Abstract
Oncogenic Ras expression is associated with activation of the DNA damage response (DDR) pathway, as evidenced by elevated DNA damage, primarily DNA double-strand breaks (DSBs), and activation of DNA damage checkpoints, which in primary human cells leads to entry into senescence. DDR activation is viewed as a physiological barrier against uncontrolled proliferation in oncogenic Ras-expressing cells, and arises in response to genotoxic stress due to the production of reactive oxygen species (ROS) that damage DNA, and to hyper-replication stress. Although oncogene-induced senescence (OIS) is considered a tumor suppressor mechanism, the accumulation of DNA damage in senescent cells is thought to cause genomic instability, eventually allowing secondary hits in the genome that promote tumorigenesis. To date, the molecular mechanisms behind DNA repair defects during OIS remain poorly understood. Here, we show that oncogenic Ras expression in human primary cells results in down-regulation of BRCA1 and 53BP1, two key factors in DNA DSBs repair by homologous recombination (HR) and non-homologous end joining (NHEJ), respectively. As a consequence, Ras-induced senescent cells are hindered in their ability to recruit BRCA1 and 53BP1 to DNA damage sites. While BRCA1 is down-regulated at transcripts levels, 53BP1 loss is caused by activation of cathepsin L (CTSL)-mediated degradation of 53BP1 protein. Moreover, we discovered a marked down-regulation of vitamin D receptor (VDR) during OIS, and a role for the vitamin D/VDR axis regulating the levels of these DNA repair factors during OIS. This study reveals a new functional relationship between the oncogene Ras, the vitamin D/VDR axis, and the expression of DNA repair factors, in the context of OIS. The observed deficiencies in DNA repair factors in senescent cells could contribute to the genomic instability that allows senescence bypass and tumorigenesis.
Keywords: Oncogenic Ras, vitamin D, vitamin D receptor, BRCA1, 53BP1, DNA repair
INTRODUCTION
Expression of oncogenic Ras in primary human cells results in a growth-arrest known as oncogene-induced senescence (OIS)8, 9, 50. This is more commonly observed in human pre-neoplasia than in neoplastic lesions, and is considered a tumor suppressor mechanism4, 19, 27. There is strong evidence that establishment of OIS is tightly dependent on persistent activation of the DNA damage response (DDR) pathway due to replication stress1, 3, 18, 39, 40 and/or increased levels of reactive oxygen species (ROS)37, 58. In fact, cells expressing oncogenic Ras but unable to activate DDR do not enter senescence4, 18. Similarly, oncogene expression in non-replicating cells does not trigger DDR. Although these findings stress the importance of DDR activation during OIS, we have limited understanding about the integrity of DNA repair mechanisms in senescent cells.
Recent studies reported that oncogenic Ras inactivates the BRCA1 DNA repair complex by dissociating BRCA1 from chromatin, coinciding with DNA damage accumulation prior to senescence55, 56. Importantly, these cells subjected to DNA damage by ionizing radiation (IR) during replication, bypass senescence and become transformed more efficiently than not irradiated cells. Thus, oncogenic Ras-induced BRCA1 loss of function was proposed to predispose senescent cells to additional hits in the genome that promote senescence bypass and eventually cellular transformation.
The reduced association of BRCA1 to chromatin could explain at least in part the accumulation of DNA damage during hyper-replication, due to inefficient DNA repair by homologous recombination (HR). Previous studies have shown that genomic instability in BRCA1-deficient cells is tightly linked to the function of 53BP1. Recruitment of 53BP1 to DNA double-strand breaks (DSBs) facilitates DNA repair by non-homologous end joining (NHEJ) while inhibiting end-resection at the break, a prerequisite for HR5, 6, 10. Thus, BRCA1 and 53BP1 seem to compete for DNA repair substrate and facilitate either HR or NHEJ, respectively. This competition is important for the maintenance of genome integrity, with loss of either BRCA1 or 53BP1 resulting in genomic instability and cancer susceptibility35, 42. In contrast, loss of 53BP1 alleviates HR defects in BRCA1-deficient cells. While loss of BRCA1 function in mice causes embryonic lethality, depletion of 53BP1 in this context rescues lethality10. Thus, 53BP1 loss is synthetically viable with the loss of BRCA12, 34, 38. Moreover, reduced 53BP1 expression is observed in triple negative breast cancers (TNBC) and BRCA1-mutated tumors, suggesting that 53BP1 loss contributes to the survival of BRCA1-deficient cells5, 29. Whether or not the function of 53BP1 is altered during Ras-induced senescence remains unknown.
Our studies showed a correlation between 53BP1 and BRCA1 levels in a variety of contexts. Human fibroblasts and cancer cells (MCF7) growth-arrested by contact inhibition or serum deprivation exhibit reduced BRCA1 and 53BP1 levels14. Growth-arrested cells down-regulate BRCA1 expression and up-regulate the protease cathepsin L (CTSL), which is responsible for 53BP1 protein degradation. In addition, BRCA1 depletion in MCF7 cells results in genomic instability and growth arrest. However, after approximately two to three weeks in growth arrest, BRCA1-deficient cells activate CTSL-mediated degradation of 53BP1 as a means to overcome the proliferation arrest induced by BRCA1 loss29. Similarly, depletion of lamin A/C proteins results in reduced BRCA1 expression and activation of CTSL-mediated degradation of 53BP1, concomitant with DNA damage accumulation25, 45, 46. Altogether, these studies suggest that BRCA1 and 53BP1 levels are down-regulated in response to a variety of stress conditions that cause growth arrest. Whether the loss of BRCA1 function during Ras-induced senescence55 impacts on 53BP1 levels is unknown.
Our analysis of tissue microarrays from breast cancer patients revealed nuclear accumulation of CTSL primarily in TNBC and BRCA1-mutated tumors29, and an inverse correlation with 53BP1 levels. These data suggest that nuclear CTSL could contribute to 53BP1 loss. Moreover, subsets of TNBC and most BRCA1-deficient tumors analyzed exhibit reduced VDR levels, which correlate with accumulation of nuclear CTSL, and reduced 53BP1 levels29. These results suggest that vitamin D/VDR might regulate CTSL-mediated degradation of 53BP1. In support of this notion, vitamin D treatment inhibits CTSL-mediated degradation of 53BP1 in a variety of contexts, including BRCA1-deficient and lamin A/C-deficient cells25, 29.
Overall, these studies suggest a functional relationship between BRCA1, CTSL-mediated degradation of 53BP1, and the vitamin D/VDR axis. Given that Ras-induced senescence causes BRCA1 dissociation from chromatin55 and that Ras expression up-regulates CTSL13, 17, 28, 57, we determined whether 53BP1 is down-regulated during Ras-induced senescence, and whether vitamin D/VDR regulate this process. Here, we show that expression of oncogenic Ras (H-RasG12V) in primary human fibroblasts results in down-regulation of BRCA1 expression and activation of CTSL-mediated degradation of 53BP1. In addition, we discover a marked down-regulation of VDR during Ras-induced senescence. Importantly, stabilization of VDR via vitamin D treatment restores near normal levels of BRCA1 and 53BP1, and their recruitment to sites of DNA damage. This study demonstrates a functional relationship between the oncogene Ras, the vitamin D/VDR axis, and the expression of DNA repair factors, in the context of OIS.
RESULTS
Ras-induced senescence leads to up-regulation of CTSL, reduced levels of DNA repair factors, and down-regulation of VDR
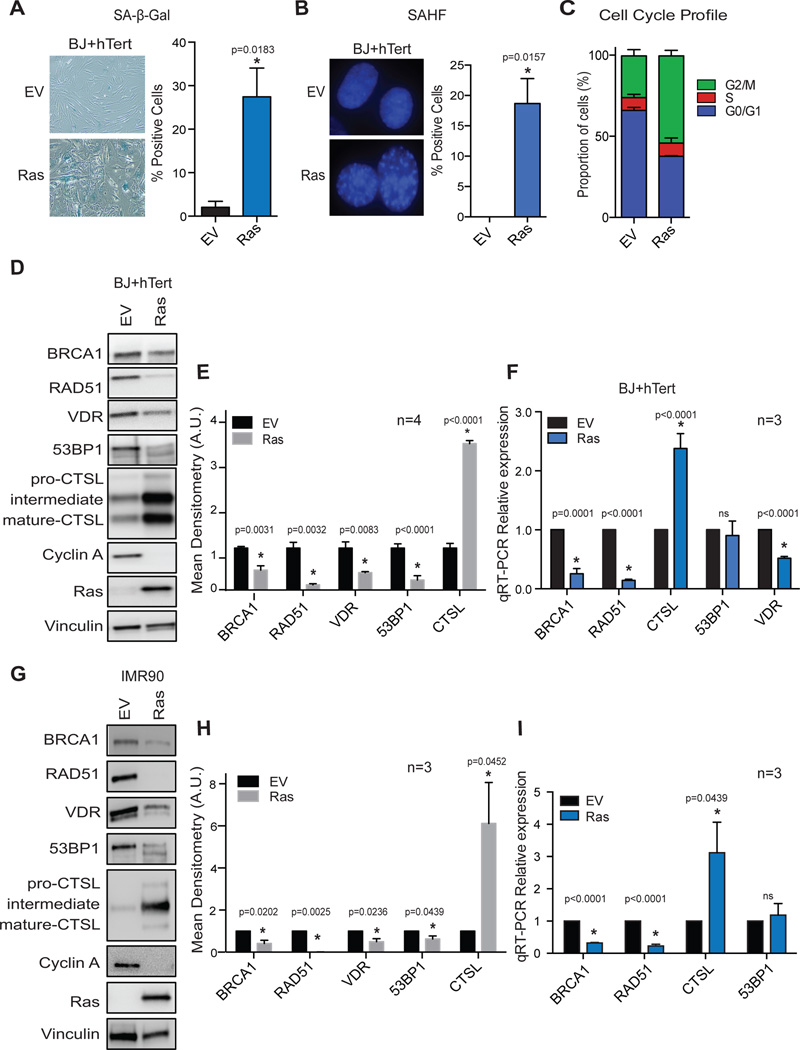
Given our findings that CTSL regulate the levels of 53BP1 in tumor and normal cells14, 25, 29, we determined whether over-expression of oncogenic Ras results in the activation of this regulatory mechanism in human cells. As previously reported, retroviral transduction of the constitutive active form of H-Ras (H-RasG12V) in human primary fibroblasts immortalized with telomerase (BJ+hTert) results in the establishment of senescence8, 9, 50, as shown by the typical flat morphology, positivity for β-galactosidase (SA-β-Gal) activity (Fig 1A), presence of senescence-associated heterochromatic foci (SAHF) (Fig 1B), accumulation of cells in G2 phase of the cell cycle (Fig 1C), and loss of Cyclin A expression (Fig 1D). These senescent cells display a marked up-regulation of CTSL at protein and transcript levels as assessed by immunoblottting (Fig 1D&1E) and qRT-PCR, respectively (Fig 1F). Interestingly, CTSL upregulation correlates with a marked decrease in 53BP1 protein (Fig 1D&1E), but not in transcripts levels (Fig 1F), suggesting that CTSL-mediated degradation of 53BP1 might be activated during OIS.
