Abstract
Objective
To describe baseline headache characteristics of children and adolescents participating in a multicenter, randomized, double-blinded, placebo-controlled, comparative effectiveness study of amitriptyline, topiramate, and placebo for the prevention of migraine (CHAMP Study).
Study Design
Children and adolescents (age 8 to 17 years old, inclusive) diagnosed with migraine with or without aura, having headaches at least 4 times per month were enrolled from 2012 through 2014. The trial involved a baseline period (minimum of 28 days) during which prospective diaries were completed and demographics and headache features obtained.
Results
A total of 488 children and adolescents (mean age 14.0 ± 2.4 years) agreed to participate in the trial, with 361 randomized and 127 not randomized. Randomized subjects had a 5.5 ± 3.1 year history of headaches, with 15.1 ± 7.1 headache days per month (based upon retrospective report at screening visit). Prospective diaries reported 11.5 ± 6.1 headache days per 28 day baseline. Across this 28 day period, reported headache days per week were stable (about 3 headache days per week). Recording of individual headache features by diary (n=4136 headache days) showed characteristics consistent with migraine (mean duration 10.5 ± 8.1 hours, mean severity 6.0 ± 2.1, 60% throbbing, 55% with activity worsening headaches, 55% with photophobia, 47% with phonophobia).
Conclusions
Baseline data from the CHAMP Study suggested that the randomized sample was representative of the real world population of children and adolescents that present for treatment of migraine (1, 2). Headaches in children and adolescents recorded during a 28 day prospective baseline period in this multi-site comparative effectiveness study did not change over the course of the baseline period, even though a clear diagnosis, recommendation for effective acute treatment, and standardized education about healthy habits occurred prior to the diary collection period.
List of Key Words: randomized clinical trial, pediatrics, headache characterization, headache days, preventive medication
INTRODUCTION
Migraine is one of the 5 most prevalent chronic diseases of childhood, affecting up to 10% of children age 5–15 years (3) and up to 28% of older adolescents age 15–19 years (4). Despite the high prevalence, migraine is often underdiagnosed and undertreated in children and adolescents due to the combination of a lack of recognition and a very limited number of well-controlled treatment studies. Migraine frequency can vary from episodic to chronic, and the attacks are quite often disabling. The negative impact of migraine on overall quality of life is similar to childhood cancer, heart disease, and rheumatic disease (5). Migraine disability can lead to decreased school functioning and missed school days, in addition to limiting participation in organized activities and socialization with friends (6). Migraines can have a significant impact on family members, often requiring caregivers to miss work and to adjust schedules to care for the child.
In current practice, treatment of pediatric migraine is primarily based upon cumulative experience versus evidence based care. Intervention involves a multi-tiered approach including pharmacological – acute and preventive medications, and biobehavioral interventions. Acute treatments for migraine may include over-the-counter nonsteroidal anti-inflammatory drugs (NSAIDs) and migraine-specific treatments (triptans) or a combination. Non-pharmacologic management includes healthy lifestyle (i.e., hydration, diet interventions – not skipping meals, exercise, and sleep maintenance) and biobehavioral interventions (i.e., biofeedback assisted relaxation therapy, cognitive behavioral therapy). When the headaches become frequent or disabling, preventive medication and/or cognitive behavioral therapy may be indicated (7, 8). The medication topiramate was approved by the United States FDA for migraine prevention in adolescents aged 12–17 years in 2013, mid-way through the study. It remains the only drug approved for migraine prevention in youth.
Presently, there are only a very limited number of randomized, double-blind, placebo controlled studies of medications for the prevention of migraine in children and adolescents. The CHAMP Study (a randomized, double-blind, placebo-controlled, comparative effectiveness study of amitriptyline, topiramate, and placebo for the prevention of migraine in children and adolescents) was designed to mirror real world practice with the goal of identifying the best prevention medication for children and adolescents with migraine (9). To date, it has been the largest enrolling study of migraine prevention in youth.
This paper describes the demographics of the CHAMP Study participants, headache characteristics, associated symptoms, study sites, and baseline pattern of headache days as measured by a prospective 28-day diary. The aims were to examine if the characteristics of the randomized sample for the CHAMP Study could be viewed as representative of the types of patients that present to headache practices; determine if the trajectory of headache days over the 28-day baseline assessment was stable; and explore if the CHAMP Study randomized sample was comparable to those subjects that were not randomized.
METHODS
Study design
This study was a phase III, multicenter, randomized, double blinded, placebo controlled comparative effectiveness trial of amitriptyline, topiramate, and placebo in the prevention of childhood and adolescent migraine (CHAMP Study) (9). The trial was intended to represent a real world approach that incorporated acute, preventive, and biobehavioral therapy. The overall study was approved by the Cincinnati Children’s Hospital Medical Center (CCHMC) Institutional Review Board (IRB) with each site using either reliance CCHMC IRB or a site specific IRB.
In order to establish eligibility, subjects completed a baseline assessment that included a physical and neurological exam, structured headache history interview, psychosocial screening, ECG, demographic questionnaire, and urine pregnancy test. After consenting to participate, subjects were instructed about effective acute medication treatment as well as healthy habits (adequate hydration, sleep, exercise, regular meals with healthy eating), then completed a minimum of a 28 day prospective headache calendar and diary. This treatment approach was applied in a uniform fashion across study sites.
Subjects
Between July 2012 and November 2014, 488 children and adolescents (aged 8 to 17 years, inclusive) were enrolled in this trial from sites across the United States. The study sites included both academic centers and private practices, representing pediatric headache medicine specialty, pediatric neurology, and adult headache medicine and neurology groups that treat children and adolescents. The protocol for the study with the inclusion and exclusion criteria have been previously published (9). Of note, at time of screening, subjects were required to meet the International Classification of Headache Disorders, 2nd Edition (ICHD-II)(10) for migraine with aura, migraine without aura, and/or chronic migraine without continuous headaches or medication overuse and a PedMIDAS score of >10 and <140(6).
