Abstract
The hippocampal formation is traditionally viewed as having a feed-forward, unidirectional circuit organization which promotes propagation of excitatory processes. While the substantial forward projection from hippocampal CA1 to the subiculum has been very well established, accumulating evidence supports the existence of a significant back-projection pathway comprised of both excitatory and inhibitory elements from the subiculum to CA1. Based on these recently updated anatomical connections, such a back projection could serve to modulate information processing in hippocampal CA1. Here we review the published anatomical and physiological studies on the subiculum to CA1 back-projection, and present recent conclusive anatomical evidence for the presence of non-canonical subicular projections to CA1. New insights into this under-studied pathway will improve our understanding of reciprocal CA1-subicular connections and guide future studies on how the subiculum interacts with CA1 to regulate hippocampal circuit activity and learning and memory behaviors.
Keywords: hippocampus, back projection, viral tracing, genetic targeting, learning and memory
Graphical Abstract
In this article, we revisit the published studies on non-canonical connections from the subiculum to CA1, and review recent conclusive evidence for the presence of excitatory and inhibitory subicular projections to CA1. New insights into this under-studied pathway will improve our understanding of reciprocal CA1-subicular connections and guide future studies.
Introduction
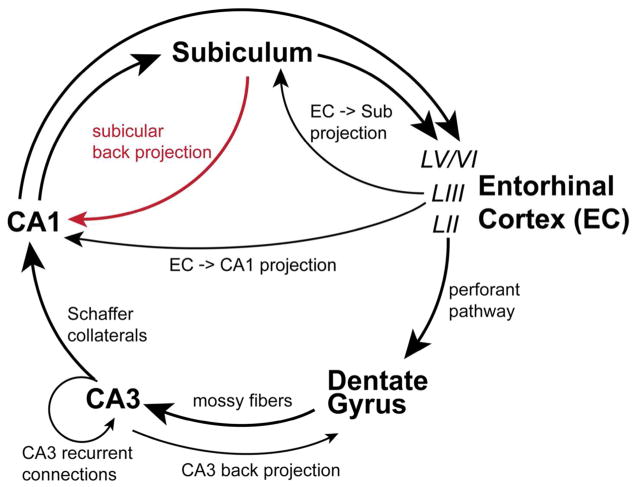
It has been generally considered that the hippocampal formation 1 is strongly feed-forward in terms of the directionality of its information flow (Figure 1). Within this framework, the subiculum is viewed as one of the two major output targets of the hippocampus proper, Cornu Ammonis (CA) (O’Mara et al., 2001, O’Mara, 2005, Witter, 2006, Cenquizca and Swanson, 2007), with the other major output target of the hippocampus being the entorhinal cortex (EC). However, accumulating evidence starts to challenge the traditional view of the unidirectional projection between hippocampal CA1 and the subiculum. While this characterization of feed-forward circuit connections has been conceptually useful for our understanding of learning and memory processes, but it is now time to consider the largely unexplored role of the subicular back-projection to the hippocampus.
Figure 1. Canonical hippocampal circuitry and the non-canonical subicular-CA1 pathway.
The diagram depicts the non-canonical and canonical circuitry of the hippocampal formation. Evidence herein describes non-canonical back projections from the subiculum to CA1 (red line). Feed-forward and unidirectional canonical projections are depicted as black lines with large directional arrows. The trisynaptic circuit connections are made up of layer II (LII) entorhinal cortex (EC) projections to the dentate gyrus via the perforant pathway, projections of the dentate granule cells to area CA3 pyramidal neurons via mossy fibers, and CA3 projections to area CA1 pyramidal neurons via Schaffer collaterals. CA1 transfers excitatory information out of the hippocampus proper via direct projections to deep layers (layers V and VI, LV/VI) of the entorhinal cortex or to the subiculum. CA2 is described in the text but is not depicted in this diagram. Additional excitatory projections within the hippocampal formation include layer III (LII) entorhinal neurons projections to CA1 and the subiculum (the temporoammonic pathway), and local recurrent collaterals of CA3 pyramidal cells onto other CA3 pyramidal cells. Also the back-projection of CA3 pyramidal neurons to the dentate gyrus has been described.
