Abstract
BACKGROUND
The incubation of cue-induced drug craving in rodents provides a model of persistent vulnerability to craving and relapse in human addicts. After prolonged withdrawal, incubated cocaine craving depends on strengthening of nucleus accumbens (NAc) core synapses through incorporation of Ca2+-permeable AMPA receptors (CP-AMPARs). Through mGlu1-mediated synaptic depression, mGlu1 positive allosteric modulators (PAMs) remove CP-AMPARs from these synapses and thereby reduce cocaine craving. This study aimed to determine if similar plasticity accompanies incubation of methamphetamine craving.
METHODS
Rats self-administered saline or methamphetamine under extended-access conditions. Cue-induced seeking tests demonstrated incubation of methamphetamine craving. After withdrawal periods ranging from 1 to >40 days, rats underwent one of the following procedures: 1) whole-cell patch clamp recordings to characterize AMPAR transmission, 2) intra-NAc core injection of the CP-AMPAR antagonist 1-napthyl acetyl spermine (naspm) prior to a seeking test, or 3) systemic administration of an mGlu1 PAM prior to a seeking test.
RESULTS
Incubation of methamphetamine craving was associated with CP-AMPAR accumulation in NAc core, and both effects were maximal after ~1 week of withdrawal. Expression of incubated craving was decreased by intra-NAc naspm injection or systemic mGlu1 PAM administration.
CONCLUSIONS
These results are the first to demonstrate a role for the NAc in the incubation of methamphetamine craving and describe adaptations in synaptic transmission associated with this model. They establish that incubation of craving and associated CP-AMPAR plasticity occur much more rapidly during withdrawal from methamphetamine than cocaine. However, a common mGlu1-based therapeutic strategy may be helpful for recovering cocaine and methamphetamine addicts.
Keywords: Ca2+-permeable AMPA receptors, extended-access drug self-administration, metabotropic glutamate receptor 1 (mGlu1), incubation of craving, methamphetamine, nucleus accumbens
Introduction
Methamphetamine addiction is a serious public health problem. Its long-term abuse leads to alterations in brain circuitry and cognitive function that are associated with a high likelihood of relapse, even after prolonged abstinence (1,2). In a rodent model of this persistent vulnerability to relapse, termed the incubation model, cue-induced drug craving progressively increases (incubates) during abstinence from drug self-administration and can remain high for months. Incubation of cue-induced craving has been demonstrated in rodents during forced abstinence/withdrawal (the terms will be used interchangeably) following extended-access self-administration of methamphetamine as well as cocaine, heroin, ethanol and nicotine (3–5). Incubation of craving also occurs during forced abstinence in humans addicted to methamphetamine (6), nicotine (7), and alcohol (8). Recently, rodent studies have shown that incubation of methamphetamine craving occurs not only during forced abstinence, but also when abstinence is self-imposed, either because drug-taking is punished (9) or because animals are forced to choose between methamphetamine and a food reward (10). Together, these findings establish that the incubation of craving model is relevant to understanding why methamphetamine users remain vulnerable to cue-induced craving and relapse long after achieving abstinence, regardless of whether abstinence results from incarceration or hospitalization (forced abstinence), a desire to avoid negative consequences associated with drug use, or a transition to alternative sources of reinforcement.
While the cellular basis of incubation of cocaine craving has been extensively studied (5), less is known about mechanisms mediating the incubation of methamphetamine craving (see Discussion). Regarding the underlying neural circuitry, it was found that reversible inactivation of the central nucleus of the amygdala (CeA), but not other regions tested [dorsal medial prefrontal cortex (mPFC), ventral mPFC, and orbitofrontal cortex], impaired the expression of incubated methamphetamine craving (11). Thus, there is partial overlap with incubation of cocaine craving, which requires both CeA and mPFC activation for its expression (12–14). Another study found that D1 dopamine receptor transmission in the dorsal striatum was required for the expression of incubated methamphetamine craving (15). Surprisingly, the nucleus accumbens (NAc), which plays a critical role in the expression of incubated cocaine craving after prolonged withdrawal (see next paragraph), has yet to be evaluated for a role in the incubation of methamphetamine craving. While inactivation of the NAc core was shown to eliminate cue-and methamphetamine-induced reinstatement of drug-seeking after extended-access methamphetamine self-administration and extinction training (16), extinction training and forced abstinence can engage different circuits and produce different neuroadaptations (see Discussion).
