Abstract
Purpose
Glucagon-like peptide-1 (GLP-1) is originally identified in the gut as an incretin hormone, and it is potent in stimulating insulin secretion in the pancreas. However, increasing evidence suggests that GLP-1 is also produced locally within pancreatic islets. This review focuses on the past and current discoveries regarding intra-islet GLP-1 production and its functions.
Main findings
There has been a long-standing debate with regard to whether GLP-1 is produced in the pancreatic α cells. Early controversies lead to the widely accepted conclusion that the vast majority of proglucagon is processed to form glucagon in the pancreas, whereas an insignificant amount is cleaved to produce GLP-1. With technological advancements, recent studies have shown that bioactive GLP-1 is produced locally in the pancreas, and the expression and secretion of GLP-1 within islets are regulated by various factors such as cytokines, hyperglycemia, and β cell injury.
Conclusions
GLP-1 is produced by the pancreatic α cells, and it is fully functional as an incretin. Therefore, intra-islet GLP-1 may exert insulinotropic and glucagonostatic effects locally via paracrine and/or autocrine actions, under both normal and diabetic conditions.
Keywords: GLP-1, alpha cells, islets, diabetes, PC1/3
1. Introduction
Originally discovered in the gut, Glucagon-like peptide-1 (GLP-1) is one of a few peptide hormones encoded by the proglucagon gene (Bell, et al. 1983; Drucker, et al. 1986; Heinrich, et al. 1984; Lopez, et al. 1983; Mojsov, et al. 1986). Derived from the same precursor—proglucagon, GLP-1 and glucagon share ~50% homology. The diversification of the two peptides takes place at the post-translational level in a tissue-specific manner (Fig. 1). In the α-cells of pancreatic islets, proglucagon is primarily cleaved by prohormone convertase 2 (PC2), resulting in the production of glucagon, the major proglucagon fragment (MPGF), glicentin-related polypeptide (GRPP), and intervening peptide-1 (IP-1) (Patzelt and Schiltz 1984; Patzelt, et al. 1979; Rouille, et al. 1994b). In enteroendocrine L-cells, proglucagon is mostly processed by prohormone convertase 1/3 (PC1/3), giving rise to GLP-1, GLP-2, and several other small peptides (Dhanvantari and Brubaker 1998; Rouille, et al. 1995).
Fig. 1. Diagram of posttranslational processing of proglucagon mediated by PC1/3 and PC2.
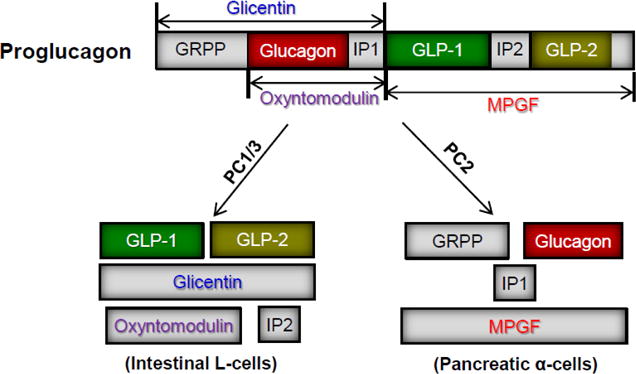
GRPP: glicentin-related polypeptide; IP: intervening peptide; MPGF: major proglucagon fragment.
GLP-1 exists in three forms: the–full length GLP-1 containing 37 amino acid residues (aa), the N-terminally truncated GLP-1 (7–37aa), and an amidated version of the truncated form—GLP-1 (7–36)amide. Among them, GLP-1 (7–36)amide has the highest biological activity (Drucker et al. 1986; Orskov, et al. 1989), although some other studies have shown the non-amidated and amidated forms of the truncated GLP-1 peptide are equally effective in stimulating insulin release from perfused pancreas (Suzuki, et al. 1989; Weir, et al. 1989).
GLP-1 has many effects on multiple organ systems related to nutrient ingestion and blood glucose homeostasis. In the gastrointestinal system, GLP-1 has been shown to reduce gastric acid secretion, inhibit gastrointestinal motility, and slow gastric emptying (Meier, et al. 2003; Salehi, et al. 2008). GLP-1 also suppresses appetite in both normal and obese individuals through the nervous system, thereby reducing food intake (Turton, et al. 1996; Williams, et al. 2009). In the pancreas, GLP-1 stimulates insulin secretion from islet β cells in a blood-glucose dependent manner, while simultaneously inhibiting glucagon secretion in α cells, thus playing an essential role in blood glucose regulation (Fehmann and Habener 1992; Holst 2007; Kieffer and Habener 1999; Mojsov, et al. 1987).
Because of its origin within the intestine and its vital role in stimulating insulin secretion, GLP-1 is deemed a member of the incretin family. However, increasing evidence suggests that GLP-1 is not only produced in the intestinal L-cells, but also in pancreatic α-cells, although normally at much lower levels than glucagon. Of note, the term “α cells” herein represents all endocrine cells expressing proglucagon (instead of glucagon) in the pancreas. There are a number of outstanding and comprehensive reviews regarding general GLP-1 biology, physiology, and its therapeutic use (Baggio and Drucker 2007; Guo, et al. 2016; Holst 2007; Kieffer and Habener 1999; Marathe, et al. 2013; Nauck 2016; Sandoval and D’Alessio 2015). This review, however, will focus on locally produced GLP-1 and its functions within the pancreatic tissue.
2. A history of debates on tissue-specific expression of GLP-1
GLP-1 and its fellow proglucagon-derived peptide, GLP-2, are first identified during investigation of the gene encoding the glucagon precursor, proglucagon (Bell et al. 1983; Lopez et al. 1983; Lund, et al. 1982). Sequence analysis at that time has revealed that, in addition to glucagon, there are two additional segments homologous to glucagon in the precursor. The two segments, termed glucagon-like peptide-1 and 2 (GLP-1 and GLP-2), are flanked by pairs of basic amino acids that are typically associated with the processing of hormones. It was thus suggested that the two peptides and glucagon must have resulted from the posttranslational processing of proglucagon (Bell et al. 1983; Lopez et al. 1983). Since then, studies have discovered that different prohormone convertases, namely PC1/3 and PC2, are responsible for the posttranslational processing of proglucagon (Mineo, et al. 1995; Rothenberg, et al. 1995; Rouille, et al. 1994a; Scopsi, et al. 1995). In particular, the production of glucagon is a result of PC2-mediated proglucagon process, whereas GLP-1 is resulted from PC1/3-mediated cleavage. Therefore, the decision of which hormones are produced from the proglucagon precursor is ultimately a result of differential expression of the PCs in their respective tissues.
The debate regarding tissue-specific GLP-1 expression began when researchers first attempted to identify the tissues and cell types responsible for its production. The original evidence suggesting the existence of GLP-1 comes from cDNA analysis using pancreatic extracts. However, when pancreatic extracts were separated by High Performance Liquid Chromatography (HPLC) and examined by immunoassays, GLP-1 immunoreactivity was detected within a single large peptide, not as a smaller individual peptide, which would have been expected if proglucagon were cleaved to free GLP-1 in the pancreas (George, et al. 1985; Ørskov, et al. 1987; Ørskov, et al. 1986). On the other hand, experimental evidence of GLP-1 expression was reported in studies involving human gastrointestinal mucosa (Baldissera and Holst 1984; Heinrich et al. 1984). Using immunohistochemistry (IHC), radioimmunoassay and chromatography assays, investigators soon found that GLP-1 production was largely limited to the intestinal tract (George et al. 1985; Ørskov et al. 1987; Ørskov et al. 1986; Uttenthel, et al. 1985). Furthermore, a series of secretion studies were performed using isolated porcine pancreas and ilium. The results have shown that, while GLP-1 is secreted as an individual peptide from the intestinal cells, it is not cleaved into individual products in the pancreas—instead, it is secreted as a single large peptide containing GLP-1, GLP-2 and IP-2 (George et al. 1985; Ørskov et al. 1987; Ørskov et al. 1986; Uttenthel et al. 1985).
