Abstract
Nanoparticle-based magnetic resonance imaging (MRI) contrast agents have received much attention over the past decade. By virtue of a high payload of magnetic moieties, enhanced accumulation at disease sites, and a large surface area for additional modification with targeting ligands, nanoparticle-based contrast agents offer promising new platforms to further enhance the high resolution and sensitivity of MRI for various biomedical applications. T2* superparamagnetic iron oxide nanoparticles (SPIONs) first demonstrated superior improvement on MRI sensitivity. The prevailing SPION attracted growing interest in the development of refined nanoscale versions of MRI contrast agents. Afterwards, T1-based contrast agents were developed, and became the most studied subject in MRI due to the positive contrast they provide that avoids the susceptibility associated with MRI signal reduction. Recently, chemical exchange saturation transfer (CEST) contrast agents have emerged and rapidly gained popularity. The unique aspect of CEST contrast agents is that their contrast can be selectively turned “on” and “off” by radiofrequency (RF) saturation. Their performance can be further enhanced by incorporating a large number of exchangeable protons into well-defined nanostructure. Besides activatable CEST contrast agents, there is growing interest in developing nanoparticle-based activatable MRI contrast agents responsive to stimuli (pH, enzyme, etc.), which improves sensitivity and specificity. In this review, we summarize the recent development of various types of nanoparticle-based MRI contrast agents, and have focused our discussions on the key advantages of introducing nanoparticles in MRI.
Introduction
Magnetic resonance imaging (MRI), developed on the principle of nuclear magnetic resonance (NMR), is one of the most powerful tools extensively used for noninvasive molecular and cellular imaging1. With superb spatial resolution and tissue contrast, MRI provides anatomic images of soft tissues and is considered as one of the most important diagnostic modalities for imaging of the brain2, cartilage, heart, blood vessels and for tumor detection3. Unlike other imaging platforms, such as computerized axial tomography (CAT), positron emission tomography (PET) and single-photon-emission computed tomography (SPECT), MRI techniques do not require the use of radioactive agents and ionizing radiation. Although MRI itself is able to provide detailed images of soft tissues, its intrinsic low sensitivity makes it hard to differentiate normal tissues from lesions. The introduction of supplements, called contrast agents, can enhance the contrast effect at regions of interest by accelerating magnetic relaxation4, 5. These MRI contrast agents can be divided into three groups based on the mechanism by which contrast is generated. T1 agents provide positive contrast by shortening the longitudinal relaxation time of surrounding water molecules, whereas T2 agents shorten the transverse relaxation time of water protons (basic principles will be discussed in Section 1). The third group relies on chemical exchange saturation transfer (CEST) and represents a relatively new approach to enhance MRI contrast. CEST agents exchange their presaturated exchangeable protons with those of bulk water, with the MRI contrast capable of being switched “on” and “off” by irradiation with radiofrequency (RF) pulses.
Among the various contrast agents, nanoparticle-based contrast agents (especially nanoparticles with diameters of 1–100 nm) have become extremely attractive due to their unique features. Firstly, nanoparticles can be loaded with up to hundreds of thousands of imaging moieties per structure, providing superb signal amplification that enables good imaging contrast at a low dose of contrast agent, and reduces the potential for any cytotoxicity associated with the contrast agent6. For example, a 150 nm dendrimer nanocluster possesses a loading capacity of ~300,000 gadolinium ions, giving a r1 relaxivity value of 12.3 mM−1sec−1 per gadolinium ion and 3,600,000 mM−1sec−1 per particle (1.41 T, 40 °C)7, while the r1 relaxivity value of small gadolinium chelates is only 3.5 mM−1sec−1. Secondly, compared to bulk counterparts, nanoparticles possess a relatively large surface area that offers improved reactivity and an ability to be tailored with additional surface moieties to either improve targeting8 or introduce additional functionality (such as therapeutic features9 or fluorescence10). Multimodal MRI contrast agents are even more prevalent since they can reveal several properties at the same site by applying a single contrast agent. Thirdly, nanoparticles tend to accumulate at tumor sites through the enhanced permeability and retention (EPR) effect11, thereby rendering a higher signal-to-noise ratio in tumors12.
Researchers have developed various types of nanoparticle-based MRI contrast agents of T1, T2 and CEST modalities to fulfill different purposes. Among them, T1 nanoparticle-based contrast agents have proven the most popular due to the positive contrast provided. Gadolinium (GdIII) complexes like Gd-DTPA are widely used to detect the breakage of the blood brain barrier (BBB) and characterize changes in vascularity13. However, GdIII chelates have a short circulation time and the relaxivity exhibited is relatively low, requiring a large dose of ion chelates to reach a useful detection level. Moreover, the non-biocompatible gadolinium has the potential to introduce toxicity into cells upon dechelation from its complexes. Alternatively, nanostructures (like dendrimers14, liposomes15, quantum dots16, mesoporous silica17 and carbon nanotubes18) can be employed as carriers for GdIII chelates, which significantly improves relaxivity, and the nanoparticle surface can be facilely modified with additional functional groups to create multimodal contrast agents. Since the number of GdIII chelates in these type of systems is limited by the available anchoring sites on the surface, and the size of ionic nanoparticle-based contrast agents are usually too large to avoid rapid excretion by the reticuloendothelial system (RES)19, smaller-sized inorganic nanoparticles composed of gadolinium oxide (Gd2O3)10, gadolinium fluoride (GdF3)20, gadolinium phosphate (GdPO4)21 or manganese oxide (MnO)22 have become more attractive, and relevant research has been conducted on functionalized inorganic nanoparticles as MRI contrast agents. Unlike T1 contrast agents, the negative contrast provided by T2 or T2* contrast agents is insufficient to fully differentiate pathogenic tissues from normal tissues, and the susceptibility generated distorts surrounding normal tissues. The intrinsic high magnetization and biocompatible features still lead to intensive research on iron oxide nanoparticles (extensively used T2* contrast agents) as MRI contrast agents, although the image contrast has been compromised to some degree. Superparamagnetic iron oxide nanoparticles (SPION) and ultra-small superparamagnetic iron oxide nanoparticles (USPION) are representative commercial products of T2 contrast agents that are widely used as liver and lymph node imaging contrast agents. As both core and shell affect magnetic properties of contrast agents, improvement on each aspect has been studied. As an alternative to always “on” MRI contrast agents, MRI nanoprobes that are responsive to various stimuli (i.e. pH, enzyme, RF saturation, etc.) have become attractive to researchers, since they could provide better sensitivity and specificity. Numerous studies have been conducted and reported on CEST contrast agents, as they are easily controlled to be “on” and “off” by RF saturation. Since the sensitivity is strongly dependent on the number of exchangeable protons, the incorporation of CEST contrast agents into nanoparticles can greatly increase the amount of exchangeable protons.
Herein, we summarize representative nanoparticle-based MRI contrast agents on the basis of the three operating mechanisms: T1, T2, and CEST. Advances in nanotechnology enables researchers to develop nanoparticle-based MRI contrast agents with higher magnetization and the desired surface characteristics to meet the various needs for effective biodistribution. In this review, we will also discuss improvements focusing on magnetic materials and nanocarriers used to load magnetic materials.
1. Basic principles of MRI contrast agents
As illustrated in Figure 1a, upon exposure to an external magnetic field (B0), a greater proportion of protons will prefer to align parallel to the magnetic field (lower energy state), while the remainder aligns antiparallel (higher energy state). Therefore, a net magnetization (Mz) is produced along the direction of magnetic field (z-axis), and protons spin around the z-axis at a precession rate named the Larmor frequency (ω0). During this period, protons precess separately (out of phase). When an RF pulse is applied to the nuclei with the same precession frequency, some protons in the lower energy state are flipped to the higher state by absorbing the RF energy (Figure 1b). Furthermore, the protons become synchronized and precess together (in phase). Consequently, a transverse magnetization (Mxy) perpendicular to the static magnetic field is generated.
Figure 1.

The principles of magnetic resonance imaging. (a) Protons align either parallel (majority) or antiparallel (minority) and precess under external magnetic field B0. (b) Upon the introduction of RF pulses, protons are excited, with relaxation occurring following removal of the RF pulses. Graphical representation of (c) T1 relaxation (eqn (1)) and (d) T2 relaxation (eqn (2)). (Reproduced with permission from 19. Copyright 2009 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim)
After removal of the RF pulse, the protons relax to their equilibrium state via two relaxation pathways: longitudinal relaxation (T1 relaxation) and transverse relaxation (T2 relaxation). In T1 relaxation, antiparallel protons jump back to the parallel state and give up energy to molecules in the surrounding environment (lattice), so that T1 relaxation is known as spin-lattice relaxation. The recovery of Mz is described in equation (1), where the T1 relaxation time is defined as the time taken to recover 63% of the original longitudinal magnetization. The T1 relaxation rate, R1, is given by the reciprocal of T1 (1/T1), which is a function of r1 (T1 relaxivity), an intrinsic property of T1 contrast agents, and contrast agent concentration (see equation (2)). The ideal T1 contrast agent should be able to effectively shorten the T1 relaxation time at low concentration. In terms of T2 relaxation (spin-spin relaxation), protons that are in phase begin to dephase, with the transverse magnetization (Mxy) decaying as a result. This decay process follows equation (3), where T2 refers to the time taken to decay to 37% of the original Mxy value. T2 relaxivity (r2), an intrinsic property of T2* contrast agents, affects the T2 relaxation rate, R2, as described in equation (4). Generally, spins decay faster than T2 due to the magnetic field inhomogeneity generated by T2 contrast agents. After taking this inhomogeneity into consideration, the effective relaxation time (T2*) is given by equation (5), where γ is the gyromagnetic ratio and ΔBi is the difference in the local magnetic field strength due to the inhomogeneity.
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
2. T1 contrast agents
The signal enhancing nature of T1 contrast agents generates a favorably positive contrast effect, making them the most popular contrast agents for clinical use, especially small molecular gadolinium (GdIII) chelates. The utility of GdIII lies in its electron configuration, possessing seven unpaired electrons in f orbitals that give a symmetric S electronic state. This combination bestows a large magnetic moment on the ion and generates a high relaxivity23. Since gadolinium is toxic as free ions, the chelation of multi-dentate ligands GdIII ions can help minimize toxicity, and GdIII complexes are divided into linear chelates (Gd-DTPA, Gd-BOPTA, and MS-325) and macrocyclic chelates (Gd-DOTA, etc.), where macrocyclic GdIII chelates exhibit stronger thermodynamic and kinetic stability than their linear counterparts24. Among them, Gd-DTPA (Magnevist®) and Gd-DOTA (Dotarem®) possess similar r1 relaxivities around 3.5 mM−1sec−1 (measured at 20 MHz and 37 °C), while protein binding contrast agents like Gd-BOPTA (Multihance®) and MS-325 (Ablacar®) have higher relaxivity of 9.2 mM−1sec−1 (measured at 0.7 T and 37 °C), attributed to the slower molecular rotation caused by non-covalent binding to albumin25. Although GdIII chelates exhibit invaluable utility in diagnosing cancer and sclerosis, they experience rapid renal clearance that limits the time window for MRI26. Unlike iron oxide nanoparticles (T2* contrast agents) that can remain at the imaging site for several months, 95% of GdIII chelates are excreted intact within 24 h of administration27. Regarding the intrinsic low sensitivity nature associated with MRI, the relatively small relaxivity of small molecular GdIII chelates need further improvement. Additionally, the nonspecific extravasating behavior of small molecular GdIII chelates in both normal tissue and at pathogenic sites is an obstacle to clinical application. Therefore, new platforms with specific targets should be incorporated to tackle these problems13. Intensive research has been devoted to developing nanoparticle-based T1 contrast agents, focusing on two methods in particular. The first involves either anchoring small molecular GdIII chelates onto the surface of the nanoparticles or loading GdIII chelates into the interior cavity of the nanostructure. The second method is the creation of paramagnetic inorganic nanoparticles composed of Gd2O3, Gd2F3 and GdPO4. Both methods enhance the potential relaxation by increasing the number of GdIII ions per unit volume, with the high payload of imaging moieties increasing the T1 relaxivity and lowering the dose of contrast agent required for proton relaxation. Furthermore, an additional benefit is derived from the preferential accumulation of nanoparticles at tumor sites due to the leaky vasculature of abnormal tissue, and the targeting efficiency can be further improved by modifying the nanoparticle surface with targeting ligands.
Nano-sized carriers can be employed to load GdIII chelates, creating nanoparticle-based T1 contrast agents that not only incorporate advantages of nanoparticles for image diagnosis, but also demonstrate a positively enhanced contrast effect. Commonly used carriers include dendrimers28, liposomes, quantum dots, silica and supramolecular self-assembly29.
2.1 Soft nanoparticles
Dendrimers are highly branched synthetic spherical polymers, which are fabricated as the core in nanostructure-based GdIII contrast agent. Previous research revealed that polyamidoamine (PAMAM) dendrimer-DOTA- GdIII chelates could increase T1 relaxivity up to 45 mM−1sec−1 (for dendrimer generations (G) of 9 or 10), dramatically improving T1 sensitivity compared with small molecular GdIII chelates (r1 ~ 3.5 mM−1sec−1). Although dendrimer nanoclusters (DNCs) enhance r1 relaxivity, the slow and/or incomplete excretion of such large-size molecules hampers their potential for clinical translation as the accumulation of free GdIII ions from dechelation may cause nephrogenic systemic fibrosis (NSF) that impairs kidney function, especially those released from linear GdIII chelates (such as Gd-DTPA). Furthermore, gadolinium chelates can undergo transmetallation with copper and zinc ions in the body, followed by the onset of toxicity from the released GdIII ions30. Hence, it is important for researchers to focus on developing biodegradable DNCs with stable ligands. Recently, Tsourkas and co-workers integrated polydisulfide linkages between individual PAMAM (G-3) dendrimers (figure 2A), enabling DNCs to further degrade through thiol-disulfide exchange reactions into smaller molecules that were readily excreted through renal clearance26. Unlike the traditional method of labeling GdIII onto dendrimers with chelating ligands, Tsourkas and co-workers prepared [Gd-C-DOTA]−1first, and then reacted dendrimers with the pre-metallated ion complexes. By doing so, the potential toxicity stemming from free GdIII ions trapped within dendrimer cavities was reduced. The relaxivities of 59, 91 and 142 nm sized polydisulfide DNCs were 11.7, 18.4 and 22.4 mM−1sec−1 per GdIII ion, respectively, which were higher than PAMAM (G-3)–[Gd-C-DOTA] −1 without polydisulfide bonds (r1 = 10.9 mM−1sec−1)31 and other polydisulfide gadolinium chelates that have previously been reported as blood pool agents (r1 = 4.4–16.3 mM−1sec−1)32–34.
Figure 2.