In drug-naïve or saline treated animals, GluA2-containing Ca2+-impermeable AMPA receptors (CI-AMPARs) are largely responsible for excitatory transmission onto medium spiny neurons (MSN), the output neurons of the NAc (17,18). However, after ~1 month of withdrawal from extended-access cocaine self-administration, high conductance Ca2+-permeable AMPARs (CP-AMPARs) accumulate in NAc core synapses (18–23) and thereafter their activation is required for the expression of incubated cocaine craving (18,23). This is consistent with other evidence that enhanced activation of NAc core MSN is critical for incubation (24–26), as well as cocaine seeking in other models (e.g., 27,28). CP-AMPAR plasticity occurs in concert with plasticity of group I mGlu-mediated synaptic depression in the NAc core. Control rats exhibit mGlu5-dependent synaptic depression that is expressed presynaptically via cannabinoid receptor 1 stimulation (29). In “cocaine incubated rats”, this is impaired and instead we observe mGlu1-dependent synaptic depression that is expressed postsynaptically via CP-AMPAR endocytosis (20,30). Using mGlu1 positive allosteric modulators (PAMs), this mGlu1-dependent synaptic depression can be targeted to remove CP-AMPARs from NAc synapses and thus reduce cue-induced cocaine craving (23), offering a potential strategy to help recovering users maintain abstinence (5,31). Here we determined whether elevated CP-AMPAR levels and mGlu1-mediated synaptic depression can be demonstrated in the NAc core after incubation of methamphetamine craving and whether the latter mechanism can be targeted to reduce methamphetamine craving.
Methods and Materials
Subjects and Surgery
All experimental procedures were approved by the Rosalind Franklin University Institutional Animal Care and Use Committee in accordance with the USPHS Guide for Care and Use of Laboratory Animals. Adult male Sprague-Dawley rats (Harlan, Indianapolis, IN), 250–275 g upon arrival, were housed 3/cage under a reverse 12-h light-dark cycle. Jugular catheter surgery was performed as described previously (18) (see Supplemental Methods). For rats destined for the experiment using 1-naphthyl acetyl spermine (naspm), following jugular catheterization, guide cannulae (23-gauge, Plastics One) were implanted bilaterally 1.5 mm above the NAc core. Coordinates were: AP −1.4 mm, ML ±2.5 mm (6° angle), DV −5.5 mm (32).
Drug self-administration training
Rats were trained to self-administer methamphetamine or saline (control condition) during a total of 10 daily sessions, each lasting 6 h, conducted over 11–12 days with 1–2 days off, under a fixed-ratio-1 reinforcement schedule. Sessions began at the onset of the dark cycle. Methamphetamine (dissolved in saline) was self-administered at a dose of 0.1 mg/kg/infusion (0.065 mL/infusion). Control rats self-administered saline (0.065 mL/infusion) under the same schedule. Self-administration was conducted in operant chambers equipped with two nose-poke holes. Active hole responses activated the infusion pump and led to the delivery of a 20-sec light cue (white light illuminating the active hole). Each infusion was followed by a 20-sec timeout period. Nose poking in the inactive hole had no consequences. For additional details, see Supplemental Methods.