Measurements/Assessments
Screening Assessments
Using a standardized, structured interview, a complete headache history was obtained and demographic characteristics were assessed. Data from this initial study visit were used to assess for trial eligibility and to provide a description of the study population so representativeness and generalizability could be evaluated.
Prospective Headache Calendar and Diary
Subjects were trained to complete a prospective 28 day headache calendar and recorded a more detailed daily diary for all days in which they experienced a headache. For each day on the calendar, subjects were asked to document if a headache occurred (recording “yes” or “no”). Subjects were instructed on the diagnostic criteria for migraine (ICHD-II) and based on their personal interpretation of these criteria, the subject recorded if this headache was a migraine (“yes” or “no”). Subsequently, when a subject had a headache, an individual diary was completed. The diary collected information on headache duration, severity, location, disability, features and associated symptoms.
After the 28 day screening period was completed, the subject would meet with the study coordinator to review the calendar and diary to ensure completeness. The CHAMP Study database required that the 28 days of calendars immediately prior to the day of randomization be entered in order for the study database to randomize the subject.
A headache day was defined as any day during which a headache occurred within a 24-hour period starting and ending at midnight. This approach is consistent with the Common Data Elements of the National Institute of Neurological Disorders and Stroke (11).
Study Staff Training
Site investigators and study staff received extensive training from the Clinical Coordinating Center (CCC) at Cincinnati Children’s Hospital Medical Center on the demographic questionnaire, structured screening headache history, subject calendar and diary completion, plus review of the completed calendars and diaries. The Data Coordinating Center (DCC), Clinical Trials Statistical and Data Management Center at the University of Iowa, trained study staff on data entry using an internet based data entry system which included pre-defined required fields to ensure data entry completeness and prevent random entries.
Statistical Analysis
Continuous variables were summarized by means and standard deviations. Categorical variables were summarized by percentages. Comparisons of continuous variables were performed using two sample t-tests. Comparisons of categorical variables were performed using chi-square tests. All statistical tests were two-sided and used a significance level of 0.05. No adjustments for multiple comparisons were made. All analyses were performed using SAS® version 9.3 or later.
RESULTS
Study sites
A total of 34 sites participated in the CHAMP Study, with 31 sites enrolling at least one subject. Of the 31 sites, 22 were academic centers and 9 were private practices, with 19 site principal investigators certified in Headache Medicine by the United Council for Neurologic Subspecialties (UCNS).
Demographics
488 subjects met initial screening criteria and agreed to participate in the study. The mean age at initial screening was 14.0 ± 2.4 years. The gender ratio was 2.01:1.00 - female: male. Demographic characteristics were typical of the US population, representing a clinically generalizable population (Table 1).
Table 1.
Demographic Characteristics
| Variable | Consented N = 488 |
Randomized N = 361 |
Non-Randomized N = 127 |
|---|---|---|---|
| Age in years, Mean (SD)* | 14.0 (2.4) | 14.2 (2.4) | 13.3 (2.5) |
| Gender (% female) | 326 (67%) | 247 (68%) | 79 (62%) |
| Race (% white) | |||
| White | 345 (71%) | 253 (70%) | 92 (72%) |
| Black or African American | 83 (17%) | 67 (19%) | 16 (13%) |
| Asian | 6 (1%) | 6 (2%) | 0 (0%) |
| American Indian or Alaska Native | 39 (8%) | 27 (7%) | 12 (9%) |
| Native Hawaiian or other Pacific Islander | 2 (0%) | 1 (0%) | 1 (1%) |
| Not reported/unknown | 13 (3%) | 7 (2%) | 6 (5%) |
| Ethnicity (% non-Hispanic/Latino) | 424 (87%) | 316 (88%) | 108 (85%) |
| Maternal Education | |||
| Less than high school | 19 (4%) | 12 (3%) | 7 (6%) |
| High school diploma | 69 (14%) | 51 (14%) | 18 (14%) |
| Some college or technical school | 157 (32%) | 117 (32%) | 40 (31%) |
| College degree | 170 (35%) | 135 (37%) | 35 (28%) |
| Graduate degree | 41 (8%) | 32 (9%) | 9 (7%) |
| Unknown | 32 (7%) | 14 (4%) | 18 (14%) |
| Paternal Education | |||
| Less than high school | 24 (5%) | 16 (4%) | 8 (6%) |
| High school diploma | 114 (23%) | 85 (24%) | 29 (23%) |
| Some college or technical school | 129 (26%) | 99 (27%) | 30 (24%) |
| College degree | 122 (25%) | 97 (27%) | 25 (20%) |
| Graduate degree | 42 (9%) | 35 (10%) | 7 (6%) |
| Unknown | 57 (12%) | 29 (8%) | 28 (22%) |
| Annual Income* | |||
| < $35,000 | 117 (24%) | 78 (22%) | 39 (31%) |
| $35,000 to $99,999 | 184 (38%) | 147 (41%) | 37 (29%) |
| $100,000 or more | 107 (22%) | 81 (22%) | 26 (20%) |
| Not reported/unknown | 80 (16%) | 55 (15%) | 25 (20%) |
P-value comparing randomized vs. non-randomized < 0.05
Headache characteristics at screening visit for the Randomized Sample
The average age of onset of headaches was 8.7 ± 3.1 years of age. Subjects reported their average history of having headaches for 5.5 ± 3.1 years prior to the screening visit. The mean frequency of headaches was 15.1 ± 7.1 days per month with the subjects reporting having 7.1 ± 4.7 bad headache days per month. The subjects reported an average duration of the headaches of 6.2 ± 7.7 hours, with the shortest headaches lasting 1.9 ± 3.1 hours and the longest headaches were 23.2 ± 25.4 hours.