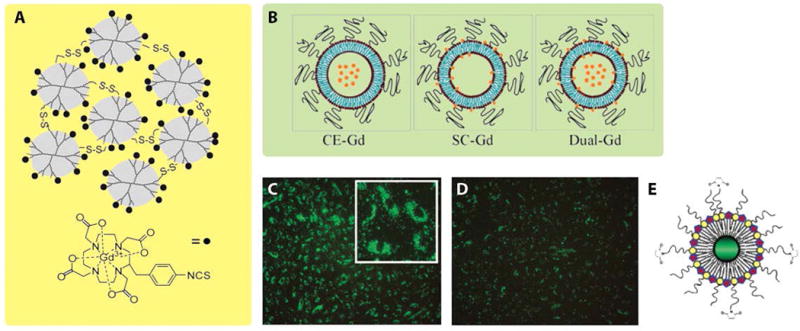
Illustration of Gd-based nanoparticles, including dendrimer nanoclusters, liposomal-Gd agents, and quantum dots. (A) Schematic illustration of PAMAM (G-3)–[Gd-C-DOTA]−1 with disulfide bonds. (B) Schematic core encapsulated GdIII liposomes (CE-Gd), surface conjugated GdIII liposomes (SC-Gd) and dual-Gd liposomes (both surface and core are loaded with GdIII chelates, represented by orange stars). (C) Fluorescence image of human umbilical vein endothelial cells (HUVEC) incubated with green emitting RGD-pQDs, which were internalized and localized in the perinuclear region. (D) Fluorescence image of HUVEC incubated with pQDs. Compared with panel C, panel D displayed much less green fluorescence, which was attributed to non-specific cellular uptake of pQDs. (E) Schematic illustration of quantum dots coated with GdIII chelates and PEG-lipids (pQDs). (Reproduced with permission from 26, 38 and 16. Copyright 1999 Wiley-Liss Inc., 2009 PLoS ONE, and 2006 American Chemical Society)
Liposomes are biocompatible vesicles formed by either natural or synthetic amphiphilic lipids, with examples of commonly used materials being phosphatidyl choline (PC), phosphatidyl glycerol (PG), and cholesterol. Confining the liposomal diameter to between 60–500 nm, and the introduction of additional surface coatings has been shown to prolong circulation time and improve stability in vivo35. Intensive research has been devoted to investigate liposomes as possible carriers of Gd-chelates, and liposomal contrast agents could be synthesized through two approaches: core-encapsulated GdIII (CE-Gd) liposomes or surface-conjugated GdIII (SC-Gd) liposomes36. Ghaghada studied how liposome size and internal gadolinium concentration would affect T1 relaxivity, determining that the T1 relaxivity increased as the size of the liposomal nanoparticles decreased (highest r1 = 1.85 mM−1sec−1 at 2 T magnetic field strength), and T1 relaxivity did not change significantly with core GdIII concentration over the measured concentration range37. Annapragada et al. compared relaxivity among three liposomal formulations (Figure 2B): CE-Gd liposomes, SC-Gd-liposomes and dual-Gd liposomes (where GdIII chelates were encapsulated within the core and conjugated onto the surface)38. Since dual-Gd liposomes possessed the highest number of GdIII ions per liposome, they demonstrated the highest T1 relaxivity on an individual nanoparticle basis, which was more than 2000–8000 fold that of small molecular GdIII chelates. Furthermore, the dual-Gd liposomes demonstrated the highest signal-to-noise and contrast-to-noise ratios in CE-MRA studies.
Very recently, supramolecular MRI contrast agents have quickly drawn researchers’ attention since their first introduction by Stupp and Meade39, where self-assembling peptide amphiphiles (PAs) were conjugated with macrocyclic GdIII chelates to furnish peptide amphiphile contrast agents (PACAs). Nanostructures self-assembled from PAs can emulate extracellular matrices, a biomimetic strategy widely applied in the field of regenerative medicine40. Depending on the design rationale and assembly environment, various one-dimensional morphologies with different physical properties can be obtained upon self-assembly of PAs, which renders PACAs a versatile platform for imaging purposes41. Most importantly, it is well known that the relaxivity of contrast agents will be enhanced by conjugation to proteins and polymers with large molecular weight, or by preparation of micellar structures42. Similarly, the structure of self-assembled PAs allows the increase in rotational correlation time (τr) that subsequently enhances relaxivity. Bull et al. reported the relaxivity difference that arose from the varying morphology (see Figure 3A–B) of two self-assembled PAs, where the relaxivity of nanofibers was 14.7 mM−1sec−1, while the relaxivity of spherical micelles was 22.8 mM−1sec−1 before cross linking (pH = 7.41)43. Later, Bull et al. fabricated three molecules (1, 2, 3) with similar sequences, with PA 1 able to form self-supporting hydrogels, while PACAs 2 and 3 possessed Gd-DOTA positioned at different distances from the hydrophobic alkyl tails (Figure 3C). Both PACAs self-assembled into nanofibers at pH greater than 7.0, though cannot form hydrogels by themselves39. Upon mixing PACA 2 or 3 with filler PA 1, a homogeneous hydrogel was formed that allowed MR images to be obtained. From the MR images of these phantom gels, the mixture of PACA 3 and PA 1 exhibited the greatest contrast, implying that positioning GdIII chelates closer to the hydrophobic end of PAs would result in higher relaxivity. The postulated reason behind this was due to the decreased internal flexibility and increased steric hindrance of GdIII chelates that occurs upon self-assembly. Since the highest contrast was generated with PACA 3, it was further mixed with various epitope-bearing PAs (such as the IKVAV or YIGSR epitopes for neuronal stem cell differentiation, and the RGD epitope for cell adhesion) to form hydrogels (doping with an equal amount of 3). The T1 values of each mixed hydrogel were found to be similar, proving the ability to use PACAs with various PA gels.
Figure 3.
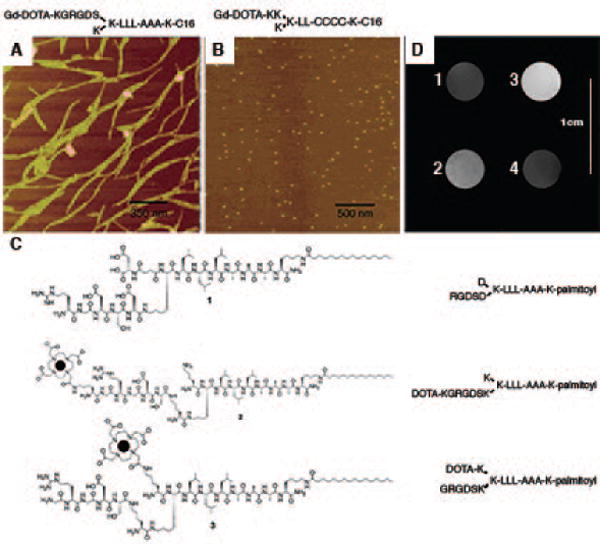
Self-assembled peptide amphiphile nanostructures containing Gd-based magnetic moieties. (A) AFM image of nanofibers. (B) Atomic force microscopy (AFM) image of nanospheres. (C) Molecular design of 1, 2, 3. (D) MR images of phantom gels formed from (1) the control PA of 1, (2) a mixture of 1 and 2, (3) a mixture of 1 and 3, and (4) Gd-DTPA. The mixture of 1 and 3 demonstrated the highest contrast due to the increase in τr of GdIII chelates. (Reproduced with permission from 43 and 39. Copyright 2005 American Chemical Society)
The feasibility of employing supramolecular dual-modality nanoprobes (self-assembly of amphiphilic peptide conjugates containing the fluorophore 5-FAM and Gd-DOTA) as contrast agents for MR and fluorescence imaging was assessed by Cui and co-workers (Figure 4)44. The live-cell fluorescence imaging of two self-assembled nanoprobes (one with single hydrocarbon tail, and the other with two hydrocarbon tails) was studied in KB-3-1 human cervical cells to evaluate cell viability of PACAs. The dual-tailed PACA 2 (50 μM) showed 25-fold higher cellular uptake than PACA 1 (200 μM), which was due to membrane insertion through the two hydrophobic alkyl chains. Before self-assembly, the relaxivity, r1, of PACA 1 and PACA 2 were 4.3 mM−1sec−1 and 4.2 mM−1sec−1 (pH = 7.4, room temperature), respectively, which were similar to the relaxivity of small molecular Gd-DOTA contrast agents (3.5–4.8 mM−1sec−1)23. However, upon self-assembly, the nanospheres formed by PACA 1 and PACA 2 had relaxivities of 7.8 mM−1sec−1 and 14.3 mM−1sec−1 that were higher than the monomeric forms. The higher relaxivity of self-assembled PACA 2 stems from the denser packing of PACA 2 in its self-assembled state, which led to a higher aggregation number of molecules and effective molar mass than self-assembled PACA 1.
Figure 4.
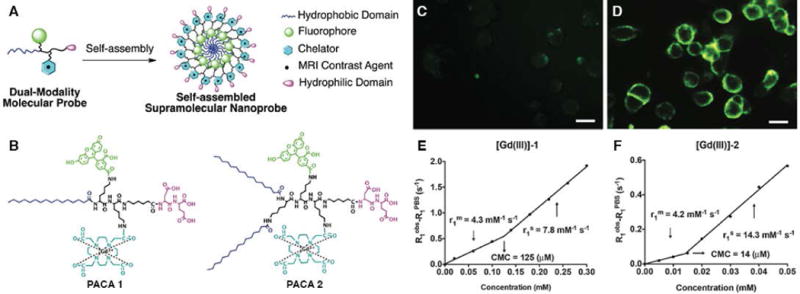
Design and Characterization of dual-modal supramolecular nanoprobes. (A) Rational design of supramolecular dual-modality nanoprobes, composed of a hydrophobic domain to promote self-assembly, a fluorophore for optical imaging, a GdIII chelator for MR contrast, and a hydrophilic headgroup. (B) Chemical structures of PACA 1 and PACA 2. Fluorescence images of KB-3-1 cells after incubation (2h) with (C) PACA1 (200 μM) and (D) PACA2 (50 μM). Scale bars were 20 μm. (E)–(F) The plots of 1/T1 versus concentration for [Gd (III)]-1 and [Gd (III)]-2. Slopes as r1. (Reproduced with permission from 44. Copyright 2015 The Royal Society of Chemistry)
As we described above, self-assembling PAs have the propensity to enhance T1 relaxivity, and one advantage associated with fibrous PA structures is their potential for biodegradation into natural building blocks, so that it is of importance to track the fate of biomaterials after implanted in vivo29. There are, however, limited studies that shed light on the degradation process in vivo. By incorporating MRI modality to image PACAs, we could rationalize using PACAs to enhance MRI contrast, and possibly allow us to track the fate of PACAs in vivo, thus laying a critical foundation for future development as therapies45. Stupp, Meade and co-workers have reported on the in vivo biomaterial localization with Gd(HPN3DO3A) labeled peptide nanofibers (Figure 5). These PAs were designed to have one chelate next to the C-terminal (PA1 and PA2), three chelates at the C-terminal end (PA3), or one chelate relatively far away from the C-terminus (PA4)29. Small-angle X-ray scattering (SAXS) experiments revealed that the bulky Gd (HPN3DO3A) conjugated to the outermost residue of the PAs exhibited β-sheet character and retained high-aspect-ratio structures. The in vivo degradation was evaluated with the mixture (gels) of PA (1 or 3) and filler C16V3A3E3-NH2, of which the PA1 gel produced positive contrast in T1-weighted MRI and PA3 gel produced negative contrast. Subsequent ICP-MS analysis was conducted to measure GdIII retention. The result showed that 62 ± 8% of PA1 and 54 ± 9% of PA3 remained in the mouse leg after 4 days, and the similar T1 relaxation time at day 0 and day 4 indicated the PA concentration did not change significantly, verifying that the approach of using MRI to track the fate of biomaterials is practicable.
Figure 5.
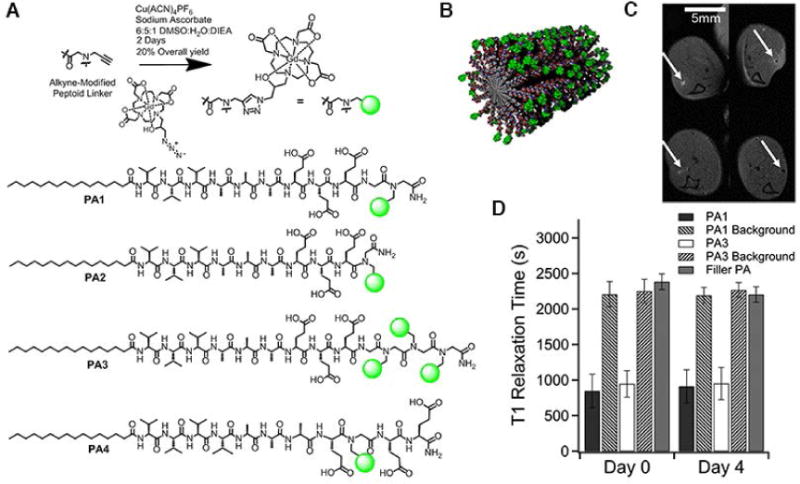
In vivo biomaterial localization with Gd-labeled peptide nanofibers. (A) Schematic illustration of the ‘clicking’ of Gd(HPN3DO3A) to the alkyne peptoid. Chemical structure of the self-assembling MRI contrast agents PA1–PA4. (B) Cartoon of self-assembled nanofibers of PA1. In vivo evaluation of PA1 and PA3: (C) 4 uL of PA gels were injected into each of six wild-type mice (injection point indicated by white arrows), and anatomical scan of mouse legs was performed immediately upon injection (top row) and after 4 days (bottom row). PA1 produced positive contrast in T1 weighted MRI, while PA3 produced negative contrast in T2 weighted MRI. (D) Average T1 times from the region of interest and the background measured several millimeters from the PA injection site). PA1 possessed the shortest T1 time (highest r1 relaxivity). (Reproduced with permission from 29. Copyright 2014 American Chemical Society)
2.2 Inorganic nanoparticles
Quantum dots (diameters of 2–6 nm) are of interest as imaging modalities due to their bright fluorescence, phosphorescence, and narrow emission spectrum. Incorporating such semiconductor nanocrystals into the design of GdIII-chelated nanoparticles imparts multimodal functionality for both MRI and fluorescence microscopy. Mulder et al. fabricated quantum dots with GdIII chelates as well as a PEG-lipid coating (pQDs), which endowed these contrast agents as bimodal molecular imaging probes (see figure 2C–E)16. The PEGylated lipid coating protected the contrast agents from interacting with plasma proteins, so that they went through less rapid hepatic clearance. The T1 relaxivity of pQDs was more than 12 mM−1sec−1, three times higher than those of small molecular GdIII chelates. If pQDs were further functionalized with the RGD peptide sequence, a cell adhesion epitope, RGD-pQDs could be employed to detect angiogenesis (new blood vessel formation), an important pathological process in cancer and atherosclerosis46.