On the other hand, the argument that GLP-1 is produced not only in the intestine, but also in the pancreas, has also gained support. For instance, GLP-1 immuno-reactivity has been detected in both pancreatic α cells and intestinal L-cells of all species in IHC studies using various monoclonal and polyclonal antibodies against GLP-1 (Eissele, et al. 1992; Kauth and Metz 1987; Varndell, et al. 1985). Several HPLC studies with extracts from human pancreas, glucagonoma, and intestine, have reported the presence of free GLP-1 in these tissues, although its concentration in the pancreas is considerably lower than that of glucagon (George et al. 1985; Uttenthel et al. 1985). Moreover, fully cleaved GLP-1 has been identified from rat, human, and porcine pancreas lysates using HPLC, mass spectrometry and amino acid sequencing (Holst, et al. 1994; Mojsov, et al. 1990), providing additional evidence for the GLP-1 production in the pancreas. Furthermore, other secretion studies suggest that, not only is GLP-1 produced within the pancreas, but is also secreted (Hirota, et al. 1987; Shima, et al. 1987). The biological activity of the secreted GLP-1, however, was questionable, because what detected was mostly the full-length GLP-1, GLP-1 (1–37), in the secretions of perfused pancreas (Hirota et al. 1987; Shima et al. 1987). Based on these early studies, it has been concluded that, although a small amount of proglucagon is cleaved to produce GLP-1, the majority is processed to form glucagon in the pancreas (George et al. 1985; Hirota et al. 1987; Holst et al. 1994; Shima et al. 1987; Uttenthel et al. 1985).
3. Emerging evidence supporting GLP-1 production in pancreatic islets
As antibodies are developed with increasing specificity, and with technology advancements, researchers have been able to determine whether bioactive GLP-1 is produced within pancreatic islets with greater precision and accuracy. Anti-GLP-1 antibodies generated specifically for the truncated and C-terminally amidated GLP-1 are now commercially available (Shown in Fig. 2 is an example). Additional evidence supporting the production of GLP-1 in pancreatic α cells has emerged as a result of these improvements.
Fig. 2. Expression of Glucagon and GLP-1 in human pancreas.
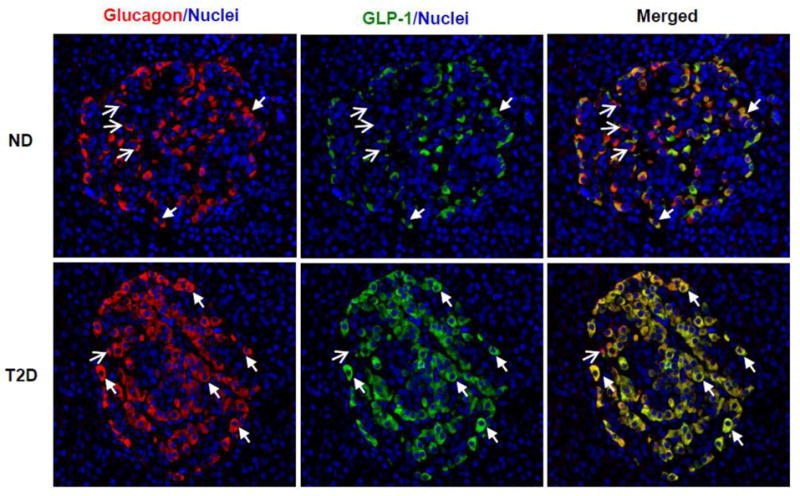
Human pancreatic slices from non-diabetic (ND) and type 2 diabetic (T2D) donors were obtained from the network for Pancreatic Organ Donors (nPOD), and co-stained with anti-glucagon (Cell Signaling Technology, Danvers, MA) (red) and anti-amidated GLP-17–36 (Abcam, Cambridge, MA) (green) antibodies, as well as nuclei dye DAPI (blue). Bioactive GLP-1 was readily detected in non-diabetic islets, mostly co-expressed with glucagon in the same cells but at lower intensity. Note that there were more red signals (glucagon) than green signals (GLP-1) in top panel. In the T2D islets, GLP-1 expression was up-regulated, and its staining (green) almost overlapped completely with glucagon staining (red) as the merged color became yellow (lower panel). The closed arrows mark examples of α cells co-expressing glucagon and GLP-1; and open-head arrows mark those expressing only glucagon. Detection of glucagon+/GLP-1− cells suggests the anti-GLP-1 antibody is specific for the fully cleaved bioactive GLP-1.
3.1. GLP-1 is produced in α-cell lines
GLP-1 production in α cells is clearly supported using immortalized α cell lines. Multiple studies have yielded results demonstrating that bioactive GLP-1 is not only expressed in α cells, but also secreted from these cells into the culture media (Hansen, et al. 2011; Nie, et al. 2000; Whalley, et al. 2011). Further support for these findings lay in PC1/3, the prohormone convertase responsible for GLP-1 cleavage from the precursor proglucagon. PC1/3 has been detected in these α cells, although its expression is considerably lower than that of PC2, which is responsible for glucagon cleavage (Hansen et al. 2011; Nie et al. 2000; Whalley et al. 2011). In addition, the secretion of GLP-1 in these α cells is dependent on glucose levels, and high-glucose up-regulates both its expression and secretion (Hansen et al. 2011; McGirr, et al. 2005; Nie et al. 2000; Whalley et al. 2011).
3.2. GLP-1 is produced and secreted by isolated pancreatic islets
GLP-1 has also been detected in isolated primary human and rodent islets, generally in the lysates, regardless of the ex vivo culturing condition (Hansen et al. 2011; Heller and Aponte 1995; Marchetti, et al. 2012; Masur, et al. 2005; Whalley et al. 2011). However, detection of GLP-1 secretion into the culture media is both less frequent and more complicated. For example, in one study, GLP-1 was detected in the human islet extracts which had been cultured for 24 hours in media containing 5 mM glucose, but not in the culture supernatant (Whalley et al. 2011). This suggested that either GLP-1 was not secreted into the media, or the concentration of GLP-1 in the medium did not reach the detection limits. Data from other studies have suggested the latter is the case; Namely, studies where human islets were cultured for 3 or more days detected considerably larger amounts of GLP-1 in the supernatants (Hansen et al. 2011; Masur et al. 2005). In addition, both high glucose concentration and β cell damage appear to stimulate GLP-1 secretion from cultured islets, allowing it to be readily detected. Using islets isolated from normal and type 2 diabetic (T2D) donors, Marchetti et al performed comprehensive experiments to analyze GLP-1 expression in both intact human islets and FACS-sorted α and β cell fractions of the islets by using confocal microscopy, western blotting and mass spectrometry assays (Marchetti et al. 2012). Their results confirmed that GLP-1 was produced in and secreted from human islets, specifically the α cells. In a more recent study, Taylor et al (2013) employed Peptide Hormone Acquisition through Smart Sampling Technique-Mass Spectrometry (PHASST-MS), an advanced peptidomics platform that employs high resolution liquid chromatography-mass spectrometry (LC-MS), to identify secreted peptide hormones, and reported that both full-length GLP-1(1–37) and bioactive GLP-1(7–37) were detected in the media of cultured human islets (Taylor, et al. 2013). All of these studies support the presence of a local GLP-1 production system within the pancreatic islets.
