicated, and/or the patient already had vascular access established (for example if the patient had a catheter for extra-corporeal membrane oxygenation). We excluded the use of the Aquadex system for any infants who needed CRRT because of inborn errors of metabolism, as they would require much higher clearance rates during CRRT [6].
The characteristics of the 12 subjects who received CVVH are listed in Table 1. For the most part, these were very critically ill patients in whom the only options that we had for renal support were CVVH with Aquadex™ or CVVHDF with Prismaflex™. The median age at initiation was 30 days (minimum = 4 days; maximum = 4 years; IQR = 13, 38 days). The median weight at initiation was 3.4 kg (minimum = 2.7 kg; maximum = 12.4 kg; IQR = 3.0, 4.3 kg). The indications for CRRT were severe congenital chronic kidney disease (with a contraindication for peritoneal dialysis; n = 3) and acute kidney injury (n = 9). The median percentage fluid overload (defined as fluid intake – fluid output/ICU admission weight) at CRRT initiation was 25.5 % (IQR = 9.5, 38.5). Infants received care in the CICU (n = 5) or the NICU (n = 7). Most children had double lumen vascular access (n = 9) (4 used 8F; 4 used 7F; 1 used 6F), while 3 infants with congenital heart disease had 2 × 4F single lumen catheters. The median CRRT duration was 14.5 days (minimum 4 days; maximum 61 days; IQR = 10, 22.8).
Table 1.
Patient Characteristics
| Patient | Wt (kg) |
Age (days) |
% FO @ start |
CVVH days |
Primary Dx | Comorbidities | Reaso n |
Access | Complication | Survival | kidney outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4.7 | 17 | 8 | 20 | Meconium aspiration | PPHN, s/p ECMO | AKI | 8F RIJ DLC | none | Survived | eGFR = 85.4 ml/min/1.73m2 at 1 year of age |
| 2 | 3.4 | 15 | 43 | 17 | Prune Belly syndrome | Pulmonary hypoplasia, Imperforated anus | CKD | 8F RIJ DLC | Hypothermia | Died | n/a |
| 3 | 3.0 | 4 | 4 | 4 | PUV | Pulmonary hypoplasia, thrombocytopenia | CKD | 8F RIJ DLC | none | Died | n/a |
| 4 | 3.4 | 30 | 35 | 14 | Congenital Heart Disease | Heart Transplant, IVC Stenosis, Liver Failure, TAMOF, Pneumothorax | AKI | 5F RIJ and 5F Fem | None | Died | n/a |
| 5 | 3.3 | 8 | 30 | 7 | Congenital Heart Disease | PPHN, Omphalocele, s/p ECMO | AKI | 8F LIJ and 5F Rfem | None | Died 5 mo after CRRT | eGFR = 90.1 ml/min/1.73m2 at 6 mo of age |
| 6 | 2.7 | 31 | 37 | 60 | Renal dysplasia | PPHN, omphalocele, s/p ECMO, imperforate anus, Cloacal extrophy, | AKI | 7F RIJ DLC | Bleeding Clot in Right Atrium | Survived | eGFR = 23.4 ml/min/1.73m2 at 6 mo of age |
| 7 | 4.2 | 39 | 21 | 37 | Congenital diaphragmatic hernia | Pulm hypertension, liver failure, s/p ECMO, | AKI | 8F RIJ DLC | none | Survived | eGFR = 50.0 ml/min/1.73m2 at 6 mo of age |
| 8 | 3.0 | 32 | 20 | 11 | Renal vein thrombosis/ HIE | Systemic Hypertension, PPHN, sepsis, s/p ECMO | AKI | 7F RIJ DLC | none | Survived | eGFR = 55 ml/min/1.73m2 at 6 mo of age |