Figure 1. Deficiencies in DNA repair factors and VDR in Ras-induced senescent cells.
Human foreskin fibroblasts immortalized with telomerase (BJ+hTert) were retrovirally transduced with oncogenic Ras (H-RasG12V) or an empty vector control (EV), and 6 days post-infection cells were monitored for markers of senescence such as positivity for SA-β-Gal activity (A), presence of SAHF (B), and cell-cycle arrest (C). Graphs show average±sem of 3 independent experiments, each from a biological repeat. (D) Immunoblotting analysis comparing the levels of a variety of proteins between control (EV) and Ras-senescent cells (6 days post-infection). The blots are representative of at least 4 biological repeats. (E) Densitometry of immunoblots showing average±sem from 4 biological repeats in BJ+hTert fibroblasts. (F) qRT-PCR of control and Ras-senescent cells to determine if changes in protein levels in (D) are observed at transcripts levels. Graph shows average±sem of 3 independent experiments, each from a biological repeat. (G) Immunoblotting analysis of lung fibroblasts immortalized with telomerase (IMR90+hTert) to compare levels of a variety of proteins between control (EV) and Ras-induced senescent cells. (H) Densitometry of immunoblots showing average±sem from 3 biological repeats in IMR90+hTert fibroblasts. (I) qRT-PCR of control and Ras-senescent IMR90+hTert cells to determine relative expression of the different factors. Graph shows average±sem of 3 independent experiments. * p value of statistical significance (*p≤0.05).
In addition, oncogenic Ras-induced senescent cells exhibit deficiencies in the DNA repair factors BRCA1 and RAD51, at both protein and transcript levels (Figs 1D–1F). RAD51 decrease might be explained by the fact that this factor is regulated during the cell cycle36, 60. In addition, a progressive reduction of RAD51 expression has been reported during replicative senescence41. In contrast, there is no evidence for down-regulation of BRCA1 or 53BP1 levels during OIS. One study showed however that BRCA1 association with chromatin is reduced during Ras-induced senescence, although the global protein levels did not change55. In our hands, we find down-regulation of BRCA1 at transcript and protein levels in senescent cells expressing H-RasG12V (Figs 1D–1F). Importantly, the same upregulation of CTSL and downregulation of DNA repair factors BRCA1, RAD51, and 53BP1 were observed in IMR90 cells transduced with oncogenic Ras (Figs 1G–1I), indicating that this is not a cell type-specific phenotype.
Previous studies showed that Ras activation induces VDR mRNA instability in tumor cells and reduces nuclear VDR activity in mouse fibroblasts48, 54. Thus, we determined whether VDR levels are altered during OIS. We show that VDR is down-regulated at protein and transcripts levels during Ras-induced senescence (Figs 1D–1F).
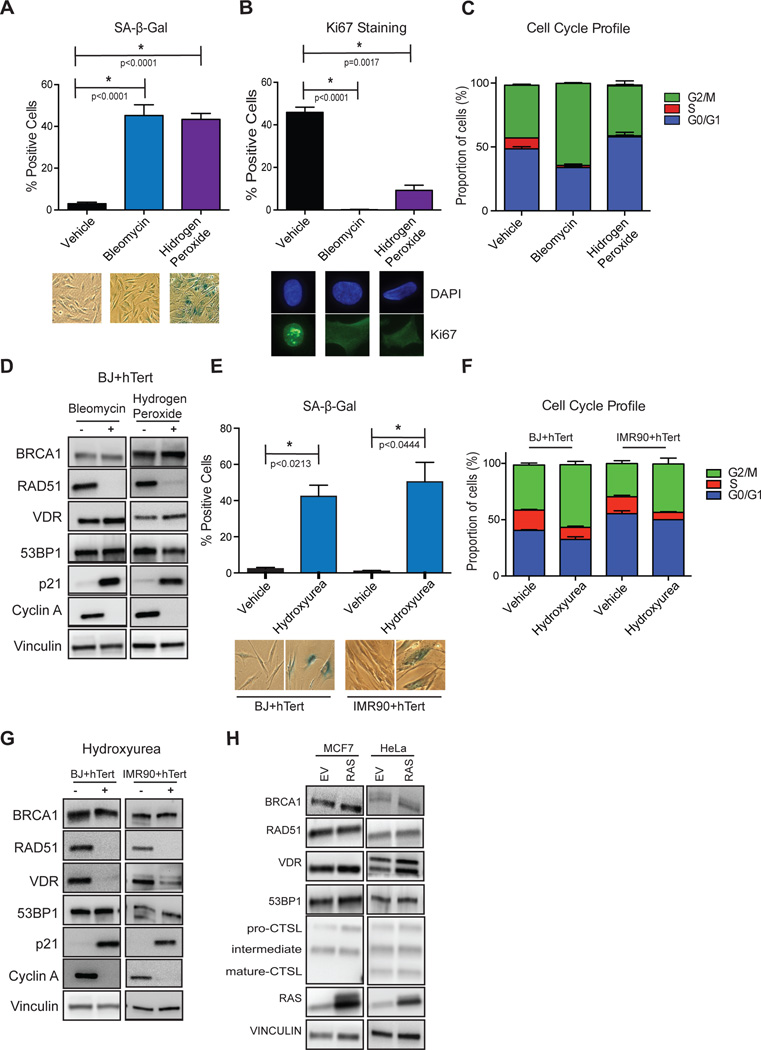
Overall, these studies reveal a marked reduction in the levels of key factors in DNA repair (53BP1 and BRCA1), and in VDR expression, during Ras-induced senescence. To determine if these deficiencies are due to growth arrest per se, we used different strategies to induce senescence. BJ+hTert fibroblasts were subjected to bleomycin or hydrogen peroxide treatments and senescence evaluated by positivity for SA-β-Gal activity (Fig 2A), decreased Ki67 levels (Fig 2B), accumulation of cells in G1/G2 phases (Fig 2C), and changes in Cyclin A and p21 (Fig 2D). Importantly, these strategies to induce senescence do not result in down-regulation of BRCA1, 53BP1, or VDR (Fig 2D), indicating that expression of oncogenic Ras, and not only senescence, is responsible for the deficiencies in these factors during OIS. In addition, we treated human fibroblasts (BJ and IMR90) with hydroxyurea (HU) to induce replication stress-induced senescence (Fig 2E–2G). We observed down-regulation of VDR expression in response to HU, but not of DNA repair factors 53BP1 and BRCA1 (Fig 2G). These data suggest that VDR deficiency might be linked to replication stress during OIS, but not the DNA repair deficiencies, which are only observed upon expression of oncogenic Ras. In contrast, expression of oncogenic Ras in tumor cell lines (MCF7 and HeLa) did not result in growth arrest or downregulation of VDR or DNA repair factors (Fig 2H), suggesting that these alterations might involve tumor suppressor mechanisms, which are lost in tumor cells.
Figure 2. Senescence induced by bleomycin, hydrogen peroxide, or hydroxyurea does not result in deficiencies in DNA repair factors.
BJ+hTert fibroblasts were treated with bleomycin or hydrogen peroxide to induce senescence, and with vehicle control, as described in methods. After the treatments, markers of senescence were monitored, including positivity for SA-β-Gal activity (A) and negativity for Ki67 (B), as well as accumulation of cells in G1/G2 phases of the cell cycle (C). Graphs show average±sem of 5 bleomycin treatments and 3 H2O2 treatments. (D) Cells treated with bleomycin or hydrogen peroxide that entered senescence were collected and processed for immunoblotting to monitor levels of DNA repair factors and VDR. Note how these treatments did not result in deficiencies in VDR, 53BP1, or BRCA1. Blots are representative of at least 3 biological repeats. (E) BJ+hTert and IMR90+hTert fibroblasts were treated with hydroxyurea to induce senescence, and with vehicle control. After the treatment, positivity for SA-β-Gal activity was quantitated. (F) Cell cycle shows accumulation in cells in G1/G2 phases of the cell cycle upon HU treatment. (G) Immunoblots of cells that entered senescence upon HU treatment. Experiments are representative of 2 biological repeats.