Tests for cue-induced methamphetamine seeking
For experiments involving seeking tests, rats were divided into groups matched for mean number of infusions and active hole responses during training. They were returned to the self-administration chambers on the specified withdrawal day during the dark cycle and tested for 30 min under extinction conditions, i.e., nose pokes in the previously active hole resulted in presentations of the light cue previously paired with methamphetamine, but methamphetamine was not delivered. The number of responses in the previously active hole was used as a measure of methamphetamine seeking or craving. For experiments that evaluated the effect of the systemically active mGlu1 PAM SYN119 (33) on cue-induced methamphetamine seeking, rats received an injection of vehicle or SYN119 [10 mg/kg, intraperitoneal (i.p.)] 20 min before placement in the operant chamber. During the 20-min post-injection period, they were returned to their home cage. This 20-min period was determined on the basis of the half-life of SYN119 (55 min; ref 34) and on the basis of our prior positive results in similar cocaine studies (23). For the WD1 seeking tests, each rat received one seeking test and one injection (SYN119 or vehicle). For the late withdrawal seeking tests, vehicle and SYN119 injection groups were counterbalanced, with all rats receiving 2 seeking tests and 2 injections (SYN119 and vehicle), 8 days apart (on WD44 and WD52). Counterbalancing was not feasible in early withdrawal. For the experiment that evaluated the effect of intra-NAc core infusion of naspm (on WD45), intracranial injections of naspm (40 μg/site, injection volume 0.5 μl) were made as described previously (18), 15 min before the start of the 30-min seeking test (see Supplemental Methods). Each rat received only one intra-NAc infusion (naspm or vehicle).
Whole-cell patch-clamp recordings
Electrophysiological procedures were described previously (18–23) and are detailed in Supplemental Methods. All MSN were recorded from the NAc core subregion.
Reagents and vehicles
See Supplemental Methods.
Statistical analyses
Data are expressed as mean ± SEM. Student’s t-tests (unpaired unless otherwise indicated) were used for comparing two groups whereas ANOVA was used for comparing multiple groups. Differences between experimental conditions were considered statistically significant when p<0.05.
Results
Incubation of methamphetamine craving
Rats were trained to self-administer methamphetamine (10 total sessions of 6 h/day) and tested for cue-induced methamphetamine craving on either WD1 (n=13) or WD45 (n=13) (Figure 1A). Self-administration training was staggered so that the WD1 group was tested on the same day as the WD45 group. Rats assigned to these groups did not differ during training (Figure 1B). We found a significant increase in the number of nose-pokes in the active hole during the seeking test on WD45 compared to WD1 (t24=3.48, p=0.002), but no significant difference for inactive hole responding (p>0.05) (Figure 1C). These results establish that our methamphetamine regimen leads to incubation of methamphetamine craving, as found with similar regimens (11,15,35).
Figure 1.
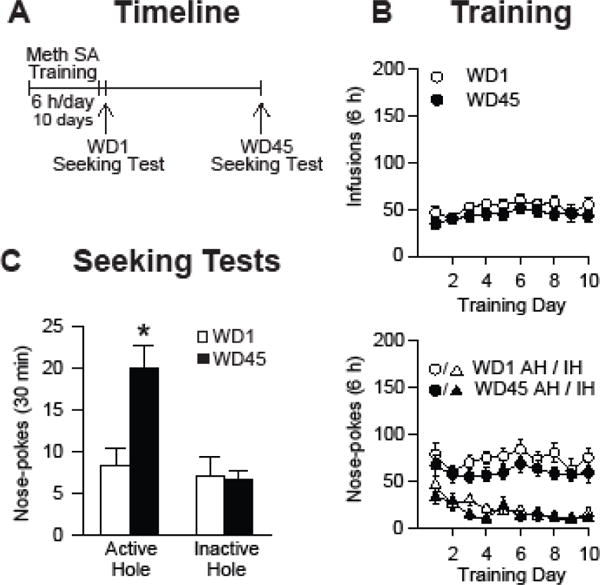
Incubation of methamphetamine craving. (A) Timeline of the experimental procedure. Rats were trained to self-administer methamphetamine for 6 h/day for a total of 10 days, and tested for cue-induced methamphetamine craving on either WD1 or WD45. (B) Training phase: Mean ± SEM number of methamphetamine infusions (top) and number of active hole (AH) and inactive hole (IH) nose-pokes (bottom) over the ten, 6-h daily self-administration training sessions (n=26 total rats). (C) Seeking tests: Data are mean ± SEM nose-pokes in the previously active hole and in the inactive hole during the seeking tests on WD1 (n=13 rats) and WD45 (n=13 rats). During the seeking tests, active hole nose-pokes led to contingent presentation of a 20-sec light cue previously paired with each methamphetamine injection. Meth, methamphetamine; SA, self-administration; WD, withdrawal day.