| 9 | 12.4 | 1460 | 45 | 15 | Hypoplastic left heart syndrome | Heart Transplant, TAMOF, s/p ECMO IVC thrombosis, Mediastinal Infection, | AKI | 6F Rfem DLC | Bleeding | Died | n/a |
| 10 | 2.9 | 8 | 10 | 4 | Renal hypoplasia/ dysplasia | Pulmonary hypoplasia | CKD | 7F RIJ DLC | Bleeding | Survived | Transitioned to peritoneal dialysis |
| 11 | 3.0 | 38 | 75 | 14 | Congenital Heart Disease | s/p ECMO | AKI | 5F RFem and 5F LFem | Hypothermia | Died | n/a |
| 12 | 6.0 | 86 | 3 | 18 | Congenital Heart Disease | AKI | 7F RIJ | Bleeding Hypothermia | Survived | Transitioned to peritoneal dialysis |
Wt – weight; FO – Fluid overload; CVVH (continuous veno-venous hemofiltration); PUV – posterior uretheral valves; PPHN – persistent pulmonary hypertension; ECMO – extra-corporeal membrane oxygenation; AKI – acute kidney injury; CKD – chronic kidney disease; IVC = inferior vena cava; RIJ – Right internal jugular; TAMOF – thrombocytopenia associated multi-organ failure; DLC – double lumen catheter; eGFR – estimated glomerular filtration rate. eGFR was calculated using Schwartz formula (0.41 × ht (cm)/creatinine (mg/dl))
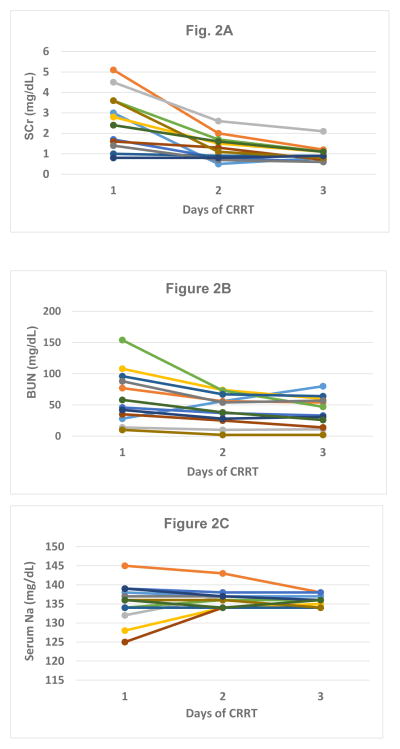
Pre-filter convective clearance dose was initiated at 30 mL/kg with Prismasol™ 2K/3.5Ca plus 1 meq/L KCL, 1 meq/L KPhos and 0.5 meq/L magnesium sulfate. In CVVH, clearances of small and middle molecules are dependent on ultrafiltration dose because the sieving coefficient of urea and other similar toxins approaches 1 (as opposed to hemodialysis, which is dependent on blood flow and treatment time). For this reason, we do not routinely perform formal clearance studies in infants on any CRRT device at our Institution. Instead, we determine clearance by final effluent volume. Achievement of metabolic control was obtained in all subjects during the first few days of therapy, and is shown in Fig. 2 as changes in blood urea nitrogen (BUN) and serum creatinine (SCr). There was one infant who had a rise in BUN; this was an anuric patient who prior to Aquadex™ CVVH was on CVVHDF in conjunction with extra-corporeal membrane oxygenation with an effluent dose of 82 mL/kg/h. The reason for the rise in BUN was that once on Aquadex™, this patient was on lower prescribed clearance rates (30 ml/kg/hr). Although the prescribing clinician had ample room to increase clearance (if clinically indicated) by simply increasing the pre-replacement infusion dose, this was not necessary in any of the 12 patients. Even in children who were anuric during the CVVH course, their SCr was typically between 0.8 to 1.2 mg/dL throughout the procedure.
FIGURE 2. Changes in serum creatinine (SCr; Fig 2A), blood urea nitrogen (BUN; Fig. 2B), and serum Na during the first three days of continuous renal replacement therapy (CRRT).
Serum creatinine (Fig. 2a) and blood urea nitrogen (Fig. 2b) changed on the day of initiation (day 1) and the following two days for the 12 patients on CVVH using the adapted Aquadex™ circuit.