CTSL is responsible for 53BP1 loss during Ras-induced senescence
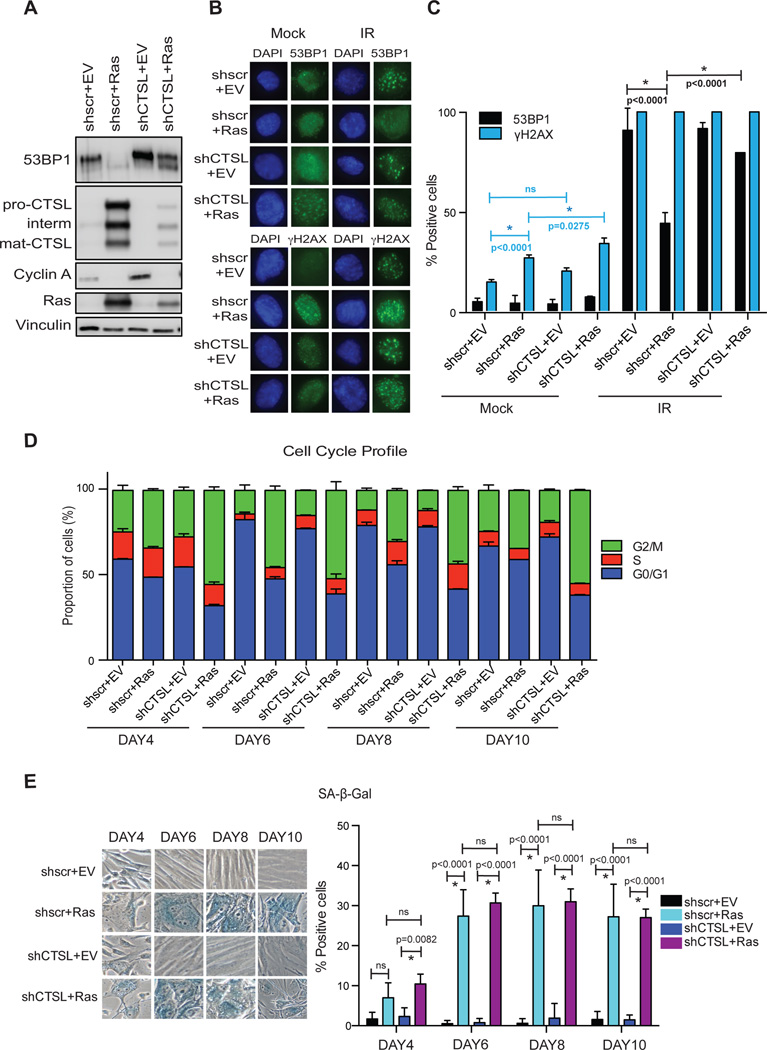
During Ras-induced senescence, we found a correlation between up-regulation of CTSL expression and reduced 53BP1 protein levels, without changes in 53BP1 transcript levels (Figs 1D–1G). To determine if CTSL is responsible for 53BP1 degradation in this context, we depleted CTSL prior to expression of oncogenic Ras in BJ+hTert cells. Depletion of CTSL prior to Ras expression abrogates 53BP1 loss (Fig 3A). Of note, expression of Ras in CTSL-depleted cells results in a slight increase in the levels of the protease, reflecting the dramatic effect that Ras exerts on its expression. In fact, we detected a 53BP1 protein of lower molecular weight in these cells, possibly indicating that some degradation is taking place. These results demonstrate that CTSL is required for the degradation of 53BP1 during Ras-induced senescence.
Figure 3. Depletion of CTSL prevents 53BP1 loss in Ras-senescent cells.
BJ+hTert fibroblasts were lentivirally transduced with shRNA specific for CTSL (shCTSL) or a control shRNA scrambled (shscr). Immediately after puromycin selection, cells were retrovirally transduced with oncogenic Ras (H-RasG12V) or empty vector control (EV). (A) Immunoblotting shows a marked depletion of CTSL, which in turn prevents the decrease in 53BP1 levels upon Ras expression. Immunoblot is representative of 3 biological repeats. (B) The four lines generated were irradiated with 3 Gy and the formation of γH2AX and 53BP1 foci monitored 3 hours post-irradiation. (C) Graph shows average±sem of 2 independent experiments, one from each biological repeat, and relative frequencies calculated. Note how the decreased ability to form 53BP1 foci in Ras-expressing cells is rescued by depletion of CTSL. (D) Cell cycle profile of the four cell lines generated was monitored at different times after transduction with oncogenic Ras or empty vector control. (E) Graph shows average±sem of 2 biological repeats monitoring positivity for SA-β-Gal activity in the four cell lines generated at different times after expression of oncogenic Ras.
Next, we determined if the decrease in 53BP1 has functional consequences, by assessing the ability of Ras-senescent cells to recruit 53BP1 protein to ionizing radiation (IR)-induced DNA damage sites. Irradiation of control and Ras-senescent cells results in proper activation of the DDR pathway, with 100% of cells being positive for γH2AX ionizing radiation-induced foci (IRIF) formation (Figs 3B&3C). Interestingly, while the majority of control cells are positive for 53BP1 IRIF formation, Ras-senescent cells exhibit a decreased ability to form 53BP1 IRIF. This is consistent with the reduction in 53BP1 global levels. Importantly, depletion of CTSL rescues the formation of 53BP1 IRIF in Ras-senescent cells. These data indicate that CTSL-mediated degradation of 53BP1 is activated during Ras-induced senescence, hindering the recruitment of this important DNA repair factor to sites of DNA damage.
To determine if the stabilization of 53BP1 has consequences for OIS, we monitored the entry into senescence of cells depleted of CTSL. As shown in Fig 3D, expression of oncogenic Ras in CTSL-depleted cells results in a more robust G2 arrest, when compared to CTSL-proficient cells expressing Ras, and at all times tested. However, positivity for SA-β-Gal activity was similar in both contexts (Fig 3E). These results suggest that although stabilization of 53BP1 promotes a growth arrest in G2, it does not affect the overall extent of OIS.
Down-regulation of VDR leads to an imbalance between BRCA1 and 53BP1, which is responsible for premature entry into senescence
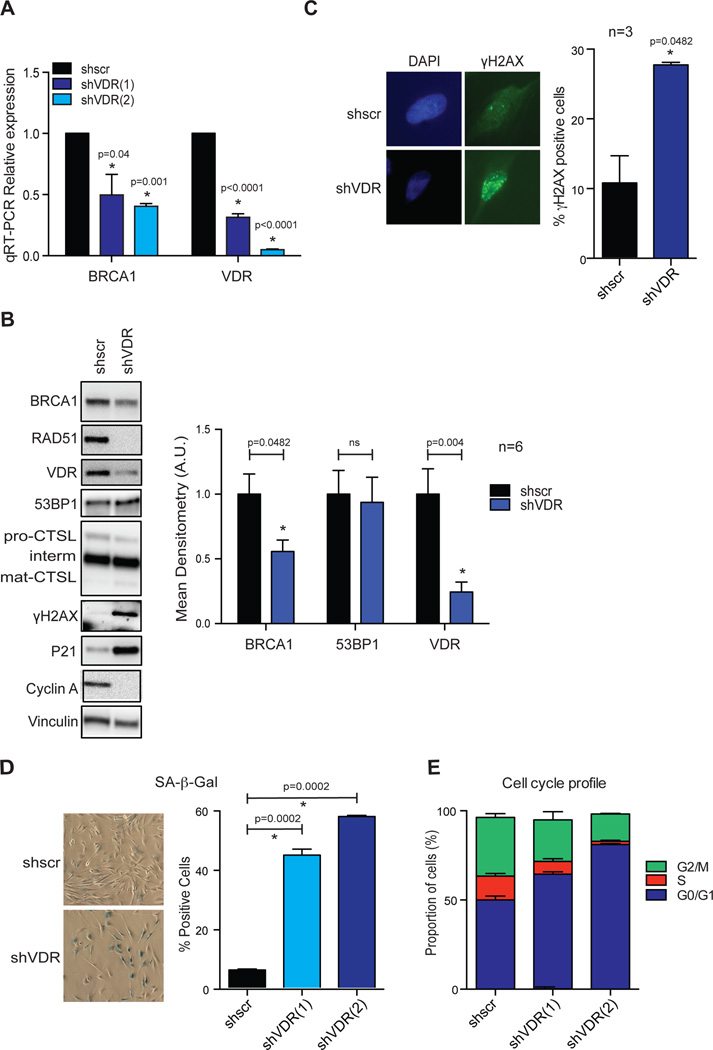
A functional relationship between BRCA1 and the vitamin D/VDR axis has been previously suggested. Studies showed that vitamin D/VDR regulates indirectly the expression of BRCA17, and that BRCA1 interacts with VDR16. Our own studies in normal fibroblasts and fibroblasts derived from patients with Hutchinson Gilford Progeria Syndrome revealed that depletion of VDR results in decreased levels of BRCA1, and accumulation of DNA damage (γH2AX foci) (Kreienkamp et al. unpublished). Given our findings that VDR levels are decreased during Ras-induced senescence, we determined whether VDR deficiency could contribute to DNA repair defects.
As shown in Fig 4A, depletion of VDR in BJ+hTert fibroblasts via lentiviral transduction with two independent shRNAs results in down-regulation of BRCA1 transcripts, concomitant with decreased protein levels (Fig 4B), mirroring Ras-induced senescent cells. In contrast, we did not find alterations in CTSL or 53BP1 protein levels upon VDR depletion (Fig 4B). Importantly, VDR depletion leads to accumulation of DNA damage, as shown by increased global levels of γH2AX by immunoblotting (Fig 4B), and increased percentage of γH2AX-positive cells by immunofluorescence (Fig 4C). Moreover, VDR depletion results in growth arrest and establishment of senescence, as shown by positivity for SA-β-Gal activity (Fig 4D), accumulation of cells in G1 phase (Fig 4E), and high expression of p21 (Fig 4B). The effect of VDR depletion triggering senescence was more robust than oncogenic Ras expression. While growth arrest is established over the course of the first week upon H-RasG12V transduction, VDR loss-induced growth arrest is established within the third day after VDR depletion.
Figure 4. VDR depletion causes BRCA1 deficiency, DNA damage, and senescence.
(A) BJ+hTert fibroblasts were lentivirally transduced with 2 independent shRNAs targeting VDR (shVDR) and a control shRNA scrambled (shscr), and the levels of VDR and BRCA1 transcripts monitored by qRT-PCR after puromycin selection. Note the marked downregulation of BRCA1 upon VDR depletion in 2 independent experiments with each of the hairpins, each from a different biological repeat. (B) Immunoblotting analysis in VDR-depleted and control cells to monitor the levels of DNA repair factors (53BP1 and BRCA1), VDR, cell cycle regulators (p21 and Cyclin A), as well as basal DNA damage (γH2AX). Graph shows densitometry as average±sem of 6 biological repeats. (C) Immunofluorescence with γH2AX was performed in VDR-depleted (shVDR) and control (shscr) cells to monitor basal levels of DNA damage. Cells were counterstained with DAPI to label nuclei. Graph shows quantitation of percentage of cells positive for γH2AX foci formation (≥5 foci). Average±sem of 3 independent experiments is represented, with at least 200 cells counted per condition in each experiment. (D) Graph shows quantitation of percentage of cells positive for SA-β-Gal in VDR-depleted cells, compared to control cells (n=3 for shVDR(1) and n=2 for shVDR(2)). Representative images of SA-β-Gal staining are shown. (E) Cell cycle analysis shows accumulation of cells in G1 phase of the cell cycle after VDR depletion (n=3 for shVDR(1) and n=2 for shVDR(2)).