CP-AMPARs and mGlu1-mediated synaptic depression emerge after incubation
To determine whether the incubation of methamphetamine craving is associated with CP-AMPAR accumulation, whole-cell patch-clamp recordings of NAc core MSN were conducted following >40 days of withdrawal from extended-access methamphetamine or saline self-administration (Figure 2A). This timing was chosen because elevation of CP-AMPAR levels in the NAc is stable from WD35 through at least WD90 in rats that have undergone incubation of cocaine craving (36). Recordings were performed using different rats from those depicted in Figure 1, but self-administration training data for these rats were very similar to data in Figure 1B. The contribution of CP-AMPARs to synaptic transmission was determined by bath application of the CP-AMPAR blocker naspm (100μM). Naspm produced a small inhibition of the EPSC−70mV in MSN from saline controls (8.8%, n=6 cells/4 rats) (Figure 2B), consistent with our previous results indicating that CP-AMPARs are responsible for ~5–10% of the evoked EPSC in drug-naïve rats (18,21,22). In contrast, naspm application produced a significantly larger reduction in the EPSC−70mV in MSN recorded from methamphetamine rats on >WD40 (24.3%, 8 cells/5 rats; t12=3.3, p=0.006, methamphetamine group versus saline controls) (Figure 2B). Thus, after >WD40, incubation of methamphetamine craving is accompanied by an increase in the CP-AMPAR contribution to the EPSC−70mV at excitatory synapses onto NAc core MSN, as reported previously for incubation of cocaine craving (18–23).
Figure 2.
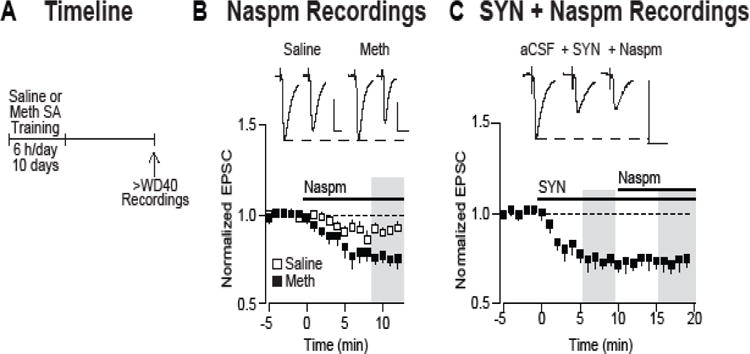
The incubation of methamphetamine craving is accompanied by accumulation of CP-AMPARs in NAc core synapses and emergence of robust mGlu1-mediated synaptic depression. (A) Timeline of the experimental procedure (see legend to Figure 1 for details). (B) Bath application of the CP-AMPAR antagonist naspm (100 μM) produced a significantly greater reduction in EPSC−70mV in MSN from methamphetamine rats (WD>40) as compared to saline controls (24.3% vs. 8.8%; calculated from min 9–12 after naspm application, as shown in shaded region; p<0.05) (methamphetamine, n=8 cells/5 rats; saline, n=6 cells/4 rats). (C) In MSN from methamphetamine rats (>WD40), application of the mGlu1 positive allosteric modulator SYN119 (SYN; 1μM) induced a robust depression of the EPSC−70mV (28%), and subsequent application of naspm had no further effect (based on comparison of shaded regions: last 4 min of SYN alone versus last 4 min of naspm). Scale bars: 40 ms × 100 pA.