Furthermore, studies have demonstrated that GLP-1 forms secreted from the pancreatic islets are functionally active, where their biological functionality is evaluated on whether it enhances insulin secretion from islet β cells or whether it activates the GLP-1 receptor (GLP-1R) (Hansen et al. 2011; Heller and Aponte 1995; Marchetti et al. 2012; Masur et al. 2005; Mojsov et al. 1990). For instance, Marchetti et al examined the bioactivity of islet-released GLP-1 by measuring insulin secretion from isolated islets that were exposed to conditioned media containing human islet culture supernatant. They found that the islets cultured in the conditioned media showed significantly greater insulin release in response to glucose than the controls, and this effect was blocked by exendin (9–39), a GLP-1R antagonist, demonstrating the presence of bioactive GLP-1 in the culture supernatant of human islets that was used in the condition media (Marchetti et al. 2012). Another line of evidence comes from studies using GLP-1R activation as a functional sensor. For example, using GLP-1R cDNA-transfected COS-7 cells, Masur et al have shown that the addition of islet culture supernatants to these cells led to significant increase in the formation of cAMP, the second messenger of GLP-1R activation, in comparison to the controls (Masur et al. 2005). Taken together, these studies have demonstrated that the bioactive GLP-1 is secreted from the isolated islets ex vivo.
3.3. GLP-1 expression is detected in pancreatic islets in vivo
In addition to the ex vivo investigations of isolated islets, the production of GLP-1 in the pancreas has also been verified by a number of other studies using both rodent and human pancreas. The first line of evidence lay in the earlier biochemical studies, in which various forms of GLP-1, including the inactive full-length GLP-1 (1–37), and the active forms GLP-1 (7–37) and GLP-1 (7–36)amide, could be isolated directly from pancreatic lysates of various species (Heller and Aponte 1995; Holst et al. 1994; Mojsov et al. 1990; Shima et al. 1987). In addition, with the successful development of monoclonal antibodies that specifically recognize the amidated GLP-1, multiple studies have been able to show co-localization of GLP-1 (7–36)amide with glucagon in α cells of pancreatic islets (Heller and Aponte 1995; Kilimnik, et al. 2010; O’Malley, et al. 2014) (Fig. 2). Double immunogold staining and electron microscopy (EM) have revealed that the active form of GLP-1 co-localizes with glucagon in the same granules in pancreatic α cells of murine models (Kilimnik et al. 2010).
Bioactive GLP-1 has also been detected in the pancreas of various diabetes models. For instance, studies using T2D models, Psammomys obesus or db/db mice, have shown the presence of biologically active GLP-1 within α cells and its upregulation with the development and progression of diabetes (Hansen et al. 2011; O’Malley et al. 2014). Significant amount of biologically active GLP-1 has also been found in the pancreatic lysates of streptozotocin (STZ)-treated rats using radioimmunoassay specific for amidated GLP-1 (Nie et al. 2000). Immunohistochemistry revealed strong GLP-1 immuno-reactivity throughout the pancreatic islets of these rats, whereas only weak signals were detected from the control animals (Nie et al. 2000).
More recently, it has been proposed that the production of GLP-1 in α cells is a characteristic of undifferentiated α cells, namely pro-α-cells (Habener and Stanojevic 2012). This hypothesis is mainly based on the fact that the GLP-1 processing enzyme PC1/3 is co-expressed with GLP-1 in the α cells of developing endocrine and in the models of α-cell hyperplasia (Habener and Stanojevic 2012). However, the hypothesis is debatable because it does not explain the detection of bioactive GLP-1, but not PC1/3, in α cells of normal adult islets. In addition, a key element is missing in this hypothesis— the marker for a pro-α-cell. Without such a marker, the definition of pro-α-cells is vague.
4. PC1/3 expression and its role in pancreatic islets
Several lines of evidence support that the prohormone convertase PC1/3 is responsible for the post-translational cleavage of GLP-1 from proglucagon in the intestinal L-cells (Dhanvantari, et al. 1996; Mineo et al. 1995; Neerman-Arbez, et al. 1994; Rothenberg et al. 1995; Scopsi et al. 1995; Tucker, et al. 1996). First, PC1/3 and GLP-1 are co-expressed in the intestinal cells. In addition, transfecting proglucagon cDNA into PC1/3-expressing cell lines has led to the production of free GLP-1, while transfecting proglucagon cDNA into PC2-expressing cell lines has resulted in glucagon production (Mineo et al. 1995). Similarly, GLP-1 is produced when PC1/3 cDNA is transfected into proglucagon-expressing cell lines, whereas glucagon is produced when PC2 cDNA is transfected into the same cells (Dhanvantari et al. 1996).
Building on these findings, studies have been performed to determine if α cells also express PC1/3. In an early study using Northern blotting analysis, Rouille et al found that, unlike PC2 that was easily detected, PC1/3 was not detected in the α-cell line, αTC1.6 (Rouille et al. 1994a). However, immunohistochemistry studies with antibodies against PC1/3 and PC2 revealed the presence of both PC1/3 and PC2 in the αTC1.6 cells (Rothenberg et al. 1995). In addition, with prolonged exposure time in the Northern blotting assays, PC1/3 mRNA was successfully detected, although the signal was considerably weaker than PC2 mRNA (Rothenberg et al. 1995). These results demonstrate that PC1/3 is expressed in αTC1.6 cells at very low levels, which corresponds to the detection of low levels of GLP-1 in these cells.
PC1/3 is abundant in the pancreatic islets, but it is typically associated with β cells because PC1/3 is required for proinsulin processing (Steiner, et al. 1996). Little, if any, PC1/3 is detected within α cells in normal pancreas (Hansen et al. 2011; Kilimnik et al. 2010; Liu, et al. 2011; Nie et al. 2000; O’Malley et al. 2014). However, studies have suggested that PC1/3 expression is upregulated in α cells under β cell stress or regeneration (Kilimnik et al. 2010; Nie et al. 2000; O’Malley et al. 2014; Whalley et al. 2011). For instance, there was a notable increase in PC1/3 expression in rat islets when cultured in media containing higher glucose levels (11 mM and 25 mM) compared to that at normal glucose concentration (5 mM). GLP-1 secretion increased with glucose concentrations in accordance (Whalley et al. 2011). In diabetic rats induced by multiple low-doses of streptozotocin (STZ), both PC1/3 and GLP-1 expression significantly increased within α cells (Nie et al. 2000). In the study, PC1/3 expression was detected in 95% of α cells in the STZ-treated animals, and only in 9% of α cells in normal rats, which is in agreement with the production of amidated GLP-1 in these animals. On the other hand, PC1/3 expression in β cells did not show such an increase in the STZ-treated rats (Nie et al. 2000), implying that the upregulation of PC1/3 in the α cells acts to increase GLP-1 production and thus provides a means to help alleviate hyperglycemia.