The hippocampal trisynaptic excitatory pathway consists of layer II EC stellate neuronal projections to the dentate gyrus, projections of the dentate granule cells to area CA3 pyramidal neurons, and CA3 projections to area CA1 pyramidal neurons. As a relatively small area interposed between CA3 and CA1, area CA2 receives inputs from CA3 and EC layer II/III neurons (Chevaleyre and Siegelbaum, 2010, Hitti and Siegelbaum, 2014, San Antonio et al., 2014), and makes strong projections to stratum oriens and weaker projections to stratum radiatum of CA1 (Hitti and Siegelbaum, 2014). CA1 transfers excitatory information out of the hippocampus either directly or via a dense projection to the subiculum (Amaral and Witter, 1989, Amaral, 1993, Naber et al., 2001). With respect to EC projections, the subiculum can either project directly into the deep layers of entorhinal cortex, or project to the adjacent presubiculum and parasubiculum which then projects to the entorhinal cortex (Canto et al., 2012, O’Reilly et al., 2013). While these circuit connections contribute to the predominantly unidirectional entorhinal –> hippocampal -> entorhinal loop, there are other alternative circuits that provide different pathways for information flow (Figure 1). For example, layer III entorhinal pyramidal neurons project directly onto the pyramidal neurons of the subiculum and CA1 (the temporoammonic pathway). Recurrent collaterals of CA3 pyramidal cells onto other CA3 pyramidal cells mediate synchronous firing within the CA3 region (Kali and Dayan, 2000, Le Duigou et al., 2014). Both morphological and physiological evidence suggests that CA3 and the dentate are synchronized via a back-projection from CA3 to dentate granule cells. (Li et al., 1994, Scharfman, 2007, Shi et al., 2014). Moreover, it has been demonstrated that in contrast to the typical notion of feed-forward unidirectional communication from CA3-CA1 to the subiculum, hippocampal theta network activity can flow ‘in reverse’ from the subiculum to CA1 and CA3 to actively modulate spike timing and local network rhythms in these subregions (Jackson et al., 2014).
Within this context, we revisit the under-described non-canonical connections from the subiculum to CA1 (Figure 1). In this article, we first summarize the earlier anatomical and physiological studies supporting the feedback projections from the subiculum to CA1. We then present results of new viral tracing studies that firmly establish this pathway and provide a foundation for further investigation. Specifically, we will discuss below whether excitatory feedforward signaling from CA1 to the subiculum may be modulated by inhibitory or excitatory feedback to CA1, as determined by subiculum activity. Last, we will discuss outstanding questions and directions for further analysis of subicular projections to CA1, and propose future studies to examine functional implications of non-canonical subicular projections.
Existing evidence of subicular projections to CA1
The earliest evidence for a backward projection from the subiculum to area CA1 came from conventional anatomical tracing studies in the 1980s. Berger et al. (1980) first reported the projections from the subicular region to hippocampal CA1. They examined anatomical connections between the dorsal hippocampus and subiculum in the rabbit, using then state-of-the-art horseradish peroxidase (HRP) and autoradiographic tracing methods. They found that HRP injections into hippocampal CA1 resulted in retrograde labeling in the subiculum, suggesting a direct connection from the subiculum to the CA1 (Berger et al., 1980). In the same paper, they performed further experiments with injections of tritiated amino acids into the subiculum to show anterograde label in CA1 stratum oriens and stratum moleculare. Their study also suggests that a localized subset of cells within the subiculum may project to CA1 rather than the projection originating throughout the entire subiculum. Using intracellular injection of HRP, Finch et al. (1983) demonstrated axonal projections of select subicular pyramidal neurons to hippocampal CA1 stratum oriens in the rat (Finch et al., 1983). Similarly, axons of neurobiotin-labeled subicular pyramidal neurons (5 out of 50 cells) were visualized in the apical dendritic region of CA1 by Harris and Stewart (2001). In addition, Kohler (1985) studied the intrahippocampal projections of the subicular complex with the aid of the anterogradely transported lectin, Phaseolus vulgaris leucoagglutinin (PHA-L). He found that a rostrally directed projection from the subiculum innervates all layers of CA1 and the molecular layer of CA2/CA3, as well as the molecular layer of the dentate gyrus. These tracing results were supported by Witter et al. (1990), which showed that PHA-L injections in the proximal part of the subiculum labels axonal fibers in CA1 (Witter et al., 1990).
The anatomical evidence for subicular projections to area CA1 is supported functionally by several electrophysiological studies. Shao and Dudek (2005) used focal flash photolysis of caged glutamate in adult rat slices and showed that CA1 pyramidal cells receive excitatory synaptic input originating from the subiculum. In slices disinhibited with bicuculline block of GABAergic inhibition, 25% (6 of 24) of the recorded CA1 pyramidal cells consistently generated repetitive excitatory postsynaptic currents (EPSCs) in response to glutamate stimulation in the subiculum. The responsive neurons are located 200–500 μm from the distal end of CA1 and 400–1100 μm from the stimulation sites in the subiculum. Repeated glutamate stimulation robustly evoked repetitive EPSCs lasting for a few hundred milliseconds. Given that glutamate photolysis cannot stimulate axonal fibers of passage, this study provided strong evidence that there are excitatory synaptic projections from the subicular neurons to CA1 pyramidal cells. The relatively short latency difference between the first action potential in the subiculum and the first EPSC recorded in CA1 in response to flash glutamate stimulation strongly suggests that these EPSCs are not mediated by polysynaptic intervening connections between the subiculum and CA1 (i.e., subiculum → EC → hippocampus).
