Abstract
We used a flow cytometry assay to measure proliferation and cytokine production of self-antigen-specific T cells in individual patients during the clinical course of multiple sclerosis (MS). Myelin-associated oligodendrocytic basic protein (MOBP) was selected for proof of principles in the assay, along with myelin basic protein (MBP) to assess specific activated T cells in 10 MS patients over an 18-month period, in parallel with brain magnetic resonance imaging (MRI) scans and clinical rating scale. A positive correlation occurred between antigen-specific T cell proliferation and interferon-γ production with clinical relapses and MRI lesion activity that was absent when the same patients were in remission.
Keywords: Autoimmunity, Central nervous system, T cell, Self-antigen, Flow cytometry
1. Introduction
Both CD4+ and CD8+ T cells and/or their products have been shown to play critical roles in tissue damage of autoimmune diseases (Marrack et al., 2001). Although a single epitope may be targeted during the initial stage of these diseases, the resultant activation broadens and involves many epitopes later in the pathological process (Marrack et al., 2001). The identification of the self-epitopes targeted by the activated immune responses at different time points during these disease processes should allow a better understanding of the pathogenesis and may lead to the design of molecules to specifically block aberrant responses.
Multiple sclerosis (MS), a disease of the human central nervous system (CNS) is characterized by the presence of infiltrating immune cells in the CNS, focal demyelination and axonal loss (Noseworthy et al., 2000). Associated with MS are active immune responses against many myelin antigens (reviewed in Marrack et al., 2001). Since T and B lymphocytes, macrophages and the immune mediators they secrete are found in MS lesions, they likely participate in the related demyelination (Cannella and Raine, 1995; Genain et al., 1999; Hofman et al., 1989; Lucchinetti et al., 2000; Prineas and Graham, 1981; Selmaj et al., 1991; Storch et al., 1998; Wucherpfennig et al., 1992). Within MS lesions, both CD8+ and CD4+ T cells are present, undergo clonal expansion (Babbe et al., 2000), therefore supporting the hypothesis that pathogenic T cell responses directed against self-myelin antigens are involved in the immune attack (Steinman, 1996; Stinissen et al., 1997).
Many cytokines, mainly of the Th1 type such as interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α) have been implicated in MS (Benvenuto et al., 1991; Calabresi et al., 1998b; Hofman et al., 1989; Killestein et al., 2001; Pouly et al., 2000; Selmaj et al., 1991; Selmaj and Raine, 1988). A role for IFN-γ is suggested by the following three observations. First, administration of IFN-γ to MS patients worsens the disease (Panitch et al., 1987); second, the proportion of CD4+ and CD8+ T cells from peripheral blood expressing IFN-γ increases significantly in patients with progressive MS compared to control group (Becher et al., 1999). Finally, CNS expression of IFN-γ in transgenic mice induces primary demyelination (Horwitz et al., 1997).
Utilizing the above observations, investigators from many laboratories have evaluated self-reactive myelin-specific T cell responses while seeking to identify cells involved in MS-associated CNS demyelination. However, auto-reactive T cells are also frequently part of the mature immune repertoire of healthy non-MS humans (Burns et al., 1983, 1986; Jingwu et al., 1992; Kerlero de Rosbo et al., 1993; Lindert et al., 1999; Markovic-Plese et al., 1995; Ota et al., 1990) as well as of nonimmunized animals (Schluesener and Wekerle, 1985). Thus, humans often show comparable frequencies of myelin [myelin basic protein (MBP), proteolipid protein (PLP) and myelin oligodendrocyte glycoprotein (MOG)]-reactive T cells in their blood, whether they are MS patients or healthy controls (Burns et al., 1983, 1986; Jingwu et al., 1992; Kerlero de Rosbo et al., 1993; Lindert et al., 1999; Markovic-Plese et al., 1995; Ota et al., 1990). These findings suggest that the more important factor in disease development may be activation; that is a greater frequency of activated myelin-reactive cells in MS patients as compared to healthy individuals (Zhang et al., 1994). Several studies support this concept, as MBP- and PLP-specific cells derived from MS patients have an increased frequency of hypoxanthine–guanine phosphoribosyltransferase reporter gene mutations (Allegretta et al., 1990), undergo in vivo activation and expansion in blood and accumulate in the cerebrospinal fluid (CSF) of these patients (Allegretta et al., 1990; Chou et al., 1992; Vandevyver et al., 1995; Zhang et al., 1994). In addition, an important proportion of auto-reactive cells derived from MS patients’ peripheral blood and CSF secrete pro-inflammatory cytokines such as interleukin-2 (IL-2), IFN-γ and TNF (Sharief and Thompson, 1993; Strunk et al., 2000).
We selected to study myelin-associated oligodendrocytic basic protein (MOBP) antigen-specific responses in MS patients for many reasons. First, MOBP is exclusively expressed by oligodendrocytes in brain and spinal cord (Holz et al., 1996; Yamamoto et al., 1994) and is the third most abundant myelin protein in the CNS after MBP and PLP (Yamamoto et al., 1994). Rodent MOBP, either as whole protein or peptides, could induce experimental autoimmune encephalomyelitis in susceptible mice (Holz et al., 2000; Maatta et al., 1998). Analysis of the human MOBP sequence (Yamamoto et al., 1994) identified the presence of putative peptide-binding motifs for the MS-associated human leucocyte antigen (HLA)-DR alleles (Holz et al., 2000). Further, peripheral blood mononuclear cells (PBMC) from several MS patients display a specific proliferative response to human MOBP peptides, when compared to healthy donors (Holz et al., 2000).
We show in this paper that activation of T cells specific for MOBP coincide with increased clinical disability and visualization of new plaques by brain magnetic resonance imaging (MRI). Activated T cells were analyzed and quantified using a flow cytometry-based assay that evaluated both proliferation of myelin-specific T cells and their activation (i.e., capacity to secrete IFN-γ). Following 10 relapsing–remitting MS patients over an 18-month period, who did not receive immunomodulatory therapy during this period of time, we show a direct correlation between clinical neurologic disease, enhancing MRI lesions and MOBP-specific T cell proliferation and activation. In contrast, a loss of such MOBP-specific T cell responses occurred when patients entered clinical remissions.
2. Materials and methods
2.1. Patients
Ten patients with clinically diagnosed relapsing–remitting MS and not on immunosuppressive or immunomodulatory drugs were selected. These patients did not receive medications for at least 3 months prior to the study and comprised a placebo group enrolled in a previous study (Romine et al., 1999). The studies reported here were approved by the Human Subjects Committee of Scripps Clinic, Scripps Green Hospital and The Scripps Research Institute (TSRI). Patients were studied each by the same neurologist who graded their clinical status as to Expanded Disability Status Score (EDSS). Concurrently, blood was taken and brain MRI performed. PCR-based major histocompatibility complex (MHC) class II typing was done by the Immunogenetics and Transplantation Laboratory of the University of California at San Diego Healthcare, and results are displayed in Table 1.
Table 1.
Description of patients with relapsing–remitting MS
| Patient | Gender | Date of birth | HLA-DR | HLA-DQ |
|---|---|---|---|---|
| 1 | F | May 1953 | 1, 7 | 5, 9 |
| 2 | M | June 1949 | 15, 17 | 2, 6 |
| 3 | M | February 1958 | 7, 15 | 2, 6 |
| 4 | M | February 1948 | 8, 15 | 4, 6 |
| 5 | F | July 1953 | 04, 15 | 3, 6 |
| 6 | F | December 1941 | 1, 11 | 3, 5 |
| 7 | F | February 1951 | 03, 15 | 2, 6 |
| 8 | F | June 1955 | 15 | 6 |
| 9 | F | August 1962 | 7, 13 | 3, 6 |
| 10 | F | June 1949 | 1, 15 | 5, 6 |
2.2. Magnetic resonance imaging
MRI was performed using a 1.5-T General Electric Signa scanner (Romine et al., 1999). T2 and proton density-weighted images were obtained by conventional spin-echo sequence with repetition times of 2500 ms and echo delay of 30 and 90 ms. Axial scans of 3-mm thickness and without interslice gap were obtained about 10 min after gadopentetate dimeglumine (GD) injection to assure optimal time for transmigration of the contrast agent across the blood–brain barrier.