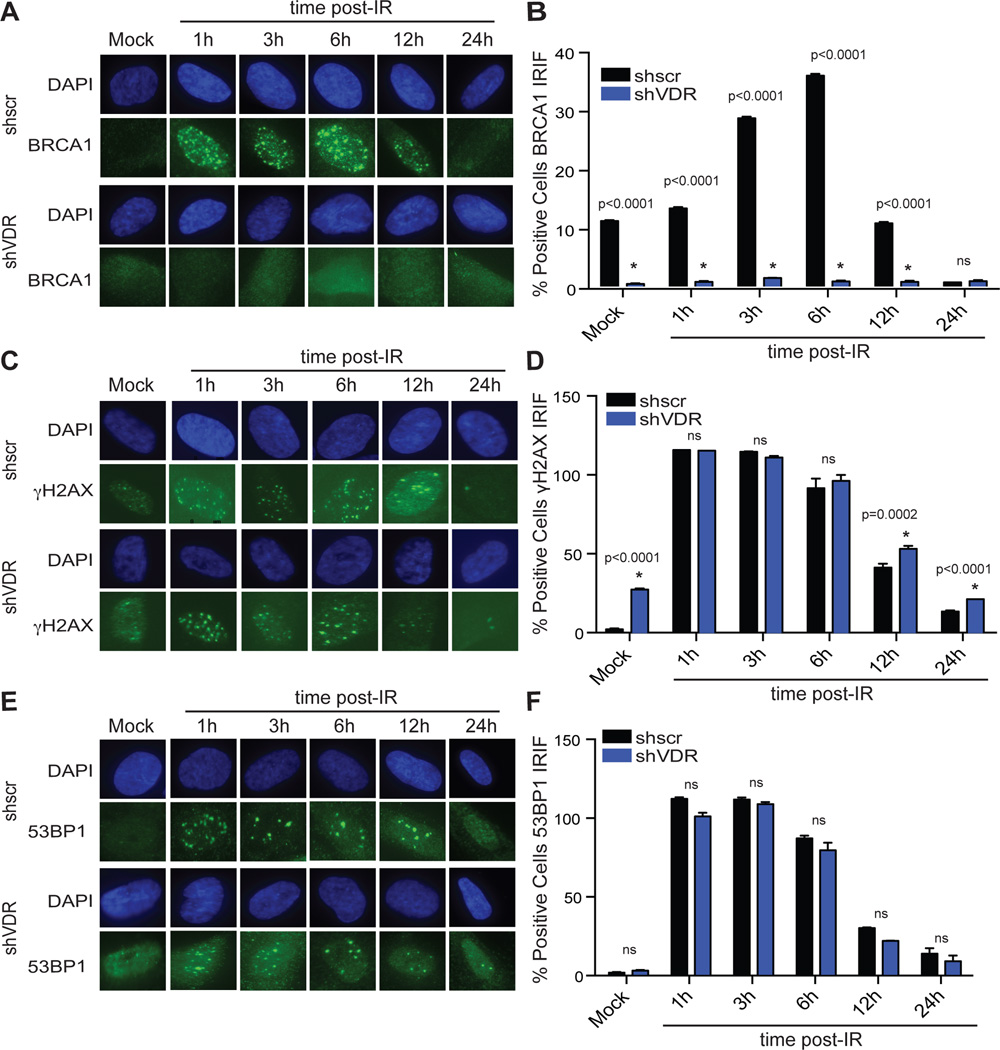
Consistent with the decrease in BRCA1 levels, VDR-depleted cells were hindered in their ability to form BRCA1 IRIF (Figs 5A&5B), suggesting that VDR deficiency could contribute to defects in BRCA1-mediated DNA repair by HR during OIS. VDR-depleted cells were able to activate the DDR in response to IR, as shown by positivity for γH2AX IRIF formation (Figs 5C&5D), and did not show deficiencies in 53BP1 IRIF (Figs 5E&5F).
Figure 5. VDR depleted cells exhibit defects in BRCA1 recruitment to DSBs and HR defects.
VDR-depleted BJ+hTert cells were irradiated with 1 Gy immediately after selection and processed at different times post-irradiation for immunofluorescence with BRCA1 (A), γH2AX (C), or 53BP1 (E). (B) Graph shows quantitation of percentage of cells positive for formation of BRCA1-labeled IRIF. (D) Quantitation of percentage of cells positive for formation of γH2AX IRIF. (F) Quantitation of percentage of cells positive for formation of 53BP1 IRIF. Note how VDR-depleted cells exhibit deficiencies in formation of BRCA1 IRIF, but not in γH2AX or 53BP1 foci formation. All graphs represent two biological repeats with relative frequencies calculated.
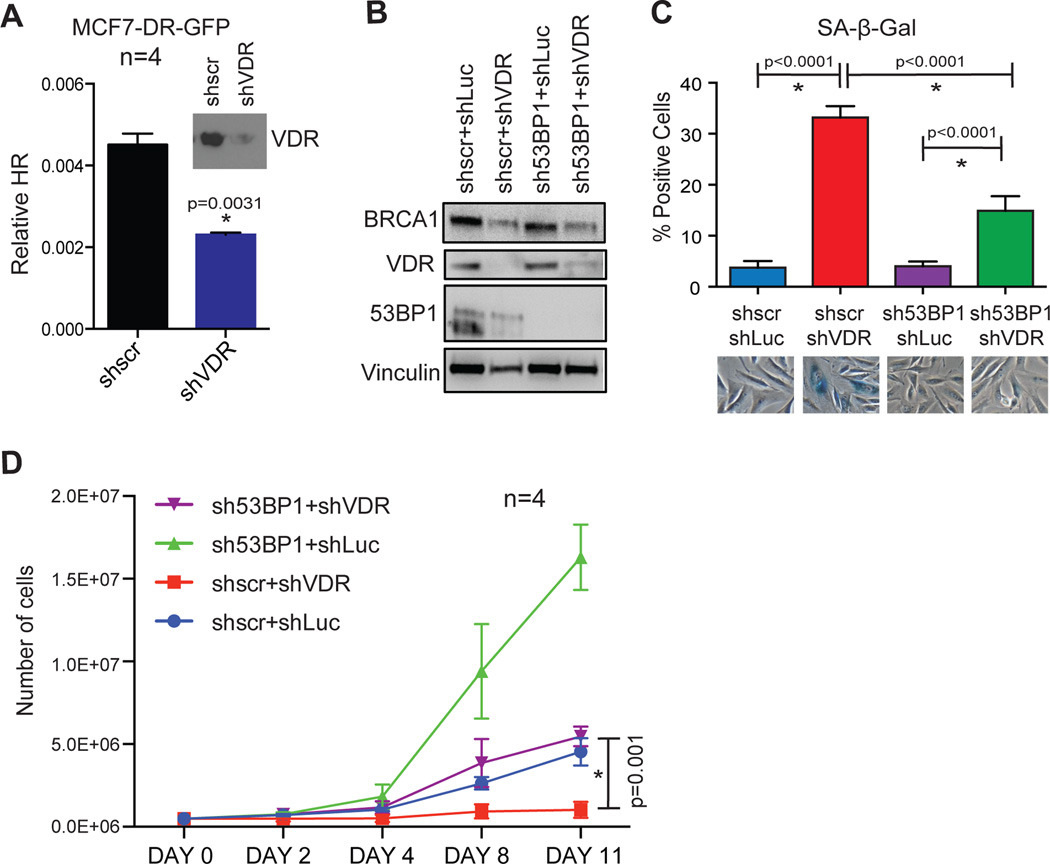
To test directly if VDR loss affects DNA repair by HR, we depleted VDR in MCF7 cells carrying an HR reporter construct. Depletion of VDR results in a marked decrease in GFP-positive cells, indicating that VDR deficiency hinders DNA repair by HR (Fig 6A). Altogether, these data demonstrate that VDR depletion leads to an imbalance between BRCA1 and 53BP1. This scenario is known to induce profound genomic instability and proliferation arrest; phenotypes that are rescued by lowering 53BP1 levels29. Thus, one possible explanation for the most robust growth arrest when compared to Ras-expressing cells is that VDR-depleted cells express normal levels of 53BP1, in a context of BRCA1 deficiency. To test this hypothesis, we depleted 53BP1 prior to depletion of VDR in BJ+hTert cells. As shown in Fig 6B, we achieved a marked depletion of both VDR and 53BP1, and BRCA1 levels were maintained in 53BP1-depleted cells, but not in VDR-depleted cells, as expected. Interestingly, depletion of 53BP1 abrogates the ability of BJ+hTert cells to growth arrest upon depletion of VDR, as shown by decreased positivity for SA-β-Gal activity (Fig 6C), and by proliferation rate (Fig 6D). These data uncover a critical role for 53BP1 in the establishment of senescence due to VDR loss, as previously shown in the context of BRCA1 depletion29.
Figure 6. 53BP1 depletion rescues growth arrest in VDR-depleted cells.
(A) MCF-7 cells carrying an HR reporter construct (DR-GFP) were lentivirally transduced with shRNA targeting VDR or shRNA control (shscr). We monitored the percentage of GFP-positive cells resulting from HR of I-SceI-induced DSBs by FACS in 4 independent experiments. Note how depletion of VDR (immunoblotting) results in decreased HR efficiency. (B) BJ+hTert fibroblasts were depleted of 53BP1 via lentiviral transduction with specific shRNA (sh53BP1) prior to depletion of VDR (shVDR). Hairpins shscr and shLuc were used as control for the depletions. After dual selection, cells were processed for immunoblotting to monitor the levels of BRCA1, VDR, and 53BP1. Vinculin was used as loading control. (C) Percentage of cells positive for SA-β-Gal activity was calculated 6 days after the second lentiviral transduction with shRNAs. Graph shows average±sem of 2 biological repeats. (D) Proliferation of the different cell lines generated was evaluated by counting cells days 2–11 after dual selection, as described in methods. The graph shows average±sem of 4 biological repeats. Note how depletion of 53BP1 prevents growth arrest in VDR-depleted cells (Day 11, *p=0.001).
In summary, our results are consistent with VDR transcription factor playing a role in the maintenance of genomic stability by being involved in the regulation of BRCA1 expression, and with VDR loss contributing to DNA repair defects during Ras-induced senescence. In contrast, CTSL up-regulation and 53BP1 decrease are not observed during VDR loss-induced senescence suggesting that Ras regulates these processes independently of VDR. We also provide evidence for the importance of a balance between 53BP1 and BRCA1 to maintain genome stability and normal proliferation.