Using isolated brain slices containing only CA1 and the subiculum, Harris and Stewart (2001) reported backward propagation of synchronous epileptiform events (produced by bathing slices in a reduced magnesium solution) from the subiculum into area CA1, with events in CA1 always following events in the proximal subiculum at a conduction velocity of 0.04 m/s. Full transections between CA1 and the subiculum were required to block the propagation. Following complete transections, spontaneous events were measured in all subiculum fragments but absent in CA1 fragments. The authors concluded that a subiculum - CA1 circuit serves to synchronize activities between both regions and maintains enhanced activation of subicular and CA1 pyramidal neurons. More recently, Jackson et al. (2014) showed in multi-electrode array recordings that in the intact isolated rat hippocampus (removed from subcortical and cortical afferent inputs to the hippocampus while keeping all intrinsic hippocampal circuits intact), spontaneous theta rhythms generated in the subiculum back-propagate to CA1 and CA3. This work reveals a previously undescribed form of reversed intra-hippocampal theta rhythm signaling. Their local field recordings in behaving rats also suggests that this reversed theta rhythm influences the majority of theta epochs in vivo (especially during REM sleep) and modulates the timing of theta and spiking within CA3. These findings are supported by an earlier in vivo physiological study (Commins et al., 2002) with stimulating electrodes implanted in the dorsal subiculum of the rat to elicit CA1 responses which show a possible projection from the subiculum to hippocampal area CA1. However, Commins et al. (2002) were unable to record LTP in CA1 following high-frequency stimulation in the subiculum. This prompted the proposal that the subiculum-CA1 backward projection may have an inhibitory effect on area CA1. This suggestion gained support from a more recent slice physiology study (Craig and McBain, 2015) which demonstrated that disconnecting the subiculum increased gamma frequencies pharmacologically induced in rat CA1. Again it was proposed that this is perhaps due to removal of an inhibitory feedback mechanism from the subiculum to CA1.
Why wasn’t the subiculum-CA1 pathway determined earlier with greater certainty? The previously cited anatomical studies (which were done in rabbits and rats) that indicated back projections from the subiculum to CA1 were affected by potential technical limitations of conventional tracer injections, which limited reliability and interpretation of data. These injections can be leaky and lead to off target uptakes as axons of passage can readily take up these tracers. Moreover, PHA-L and HRP tracing techniques are limited in that they cannot provide information regarding the restricted molecular or functional make-up of the neurons involved. Further, in the previous electrophysiological studies, extracellular electrical stimulation can activate axonal fibers of passage and local field recordings do not have the spatial resolution needed for studying topographic mapping of projections. Together, these methodological caveats have tempered the interpretations of findings that indicate subicular back-projections to area CA1. Thus, further studies of this pathway using new methods that overcome previous technical limitations are warranted.
Monosynaptic rabies tracing establishes direct subicular connections to the hippocampus
New advances in virology and genetic technology offer powerful tools for mapping neural circuit connectivity and function to complement more traditional approaches. Among them, genetically modified rabies tracing has proved to be a useful mapping tool for identifying direct circuit inputs to specific neuronal types (Wickersham et al., 2007b, Marshel et al., 2010, Wall et al., 2010, Nakashiba et al., 2012). The modifications of rabies virus allows the initial infection with rabies to be restricted to particular genetically targeted neurons, and restrict retrograde spread of the virus to monosynaptic connections. In order to map direct synaptic connections to selected neurons, this approach requires that a starter population expresses both the EnvA receptor, TVA, and rabies glycoprotein (RG). This population is then selectively infected with an EnvA-pseudotyped, RG-deleted rabies virus. Together, this conditional intersection results in transcomplementation and monosynaptic retrograde spread of the rabies virus to presynaptic neurons of the starter population. Because rabies replicates its core within infected cells, this enables intense fluoresce with strong transgene expression and reveals fine dendritic and axonal structures. This approach does have temporal limits. Despite the relatively low initial cytopathic effects, rabies infection beyond two weeks impairs the health of infected neurons (Wickersham et al., 2007a). Thus within two weeks following infection, genetically modified rabies virus is an effective tool for labeling trans-synaptic neuronal circuits (Wickersham et al., 2007a, Osakada et al., 2011).
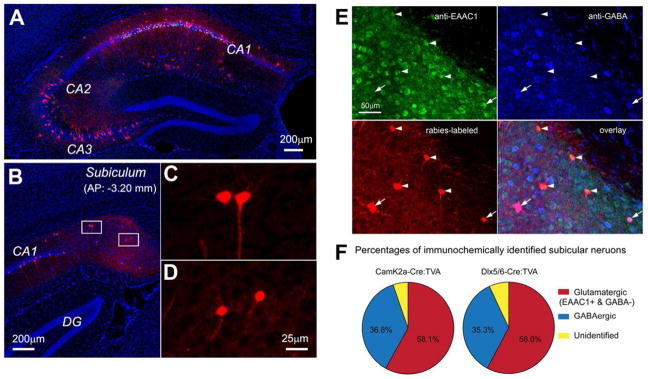
Our group developed and applied a Cre-dependent, genetically modified rabies-based tracing system to map local and long-range monosynaptic connections to specific types of neurons in the intact brain. This is achieved by genetically targeting neuron types defined by their Cre expression, including excitatory pyramidal cells (Camk2a-Cre) and inhibitory cell types (Dlx5/6-Cre) in CA1 of the mouse hippocampus (Sun et al., 2014). Using this approach, we generated a map of circuit connections of excitatory and inhibitory cell types in the mouse hippocampus. To establish that the “leak” is not an issue, our first level of analysis was to visualize the injection site and ensure that double-labeled starter cells were restricted to CA1. Starter CA1 cells in brain sections were unambiguously identified by their GFP and mCherry expression from the helper AAV and G-mCherry rabies genomes, respectively (Figures 2A, 2C, and 2D). For mapping rabies-labeled presynaptic neurons (expressing mCherry only), every section was examined to identify and mark the locations of mCherry-expressing cell bodies. These labeled cells were assigned to specific anatomical structures for regional input quantification.