Recently, researchers have been interested in investigating inorganic nanostructures like porous or mesoporous silica with high loading capacity. High relaxivities have been reported for GdIII chelates embedded in mesoporous silica. Lin and co-workers grafted GdIII chelates onto mesoporous silica nanospheres (MSN-Gd), which exhibited high T1 relaxivities of 28.8 mM−1sec−1 and T2 relaxivity of 65.5 mM−1sec−1 (determined with a magnetic field strength of 3.0 T)17. Considering its high r1 and r2 values, MSN-Gd is a promising candidate for intravascular MR imaging (T1) and depicting soft tissues (T2). Tsai et al. described multifunctional mesoporous silica nanorods loaded with Gd-DTPA and green fluorescing dye47. The magnetic nanorods exhibited no short-term cytotoxicity, and displayed high cellular uptake efficiency without the need for transfection agents. For the GdIII chelates grafted inside silica pores, however, the access of water molecules may be hindered. At the other extreme, the presentation of GdIII chelates on the surface of nonporous nanoparticles does not hinder the interaction of water molecules with each Gd site, thus allowing better contrast. For example, fluorescent quantum dots or colloidal gold nanocrystals were coated with a thin silica shell, onto which multiple Gd-DOTA molecules were covalently attached without negative influence on optical properties of the nanoparticle cores48. The particulate relaxivity was dependent on the number of gadolinium ions on the silica surface, and the number of Gd-DOTA molecules can be easily tuned from 20 to 320 GdIII ions per quantum dot. Moreover, due to the reduced rotational motion of Gd-DOTA molecules conjugated to the silica, both the r1 and r2 relaxivities were 6 to 15 times larger than free Gd-DOTA. One limitation associated with quantum dots or gold nanoparticles is that the light penetration depth in living tissue is just a few centimeters in an optimized scenario, which makes them uncompetitive as multimodal MRI contrast agents. The application of silica coatings and Gd-DOTA attachment endows high r1 and r2 relaxivities, and preliminary in vivo tests have verified the contrast enhancement provided by paramagnetic silica-coated nanoparticles.
Although nanostructures labeled with ionic T1 contrast agents exhibit higher relaxivity than small molecular GdIII chelates, the number of paramagnetic ions attached to the nanostructure surface is limited by the total number of available anchoring sites. Since the number of paramagnetic ions intrinsically determines the magnetism of nanoparticles, a higher number of imaging moieties per nanoparticle is always preferred. Most significantly, the size of ionic T1 nanoparticles is usually larger than 100 nm, making them susceptible to excretion by the RES system19. Alternatively, transition metal and lanthanide metal based inorganic nanoparticles composed of gadolinium oxide (Gd2O3), gadolinium fluoride (GdF3)20, gadolinium phosphate (GdPO4)49 or manganese oxide (MnO) have drawn researcher’s attention as a second type of T1 nanostructure-based contrast agent, since they have increased magnetic moments stemming from the abundance of paramagnetic ions at the surface, and the overall size is smaller than 100 nm.
Early research led to the development of paramagnetic ultrasmall Gd2O3 nanoparticles with an optimal particle diameter of 1 nm and r1 of 9.9 mM−1sec−1, which was higher than small molecular GdIII chelates50. This may be due to the induced longitudinal relaxation of water protons by surface GdIII ions of the Gd2O3 nanoparticles. A biocompatible PEG coating can enhance the steric repulsion of nanocrystals to avoid undesired aggregation, but conventional procedures to graft PEG onto Gd2O3 are time consuming. Fortin and co-workers reported a new, fast and efficient one-pot technique to prepare PEGylated nanoparticles with small sizes of 1.3 nm51. The PEGylated nanoparticles were colloidally stable in aqueous media, ideal for T1-weighted MRI since the ratio of r2/r1 was small enough (r2/r1 = 1.20 at 60 MHz). Furthermore, these ultrasmall PEG-Gd2O3 nanoparticles allowed the visualization of labeled cells implanted in vivo. Labeled F98 brain cancer cells were implanted into animals with brain tumors, and positive contrast was observed up to at least 48 h after implantation. Multimodal contrast agents are attracting increasing attention because they combine several complementary properties into a single object. Bridot et al. encapsulated Gd2O3 within a polysiloxane shell that carried organic fluorophores, and the surface of the nanoparticles was further coated with PEG-COOH to avoid agglomeration and precipitation10. Designed Gd2O3 nanoparticles with 2.2 nm core exhibited higher r1 and r2 values than small molecular GdIII chelates (r1 = 8.8 mM−1sec−1, r2 = 11.4 mM−1sec−1), and the small r2/r1 ratio rendered the nanoparticle as ideal T1 contrast agents (r2/r1 = 1.3). Fluorescence reflectance imaging after 3 h injection revealed no undesired accumulation in lungs and liver, which was due to both the small size and PEG coating. T1-weighted MRI of rats 1 h after injection and ICP-MS of some organs and urine confirmed that the uptake of Gd2O3 nanoparticles in lung, spleen and heart was insignificant.
Since relaxivity is inversely proportional to the sixth power of the ion–nuclear distance, metal ions like GdIII with seven unpaired electrons are the most predominantly used paramagnetic contrast agents52. However, GdIII ions are not naturally present in the human body. Alternatives based on transition metals, like manganese (five unpaired electrons), have progressed since there are natural processes that utilize these ions and could potentially be exploited. For instance, MnII ions are natural cellular constituents that play an important role as cofactors of enzymes and receptors. Moreover, the uptake of manganese is highly dependent on mitochondrial density that makes MnII a very promising contrast agent for mitochondria-rich organs like the liver, pancreas and kidney53. It is this combination of unique features that make MnII an attractive study object. Cai et al. designed hollow mesoporous Prussian blue (HMPB) nanoparticles loaded with a Mn-containing Prussian blue analogue (MnPBA), which also acted as an excellent drug carrier (high loading efficiency of 97.5% and loading capacity of 62.3%) and a photothermal agent, and most importantly had a high relaxivity (r1 = 7.43 mM−1sec−1) at pH 5.0 (tumor site conditions)54. This pH-responsive theranostic nanoplatform could concurrently release MnII ions and doxorubicin (DOX) at the tumor site. The positive correlation between MRI intensity and DOX release allowed controlled drug release (Figure 6). Additionally, the combination of strong absorbance in the NIR and chemotherapy of DOX realized a synergistic chemothermal therapy that achieved enhanced tumor therapeutic efficacy.
Figure 6.
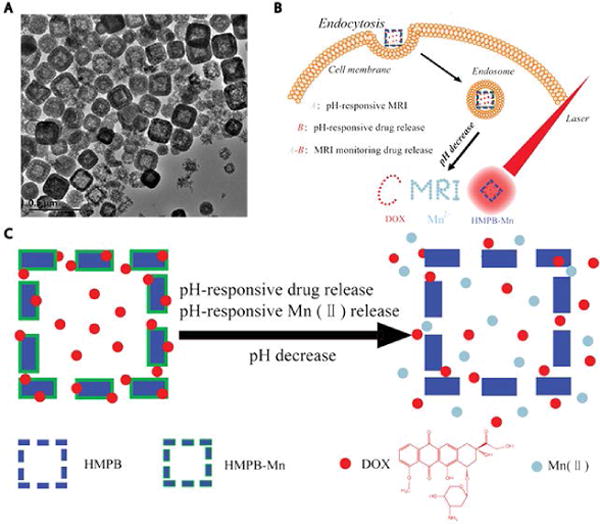
Smart theranostic agents of Prussian blue-based core-shell hollow mesoporous nanoparticles. (A) Transmission electron microscopy (TEM) images of HMPB-Mn. (B) HMPB-Mn loaded with DOX enters cell via endocytosis. Upon reaching a low pH site, such as an endosome, the nanocarrier released DOX and MnII simultaneously, which acted as both T1 weighted MRI contrast agents and chemotherapy agent for cancer treatment. (C) Schematic illustration of pH triggered release of DOX and MnII. (Reproduced with permission from 54. Copyright 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim)
One drawback, however, is that MnII chelates can cause hepatic failure and cardiac toxicity, severely limiting their application in animal studies. Hence, studies on functionalized MnO/Mn3O4-based nanoparticles have been undertaken. Hyeon, Gilad and co-workers studied mesoporous silica-coated hollow manganese oxide nanoparticles (HMnO@mSiO2) with a relaxivity of 0.99 mM−1sec−1 at 11.7 T55, a value much higher than existing MnO nanoparticle-based T1 contrast agents (MnO encapsulated within PEG-phospholipids, MnO coated with dense silica, and non-etched mesoporous silica-coated MnO)56, 57, simply because the mesoporous structure allowed the rapid penetration of water into the manganese core, and hence greater exposure of the MnII ions to water molecules (Figure 7). Although the relaxivity of these MnO nanoparticle-based contrast agents was compromised compared to small molecular MnII compounds, they were specifically designed to label and track cells that MnII compounds could not. With electroporation, HMnO@mSiO2 nanoparticles exhibited high cellular uptake (more than 75% in 24 h) by mouse adipose-derived mesenchymal stem cells (MSCs), and the applied electroporation only minimally impacted cell viability. In mice transplanted with labelled MSCs, the feasibility of HMnO@mSiO2 nanoparticles for long-term (over 14 days) in vivo cell tracking was studied, and the results revealed a hyperintense region at the transplantation site. The sustained contrast in vivo over a prolonged time period rendered HMnO@mSiO2 nanoparticles as competitive candidates for noninvasively monitoring the fate of transplanted cells in vivo.
Figure 7.
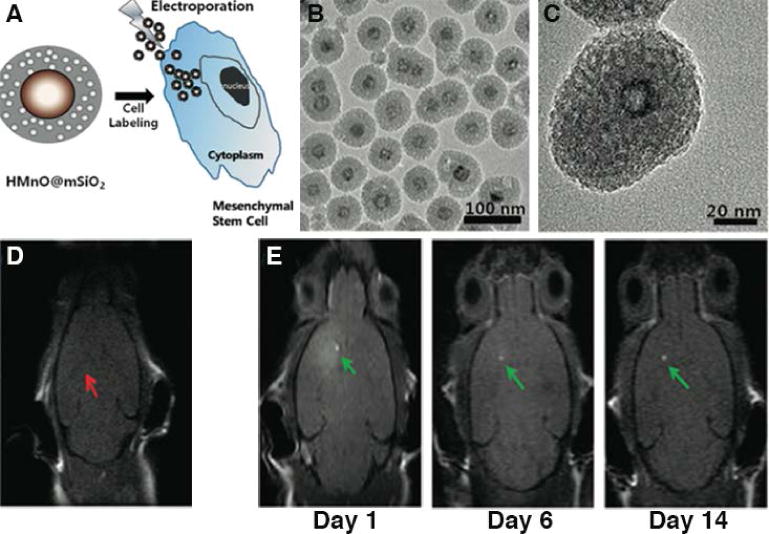
In vivo MRI tracking of adipose-derived mesenchymal stem cells labelled with mesoporous silica-coated hollow manganese oxide. (A) Schematic illustration of HMnO@mSiO2 nanoparticles and labeling of MSCs. (B) TEM image of HMnO@mSiO2 nanoparticles. Particle diameter = 65 nm, MnO core size = 15 nm. (C) High resolution TEM image of a single nanoparticle, clearly showing the mesoporous silica shell and hollow MnO core structures. In vivo MRI of transplanted MSCs: (D) Unlabeled MSCs. No hyperintense signal was detected (red arrow). (E) Hyperintense signals (green arrows) resulting from transplantation of HMnO@mSiO2 nanoparticles labeled MSCs were still visible 14 days after injection. (Reproduced with permission from 55. Copyright 2011 American Chemical Society)
3. T2 MRI contrast agents
3.1 Superparamagnetic Iron Oxide Nanoparticles
Shortly after the introduction of GdIII chelates, increased investigations were conducted on iron oxide nanoparticles as T2-weighted MRI contrast agents, whose characteristics cannot be replaced by bulk counterparts or atoms. Contrary to the T1 contrast effect, T2 contrast agents generate a negative contrast effect due to a signal reduction mechanism that at times can make it difficult to differentiate normal tissue from lesions. It is also known that T2 contrast agents induce high susceptibility that can induce local magnetic field inhomogeneities in normal tissues (blooming effect)6. As a result, the background contrast of tissues surrounding lesions would be distorted, giving obscured images. Superparamagnetic iron oxide nanoparticles (SPIONs), composed of magnetite (Fe3O4) and/or maghemite (γ-Fe2O3; an oxidized form of magnetite), have been popularly used as T2 MRI contrast agents. Although the limitations of the T2 mechanism compromise the image contrast provided by SPIONs, they still possess many advantages that attract researchers’ interest, such as an intrinsic high sensitivity and biological tolerance. Usually, SPIONs are composed of a core containing thousands of iron atoms and a surface coating to stabilize the magnetic nanoparticles. This feature of the core content endows higher sensitivity over gadolinium complexes in the micromolar and nanomolar ranges, rendering SPIONs as competitive MRI contrast agents, particularly for T2-weighted MR imaging. Moreover, iron, as one of the metallic elements in living organisms, is extensively involved in biological processes, with oxygen transport being one such example. After intravenous injection, iron oxide nanoparticles that contain biodegradable iron will be cleared by macrophages of the RES, and further degraded by lysosomes following recycling and red blood cell reproduction processes58. Additionally, the magnetic properties of SPIONs can be easily manipulated by controlling nanoparticle size and shell thickness, where the shell may be functionalized by biological and targeting probes to realize multifunctional MRI.
Dextran, a biocompatible material, is commonly employed as a surface coating for SPIONs, with several dextran-coated commercial products approved for clinical use. Feridex (diameter of 120–180 nm) was the first commercial product approved by FDA for the detection of liver lesions, and was intravenously administrated through slow diffusion. It allowed the differentiation of normal hepatic cells from abnormal ones through their selective uptake by phagocytic Kupffer cells in the reticuloendothelial system (RES) and spleen. Since malignant liver cells lack these Kupffer cells, no Feridex would be taken up and there was no subsequent reduction in the MR signal intensity of this region6. In other words, the signal intensity of normal regions was lowered since iron oxide nanoparticles shorten T2 relaxation, and a contrast was thus generated between malignant cells and normal cells. Another in vivo application of Feridex was studied by Zhu et al. (Figure 8), showing the feasibility of Feridex I.V. to noninvasively track stem-cell engraftment and migration after implantation with the use of MRI59. The autologous cultured neural stem cells from humans were labelled with Feridex I.V. and Effectene (a lipofection reagent) one day before implantation, and then stereotactically implanted around the region of brain trauma. A hypointense signal was found at the injection sites on the first day after implantation, and faded thereafter. However, the signal intensified at the periphery region around the lesion, indicating the migration of neural stem cells from the site of injection to the border of damaged tissue. In this pilot case, superparamagnetic iron oxide nanoparticles were proven to successfully label and track neural stem cells in vivo in patients with brain trauma. There are, however, numerous side effects, like hypotension and body pains, associated with Feridex administration60. Resovist is a carboxydextran-coated SPION that replaced the use of Feridex, as Resovist can be injected in a bolus fashion and causes weaker side effects.