Vitamin D treatment rescues the levels of DNA repair factors during oncogenic Ras-induced senescence
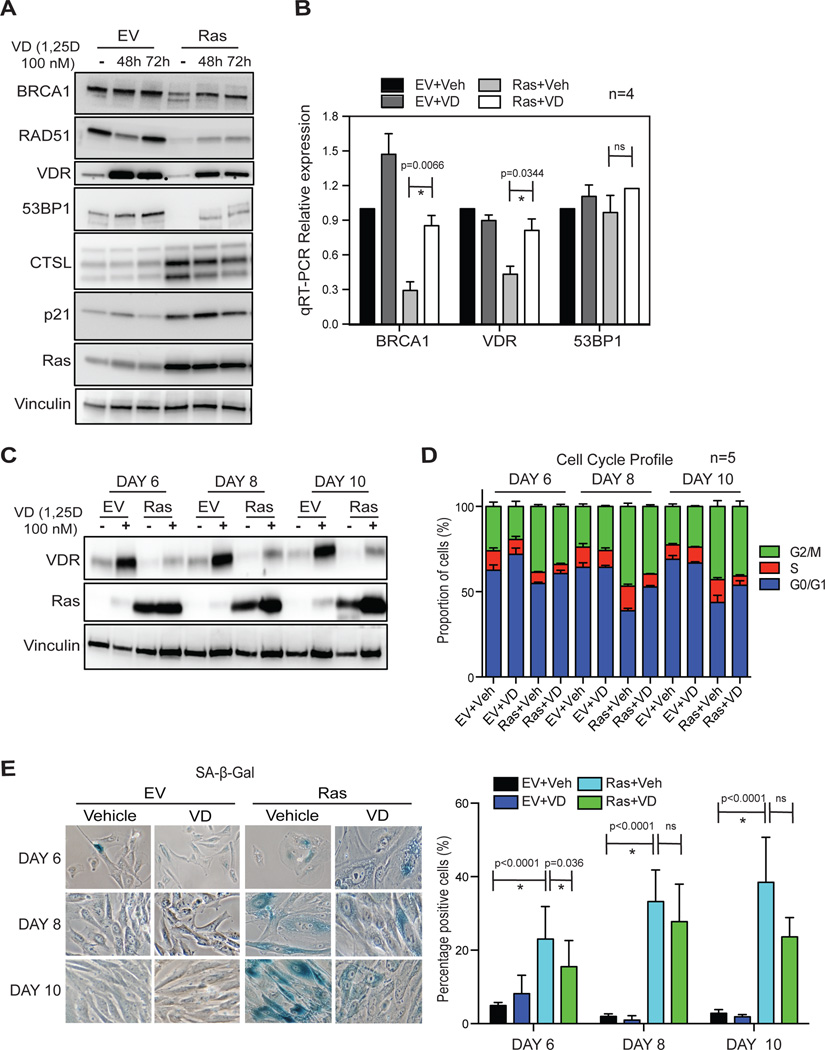
To investigate further the functional relationship between vitamin D/VDR and DNA repair factors during OIS, we monitored the effect of activation of VDR by vitamin D in this context. Vitamin D (1α,25-dihydroxy-vitamin D3) or vehicle were added to BJ+hTert cells on day 4 after transduction with oncogenic Ras, and cells collected 48–72 hours later for immunoblotting and qRT-PCR analyses. At this point, cells exhibit all the characteristics of senescence. As expected, vitamin D treatment increases VDR levels20, demonstrating that the treatment was effective (Fig 7A). Interestingly, vitamin D treatment increases BRCA1 protein (Fig 7A) and transcripts (Fig 7B) levels in senescent cells, when compared to vehicle-treated cells. These data support the notion that VDR down-regulation contributes to BRCA1 reduction during Ras-induced senescence. In addition, vitamin D stabilizes, at least partially, the levels of 53BP1 protein in senescent cells (Fig 7A), without affecting 53BP1 transcripts levels (Fig 7B), which could be explained by the role that vitamin D plays inhibiting CTSL activity25. These results, and data showing that VDR depletion does not activate CTSL-mediated degradation of 53BP1 suggest that VDR deficiency during OIS does not contribute to 53BP1 loss. However, once this mechanism is activated by Ras expression, vitamin D/VDR can inhibit 53BP1 degradation by regulating the levels/activity of CTSL, as previously reported in other contexts25, 29. Overall, our results support the notion that VDR, the main effector of vitamin D’s genomic actions, is involved in the regulation of DNA repair factors such as 53BP1 and BRCA1, which play critical roles in the maintenance of genomic stability. During OIS, the expression of Ras activates CTSL-mediated degradation of 53BP1, and the concomitant decrease in VDR expression could contribute to BRCA1 loss.
Figure 7. Vitamin D (1,25D) treatment rescues the levels VDR and DNA repair factors during Ras-induced senescence.
(A) Oncogenic Ras-expressing cells in the senescence phase were treated with 1,25D (100 nM) or vehicle control for 48 or 72 hours, and collected for analysis by immunoblotting. The levels of DNA repair factors, VDR, CTSL, Ras, and cell cycle regulators were monitored. Note how 1,25D treatment partially rescues the levels of BRCA1 and 53BP1, while stabilizing VDR. The experiment is representative of 4 biological repeats. (B) qRT-PCR shows decreased levels of BRCA1 transcripts in Ras-senescent cells, which are rescued by 1,25D treatment. Levels of 53BP1 transcripts are not altered by 1,25D treatment. Graph shows average±sem of 4 independent experiments. (C) 1,25D treatment of BJ+hTert fibroblasts was initiated concomitant with retroviral transduction with oncogenic Ras or empty vector control. Samples were collected 6, 8, and 10 days after viral transduction and processed for immunoblotting to monitor Ras and VDR expression. Vinculin was the loading control. Note how 1,25D treatment restores normal levels of VDR in Ras-expressing cells. (D) The same experimental protocol as in (C), followed by monitoring of cell cycle profile. Graph shows average of 5 independent experiments. (E) The same experimental protocol as in (C), followed by quantitation of percentage of SA-β-Gal positive cells. Representative images of SA-β-Gal staining are shown from 2 biological repeats.
Next, we tested whether vitamin D supplementation started concomitant with oncogenic Ras expression would have an impact on the onset of senescence (Figs 7C–7E). As shown in Fig 7C, Ras-expressing cells show decreased levels of VDR, which are rescued by 1,25D treatment. We found modest decreases in the percentage of G2-arrested cells (Fig 7D) and of SA-β-Gal positive cells (Fig 7E) at all times tested, however vitamin D treatment was not sufficient to prevent OIS. Thus, we speculate that although activation of vitamin D/VDR signaling does not impact the onset of OIS, it might improve DNA repair capabilities in senescent cells, reducing the occurrence of secondary hits in the genome that promote malignant transformation.
Vitamin D treatment improves DNA repair in Ras-induced senescent cells
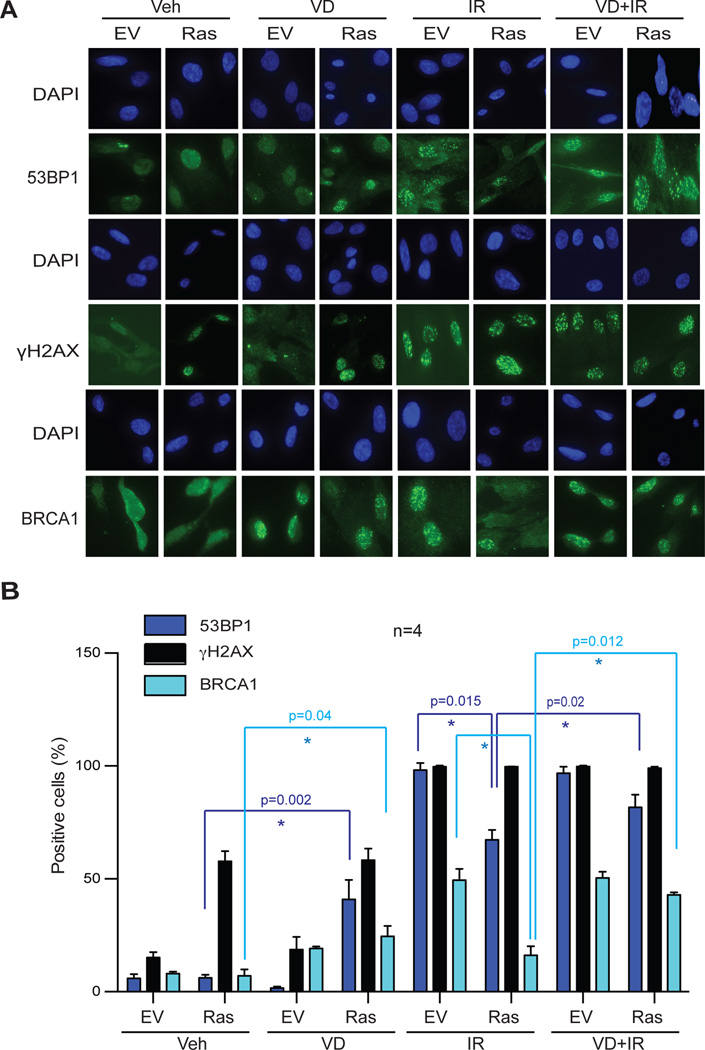
Given the reduced BRCA1 and 53BP1 levels observed in Ras-induced senescent cells, we investigated the ability of these factors to be recruited to sites of DNA damage induced by IR. Senescent cells were irradiated, and the formation of DNA repair foci labeled with γH2AX, BRCA1, or 53BP1 monitored by immunofluorescence. As expected, Ras-senescent cells accumulate basal levels of DNA damage (58% γH2AX-positive), when compared to control cells (15% γH2AX-positive) (Fig 8A&8B). In contrast, Ras-senescent cells are hindered in their ability to recruit 53BP1 to sites of basal DNA damage (6% 53BP1-positive). This is consistent with the global reduction in 53BP1 levels, which could hinder DNA repair by NHEJ. Importantly, while 100% of Ras-senescent cells are positive for γH2AX IRIF formation, and thus able to activate DDR, only 67% show positivity for 53BP1 IRIF, supporting the idea that these cells exhibit DNA repair deficiencies. Significantly, vitamin D treatment of Ras-senescent cells increases 53BP1 foci formation both under basal conditions and in response to IR. Similarly, we found a low percentage of BRCA1-positive cells in irradiated Ras-senescent cells, indicating defects in BRCA1 recruitment to sites of DNA damage, which is indicative of HR defects. Importantly, vitamin D treatment rescues the ability of Ras-senescent cells to recruit BRCA1 to IRIF (Fig 8A&8B).