Figure 2. New monosynaptic rabies tracing firmly demonstrates the non-canonical subicular projections to CA1.
A–D. Example data images illustrating monosynaptic rabies tracing of presynaptic connections to excitatory neurons in hippocampal CA1 of the Camk2a-Cre; TVA mouse. A: Ipsilateral section image of the rabies injection site; mCherry labeling of excitatory neurons is seen throughout CA1. Note that much of the CA1 label is due to primary rabies infection as pyramidal neurons expressing TVA can be directly infected by local injection of EnvA- G rabies. The extent of the CA1 label under this experimental condition is not due to strong recurrent connections within CA1.
B: Ipsilateral image of presynaptic retrograde tracing in the subiculum (Sub) showing non-canonical circuit connections between the subiculum and CA1. C and D: Enlarged views of the boxed areas in B showing the subicular neurons that project axons to CA1. E–F. Immunochemical analysis of subicular neurons projecting to CA1. E: Immunochemical characterization of rabies-labeled, CA1-projecting subicular neurons. The representative subicular section was from monosynaptic rabies tracing of CA1 excitatory pyramidal neurons in the Camk2a-Cre; TVA mouse. The section was stained immunochemically against excitatory amino acid transporter (EEAC1) with arrowheads indicating subicular excitatory neurons projecting to CA1, and against GABA with arrowheads indicating subicular inhibitory interneurons projecting to CA1. The CA1 projecting subicular neurons were labeled by mCherry-expressing rabies. F: Percentage quantification of glutamatergic versus GABAergic CA1 projecting subicular cells revealed by rabies tracing in Camk2a-Cre; TVA (targeting CA1 excitatory neurons) and Dlx5/6-Cre; TVA (targeting CA1 inhibitory neurons) mice. This figure is modified from data figures presented in Sun et al. (2014).
Using genetically targeted rabies tracing, we found unambiguously that there are significant direct inputs from the subiculum to both CA1 excitatory and inhibitory cell types (Sun et al., 2014). This confirms the existence of a subicular-CA1 back-projection pathway in the mouse (Figure 2), as previously suggested in other mammalian species using less strict mapping methods (Berger et al., 1980, Kohler, 1985, Shao and Dudek, 2005). Further, monosynaptic rabies tracing shows that both excitatory and inhibitory subicular neurons project to CA1 (Figure 2B–E), and that both CA1 excitatory neurons and inhibitory interneurons are innervated by subicular inputs (Figure 2F). Immunochemical analysis indicates that similar proportions of subicular excitatory versus inhibitory cells innervate either CA1 excitatory or inhibitory neurons (Figure 2E–F). These findings suggest new possibilities for understanding subiculum-hippocampal functional circuitry. We propose that the back-projection from the subiculum to CA1 is comprised of a potential balance between both excitatory and inhibitory elements. This allows consideration of a hypothesis that such a back projection could serve to modulate information processing in hippocampal CA1 by acting as an activity-dependent network switch between dampening and amplifying CA1 activity. The modulatory effects proposed by this hypothesis would depend on whether the subicular inhibitory or excitatory components are dominant, thus promoting different learning or spatial navigational behaviors.
The subicular inputs to CA1 are relatively strong in terms of circuit connection strengths (Sun et al., 2014). We operationally define input connection strength index (CSI) as the ratio of the number of presynaptic neurons versus the number of targeted postsynaptic (starter) neurons. The overall strength of subicular back-projections to CA1 inhibitory interneurons (CSI: 1.00 ± 0.20) is comparable to CA1 excitatory pyramidal neurons (CSI: 0.81 ± 0.01) (Sun et al., 2014). The connectivity strength of subicular inputs to CA1 is lower than the connectivity strength between CA1 and CA3 (CA3 provides the strongest input to CA1). The CSIs of CA1 pyramidal neurons for their presynaptic CA3 excitatory cells are 7.10 ± 0.28 (ipsilateral) and 3.44 ± 0.22 (contralateral). The strength of subicular-CA inputs is close to that of the medial septum and diagonal band (MS-DB) projections to CA1. The overall CSI for MS-DB neurons to CA1 neuron is between 0.95 and 1.17. This suggests that non-canonical back projections from the subiculum to CA1 are modulatory, similar to the MS-DB projections to CA1.