Whereas mGlu1 activation has no significant effect on excitatory synaptic transmission in NAc core MSN of drug-naïve rats, it produces synaptic depression in NAc core MSN recorded after incubation of cocaine craving (20,23); this synaptic depression is expressed postsynaptically via removal of CP-AMPARs that accumulate during incubation (20,23,30). To determine whether mGlu1-mediated synaptic depression was similarly observed in NAc core MSN after incubation of methamphetamine craving, the mGlu1 PAM SYN119 (SYN; 1μM) was bath-applied and found to produce a significant depression of the EPSC−70mV (28%, 6 cells/3 rats; t5=9.96, p=0.00018, paired t-test, last 4 min of SYN alone versus last 4 min of pre-SYN baseline) (Figure 2C). Subsequent application of naspm failed to produce a further decrease (1.2%, n=6 cells/3 rats; t5=0.436, p>0.05, paired t-test, last 4 min of SYN alone versus last 4 min of naspm), which we interpret to indicate that SYN had already removed CP-AMPARs from NAc synapses. Therefore, we conclude that mGlu1 activation eliminates the elevated contribution of CP-AMPARs to the EPSC–70mV that is observed in “methamphetamine incubated rats”.
Systemic administration of an mGlu1 PAM reduces incubated cue-induced methamphetamine seeking after >WD40
Having shown that mGlu1 activation attenuates CP-AMPAR-mediated transmission in vitro (Figure 2), we assessed whether enhancing NAc mGlu1 function in vivo would reduce incubated cue-induced methamphetamine craving. A new group of rats (n=20) was trained to self-administer methamphetamine as described above (10 total sessions of 6 h/day) (Figure 3A,B). Rats then underwent withdrawal in their home cages and, after >40 days of withdrawal, received an injection of SYN119 (10 mg/kg, i.p.) or vehicle 20 min prior to a 30-min seeking test, as described previously (23). Vehicle and SYN119 injection groups were counterbalanced, with all rats receiving 2 seeking tests, 8 days apart. Our previous results establish that residual effects of a single SYN119 injection dissipate within 2 days (23). Rats that received SYN119 prior to the seeking test showed a significant reduction in active hole responding compared to vehicle-injected controls (paired t-test, t19=2.44, p=0.025), with no group differences in inactive hole responding (p>0.05) (Figure 3C). Thus, systemic injection of an mGu1 PAM reduces the expression of incubated methamphetamine craving, as shown previously for cocaine (23).
Figure 3.
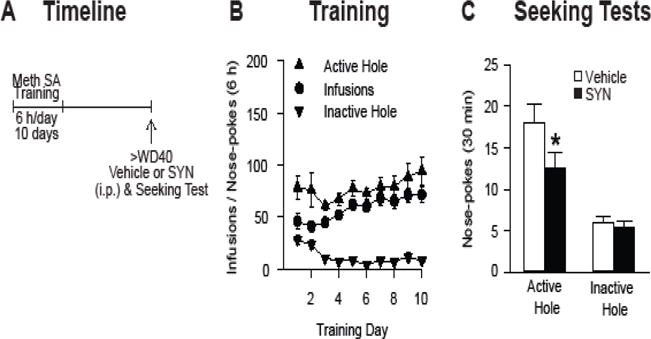
Systemic administration of the mGlu1 positive allosteric modulator SYN119 reduces incubated cue-induced methamphetamine seeking. (A) Timeline of the experimental procedure (see legend to Figure 1 for details). (B) Training phase: Mean ± SEM number of methamphetamine infusions, active hole and inactive hole nose-pokes over the ten, 6-h daily self-administration training sessions (n=20 total rats). (C) Seeking test: Data are mean ± SEM nose-pokes in the previously active hole and in the inactive hole during seeking tests on or after WD40. SYN119 or vehicle was injected (10 mg/kg, i.p.) 20 min prior to the seeking test. SYN119 significantly reduced cue-induced methamphetamine seeking compared to vehicle-injected controls (*p<0.05).