2.3. Flow cytometry-based assay
To obtain information about antigen-specific functional responses of individual lymphocytes in CD4 and CD8 T cell subsets, we devised a flow cytometry-based assay that combined a quantitative assay of cell proliferation using the vital dye 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE) (Lyons, 2000) with the detection of individual cells expressing IFN-γ in the CD4 or CD8 T cell compartment. CFSE has been used to follow human T lymphocyte proliferation by others (Karandikar et al., 2002; Matthews et al., 2000; Wen et al., 2002). CFSE-labeled PBMC were cultured with the appropriate stimulus and at every cell division thereafter lost 50% of the CFSE intensity. After 10 days of culture, these cells were stimulated with phorbol 12-myristate 13-acetate (PMA), ionomycin and brefeldin A for 5 h before surface and intracellular labeling. The PMA and ionomycin combination, which broadly activates lymphocytes, generally represents the balance of T cell type 1 and type 2 responses at the cellular level (Maino and Picker, 1998). In addition, brefeldin A blocks protein secretion, leading to cytokine accumulation in the cell cytoplasm. With flow cytometry, we could then simultaneously evaluate individual cells for proliferation (CFSE), surface markers (CD4, CD8) and intracellular cytokine (IFN-γ).
Initial studies used PBMC from healthy controls stimulated with tetanus toxoid to optimize the assay (data not shown). After an incubation of 10 days, sufficient cells proliferated and were easily distinguished from the nonproliferated cells. In addition, for few samples, we compared thymidine incorporation and CFSE-labeling assay and observed that peptide(s) inducing a significant proliferation above the background by CFSE-labeling measurement also showed a number of wells above a stimulation index of 2 compared to the negative controls. A subsequent 4–6-h incubation with PMA and ionomycin in the presence of brefeldin A allowed optimal assessment of cytokines (Maino and Picker, 1998). The results obtained were both robust and reproducible, as duplicate cultures and experiments gave similar results, as did different aliquots of the same PBMC tested on different days (data not shown). Samples showing a percentage of CD4+ T cells positive for proliferation and for IFN-γ at least 1% over the background were repeatedly robust. The detection of IFN-γ was similar to TNF-α detection; that is, the majority of samples that were positive for IFN-γ were also positive for TNF-α and vice versa. However, the background production of TNF-α (cells without any peptide stimulation) was greater for most donors tested than for IFN-γ. As IFN-γ is still the most commonly measured Th1 cytokine (Hellings et al., 2001; Vergelli et al., 2001; Weissert et al., 2002) and has been shown to be involved in MS pathogenesis (Panitch et al., 1987; Woodroofe and Cuzner, 1993), we limited our results presented here to IFN-γ production by myelin-specific T cells. To avoid bias, analysis of the flow cytometry-based assay was performed without the evaluator knowing the patients’ clinical status.
2.4. Isolation and stimulation of human peripheral mono-nuclear cells
PBMC were isolated by density gradient using Ficoll-Paque Plus (Pharmacia Biotech, Uppsala, Sweden) and then frozen in RPMI containing 10% (v/v) dimethylsulfoxide (DMSO) (Sigma, St. Louis, MO) and 10% (v/v) fetal bovine serum (Hyclone, Logan, UT). For the present study, PBMC were rapidly thawed, washed three times with RPMI and labeled with 2.5 μM 5- (and 6-) carboxyfluorescein succinimidyl ester (CFSE) (Molecular Probes, Eugene, OR) for 10 min at 37°C in RPMI containing 0.5% human AB serum (Sigma). Cells were then washed twice with cold PBS, resuspended in RPMI (Gibco Life-Technologies, Rockville, MD) containing 10% normal AB human serum, 2 mM glutamine (Gibco), 10 mM HEPES (Gibco), 100 U/ml penicillin (Gibco), 100 μg/ml streptomycin (Gibco), 1 mM sodium pyruvate (Gibco), 0.1 mM nonessential amino acids (Gibco), 50 μM 2-mercaptoethanol (Sigma), incubated for at least 4 h at 37 °C and 5% CO2, counted and seeded in 48-well plates (5 × 105 cells/ml) containing the appropriate stimulus: human MOBP peptides (MOBP amino acids (aa) 1–19, MOBP aa 11–29, MOBP aa 20–38, MOBP aa 31–49, MOBP aa 41–59, MOBP aa 51–70) at 10 μM or bovine MBP (Sigma) at 50 μg/ml or in the absence of stimulus for the baseline control. MOBP peptides were synthesized at TSRI peptide core facility by standard FMOC synthesis chemistry and were >95% pure as judged by HPLC and by mass spectrometry. Earlier report showed that these selected overlapping MOBP peptides induced a lymphoproliferative response of MS patients’ PBMC (Holz et al., 2000). Recombinant human IL-2 (50 U/ml) (Roche, Nutley, NJ) was added 5 days after the beginning of the incubation. At day 10, half of the supernatant was collected and stored at −20 °C until used to measure by ELISA. All samples from a patient were analyzed at the same time using the Duo set from R&D Systems (Minneapolis, MN) for IFN-γ according to the manufacturer’s instruction. ELISA readout was 10 pg/ml of IFN-γ over the background as the lower limit of detection was 7.8 pg/ml. After the 10-day culture, cells were stimulated to produce cytokine with 20 ng/ml PMA (Sigma), 1 μg/ml ionomycin (Sigma) and 5 μg/ml brefeldin A (Sigma) for 5 h before flow cytometry analysis.
2.5. Flow cytometry
PBMC were labeled for surface antigens using anti-CD8 antibody conjugated to Cychrome and anti-CD4 antibody conjugated to allophycocyanin (BD PharMingen, La Jolla, CA). After staining for 20–30 min on ice in PBS (Gibco) containing 1% (v/v) fetal bovine serum (FBS) and 0.1% (w/v) NaN3 (Sigma), cells were washed twice, fixed and permeabilized in 4% (w/v) paraformaldehyde (Sigma) with 0.1% (w/v) saponin (Sigma) in Hank’s Balanced Salt Solution (Gibco). Intracellular cytokine staining was accomplished by incubating PBMC with antibody to IFN-γ conjugated to phycoerythrin (PE) for 30 min on ice in 0.1% (w/v) saponin, 1% FBS and 0.1% (w/v) NaN3 PBS, followed by two washes and resuspension of cells in 1% (v/v) FBS and 0.1% (w/ v) NaN3 PBS. Cells were acquired on a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA) and analyzed with Cell Quest (Becton Dickinson) and FlowJo (Treestar, San Carlos, CA) software.