Because during CRRT, the composition of the replacement fluid will approximate the patient’s blood chemistries after equilibrium, we did not have any major issues with electrolytes. We achieved excellent metabolic control and only very minor adjustments to the fluid compositions were necessary. Fig. 2c depicts sodium concentrations during the first 3 days of therapy for all 12 infants.
We used 101 circuits over 261 days (mean number of days/circuit = 2.6 days). Of the 101 circuits, 12 were initiated for new start. Of the remaining 89 circuits, 12/89 (13.5 %) were planned discontinuations (seven circuits were stopped due to patient issues — including discontinuation of cardiopulmonary support; four stopped for elective therapeutic plasmapheresis; and one, for trial off CVVH). Of the remaining 77 circuits, 54/77 (70 %) were stopped for routine change after 72 hours, while 15/77 (19.4 %) stopped because of clotting issues, 4/77 (5 %) because of access issues, and 4/77 (5 %) due to system alarms.
Blood was used to prime 80/101 (79 %) circuits; all others were primed with saline. If a blood prime was used, circuits that lasted 72 hours were cross-primed to reinitiate the next circuit (whereby the blood from the running machine was used to prime the blood circuit of the new machine). We used 11.5 mL of blood for the blood prime (mixed 1:1 with sodium bicarbonate), and as per our Institution policies, we used blood that the infant was already exposed to when available. We found no episodes of thrombocytopenia, anemia or coagulopathy associated with circuit initiation.
Only 5/101 (5 %) circuit initiations were associated with any interventions to maintain blood pressure. Of the five circuit starts that received interventions, the hemodynamic changes and the interventions needed were very mild (four had a <10 % increase in the dose of dopamine that they were already receiving, and one received a 10 mL/kg of 5 % albumin). One of these circuits was initiated with a saline prime, while the other four were blood primed circuits. All of these patients had a prompt response to interventions. No patients had severe decompensation associated with circuit initiation.
Complications were few, considering that these patients are exposed to risks of therapy 24 hours a day. Hypothermia (as low as 35.2 °C) occurred three times; the first was on the first infant on this therapy (before we started using an in-line blood warmer); the second occurred when the patient was taken off the unit for a radiologic procedure without the in-line blood warmer, and the third occurred when the in-line blood line warmer was on, but not appropriately set. The complication rate for hypothermia on any given day was 3/261 days = 11 episodes/1000 patient-days.
Bleeding occurred in four patients; three had a small amount of bleeding from venous lines (which improved with decreased heparin administration), while one patient developed significant bleeding in his thoracic cavity. This patient was a heart transplant recipient who had an open chest, had severe transplant rejection, was coagulopathic, thrombocytopenic, had fungal sepsis and a life-threatening inferior vena cava thrombus. His anti-coagulation was being managed by the cardiac intensive team who was treating him with aspirin and systemic heparin (target antiXa levels of 0.5 to 1.0 U/mL) for thrombus when he developed the bleeding. The bleeding improved by stopping aspirin, provision of fresh frozen plasma/platelets, and decrease in heparin dose. No children developed intra-cerebral or pulmonary hemorrhage. The incidence of bleeding was 4/261 = 15 episodes/1000 patient-days. In comparison, in the ppCRRT registry, 9/93 (10 %) of patients receiving heparin anticoagulation developed significant bleeding (epistaxis, intra-cerebral, pulmonary, vaginal or bladder hemorrhage), and mild bleeding at venous sites was not documented.
Most patients, seven out of 12 (58 %) survived to come off renal support or changed to peritoneal dialysis (n = 2) for chronic therapy associated with end-stage renal disease. Of the patients who survived, six out of 12 (50 %) survived to hospital discharge; while there was a patient who died 6 months after CVVH and had an eGFR of 91 mL/min/1.73 m2 prior to death. For those who died while on CRRT (n = 5), the family elected to stop support for progressive pulmonary or cardiac failure. No child’s therapy was discontinued due to the inability to perform CVVH or complications of CVVH. Although we did not do formal fluid precision studies in children while on CVVH, we did not have any major concerns (i.e., excessive fluid loss or gain) in any of our patients.