The subjects reported headache characteristics consistent with their diagnosis of migraine (Table 2). The average severity on a 0–10 point scale was 6.8 ± 1.6 with exclusively bilateral headaches in 38%, unilateral in 12% and a combination of bilateral or unilateral in 51%; the most common being frontal and bitemporal, as expected for this age range. The pain quality was throbbing in 84% with a wide variety of additional descriptive features. Activity worsened the headache in 85%, with 89% of subjects reporting activity reduction due to the headaches.
Table 2.
History of Headache Characteristics at Screening Visit1
| Variable | Consented N = 4762 |
Randomized N = 361 |
Non-Randomized N = 1152 |
|---|---|---|---|
| Age at Onset | 8.5 (3.1) | 8.7 (3.1) | 8.1 (3.2) |
| Years Since Onset mean (SD) | 5.5 (3.0) | 5.5 (3.1) | 5.2 (2.7) |
| Frequency (days per month) | |||
| Headache Days3 | 14.6 (7.1) | 15.1 (7.1) | 13.1 (6.8) |
| Bad Headache Days3 | 6.9 (4.6) | 7.1 (4.7) | 6.1 (3.9) |
| Duration (hours) | |||
| Shortest | 1.9 (2.9) | 1.9 (3.1) | 1.6 (1.8) |
| Longest | 22.9 (25.1) | 23.2 (25.4) | 21.9 (24.0) |
| Average | 6.1 (7.6) | 6.2 (7.7) | 5.8 (7.6) |
| Severity mean (SD) | 6.8 (1.7) | 6.8 (1.6) | 6.6 (1.7) |
| Side of Head Pain Occurs | |||
| Both Sides | 181 (38%) | 136 (38%) | 45 (39%) |
| One Side | 55 (12%) | 42 (12%) | 13 (11%) |
| Sometimes one side, Sometimes both sides | 240 (50%) | 183 (51%) | 57 (50%) |
| Location of Pain | |||
| Both Temples | 251 (53%) | 197 (55%) | 54 (47%) |
| Left Temple | 137 (29%) | 103 (29%) | 34 (30%) |
| Right Temple | 144 (30%) | 108 (30%) | 36 (31%) |
| Front | 279 (59%) | 215 (60%) | 64 (56%) |
| Top | 155 (33%) | 120 (33%) | 35 (30%) |
| Back | 144 (30%) | 104 (29%) | 40 (35%) |
| Around Eyes | 170 (36%) | 135 (37%) | 35 (30%) |
| Behind Eyes | 193 (41%) | 154 (43%) | 39 (34%) |
| All Over | 128 (27%) | 98 (27%) | 30 (26%) |
| Other | 80 (17%) | 60 (17%) | 20 (17%) |
| Pain | |||
| Throbbing | 396 (83%) | 304 (84%) | 92 (80%) |
| Constant3 | 263 (55%) | 210 (58%) | 53 (46%) |
| Pressing/Squeezing | 188 (39%) | 143 (40%) | 45 (39%) |
| Sharp | 232 (49%) | 181 (50%) | 51 (44%) |
| Stabbing | 146 (31%) | 111 (31%) | 35 (30%) |
| Other (includes pinching, burning, dull, other) | 375 (77%) | 284 (79%) | 91 (72%) |
| Symptoms | |||
| Nausea | 331 (70%) | 253 (70%) | 78 (68%) |
| Vomiting | 178 (37%) | 128 (35%) | 50 (43%) |
| Sensitivity to Light | 421 (88%) | 315 (87%) | 106 (92%) |
| Sensitivity to Sound3 | 399 (84%) | 295 (82%) | 104 (90%) |
| Sensitivity to Smells | 151 (32%) | 109 (30%) | 42 (37%) |
| Lightheadedness | 304 (64%) | 238 (66%) | 66 (57%) |
| Spinning Sensation | 175 (37%) | 132 (37%) | 43 (37%) |
| Tearing Eyes | 97 (20%) | 71 (20%) | 26 (23%) |
| Runny Nose | 43 (9%) | 33 (9%) | 10 (9%) |
| Decreased Appetite | 240 (50%) | 186 (52%) | 54 (47%) |
| Stomach Pain | 158 (33%) | 117 (32%) | 41 (36%) |
| Fatigue | 304 (64%) | 237 (66%) | 67 (58%) |
| Ringing in the Ears | 127 (27%) | 94 (26%) | 33 (29%) |
| Changes in Vision | 170 (36%) | 133 (37%) | 37 (32%) |
| Difficulty Thinking/Walking/Using Arms/Talking | 249 (52%) | 187 (52%) | 62 (54%) |
| Confusion | 104 (22%) | 75 (21%) | 29 (25%) |
| Other: | 45 (9%) | 36 (10%) | 9 (8%) |
| No Symptoms | 1 (0%) | 0 (0%) | 1 (1%) |
| Headache Changes Activity Level | 423 (89%) | 321 (89%) | 102 (89%) |
| Activity or Play Made Headache Worse3 | 413 (87%) | 307 (85%) | 106 (93%) |
Reported Through Clinical Interview
Number of non-randomized subjects with incomplete history of migraine at baseline eCRF = 12
P-value comparing randomized vs. non-randomized < 0.05
The most frequent associated symptoms were photophobia (87%), phonophobia (82%) and nausea (70%). Vomiting was reported by 35% of the subjects, while lightheadedness was reported in 66% and fatigue was reported in 66%.