Kilimnik et al. came to a similar conclusion while studying PC1/3 expression in common murine models of β cell regeneration. Specifically, they examined PC1/3 expression in normal, ob/ob, db/db, and NOD mice at time points typically associated with islet cell expansion such as during pregnancy or at neonatal stages, and found that PC1/3 expression could be detected within a subset of α cells (Kilimnik et al. 2010). In all of the models, α cell hyperplasia was also a key feature, albeit in varying degrees. Because α cell regeneration and the ensuing activation of PC1/3 expression can trigger the biosynthesis and secretion of GLP-1 in α cells, these cells may play an important role in supporting β cell proliferation, underlining the importance of the intra-islet GLP-1 (Kilimnik et al. 2010). Moreover, similar observations were made in a study involving diabetes development and progression in db/db and NOD mice. In α cells of normal mice, PC1/3 expression level was too low to be detected by IHC staining, even in GLP-1-positive cells (O’Malley et al. 2014). However, with the onset and progression of diabetes, PC1/3 expression became readily detectable in α cells (O’Malley et al. 2014). The ratio of GLP-1+ : glucagon+ α cells significantly and progressively increased with the development of diabetes in db/db mice, providing additional evidence that α cells may switch from primarily glucagon production to GLP-1 production, via up-regulation of PC1/3 expression, in an attempt to ameliorate hyperglycemia (O’Malley et al. 2014).
5. The functionality of intra-islet produced GLP-1
GLP-1 not only stimulates insulin secretion, but also promotes β cell survival and proliferation, thus is essential for maintaining β cell function within the pancreas. However, the source of GLP-1 that acts on the β cells remains to be debated. Historically, the insulinotropic and glucagonostatic effects have been attributed to the GLP-1 in circulation, which is believed to be largely produced in the intestinal tract, whereas α cell-produced GLP-1 has not been recognized or appreciated. With recent confirmation of GLP-1 production within islets, researchers have begun to determine the functionality of this locally produced incretin using isolated islets as the source of GLP-1. This is in contrast to previous studies that have identified the functions of GLP-1, which are mainly based on applications of exogenous GLP-1, GLP-1R agonists or antagonists. Increasing evidence suggests that locally produced GLP-1 within islets plays active roles in maintaining β cell function.
5.1. Islet produced GLP-1 stimulates insulin secretion
Through various studies utilizing isolated islets in culture, α cell-produced GLP-1 have been shown to promote insulin secretion. It has been demonstrated that islets cultured in conditioned media containing human islet supernatants display significantly greater glucose-stimulated insulin secretion (GSIS) responses than that in controls, and the effects are blocked by GLP-1R antagonists (Marchetti et al. 2012). When normal human islets are cultured in the conditioned media derived from islets cultured in high-glucose media—and thus containing more islet-produced GLP-1, they show higher GSIS than controls using conditioned media from low-glucose culture (Marchetti et al. 2012). In addition, forced PC1/3 expression in α cells provides further support for the role of α cell produced GLP-1 in insulin secretion (Wideman, et al. 2006). When PC1/3 cDNA is introduced into the α cells, but not the β cells, in the isolated islets, it not only leads to a significant increase in GLP-1 production by the transduced α cells, but also results in a significant increase in insulin production and peak insulin output when challenged with high glucose, in comparison to that in control islets (Wideman et al. 2006). These results demonstrate that GLP-1 produced in α cells is capable of stimulating insulin secretion from β cells in response to high glucose.
5.2. Islet produced GLP-1 promotes β cell survival
The islet produced GLP-1 may be vital for the maintenance of β cell function. Indeed, induction of GLP-1 expression and secretion within islets has been widely observed in situations of β cell damage or stress, suggesting the locally produced GLP-1 may be recruited to help β cells survive (Hansen et al. 2011; Huang, et al. 2015; Liu et al. 2011; Whalley et al. 2011). For example, when treated with cytokine cocktail containing IL-1β, IFNγ, and TNFα, which induces β cell stress and apoptosis, isolated mouse islets exhibit robust GLP-1 secretion (Liu et al. 2011). Similarly, when primary rat islets are treated with the β cell toxin STZ, GLP-1 release from these islets significantly increases whereas glucagon secretion remains constant (Whalley et al. 2011). In addition, it has been reported that chronic hyperglycemia as result of diabetes leads to increased secretion of GLP-1 as assessed with islets isolated from diabetic versus normal animals (Hansen et al. 2011; Nie et al. 2000). Furthermore, incubation of mouse islets with palmitate at a concentration mimicking lipotoxicity markedly induces GLP-1 release from the islets (Huang et al. 2015). In accordance, high-fat diet feeding has been shown to significantly increase PC1/3 expression and GLP-1 production in islet α cells in vivo (Huang et al. 2015).
Mechanistically, it appears that lipid overload leads to oxidative stress that triggers the activation of intra-islet GLP-1 system, which acts as a self-defense mechanism to enhance β cell survival (Huang et al. 2015). Indeed, following cellular injury, the stromal cell-derived factor-1 (SDF-1), a chemokine involved in mediating tissue repair, has been shown to enhance β cell survival through induction of GLP-1 production and secretion by islet α cells (Liu et al. 2011). Additional evidence supporting the protective effect of islet-produced GLP-1 on β cells arose from studies related to PC1/3 expression in α cells (Wideman, et al. 2007; Wideman et al. 2006). PC1/3-transduced islets contain significantly increased amount of GLP-1, and they are protected from apoptosis when treated with the cytokine IL-1β, whereas the control islets display significantly more cell death (Wideman et al. 2006).
Taken together, these results have demonstrated that GLP-1 production within the islets increases in response to β cell damage, which may serve as a protective mechanism in the attempts to overcome impaired β cell function and hyperglycemia.
5.3. Islet produced GLP-1 enhances β cell proliferation
Using mouse models of hyperglycemia, studies have shown that GLP-1 produced within islets plays an important role in the regeneration or proliferation of β cells. Up-regulation of GLP-1 expression in α cells has been observed in models of β cell expansion and regeneration, such as neonatal and pregnant mice, suggesting GLP-1 produced within the islets likely promotes β cell proliferation (Kilimnik et al. 2010; Moffett, et al. 2014; Vasu, et al. 2014). Indeed, GLP-1 receptor knockout mice have been shown to have impaired β cell mass adaptation to pregnancy or in response to insulin resistance (Moffett et al. 2014; Vasu et al. 2014). Analysis of circulating GLP-1 concentration and intra-islet GLP-1 content indicates pregnancy or metabolic stress-induced β cell mass compensation is mediated by locally produced GLP-1 (Moffett et al. 2014; Vasu et al. 2014). This view is further supported by studies showing PC1/3 overexpression in α cells results in increased β cell proliferation and improved protection against STZ-induced diabetes. Specifically, the mice transplanted with PC1/3-transduced α cells tend to have, on average, greater insulin-positive area and larger islets, while displaying an increased number of replicating β cells in comparison to that of controls (Wideman et al. 2007).
Additional evidence regarding the effects of α cell produced GLP-1 on β cell proliferation comes indirectly from studies involving the cytokine IL-6. IL-6 has been shown to elevate during obesity or exercise, which improves insulin secretion and glucose tolerance. It has been demonstrated that IL-6 acts on α cells by stimulating GLP-1 production and α cell mass expansion, a process appears to be required for β cell compensation in response to high-fat diet or obesity (Ellingsgaard, et al. 2008; Ellingsgaard, et al. 2011). Together, these studies support that islet produced GLP-1 promotes β cell proliferation.
6. Factors regulating intra-islet GLP-1 production
Current studies have suggested that GLP-1 expression within islets may be influenced by many factors. In addition to PC1/3 expression, the signals responsible for regulating GLP-1 versus glucagon expression in the islets include cytokines and metabolic stress.