Figure 8.
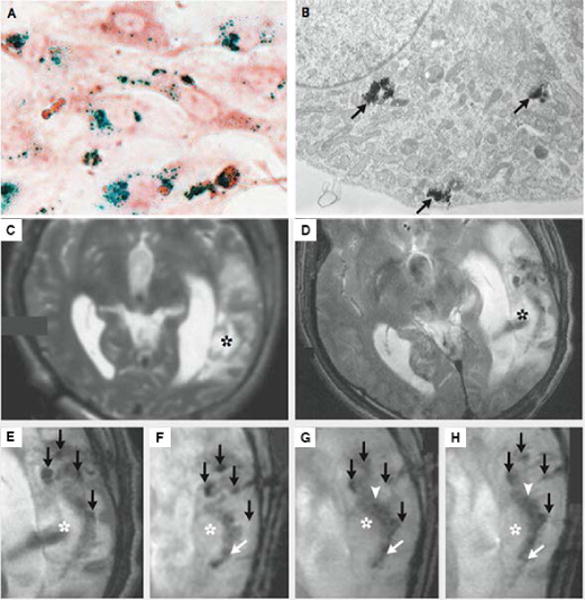
Tracking neural stem cells in patients with brain trauma. (A) Photomicrograph of neural stem cells labeled with iron oxide nanoparticles (Prussian blue staining, and neutral red counterstaining). (B) TEM image of neural stem cells labelled with iron oxide nanoparticles. Cluster of iron oxide nanoparticles were close to the Golgi apparatus (black arrow), which confirmed iron oxide nanoparticles entering cells. (C)–(H) MRI scanning of patient receiving neural stem cells labeled with Feridex I.V. (C) Scan before implantation. No pronounced hypointense signal was found around the lesion in the left temporal lobe (asterisk). (D–E) Scan on day 1 after implantation. The magnified image of E showed four hypointense signals (black arrows) at injection site. (F–H) Magnified images taken on day 7, day 14 and day 21. By day 7, dark signals posterior to the lesion were observed (white arrow). By day 14, hypointense signals at the injection site faded, while another dark signal intensified at the border of damaged tissues. (Reproduced with permission from 59. Copyright 2006 Massachusetts Medical Society)
On the other hand, ultrasmall superparamagnetic iron oxide (USPIO) nanoparticles with diameters of <20 nm experience longer intravascular circulation due to their smaller sizes that allow them to avoid rapid accumulation in the RES. Ferumoxtran-10 (also named Combidex) is a dextran-coated USPIO employed for lymph node imaging (Figure 9)61. Since early histological changes of lymph nodes are one of the prognostic factors in tumor staging and help monitoring future therapeutics, it is crucial for clinicians to accurately detect lymph-node status. Compared with traditional surgical dissection, Ferumoxtran-10 offers a noninvasive approach to identifying malignant lymph nodes. After administration, Ferumoxtran-10 is slowly extravasated from blood vessels to the interstitial space following phagocytosis by macrophages, and is finally transported to normal lymph nodes62. Accumulation of USPIOs within normal lymph nodes induce a significant susceptibility effect, resulting in a dramatic signal loss in T2*-weighted MRI62. In other words, nodes infiltrated by malignant cells would fail to uptake USPIOs and retain a high intensity, and those regions would be suggested as possible metastasis sites. Heesakkers et al.63 explored the feasibility of using Ferumoxtran-10 to detect lymph nodes metastases in 296 patients with prostate cancer. Fifty-eight patients were identified with positive results, but pathologic nodes were not found in 14 patients upon histologic examination, giving a 24.1% false positive rate63. Due to this relatively high false positive rate, Ferumoxtran-10 remains at the phase III stage of clinical trials. Since the intrinsic features of Ferumoxtran-10 fit with MRI contrast agents, further investigations could be conducted to solve the issue of high false positive rate.
Figure 9.
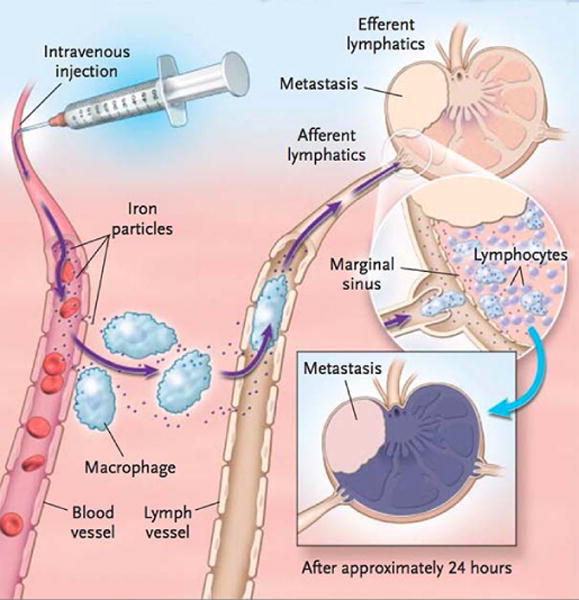
Schematic illustration of the Ferumoxtran-10 transport pathway. Extravasation from blood vessels to the interstitial space occurred following phagocytosis by macrophages, and was then transported to normal lymph nodes. (Reprinted with permission from 61. Copyright 2003 Massachusetts Medical Society)
3.2 New nanoparticles with improved core/shell (surface)
Recently, a large number of studies have focused on developing advanced MRI contrast agents based on nanoparticles with stronger contrast capabilities and biocompatible surfaces. The capability of MRI contrast effects stem from core size and material, while the chemical composition and thickness of the outer surface coating determine a nanoparticles’ biodistribution. Additionally, more factors may be taken into consideration, such as the interaction between the surface material and water molecules.
3.2.1 Improvement on core property
It has been shown that the R2 relaxation rate is proportional to the effective radius of nanoparticles. With increased nanoparticle size, T2 relaxivity increases58. Suh, Cheon and coworkers tailored water-soluble iron oxide nanoparticles with sizes of 4, 6, 9 and 12 nm, and the corresponding mass magnetization value at 1.5 T increased from 25 to 43, 80 and 102 emu per gram of iron, respectively64. Aside from extrinsic properties like particle size, the intrinsic properties of the materials can also affect the magnetism. For instance, although iron oxide nanoparticles serve as promising MRI contrast agents, they are still far from optimal for ultra-sensitive molecular imaging due to limited saturation magnetization of the oxidized state65. To address this, other materials have been explored as possible candidates for T2* MRI contrast agents. Hadjipanayis reported that iron nanoparticles coated with biocompatible bis-carboxyl-terminated poly (ethylene glycol) can offer much higher magnetization and coercivity than iron oxide nanoparticles of comparable size (10 nm)65. Suh and Cheon fabricated various metal-doped spinel MFe2O4 (M = Mn, Fe, Co or Ni). These bimetallic alloy nanoparticles exhibit high magnetization values, particularly those of MnFe2O4 that enable the in vivo imaging of small tumors64.
3.2.2 Improvement on shell property and surface modification
In addition to the core, the surface coating also plays important role in signal enhancement. The R2 relaxation rate could be described by66:
| (6) |
where γ is the proton gyromagnetic ratio, V* is the volume fraction, Ms is the saturation magnetization, a is the core radius, D refers to the diffusivity of water molecules, and L is the thickness of the surface coating. The equation above tells that R2 is not only proportional to the square of the core radius, but is also inversely related to the coating thickness. Bao et al. confirmed the relationship between the T2 relaxivity, core dimension and coating thickness by comparing phospholipid-PEG-encapsulated iron oxide nanoparticles with core sizes of 5 nm and 14 nm, and varying PEG chain lengths66. When they shared the same PEG chain length, the 14 nm SPION exhibited a higher relaxivity than the 5 nm SPION, consistent with the theoretical equation. The T2 relaxivity of the 14 nm SPION increased 2.54-fold upon decreasing the PEG molecular weight from 5 to 1 kDa, whereas the relaxivity of the 5 nm SPION exhibited a 7.79-fold increase. However, the ratio of core/shell value was only a partial reason contributing to T2 relaxivity change due to two reasons. Firstly, the changes in T2 relaxivity were simply calculated using equation (6), and the results were 1.66- and 1.97-fold increases for 14 nm and 5 nm SPIOs, respectively, which were not consistent with experimental results. Secondly, the T2 relaxivity didn’t further increase when the molecular weight of PEG decreased to 750 and 550 Da. A possible explanation was that the PEG coating may attract and immobilize water molecules via hydrogen bonding, which would slow down the water diffusion around the magnetic nanoparticles. Since the relaxation of water protons was perturbed, the T2 relaxivity will be affected. In other words, the motion of water molecules also governs the change in T2 relaxivity.
In order to achieve real time visualization of soft tissues, nanoparticle-based contrast agents are required to be biocompatible. Conventional co-precipitation methods to synthesize SPIONs and USPIOs yield nanoparticles with polydisperse sizes (deviations up to 25%67), which can lead to inferior magnetic properties that weaken the MRI contrast effect. A new method called thermal decomposition has been developed to overcome disadvantages associated with traditional co-precipitation. Magnetic nanoparticles with uniform size and high crystallinity can be fabricated using this thermal decomposition technique, but a hydrophobic surface is created due to the use of organic solvents, hampering their biomedical applications in aqueous media. Consequently, surface modification is necessary to endow the magnetic nanoparticles with a hydrophilic surface and to impart further functional capabilities. Recently, various surface-modification methods have been developed, with the most representative being ligand exchange68, shell formation69 and micellar encapsulation70.
During the ligand exchange process, the oleic acid ligands on nanoparticles resulting from thermal decomposition are replaced with bifunctional hydrophilic ligands. Bifunctional ligands are composed of strong binding moieties that anchor to the nanoparticle surface and relatively low binding hydrophilic tails that remain exposed to the aqueous media, affording water-dispersible nanoparticles. Ligand exchange is performed via a simple procedure, and nanoparticles modified by this method possess the advantages of thin passivating layers and small overall size. As this process is an equilibrium in nature, an excess amount of ligands are added to ensure successful exchange. Conventionally, thiolated organic molecules are extensively used to link functional molecules onto magnetic nanoparticles with a metal surface. However, few ligand systems are well developed for strong binding to metal oxide surfaces, especially that of iron oxide71. Recently published work has reported several ligands used to functionalize ferrite nanoparticles, such as sulfonates72, carboxylates73, phosphonates74, and dopamine75 and its derivatives76. There is, however, a lack of investigation into how the anchoring groups and ligand exchange methods affect the magnetic properties. Typically, the ligand exchange process follows either a stripping or biphasic protocol77. The stripping protocol requires two steps, the first being removal of the oleic acid on the nanoparticle surface, followed by the addition of new hydrophilic ligands. On the other hand, the biphasic protocol relies on the competition between two ligands, and only requires one step. Pierre et al. studied the magnetic properties of magnetic iron oxide nanoparticles (MION) that had been functionalized with a library of hydrophilic ligands, utilizing one of four chelating groups—2,3-dihydroxybenzamide, phosphonic acid, carboxylic acid or dopamide—to anchor a PEG chain to the surface. Additionally, the effect of the stripping protocol and biphasic protocol on the MION magnetic properties was also explored. After careful study, three noteworthy conclusions were drawn: Firstly, phosphonic acid and carboxylic acid dramatically decreased saturation magnetization and relaxivity, but catechol-type anchors like dopamide-PEG and 2.3-dihydroxybenzamide-PEG yielded magnetic properties comparable to control molecules; secondly, MIONs subjected to ligand exchange via the biphasic protocol possessed higher relaxivities than nanoparticles prepared by the stripping protocol; lastly, the biphasic method produced nanoparticles of smaller size and higher monodispersity than the stripping method. In summation, the influence of the organic coating on magnetic behavior should not be ignored, and the choice of anchoring moieties should also be taken into careful consideration.
Inorganic shell formation is another strategy to modify the nanoparticle surface, protecting magnetic nanoparticles from undesired oxidation or reactive species, and enabling further facile surface functionalization. Pierre et al. fabricated Fe3O4@organic@gold nanocomposites as MRI contrast agents with high saturation magnetization78. The iron oxide core went through ligand exchange with either phosphonate or catecholate anchoring groups, and the surface was then coated with gold. Gold nanoparticles are well known for their plasmonic properties that provide additional modes of study, such as dark field spectroscopy and surface-enhanced Raman spectroscopy (SERS)79. The combination of iron oxide and gold therefore endows the single nanocomposite with both magnetic and plasmonic properties. The high relaxivity that Fe3O4@organic@gold nanocomposites display makes them attractive candidates for multimodal cell imaging. Crosslinked amorphous silica shells are also widely used to stabilize magnetic nanoparticles. Although silica’s toxicity remains controversial, several promising in vivo and in vitro experiments have been conducted on silica-coated magnetic nanoparticles. A reverse-micelle sol-gel technique provides a way to prepare silica-coated SPIONs80. The selection of multicore iron oxide nanoparticles with etched silica shells displayed enhanced T2 relaxivity. One issue with the silica surface was that it may carry negative charges due to the presence of silanol groups, which can cause precipitation and gel formation. Another layer of biocompatible polymer such as PEG was therefore required to improve colloidal stability81. Moon and Hyeon demonstrated the multifunctional capability of magnetite nanoparticles coated with a mesoporous silica shell, where the silica was doped with a fluorophore, and the porous structure can serve as drug carrier82.
As an alternative, hydrophobic nanoparticles can achieve good colloidal stability through encapsulation by micelles and vesicles assembled from block copolymers, which can also function as promising nanocarriers for hydrophobic anticancer drugs, such as doxorubicin83, paclitaxel and camptothecin. A relaxivity comparison between ligand exchange, amphiphilic polymer encapsulation and lipid micelle encapsulation has been studied for superparamagnetic MnFe2O4 nanoparticles70. Unlike the other two methods, the encapsulation by lipid micelles exhibited an enhanced contrast in T2* weighted images. Possible reasons for this include the controlled particle aggregation and/or the weak diffusion effect of lipid molecules, as well as water molecules in the surrounding environment.
4. Nanoparticle-based activatable MRI contrast agents
Most of the MRI contrast agents described above possess a constant relaxivity, i.e. the relaxation properties are not sensitive to the surrounding physiological environments. These “always on” imaging probes emit constant signals regardless of the proximity and interaction with target tissues and cells, suffering a low target-to-background ratio (TBR)84, which in turn makes the target of interest hard to distinguish. Recently, MRI contrast agents have emerged that can be turned from “off” to “on” by various stimuli, including the CEST contrast agents that are turned on and off by RF radiation (detailed discussion in Section 5). These new MRI contrast agents potentially allow MRI to probe biochemical microenvironments for variations in conditions such as pH, metal ion and enzyme concentrations, and redox processes. Besides sensing biological processes, activatable MRI contrast agents, which respond to specific stimuli, can maximize the signal at target sites and minimize the signal from background. As a result, both sensitivity and specificity of imaging probes are improved. With the merits of nanomaterials, nanoparticle-based activatable MRI contrast agents have been the subjects of intensive research. Here, we will briefly introduce several types of nanoparticle-based activatable MRI contrast agents, and the stimuli range from hydrogen peroxide (H2O2) to pH and enzyme.