3. Results
3.1. Assay developed to measure T cell reactivity to myelin antigens
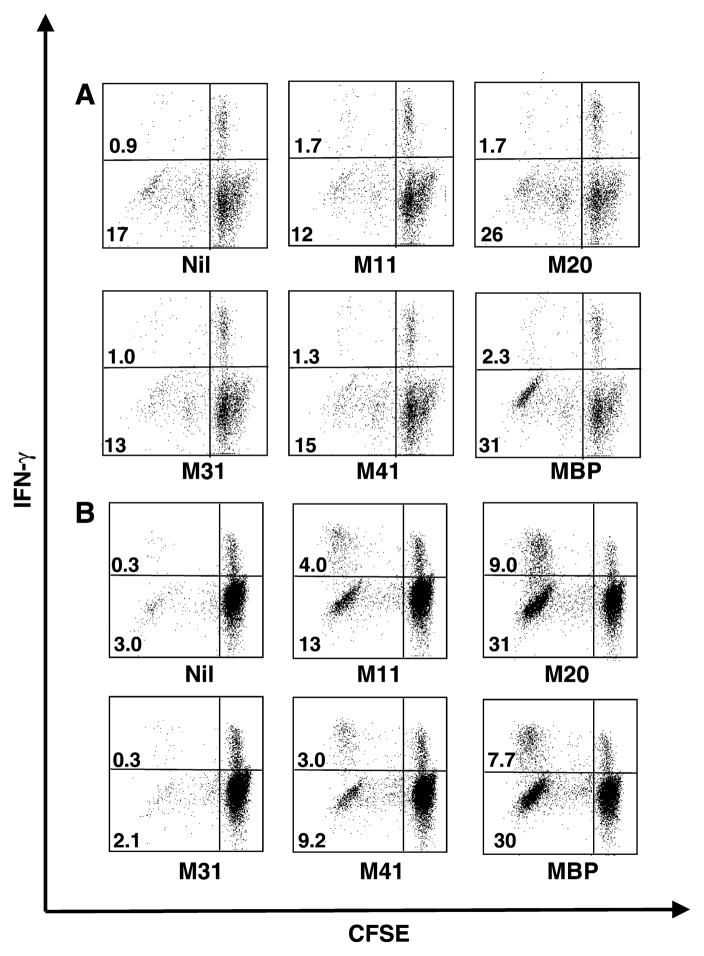
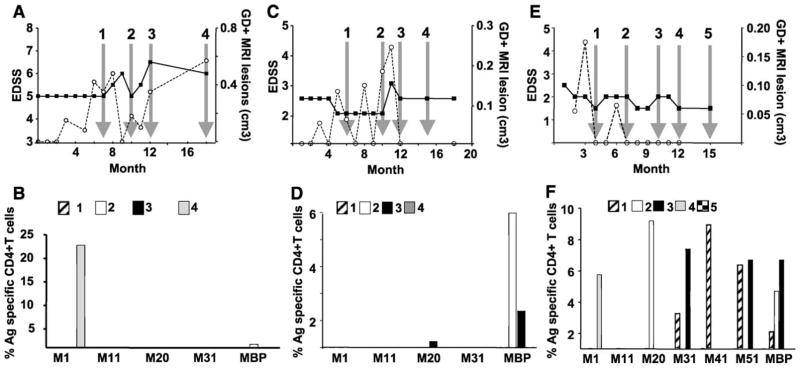
We first developed an assay to measure T cell reactivity to various myelin antigens in MS patients. Figs. 1 and 2 illustrate flow cytometry results from two MS patients (3 and 5): at two time points, CD4+ lymphocytes were analyzed for proliferation and IFN-γ production. Since CD8+ T cells did not significantly proliferate above the background for the antigens tested, no result for this subpopulation of lymphocytes is shown. PBMC obtained from patient 3 at the 12th month of the follow-up (Fig. 1, panel B) proliferated (left quadrants) and produced IFN-γ (upper left quadrants) when stimulated with myelin antigens: MOBP aa 11–29, MOBP aa 20–38, MOBP aa 41–59 and bovine MBP. Compared to a background level (Nil, upper left quadrant) of 0.3% of CD4+ T cells, a greater than 10–25-fold enhancement of IFN-γ-producing cells was observed. These are antigen-specific reactive T cells. The first dot plot shows cells cultured without antigen (Nil) and represents the background level. Clearly, most CD4+ T cells were IFN-γ negative and CFSEhigh (lower right quadrant), denoting that they did not proliferate and did not produce IFN-γ. Defining this low background allowed us to focus on cells that proliferated and produced IFN-γ, as displayed in the upper left quadrant of the dot plots. Only the samples demonstrating a CD4+ T cell proliferation, regardless of the cytokine production, above the background were considered in our analysis. Among the CD4+ T cells that proliferated (CFSElow), a fraction produced IFN-γ (upper left quadrant); 4.0%, 9.0%, 3.0% and 7.7% to MOBP aa 11–29, MOBP aa 20–38, MOBP aa 41–59 and MBP antigens, respectively. Among proliferating (CFSElow) CD4+ T cells, only a small fraction produced IFN-γ (upper left quadrant: 0.3% of CD4+ T cells), whereas most cells did not (lower left quadrant: 3.0% of CD4+ T cells), when no antigen was added. Since MOBP peptides were overlapping, the observed reactivity to MOBP aa 11–29 and MOBP aa 20–38 may represent a shared epitope present in both peptides. In fact, 17%, 40%, 12.2% and 37.7% of antigen-specific CD4+ T cell proliferation (including IFN+ or IFN − proliferation + CD4+ T cells) were observed for MOBP aa 11–29, MOBP aa 20–38, MOBP aa 41–59 and MBP, respectively. Interestingly, myelin antigens that showed a significantly greater percentage of proliferation + IFN-γ + CD4+ T cells also induced a greater proliferation of CD4+ T cells regardless of T cells’ ability to secrete IFN-γ. The total percentage of CD4+ T cells that proliferated in the sample without peptide was 3.3 (0.3% CFSElowIFN-γ + added to 3.0% CFSElowIFN-γ −). The MOBP aa 31–49 did not induce proliferation and/or IFNγ production. At the 7th month of the follow up (Fig. 1 panel A), only the sample stimulated with bovine MBP showed a response greater than the background.
Fig. 1.
Proliferation and cytokine production by CD4+ T cells from patient 3 after myelin antigen stimulation. CFSE-labeled human PBMC from patient 3 were incubated either with or without different myelin antigens for 10 days and then stimulated for 5 h in the presence of PMA, ionomycin and brefeldin A before labeling for surface CD4+ T cells and intracellular IFN-γ. Dot plots from the flow cytometry assays represent gated cells for CD4+ lymphocytes. The antigen added to the culture is written underneath each dot plot: Nil: no antigen, M1: MOBP aa 1–19, M11: MOBP aa 11–29, M20: MOBP aa 20–38, M31: MOBP aa 31–49, M41: MOBP aa 41–59, MBP: myelin basic protein. Percentage of cells that proliferated is indicated in appropriate quadrant either IFN-γ+ (upper left quadrant) or IFN-γ − cells (lower left quadrant). (A) Cells taken at the 7th month of the follow-up; (B) cells taken at the 12th month of the follow-up.
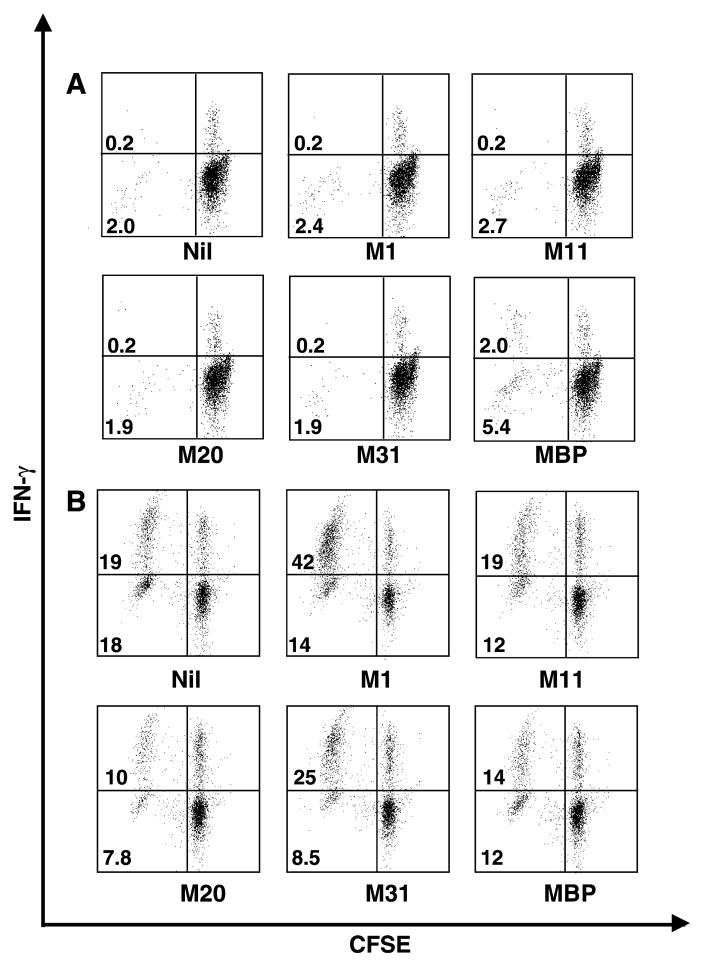
Fig. 2.
Proliferation and cytokine production by CD4+ T cells from patient 5 after myelin antigen stimulation. (A) Cells taken at the 10th month of the follow up; (B) cells taken at the 18th month of the follow-up.