6.1. Glucose
Glucose appears to be a key regulator for intra-islet GLP-1 production and secretion. In cultured primary islets, higher glucose concentration in the media appears to induce more GLP-1 secretion from the isolated islets (Marchetti et al. 2012; Masur et al. 2005; Whalley et al. 2011). In addition, islets purified from T2D patients and hyperglycemic animals showed significantly higher GLP-1 concentrations in culture supernatant compared to those from control animals (Hansen et al. 2011; Marchetti et al. 2012). In vivo, hyperglycemia and β cell damage were found to increase GLP-1 production by islet α cells. Up-regulation of GLP-1 in α cells has been observed in most diabetic animal models such as db/db mice, ob/ob mice, STZ-induced diabetic mice, and human T2D patients (Kilimnik et al. 2010; Marchetti et al. 2012; Nie et al. 2000; O’Malley et al. 2014; Whalley et al. 2011). For instance, GLP-1 production in α cells of db/db mice progressively increases with the development of diabetes (O’Malley et al. 2014). Furthermore, it appears that chronic hyperglycemia is required for the upregulation of GLP-1 secretion by α cells (Hansen et al. 2011; Heller and Aponte 1995). For example, GLP-1 secretion from islets did not show significant changes with increasing glucose concentrations (3, 12, and 25 mM) in the media when cultured for 24 hours. However, a significant glucose dose-dependent increase in GLP-1 release was observed when islets were cultured for 6 days (Hansen et al. 2011; Heller and Aponte 1995). All of these studies support that glucose is a key regulator for intra-islet GLP-1 production and secretion.
6.2. IL-6
Primarily produced by adipose tissue, the cytokine IL-6 is elevated during obesity and is believed to contribute to the induction of insulin resistance during T2D development. On the other hand, it has been shown that IL-6 concentrations in circulation increases during exercise, which promotes efficient nutrient uptake and whole-body insulin sensitivity (Carey, et al. 2006; Febbraio, et al. 2003; Lazar 2005). Further studies have demonstrated that the direct target of IL-6 in pancreas is α cells because these cells express the highest levels of IL-6 receptors among all cell types within the endocrine pancreas (Ellingsgaard et al. 2008). IL-6 stimulates α cell proliferation, inhibits α cell apoptosis, and upregulates proglucagon expression in the islets in response to high-fat diet induced expansion of α cell mass (Ellingsgaard et al. 2008). In addition, it has been shown that systemic administration of IL-6 improved glucose-stimulated insulin secretion in normal mice, but not in GLP-1R knockout mice, suggesting IL-6 affects insulin secretion through GLP-1 (Ellingsgaard et al. 2011). Indeed, GLP-1 content in mouse pancreas increases following IL-6 administration, confirming that IL-6 promotes GLP-1 production within islets. Using isolated islets, researchers have shown IL-6 significantly increases both the constitutive and the acute arginine-stimulated GLP-1 release from the islets. Moreover, FACS-sorted α cells from the dissociated human islets have been used to address whether IL-6 directly acts on α cells (Ellingsgaard et al. 2011). When exposed to IL-6, these α cells exhibit significant increases in GLP-1 production and secretion at both low and high glucose levels, while glucagon secretion is only increased at low glucose level. Further gene expression analysis has revealed upregulation of both preproglucagon mRNA and PC1/3 mRNA in these α cells in response to IL-6 exposure, suggesting IL-6 induces a shift from glucagon towards GLP-1 in the processing of proglucagon (Ellingsgaard et al. 2011). Taken together, these results have demonstrated that IL-6 enhances β cell function by stimulating GLP-1 secretion from α cells, especially during times of β cell stress (Ellingsgaard et al. 2011).
Interestingly, recent studies have discovered that inflammatory stimuli such as endotoxin and IL-1β stimulate GLP-1 secretion in an IL-6-dependent manner in both mice and human (Kahles, et al. 2014; Lebherz, et al. 2016; Nguyen, et al. 2014). In addition, glucose-dependent insulintropic peptide (GIP) appears to stimulate GLP-1 production in pancreatic islets via induction of IL-6 (Timper, et al. 2016). Therefore, IL-6 may be a major mediator in regulating GLP-1 production and secretion within islet α cells.
6.3. SDF-1
The stromal cell-derived factor-1 (SDF-1) is a chemokine involved in mediating tissue repair following cellular injury. It has been shown that the injury-induced expression of SDF-1 by β cells acts in a paracrine manner to regulate GLP-1 expression in α cells (Liu et al. 2011). Using both isolated primary islets and immortalized cell lines, researchers have discovered that β cell injury induces SDF-1 expression, which subsequently activates its receptor CXCR4 that is expressed on α cells. Activation of the SDF-1/CXCR4 signaling cascade in α cells stimulates α cell proliferation and induces the expression of PC1/3, resulting in the switch of proglucagon processing from primarily glucagon production to GLP-1 production in the α cells. GLP-1 in turn promotes β cell growth and survival via GLP-1R that is localized on β cell surface, and acts in a concerted manner with SDF-1 to increase β cell mass following injury (Liu et al. 2011).
7. Conclusion
It has become increasingly clear that bioactive GLP-1 is produced locally in the pancreatic islets. The expression of GLP-1 and its processing enzyme, PC1/3, in α cells increases in the situations of β cell injury or stress, likely acting as a “self-defense” mechanism that is adopted by the islets to help preserve β cells and alleviate hyperglycemia. The α cell-produced GLP-1 is fully functional regarding the “incretin” effect—it enhances insulin secretion, promotes β cell survival and stimulates β cell proliferation. Therefore, intra-islet GLP-1 may play a major role in regulating insulin and glucagon secretion in pancreas, under both normal and diabetic conditions.
With the gradual acceptance that GLP-1 is indeed produced locally in the islets, many questions remain to be answered. For instance, because GLP-1 has a very short half-life in circulation (1–2 minutes), the GLP-1 molecules produced in intestinal L-cells is expected to be mostly degraded before they reach pancreas. An emerging hypothesis is that the GLP-1 molecules exerting the insulinotropic and glucagonostatic effects are produced within the islets. This makes biological sense because it is much more efficient. However, how do we prove this is the case? In addition, GLP-1 acts not only locally in the intestine and pancreas, but also systemically as a hormone. How much does the islet-produced GLP-1 contribute to the systemic GLP-1 hormonal effect in vivo? Furthermore, GLP-1 and glucagon are produced form the same precursor, often in the same cell, and their relative production can clearly change under different situations. So, what is the mechanism that regulates their production? Is there a receptor-mediated feed-back mechanism to fine tune and balance their production? Finally, since GLP-1-based incretins have been used in clinic for the treatment of T2D patients, do these drugs affect endogenous GLP-1 production in pancreas?
Apparently, all of these questions require stringent investigations. A key study would involve tissue-specific elimination of GLP-1 production, which may be achieved by genetically knocking out PC1/3 from either pancreatic α-cells or intestinal L-cells. This will allow us to isolate the contributions of the islet-produced versus gut-produced GLP-1 in its overall incretin effects. In addition, to fully understand the autocrine and paracrine effects of α-cell-produced GLP-1, it is necessary to re-examine the expression of its receptor, GLP-1R, in endocrine cells other than β-cells, such as α- and δ-cells, which has been controversial largely due to GLP-1R antibody issues (Panjwani, et al. 2013). Indeed, another study has suggested that GLP-1 suppresses glucagon secretion via somatostatin (de Heer, et al. 2008). If so, the islet-produced GLP-1 would have a paracrine effect on somatostain-producing δ cells. Should GLP-1R expression be detected in non-β islet cells, it would argue for an autocrine and/or broader paracrine effects of GLP-1 within pancreatic islets. Further studies, such as generation of cell-specific GLP-1R knockout mice, should then be undertaken to determine the mechanisms regarding these effects. Moreover, primary islets and the cell-specific PC1/3 and GLP-1R knockout mouse models will provide valuable tools to study whether exogenous incretin therapy affects intra-islet GLP-1 production. These investigations will not only be important scientifically, but also have an impact on the relevant clinical practices.