4.1 H2O2 as a stimulus
As a reactive oxygen species (ROS), H2O2 plays a vital role in regulating numerous physiological processes, and its overabundant expression may result in oxidative stress. Almutairi and co-workers reported highly efficient activatable MRI contrast agents by encapsulating ultrasmall gadolinium oxide particles in responsive polymers sensitive to hydrogen peroxide84. Ultrasmall gadolinium oxide nanoparticles (diameter < 5 nm) possess higher relaxivity than gadolinium chelates, considered to have the highest Gd density among all paramagnetic contrast agents50. In Almutairi’s design, the matrix of hydrophobic polymer shielded gadolinium oxide from the aqueous environment, resulting in quenched T1-weighted signal enhancement (0.5 mM−1sec−1). Upon triggered disassembly of the polymer matrix by the presence of hydrogen peroxide, gadolinium oxide nanoparticles were released (Figure 10), and a significant MRI contrast enhancement was activated (11-fold increase in r1). The H2O2 MRI biosensors developed in this case have better sensitivity and spatial integrity than what has been reported before, making these activatable agents suitable for monitoring metabolic reactions.
Figure 10.
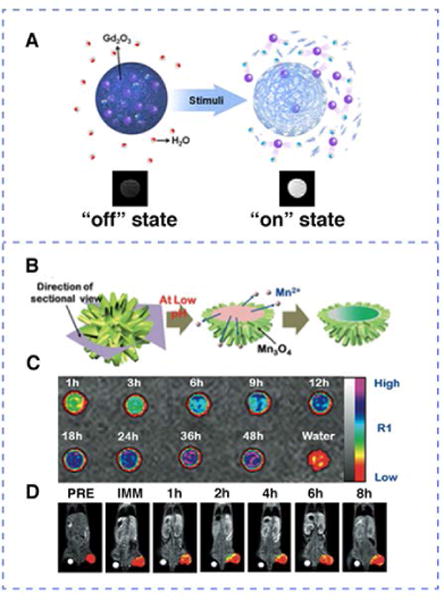
Examples of stimuli-repsonsive MRI contrast agents. H2O2 responsive contrast agent: (A) Schematic representation of activation by hydrogen peroxide: gadolinium oxide nanoparticles (purple spheres) were released upon polymer degradation triggered by hydrogen peroxide. pH responsive contrast agent: (B) Representation showing the core MnO content unloading from the urchin-shaped MnO@Mn3O4 nanoparticle at low pH. (C) Magnetic resonance images of leached out MnII ions from pH 5 PBS buffer (color coded). (D) Temporal color-coded T1-ultrashort echo time MR images of a tumor after intravenous injection of hollow MnO@Mn3O4 nano-urchin. (Reproduced with permission from 84 and 86. Copyright 2013 American Chemical Society and 2011 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim)
4.2 pH as a stimulus
Due to the altered metabolic profile of malignancy, the tumor microenvironment is different from that of normal tissues, exhibiting a decreased extracellular pH85. The pH responsive MRI contrast agents provide noninvasive assessment of tissue pH, which could be applied to tumor detection, imaging and therapy. The urchin-shaped manganese oxide nanoparticles fabricated by Kim et al., with a considerable surface area, functioned as pH-responsive activatable T1 contrast agents for MRI86. As illustrated in Figure 10, MnO nanoparticles were trapped inside a thin shell of Mn3O4 that gives the appearance of an urchin. The nano-urchin (MnO@ Mn3O4) was stable at pH values higher than 7, wheras the MnO core was dissolved and released as MnII upon reaching tumor sites with lower pH conditions (pH 4–6). Within 48h, the leached content of MnII constituted 16% of the total amount of Mn content in nano-urchin, and the accumulation of released MnII ions as well as the remaining MnO together greatly enhanced the T1 contrast. The ability of using pH-responsive activatable nano-urchin to image tumors was evaluated by in vivo MRI. After the injection of nano-urchins modified with anti-HER2 antibodies (tail veil injection into the mouse model with implanted NIH3T6.7 cells in the proximal thigh region), the T1 contrast was much enhanced after 4 h.
4.3 Enzymes as a stimulus
Caspases, which belong to the family of cysteine proteases, are ideal apoptosis-imaging targets, as they play vital roles in initiating, regulating, and executing downstream proteolytic events in the process of programmed cell death87. Predictive information, such as therapeutic efficacy and anti-cancer drug selection, could therefore be obtained from the imaging of tumor apoptosis. A number of activatable MRI probes have been developed to image caspase-3/7 activity in apoptotic cells and living mice, i.e. a thulium-based PARACEST MRI probe88 and 19F MRI agents89, but their potential as in vivo MRI contrast agents are compromised due to relatively low detection sensitivities. Alternatively, Ye et al. reported Gd-based MRI probe C-SNAM to image chemotherapy (Doxorubicin) induced tumor apoptosis in mice87. More specifically, the designed molecules were composed with 2-cyano-6-hydroxyquinoline (CHQ), D-cysteine residue, a DEVE peptide sequence sensitive to caspase-3/7, Gd-DOTA as MRI reporter, and a disulphide bond potentially reduced by glutathione (GSH). Upon disulphide reduction and DEVD sequence cleavage by caspase-3/7, the C-SNAM molecule would trigger intramolecular cyclization and subsequently self-assembled into Gd nanoparticles (GdNPs) (Figure 11). At the same time, an 86% increase in r1 relaxivity (10.2 mM−1sec−1 to 19.0 mM−1sec−1, measured at 1 T) was observed, as the molecular tumbling time (τr) increased. To image caspase-3/7 activity in chemotherapy induced apoptotic tumor cells in vivo, small molecule C-SNAM was intravenously injected into nude mice model bearing subcutaneous HeLa tumors, undergoing rapid extravasation and penetration into the tumor site. The C-SNAM would quickly diffuse away from the tumor in viable tumor cells, since dominant pro-caspase-3/7 was not able to cleave the DEVD peptide sequence. In apoptotic tumor cells, on the other hand, the active caspase-3/7 cleaved DEVD and GSH in the intracellular microenvironment reduced the disulphide bonds. As a result, the combination of caspase-3/7 cleavage and GSH reduction induced intramolecular cyclization and nanoparticle aggregation. It is worth noting that the GdNPs exhibited prolonged retention at the tumor site due to their large size. After 40 min post-injection, an enhanced contrast was observed in treated tumors as compared to the baseline. The self-assembly triggered by caspase-3/7 was controllable, and may have great potential to be generalized as activatable MRI probes for imaging enzyme activity in vivo.
Figure 11.
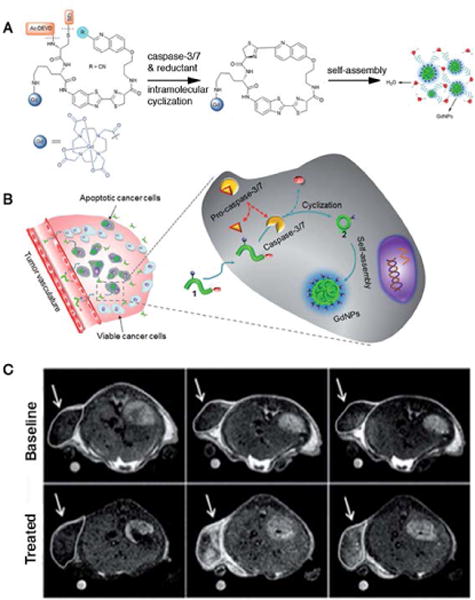
An example of caspase responsive MRI contrast agent. (A) Chemical structures of the C-SNAM probe and the proposed intramolecular cyclization upon disulfide reduction and caspase-3/7 triggered DEVD peptide cleavage, and the resulting self-assembly into nanoparticles. (B) Mechanism of C-SNAM for in vivo MR imaging of caspase-3/7 activity in a chemotherapy responsive tumor. (C) T1-weighted MR imaging of HeLa tumors prior to injection and 40 min, 12 min post-injection. Baseline group was not treated with chemotherapy, while treated group was. (Reproduced with permission from 87. Copyright The Royal Society of Chemistry 2014)
5. Chemical Exchange Saturation Transfer (CEST) contrast agents
5.1 Principles behind CEST
The contrast of chemical exchange saturation transfer (CEST) is generated through chemical exchange between solute and solvent, and can be proton or small molecule exchange (Figure 12e–i)90. The simplest model used to describe this process is the two-pool model, shown in Figure 1291. The exchangeable solute protons are selectively saturated by radiofrequency (RF) irradiation, and the saturated solute protons are subsequently transferred to bulk water protons by the exchange process, so that the signal of bulk water is attenuated. Since the water pool (concentration of about 110 M) is much larger than the solute pool (concentration in micromolar to millimolar range), each presaturated solute proton will be replaced by a non-saturated bulk water proton, and therefore another exchange occurs. With a fast exchange rate and long saturation time, this process will be continually repeated, with the bulk water signal becoming increasingly attenuated.
Figure 12.
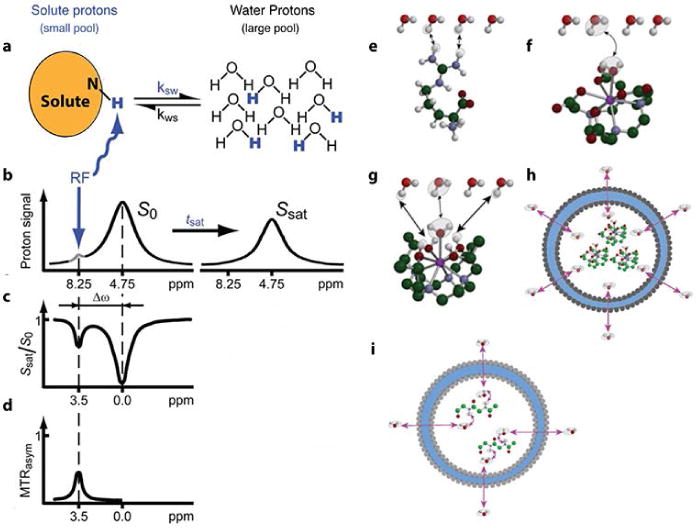
CEST principle: (a) Exchangeable solute protons are selectively saturated at a specific resonance frequency (RF) (8.25 ppm for amide protons), and the saturation is subsequently transferred to water (4.75 ppm). Non-saturated protons (black) are replaced by saturated solute protons (blue), and the process will be repeated to generate a discernable effect on the water signal intensity. (b) The saturation transfer causes water signal attenuation after tsat. (c) Z spectrum (or CEST spectrum, MT spectrum). When RF irradiates at 4.75 ppm, the water signal disappears because of direct saturation (DS), and the frequency is set to 0 ppm in Z spectrum. After a period of RF saturation (tsat), CEST effect becomes obvious, and the frequency of solute protons (amide protons) is 8.25−4.75 = 3.5 ppm. (d) MTRasym spectrum: asymmetry analysis of Z spectrum to remove DS effect. Various exchange pathways: (e) proton exchange, (f) molecule exchange, (g) proton and molecule exchange, (h) compartment exchange, and (i) molecule-mediated compartment exchange. (Reproduced with permission from 91 and 90. Copyright 2011 Wiley-Liss, Inc. and 2013 John Wiley and Sons, Ltd)
5.2 Nanoplatforms applied in CEST
Chemical exchange saturation transfer (CEST) agents are a relatively new class of MRI contrast agents. Endogenous CEST agents in biological systems possess exchangeable protons such as OH, NH2 and other labile molecules, whereas exogenous CEST agents can be designed to respond to versatile physiological signals like pH92, temperature93, 94, enzyme activity95–97 and metabolite levels98–100. CEST agents are called “negative” contrast agents because the CEST signal is achieved by attenuating the signal of bulk water, and renders the targeted region darker than the surrounding tissue91. After the first introduction by Balaban and coworkers in 2000, the CEST approach became tremendously popular, because it offers a metal-free approach to imaging metabolites in biological tissues as well as anatomical features101, and the contrast is easy to switch “on” and “off” by using frequency-selective RF presaturation pulses. However, exogenous contrast agents usually have low sensitivity, and a large dose (1–10 mM) is needed for detection. The high concentration requirement makes them difficult to translate to clinical applications and current efforts are focused on improving the sensitivity of CEST contrast agents. It has been demonstrated that cationic diamagnetic macromolecular systems (such as polypeptides and dendrimers) can be used to enhance CEST signals since they possess a large amount of exchangeable amide groups102. Also, paramagnetic complexes can improve CEST contrast due to the slow exchange between metal ion-bound water molecules and bulk water in lanthanide (III) complexes that results in a large chemical shift difference relative to bulk water. This large difference can minimize the direct saturation of bulk water. In order to assemble paramagnetic complexes as CEST contrast agents, nanostructures with the capacity to load large number of protons were introduced to confine paramagnetic complexes into a compartment where only a limited number of water molecules are present. The sensitivity can be improved by doing so. Commonly used nanocarriers include liposomes, micelles, and proteins such as apoferritin. Figure 13 compares various CEST probes in terms of their size and sensitivity.
Figure 13.