Naspm injection into the NAc core reduces incubated cue-induced methamphetamine seeking on WD45
To investigate whether the reduction in cue-induced methamphetamine craving elicited by SYN119 was mediated by removal of CP-AMPARs from NAc synapses, as suggested by our electrophysiological results (Figure 2C), we asked whether directly blocking CP-AMPARs in the NAc core with naspm would produce a similar reduction. We targeted the core subregion because of its critical role in drug-seeking (see Introduction) and because naspm or mGlu1 PAM injection into the core decreased the expression of incubated cue-induced cocaine seeking (18,23). For this experiment, a new group of rats was implanted with bilateral guide cannulae over the NAc core at the same time as surgery for jugular catheter implantation. After recovery, rats (n=26) were trained to self-administer methamphetamine (Figure 4A). There was no significant difference between rats destined for the naspm group (n=14) versus the vehicle group (n=12) (Figure 4B). On WD45, we injected naspm or vehicle into the NAc core bilaterally 15 min prior to a 30-min seeking test. The naspm group showed significantly less responding in the active hole (t24=2.60, p=0.016), whereas no significant difference was found for inactive hole responding (p>0.05) (Figure 4C). For this study, two separate experiments were run at different times. Due to differences in raw data values between these experiments, data were normalized to the vehicle control group within each experiment and then combined. This explains why inactive hole responses in the vehicle group appear as high as active hole responses in Figure 4C; both are set at 100%. Histological analysis was performed to confirm cannulae placement in the NAc core (not shown), and only rats with confirmed placements were included in the analysis.
Figure 4.
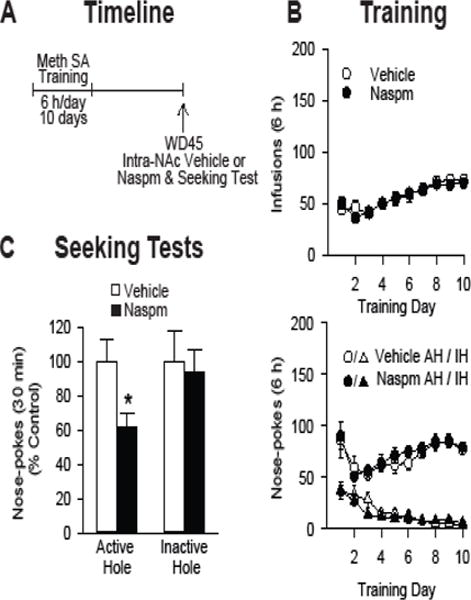
Blockade of CP-AMPARs in the NAc core with naspm reduces incubated cue-induced methamphetamine seeking. (A) Timeline of the experimental procedure (see legend to Figure 1 for details). (B) Training phase: Mean ± SEM number of methamphetamine infusions (top) and number of active hole (AH) and inactive hole (IH) nose-pokes (bottom) over the ten, 6-h daily self-administration training sessions (n=26 total rats). (C) Seeking test: Shown are nose-pokes in the previously AH and in the IH during a seeking test on WD45. Naspm (n=14 rats) or vehicle (n=12 rats) was injected into the NAc core 15 min prior to the seeking test. Naspm significantly reduced cue-induced methamphetamine seeking compared to vehicle-injected controls (*p<0.05). Data are expressed as percent of vehicle group (mean ± SEM); note that both AH and IH responses for the vehicle group are set at 100%. Data are presented in this manner because we combined results from two different experiments (done years apart) with substantially different raw values for AH and IH nose-pokes during the seeking test [Experiment 1: veh group (n=7), AH: 40.4 ± 9.1, IH: 10.0 ± 3.1; naspm group (n=10), AH: 23.4 ± 3.2, IH: 9.8 ± 1.7; Experiment 2: veh group (n=5), AH: 16.4 ± 1.3, IH: 4.8 ± 0.6; naspm group (n=4), AH: 11.8 ± 3.2, IH: 4 + 0.9].