Results for patient 5 at the 10th and 18th months are shown in Fig. 2. At the 10th month (panel A), bovine MBP elicited an antigen-specific response. In contrast, MOBP aa 1–19 induced a significant antigen-specific response when PBMC from the 18th month were assessed (panel B): 56% of CD4+ T cells proliferated and 42% proliferated and produced cytokine, compared to 47% of total CD4+ T cell proliferation and 19% for both proliferation and cytokine production when the cells were not stimulated with a myelin antigen. Although PBMC from patient 5 showed variable background level: 0.2% of CD4+ T cells were CFSElow and IFN-γ+ at the 10th month and 19% at the 18th month when cultured without any peptide, antigen-specific response could still be detected above background levels. Since humans are constantly exposed to a number of potential immune activators (i.e., microbial infections), this variation in the background likely represents immune responses in a normal environment in contrast to a pathogen-free environment.
Intracellular IL-4 was measured in some samples. The production of IL-4, a Th2 cytokine, was low compared to IFN-γ and TNF-α, Th1 cytokines, both in percentage of CD4+ T cells and in number of positive samples. Among the samples that were positive for IFN-γ, only a fraction (1/3 to 2/3 depending on the donor and time point) was also positive for IL-4. In no instance was IL-4 detectable in samples that were below the background for IFN-γ production. Hence, as Th1 rather than Th2 cells have been shown to be involved in the pathogenesis of animal models and MS, and as IL-4 levels were low or absent, we focused on IFN-γ for all donors and time points.
3.2. Reactivity to myelin antigens is associated with MS relapses
Having defined the parameters of the assay, we next measured the reactivity of MS patients’ T cells to myelin antigens over an 18-month period using each MS patient as his/her own control. All patients were characterized as remitting–relapsing MS patients and information on their date of birth, sex and HLA typing are provided in Table 1.
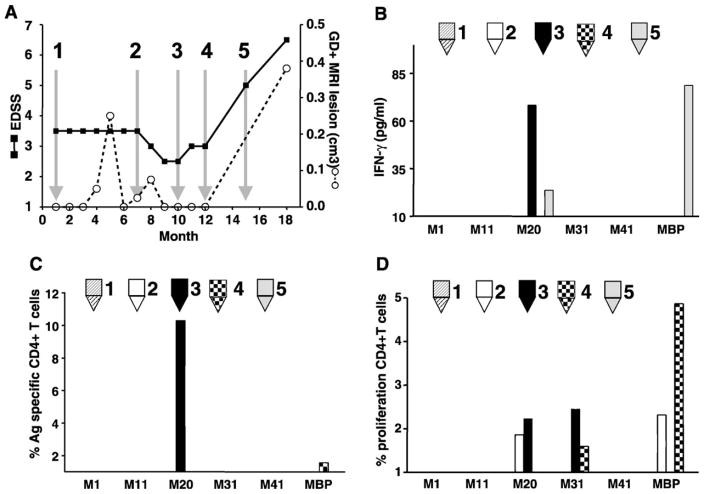
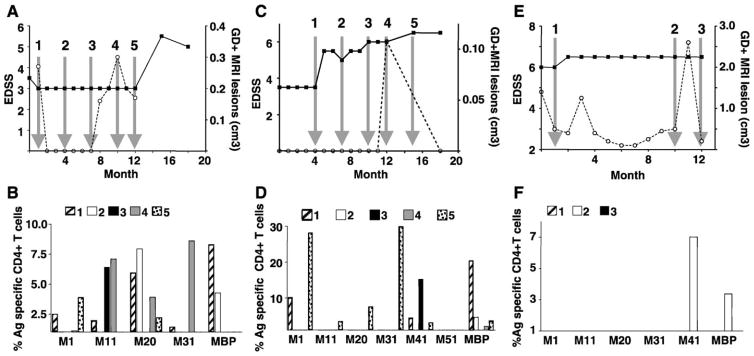
Data from 10 MS patients are presented below. Results obtained for patient 1 are shown in Fig. 3. This patient was clinically stable during the first 7 months, but then experienced a sudden severe increase in disability accompanied by an MRI evidence of a new brain lesion (Fig. 3 panel A). PBMC displayed a significant CD4+ T cell response to MOBP aa 20 –38 occurring immediately before both increase in clinical disease and gadolinium-enhanced MRI lesions (arrows 3, 4 and 5). The antigen-specific T cell response was confirmed both by the flow cytometry (panel C) and by the IFN-γ-specific ELISA (panel B). Total CD4+ T cell proliferation (Fig. 4 panel D) to MOBP aa 20–38, MOBP aa 31–49 and bovine MBP was observed at time points indicated by arrows 2, 3 and 4. In contrast, in the absence of either new brain lesions or enhanced clinical disease (arrow 1), there was no detection of antigen-specific MOBP or of MBP T cells, no measurable release of IFN-γ in the supernatant (panel B), no significant CD4+ proliferation (panel D) nor CD4+ proliferation accompanied by IFN-γ production (panel C). Overall, samples that yielded a positive signal detected by the flow cytometry assay were also positive for IFN-γ by ELISA. In rare instances (< 10% of cases), the amount of IFN-γ measured in the supernatant was not proportional to the percentage of cells that proliferated and expressed IFN-γ by flow cytometry. This is likely due to IFN-γ-producing cells not secreting the same cytokine amount. The amounts of IFN-γ found were similar to earlier reports for myelin-specific T cell lines (Correale et al., 1997a) and slightly higher to the level observed in MS serum (Nicoletti et al., 2000).
Fig. 3.
Clinical data and myelin reactivity of T cells from patient 1. At the time points indicated, patient 1 was examined for EDSS and for gadolinium-enhanced brain MRI scans. Simultaneously, this patient gave blood from which PBMC were isolated and frozen for later analysis. Arrows labeled 1, 2, 3, 4 and 5 indicate times when CFSE-labeled PBMC were cultured for 10 days with and without different myelin antigens specified. Supernatants were harvested for ELISA, then cells were stimulated for 5 h with the combined PMA, ionomycin and brefeldin A before labeling for surface CD4 and intracellular IFN-γ. (A) Clinical data from donor: EDSS (■, solid line) and gadolinium-enhanced brain MRI lesion (○ dashed line). (B) Amount of IFN-γ over the background measured by ELISA in the supernatant after 10-day culture with different myelin antigens M1: MOBP aa 1–19, M11: MOBP aa 11–29, M20: MOBP aa 20–38, M31: MOBP aa 31–49, M41: MOBP aa 41–59. (C) Percentage of CD4+ IFN-γ-producing T cells that proliferated over the background after culture with different myelin antigens and PMA, ionomycin and brefeldin A stimulation (time points are indicated by the arrows on panel A). (D) Percentage of CD4+ T cells over the background that proliferated after culture with different myelin antigens (time points are indicated by the arrows on panel A).
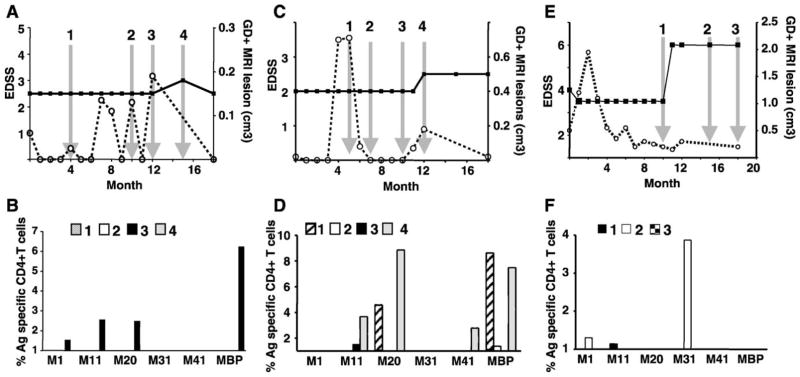
Fig. 4.