The inclusion criteria for the study required a diagnosis of migraine without aura, migraine with aura and/or chronic migraine. Subjects were allowed to be diagnosed with multiple headache types. At screening, 94.7% reported migraine without aura (n=462), 18.4% reported migraine with aura (n=90) and 34.8% reported chronic migraine (n=170).
Prospective Headache Calendar and Diary Information for the Randomized Sample
Trajectory of Headache Days over the 28-day Baseline Period
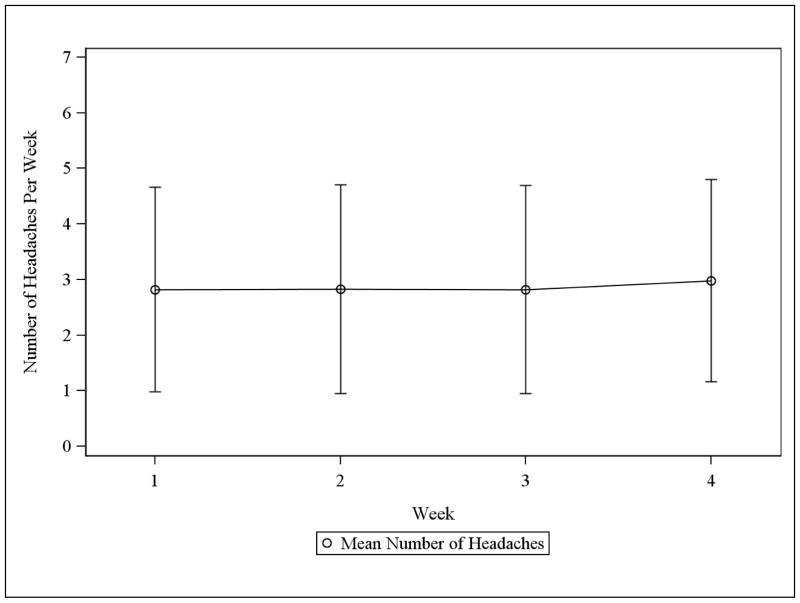
When the trajectory of headache days was examined per week during the 28-day baseline period for the randomized sample, the pattern was stable (See Figure 1). The average number of headache days per week was about 3 (leading to a mean over 28 days of 11.5 headache days). There were 100% complete data for Headache calendars during the study baseline period.
Figure 1.
Mean Number of Headaches Per Week in 28 Day Baseline Period
Headache Characteristics
During the 28 day baseline, 4136 headaches were recorded by the 361 randomized subjects. Table 3 presents information on the headache characteristics and associated features of these 4136 headaches and the percentage of participants that reported at least 1 headache during the 28 day baseline with each characteristic and associated symptom.
Table 3.
Headache Characteristics Based Upon Prospective 28 Day Headache Diary
| Variable | Percent of Total Number of Headaches1 N=41362 |
Percent of Participants Reporting Anytime
During 28 Day Period3 N = 361 |
|---|---|---|
| Duration (hours) | 10.5 (8.1) | |
| Severity (Scale 0–10) | 6.0 (2.1) | |
| Severity Grade | ||
| Mild | 25% | 75% |
| Moderate | 52% | 97% |
| Severe | 23% | 46% |
| Description of Pain | ||
| Throbbing | 60% | 91% |
| Pressing/Squeezing | 49% | 81% |
| Stabbing | 18% | 55% |
| Constant | 54% | 82% |
| Sharp | 25% | 64% |
| Other | 3% | 12% |
| Location of Pain | ||
| One Side of Head | 27% | 65% |
| Both Sides of Head | 71% | 93% |
| Area Pain Located | ||
| Both Temples/Sides | 39% | 71% |
| Left Temple/Side | 15% | 50% |
| Right Temple/Side | 15% | 51% |
| Around Eyes | 23% | 56% |
| Behind Eyes | 20% | 50% |
| Top | 16% | 48% |
| Front | 35% | 70% |
| Back | 12% | 37% |
| All Over | 20% | 43% |
| Other | 3% | 12% |
| Symptoms | ||
| Nausea | 26% | 65% |
| Vomiting | 3% | 18% |
| Sensitivity to Light | 55% | 90% |
| Sensitivity to Sound | 47% | 83% |
| Headache Changed Activity Level | 49% | 96% |
| Headache Worsened by Activity | 55% | 93% |
| Missed School | ||
| Full Day | 4% | 25% |
| Half or Part of the Day | 3% | 21% |
| Attended Full Day of School | ||
| Headache Affected Performance | 20% | 65% |
Percent of all headaches reported by all participants during 28 day baseline.
Total number of headaches includes those with a returned headache record eCRF. Number of incomplete records = 132.
Percent of number of participants that reported at least 1 headache during the 28 day baseline with these characteristics and associated symptoms.
When all of the 4136 headaches were analyzed, the mean duration of a headache during a headache day was 10.5 ± 8.1 hours. The mean severity was 6.0 ± 2.1 on a 0–10 point scale, with 25% of the headaches described as mild, 52% as moderate, and 23% as severe. Nearly two-thirds (60%) of the individual headaches had a throbbing quality. The location of the pain for the individual headache was most likely to be bilateral (71%). The activity level of the subjects was reduced by 49% due to headaches, while activity worsened the headaches in 55%.
When the diary data was examined per subject, the headache characteristics and associated symptoms aligned closely with those expected for migraine. A throbbing quality for at least 1 headache was described by 91% of the subjects, with a bilateral location in 93% and unilateral location in 65%. Bitemporal (71%) and frontal (70%) remained the most common location; while 96% recorded headaches changed their activity level and 93% recorded activity worsened their headaches. Photophobia was the most common associated symptom (90%) with phonophobia in 83% and vomiting recorded by 18% of the subjects.