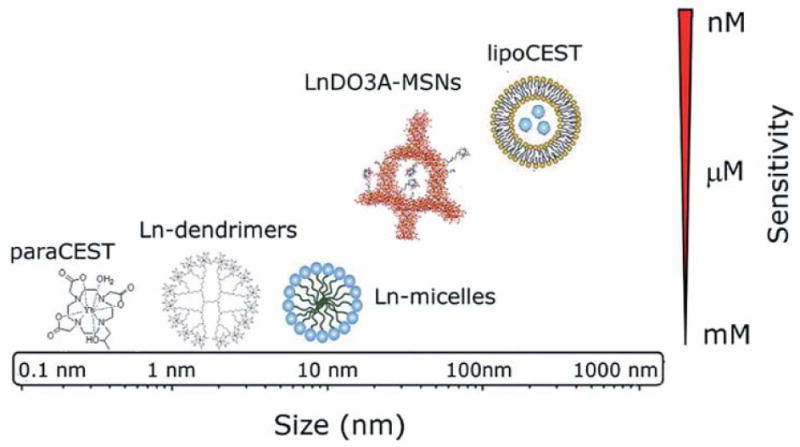
Comparison among different nanoparticle based CEST probes with the same Ln metal in terms of size and sensitivity. As shown, lipoCEST demonstrated the highest sensitivity, while Ln-dendrimers and Ln-micelles exhibited the lowest sensitivity. (Reprinted with permission from 111. Copyright 2014 The Royal Society of Chemistry)
5.2.1 Liposomes
The first nanoplatform we will discuss here is based on the entrapment of paramagnetic complexes within liposomes. As discussed above, paramagnetic lanthanide (Ln) complexes, especially gadolinium complexes, are a well-established class of T1 contrast agents. Several nanocarriers, such as dendrimers, micelles, liposomes, silica-like particles, and biologically systems (apoferritin and viral capsids) have been used to load tens to hundreds of thousands number of lanthanide (III) chelates, which exhibit high payload of GdIII chelates103. Besides their role as T1 contrast agents, lanthanide (III) complexes are also an emerging class of CEST contrast agents. A novel strategy to enhance CEST sensitivity is to entrap water molecules inside the liposomal cavity as a source of exchangeable protons to enhance the sensitivity (dubbed LipoCEST). As the membrane of liposomes is water-permeable, the exchange rate of trapped water molecules could be facilely tuned by changing lipid membrane composition. By encapsulating lanthanide (III)-based shift reagents (SR), the resonance frequency of inside water molecules would be differentiated from outside bulk water. SRs with a highly symmetric macrocyclic structure, such as [LnDOTMA]−, [LnDOTA]− and [LnHPDO3A], are preferred to maximize the paramagnetic shift that is dominated by the dipolar constant. TmIII and DyIII, in virtue of their high dipolar constants, are the most commonly used lanthanide metals. Liposomes containing these SRs with a concentration of 200 mM are able to induce high sensitivity104 (saturation percentage of ca. 10% in vitro is generated by subnanomolar amounts of vesicles) and a paramagnetic shift offset of ± 4 ppm. Since the shape of liposomes is sensitive to the osmotic force, liposomes suspended in hyperosmotic medium will shrink, and the SRs inside will be concentrated. As a result, the paramagnetic shift will be increased. Aime and coworkers demonstrated this effect by encapsulating [GdHPDO3A] with null chemical shift, and the signal of inside water protons was shifted by 7 ppm with respect to the bulk water. A further increase in paramagnetic shift can be obtained by incorporating amphiphilic SRs105 or neutral polynuclear SRs106 in the bilayer structure of liposomes. The highest shift achieved was ± 60 ppm, which significantly improved MRI sensitivity.
5.2.2 Micelles
Micelle formation is another approach to prepare CEST probes. Paramagnetic complexes are coupled to micelle-forming cationic polymers through ion-pair attractions. Gao and coworkers investigated the feasibility of applying polymer-based CEST probes that were able to form micelles and reversibly dissociate into unimers in different pH environments to turn the CEST contrast “on” and “off”. They reported tertiary amine-based block copolymers, such as poly[2-(diisopropylamino)ethyl methacrylate] (PEG-b-PDPA), self-assembled into micelles at neutral pH, with the CEST signal remaining silent. In acidic conditions the micelles dissociated into protonated unimers, turning on the CEST signal107. This successful result demonstrates the uniqueness of a pH-activable micelle platform as a CEST contrast agent, though its potential may need to be further investigated for the in vivo MRI of acidosis-related metabolic diseases, as well as pH-responsive drug carriers.
5.2.3 Apoferritin
Apoferritin is the proteinaceous part of the iron-storage protein ferritin, which usually forms a shell around iron oxide core. Apoferritin can self-assemble into a spherical structure that possess eight hydrophilic and six hydrophobic channels are in diameters of 3–4 Å. Small molecules like water can pass between inside cavity and outside bulk water, while large molecules like lanthanide complexes would not escape from the cavity once entrapped. Aime108 reported that the loading of GdHPDO3A within an apoferritin ‘cage’ generated a water relaxivity as high as 80 mM−1s−1. It is difficult for a single water molecule to achieve such a high relaxivity, and indicated that the inside surface of apoferritin may undergo catalytic prototropic exchange of water molecules to enhance the relaxivity. Vasalatiy designed apoferritin-encapsulated EuIII complexes, because EuIII chelates exchange water very slowly, and the tri-cationic feature endowed complexes rich with exchangeable protons109.
5.2.4 Mesoporous Silica
By virtue of their uniform mesopores, large surface area, good biocompatibility, and chemical and thermal stability, mesoporous silica nanoparticles (MSNs) have been extensively used as nanocarriers for ion complexes. The extraordinary efficiency of MSNs as T1-weighted contrast agents has been reported110, with r1 values as high as 80 mM−1sec−1. Very recently, Ferrauto demonstrated that Ln (III) chelates anchored on the surface of MSNs exhibited higher sensitivity than dendrimers and micelles with the same concentration111. The rationale behind the design is illustrated in Figure 14, where LnHPDO3A complexes (Ln = Eu, Tm, Tb or Gd) were anchored to MCM-41 silica surface (particle size in the range of 20–50 nm). The chemical shift between Eu-, Tm-, Tb-silica and control silica was compared with ST-spectra (ST stands for percent of saturation, where ST = (1−Mz/Mo)*100%; Mz stands for longitudinal magnetization; Mo is equilibrium value of the longitudinal magnetization). Control silica exhibited no CEST effect, while the chemical shifts of Eu-, Tm- and Tb-silica were 5.5, 7.5 and 15 ppm, respectively. As Tb-silica was most efficient among these chelates, the pH and temperature effect were studied on this entity. For a pH in the range of 6.0–8.5 and a temperature in the range of 29–45 °C, the ST effect remained unperturbed. When the pH was lower than 6.0, ST effect rapidly diminished, which may be due to slow exchange between Ln-chelates and the water pool, or substantial alternation of the silica surface. Furthermore, in the silica framework, the outer surface and inner pores provide a large space for the attachment of specific probes, or for loading with drugs and fluorescent dyes to fulfil the functions of controlled drug delivery and multimodal imaging, respectively.
Figure 14.
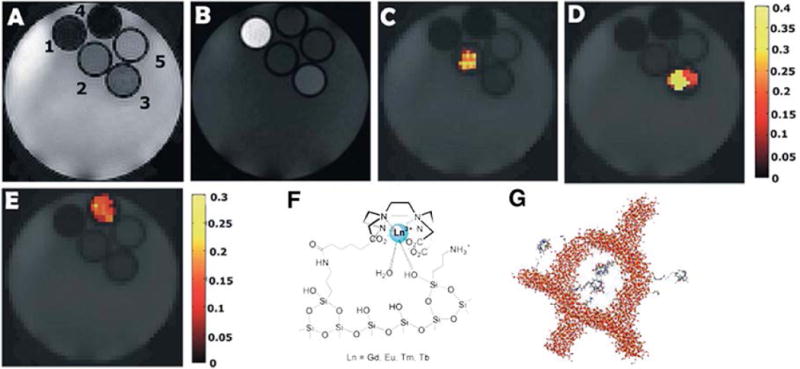
CEST of Ln (III) chelates anchored on the surface of mesoporous silica nanoparticles. (A) T2w, (B) T1w, and (C)–(E) STmap at 5.5 ppm, 7.5 ppm and 15 ppm, respectively; Labeled capillary positions 1–5 correspond to (1) GdDO3A–MCM-41, (2) EuDO3A–MCM-41, (3) TmDO3A–MCM-41, (4) TbDO3A–MCM-41, (5) unlabeled MCM-41; Schematic representation of: (F) the interaction between LnDO3A-like chelates and the surface of the organo-modified MCM-41; (G) Ln (III) chelates anchored on organo-modified mesoporous silica nanoparticles. (Reproduced with permission from 111. Copyright 2014 The Royal Society of Chemistry)
Conclusions and future perspectives
Nanoparticle-based MRI contrast agents play important roles in improving MRI sensitivity, and they are applied in broad criteria from T1-weighted imaging, T2-weighted imaging and CEST contrast. Their high surface-to-volume ratio enables facile surface modification. Negative contrast originates from T2 contrast agents make it hard to differentiate normal tissues and pathogenic tissues. On the contrary, T1 contrast agents are so called positive contrast agents, which can enhance the high-resolution of MRI. The first class of T1 contrast agents is focused on metal ion chelates, with lanthanide elements like gadolinium the most popular metals used in ion chelates. Since gadolinium chelates have short circulation time, they are not suitable for long term tracking of MRI, and there have been reports of high dose injections of GdIII complexes causing renal NFS. As an extension to the development of ion chelates, many researchers have focused on ionic nanostructure-based T1 contrast agents that incorporate ion chelates onto or into nano-sized carriers to increase the relaxivity. An underlying disadvantage associated with ionic nanostructures, however, is their large size, which makes them susceptible to rapid excretion by the RES. Furthermore, the synthesis of such nanoparticles is usually complicated. Another way to improve T1 contrast is the use of metal oxide nanoparticles, which not only avoid the “blooming” effect of T2 contrast agents, but also enhance the advantage of nanoparticle-based platforms. In the context of T2 contrast agents, superparamagnetic iron oxides are frequently exploited for molecular and cellular imaging applications. Dextran-coated SPIONs (such as Feridex and Resovist) are FDA approved clinical contrast agents used for imaging of the liver, spleen and bone marrow, due to their selective uptake by Kupffer cells in the RES. Smaller-sized USPIOs escape from phagocytosis by the RES, and are specifically uptaken by malignant lymph node regions, rendering pathogenic regions darker than the surrounding area. The conventional co-precipitation method used to prepare iron oxide nanoparticles suffered from dimensional polydispersity, leading to inferior magnetization. The alternate method of thermal decomposition fabricates iron oxide nanoparticles in organic solution with a uniform size. However, iron oxide nanoparticles prepared from thermal decomposition have solubility issues arising from the hydrophobic ligands that attach to nanoparticle surface during synthesis. Surface modification is required to endow nanoparticles with a biocompatible surface and to impart additional functions. Common methods of surface modification include ligand exchange, inorganic shell coating and micelle encapsulation. Improvements on core and shell to enhance magnetic property of iron oxide nanoparticles are described separately. Compared with always “on” MRI contrast agents, an emerging class of activatable MRI contrast agents with improved sensitivity and specificity have quickly gained popularity, since they are activated by specific stimuli, such as pH, enzyme, RF saturation, etc. Combined with the benefits of nanomaterials, the activatable nanoparticle-based MRI contrast agents could generate higher relaxivity values. Recently, CEST contrast agents, as a member of activatable contrast agents, have become increasingly more attractive. Nanostructure-based CEST contrast agents incorporate paramagnetic ion chelates into nanocarriers, such as liposomes, micelles, mesoporous silica and apoferritin, and the CEST signal can be easily switched “on” and “off” by simply changing RF pulses.
Currently, the main challenge of nanoparticle-based MRI contrast agents is how to bring bench-top developed agents into clinical application, a challenge that requires multidisciplinary collaboration between scientists and doctors in the fields of nanotechnology, imaging and medicine. To facilitate the translation of T1 or T2 nanoparticle-based MRI contrast agents from in vitro study to in vivo applications, researchers may employ more biocompatible nanomaterials to coat or encapsulate magnetic moieties, so that toxicity would be minimized. Micelles may be good choices, since they have been approved by the FDA for clinical trials and use in humans. Another challenge regarding T1 or T2 nanoparticle-based MRI contrast agents is how to improve the sensitivity and specificity for in vivo imaging. Activatable MRI nanoprobes are designed to amplify imaging signals upon the presence of specific stimulus, and therefore greatly enhance imaging sensitivity and specificity. Although these activatable MRI nanoprobes are still in their infancy and only a few clinical successes have been achieved, the remarkable merits would reinforce the appeal of using activatable MRI nanoprobes for in vivo application. The metal-based MRI contrast agents currently used in the clinic pose some risks in introducing undesired toxicity. Hence, the development of non-metallic biocompatible contrast agents for clinical MRI is highly desired in future research. Very recently, there is a growing interest in developing CEST contrast agents based on either clinically approved agents or natural compounds, i.e. iopamidol or glucose; the feasibility of which in human imaging has been successfully tested. Nanoparticle-based MRI contrast agents with low risk and complementary contrast would be alternatives for future cancer studies. Additionally, we believe cell tracking and the determination of cell functions would be involved in the function of CEST contrast agents.
Acknowledgments
We acknowledge financial support from the National Science Foundation (DMR 1255281, DMR 1506937, and CHE 1412985) and the National Institutes of Health (NIH/R21CA191740).