Relevant to the subicular back projection to the hippocampus, adult-born dentate granule neurons were found to receive an input from the subiculum (Deshpande et al., 2013). This subicular connection to the dentate gyrus was not previously identified, but was supported by Kohler (1985). He found that the subicular complex sends a minor projection to the molecular layer of the dentate gyrus using PHA-L tracing injections. Considering that hippocampal activity can flow ‘in reverse’ (i.e., reversed theta signaling) from the subiculum to CA3 (Jackson et al., 2014), it would be worth performing rabies tracing to test if hippocampal CA3 receives direct subicular inputs as does CA1.
Outstanding questions guiding further analysis of subicular projections to CA1
The new rabies tracing study in the mouse indicates that both excitatory and inhibitory cell types are involved and suggests previously unconsidered potential roles for direct subicular modulation of hippocampal CA1 circuit activity. To gain a deeper understanding of functional implications of the subiculum-CA1 back-projection pathway, the following questions need to be addressed. (1) How do different subclasses of CA1-projecting subicular neurons provide synaptic inputs to specific CA1 neuronal types? (2) What are the topographical and functional relationships of projections from the subiculum to CA1? (3) Do the CA1-projecting subicular neurons exhibit distinct physiological characteristics? (4) Do the subiculum to CA1 projections modulate rhythm synchrony and amplitude within and between hippocampal regions? (5) Do subiculum -> CA1 projections modulate hippocampus-associated learning and memory behaviors?
As a first step, direct subicular-CA1 synaptic inputs need to be physiologically validated. Subcellular ChR2-assisted circuit mapping (sCRACM) analysis (Petreanu et al., 2009, Mao et al., 2011) could be used to map subicular inputs to CA1 neurons. Given the differential roles of diverse CA1 inhibitory interneurons involved in feedforward and feedback operations, it will be important to determine how excitatory pyramidal neurons and major inhibitory CA1 cell types differ in spatiotemporal properties of their subicular inputs.
Addressing the question of whether the subiculum-CA1 projection shows a specific topographic relationship as seen in the CA1-subiculum projection is important. It is known that the CA1 projection to the subiculum has a mirrored topography so that distal CA1 projects to proximal subiculum, and proximal CA1 projects to distal subiculum (Amaral et al., 1991, Amaral, 1993). However, it remains to be seen if the subiculum shows a similar transverse topography in its projection to CA1. Given that intrahippocampal connections are dense and largely contained in some planes of sections oriented perpendicularly to the long axis of the hippocampus, this would allow for the examination of topographic subiculum-CA1 connections in brain slice preparations. The notion of a topographic projection from the subiculum to CA1 is supported by previous slice mapping experiments (Shao and Dudek, 2005). They showed that CA1 responses were limited to a particular range from the CA1-Sub border (200–500μm) (Shao and Dudek, 2005). Further, CA1 responses reliably follow stimulation of the subiculum, particularly proximal subiculum, which supports the idea that there is a shared topography between CA1 and subiculum interactions (Harris and Stewart, 2001). It will be important to follow up the initial rabies-based mapping study (Sun et al., 2014) to investigate topographical relationships of subiculum-CA1 projections in vivo. Monosynaptic rabies tracing from different CA1 segments (i.e., proximal, intermediate and distal CA1) would allow examining differential distributions of CA1-projecting subicular neurons along the proximal-distal axis. This will test if there are any specific projection relationships, and show whether distal and proximal CA1 regions have differential strengths of subicular innervation.
In addition, it is not yet known whether CA1-projecting subicular neurons are a unique neuronal group with distinct physiological properties and circuit connections as compared to other subicular neuronal populations. This is particularly relevant as subicular pyramidal neurons are categorized into two subpopulations based on their firing rates, regular-spiking (RS) and burst-firing (BF) neurons (Stewart and Wong, 1993, Taube, 1993, Sharp and Green, 1994, Staff et al., 2000, Kim and Spruston, 2012). Although it is reported that RS outnumber BF cells 2:1 across the entire subiculum (Menendez de la Prida et al., 2003, Knopp et al., 2005), BF neurons are concentrated in the distal region of the subiculum while RS neurons are preferentially distributed in the region proximal to the CA1/subiculum border (Staff et al., 2000, Menendez de la Prida et al., 2003). The relationship between the firing properties and the targets of subicular pyramidal neurons has been examined by Kim and Spruston (2012). They used in vivo injections of retrogradely transported fluorescent beads into each of nine different regions and conducted whole-cell current-clamp recordings from the bead-containing subicular neurons in acute brain slices. Their study found that neurons projecting to amygdala, lateral entorhinal cortex, nucleus accumbens, and medial/ventral orbito-frontal cortex are located primarily in the proximal subiculum and consist mostly of regular-spiking neurons (~80%). In contrast, neurons projecting to medial EC, presubiculum, retrosplenial cortex, and ventromedial hypothalamus are located primarily in the distal subiculum and consist mostly of bursting neurons (~80%). Ishizuka (2001) also reported the differences in connectional targets among subicular pyramidal neurons, as nucleus accumbens-projecting cells are more prominent in the proximal half of the subiculum, whereas thalamic nucleus-projecting cells are distributed toward the distal half of the subiculum (Ishizuka, 2001).