In vitro tests
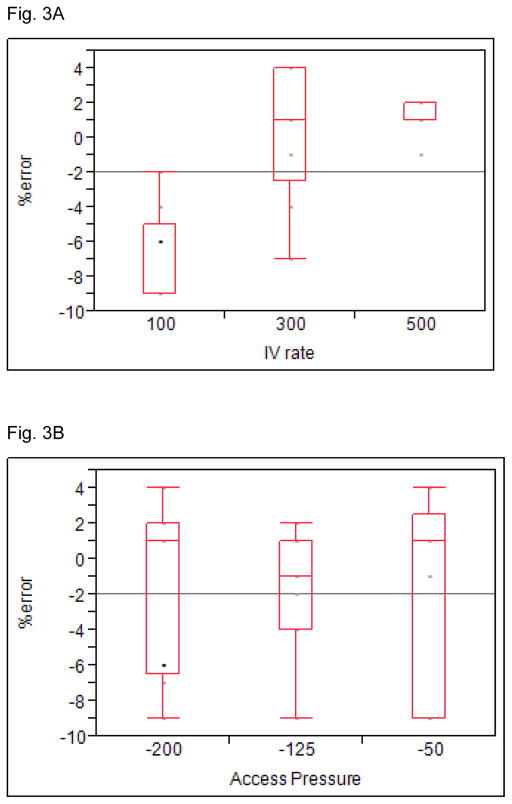
The median % error rate for all 27 experiments was −1 % (maximum 4 %; minimum −9 %; IQR 1 %, −6 %). Fig. 3a shows differences in % error by replacement fluid rates (overall p value <0.001). There was a significant difference between 100 mL/h vs. 300 mL/h (p <0.01) and between 100 and 500 mL/h (p <0.01), but no difference was detected between 300 and 500 mL/hr. Fig. 3b shows differences by access pressures; and no statistically significant differences were detected across pressures, nor were we ever concerned that excessive fluid removal was causing cardiovascular instability due to imprecise fluid removal.
Figure 3. In-vitro assessment of percentage error between prescribed vs. infusion of fluid.
In vitro experiments to assess the percentage error between prescribed vs. infusion of fluid as a function of flow rate (Fig. 3a) and access pressure (Fig. 3b).
Discussion
Here we report a case series of 12 patients who underwent CVVH over 261 days of therapy using the Aquadex™ machine adapted for CVVH. This procedure was performed in critically ill patients who otherwise would have not been able to receive renal support, or who would have been treated with a CRRT machine that had an ECV three times larger than the Aquadex™. We were able to support seven out of 12 infants to come off CVVH, and 6 out 12 infants in this series survived to hospital discharge. None of the infants died as a consequence of the renal failure, or as a complication of CVVH. Although difficult to attribute improved survival compared to other cohorts when using such a small sample size, the survival rates in this cohort were higher than that reported in children <10 kg in the ppCRRT registry, where they had a 67 % mortality rate [2].
Compared to our experience using other circuits available in the United States, the most striking difference that we observed with the adapted Aquadex™ CVVH system is the tremendous ease in getting these infants on circuit without the need for intensive cardiovascular, respiratory and blood product support. To our knowledge, the incidence of need to intervene with cardiopulmonary support during circuit initiation in small children has not been reported for small critically ill patients. However, as most pediatric nephrologists who have started CRRT on small infants can attest, the initiation procedure usually involves tremendous anxiety and high risk of complications during the initiation, particularly in critically ill infants, and those whose percentage ECV/blood volume approaches 25 % or higher. We certainly have been at the bedside on numerous occasions when cardiopulmonary resuscitation is initiated shortly after CRRT initiation. At our Institution, prior to Aquadex™, our policy dictated that two attendings had to be present at bedside, and a concise plan of action for potential hypotension had to be agreed upon before initiation of CRRT in any small infant requiring a blood prime.