Figure 8. Defects in recruitment of DNA repair factors to IRIF in Ras-senescent cells are rescued by 1,25D treatment.
Ras-induced senescent cells and control cells were treated with 1,25D (100 nM) or vehicle control for 48 hours prior to being irradiated with 3 Gy or mock irradiated. Cells were processed for immunofluorescence to detect the formation of γH2AX, BRCA1, or 53BP1 IRIF. (A) Representative images of formation of IRIF labeled with γH2AX and 53BP1 3 hours post-irradiation, and of IRIF labeled with BRCA1 6 hours post-irradiation. (B) Graph shows the quantification of percentage of positive cells for formation of IRIF. Average±sem of 4 independent experiments is shown. Note how the deficiencies in 53BP1 and BRCA1 foci formation in Ras-senescent cells are rescued by 1,25D treatment. * p value of statistical significance (*p≤0.05).
These results suggest that vitamin D, by stabilizing 53BP1 and BRCA1 levels, restores the function of these important DNA repair factors during Ras-induced senescence. To the best of our knowledge, this is the first report showing the down-regulation of DNA repair factors during OIS, and a mechanism through which the DNA repair deficiencies might be, at least in part, rescued.
Discussion
Our study reveals new molecular mechanisms contributing to OIS (Fig 9). In particular, expression of oncogenic Ras results in down-regulation of VDR and DNA repair factors such as BRCA1 and 53BP1, which are essential for the maintenance of genome stability. Consequently, recruitment of these factors to DNA damage sites is impaired in Ras-induced senescent cells. These alterations are not observed in other types of senescence such as that induced by bleomycin or hydrogen peroxide treatments, indicating that they are not solely the result of growth arrest. Importantly, we uncover an unprecedented role for the vitamin D/VDR axis regulating DNA repair factors during OIS. We find that vitamin D treatment stabilizes VDR, BRCA1 and 53BP1 levels, rescuing at least partially the recruitment of these DNA repair factors to sites of damage in senescent cells. Altogether these studies suggest that Ras-induced senescent cells could be hindered in their ability to deal with endogenous and exogenous DNA damaging insults, which could facilitate the acquisition of secondary hits in the genome that allow senescence bypass and cellular transformation. Maintaining the levels of VDR in senescent cells could potentially improve their DNA repair capabilities and ameliorate the genomic instability that drives malignancy.
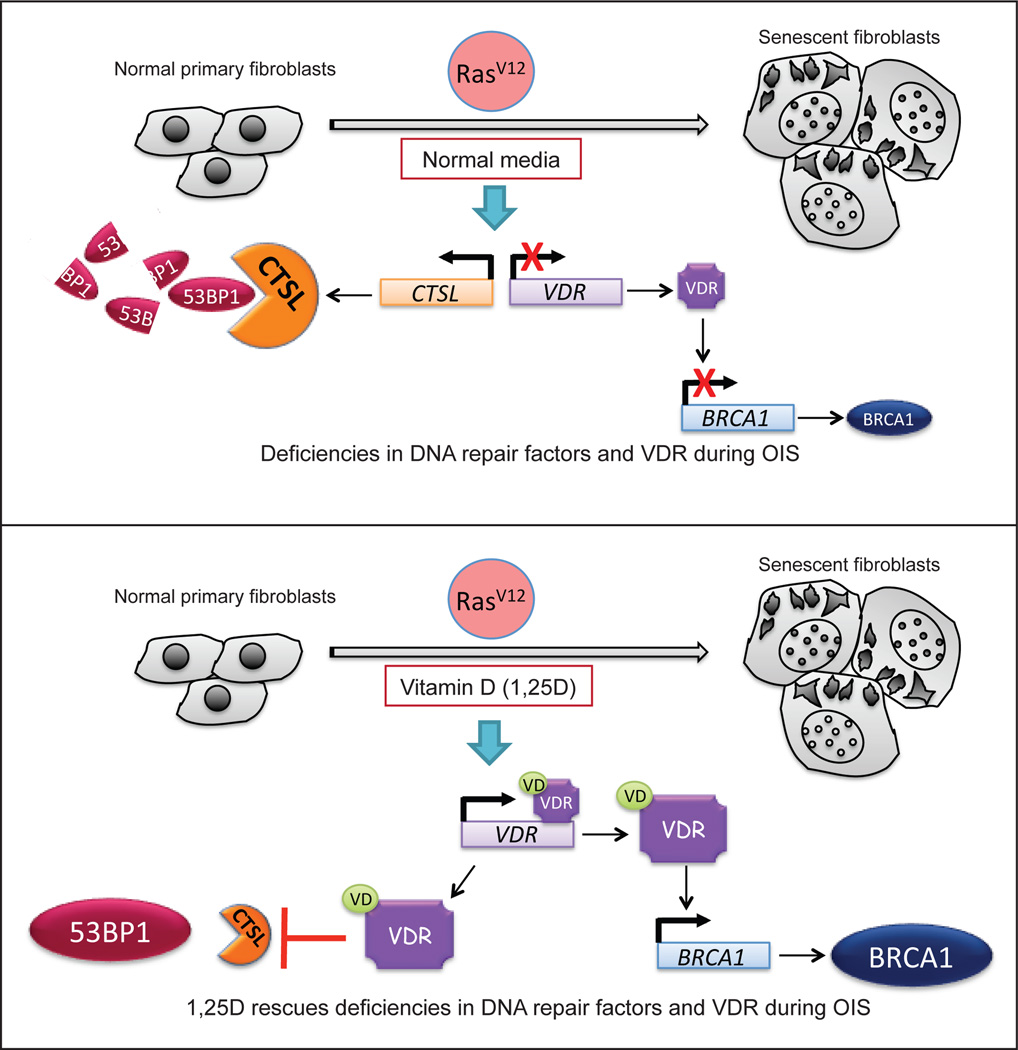
Figure 9. Model of functional relationship between vitamin D/VDR axis and DNA repair factors during Ras-induced senescence.
We propose a model whereby the vitamin D/VDR signaling axis affects DNA repair capabilities by regulating the expression of DNA repair factors during OIS. (A) Expression of oncogenic Ras in human primary fibroblasts results in up-regulation of the cysteine protease CTSL, which in turn is responsible for the degradation of 53BP1 protein during OIS. In addition, oncogenic Ras expression leads to down-regulation of VDR, which in turn contributes to a reduction in BRCA1 expression. VDR deficiency and loss of DNA repair factors during OIS could contribute to the genomic instability that drives senescence bypass and cellular transformation over time. (B) Vitamin D (1,25D) treatment increases VDR expression, as previously reported in other contexts30. Increased vitamin D/VDR signaling results in up-regulation of BRCA1 expression and inhibition of CTSL-mediated degradation of 53BP1, as reported25. As a consequence, vitamin D treatment rescues DNA repair defects in senescent cells. Maintaining vitamin D/VDR levels could ameliorate the occurrence of secondary hits in the genome that promote senescence bypass and malignant transformation.
Mechanistically, we propose that reduced expression of VDR during OIS contributes to the down-regulation of BRCA1, since depletion of VDR results in decreased BRCA1 transcripts and protein levels, defects in BRCA1 recruitment of DNA damage sites, and deficiency in DNA repair by HR. Ligand binding to VDR increases VDR expression, as previously reported in other contexts30. This counteracts VDR deficiency during OIS, which in turn increases BRCA1 expression. However, it is likely that activation of vitamin D/VDR signaling impacts other cellular pathways, with consequences for proliferation, senescence, and potentially for transformation. Future studies are needed to understand in depth the consequences of VDR deficiency in genomic instability during tumorigenesis.
Importantly, we show for the first time that VDR depletion leads to a prompt growth arrest with characteristics of senescence, which is dependent upon the presence of 53BP1. As such, depletion of 53BP1 abrogates growth arrest in VDR-depleted cells. These results support our previous findings that MCF7 cells depleted of BRCA1 undergo growth arrest due to the aberrant up-regulation of 53BP1-mediated NHEJ, which causes chromosomal instability29. In this context, depletion of 53BP1 also prevents the proliferation arrest. Our findings are also consistent with landmark studies showing that 53BP1 loss is synthetically viable with the loss of BRCA1, and that the imbalance between 53BP1 and BRCA1 causes genomic instability and growth arrest5, 6, 10, 34, 38. Our data suggest that 53BP1 plays a major role in the growth arrest imposed by VDR loss and HR deficiency, acting as a tumor suppressor mechanism. Consistent with this notion, reduced expression of 53BP1 has been observed in breast tumors from patients carrying germ-line mutations in BRCA1, and in TNBC, which are often deficient in DNA repair by HR5, 29. Based on all these data, we propose that 53BP1 plays an active role in senescence induced by genomic instability due to HR defects, such as in BRCA1- and VDR-depleted cells, and that loss of 53BP1 allows evasion of this barrier to tumor progression. Importantly, the fact that Ras-induced senescent cells reduce 53BP1 levels during the senescence phase in the context of BRCA1 deficiency, support the idea that these cells could be more prone to transformation, when compared to other senescent cells where BRCA1 and 53BP1 remain highly expressed. In tissues, OIS is thought to arrest tumor growth before cells become malignant12. Thus, senescent cells are typically observed in benign neoplastic lesions, but not in malignant cancers. Interestingly, analysis of several benign and malignant human neoplasms revealed a clear abundance of 53BP1-labeled DNA repair foci in benign lesions, and a marked decrease in 53BP1 positivity in cancerous lesions, including melanomas and invasive breast and colon carcinomas53.