Acknowledgments
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases [R01DK081463].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None.
References
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Holst J. Glucagon-related peptides in the human gastrointestinal mucosa. Diabetologia. 1984;26:223–228. doi: 10.1007/BF00252412. [DOI] [PubMed] [Google Scholar]
- Bell GI, Santerre RF, Mullenbach GT. Hamster preproglucagon contains the sequence of glucagon and two related peptides. Nature. 1983;302:716–718. doi: 10.1038/302716a0. [DOI] [PubMed] [Google Scholar]
- Carey AL, Steinberg GR, Macaulay SL, Thomas WG, Holmes AG, Ramm G, Prelovsek O, Hohnen-Behrens C, Watt MJ, James DE. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes. 2006;55:2688–2697. doi: 10.2337/db05-1404. [DOI] [PubMed] [Google Scholar]
- de Heer J, Rasmussen C, Coy DH, Holst JJ. Glucagon-like peptide-1, but not glucose-dependent insulinotropic peptide, inhibits glucagon secretion via somatostatin (receptor subtype 2) in the perfused rat pancreas. Diabetologia. 2008;51:2263–2270. doi: 10.1007/s00125-008-1149-y. [DOI] [PubMed] [Google Scholar]
- Dhanvantari S, Brubaker PL. Proglucagon processing in an islet cell line: effects of PC1 overexpression and PC2 depletion. Endocrinology. 1998;139:1630–1637. doi: 10.1210/endo.139.4.5936. [DOI] [PubMed] [Google Scholar]
- Dhanvantari S, Seidah NG, Brubaker PL. Role of prohormone convertases in the tissue-specific processing of proglucagon. Molecular endocrinology (Baltimore, Md) 1996;10:342–355. doi: 10.1210/mend.10.4.8721980. [DOI] [PubMed] [Google Scholar]
- Drucker D, Mojsov S, Habener JF. Cell-specific post-translational processing of preproglucagon expressed from a metallothionein-glucagon fusion gene. Journal of Biological Chemistry. 1986;261:9637–9643. [PubMed] [Google Scholar]
- Eissele R, Göke R, Willemer S, HARTHUS HP, Vermeer H, Arnold Re, Göke B. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. European journal of clinical investigation. 1992;22:283–291. doi: 10.1111/j.1365-2362.1992.tb01464.x. [DOI] [PubMed] [Google Scholar]
- Ellingsgaard H, Ehses JA, Hammar EB, Van Lommel L, Quintens R, Martens G, Kerr-Conte J, Pattou F, Berney T, Pipeleers D, et al. Interleukin-6 regulates pancreatic alpha-cell mass expansion. Proc Natl Acad Sci U S A. 2008;105:13163–13168. doi: 10.1073/pnas.0801059105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingsgaard H, Hauselmann I, Schuler B, Habib AM, Baggio LL, Meier DT, Eppler E, Bouzakri K, Wueest S, Muller YD. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nature medicine. 2011;17:1481–1489. doi: 10.1038/nm.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio MA, Steensberg A, Keller C, Starkie RL, Nielsen HB, Krustrup P, Ott P, Secher NH, Pedersen BK. Glucose ingestion attenuates interleukin-6 release from contracting skeletal muscle in humans. The Journal of physiology. 2003;549:607–612. doi: 10.1113/jphysiol.2003.042374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehmann HC, Habener JF. Insulinotropic hormone glucagon-like peptide-I(7–37) stimulation of proinsulin gene expression and proinsulin biosynthesis in insulinoma beta TC-1 cells. Endocrinology. 1992;130:159–166. doi: 10.1210/endo.130.1.1309325. [DOI] [PubMed] [Google Scholar]
- George S, Uttenthal L, Ghiglione M, Bloom S. Molecular forms of glucagon-like peptides in man. FEBS letters. 1985;192:275–278. doi: 10.1016/0014-5793(85)80124-1. [DOI] [PubMed] [Google Scholar]
- Guo X, Yang Q, Dong J, Liao L, Zhang W, Liu F. Tumour Risk with Once-Weekly Glucagon-Like Peptide-1 Receptor Agonists in Type 2 Diabetes Mellitus Patients: A Systematic Review. Clin Drug Investig. 2016 doi: 10.1007/s40261-016-0389-8. [DOI] [PubMed] [Google Scholar]
- Habener JF, Stanojevic V. alpha-cell role in beta-cell generation and regeneration. Islets. 2012;4:188–198. doi: 10.4161/isl.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen A, Bödvarsdottir T, Nordestgaard D, Heller R, Gotfredsen C, Maedler K, Fels J, Holst JJ, Karlsen A. Upregulation of alpha cell glucagon-like peptide 1 (GLP-1) in Psammomys obesus—an adaptive response to hyperglycaemia? Diabetologia. 2011;54:1379–1387. doi: 10.1007/s00125-011-2080-1. [DOI] [PubMed] [Google Scholar]
- Heinrich G, Gros P, Habener JF. Glucagon gene sequence. Four of six exons encode separate functional domains of rat pre-proglucagon. Journal of Biological Chemistry. 1984;259:14082–14087. [PubMed] [Google Scholar]
- Heller RS, Aponte GW. Intra-islet regulation of hormone secretion by glucagon-like peptide-1-(7–36) amide. American Journal of Physiology-Gastrointestinal and Liver Physiology. 1995;269:G852–G860. doi: 10.1152/ajpgi.1995.269.6.G852. [DOI] [PubMed] [Google Scholar]
- Hirota M, Shimizu I, Ohboshi C, Nishino T, Shima K. A large molecular form of glucagon-like peptide-1 (GLP-1) immunoreactivity is co-released with glucagon from pancreas by arginine in normal subjects. Clinica chimica acta. 1987;167:293–302. doi: 10.1016/0009-8981(87)90349-4. [DOI] [PubMed] [Google Scholar]
- Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- Holst JJ, Bersani M, Johnsen AH, Kofod H, Hartmann B, Orskov C. Proglucagon processing in porcine and human pancreas. Journal of Biological Chemistry. 1994;269:18827–18833. [PubMed] [Google Scholar]
- Huang C, Yuan L, Cao S. Endogenous GLP-1 as a key self-defense molecule against lipotoxicity in pancreatic islets. Int J Mol Med. 2015;36:173–185. doi: 10.3892/ijmm.2015.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahles F, Meyer C, Mollmann J, Diebold S, Findeisen HM, Lebherz C, Trautwein C, Koch A, Tacke F, Marx N, et al. GLP-1 secretion is increased by inflammatory stimuli in an IL-6-dependent manner, leading to hyperinsulinemia and blood glucose lowering. Diabetes. 2014;63:3221–3229. doi: 10.2337/db14-0100. [DOI] [PubMed] [Google Scholar]
- Kauth T, Metz J. Immunohistochemical localization of glucagon-like peptide 1. Histochemistry. 1987;86:509–515. doi: 10.1007/BF00500625. [DOI] [PubMed] [Google Scholar]
- Kieffer TJ, Habener JF. The glucagon-like peptides. Endocr Rev. 1999;20:876–913. doi: 10.1210/edrv.20.6.0385. [DOI] [PubMed] [Google Scholar]