When the impact on school was assessed, 4% of the headaches resulted in a lost school day, with 3% resulting in missing a partial day of school. This represents 165 lost full days of school and 124 days of partially missed school during the baseline period. From all of the randomized subjects, one-fourth reported missing at least 1 day of school during the 28 day baseline, while nearly one-fourth reported missing a partial day. For those headaches that did occur in school, school performance was reduced during 20% of the headaches with 65% of the subjects having at least 1 headache that reduced school performance.
During the 28 day baseline period, the frequency of headaches for the subjects that were randomized was 11.5 ± 6.1 with the subjects defining 6.4 ± 4.7 as migraine (Table 4). Using the frequency of headaches during this 28 day baseline, 73.7% of the subjects could be classified as episodic migraine, while 26.3% could be classified as chronic migraine. This fraction of subjects with chronic migraine by calendar closely approximates the percentage of subjects diagnosed with chronic migraine at time of screening (34.8%).
Table 4.
Headache Days by Age Based Upon Prospective 28 Day Headache Diary
| Age | Overall (N=361) | ||
|---|---|---|---|
| 8–12 years (N=107) | 13–17 years (N=254) | ||
| Headache Days | 9.7 (5.7) | 12.2 (6.2) | 11.5 (6.1) |
| Episodic (4–14 days) | 88 (82.2%) | 178 (70.1%) | 266 (73.7%) |
| Chronic (≥ 15 days) | 19 (17.8%) | 76 (29.9%) | 95 (26.3%) |
| Migraine Days | 5.6 (4.6) | 6.8 (4.7) | 6.4 (4.7) |
Headache Characteristics by Age Strata
The frequency of headaches was examined by age strata comparing children (age 8–12 years) and adolescents (age 13–17 years). Based on the 28 consecutive calendar days prior to the day of randomization, the mean headache day frequency for 8–12 year olds was 9.7 ± 5.7, and the mean self-identified migraine day frequency was 5.6 ± 4.6, with 82.2% having episodic headaches and 17.8% having chronic headaches. The mean headache day frequency for 13–17 year olds was 12.2 ± 6.2 and the mean self-identified migraine day frequency was 6.8 ± 4.7, with 70.1% having episodic headaches and 29.9% having chronic headaches. The mean headache day frequency was significantly less for 8–12 year olds than for 13–17 year olds (p = 0.004). The percent of 8–12 years olds having episodic headaches was significantly larger than the percent of 13–17 year olds (p = 0.0165).
Randomized Sample compared to Not Randomized Group
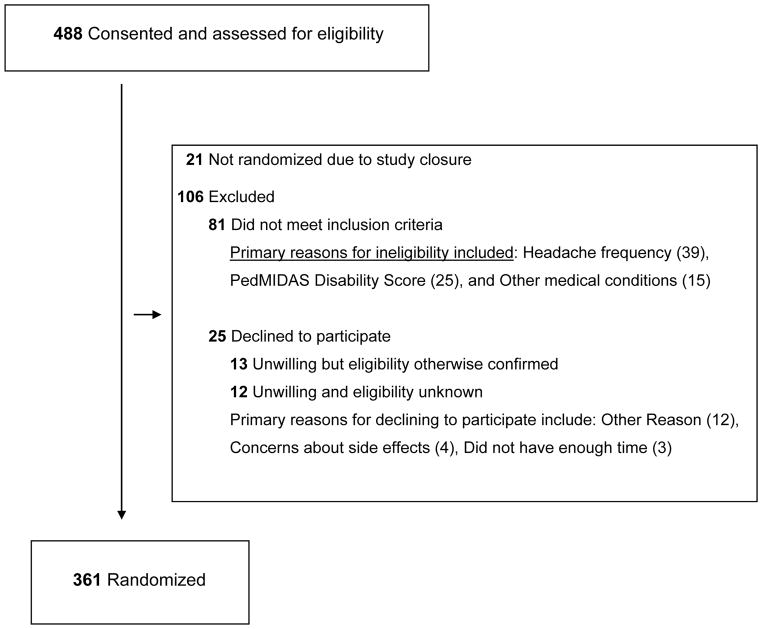
Figure 2 presents the flow of participants from consent to randomization. Of the 127 subjects that enrolled but were not randomized, 81 (16.6% of consented, 63.8% of those not randomized) did not meet inclusion criteria; 25 (5.1% of consented, 19.7% of those not randomized) elected not to continue participation in the study; and 21(4.3% of consented, 16.5% of those not randomized) were excluded due to early study closure. The most common reasons for ineligibility were low headache frequency, low PedMIDAS Score, and other medical conditions. The most common reasons for not completing all the baseline assessments were concerns about side effects and time constraints for diary completion or study visits. Table 1 presents demographic characteristics for the randomized sample and the non-randomized group and Table 2 presents data from the headache history component of the screening visit for both of these groups. In exploratory analyses (52 comparisons in total), the two groups were similar on demographic and headache characteristics. Exceptions included age and income level (demographics), headache days, bad headache days, constant pain, sensitivity to sound, and play makes headaches worse derived from the structured headache history assessment. The application of the inclusion and exclusion criteria may have affected the age, headache frequency, and pain quality variables, with low frequency and low disability making up a majority of those subjects that met exclusion criteria.
Figure 2.