The role of 53BP1 in tumor suppression stresses the importance of identifying molecular mechanisms responsible for its loss in tumor cells and in senescent cells, as shown in this study. These mechanisms could represent novel therapeutic targets. Our previous studies identified one molecular mechanism regulating 53BP1 levels. We found that upregulation of CTSL and its entry into the nucleus is responsible for 53BP1 protein degradation in a variety of contexts, i.e. mouse embryonic fibroblasts deficient in lamin A/C25, MCF7 cells depleted of BRCA129, and primary human fibroblasts and MCF7 cells growth arrested by contact inhibition or serum deprivation14. As such, depletion of CTSL in all these contexts results in stabilization of 53BP1, rescuing its targeting to DNA repair sites. Here, we show that this mechanism of regulation of 53BP1 is also activated during Ras-induced senescence. This finding is highly significant, since up-regulation of CTSL has been observed in a variety of cancers and associated with increased invasiveness, metastasis, and overall degree of malignancy23, 33, 51. Future studies need to determine whether activation of CTSL-mediated degradation of 53BP1 in senescent cells contributes to senescence bypass and transformation. Importantly, we demonstrate the ability to regulate 53BP1 protein levels by vitamin D treatment in senescent cells. Our study reveals a new potential therapeutic strategy to maintain 53BP1 levels in senescent cells as well as in cells already transformed. In particular, maintaining high levels of 53BP1 could prevent proliferation and survival of senescent or transformed cells that exhibit genomic instability due to defects in HR.
The importance of the vitamin D/VDR axis in tumor suppression has been extensively reported. Epidemiological studies showed that vitamin D/VDR deficiency enhances cancer risk, and that vitamin D treatment has anti-proliferative effects in a variety of cancers15, 43. In addition, VDR expression has been associated with improved overall survival in cancer patients, including patients with tumors of poor prognosis such as human glioblastoma multiforme49, and non-small cell lung cancer52. Moreover, a high prevalence of vitamin D deficiency has been reported among African-American women, who are disproportionately affected by TNBC11, 44, 59. The TNBC phenotype is associated with mutations in BRCA1 and defects in HR, thus suggesting that VDR deficiency could be linked to the high incidence of these tumors in African-American women. However, the molecular mechanisms by which deficiencies in the vitamin D/VDR axis contribute to malignancy remain poorly understood. This study and our previous findings25, 26, 29 support a role for vitamin D/VDR regulating DNA repair factors with key roles in the maintenance of genome stability. Here, we demonstrate that VDR depletion results in BRCA1 reduction and HR defects, which in turn results in a prompt growth arrest of primary human cells. Activation of CTSL-mediated degradation of 53BP1 in senescent cells, either due to direct upregulation of CTSL, such as in Ras-induced senescent cells, or via alternative unknown mechanisms, could prime cells for senescence bypass. In the context of tumor cells, VDR and BRCA1 deficiencies would in principle sensitize tumor cells to DNA-damaging strategies. However, we also found a correlation between VDR deficiency and loss of 53BP129, a well-known mechanism of therapy resistance, in BRCA1-related tumors and TNBCs21, 22, 31, 32, 47. Thus, we hypothesize that vitamin D/VDR deficiency promotes malignancy by causing DNA repair defects, while allowing the activation of mechanisms of therapy resistance. Future studies need to determine if vitamin D treatment could represent a new strategy to stabilize 53BP1 in the context of BRCA1 deficiency, restoring the sensitivity of specific tumor cells to DNA-damaging strategies currently used in the clinic for cancer treatment.
Materials and Methods
Cell Culture
Human foreskin (BJs) and lung (IMR90) fibroblasts expressing telomerase (hTERT) were cultured in DMEM supplemented with 10% FBS+1% antibiotics/antimycotics.
Bleomycin treatment
Bleomycin was used to induce senescence of fibroblasts (BJ+hTert). Cells were incubated with 100µg/ml Bleomycin for 24 hours in complete medium. Bleomycin was then removed by extensive washing with PBS, and cells were grown for 72 hours in complete medium, and collected for different assays.
H2O2 treatment
Hydrogen Peroxide was used to induce senescence of fibroblasts (BJ+hTert). Cells were incubated with 500µM H2O2 for 1 hour in complete medium, washed extensively in PBS, and cultured in complete medium for 4 days. Cells were then treated with 200µM H2O2 for 2 hours, washed with PBS and cultured for additional 4–6 days. After two weeks from the first treatment cells were collected and processed for different assays.
Hydroxyurea treatment
Cells were grown in complete medium supplemented with 600µM HU (or vehicle), freshly replaced every two days. After two weeks from the first treatment cells were collected and processed for different assays.
Vitamin D treatment
In all the cases, the active form of Vitamin D, 1α,25-dihydroxyvitamin D3 (Sigma-Aldrich), was administrated to cells. Aliquots of 1nmol 1,25D were re-suspended in 500µl FBS and diluted to 100nM in DMEM supplemented with FBS at a final concentration of 10−7M. Short treatment: Ras-expressing cells at day 4 post-infection were treated with 1,25D for 48 or 72 hours, replacing fresh 1,25D-containing medium daily. Prolonged treatment: 1,25D was administrated to cells concomitantly with the expression of oncogenic Ras, replacing fresh 1,25D-containing medium every two days.
Ionizing radiation
For assessing the ability of Ras-senescent cells to form DNA-repair foci, cells were irradiated with 3Gy and fixed and processed for immunofluorescence 3 or 6 hours post-irradiation to monitor 53BP1 and BRCA1 IRIF foci formation, respectively. As control for DNA damage, γH2AX IRIF were monitored at both time points as well. For the time course experiment of DNA-repair foci formation/dissolution, cells were irradiated with 1Gy and fixed at different time points (1–24 hours) post-irradiation.
Constructs and Viral Transduction
Retroviral and lentiviral transductions were performed as previously described24. Details in supplemental information.
Senescence markers
To monitor senescence, we performed analyses of cell cycle profile, positivity for SA-β-Gal activity, presence of SAHF, and expression of cyclin A and p21. Assays are described in detail in supplemental information.
Proliferation assay
To monitor rate of proliferation, cells were plated in triplicate at 500,000 cells/10-cm plate and counted every two days utilizing Trypan Blue Viability Assay on the Nexcelom Cellometer Vision CBL. To extrapolate proliferation to the respective time periods, we used the equation Nf=Noekt, where Nf is the final number of cells, No is the starting number of cells, k is ln2/DT (doubling time), and t is time in days. For each period we calculated the doubling time and used it to estimate the number of cells (Nf) that would result from initially plating 500,000 (N0) and culturing them for a given period of time.
Immunoblotting, immunofluorescence, and qRT-PCR
These were performed using standard protocols described before14, 29, 46. Details in supplemental information.
HR assays
HR was measured in MCF7-pDR-GFP cells by flow cytometry as described61
Microscopy
Microscopy and photo capture was performed on a Leica DM5000 B microscope using 40× or 63× oil objective lenses with a Leica DFC350FX digital camera and the Leica Application Suite (Version 4.1.0).
Statistical analysis
A standard “two-tailed” student’s t-test was used to calculate statistical significance of the observed differences between two independent samples. When comparisons were made among more than two samples, we used one-way ANOVA. All graphs show average±sem of at least 3 biological repeats. GraphPad Prism 6 was used for the calculations. Differences were considered statistically significant when p<0.05.
Supplementary Material
References
- 1.Aird KM, Zhang G, Li H, Tu Z, Bitler BG, Garipov A, et al. Suppression of nucleotide metabolism underlies the establishment and maintenance of oncogene-induced senescence. Cell Rep. 2013;3:1252–1265. doi: 10.1016/j.celrep.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aly A, Ganesan S. BRCA1, PARP, and 53BP1: conditional synthetic lethality and synthetic viability. J Mol Cell Biol. 2011;3:66–74. doi: 10.1093/jmcb/mjq055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartek J, Bartkova J, Lukas J. DNA damage signalling guards against activated oncogenes and tumour progression. Oncogene. 2007;26:7773–7779. doi: 10.1038/sj.onc.1210881. [DOI] [PubMed] [Google Scholar]
- 4.Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–637. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- 5.Bouwman P, Aly A, Escandell JM, Pieterse M, Bartkova J, van der Gulden H, et al. 53BP1 loss rescues BRCA1 deficiency and is associated with triple-negative and BRCA-mutated breast cancers. Nat Struct Mol Biol. 2010;17:688–695. doi: 10.1038/nsmb.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunting SF, Callen E, Wong N, Chen HT, Polato F, Gunn A, et al. 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. Cell. 2010;141:243–254. doi: 10.1016/j.cell.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell MJ, Gombart AF, Kwok SH, Park S, Koeffler HP. The anti-proliferative effects of 1alpha,25(OH)2D3 on breast and prostate cancer cells are associated with induction of BRCA1 gene expression. Oncogene. 2000;19:5091–5097. doi: 10.1038/sj.onc.1203888. [DOI] [PubMed] [Google Scholar]
- 8.Campisi J. Suppressing cancer: the importance of being senescent. Science. 2005;309:886–887. doi: 10.1126/science.1116801. [DOI] [PubMed] [Google Scholar]
- 9.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Cao L, Xu X, Bunting SF, Liu J, Wang RH, Cao LL, et al. A selective requirement for 53BP1 in the biological response to genomic instability induced by Brca1 deficiency. Mol Cell. 2009;35:534–541. doi: 10.1016/j.molcel.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 12.Collado M, Serrano M. Senescence in tumours: evidence from mice and humans. Nature reviews Cancer. 2010;10:51–57. doi: 10.1038/nrc2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collette J, Ulku AS, Der CJ, Jones A, Erickson AH. Enhanced cathepsin L expression is mediated by different Ras effector pathways in fibroblasts and epithelial cells. Int J Cancer. 2004;112:190–199. doi: 10.1002/ijc.20398. [DOI] [PubMed] [Google Scholar]