References
- 1.Brown MA, Semelka RC. MRI: Basic Principles and Applications. Wiley; 2011. [Google Scholar]
- 2.Tang H, Wu EX, Ma QY, Gallagher D, Perera GM, Zhuang T. MRI brain image segmentation by multi-resolution edge detection and region selection. Computerized Medical Imaging and Graphics. 2000;24:349–357. doi: 10.1016/s0895-6111(00)00037-9. [DOI] [PubMed] [Google Scholar]
- 3.Sipkins DA, Cheresh DA, Kazemi MR, Nevin LM, Bednarski MD, Li KCP. Detection of tumor angiogenesis in vivo by αvβ3-targeted magnetic resonance imaging. Nature Medicine. 1998;4:623–626. doi: 10.1038/nm0598-623. [DOI] [PubMed] [Google Scholar]
- 4.Hung AH, Duch MC, Parigi G, Rotz MW, Manus LM, Mastarone DJ, Dam KT, Gits CC, Macrenaris KW, Luchinat C, et al. Mechanisms of Gadographene-Mediated Proton Spin Relaxation. J Phys Chem C Nanomater Interfaces. 2013;117 doi: 10.1021/jp406909b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matosziuk LM, Leibowitz JH, Heffern MC, MacRenaris KW, Ratner MA, Meade TJ. Structural optimization of Zn(II)-activated magnetic resonance imaging probes. Inorg Chem. 2013;52:12250–12261. doi: 10.1021/ic400681j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bulte JW, Kraitchman DL. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed. 2004;17:484–499. doi: 10.1002/nbm.924. [DOI] [PubMed] [Google Scholar]
- 7.Huang CH, Tsourkas A. Gd-based Macromolecules and Nanoparticles as Magnetic Resonance Contrast Agents for Molecular Imaging. Current Topics in Medicinal Chemistry. 2013;13:411–421. doi: 10.2174/1568026611313040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tassa C, Duffner JL, Lewis TA, Weissleder R, Schreiber SL, Koehler AN, Shaw SY. Binding affinity and kinetic analysis of targeted small molecule-modified nanoparticles. Bioconjug Chem. 2010;21:14–19. doi: 10.1021/bc900438a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janib SM, Moses AS, MacKay JA. Imaging and drug delivery using theranostic nanoparticles. Adv Drug Deliv Rev. 2010;62:1052–1063. doi: 10.1016/j.addr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bridot JL, Faure AC, Laurent S, Riviere C, Billotey C, Hiba B, Janier M, Josserand V, Coll JL, Elst LV, et al. Hybrid gadolinium oxide nanoparticles: multimodal contrast agents for in vivo imaging. J Am Chem Soc. 2007;129:5076–5084. doi: 10.1021/ja068356j. [DOI] [PubMed] [Google Scholar]
- 11.Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu Rev Med. 2012;63:185–198. doi: 10.1146/annurev-med-040210-162544. [DOI] [PubMed] [Google Scholar]
- 12.Gao J, Xu B. Applications of nanomaterials inside cells. Nano Today. 2009;4:37–51. doi: 10.1016/j.nantod.2008.10.009. [DOI] [Google Scholar]
- 13.Zhou Z, Lu ZR. Gadolinium-based contrast agents for magnetic resonance cancer imaging. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2013;5:1–18. doi: 10.1002/wnan.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konda SD, Aref M, Wang S, Brechbiel M, Wiener EC. Specific targeting of folate–dendrimer MRI contrast agents to the high affinity folate receptor expressed in ovarian tumor xenografts. Magma: Magnetic Resonance Materials in Physics, Biology, and Medicine. 2001;12:104–113. doi: 10.1007/bf02668091. [DOI] [PubMed] [Google Scholar]
- 15.Saito R, Krauze MT, Bringas JR, Noble C, McKnight TR, Jackson P, Wendland MF, Mamot C, Drummond DC, Kirpotin DB, et al. Gadolinium-loaded liposomes allow for real-time magnetic resonance imaging of convection-enhanced delivery in the primate brain. Exp Neurol. 2005;196:381–389. doi: 10.1016/j.expneurol.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Mulder WJ, Koole R, Brandwijk RJ, Storm G, Chin PT, Strijkers GJ, de Mello Donega C, Nicolay K, Griffioen AW. Quantum dots with a paramagnetic coating as a bimodal molecular imaging probe. Nano Lett. 2006;6:1–6. doi: 10.1021/nl051935m. [DOI] [PubMed] [Google Scholar]
- 17.Taylor KM, Kim JS, Rieter WJ, An H, Lin W, Lin W. Mesoporous silica nanospheres as highly efficient MRI contrast agents. J Am Chem Soc. 2008;130:2154–2155. doi: 10.1021/ja710193c. [DOI] [PubMed] [Google Scholar]
- 18.Ananta JS, Godin B, Sethi R, Moriggi L, Liu X, Serda RE, Krishnamurthy R, Muthupillai R, Bolskar RD, Helm L, et al. Geometrical confinement of gadolinium-based contrast agents in nanoporous particles enhances T1 contrast. Nat Nanotechnol. 2010;5:815–821. doi: 10.1038/nnano.2010.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Na HB, Song IC, Hyeon T. Inorganic Nanoparticles for MRI Contrast Agents. Advanced Materials. 2009;21:2133–2148. doi: 10.1002/adma.200802366. [DOI] [Google Scholar]
- 20.Evanics F, Diamente PR, van Veggel FCJM, Stanisz GJ, Prosser RS. Water-Soluble GdF3and GdF3/LaF3NanoparticlesPhysical Characterization and NMR Relaxation Properties. Chemistry of Materials. 2006;18:2499–2505. doi: 10.1021/cm052299w. [DOI] [Google Scholar]
- 21.Dumont MF, Baligand C, Li Y, Knowles ES, Meisel MW, Walter GA, Talham DR. DNA surface modified gadolinium phosphate nanoparticles as MRI contrast agents. Bioconjug Chem. 2012;23:951–957. doi: 10.1021/bc200553h. [DOI] [PubMed] [Google Scholar]
- 22.Shin J, Anisur RM, Ko MK, Im GH, Lee JH, Lee IS. Hollow manganese oxide nanoparticles as multifunctional agents for magnetic resonance imaging and drug delivery. Angew Chem Int Ed Engl. 2009;48:321–324. doi: 10.1002/anie.200802323. [DOI] [PubMed] [Google Scholar]
- 23.Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Gadolinium(III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications. Chemical Reviews. 1999;99:2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 24.Wiener E, Brechbiel MW, Brothers H, Magin RL, Gansow OA, Tomalia DA, Lauterbur PC. Dendrimer-based metal chelates: A new class of magnetic resonance imaging contrast agents. Magnetic Resonance in Medicine. 1994;31:1–8. doi: 10.1002/mrm.1910310102. [DOI] [PubMed] [Google Scholar]
- 25.Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann HJ. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Investigative Radiology. 2005;40:715–724. doi: 10.1097/01.rli.0000184756.66360.d3. [DOI] [PubMed] [Google Scholar]
- 26.Huang CH, Nwe K, Al Zaki A, Brechbiel MW, Tsourkas A. Biodegradable polydisulfide dendrimer nanoclusters as MRI contrast agents. ACS Nano. 2012;6:9416–9424. doi: 10.1021/nn304160p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perazella MA. Current status of gadolinium toxicity in patients with kidney disease. Clin J Am Soc Nephrol. 2009;4:461–469. doi: 10.2215/CJN.06011108. [DOI] [PubMed] [Google Scholar]
- 28.Bryant LH, Jr, Brechbiel MW, Wu C, Bulte JW, Herynek V, Frank JA. Synthesis and relaxometry of high-generation (G = 5, 7, 9, and 10) PAMAM dendrimer-DOTA-gadolinium chelates. J Magn Reson Imaging. 1999;9:348–352. doi: 10.1002/(sici)1522-2586(199902)9:2<348::aid-jmri30>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 29.Preslar AT, Parigi G, McClendon MT, Sefick SS, Moyer TJ, Haney CR, Waters EA, MacRenaris KW, Luchinat C, Stupp SI, et al. Gd(III)-labeled peptide nanofibers for reporting on biomaterial localization in vivo. ACS Nano. 2014;8:7325–7332. doi: 10.1021/nn502393u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li A, Wong CS, Wong MK, Lee CM, Au Yeung MC. Acute adverse reactions to magnetic resonance contrast media–gadolinium chelates. Br J Radiol. 2006;79:368–371. doi: 10.1259/bjr/88469693. [DOI] [PubMed] [Google Scholar]
- 31.Cheng Z, Thorek DL, Tsourkas A. Gadolinium-conjugated dendrimer nanoclusters as a tumor-targeted T1 magnetic resonance imaging contrast agent. Angew Chem Int Ed Engl. 2010;49:346–350. doi: 10.1002/anie.200905133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu ZR, Mohs AM, Zong Y, Feng Y. Polydisulfide Gd(III) chelates as biodegradable macromolecular magnetic resonance imaging contrast agents. International Journal of Nanomedicine. 2006;1:31–40. doi: 10.2147/nano.2006.1.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ke T, Feng Y, Guo J, Parker DL, Lu ZR. Biodegradable cystamine spacer facilitates the clearance of Gd(III) chelates in poly(glutamic acid) Gd-DO3A conjugates for contrast-enhanced MR imaging. Magn Reson Imaging. 2006;24:931–940. doi: 10.1016/j.mri.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Zong Y, Wang X, Jeong EK, Parker DL, Lu ZR. Structural effect on degradability and in vivo contrast enhancement of polydisulfide Gd(III) complexes as biodegradable macromolecular MRI contrast agents. Magn Reson Imaging. 2009;27:503–511. doi: 10.1016/j.mri.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allen TM, Hansen C, Martin F, Redemann C, Yau-Young A. Liposomes containing synthetic lipid derivatives of poly(ethylene glycol) show prolonged circulation half-lives in vivo. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1991;1066:29–36. doi: 10.1016/0005-2736(91)90246-5. [DOI] [PubMed] [Google Scholar]
- 36.Mulder WJ, Strijkers GJ, Griffioen AW, van Bloois L, Molema G, Storm G, Koning GA, Nicolay K. A liposomal system for contrast-enhanced magnetic resonance imaging of molecular targets. Bioconjug Chem. 2004;15:799–806. doi: 10.1021/bc049949r. [DOI] [PubMed] [Google Scholar]
- 37.Ghaghada K, Hawley C, Kawaji K, Annapragada A, Mukundan S., Jr T1 relaxivity of core-encapsulated gadolinium liposomal contrast agents–effect of liposome size and internal gadolinium concentration. Acad Radiol. 2008;15:1259–1263. doi: 10.1016/j.acra.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghaghada KB, Ravoori M, Sabapathy D, Bankson J, Kundra V, Annapragada A. New dual mode gadolinium nanoparticle contrast agent for magnetic resonance imaging. PLoS One. 2009;4:e7628. doi: 10.1371/journal.pone.0007628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bull SR, Guler MO, Bras RE, Venkatasubramanian PN, Stupp SI, Meade TJ. Magnetic resonance imaging of self-assembled biomaterial scaffolds. Bioconjug Chem. 2005;16:1343–1348. doi: 10.1021/bc050153h. [DOI] [PubMed] [Google Scholar]
- 40.Webber MJ, Kessler JA, Stupp SI. Emerging peptide nanomedicine to regenerate tissues and organs. J Intern Med. 2010;267:71–88. doi: 10.1111/j.1365-2796.2009.02184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ortony JH, Newcomb CJ, Matson JB, Palmer LC, Doan PE, Hoffman BM, Stupp SI. Internal dynamics of a supramolecular nanofibre. Nat Mater. 2014;13:812–816. doi: 10.1038/nmat3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nicolle GM, Toth E, Eisenwiener KP, Macke HR, Merbach AE. From monomers to micelles: investigation of the parameters influencing proton relaxivity. J Biol Inorg Chem. 2002;7:757–769. doi: 10.1007/s00775-002-0353-3. [DOI] [PubMed] [Google Scholar]
- 43.Bull SR, Guler MO, Bras RE, Meade TJ, Stupp SI. Self-assembled peptide amphiphile nanofibers conjugated to MRI contrast agents. Nano Lett. 2005;5:1–4. doi: 10.1021/nl0484898. [DOI] [PubMed] [Google Scholar]
- 44.Liu S, Zhang P, Banerjee SR, Xu J, Pomper MG, Cui H. Design and assembly of supramolecular dual-modality nanoprobes. Nanoscale. 2015;7:9462–9466. doi: 10.1039/c5nr01518a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Appel AA, Anastasio MA, Larson JC, Brey EM. Imaging challenges in biomaterials and tissue engineering. Biomaterials. 2013;34:6615–6630. doi: 10.1016/j.biomaterials.2013.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Griffioen AW. Anti-angiogenesis: making the tumor vulnerable to the immune system. Cancer Immunol Immunother. 2008;57:1553–1558. doi: 10.1007/s00262-008-0524-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsai CP, Hung Y, Chou YH, Huang DM, Hsiao JK, Chang C, Chen YC, Mou CY. High-contrast paramagnetic fluorescent mesoporous silica nanorods as a multifunctional cell-imaging probe. Small. 2008;4:186–191. doi: 10.1002/smll.200700457. [DOI] [PubMed] [Google Scholar]
- 48.Gerion D, Herberg J, Bok R, Gjersing E, Ramon E, Maxwell R, Kurhanewicz J, Budinger TF, Gray JW, Shuman MA, et al. Paramagnetic Silica-Coated Nanocrystals as an Advanced MRI Contrast Agent. Journal of Physical Chemistry C. 2007;111:12542–12551. doi: 10.1021/jp074072p. [DOI] [Google Scholar]
- 49.Hifumi H, Yamaoka S, Tanimoto A, Akatsu T, Shindo Y, Honda A, Citterio D, Oka K, Kuribayashi S, Suzuki K. Dextran Coated Gadolinium Phosphate Nanoparticles for Magnetic Resonance Tumor Imaging. Journal of Materials Chemistry. 2009;19:6393. doi: 10.1039/b902134e. [DOI] [Google Scholar]
- 50.Park JY, Baek MJ, Choi ES, Woo S, Kim JH, Kim TJ, Jung JC, Chae KS, Chang Y, Lee GH. Paramagnetic ultrasmall gadolinium oxide nanoparticles as advanced T1 MRI contrast agent: account for large longitudinal relaxivity, optimal particle diameter, and in vivo T1 MR images. ACS Nano. 2009;3:3663–3669. doi: 10.1021/nn900761s. [DOI] [PubMed] [Google Scholar]
- 51.Faucher L, Tremblay M, Lagueux J, Gossuin Y, Fortin MA. Rapid synthesis of PEGylated ultrasmall gadolinium oxide nanoparticles for cell labeling and tracking with MRI. ACS Appl Mater Interfaces. 2012;4:4506–4515. doi: 10.1021/am3006466. [DOI] [PubMed] [Google Scholar]
- 52.Pan D, Schmieder AH, Wickline SA, Lanza GM. Manganese-based MRI contrast agents: past, present and future. Tetrahedron. 2011;67:8431–8444. doi: 10.1016/j.tet.2011.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin DR, Krishnamoorthy SK, Kalb B, Salman KN, Sharma P, Carew JD, Martin PA, Chapman AB, Ray GL, Larsen CP, et al. Decreased incidence of NSF in patients on dialysis after changing gadolinium contrast-enhanced MRI protocols. J Magn Reson Imaging. 2010;31:440–446. doi: 10.1002/jmri.22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cai X, Gao W, Ma M, Wu M, Zhang L, Zheng Y, Chen H, Shi J. A Prussian Blue-Based Core-Shell Hollow-Structured Mesoporous Nanoparticle as a Smart Theranostic Agent with Ultrahigh pH-Responsive Longitudinal Relaxivity. Adv Mater. 2015 doi: 10.1002/adma.201503381. [DOI] [PubMed] [Google Scholar]