Incubation and CP-AMPAR elevation develop rapidly after discontinuing methamphetamine self-administration
After cocaine self-administration, incubation of craving begins within the first week of abstinence (37), whereas CP-AMPAR elevation is first detected after ~1 month of abstinence (36). We determined whether these changes occur with a similar time-course during methamphetamine withdrawal, beginning with CP-AMPAR plasticity. A new group of rats was used. On WD1, the reduction in EPSC−70mV produced by naspm (4.2 ± 4.0%, n=6 cells/4 rats, black circles in Figure 5B) did not differ from that observed in saline controls (8.8 ± 2.0%, n=6 cells/4 rats, open squares in Figure 2B; t10=1.04, p>0.05, methamphetamine WD1 versus saline) and was in the range reported previously for other saline controls (18,21,22). Furthermore, systemic administration of the mGlu1 PAM SYN119 had no effect on cue-induced seeking on WD1 (vehicle, 11.9±1.3, n=14 rats; SYN, 8.5±1.7, n=15 rats; t27=1.5, p>0.05; vehicle versus SYN, active hole responses). Thus, as expected, CP-AMPARs remain at control levels on WD1 from methamphetamine self-administration. However, to our surprise, CP-AMPARs accumulated to maximal levels by WD7–8. This was determined by comparing naspm sensitivity in groups of rats recorded on WD1, WD2–4, WD7–8, and WD>40 (Figure 5B). A significant group effect was found (F3,20=6.03, p=0.004) and LSD post-hoc tests revealed significantly greater naspm sensitivity on WD7–8 (20.6% reduction in EPSC−70mV; n=5 cells/4 rats) and WD>40 (24.3%; n=8 cells/5 rats, black squares in Figure 2B) compared to WD1 (4.2%; see above), and no significant difference between WD7–8 and WD>40 (p>0.05). The latter group included rats tested as late as WD53. If we binned the >WD40 rats into two groups, WD40–46 and WD46–53, a t-test revealed no significant difference between these groups (p>0.05). A trend towards increased naspm sensitivity was found on WD2–4 (15.2%; n=5 cells/4 rats; p>0.05 versus WD1). We then compared cue-induced methamphetamine seeking using a within-subjects design (n=12 rats) on WD7 and WD30 and found that it also reached maximal levels by WD7 (t11=0.04, p>0.05, WD7 versus WD30, paired t-test, active hole responses; Figure 5E; seeking was also comparable to WD45 data in Figure 1 and the >WD40 vehicle group in Figure 3C). Thus, incubation of craving and CP-AMPAR accumulation in the NAc core occur rapidly and in parallel after discontinuing methamphetamine self-administration.
Figure 5.
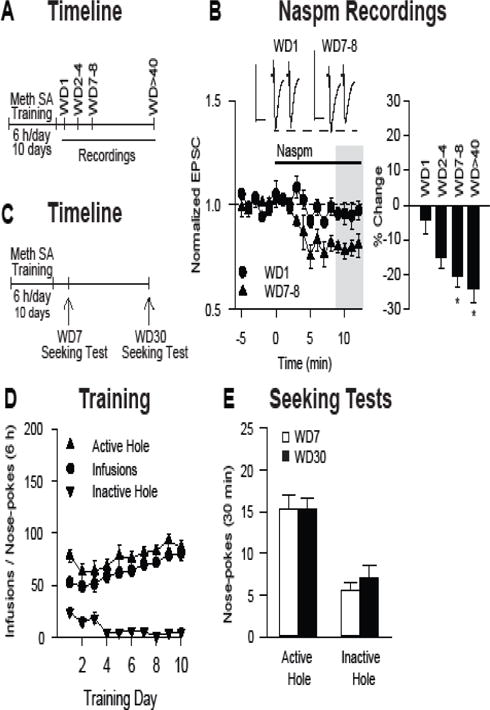
Incubation of methamphetamine craving and CP-AMPAR accumulation in NAc core synapses reach maximal levels by withdrawal day 7–8. (A) Timeline of experimental procedures for results shown in panel B (see legend to Figure 1 for details). (B) The reduction in EPSC−70 produced by bath application of the CP-AMPAR antagonist naspm (100 μM) was used to define the contribution of CP-AMPARs to synaptic transmission in methamphetamine rats recorded on different withdrawal days (WD). The left graph compares the effect of naspm in MSN recorded on WD1 (n=6 cells/4 rats) versus WD7–8 (n=5 cells/4 rats) (data are mean ± SEM). For sample traces, scale bars indicate 40 ms × 100 pA. The bar graph on the right summarizes data from WD1 and WD78, as well as from WD2–4 (n=5 cells/4 rats) and WD>40 (n=8 cells/5 rats) (the latter data are taken from Figure 2). Data in the right graph