Clinical data and myelin-reactivity of T cells from patients 2, 3 and 4. Patient 2: (A) clinical data EDSS (■, solid line) and gadolinium-enhanced brain MRI lesion (○ dashed line). (B) Percentage of CD4+ T cells over the background that proliferated and produced IFN-γ after the culture with different myelin antigens for the time points indicated by the arrows on panel A. Patient 3: (C) clinical data EDSS and GD+ brain lesion. (D) Percentage of CD4+ T cells over the background that proliferated after culture with different myelin antigens (time points are indicated by the arrows on panel C). Patient 4: (E) clinical data EDSS and GD+ brain lesion. (F) Percentage of CD4+ T cells over the background that proliferated after culture with different myelin antigens (time points are indicated by the arrows on panel E).
A second patient (Fig. 4, panels A and B) and a third patient (Fig. 4, panels C and D) displayed multiple new gadolinium-positive brain lesions occurring during the 18-month follow-up, but only a small increase in clinical disability at the 15th month for patient 2 and at the 12th month for patient 3. PBMC tested at MRI peaks (arrow 3 for patient 2 and arrows 1 and 4 for patient 3) showed a direct correlation with an enhanced antigen-specific CD4+ T cell response for at least one of the MOBP peptides. Thus, in the absence of clinical disability but presence of new gadolinium-positive brain lesions, antigen-specific T cell responses to myelin antigens occurred. Further, when an increase in EDSS (arrow 4 for patient 3) did occur, it also directly correlated with reactivity to some MOBP peptides and to bovine MBP. For patient 3, the reactivity to MOBP aa 11–29, which was negative at the initial time points (arrows 1 and 2), increased prior to (arrows 3 and 4) the clinical relapse, which was associated with new brain lesions, again indicating that the detection of antigen-specific T cell response to myelin antigen preceded newly active disease.
The fourth patient (Fig. 4, panels E and F) was clinically stable during the first 10 months of the follow-up. However, thereafter, a dramatic increase in the EDSS score occurred, which stayed high for the remainder of the study. Just prior to the relapse (arrow 1), antigen-specific T cell response to MOBP aa 11–29 was observed, and then during the relapse (arrow 2), T cell responses to MOBP aa 1–19 and MOBP aa 31–49. Unfortunately, no blood sample was available during the prolonged clinically stable period (months 1–9) in order to compare these results with previous status.
Patients 5 (Fig. 5, panels A and B) and 6 experienced numerous new gadolinium-positive lesions as well as increases in their EDSS score. PBMC were available when some of these new MRI lesions occurred (arrows 1, 2, 3 and 4 for patient 5 and arrows 1 and 2 for patient 6). Antigen-specific T cell responses to at least one MOBP peptide (MOBP aa 1–19 for patient 5; MOBP aa 20–38 for patient 6) and to bovine MBP were directly correlated with the development of new brain lesions for each patient. No antigen response was detected while patient 6 was clinically stable (arrow 4).
Fig. 5.
Clinical data and myelin reactivity of T cells from patients 5, 6 and 7. Patient 5: (A) clinical data EDSS (■, solid line) and gadolinium-enhanced brain MRI lesion (○ dashed line). (B) Percentage of CD4+ T cells over the background that proliferated and produced IFN-γ after the culture with different myelin antigens for the time points indicated by the arrows on panel A. Patient 6: (C) clinical data EDSS and GD+ brain lesion. (D) Percentage of CD4+ T cells over the background that proliferated after culture with different myelin antigens (time points are indicated by the arrows on panel C). Patient 7: (E) clinical data EDSS and GD+ brain lesion. (F) Percentage of CD4+ T cells over the background that proliferated after culture with different myelin antigens (time points are indicated by the arrows on panel E).
Many antigen-specific T cell responses were detected for patient 7 (Fig. 5, panels E and F), patient 8 (Fig. 6, panels A and B) and patient 9 (Fig. 6, panels C and D) during the observation period. Again, responses to MOBP peptides or bovine MBP paralleled the appearance of new GD+ lesions (arrow 4 for patients 8 and 9) and increases in EDSS score (arrow 3 for patient 7; arrows 3 and 5 for patient 9) or preceded these changes by 1 month (arrow 1 for patient 7, arrows 3 and 5 for patient 8; arrows 1 and 2 for patient 9). In contrast, no antigen-specific T cell response was detected during the stable period of patient 7 (arrow 5 for patient 7). Although patient 8 was clinically stable and did not show new brain GD+ lesion at the second arrow, antigen-specific T cell responses were detected for MOBP aa 20–38 and MBP.
Fig. 6.
Clinical data and myelin reactivity of T cells from patients 8, 9 and 10. Patient 8: (A) clinical data EDSS (■, solid line) and gadolinium-enhanced brain MRI lesion (○ dashed line). (B) Percentage of CD4+ T cells over the background that proliferated and produced IFN-γ after the culture with different myelin antigens for the time points indicated by the arrows on panel A. Patient 9: (C) clinical data EDSS and GD+ brain lesion. (D) Percentage of CD4+ T cells over the background that proliferated after culture with different myelin antigens (time points are indicated by the arrows on panel C). Patient 10: (E) clinical data EDSS and GD+ brain lesion. (F) Percentage of CD4+ T cells over the background that proliferated after culture with different myelin antigens (time points are indicated by the arrows on panel E).
Antigen-specific immune responses were observed only at one time point for the last patient (10, Fig. 6 panels E and F). Responses to MOBP aa 41–59 and to MBP were observed just prior (arrow 2) to the development of the main new GD+ lesion. No antigen-specific T cell response was detectable at the two other time points tested.
As a summary of these results, most relapses of MS, disease activity as measured by the occurrence of new brain MRI lesions and/or increase in EDSS, were directly associated with enhanced antigen-specific CD4+ T cell response to at least one myelin antigen. Further, for most patients, the antigen-specific CD4+ T cell responses as measured by proliferation and IFN-γ expression preceded the clinical relapse. Hence, in nine patients (except patient 10), there was a clear variation in EDSS scores denoting enhanced clinical disability occurring at the time of or just after the antigen-specific T cell response to MOBP or MBP. Moreover, the majority of new brain lesions tested were accompanied by antigen-specific CD4+ T cell responses to at least one myelin antigen.
In contrast, during clinically stable periods, there was a lack of new brain MRI lesions and no change in EDSS, and most often no specific antigen T cell response to MOBP or MBP. In a few instances, a low antigen-specific T cell response reactivity to MBP (patient 3) or to MOBP aa 20–38 and MBP (patient 8) occurred at times when no new brain MRI lesions were detectable and the EDSS was stable.
Combining the data obtained from the 10 MS patients, our results demonstrated a significant correlation between the detection of MOBP-specific CD4+ T cell responses and new brain gadolinium lesions. Statistical analyses revealed that PBMC taken at the same time point or the month before the MRI peak showed antigen-specific T cell responses to MOBP peptides (odds ratio 12.87, 95% confidence interval 1.89, 546.01), which were minimal in the absence of MRI peak. In addition, a similar correlation was also observed between MOBP-specific responses and increased EDSS score (odds ratio: 12.00, 95% confidence interval 1.79, 506.22). This correlation was specific to MOBP as the limited study of MBP-specific responses showed that these responses were not significantly associated with clinical exacerbations. As we used bovine MBP, a more exhaustive study with human MBP peptides will be necessary to determine whether a correlation between MBP-specific T cell responses and clinical exacerbations is detectable.
4. Discussion
Use of a novel flow cytometry-based assay designed to follow individual MS patients over the 18-month course of their disease showed that detection of antigen-specific CD4+ T cell response to myelin antigens (MOBP peptides and MBP) significantly correlated directly with active disease as detected by EDSS and brain MRI (8 out of 10 patients studied). Such antigen-specific T cell responses often occurred immediately before or during a relapse of MS but were absent during clinical remission or clinically stable time. Odds ratios showed a statistically significant association between antigen-specific T cell response to MOBP and exacerbations of MS. Thus, our study extends previous work demonstrating a correlation of changes in immune responses with increased MRI activity (Calabresi et al., 1998a; Oger et al., 1988; Prat et al., 2000; Rieckmann et al., 1995). Due to sample size available, our initial studies were limited to analyses to MOBP aa 1–19, MOBP aa 11–29, MOBP aa 20–38, MOBP aa 31–49, MOBP aa 41–59 and bovine MBP. While 8 out of 10 MS patients showed a direct correlation between antigen-specific T cell response to myelin antigens and exacerbations of disease, two (patients 3 and 8) showed antigen-specific T cell response during a stable period of disease perhaps due to new spinal cord lesions, which we did not assess by MRI.