Considering that RS neurons are preferentially distributed in proximal subiculum, it is possible that RS neurons are the preferential subicular cell type that projects back to CA1. A combinatorial viral strategy could distinguish CA1-projecting subicular neurons. Specifically, canine adenovirus 2 (CAV2)-mediated retrograde Cre expression could be used to selectively label CA1-projecting subicular neurons for physiological characterization and genetic manipulation of their physiological activities (Gore et al., 2013, Schwarz et al., 2015). In addition, Cre-dependent retrograde rabies tracing and anterograde herpes simplex virus (H129 strain) (Lo and Anderson, 2011) are useful for mapping circuit connections of these subicular neurons.
The anatomy and physiology suggests that future studies on the functional roles of subiculum-CA1 projections are merited. Given the intriguing presence of both excitatory and inhibitory subicular projections to CA1 and their comparably balanced inputs, it would be interesting to examine if these back-projections amplify or dampen large network synchronies within and between these two regions in the hippocampal formation. New and emerging techniques should reveal answers to questions of how subiculum to CA1 projections potentially modulate hippocampal network activity, and what roles subiculum-CA1 projections may play in hippocampus-associated learning and memory behaviors. Ziv et al. (2013) used a miniaturized microscope for Ca2+ imaging in freely behaving mice and tracked somatic response dynamics of hundreds and thousands of CA1 pyramidal cells in one field of view per mouse. Large scale neuronal population imaging of CA1 and the subiculum at single-cell resolution in freely behaving animals combined with genetic cell targeting could resolve how CA1-projecting subicular neurons modulate CA1 place cell activities and ensemble representation of the environment.
Conclusions
In conclusion, accumulating evidence establishes that there is a non-canonical but significant set of subiculum -> CA1 projections. The under-appreciated bidirectional connections of the subiculum and hippocampal CA1 require further study to update the canonical model of hippocampal circuit organization. The non-canonical subiculum-hippocampal projections may regulate network correlation and activity synchrony events that underlie different behavioral states. Future investigations will refine our understanding of how this circuit modulates learning and spatial navigation behaviors.
Acknowledgments
This work was supported by US National Institutes of Health (NIH) grants R01 NS078434 and R01 MH105427 to X.X. TCH is supported by NIH grants R01 GM102965 and R01 GM107405.
Footnotes
In this paper, the hippocampal formation includes the dentate gyrus, hippocampus proper, subiculum, presubiculum, parasubiculum and the entorhinal cortex.
Author contributions: X.X. wrote the manuscript, Y.S. and A.J.L. helped with initial writing, conception and figure making. T.C.H. edited and revised the manuscript.
Statement of conflict of interests
All authors disclose no conflict of interests for this work.
References
- Amaral DG. Emerging principles of intrinsic hippocampal organization. Current opinion in neurobiology. 1993;3:225–229. doi: 10.1016/0959-4388(93)90214-j. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Dolorfo C, Alvarez-Royo P. Organization of CA1 projections to the subiculum: a PHA-L analysis in the rat. Hippocampus. 1991;1:415–435. doi: 10.1002/hipo.450010410. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Berger TW, Swanson GW, Milner TA, Lynch GS, Thompson RF. Reciprocal anatomical connections between hippocampus and subiculum in the rabbit evidence for subicular innervation of regio superior. Brain Res. 1980;183:265–276. doi: 10.1016/0006-8993(80)90463-1. [DOI] [PubMed] [Google Scholar]
- Canto CB, Koganezawa N, Beed P, Moser EI, Witter MP. All layers of medial entorhinal cortex receive presubicular and parasubicular inputs. J Neurosci. 2012;32:17620–17631. doi: 10.1523/JNEUROSCI.3526-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenquizca LA, Swanson LW. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res Rev. 2007;56:1–26. doi: 10.1016/j.brainresrev.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Siegelbaum SA. Strong CA2 pyramidal neuron synapses define a powerful disynaptic cortico-hippocampal loop. Neuron. 2010;66:560–572. doi: 10.1016/j.neuron.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commins S, Aggleton JP, O’Mara SM. Physiological evidence for a possible projection from dorsal subiculum to hippocampal area CA1. Exp Brain Res. 2002;146:155–160. doi: 10.1007/s00221-002-1158-x. [DOI] [PubMed] [Google Scholar]