Flow of Participants through Randomization
DISCUSSION
The Childhood and Adolescent Migraine Prevention Study (CHAMP) is a National Institutes of Health/NINDS-funded, multicenter, randomized clinical trial designed to determine if preventive medication for youth with migraine (amitriptyline and/or topiramate) is more efficacious than placebo, and to examine the relative benefit of amitriptyline versus topiramate (9). Fundamental goals of this trial were to enroll a representative and generalizable sample of children and adolescents ages 8 to 17 with migraine, migraine with aura, and/or chronic migraine and to assess efficacy on the basis of clinically meaningful endpoints (i.e., ≥50% reduction in headache days and reduction in migraine disability as measured via a reliable and valid instrument, safety metrics). This report details the baseline characteristics of the randomized sample for the CHAMP Study and presents the pattern of headache days over the course of the 28-day baseline period prior to randomization.
The participants in the CHAMP Study presented with headache frequency, disability, and characteristics that appear to be typical of patients seen in general pediatric and neurology practices for headaches. For example, a prior multicenter study across 7 pediatric headache centers reported on 913 consecutive patients seen for evaluation. The mean age was 11.9 ± 3.4 (range 3–18 years) and 60% were female. Headache days based upon initial interview report were 12.6 ± 10.4 days and PedMIDAS scores were 28.8 ± 41.1. Of the 913 patients, 16% had migraine with aura and 22% had a chronic migraine diagnosis (1). Additionally, the characteristics of the subjects in the CHAMP Study are comparable to the population of adolescents identified in the American Migraine Prevalence and Prevention (AMPP) study. (12–14) The CHAMP Study systematically provides the largest and most detailed dataset of baseline headache characteristics of 8–17 year olds diagnosed with migraine to date, with the potential to discover possible predictors of outcomes in this population. (15) Of note, compared to the few other randomized clinical trials of preventive medication in children and adolescents with migraine, the CHAMP Study included youth with headache days from 4 to 28, with an average of about 3 per week. Most prior trials limited the inclusion criteria to a maximum of 12 per month, with an average of about 1 per week (9, 16). As such, the results of the CHAMP Study have the potential to be much more generalizable to the broad population of youth who seek care for their migraines.
The similarity of those subjects that continued baseline through randomization with those that were not randomized, either due to eligibility or elective withdrawal, further supports overall generalizability and lack of selection bias. Subjects that were excluded during the baseline screening phase demonstrated that the most common reasons for a patient to fail to meet the inclusion and exclusion criteria were largely based on a low headache frequency and/or low disability scores. For subjects who chose not to continue with the study, the reasons were typically based on convenience to participate in the study and not due to headache specific factors or concerns about being involved in a research study. Distance to travel and busy lifestyles were cited often as notable reasons for discontinuing study participation in the screening period. For lower income families, these types of barriers may have a greater impact on participation. These same aspects of life for children, adolescents, and their families have an impact on being able to seek and maintain clinical treatment for their headaches (2). Overall, our findings indicate that youth are willing and able to participate in randomized clinical trials focused on prevention of migraines.
The prospective baseline calendars and corresponding diaries of individual headaches demonstrate the variability of headache features and characteristics in children and adolescents with migraine. This further substantiates that a representative, real world sample of participants was obtained in this trial. One intriguing observation is the stability of headache days over the course of the 28-day headache diary baseline period (Figure 1). Previous observations have shown that children or adolescents once seen for their headaches tend to have an immediate improvement. This is suggestive that the evaluation alone with the reassurance of a diagnosis is adequate for the treatment of pediatric headache. One early study that addressed this question demonstrated that 50% of children will have an improvement in their headaches just from completion of an initial visit with a child neurologist (17). It has been hypothesized that this may be a potential underlying reason for the higher placebo effect seen in children versus adults. In the CHAMP Study, subjects received nearly complete multidisciplinary care at screening; subjects only missed the addition of prevention medication and/or engagement in cognitive behavioral therapy. The multi-tiered approach included a thorough headache medicine evaluation using a semi-structured interview process, diagnosis using ICHD-II, and management with acute medication plan and biobehavioral plan, including adequate hydration without caffeine, exercise, avoidance of skipping meals, healthy eating, and adequate regular sleep patterns. As a component of the study, all CHAMP sites were required to use this same approach in order to minimize site variability. As a component of ensuring data quality, this aim of reducing site variability in execution of the approach was maintained throughout the study, independently of the clinical coordinating center, by the data coordinating center. The consistent odds of having a headache over the baseline period suggest that although these components may be logical and important aspects of a current multidisciplinary treatment plan, they may not be adequate for changing headache frequency. This implies that the patients and families may have an expectation of need for preventive intervention – whether pharmaceutical, nutraceutical, or cognitive behavioral, or a mixture of all.
Conclusion
An analysis of the Childhood and Adolescent Migraine Prevention Study (CHAMP Study) screening, baseline eligibility and headache diary assessments demonstrated that children and adolescents are willing to participate in a randomized clinical trial and able to sustain and maintain a headache calendar and diary over a 28-day baseline period. The randomized sample for this trial included a study population that appears to be quite representative of children and adolescents who present for clinical care across the United States. Headache features may vary from headache to headache, but overall, the presence of migraine characteristics and associated symptoms are maintained throughout a 28 day baseline when a prospective diary is utilized. Headache days were stable over the course of the baseline assessment period and were not altered through confirmation of diagnosis, acute therapy, or healthy habits during this time period. The results of the trial will likely be generalizable to the population of youth who experience migraines. The stable baseline data set that was observed will allow for a clear determination of change in headache days as a result of preventive medication and placebo interventions.
Acknowledgments
Funding/Support: Funding for the Childhood and Adolescent Migraine Prevention (CHAMP) Study was provided by the National Institute of Neurological Disorders and Stroke and the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (Grant # U01NS076788; PIs: Scott W. Powers, PhD, ABPP, FAHS and Andrew D. Hershey, MD, PhD, FAHS and Grant # U01NS077108; PI: Christopher S. Coffey, PhD), National Center for Advancing Translational Sciences of the National Institute of Health (Grant # UL1RR026314; PIs: James Heubi, MD, Joel Tsevat, MD); and Cincinnati Children’s Research Foundation.
Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke and the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health through grants # U01NS076788 and U01NS077108. We would like to thank the Clinical Coordinating Center, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; the Data Coordinating Center, Clinical Trials Statistical and Data Management Center, University of Iowa, Iowa City, Iowa; members of the American Headache Society Pediatric Adolescent Special interest section; members of the Cincinnati Children’s Office for Clinical and Translational Research; members of the Cincinnati Children’s Headache Center: J. Bush, RN, E. Chaves, PhD, L. Crobsy, PhD, A. Jordan, PhD, M.A. Kabbouche, MD, J. Kacperski, MD, D. Kraus, MSW, S.L. LeCates, MSN, CNP, P. Manning, RN, H.L. O’Brien, MD, I. Parkins, PhD, A. Segers, RN, S. Slater, PhD, P. Vaughn, MS, CNP, J. Weberding, MSN, CNP, S. White, DNP, CNP, S. Williams, PhD; and the National Institute of Neurological Disorders and Stroke Clinical Research Consortium. The members of the Childhood and Adolescent Migraine Prevention (CHAMP) Study Group are as follows: Executive Committee and Steering Group: C. Coffey, PhD, L. Chamberlin, RD, MEd, D. Ecklund, RN, MSN, MBA, A. Hershey, MD, PhD, L. Korbee, BS, L. Porter, PhD, S. Powers, PhD, S. Kashikar-Zuck, PhD. Clinical Coordinating Center, Cincinnati Children’s Hospital Medical Center: J. Allen, MS, L. Chamberlin, RD, MEd, J. Hembree, AOS, A. Hershey, MD, PhD, M. Hoffman, RPh, M. Kabbouche, MD, S. Kashikar-Zuck, PhD, T. Knilans, MD, L. Korbee, BS, J. Kroner, BA, D. LaGory, RPh, S. LeCates, MSN, CNP, S. McMahan, RN, K. Nause, BS, J. Peugh, PhD, S. Powers, PhD, K. Simmons, BS, S. Sullivan, BS, S. White, DNP, FNP. Data Coordinating Center, Clinical Trials Statistical and Data Management Center, University of Iowa: W. Clarke, PhD, C. Coffey, PhD, M. Costigan, RN, BSN, D. Ecklund, RN, MSN, MBA, T. Huff, BS, E. Klingner, MS, K. Lilli, RN, BSN, N. Logsden-Sackett, MS, B. Pearson, BA, R. Peters, BS, H. Riss, BA, J. Yankey, MS. National Institute of Neurological Disease and Stroke, Bethesda, MD: D. Hirtz, MD, L. Porter, PhD, P. Gilbert, ScM. Independent Medical Monitor, Mayo Clinic: D. Dodick, MD. The CHAMP Research Investigators: M. Kabbouche, MD, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH*; J. Aceves, MD, Scott and White Healthcare, Temple, TX*; D. Arun, MD, Saint Louis University, St. Louis, MO*; V. Atluru, MD, Winthrop University Hospital, Mineola, NY*; S. Aurora, MD, Stanford Hospitals and Clinics, Palo Alto, CA*; N. Bennett, MD, Preferred Clinical Research, Pittsburgh, PA*; F. Berenson MD, Atlanta Headache Specialists, Atlanta, GA*; J. Bickel, MD, Children’s Mercy Hospital, Kansas City, MO*; R. Bjork, MD, Colorado Springs Neurological Associates, Colorado Springs, CO*; H. Blume, MD, Seattle Children’s Hospital, Seattle, WA*; J. Cohen, MD, The Headache Institute at Roosevelt Hospital, New York, NY; D. Chrusciel, MD, Oklahoma Health Sciences, Oklahoma City, OK*; M. DiSabella, DO, Children’s National Medical Center, Washington, DC; L. Matthew Frank, MD, Eastern Virginia Medical School, Norfolk, VA*; A. Gelfand, MD, University of California-San Francisco Headache Center, San Francisco, CA*; H. Jacobs, MD, University of Maryland School of Medicine, Baltimore, MD*; S. Kedia, MD, Children’s Hospital Colorado, Aurora, CO*; L. Kerr, MD, Primary Children’s Medical Center, Salt Lake City, UT*; A. LeBel, MD, Boston Children’s Hospital, Waltham, MA*; D. Lebron, MD, LeBonheur Children’s Hospital, Memphis, TN*; S. Linder, MD, Dallas Pediatric Neurology Associates, Dallas, TX*; K. Mack, MD, Mayo Clinic, Rochester, MN; H. Markley, MD, New England Regional Headache Center, Worcester, MA*; J. McVige, MD, Dent Neurological Institute, Amherst, NY*; H. Murali, MD, Marshfield Clinic, Marshfield, WI*; A. Pakalnis, MD, Nationwide Children’s Hospital, Columbus, OH*; E. Pearlman, MD, Children’s Hospital at Memorial University Medical Center, Savannah, GA; K. Ridel, MD, Josephson Wallack Munshower Neurology Research, Indianapolis, IN*; D. Rothner, MD, The Children’s Hospital, The Cleveland Clinic, Cleveland, OH*; J. Lopez, MD, Renown Neuroscience Institute, University of Nevada, Reno School of Medicine, Reno, Nevada*; R. Simmons, MD, Schenectady Neurological Consultants, Schenectady, NY*; M. Sowell, MD, University of Louisville Health Sciences Center, Louisville, KY*; C. Szperka, MD, Children’s Hospital of Philadelphia, Philadelphia, PA; M. Victorio, MD, Akron Children’s Hospital, Akron, OH*; P. Winner, DO, Premiere Research Institute, West Palm Beach, FL*; M. Yonker, MD, Phoenix Children’s Medical Group, Phoenix, AZ*. *denotes sites that enrolled a participant.