Our assay is unique in that it employed both antigen-specific proliferation and IFN-γ expression. Typically, previous methods for assessing myelin-specific T cell activation typically measured either the proliferation of PBMC by tritiated thymidine incorporation or the secretion of specific cytokines by ELISA but not both simultaneously. Although useful, these bulk methods are deficient in that they fail to provide information about functional responses of individual lymphocyte subsets (Maino et al., 1995). While ELI-SPOT assays evaluate the relative number of myelin-specific T lymphocytes able to produce cytokine of either Th1 or Th2 type (Hellings et al., 2001; Pelfrey et al., 2000), they measure only one aspect of the reactivity of T cells to a particular antigen, the cytokine production. The strength of our assay is that it measures both antigen-specific proliferation and cytokine production while using a small number of PBMC.
The causes of the observed bursts of antigen-specific T cell responses documented here are not known, but may be due to environmental factors such as infections by human pathogens or by downregulation of suppressor T cells that restrict the autoimmune responses. Epidemiologic data have indicated occurrence of viral infections preceding attacks of MS (Panitch, 1994). Moreover, cross-reactivity (molecular mimicry) or bystander activation might cause such myelin-specific T cell reactivity. Although we have circumstantial evidence that the antigen-specific T cell responses observed were correlated with the clinical course of MS disease, we have no direct evidence that these cells play a role in the disease. Nevertheless, such reactivity could be a surrogate marker of increased CNS-specific immune responses at a specific time point indicating a new or more pronounced disease burden. When the antigen-specific response closely precedes clinical disease, one might consider therapeutic intervention at that time.
Our results expanded previous data (Holz et al., 2000; Kaye et al., 2000) showing that MOBP should also be considered as a target of CD4+ T cell responses in MS. The MS patients analyzed here had heterogeneous MHC class II types (see Table 1); yet PBMC from all of them reacted to at least one MOBP peptide at one or more time points. Since MOBP-reactive T cells presumably find their target exclusively in the CNS, the only site where MOBP is expressed, they would have the potential to proliferate and cause damage, unless they encounter a molecular mimic in the periphery. Further, as MOBP is expressed later in postnatal development than MBP and PLP (Holz et al., 1996), negative selection to remove potentially auto-reactive MOBP-specific T cells may not be as efficient as for MBP and PLP, which are transcriptionally active earlier in development (Holz et al., 1996) and, importantly, are expressed in immunological organs and by antigen-presenting cells (Pribyl et al., 1996).
In our studies, antigen-specific CD8+ T cell proliferation was limited. One potential explanation is a technical one, since we used long peptides of 19 aa whereas in contrast, smaller peptides of 8–12 aa are more efficient for stimulating CD8+ T cells (Maecker et al., 2001). Recent evidence suggests that CD8+ T cells participate in MS (Babbe et al., 2000; Biddison et al., 1997, 1998; Correale et al., 1997b), and hence, future studies should optimize peptide size and selection. Currently, MOBP processing and presentation studies are being planned to identify specific MHC class I and class II epitopes processed by antigen-presenting cells and able to activate CD8 and CD4 T cells. Lastly, the absence of antigen-specific T cells to MOBP or MBP that occurred in our studies may be due to T cells specific for other myelin antigens such as MOG, MAG or PLP. Comprehensive studies using the assay described here on lymphocytes harvested from the blood and cerebrospinal fluid of larger number of well-defined MS patients with several myelin antigens appear warranted. With the increase number of identified human myelin antigen epitopes, the use of human myelin peptides covering these target antigens will allow the precise evaluation of anti-myelin responses in MS patients over the course of their disease. Further, the assay described here should be of value in study of other auto-immune diseases.
Acknowledgments
The authors acknowledge the technical assistance of the General Clinical Research Center (GCRC) of the Scripps Clinic, Scripps Green Hospital and The Scripps Research Institute for blood drawing as well as PBMC isolation, freezing and conservation. We thank Dr. Ernerst Beutler for his contribution to the MS/Cladribine studies. We thank Phyllis Minick for critically reviewing the manuscript.
This work was supported by a NIH grant NS38719 to M.B.A.O. and NIH grant MO1RR00833 to the GCRC. N.A. was supported by a postdoctoral fellowship from the Canadian Institutes of Health Research, and A.H. by the National Multiple Sclerosis Society (RG 3335A1/T). J.C.S. was supported by a grant form the ALSAM Foundation and The Skaggs Institute for Research.
References
- Allegretta M, Nicklas JA, Sriram S, Albertini RJ. T cells responsive to myelin basic protein in patients with multiple sclerosis. Science. 1990;247:718–721. doi: 10.1126/science.1689076. [DOI] [PubMed] [Google Scholar]
- Babbe H, Roers A, Waisman A, Lassmann H, Goebels N, Hohlfeld R, Friese M, Schroder R, Deckert M, Schmidt S, Ravid R, Rajewsky K. Clonal expansions of CD8(+) T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J Exp Med. 2000;192:393–404. doi: 10.1084/jem.192.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B, Giacomini PS, Pelletier D, McCrea E, Prat A, Antel JP. Interferon-gamma secretion by peripheral blood T-cell subsets in multiple sclerosis: correlation with disease phase and interferon-beta therapy. Ann Neurol. 1999;45:247–250. [PubMed] [Google Scholar]
- Benvenuto R, Paroli M, Buttinelli C, Franco A, Barnaba V, Fieschi C, Balsano F. Tumour necrosis factor-alpha synthesis by cerebrospinal-fluid-derived T cell clones from patients with multiple sclerosis. Clin Exp Immunol. 1991;84:97–102. [PMC free article] [PubMed] [Google Scholar]
- Biddison WE, Taub DD, Cruikshank WW, Center DM, Connor EW, Honma K. Chemokine and matrix metalloproteinase secretion by myelin proteolipid protein-specific CD8+ T cells: potential roles in inflammation. J Immunol. 1997;158:3046–3053. [PubMed] [Google Scholar]
- Biddison WE, Cruikshank WW, Center DM, Pelfrey CM, Taub DD, Turner RV. CD8+ myelin peptide-specific T cells can chemoattract CD4+ myelin peptide-specific T cells: importance of IFN-inducible protein 10. J Immunol. 1998;160:444–448. [PubMed] [Google Scholar]
- Burns J, Rosenzweig A, Zweiman B, Lisak RP. Isolation of myelin basic protein-reactive T-cell lines from normal human blood. Cell Immunol. 1983;81:435–440. doi: 10.1016/0008-8749(83)90250-2. [DOI] [PubMed] [Google Scholar]
- Burns J, Krasner LJ, Rostami A, Pleasure D. Isolation of P2 protein-reactive T-cell lines from human blood. Ann Neurol. 1986;19:391–393. doi: 10.1002/ana.410190416. [DOI] [PubMed] [Google Scholar]