- Craig MT, McBain CJ. Fast gamma oscillations are generated intrinsically in CA1 without the involvement of fast-spiking basket cells. J Neurosci. 2015;35:3616–3624. doi: 10.1523/JNEUROSCI.4166-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande A, Bergami M, Ghanem A, Conzelmann KK, Lepier A, Gotz M, Berninger B. Retrograde monosynaptic tracing reveals the temporal evolution of inputs onto new neurons in the adult dentate gyrus and olfactory bulb. Proc Natl Acad Sci U S A. 2013;110:E1152–1161. doi: 10.1073/pnas.1218991110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch DM, Nowlin NL, Babb TL. Demonstration of axonal projections of neurons in the rat hippocampus and subiculum by intracellular injection of HRP. Brain Res. 1983;271:201–216. doi: 10.1016/0006-8993(83)90283-4. [DOI] [PubMed] [Google Scholar]
- Gore BB, Soden ME, Zweifel LS. Manipulating gene expression in projection-specific neuronal populations using combinatorial viral approaches. Curr Protoc Neurosci. 2013;4:4 35 31–34 35 20. doi: 10.1002/0471142301.ns0435s65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E, Stewart M. Propagation of synchronous epileptiform events from subiculum backward into area CA1 of rat brain slices. Brain Res. 2001;895:41–49. doi: 10.1016/s0006-8993(01)02023-6. [DOI] [PubMed] [Google Scholar]
- Hitti FL, Siegelbaum SA. The hippocampal CA2 region is essential for social memory. Nature. 2014;508:88–92. doi: 10.1038/nature13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka N. Laminar organization of the pyramidal cell layer of the subiculum in the rat. The Journal of comparative neurology. 2001;435:89–110. doi: 10.1002/cne.1195. [DOI] [PubMed] [Google Scholar]
- Jackson J, Amilhon B, Goutagny R, Bott JB, Manseau F, Kortleven C, Bressler SL, Williams S. Reversal of theta rhythm flow through intact hippocampal circuits. Nat Neurosci. 2014;17:1362–1370. doi: 10.1038/nn.3803. [DOI] [PubMed] [Google Scholar]
- Kali S, Dayan P. The involvement of recurrent connections in area CA3 in establishing the properties of place fields: a model. J Neurosci. 2000;20:7463–7477. doi: 10.1523/JNEUROSCI.20-19-07463.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Spruston N. Target-specific output patterns are predicted by the distribution of regular-spiking and bursting pyramidal neurons in the subiculum. Hippocampus. 2012;22:693–706. doi: 10.1002/hipo.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopp A, Kivi A, Wozny C, Heinemann U, Behr J. Cellular and network properties of the subiculum in the pilocarpine model of temporal lobe epilepsy. The Journal of comparative neurology. 2005;483:476–488. doi: 10.1002/cne.20460. [DOI] [PubMed] [Google Scholar]
- Kohler C. Intrinsic projections of the retrohippocampal region in the rat brain. I. The subicular complex. The Journal of comparative neurology. 1985;236:504–522. doi: 10.1002/cne.902360407. [DOI] [PubMed] [Google Scholar]
- Le Duigou C, Simonnet J, Telenczuk MT, Fricker D, Miles R. Recurrent synapses and circuits in the CA3 region of the hippocampus: an associative network. Front Cell Neurosci. 2014;7:262. doi: 10.3389/fncel.2013.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XG, Somogyi P, Ylinen A, Buzsaki G. The hippocampal CA3 network: an in vivo intracellular labeling study. The Journal of comparative neurology. 1994;339:181–208. doi: 10.1002/cne.903390204. [DOI] [PubMed] [Google Scholar]
- Lo L, Anderson DJ. A Cre-dependent, anterograde transsynaptic viral tracer for mapping output pathways of genetically marked neurons. Neuron. 2011;72:938–950. doi: 10.1016/j.neuron.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao T, Kusefoglu D, Hooks BM, Huber D, Petreanu L, Svoboda K. Long-range neuronal circuits underlying the interaction between sensory and motor cortex. Neuron. 2011;72:111–123. doi: 10.1016/j.neuron.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshel JH, Mori T, Nielsen KJ, Callaway EM. Targeting single neuronal networks for gene expression and cell labeling in vivo. Neuron. 2010;67:562–574. doi: 10.1016/j.neuron.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez de la Prida L, Suarez F, Pozo MA. Electrophysiological and morphological diversity of neurons from the rat subicular complex in vitro. Hippocampus. 2003;13:728–744. doi: 10.1002/hipo.10123. [DOI] [PubMed] [Google Scholar]
- Naber PA, Lopes da Silva FH, Witter MP. Reciprocal connections between the entorhinal cortex and hippocampal fields CA1 and the subiculum are in register with the projections from CA1 to the subiculum. Hippocampus. 2001;11:99–104. doi: 10.1002/hipo.1028. [DOI] [PubMed] [Google Scholar]
- Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, Rodriguez Barrera V, Chittajallu R, Iwamoto KS, McBain CJ, Fanselow MS, Tonegawa S. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell. 2012;149:188–201. doi: 10.1016/j.cell.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mara S. The subiculum: what it does, what it might do, and what neuroanatomy has yet to tell us. J Anat. 2005;207:271–282. doi: 10.1111/j.1469-7580.2005.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mara SM, Commins S, Anderson M, Gigg J. The subiculum: a review of form, physiology and function. Prog Neurobiol. 2001;64:129–155. doi: 10.1016/s0301-0082(00)00054-x. [DOI] [PubMed] [Google Scholar]