- 14.Croke M, Neumann MA, Grotsky DA, Kreienkamp R, Yaddanapudi SC, Gonzalo S. Differences in 53BP1 and BRCA1 regulation between cycling and non-cycling cells. Cell Cycle. 2013;12:3629–3639. doi: 10.4161/cc.26582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nature reviews Cancer. 2007;7:684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 16.Deng C, Ueda E, Chen KE, Bula C, Norman AW, Luben RA, et al. Prolactin blocks nuclear translocation of VDR by regulating its interaction with BRCA1 in osteosarcoma cells. Mol Endocrinol. 2009;23:226–236. doi: 10.1210/me.2008-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denhardt DT, Greenberg AH, Egan SE, Hamilton RT, Wright JA. Cysteine proteinase cathepsin L expression correlates closely with the metastatic potential of H-ras-transformed murine fibroblasts. Oncogene. 1987;2:55–59. [PubMed] [Google Scholar]
- 18.Di Micco R, Fumagalli M, Cicalese A, Piccinin S, Gasparini P, Luise C, et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–642. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
- 19.Di Micco R, Sulli G, Dobreva M, Liontos M, Botrugno OA, Gargiulo G, et al. Interplay between oncogene-induced DNA damage response and heterochromatin in senescence and cancer. Nat Cell Biol. 2011;13:292–302. doi: 10.1038/ncb2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dusso AS, Brown AJ, Slatopolsky E, Vitamin D. Am J Physiol Renal Physiol. 2005;289:F8–F28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- 21.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 22.Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 23.Gocheva V, Joyce JA. Cysteine cathepsins and the cutting edge of cancer invasion. Cell Cycle. 2007;6:60–64. doi: 10.4161/cc.6.1.3669. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Suarez I, Redwood AB, Perkins SM, Vermolen B, Lichtensztejin D, Grotsky DA, et al. Novel roles for A-type lamins in telomere biology and the DNA damage response pathway. The EMBO journal. 2009;28:2414–2427. doi: 10.1038/emboj.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez-Suarez I, Redwood AB, Grotsky DA, Neumann MA, Cheng EH, Stewart CL, et al. A new pathway that regulates 53BP1 stability implicates cathepsin L and vitamin D in DNA repair. The EMBO journal. 2011;30:3383–3396. doi: 10.1038/emboj.2011.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalo S. Novel roles of 1alpha,25(OH)D on DNA repair provide new strategies for breast cancer treatment. The Journal of steroid biochemistry and molecular biology. 2013 doi: 10.1016/j.jsbmb.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorgoulis VG, Vassiliou LV, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–913. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]
- 28.Goulet B, Sansregret L, Leduy L, Bogyo M, Weber E, Chauhan SS, et al. Increased expression and activity of nuclear cathepsin L in cancer cells suggests a novel mechanism of cell transformation. Mol Cancer Res. 2007;5:899–907. doi: 10.1158/1541-7786.MCR-07-0160. [DOI] [PubMed] [Google Scholar]
- 29.Grotsky DA, Gonzalez-Suarez I, Novell A, Neumann MA, Yaddanapudi SC, Croke M, et al. BRCA1 loss activates cathepsin L-mediated degradation of 53BP1 in breast cancer cells. J Cell Biol. 2013;200:187–202. doi: 10.1083/jcb.201204053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haussler MR, Whitfield GK, Kaneko I, Haussler CA, Hsieh D, Hsieh JC, et al. Molecular mechanisms of vitamin D action. Calcif Tissue Int. 2013;92:77–98. doi: 10.1007/s00223-012-9619-0. [DOI] [PubMed] [Google Scholar]
- 31.Helleday T, Bryant HE, Schultz N. Poly(ADP-ribose) polymerase (PARP-1) in homologous recombination and as a target for cancer therapy. Cell Cycle. 2005;4:1176–1178. doi: 10.4161/cc.4.9.2031. [DOI] [PubMed] [Google Scholar]
- 32.Jaspers JE, Kersbergen A, Boon U, Sol W, van Deemter L, Zander SA, et al. Loss of 53BP1 causes PARP inhibitor resistance in Brca1-mutated mouse mammary tumors. Cancer Discov. 2013;3:68–81. doi: 10.1158/2159-8290.CD-12-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jedeszko C, Sloane BF. Cysteine cathepsins in human cancer. Biol Chem. 2004;385:1017–1027. doi: 10.1515/BC.2004.132. [DOI] [PubMed] [Google Scholar]
- 34.Kass EM, Moynahan ME, Jasin M. Loss of 53BP1 is a gain for BRCA1 mutant cells. Cancer cell. 2010;17:423–425. doi: 10.1016/j.ccr.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 35.King MC, Marks JH, Mandell JB. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643–646. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 36.Klein HL. The consequences of Rad51 overexpression for normal and tumor cells. DNA Repair (Amst) 2008;7:686–693. doi: 10.1016/j.dnarep.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee AC, Fenster BE, Ito H, Takeda K, Bae NS, Hirai T, et al. Ras proteins induce senescence by altering the intracellular levels of reactive oxygen species. J Biol Chem. 1999;274:7936–7940. doi: 10.1074/jbc.274.12.7936. [DOI] [PubMed] [Google Scholar]
- 38.Lowndes NF. The interplay between BRCA1 and 53BP1 influences death, aging, senescence and cancer. DNA Repair (Amst) 2010 doi: 10.1016/j.dnarep.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 39.Mallette FA, Gaumont-Leclerc MF, Ferbeyre G. The DNA damage signaling pathway is a critical mediator of oncogene-induced senescence. Genes Dev. 2007;21:43–48. doi: 10.1101/gad.1487307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mannava S, Moparthy KC, Wheeler LJ, Natarajan V, Zucker SN, Fink EE, et al. Depletion of deoxyribonucleotide pools is an endogenous source of DNA damage in cells undergoing oncogene-induced senescence. Am J Pathol. 2013;182:142–151. doi: 10.1016/j.ajpath.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mao Z, Tian X, Van Meter M, Ke Z, Gorbunova V, Seluanov A. Sirtuin 6 (SIRT6) rescues the decline of homologous recombination repair during replicative senescence. Proc Natl Acad Sci U S A. 2012;109:11800–11805. doi: 10.1073/pnas.1200583109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morales JC, Xia Z, Lu T, Aldrich MB, Wang B, Rosales C, et al. Role for the BRCA1 C-terminal repeats (BRCT) protein 53BP1 in maintaining genomic stability. J Biol Chem. 2003;278:14971–14977. doi: 10.1074/jbc.M212484200. [DOI] [PubMed] [Google Scholar]
- 43.Narvaez CJ, Matthews D, LaPorta E, Simmons KM, Beaudin S, Welsh J. The impact of vitamin D in breast cancer: genomics, pathways, metabolism. Front Physiol. 2014;5:213. doi: 10.3389/fphys.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nesby-O'Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76:187–192. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 45.Redwood AB, Gonzalez-Suarez I, Gonzalo S. Regulating the levels of key factors in cell cycle and DNA repair: new pathways revealed by lamins. Cell Cycle. 2011;10:3652–3657. doi: 10.4161/cc.10.21.18201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Redwood AB, Perkins SM, Vanderwaal RP, Feng Z, Biehl KJ, Gonzalez-Suarez I, et al. A dual role for A-type lamins in DNA double-strand break repair. Cell Cycle. 2011;10:2549–2560. doi: 10.4161/cc.10.15.16531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rottenberg S, Jaspers JE, Kersbergen A, van der Burg E, Nygren AO, Zander SA, et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc Natl Acad Sci U S A. 2008;105:17079–17084. doi: 10.1073/pnas.0806092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rozenchan PB, Folgueira MA, Katayama ML, Snitcovsky IM, Brentani MM. Ras activation is associated with vitamin D receptor mRNA instability in HC11 mammary cells. The Journal of steroid biochemistry and molecular biology. 2004;92:89–95. doi: 10.1016/j.jsbmb.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 49.Salomon DG, Fermento ME, Gandini NA, Ferronato MJ, Arevalo J, Blasco J, et al. Vitamin D receptor expression is associated with improved overall survival in human glioblastoma multiforme. Journal of neuro-oncology. 2014;118:49–60. doi: 10.1007/s11060-014-1416-3. [DOI] [PubMed] [Google Scholar]
- 50.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 51.Skrzydlewska E, Sulkowska M, Koda M, Sulkowski S. Proteolytic-antiproteolytic balance and its regulation in carcinogenesis. World J Gastroenterol. 2005;11:1251–1266. doi: 10.3748/wjg.v11.i9.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Srinivasan M, Parwani AV, Hershberger PA, Lenzner DE, Weissfeld JL. Nuclear vitamin D receptor expression is associated with improved survival in non-small cell lung cancer. The Journal of steroid biochemistry and molecular biology. 2011;123:30–36. doi: 10.1016/j.jsbmb.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suram A, Kaplunov J, Patel PL, Ruan H, Cerutti A, Boccardi V, et al. Oncogene-induced telomere dysfunction enforces cellular senescence in human cancer precursor lesions. The EMBO journal. 2012;31:2839–2851. doi: 10.1038/emboj.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taber LM, Adams LS, Teegarden D. Mechanisms of nuclear vitamin D receptor resistance in Harvey-ras-transfected cells. J Nutr Biochem. 2009;20:629–637. doi: 10.1016/j.jnutbio.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tu Z, Aird KM, Bitler BG, Nicodemus JP, Beeharry N, Xia B, et al. Oncogenic RAS regulates BRIP1 expression to induce dissociation of BRCA1 from chromatin, inhibit DNA repair, and promote senescence. Dev Cell. 2011;21:1077–1091. doi: 10.1016/j.devcel.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tu Z, Aird KM, Zhang R. Chromatin remodeling, BRCA1, SAHF and cellular senescence. Cell Cycle. 2013;12:1653–1654. doi: 10.4161/cc.24986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Urbanelli L, Trivelli F, Ercolani L, Sementino E, Magini A, Tancini B, et al. Cathepsin L increased level upon Ras mutants expression: the role of p38 and p44/42 MAPK signaling pathways. Mol Cell Biochem. 2010;343:49–57. doi: 10.1007/s11010-010-0497-3. [DOI] [PubMed] [Google Scholar]
- 58.Weyemi U, Lagente-Chevallier O, Boufraqech M, Prenois F, Courtin F, Caillou B, et al. ROS-generating NADPH oxidase NOX4 is a critical mediator in oncogenic H-Ras-induced DNA damage and subsequent senescence. Oncogene. 2012;31:1117–1129. doi: 10.1038/onc.2011.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yao S, Ambrosone CB. Associations between vitamin D deficiency and risk of aggressive breast cancer in African-American women. The Journal of steroid biochemistry and molecular biology. 2013;136:337–341. doi: 10.1016/j.jsbmb.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 60.Yuan SS, Chang HL, Lee EY. Ionizing radiation-induced Rad51 nuclear focus formation is cell cycle-regulated and defective in both ATM(−/−) and c-Abl(−/−) cells. Mutat Res. 2003;525:85–92. doi: 10.1016/s0027-5107(03)00009-5. [DOI] [PubMed] [Google Scholar]
- 61.Zhang J, Ma Z, Treszezamsky A, Powell SN. MDC1 interacts with Rad51 and facilitates homologous recombination. Nat Struct Mol Biol. 2005;12:902–909. doi: 10.1038/nsmb991. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.