- Calabresi PA, Fields NS, Farnon EC, Frank JA, Bash CN, Kawanashi T, Maloni H, Jacobson S, McFarland HF. ELI-spot of Th-1 cytokine secreting PBMC’s in multiple sclerosis: correlation with MRI lesions. J Neuroimmunol. 1998a;85:212–219. doi: 10.1016/s0165-5728(98)00008-3. [DOI] [PubMed] [Google Scholar]
- Calabresi PA, Tranquill LR, McFarland HF, Cowan EP. Cytokine gene expression in cells derived from CSF of multiple sclerosis patients. J Neuroimmunol. 1998b;89:198–205. doi: 10.1016/s0165-5728(98)00139-8. [DOI] [PubMed] [Google Scholar]
- Cannella B, Raine CS. The adhesion molecule and cytokine profile of multiple sclerosis lesions. Ann Neurol. 1995;37:424–435. doi: 10.1002/ana.410370404. [DOI] [PubMed] [Google Scholar]
- Chou YK, Bourdette DN, Offner H, Whitham R, Wang RY, Hashim GA, Vandenbark AA. Frequency of T cells specific for myelin basic protein and myelin proteolipid protein in blood and cerebrospinal fluid in multiple sclerosis. J Neuroimmunol. 1992;38:105–113. doi: 10.1016/0165-5728(92)90095-3. [DOI] [PubMed] [Google Scholar]
- Correale J, McMillan M, Li S, McCarthy K, Le T, Weiner LP. Antigen presentation by autoreactive proteolipid protein peptide-specific T cell clones from chronic progressive multiple sclerosis patients: roles of co-stimulatory B7 molecules and IL-12. J Neuroimmunol. 1997a;72:27–43. doi: 10.1016/s0165-5728(96)00139-7. [DOI] [PubMed] [Google Scholar]
- Correale J, Rojany M, Weiner LP. Human CD8+ TCR-alpha beta(+) and TCR-gamma delta(+) cells modulate autologous autoreactive neuroantigen-specific CD4+ T-cells by different mechanisms. J Neuroimmunol. 1997b;80:47–64. doi: 10.1016/s0926-9851(97)00020-7. [DOI] [PubMed] [Google Scholar]
- Genain CP, Cannella B, Hauser SL, Raine CS. Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat Med. 1999;5:170–175. doi: 10.1038/5532. [DOI] [PubMed] [Google Scholar]
- Hellings N, Baree M, Verhoeven C, D’Hooghe MB, Medaer R, Bernard CC, Raus J, Stinissen P. T-cell reactivity to multiple myelin antigens in multiple sclerosis patients and healthy controls. J Neurosci Res. 2001;63:290–302. doi: 10.1002/1097-4547(20010201)63:3<290::AID-JNR1023>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Hofman FM, Hinton DR, Johnson K, Merrill JE. Tumor necrosis factor identified in multiple sclerosis brain. J Exp Med. 1989;170:607–612. doi: 10.1084/jem.170.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz A, Schaeren-Wiemers N, Schaefer C, Pott U, Colello RJ, Schwab ME. Molecular and developmental characterization of novel cDNAs of the myelin-associated/oligodendrocytic basic protein. J Neurosci. 1996;16:467–477. doi: 10.1523/JNEUROSCI.16-02-00467.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz A, Bielekova B, Martin R, Oldstone MB. Myelin-associated oligodendrocytic basic protein: identification of an encephalitogenic epitope and association with multiple sclerosis. J Immunol. 2000;164:1103–1109. doi: 10.4049/jimmunol.164.2.1103. [DOI] [PubMed] [Google Scholar]
- Horwitz MS, Evans CF, McGavern DB, Rodriguez M, Oldstone MB. Primary demyelination in transgenic mice expressing interferon-gamma. Nat Med. 1997;3:1037–1041. doi: 10.1038/nm0997-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jingwu Z, Medaer R, Hashim GA, Chin Y, van den Berg-Loonen E, Raus JC. Myelin basic protein-specific T lymphocytes in multiple sclerosis and controls: precursor frequency, fine specificity, and cytotoxicity. Ann Neurol. 1992;32:330–338. doi: 10.1002/ana.410320305. [DOI] [PubMed] [Google Scholar]
- Karandikar NJ, Crawford MP, Yan X, Ratts RB, Brenchley JM, Ambrozak DR, Lovett-Racke AE, Frohman EM, Stastny P, Douek DC, Koup RA, Racke MK. Glatiramer acetate (Copaxone) therapy induces CD8(+) T cell responses in patients with multiple sclerosis. J Clin Invest. 2002;109:641–649. doi: 10.1172/JCI14380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye JF, Kerlero de Rosbo N, Mendel I, Flechter S, Hoffman M, Yust I, Ben-Nun A. The central nervous system-specific myelin oligodendrocytic basic protein (MOBP) is encephalitogenic and a potential target antigen in multiple sclerosis (MS) J Neuroimmunol. 2000;102:189–198. doi: 10.1016/s0165-5728(99)00168-x. [DOI] [PubMed] [Google Scholar]
- Kerlero de Rosbo N, Milo R, Lees MB, Burger D, Bernard CC, Ben-Nun A. Reactivity to myelin antigens in multiple sclerosis. Peripheral blood lymphocytes respond predominantly to myelin oligodendrocyte glycoprotein. J Clin Invest. 1993;92:2602–2608. doi: 10.1172/JCI116875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killestein J, Kalkers NF, Meilof JF, Barkhof F, van Lier RA, Polman CH. TNFalpha production by CD4(+) T cells predicts long-term increase in lesion load on MRI in MS. Neurology. 2001;57:1129–1131. doi: 10.1212/wnl.57.6.1129. [DOI] [PubMed] [Google Scholar]
- Lindert RB, Haase CG, Brehm U, Linington C, Wekerle H, Hohlfeld R. Multiple sclerosis: B- and T-cell responses to the extracellular domain of the myelin oligodendrocyte glycoprotein. Brain. 1999;122:2089–2100. doi: 10.1093/brain/122.11.2089. [DOI] [PubMed] [Google Scholar]
- Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Lyons AB. Analysing cell division in vivo and in vitro using flow cytometric measurement of CFSE dye dilution. J Immunol Methods. 2000;243:147–154. doi: 10.1016/s0022-1759(00)00231-3. [DOI] [PubMed] [Google Scholar]
- Maatta JA, Kaldman MS, Sakoda S, Salmi AA, Hinkkanen AE. Encephalitogenicity of myelin-associated oligodendrocytic basic protein and 2′,3′-cyclic nucleotide 3′-phosphodiesterase for BALB/c and SJL mice. Immunology. 1998;95:383–388. doi: 10.1046/j.1365-2567.1998.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maecker HT, Dunn HS, Suni MA, Khatamzas E, Pitcher CJ, Bunde T, Persaud N, Trigona W, Fu TM, Sinclair E, Bredt BM, McCune JM, Maino VC, Kern F, Picker LJ. Use of overlapping peptide mixtures as antigens for cytokine flow cytometry. J Immunol Methods. 2001;255:27–40. doi: 10.1016/s0022-1759(01)00416-1. [DOI] [PubMed] [Google Scholar]
- Maino VC, Picker LJ. Identification of functional subsets by flow cytometry: intracellular detection of cytokine expression. Cytometry. 1998;34:207–215. doi: 10.1002/(sici)1097-0320(19981015)34:5<207::aid-cyto1>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Maino VC, Suni MA, Ruitenberg JJ. Rapid flow cytometric method for measuring lymphocyte subset activation. Cytometry. 1995;20:127–133. doi: 10.1002/cyto.990200205. [DOI] [PubMed] [Google Scholar]
- Markovic-Plese S, Fukaura H, Zhang J, al-Sabbagh A, Southwood S, Sette A, Kuchroo VK, Hafler DA. T cell recognition of immunodominant and cryptic proteolipid protein epitopes in humans. J Immunol. 1995;155:982–992. [PubMed] [Google Scholar]
- Marrack P, Kappler J, Kotzin BL. Autoimmune disease: why and where it occurs. Nat Med. 2001;7:899–905. doi: 10.1038/90935. [DOI] [PubMed] [Google Scholar]
- Matthews NC, Wadhwa M, Bird C, Borras FE, Navarrete CV. Sustained expression of CD154 (CD40L) and proinflammatory cytokine production by alloantigen-stimulated umbilical cord blood T cells. J Immunol. 2000;164:6206–6212. doi: 10.4049/jimmunol.164.12.6206. [DOI] [PubMed] [Google Scholar]