Although this machine greatly improved our ability to initiate therapy in this population, adaption of a machine designed for slow continuous ultrafiltration as a CVVH device is not ideal, adds complexity, and is not without inherent risks. Several important improvements would enhance our ability to safely care for these small children. First, further reduction in the circuit size could simplify the initiation process by allowing the use of a saline prime. At the current ECV of 33 mL, an infant under 4 kg would receive a blood prime (for ECV of >10%) according to our Institutional guideline. A circuit volume <22 mL would allow all children in this case series to be primed with saline using our guidelines. Both the recently developed NIDUS [7] and CARPEDIEM™ [3] machines have circuit volumes within this amount. The adapted system relies on a pre-replacement IV fluid pump, which does not communicate with the Aquadex™ machine. Communication between the fluid replacement pump, the heparin infusion pump and ultrafiltration pumps would greatly improve this system. Fourth, integration of a blood warmer into the machine would be ideal. Finally, exquisite precision of the pumps is greatly needed.
Other machines have been developed specifically for the care of small children who need renal support therapy. These machines are currently being tested in Europe, but are not available in the United States. The CARPEDIEM™ [3] and the NIDUS™ [7] have been designed specifically for renal support of newborns. These machines have a smaller ECV than the Aquadex™. The NIDUS provides diffusive solute clearance, while the CARPEDIEM™ provides convective solute clearance. In addition, as opposed to the Aquadex™ system, infusion of replacement/diffusive fluids are incorporated into the machine. Thus far, there has been one case report on the use of CARPEDIEM™ and 11 cases of infants treated with the NIDUS™ machine with promising results. The NIDUS machine affords tremendous fluid precision (<0.25 % error) in a circuit that requires only 6.5 mL ECV, and can be used with a single lumen 4 F catheter. The CARPEDIEM™ can be used with filter sets as low as 23 mL and has fluid precision of ±7.5 % (which is close to the small in vitro tests we performed for Aquadex™). These systems have been designed for the small child and have tremendous potential to alter the benefit/risk profile, and change how we approach neonatal critical care nephrology. Pediatric nephrologists, alongside industry partners, the FDA and funding agencies will need to work together to further develop these systems and make them available in the United States, and therefore, provide practitioners with proper tools to care for these vulnerable children.
Not only do we need machines specifically designed for small children, but the field of neonatal critical care nephrology has many other questions that the community must answer to provide the necessary therapy. For example, we have found that a lower amount of heparin and lower targets for anti-coagulation monitoring can be used within the described system. The NIDUS similarly uses lower ACT monitoring (around 160 – 180). Determining the minimum amount of heparin necessary to maintain circuit patency is necessary. Having an alternate mode to provide regional (as opposed to systemic) anticoagulation could further reduce risk. In addition, we and others have found that we can use smaller access for these smaller machines. We must work to better define the minimum access required for these small machines, and ensure that industry can provide an ample variety of catheters for different sized patients. It is likely that double lumen catheters as low as 4 F will be sufficient to provide adequate flows for these machines [8]. Furthermore, and perhaps more importantly, large cohorts will be needed to determine which children and at what time points will benefit from CRRT, especially as growing evidence suggest that early initiation of CRRT improves outcomes in children [9] and adults [10]. Finally, we need more data to assess whether the approach of using a small CVVH circuit (to bridge patients until they are ready to receive PD catheters) could yield better outcomes than trying to use PD catheters when they are at greater risk of leaking.
We acknowledge the limitations of this study. This case series relies on retrospective analysis of our clinical quality database and medical records. We have relied on the ppCRRT registry to make comparisons as it represents a similar cohort of critically ill patients who received CRRT; however we cannot make the claim that survival improved in our cohort based on such a small sample size. In our program, we do not do formal urea clearance assessments of children on CVVH as the sieving coefficient in CVVH is around 1; and the clearance dose is closely related to the effluent volume (and hence, the prescribed replacement dose) monitoring. Although we provided data from in vitro laboratory tests, where we compared the prescribed vs. the actual fluid removed with this system, we have not tested the precision of our adapted system in vivo. Even though we did not witness any severe cardiovascular changes attributed to imprecise fluid removal rates, we recommend that additional measures be considered (for example twice daily weights) when using these types of adapted machinery to ensure that excess fluid is not being delivered or removed.