- 55.Kim T, Momin E, Choi J, Yuan K, Zaidi H, Kim J, Park M, Lee N, McMahon MT, Quinones-Hinojosa A, et al. Mesoporous silica-coated hollow manganese oxide nanoparticles as positive T1 contrast agents for labeling and MRI tracking of adipose-derived mesenchymal stem cells. J Am Chem Soc. 2011;133:2955–2961. doi: 10.1021/ja1084095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gilad AA, Walczak P, McMahon MT, Na HB, Lee JH, An K, Hyeon T, van Zijl PC, Bulte JW. MR tracking of transplanted cells with “positive contrast” using manganese oxide nanoparticles. Magn Reson Med. 2008;60:1–7. doi: 10.1002/mrm.21622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang H, Zhuang Y, Hu H, Du X, Zhang C, Shi X, Wu H, Yang S. Silica-Coated Manganese Oxide Nanoparticles as a Platform for Targeted Magnetic Resonance and Fluorescence Imaging of Cancer Cells. Advanced Functional Materials. 2010;20:1733–1741. doi: 10.1002/adfm.200902445. [DOI] [Google Scholar]
- 58.Lee N, Hyeon T. Designed synthesis of uniformly sized iron oxide nanoparticles for efficient magnetic resonance imaging contrast agents. Chem Soc Rev. 2012;41:2575–2589. doi: 10.1039/c1cs15248c. [DOI] [PubMed] [Google Scholar]
- 59.Zhu J, Zhou L, XingWu F. Tracking neural stem cells in patients with brain trauma. N Engl J Med. 2006;355:2376–2378. doi: 10.1056/NEJMc055304. [DOI] [PubMed] [Google Scholar]
- 60.Wang YX. Superparamagnetic iron oxide based MRI contrast agents: Current status of clinical application. Quant Imaging Med Surg. 2011;1:35–40. doi: 10.3978/j.issn.2223-4292.2011.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harisinghani MG, Barentsz J, Hahn PF, Deserno WM, Tabatabaei S, van de Kaa CH, de la Rosette J, Weissleder R. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med. 2003;348:2491–2499. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- 62.Sankineni S, Brown AM, Fascelli M, Law YM, Pinto PA, Choyke PL, Turkbey B. Lymph node staging in prostate cancer. Curr Urol Rep. 2015;16:30. doi: 10.1007/s11934-015-0505-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heesakkers RA, Jager GJ, Hovels AM, de Hoop B, van den Bosch HC, Raat F, Witjes JA, Mulders PF, van der Kaa CH, Barentsz JO. Prostate cancer: detection of lymph node metastases outside the routine surgical area with ferumoxtran-10-enhanced MR imaging. Radiology. 2009;251:408–414. doi: 10.1148/radiol.2512071018. [DOI] [PubMed] [Google Scholar]
- 64.Jun YW, Huh YM, Choi JS, Lee JH, Song HT, Kim S, Yoon S, Kim KS, Shin JS, Suh JS, et al. Nanoscale size effect of magnetic nanocrystals and their utilization for cancer diagnosis via magnetic resonance imaging. J Am Chem Soc. 2005;127:5732–5733. doi: 10.1021/ja0422155. [DOI] [PubMed] [Google Scholar]
- 65.Hadjipanayis CG, Bonder MJ, Balakrishnan S, Wang X, Mao H, Hadjipanayis GC. Metallic iron nanoparticles for MRI contrast enhancement and local hyperthermia. Small. 2008;4:1925–1929. doi: 10.1002/smll.200800261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tong S, Hou S, Zheng Z, Zhou J, Bao G. Coating optimization of superparamagnetic iron oxide nanoparticles for high T2 relaxivity. Nano Lett. 2010;10:4607–4613. doi: 10.1021/nl102623x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoffe S, Leshuk T, Everett P, Gu F. Superparamagnetic Iron Oxide Nanoparticles (SPIONs): Synthesis and Surface Modification Techniques for use with MRI and Other Biomedical Applications. Current Pharmaceutical Design. 2013;19:493–509. [PubMed] [Google Scholar]
- 68.Wu W, He Q, Jiang C. Magnetic iron oxide nanoparticles: synthesis and surface functionalization strategies. Nanoscale Res Lett. 2008;3:397–415. doi: 10.1007/s11671-008-9174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26:3995–4021. doi: 10.1016/j.biomaterials.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 70.Tromsdorf UI, Bigall NC, Kaul MG, Bruns OT, Nikolic MS, Mollwitz B, Sperling RA, Reimer R, Hohenberg H, Parak WJ, et al. Size and surface effects on the MRI relaxivity of manganese ferrite nanoparticle contrast agents. Nano Lett. 2007;7:2422–2427. doi: 10.1021/nl071099b. [DOI] [PubMed] [Google Scholar]
- 71.Xu C, Xu K, Gu H, Zheng R, Liu H, Zhang X, Guo Z, Xu B. Dopamine as a robust anchor to immobilize functional molecules on the iron oxide shell of magnetic nanoparticles. J Am Chem Soc. 2004;126:9938–9939. doi: 10.1021/ja0464802. [DOI] [PubMed] [Google Scholar]
- 72.Yee C, Kataby G, Ulman A, Prozorov T, White H, King A, Rafailovich M, Sokolov J, Gedanken A. Self-assembled monolayers of alkanesulfonic and -phosphonic acids on amorphous iron oxide nanoparticles. Langmuir. 1999;15:7111–7115. doi: 10.1021/la990663y. [DOI] [Google Scholar]
- 73.Daou TJ, Greneche JM, Pourroy G, Buathong S, Derory A, Ulhaq-Bouillet C, Donnio B, Guillon D, Begin-Colin S. Coupling agent effect on magnetic properties of functionalized magnetite-based nanoparticles. Chemistry of Materials. 2008;20:5869–5875. doi: 10.1021/cm801405n. [DOI] [Google Scholar]
- 74.Sahoo Y, Pizem H, Fried T, Golodnitsky D, Burstein L, Sukenik CN, Markovich G. Alkyl phosphonate/phosphate coating on magnetite nanoparticles: A comparison with fatty acids. Langmuir. 2001;17:7907–7911. doi: 10.1021/la010703+. [DOI] [Google Scholar]
- 75.Shultz MD, Reveles JU, Khanna SN, Carpenter EE. Reactive nature of dopamine as a surface functionalization agent in iron oxide nanoparticles. Journal of the American Chemical Society. 2007;129:2482–2487. doi: 10.1021/ja0651963. [DOI] [PubMed] [Google Scholar]
- 76.Gao J, Gu H, Xu B. Multifunctional magnetic nanoparticles: design, synthesis, and biomedical applications. Acc Chem Res. 2009;42:1097–1107. doi: 10.1021/ar9000026. [DOI] [PubMed] [Google Scholar]
- 77.Smolensky ED, Park HY, Berquo TS, Pierre VC. Surface functionalization of magnetic iron oxide nanoparticles for MRI applications - effect of anchoring group and ligand exchange protocol. Contrast Media Mol Imaging. 2011;6:189–199. doi: 10.1002/cmmi.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smolensky ED, Neary MC, Zhou Y, Berquo TS, Pierre VC. Fe3O4@organic@Au: core-shell nanocomposites with high saturation magnetisation as magnetoplasmonic MRI contrast agents. Chem Commun (Camb) 2011;47:2149–2151. doi: 10.1039/c0cc03746j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Daniel MC, Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev. 2004;104:293–346. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 80.Tanaka K, Narita A, Kitamura N, Uchiyama W, Morita M, Inubushi T, Chujo Y. Preparation for highly sensitive MRI contrast agents using core/shell type nanoparticles consisting of multiple SPIO cores with thin silica coating. Langmuir. 2010;26:11759–11762. doi: 10.1021/la1015077. [DOI] [PubMed] [Google Scholar]
- 81.Kohler N, Fryxell GE, Zhang M. A bifunctional poly(ethylene glycol) silane immobilized on metallic oxide-based nanoparticles for conjugation with cell targeting agents. J Am Chem Soc. 2004;126:7206–7211. doi: 10.1021/ja049195r. [DOI] [PubMed] [Google Scholar]
- 82.Kim J, Kim HS, Lee N, Kim T, Kim H, Yu T, Song IC, Moon WK, Hyeon T. Multifunctional uniform nanoparticles composed of a magnetite nanocrystal core and a mesoporous silica shell for magnetic resonance and fluorescence imaging and for drug delivery. Angew Chem Int Ed Engl. 2008;47:8438–8441. doi: 10.1002/anie.200802469. [DOI] [PubMed] [Google Scholar]
- 83.Adams ML, Lavasanifar A, Kwon GS. Amphiphilic block copolymers for drug delivery. J Pharm Sci. 2003;92:1343–1355. doi: 10.1002/jps.10397. [DOI] [PubMed] [Google Scholar]
- 84.Viger ML, Sankaranarayanan J, de Gracia Lux C, Chan M, Almutairi A. Collective activation of MRI agents via encapsulation and disease-triggered release. J Am Chem Soc. 2013;135:7847–7850. doi: 10.1021/ja403167p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Crayton SH, Tsourkas A. pH-titratable superparamagnetic iron oxide for improved nanoparticle accumulation in acidic tumor microenvironments. ACS Nano. 2011;5:9592–9601. doi: 10.1021/nn202863x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim T, Cho E-J, Chae Y, Kim M, Oh A, Jin J, Lee E-S, Baik H, Haam S, Suh J-S, et al. Urchin-Shaped Manganese Oxide Nanoparticles as pH-Responsive Activatable T1 Contrast Agents for Magnetic Resonance Imaging. Angewandte Chemie. 2011;123:10777–10781. doi: 10.1002/ange.201103108. [DOI] [PubMed] [Google Scholar]
- 87.Ye D, Shuhendler AJ, Pandit P, Brewer KD, Tee SS, Cui L, Tikhomirov G, Rutt B, Rao J. Caspase-responsive smart gadolinium-based contrast agent for magnetic resonance imaging of drug-induced apoptosis. Chem Sci. 2014;4:3845–3852. doi: 10.1039/C4SC01392A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yoo B, Pagel MD. A PARACEST MRI contrast agent to detect enzyme activity. J Am Chem Soc. 2006;128:14032–14033. doi: 10.1021/ja063874f. [DOI] [PubMed] [Google Scholar]
- 89.Mizukami S, Takikawa R, Sugihara F, Shirakawa M, Kikuchi K. Dual-Function Probe to Detect Protease Activity for Fluorescence Measurement and19F MRI. Angewandte Chemie. 2009;121:3695–3697. doi: 10.1002/ange.200806328. [DOI] [PubMed] [Google Scholar]
- 90.Liu G, Song X, Chan KW, McMahon MT. Nuts and bolts of chemical exchange saturation transfer MRI. NMR Biomed. 2013;26:810–828. doi: 10.1002/nbm.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van Zijl PC, Yadav NN. Chemical exchange saturation transfer (CEST): what is in a name and what isn’t? Magn Reson Med. 2011;65:927–948. doi: 10.1002/mrm.22761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang X, Wu Y, Soesbe TC, Yu J, Zhao P, Kiefer GE, Sherry AD. A pH-Responsive MRI Agent that Can Be Activated Beyond the Tissue Magnetization Transfer Window. Angew Chem Int Ed Engl. 2015;54:8662–8664. doi: 10.1002/anie.201502497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McVicar N, Li AX, Suchy M, Hudson RH, Menon RS, Bartha R. Simultaneous in vivo pH and temperature mapping using a PARACEST-MRI contrast agent. Magn Reson Med. 2013;70:1016–1025. doi: 10.1002/mrm.24539. [DOI] [PubMed] [Google Scholar]
- 94.Delli Castelli D, Terreno E, Aime S. Yb(III)-HPDO3A: a dual pH- and temperature-responsive CEST agent. Angew Chem Int Ed Engl. 2011;50:1798–1800. doi: 10.1002/anie.201007105. [DOI] [PubMed] [Google Scholar]
- 95.Hingorani DV, Randtke EA, Pagel MD. A catalyCEST MRI contrast agent that detects the enzyme-catalyzed creation of a covalent bond. J Am Chem Soc. 2013;135:6396–6398. doi: 10.1021/ja400254e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ratnakar SJ, Viswanathan S, Kovacs Z, Jindal AK, Green KN, Sherry AD. Europium(III) DOTA-tetraamide complexes as redox-active MRI sensors. J Am Chem Soc. 2012;134:5798–5800. doi: 10.1021/ja211601k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu G, Liang Y, Bar-Shir A, Chan KW, Galpoththawela CS, Bernard SM, Tse T, Yadav NN, Walczak P, McMahon MT, et al. Monitoring enzyme activity using a diamagnetic chemical exchange saturation transfer magnetic resonance imaging contrast agent. J Am Chem Soc. 2011;133:16326–16329. doi: 10.1021/ja204701x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.De Leon-Rodriguez LM, Lubag AJ, Malloy CR, Martinez GV, Gillies RJ, Sherry AD. Responsive MRI agents for sensing metabolism in vivo. Acc Chem Res. 2009;42:948–957. doi: 10.1021/ar800237f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang S, Trokowski R, Sherry AD. A paramagnetic CEST agent for imaging glucose by MRI. J Am Chem Soc. 2003;125:15288–15289. doi: 10.1021/ja038345f. [DOI] [PubMed] [Google Scholar]
- 100.Liu GS, Li YG, Pagel MD. Design and characterization of a new irreversible responsive PARACEST MRI contrast agent that detects nitric oxide. Magnetic Resonance in Medicine. 2007;58:1249–1256. doi: 10.1002/mrm.21428. [DOI] [PubMed] [Google Scholar]
- 101.Ward KM, Aletras AH, Balaban RS. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST) J Magn Reson. 2000;143:79–87. doi: 10.1006/jmre.1999.1956. [DOI] [PubMed] [Google Scholar]
- 102.Goffeney N, Bulte JWM, Duyn J, Bryant LH, van Zijl PCM. Sensitive NMR detection of cationic-polymer-based gene delivery systems using saturation transfer via proton exchange. Journal of the American Chemical Society. 2001;123:8628–8629. doi: 10.1021/ja0158455. [DOI] [PubMed] [Google Scholar]
- 103.Aime S, Castelli DD, Crich SG, Gianolio E, Terreno E. Pushing the sensitivity envelope of lanthanide-based magnetic resonance imaging (MRI) contrast agents for molecular imaging applications. Acc Chem Res. 2009;42:822–831. doi: 10.1021/ar800192p. [DOI] [PubMed] [Google Scholar]
- 104.Aime S, Delli Castelli D, Terreno E. Highly sensitive MRI chemical exchange saturation transfer agents using liposomes. Angewandte Chemie-International Edition. 2005;44:5513–5515. doi: 10.1002/anie.200501473. [DOI] [PubMed] [Google Scholar]
- 105.Aime S, Castelli DD, Terreno E. Lanthanide-Loaded Paramagnetic Liposomes as Switchable Magnetically Oriented Nanovesicles. 2009;464:193–210. doi: 10.1016/s0076-6879(09)64010-6. [DOI] [PubMed] [Google Scholar]
- 106.Terreno E, Barge A, Beltrami L, Cravotto G, Castelli DD, Fedeli F, Jebasingh B, Aime S. Highly shifted LIPOCEST agents based on the encapsulation of neutral polynuclear paramagnetic shift reagents. Chem Commun (Camb) 2008:600–602. doi: 10.1039/b715383j. [DOI] [PubMed] [Google Scholar]
- 107.Zhang S, Zhou K, Huang G, Takahashi M, Sherry AD, Gao J. A novel class of polymeric pH-responsive MRI CEST agents. Chem Commun (Camb) 2013;49:6418–6420. doi: 10.1039/c3cc42452a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Aime S, Frullano L, Geninatti Crich S. Compartmentalization of a gadolinium complex in the apoferritin cavity: a route to obtain high relaxivity contrast agents for magnetic resonance imaging. Angew Chem Int Ed Engl. 2002;41:1017–1019. doi: 10.1002/1521-3773(20020315)41:6<1017::aid-anie1017>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 109.Vasalatiy O, Zhao P, Zhang S, Aime S, Sherry AD. Catalytic effects of apoferritin interior surface residues on water proton exchange in lanthanide complexes. Contrast Media Mol Imaging. 2006;1:10–14. doi: 10.1002/cmmi.93. [DOI] [PubMed] [Google Scholar]
- 110.Carniato F, Tei L, Arrais A, Marchese L, Botta M. Selective anchoring of Gd(III) chelates on the external surface of organo-modified mesoporous silica nanoparticles: a new chemical strategy to enhance relaxivity. Chemistry. 2013;19:1421–1428. doi: 10.1002/chem.201202670. [DOI] [PubMed] [Google Scholar]
- 111.Ferrauto G, Carniato F, Tei L, Hu H, Aime S, Botta M. MRI nanoprobes based on chemical exchange saturation transfer: Ln(III) chelates anchored on the surface of mesoporous silica nanoparticles. Nanoscale. 2014;6:9604–9607. doi: 10.1039/c4nr02753a. [DOI] [PubMed] [Google Scholar]