- Nicoletti F, Di Marco R, Patti F, Zaccone P, L’Episcopo MR, Reggio E, Xiang M, Nicoletti A, Reggio A. Short-term treatment of relapsing remitting multiple sclerosis patients with interferon (IFN)-beta1B transiently increases the blood levels of interleukin (IL)-6, IL-10 and IFN-gamma without significantly modifying those of IL-1beta, IL-2, IL-4 and tumour necrosis factor-alpha. Cytokine. 2000;12:682–687. doi: 10.1006/cyto.1999.0616. [DOI] [PubMed] [Google Scholar]
- Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- Oger J, Kastrukoff LF, Li DK, Paty DW. Multiple sclerosis: in relapsing patients, immune functions vary with disease activity as assessed by MRI. Neurology. 1988;38:1739–1744. doi: 10.1212/wnl.38.11.1739. [DOI] [PubMed] [Google Scholar]
- Ota K, Matsui M, Milford EL, Mackin GA, Weiner HL, Hafler DA. T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature. 1990;346:183–187. doi: 10.1038/346183a0. [DOI] [PubMed] [Google Scholar]
- Panitch HS. Influence of infection on exacerbations of multiple sclerosis. Ann Neurol. 1994;36:S25–S28. doi: 10.1002/ana.410360709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panitch HS, Hirsch RL, Schindler J, Johnson KP. Treatment of multiple sclerosis with gamma interferon: exacerbations associated with activation of the immune system. Neurology. 1987;37:1010–1097. doi: 10.1212/wnl.37.7.1097. [DOI] [PubMed] [Google Scholar]
- Pelfrey CM, Rudick RA, Cotleur AC, Lee JC, Tary-Lehmann M, Lehmann PV. Quantification of self-recognition in multiple sclerosis by single-cell analysis of cytokine production. J Immunol. 2000;165:1641–1651. doi: 10.4049/jimmunol.165.3.1641. [DOI] [PubMed] [Google Scholar]
- Pouly S, Becher B, Blain M, Antel JP. Interferon-gamma modulates human oligodendrocyte susceptibility to Fas-mediated apoptosis. J Neuropathol Exp Neurol. 2000;59:280–286. doi: 10.1093/jnen/59.4.280. [DOI] [PubMed] [Google Scholar]
- Prat A, Pelletier D, Duquette P, Arnold DL, Antel JP. Heterogeneity of T-lymphocyte function in primary progressive multiple sclerosis: relation to magnetic resonance imaging lesion volume. Ann Neurol. 2000;47:234–237. [PubMed] [Google Scholar]
- Pribyl TM, Campagnoni C, Kampf K, Handley VW, Campagnoni AT. The major myelin protein genes are expressed in the human thymus. J Neurosci Res. 1996;45:812–819. doi: 10.1002/(SICI)1097-4547(19960915)45:6<812::AID-JNR18>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Prineas JW, Graham JS. Multiple sclerosis: capping of surface immunoglobulin G on macrophages engaged in myelin breakdown. Ann Neurol. 1981;10:149–158. doi: 10.1002/ana.410100205. [DOI] [PubMed] [Google Scholar]
- Rieckmann P, Albrecht M, Kitze B, Weber T, Tumani H, Broocks A, Luer W, Helwig A, Poser S. Tumor necrosis factor-alpha messenger RNA expression in patients with relapsing–remitting multiple sclerosis is associated with disease activity. Ann Neurol. 1995;37:82–88. doi: 10.1002/ana.410370115. [DOI] [PubMed] [Google Scholar]
- Romine JS, Sipe JC, Koziol JA, Zyroff J, Beutler E. A double-blind, placebo-controlled, randomized trial of cladribine in relapsing–remitting multiple sclerosis. Proc Assoc Am Physicians. 1999;111:35–44. doi: 10.1046/j.1525-1381.1999.09115.x. [DOI] [PubMed] [Google Scholar]
- Schluesener HJ, Wekerle H. Autoaggressive T lymphocyte lines recognizing the encephalitogenic region of myelin basic protein: in vitro selection from unprimed rat T lymphocyte populations. J Immunol. 1985;135:3128–3133. [PubMed] [Google Scholar]
- Selmaj KW, Raine CS. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann Neurol. 1988;23:339–346. doi: 10.1002/ana.410230405. [DOI] [PubMed] [Google Scholar]
- Selmaj K, Raine CS, Cannella B, Brosnan CF. Identification of lymphotoxin and tumor necrosis factor in multiple sclerosis lesions. J Clin Invest. 1991;87:949–954. doi: 10.1172/JCI115102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharief MK, Thompson EJ. Correlation of interleukin-2 and soluble interleukin-2 receptor with clinical activity of multiple sclerosis. J Neurol Neurosurg Psychiatry. 1993;56:169–174. doi: 10.1136/jnnp.56.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman L. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell. 1996;85:299–302. doi: 10.1016/s0092-8674(00)81107-1. [DOI] [PubMed] [Google Scholar]
- Stinissen P, Raus J, Zhang J. Autoimmune pathogenesis of multiple sclerosis: role of autoreactive T lymphocytes and new immunotherapeutic strategies. Crit Rev Immunol. 1997;17:33–75. doi: 10.1615/critrevimmunol.v17.i1.20. [DOI] [PubMed] [Google Scholar]
- Storch MK, Piddlesden S, Haltia M, Iivanainen M, Morgan P, Lassmann H. Multiple sclerosis: in situ evidence for antibody- and complement-mediated demyelination. Ann Neurol. 1998;43:465–471. doi: 10.1002/ana.410430409. [DOI] [PubMed] [Google Scholar]
- Strunk T, Bubel S, Mascher B, Schlenke P, Kirchner H, Wandinger KP. Increased numbers of CCR5+ interferon-gamma- and tumor necrosis factor-alpha-secreting T lymphocytes in multiple sclerosis patients. Ann Neurol. 2000;47:269–273. [PubMed] [Google Scholar]
- Vandevyver C, Mertens N, van den Elsen P, Medaer R, Raus J, Zhang J. Clonal expansion of myelin basic protein-reactive T cells in patients with multiple sclerosis: restricted T cell receptor V gene rearrangements and CDR3 sequence. Eur J Immunol. 1995;25:958–968. doi: 10.1002/eji.1830250416. [DOI] [PubMed] [Google Scholar]
- Vergelli M, Mazzanti B, Traggiai E, Biagioli T, Ballerini C, Parigi A, Konse A, Pellicano G, Massacesi L. Short-term evolution of autoreactive T cell repertoire in multiple sclerosis. J Neurosci Res. 2001;66:517–524. doi: 10.1002/jnr.1243. [DOI] [PubMed] [Google Scholar]
- Weissert R, Kuhle J, De Graaf KL, Wienhold W, Herrmann MM, Muller C, Forsthuber TG, Wiesmuller KH, Melms A. High immunogenicity of intracellular myelin oligodendrocyte glycoprotein epitopes. J Immunol. 2002;169:548–556. doi: 10.4049/jimmunol.169.1.548. [DOI] [PubMed] [Google Scholar]
- Wen T, Bukczynski J, Watts TH. 4-1BB ligand-mediated costimulation of human T cells induces CD4 and CD8 T cell expansion, cytokine production, and the development of cytolytic effector function. J Immunol. 2002;168:4897–4906. doi: 10.4049/jimmunol.168.10.4897. [DOI] [PubMed] [Google Scholar]
- Woodroofe MN, Cuzner ML. Cytokine mRNA expression in inflammatory multiple sclerosis lesions: detection by non-radioactive in situ hybridization. Cytokine. 1993;5:583–588. doi: 10.1016/s1043-4666(05)80008-0. [DOI] [PubMed] [Google Scholar]
- Wucherpfennig KW, Newcombe J, Li H, Keddy C, Cuzner ML, Hafler DA. T cell receptor V alpha-V beta repertoire and cytokine gene expression in active multiple sclerosis lesions. J Exp Med. 1992;175:993–1002. doi: 10.1084/jem.175.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Mizuno R, Nishimura T, Ogawa Y, Yoshikawa H, Fujimura H, Adachi E, Kishimoto T, Yanagihara T, Sakoda S. Cloning and expression of myelin-associated oligodendrocytic basic protein. A novel basic protein constituting the central nervous system myelin. J Biol Chem. 1994;269:31725–31730. [PubMed] [Google Scholar]
- Zhang J, Markovic-Plese S, Lacet B, Raus J, Weiner HL, Hafler DA. Increased frequency of interleukin 2-responsive T cells specific for myelin basic protein and proteolipid protein in peripheral blood and cerebrospinal fluid of patients with multiple sclerosis. J Exp Med. 1994;179:973–984. doi: 10.1084/jem.179.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]