Abbreviations Key
- CCC
Clinical Coordinating Center
- CCHMC
Cincinnati Children’s Hospital Medical Center
- CHAMP
Childhood and Adolescent Migraine Prevention Study
- DCC
Data Coordinating Center
- ECG
Electrocardiogram
- FDA
Food and Drug Administration
- ICHD-II
International Classification of Headache Disorders, 2nd Edition
- IRB
Institutional Review Board
- NSAIDS
Non-Steroidal Anti Inflammatory Drugs
- PedMIDAS
Pediatric Migraine Disability Assessment Tool
- UCNS
United Council for Neurological Subspecialties
Footnotes
Conflict of Interest: None
Clinical Trials Registration: The Childhood and Adolescent Migraine Prevention Study (CHAMP); Clinical Trials.gov Identifier: NCT01581281 http://clinicaltrials.gov/ct2/show/NCT01581281
References
- 1.Hershey AD, Powers SW, Nelson TD, Kabbouche MA, Winner P, Yonker M, et al. Obesity in the pediatric headache population: a multicenter study. Headache. 2009;49(2):170–7. doi: 10.1111/j.1526-4610.2008.01232.x. [DOI] [PubMed] [Google Scholar]
- 2.Hershey AD, Powers SW, Winner P, Kabbouche M. Pediatric Headaches in Clinical Practice. West Sussex, UK: John Wiley & Sons, Ltd; 2009. [Google Scholar]
- 3.Abu-Arafeh I, Russell G. Prevalence of headache and migraine in schoolchildren. British Medical Journal. 1994;309:765–9. doi: 10.1136/bmj.309.6957.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Split W, Neuman W. Epidemiology of migraine among students from randomly selected secondary schools in Lodz. Headache. 1999;39:494–501. doi: 10.1046/j.1526-4610.1999.3907494.x. [DOI] [PubMed] [Google Scholar]
- 5.Powers SW, Patton SR, Hommel KA, Hershey AD. Quality of life in childhood migraines: clinical impact and comparison to other chronic illnesses. Pediatrics. 2003;112(1 Pt 1):e1–5. doi: 10.1542/peds.112.1.e1. [DOI] [PubMed] [Google Scholar]
- 6.Hershey AD, Powers SW, Vockell AL, LeCates S, Kabbouche MA, Maynard MK. PedMIDAS: development of a questionnaire to assess disability of migraines in children. Neurology. 2001;57(11):2034–9. doi: 10.1212/wnl.57.11.2034. [DOI] [PubMed] [Google Scholar]
- 7.Powers SW, Kashikar-Zuck SM, Allen JR, LeCates SL, Slater SK, Zafar M, et al. Cognitive behavioral therapy plus amitriptyline for chronic migraine in children and adolescents: a randomized clinical trial. JAMA. 2013;310(24):2622–30. doi: 10.1001/jama.2013.282533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eccleston C, Palermo TM, Williams AC, Lewandowski Holley A, Morley S, Fisher E, et al. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev. 2014;5:CD003968. doi: 10.1002/14651858.CD003968.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hershey AD, Powers SW, Coffey CS, Eklund DD, Chamberlin LA, Korbee LL, et al. Childhood and Adolescent Migraine Prevention (CHAMP) study: a double-blinded, placebo-controlled, comparative effectiveness study of amitriptyline, topiramate, and placebo in the prevention of childhood and adolescent migraine. Headache. 2013;53(5):799–816. doi: 10.1111/head.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders. Cephalagia. 2004;24(Supplement 1):1–160. [Google Scholar]
- 11.NCDE; 2015. https://commondataelements.ninds.nih.gov/Headache.aspx#tab=Data_Standards. [Google Scholar]
- 12.Bigal ME, Arruda MA. Migraine in the pediatric population--evolving concepts. Headache. 2010;50(7):1130–43. doi: 10.1111/j.1526-4610.2010.01717.x. [DOI] [PubMed] [Google Scholar]
- 13.Bigal ME, Lipton RB, Winner P, Reed ML, Diamond S, Stewart WF. Migraine in adolescents: association with socioeconomic status and family history. Neurology. 2007;69(1):16–25. doi: 10.1212/01.wnl.0000265212.90735.64. [DOI] [PubMed] [Google Scholar]
- 14.Bigal ME, Serrano D, Reed M, Lipton RB. Chronic migraine in the population: burden, diagnosis, and satisfaction with treatment. Neurology. 2008;71(8):559–66. doi: 10.1212/01.wnl.0000323925.29520.e7. [DOI] [PubMed] [Google Scholar]
- 15.Stanford EA, Chambers CT, Biesanz JC, Chen E. The frequency, trajectories and predictors of adolescent recurrent pain: A population-based approach. Pain. 2008;138:11–21. doi: 10.1016/j.pain.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 16.Lewis D, Ashwal S, Hershey A, Hirtz D, Yonker M, Silberstein S. Practice parameter: pharmacological treatment of migraine headache in children and adolescents: report of the American Academy of Neurology Quality Standards Subcommittee and the Practice Committee of the Child Neurology Society. Neurology. 2004;63(12):2215–24. doi: 10.1212/01.wnl.0000147332.41993.90. [DOI] [PubMed] [Google Scholar]
- 17.Prensky AL, Sommer D. Diagnosis and treatment of migraine in children. Neurology. 1979;29(4):506–10. doi: 10.1212/wnl.29.4.506. [DOI] [PubMed] [Google Scholar]