- Kilimnik G, Kim A, Steiner DF, Friedman TC, Hara M. Intraislet production of GLP-1 by activation of prohormone convertase 1/3 in pancreatic α-cells in mouse models of β-cell regeneration. Islets. 2010;2:149–155. doi: 10.4161/isl.2.3.11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar MA. How obesity causes diabetes: not a tall tale. Science. 2005;307:373–375. doi: 10.1126/science.1104342. [DOI] [PubMed] [Google Scholar]
- Lebherz C, Kahles F, Piotrowski K, Vogeser M, Foldenauer AC, Nassau K, Kilger E, Marx N, Parhofer KG, Lehrke M. Interleukin-6 predicts inflammation-induced increase of Glucagon-like peptide-1 in humans in response to cardiac surgery with association to parameters of glucose metabolism. Cardiovasc Diabetol. 2016;15:21. doi: 10.1186/s12933-016-0330-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Stanojevic V, Avadhani S, Yano T, Habener JF. Stromal cell-derived factor-1 (SDF-1)/chemokine (C-X-C motif) receptor 4 (CXCR4) axis activation induces intra-islet glucagon-like peptide-1 (GLP-1) production and enhances beta cell survival. Diabetologia. 2011;54:2067–2076. doi: 10.1007/s00125-011-2181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez LC, Frazier ML, Su C-J, Kumar A, Saunders GF. Mammalian pancreatic preproglucagon contains three glucagon-related peptides. Proceedings of the National Academy of Sciences. 1983;80:5485–5489. doi: 10.1073/pnas.80.18.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund PK, Goodman RH, Dee PC, Habener JF. Pancreatic preproglucagon cDNA contains two glucagon-related coding sequences arranged in tandem. Proceedings of the National Academy of Sciences. 1982;79:345–349. doi: 10.1073/pnas.79.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marathe CS, Rayner CK, Jones KL, Horowitz M. Glucagon-like peptides 1 and 2 in health and disease: a review. Peptides. 2013;44:75–86. doi: 10.1016/j.peptides.2013.01.014. [DOI] [PubMed] [Google Scholar]
- Marchetti P, Lupi R, Bugliani M, Kirkpatrick C, Sebastiani G, Grieco F, Del Guerra S, D’Aleo V, Piro S, Marselli L. A local glucagon-like peptide 1 (GLP-1) system in human pancreatic islets. Diabetologia. 2012;55:3262–3272. doi: 10.1007/s00125-012-2716-9. [DOI] [PubMed] [Google Scholar]
- Masur K, Tibaduiza EC, Chen C, Ligon B, Beinborn M. Basal receptor activation by locally produced glucagon-like peptide-1 contributes to maintaining β-cell function. Molecular endocrinology. 2005;19:1373–1382. doi: 10.1210/me.2004-0350. [DOI] [PubMed] [Google Scholar]
- McGirr R, Ejbick CE, Carter DE, Andrews JD, Nie Y, Friedman TC, Dhanvantari S. Glucose dependence of the regulated secretory pathway in alphaTC1-6 cells. Endocrinology. 2005;146:4514–4523. doi: 10.1210/en.2005-0402. [DOI] [PubMed] [Google Scholar]
- Meier JJ, Gallwitz B, Salmen S, Goetze O, Holst JJ, Schmidt WE, Nauck MA. Normalization of glucose concentrations and deceleration of gastric emptying after solid meals during intravenous glucagon-like peptide 1 in patients with type 2 diabetes. J Clin Endocrinol Metab. 2003;88:2719–2725. doi: 10.1210/jc.2003-030049. [DOI] [PubMed] [Google Scholar]
- Mineo I, Matsumura T, Shingu R, Namba M, Kuwajima M, Matsuzawa Y. The role of prohormone convertases PC1 (PC3) and PC2 in the cell-specific processing of proglucagon. Biochem Biophys Res Commun. 1995;207:646–651. doi: 10.1006/bbrc.1995.1236. [DOI] [PubMed] [Google Scholar]
- Moffett RC, Vasu S, Thorens B, Drucker DJ, Flatt PR. Incretin receptor null mice reveal key role of GLP-1 but not GIP in pancreatic beta cell adaptation to pregnancy. PLoS One. 2014;9:e96863. doi: 10.1371/journal.pone.0096863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojsov S, Heinrich G, Wilson IB, Ravazzola M, Orci L, Habener JF. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. Journal of Biological Chemistry. 1986;261:11880–11889. [PubMed] [Google Scholar]
- Mojsov S, Kopczynski M, Habener JF. Both amidated and nonamidated forms of glucagon-like peptide I are synthesized in the rat intestine and the pancreas. Journal of Biological Chemistry. 1990;265:8001–8008. [PubMed] [Google Scholar]
- Mojsov S, Weir G, Habener J. Insulinotropin: glucagon-like peptide I (7–37) co-encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. Journal of Clinical Investigation. 1987;79:616. doi: 10.1172/JCI112855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauck M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab. 2016;18:203–216. doi: 10.1111/dom.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neerman-Arbez M, Cirulli V, Halban PA. Levels of the conversion endoproteases PC1 (PC3) and PC2 distinguish between insulin-producing pancreatic islet beta cells and non-beta cells. Biochem J. 1994;300:57–61. doi: 10.1042/bj3000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AT, Mandard S, Dray C, Deckert V, Valet P, Besnard P, Drucker DJ, Lagrost L, Grober J. Lipopolysaccharides-mediated increase in glucose-stimulated insulin secretion: involvement of the GLP-1 pathway. Diabetes. 2014;63:471–482. doi: 10.2337/db13-0903. [DOI] [PubMed] [Google Scholar]
- Nie Y, Nakashima M, Brubaker PL, Li Q-L, Perfetti R, Jansen E, Zambre Y, Pipeleers D, Friedman TC. Regulation of pancreatic PC1 and PC2 associated with increased glucagon-like peptide 1 in diabetic rats. Journal of Clinical Investigation. 2000;105:955–965. doi: 10.1172/JCI7456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley TJ, Fava GE, Zhang Y, Fonseca VA, Wu H. Progressive change of intra-islet GLP-1 production during diabetes development. Diabetes Metab Res Rev. 2014 doi: 10.1002/dmrr.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov C, Bersani M, Johnsen A, Højrup P, Holst J. Complete sequences of glucagon-like peptide-1 from human and pig small intestine. Journal of Biological Chemistry. 1989;264:12826–12829. [PubMed] [Google Scholar]
- Ørskov C, Holst J, Poulsen SS, Kirkegaard P. Pancreatic and intestinal processing of proglucagon in man. Diabetologia. 1987;30:874–881. doi: 10.1007/BF00274797. [DOI] [PubMed] [Google Scholar]
- Ørskov C, Holst JJ, Knuhtsen S, Baldissera FG, Poulsen SS, Nielsen OV. Glucagon-Like Peptides GLP-1 and GLP-2, Predicted Products of the Glucagon Gene, Are Secreted Separately from Pig Small Intestine but Not Pancreas*. Endocrinology. 1986;119:1467–1475. doi: 10.1210/endo-119-4-1467. [DOI] [PubMed] [Google Scholar]
- Panjwani N, Mulvihill EE, Longuet C, Yusta B, Campbell JE, Brown TJ, Streutker C, Holland D, Cao X, Baggio LL, et al. GLP-1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE(−/−) mice. Endocrinology. 2013;154:127–139. doi: 10.1210/en.2012-1937. [DOI] [PubMed] [Google Scholar]