In conclusion, we present here data that suggests that performing CRRT in small children using a machine with small ECV can improve the care of infants who need renal support therapy by providing a safer ability to initiate the therapy. We were able to initiate therapy without the need for major interventions, even in critically ill children with significant cardiac disease and who required extensive cardiopulmonary support. The pediatric nephrology community will need to work closely with funding agencies, regulatory agencies and industry to bring these machines to the bedside, so that renal support therapy with minimal risk can be timely provided to even the smallest infants.
Supplementary Material
Acknowledgments
Statement of financial support: Research reported in this publication was supported by the Pediatric and Infant Center for Acute Nephrology (PICAN) which is sponsored by Children’s of Alabama and the University of Alabama at Birmingham’s School ofMedicine, Department of Pediatrics and Center for Clinical and Translational Science (CCTS) under award number UL1TR00165.
Footnotes
Ethical approval: All procedures performed involving human participants were in accordance with the ethical standards of the Institutional Review Board at the University of Alabama at Birmingham, as well as with the 1964 Helsinki declaration and its later amendments.
Informed consent: Informed parental consent was obtained for all individual participants included in the study.
Disclosure of potential conflicts of interest: Dr. Askenazi is a speaker for the Acute Kidney Injury (AKI) foundation.
References
- 1.Jetton JG, Askenazi DJ. Update on acute kidney injury in the neonate. Curr Opin Pediatr. 2012;24:191–196. doi: 10.1097/MOP.0b013e32834f62d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Askenazi DJ, Goldstein SL, Koralkar R, Fortenberry J, Baum M, Hackbarth R, Blowey D, Bunchman TE, Brophy PD, Symons J, Chua A, Flores F, Somers MJ. Continuous renal replacement therapy for children </=10 kg: a report from the prospective pediatric continuous renal replacement therapy registry. J Pediatr. 2013;162:587–592. e3. doi: 10.1016/j.jpeds.2012.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ronco C, Garzotto F, Brendolan A, Zanella M, Bellettato M, Vedovato S, Chiarenza F, Ricci Z, Goldstein SL. Continuous renal replacement therapy in neonates and small infants: development and first-in-human use of a miniaturised machine (CARPEDIEM) Lancet. 2014;383:1807–1813. doi: 10.1016/S0140-6736(14)60799-6. [DOI] [PubMed] [Google Scholar]
- 4.Coulthard MG, Crosier J, Griffiths C, Smith J, Drinnan M, Whitaker M, Beckwith R, Matthews JN, Flecknell P, Lambert HJ. Haemodialysing babies weighing <8 kg with the Newcastle infant dialysis and ultrafiltration system (Nidus): comparison with peritoneal and conventional haemodialysis. Pediatr Nephrol. 2014;29:1873–1881. doi: 10.1007/s00467-014-2923-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bridges BC, Askenazi DJ, Smith J, Goldstein SL. Pediatric renal replacement therapy in the intensive care unit. Blood Purif. 2012;34:138–148. doi: 10.1159/000342129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spinale JM, Laskin BL, Sondheimer N, Swartz SJ, Goldstein SL. High-dose continuous renal replacement therapy for neonatal hyperammonemia. Pediatr Nephrol. 2013;28:983–986. doi: 10.1007/s00467-013-2441-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hothi DK. Designing technology to meet the therapeutic demands of acute renal injury in neonates and small infants. Pediatr Nephrol. 2014;29:1869–1871. doi: 10.1007/s00467-014-2910-8. [DOI] [PubMed] [Google Scholar]
- 8.Garzotto F, Zanella M, Ronco C. The evolution of pediatric continuous renal replacement therapy. Nephron Clin Pract. 2014;127:172–175. doi: 10.1159/000363204. [DOI] [PubMed] [Google Scholar]
- 9.Modem V, Thompson M, Gollhofer D, Dhar AV, Quigley R. Timing of continuous renal replacement therapy and mortality in critically ill children. Crit Care Med. 2014;42:943–953. doi: 10.1097/CCM.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Jie Yuan W. Timing of initiation of renal replacement therapy in acute kidney injury: a systematic review and meta-analysis. Ren Fail. 2012;34:396–402. doi: 10.3109/0886022X.2011.647371. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.