- O’Reilly KC, Gulden Dahl A, Ulsaker Kruge I, Witter MP. Subicular-parahippocampal projections revisited: development of a complex topography in the rat. The Journal of comparative neurology. 2013;521:4284–4299. doi: 10.1002/cne.23417. [DOI] [PubMed] [Google Scholar]
- Osakada F, Mori T, Cetin AH, Marshel JH, Virgen B, Callaway EM. New rabies virus variants for monitoring and manipulating activity and gene expression in defined neural circuits. Neuron. 2011;71:617–631. doi: 10.1016/j.neuron.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreanu L, Mao T, Sternson SM, Svoboda K. The subcellular organization of neocortical excitatory connections. Nature. 2009;457:1142–1145. doi: 10.1038/nature07709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Antonio A, Liban K, Ikrar T, Tsyganovskiy E, Xu X. Distinct physiological and developmental properties of hippocampal CA2 subfield revealed by using anti-Purkinje cell protein 4 (PCP4) immunostaining. The Journal of comparative neurology. 2014;522:1333–1354. doi: 10.1002/cne.23486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE. The CA3 “backprojection” to the dentate gyrus. Prog Brain Res. 2007;163:627–637. doi: 10.1016/S0079-6123(07)63034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz LA, Miyamichi K, Gao XJ, Beier KT, Weissbourd B, DeLoach KE, Ren J, Ibanes S, Malenka RC, Kremer EJ, Luo L. Viral-genetic tracing of the input-output organization of a central noradrenaline circuit. Nature. 2015;524:88–92. doi: 10.1038/nature14600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao LR, Dudek FE. Electrophysiological evidence using focal flash photolysis of caged glutamate that CA1 pyramidal cells receive excitatory synaptic input from the subiculum. J Neurophysiol. 2005;93:3007–3011. doi: 10.1152/jn.00877.2004. [DOI] [PubMed] [Google Scholar]
- Sharp PE, Green C. Spatial correlates of firing patterns of single cells in the subiculum of the freely moving rat. J Neurosci. 1994;14:2339–2356. doi: 10.1523/JNEUROSCI.14-04-02339.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Ikrar T, Olivas ND, Xu X. Bidirectional global spontaneous network activity precedes the canonical unidirectional circuit organization in the developing hippocampus. The Journal of comparative neurology. 2014;522:2191–2208. doi: 10.1002/cne.23528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staff NP, Jung HY, Thiagarajan T, Yao M, Spruston N. Resting and active properties of pyramidal neurons in subiculum and CA1 of rat hippocampus. J Neurophysiol. 2000;84:2398–2408. doi: 10.1152/jn.2000.84.5.2398. [DOI] [PubMed] [Google Scholar]
- Stewart M, Wong RK. Intrinsic properties and evoked responses of guinea pig subicular neurons in vitro. Journal of neurophysiology. 1993;70:232–245. doi: 10.1152/jn.1993.70.1.232. [DOI] [PubMed] [Google Scholar]
- Sun Y, Nguyen AQ, Nguyen JP, Le L, Saur D, Choi J, Callaway EM, Xu X. Cell-type-specific circuit connectivity of hippocampal CA1 revealed through Cre-dependent rabies tracing. Cell Rep. 2014;7:269–280. doi: 10.1016/j.celrep.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS. Electrophysiological properties of neurons in the rat subiculum in vitro. Experimental brain research. 1993;96:304–318. doi: 10.1007/BF00227110. [DOI] [PubMed] [Google Scholar]
- Wall NR, Wickersham IR, Cetin A, De La Parra M, Callaway EM. Monosynaptic circuit tracing in vivo through Cre-dependent targeting and complementation of modified rabies virus. Proc Natl Acad Sci U S A. 2010;107:21848–21853. doi: 10.1073/pnas.1011756107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickersham IR, Finke S, Conzelmann KK, Callaway EM. Retrograde neuronal tracing with a deletion-mutant rabies virus. Nat Methods. 2007a;4:47–49. doi: 10.1038/NMETH999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickersham IR, Lyon DC, Barnard RJ, Mori T, Finke S, Conzelmann KK, Young JA, Callaway EM. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron. 2007b;53:639–647. doi: 10.1016/j.neuron.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter MP. Connections of the subiculum of the rat: topography in relation to columnar and laminar organization. Behav Brain Res. 2006;174:251–264. doi: 10.1016/j.bbr.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Witter MP, Ostendorf RH, Groenewegen HJ. Heterogeneity in the Dorsal Subiculum of the Rat. Distinct Neuronal Zones Project to Different Cortical and Subcortical Targets. Eur J Neurosci. 1990;2:718–725. doi: 10.1111/j.1460-9568.1990.tb00462.x. [DOI] [PubMed] [Google Scholar]