- Patzelt C, Schiltz E. Conversion of proglucagon in pancreatic alpha cells: the major endproducts are glucagon and a single peptide, the major proglucagon fragment, that contains two glucagon-like sequences. Proc Natl Acad Sci U S A. 1984;81:5007–5011. doi: 10.1073/pnas.81.16.5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzelt C, Tager HS, Carroll RJ, Steiner DF. Identification and processing of proglucagon in pancreatic islets. Nature. 1979;282:260–266. doi: 10.1038/282260a0. [DOI] [PubMed] [Google Scholar]
- Rothenberg ME, Eilertson CD, Klein K, Zhou Y, Lindberg I, McDonald JK, Mackin RB, Noe BD. Processing of mouse proglucagon by recombinant prohormone convertase 1 and immunopurified prohormone convertase 2 in vitro. Journal of Biological Chemistry. 1995;270:10136–10146. doi: 10.1074/jbc.270.17.10136. [DOI] [PubMed] [Google Scholar]
- Rouille Y, Martin S, Steiner DF. Differential processing of proglucagon by the subtilisin-like prohormone convertases PC2 and PC3 to generate either glucagon or glucagon-like peptide. J Biol Chem. 1995;270:26488–26496. doi: 10.1074/jbc.270.44.26488. [DOI] [PubMed] [Google Scholar]
- Rouille Y, Westermark G, Martin SK, Steiner DF. Proglucagon is processed to glucagon by prohormone convertase PC2 in alpha TC1-6 cells. Proceedings of the National Academy of Sciences. 1994a;91:3242–3246. doi: 10.1073/pnas.91.8.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouille Y, Westermark G, Martin SK, Steiner DF. Proglucagon is processed to glucagon by prohormone convertase PC2 in alpha TC1-6 cells. Proc Natl Acad Sci U S A. 1994b;91:3242–3246. doi: 10.1073/pnas.91.8.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi M, Vahl TP, D’Alessio DA. Regulation of islet hormone release and gastric emptying by endogenous glucagon-like peptide 1 after glucose ingestion. J Clin Endocrinol Metab. 2008;93:4909–4916. doi: 10.1210/jc.2008-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval DA, D’Alessio DA. Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease. Physiol Rev. 2015;95:513–548. doi: 10.1152/physrev.00013.2014. [DOI] [PubMed] [Google Scholar]
- Scopsi L, Gullo M, Rilke F, Martin S, Steiner DF. Proprotein convertases (PC1/PC3 and PC2) in normal and neoplastic human tissues: their use as markers of neuroendocrine differentiation. J Clin Endocrinol Metab. 1995;80:294–301. doi: 10.1210/jcem.80.1.7829629. [DOI] [PubMed] [Google Scholar]
- Shima K, Hirota M, Ohboshi C, Sato M, Nishino T. Release of glucagon-like peptide 1 immunoreactivity from the perfused rat pancreas. Acta endocrinologica. 1987;114:531–536. doi: 10.1530/acta.0.1140531. [DOI] [PubMed] [Google Scholar]
- Steiner D, Rouille Y, Gong Q, Martin S, Carroll R, Chan S. The role of prohormone convertases in insulin biosynthesis: evidence for inherited defects in their action in man and experimental animals. Diabetes & metabolism. 1996;22:94–104. [PubMed] [Google Scholar]
- Suzuki S, KAWAI K, OHASHI S, MUKAI H, YAMASHITA K. Comparison of the Effects of Various C-Terminal and NTerminal Fragment Peptides of Glucagon-Like Peptide-1 on Insulin and Glucagon Release from the Isolated Perfused Rat Pancreas*. Endocrinology. 1989;125:3109–3114. doi: 10.1210/endo-125-6-3109. [DOI] [PubMed] [Google Scholar]
- Taylor SW, Nikoulina SE, Andon NL, Lowe C. Peptidomic profiling of secreted products from pancreatic islet culture results in a higher yield of full-length peptide hormones than found using cell lysis procedures. J Proteome Res. 2013;12:3610–3619. doi: 10.1021/pr400115q. [DOI] [PubMed] [Google Scholar]
- Timper K, Dalmas E, Dror E, Rutti S, Thienel C, Sauter NS, Bouzakri K, Bedat B, Pattou F, Kerr-Conte J, et al. Glucose-dependent Insulinotropic Peptide Stimulates Glucagon-like Peptide 1 Production by Pancreatic Islets via Interleukin-6, Produced by alpha Cells. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Tucker JD, Dhanvantari S, Brubaker PL. Proglucagon processing in islet and intestinal cell lines. Regulatory peptides. 1996;62:29–35. doi: 10.1016/0167-0115(95)00167-0. [DOI] [PubMed] [Google Scholar]
- Turton MD, O’Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- Uttenthel L, Ghiglione M, George S, Bishop A, Polak J, Bloom S. Molecular forms of glucagon-like peptide-1 in human pancreas and glucagonomas. The Journal of Clinical Endocrinology & Metabolism. 1985;61:472–479. doi: 10.1210/jcem-61-3-472. [DOI] [PubMed] [Google Scholar]
- Varndell IM, Bishop AE, Sikri KL, Uttenthal LO, Bloom SR, Polak JM. Localization of glucagon-like peptide (GLP) immunoreactants in human gut and pancreas using light and electron microscopic immunocytochemistry. J Histochem Cytochem. 1985;33:1080–1086. doi: 10.1177/33.10.3900195. [DOI] [PubMed] [Google Scholar]
- Vasu S, Moffett RC, Thorens B, Flatt PR. Role of endogenous GLP-1 and GIP in beta cell compensatory responses to insulin resistance and cellular stress. PLoS One. 2014;9:e101005. doi: 10.1371/journal.pone.0101005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir GC, Mojsov S, Hendrick GK, Habener JF. Glucagonlike peptide I (7–37) actions on endocrine pancreas. Diabetes. 1989;38:338–342. doi: 10.2337/diab.38.3.338. [DOI] [PubMed] [Google Scholar]
- Whalley N, Pritchard L, Smith DM, White A. Processing of proglucagon to GLP-1 in pancreatic α-cells: is this a paracrine mechanism enabling GLP-1 to act on β-cells? Journal of endocrinology. 2011;211:99–106. doi: 10.1530/JOE-11-0094. [DOI] [PubMed] [Google Scholar]
- Wideman RD, Covey SD, Webb GC, Drucker DJ, Kieffer TJ. A switch from prohormone convertase (PC)-2 to PC1/3 expression in transplanted α-cells is accompanied by differential processing of proglucagon and improved glucose homeostasis in mice. Diabetes. 2007;56:2744–2752. doi: 10.2337/db07-0563. [DOI] [PubMed] [Google Scholar]
- Wideman RD, Irene L, Webber TD, Verchere CB, Johnson JD, Cheung AT, Kieffer TJ. Improving function and survival of pancreatic islets by endogenous production of glucagon-like peptide 1 (GLP-1) Proceedings of the National Academy of Sciences. 2006;103:13468–13473. doi: 10.1073/pnas.0600655103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, Baskin DG, Schwartz MW. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology. 2009;150:1680–1687. doi: 10.1210/en.2008-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]