are expressed as % reduction in EPSC−70 after naspm (average of min 9–12 after naspm; this period is indicated by gray shading in the left graph) (*p<0.05). (C) Timeline of experimental procedures for results shown in panels D and E (see legend to Figure 1 for details). (D) Training phase: Mean ± SEM number of methamphetamine infusions, active hole and inactive hole nose-pokes over the ten, 6-h daily self-administration training sessions (n=12 rats). (E) Seeking test: Each rat was tested twice, on WD7 and WD30. Data are mean ± SEM nose-pokes in the previously active hole and in the inactive hole during seeking tests.
Discussion
Our goal was to determine if the same CP-AMPAR plasticity implicated in the incubation of cocaine craving is also important for incubation of methamphetamine craving. This would help determine if common therapeutic approaches might be useful for abstinent methamphetamine and cocaine addicts.
Common AMPAR adaptations in NAc core after incubation of cocaine and methamphetamine craving
To investigate the role of CP-AMPARs in methamphetamine incubation, we began by studying “methamphetamine incubated rats” after >40 days of withdrawal because elevation of CP-AMPAR levels in the NAc core of “cocaine incubated rats” is stable from WD35 through at least WD90 (36). At this late withdrawal time, our results demonstrate similarities between mechanisms underlying incubation of cocaine and methamphetamine craving. Most notably, while CP-AMPAR levels are low in NAc core MSN of drug-naïve rats, their contribution to synaptic transmission is increased (to ~25–30% of the evoked EPSC) after ~40 days of withdrawal from extended-access self-administration of cocaine (18–23) and methamphetamine (present results). Furthermore, as observed for “cocaine incubated rats” (18,23), the expression of incubated methamphetamine craving was reduced either by blocking CP-AMPARs in the NAc core (via infusion of naspm) or by treating rats with a systemic injection of the mGlu1 PAM SYN119. The latter is a key finding as it indicates that mGlu1 PAM-based therapeutic approaches may be useful for prolonging abstinence in both cocaine and methamphetamine users. We note that SYN119 did not completely abolish incubated methamphetamine craving (present results) or cocaine craving (23), consistent with evidence that incubation involves multiple mechanisms and brain regions (3–5).
For “cocaine incubated rats”, we have shown that the ability of systemic SYN119 to reduce craving was due to removal of CP-AMPARs from NAc synapses (23). Electrophysiological studies revealed that this removal involves dynamin-dependent CP-AMPAR internalization (30). It is likely that systemic SYN119 reduces incubated methamphetamine craving through the same mechanism, based on slice recordings in the present study demonstrating that bath-applied SYN119 removes CP-AMPARs from NAc MSN of “methamphetamine incubated rats”. Furthermore, in other synapses that contain high levels of CP-AMPARs, mGlu1 PAMs similarly produce an inhibition of CP-AMPAR transmission that, in all cases tested, has been found to depend upon CP-AMPAR endocytosis (38–43). We note that effects of SYN119 in the ventral tegmental area may also contribute to decreased cocaine and methamphetamine seeking (44). Future studies should determine if elevated CP-AMPAR levels in the NAc of “methamphetamine incubated rats” result from increased GluA1 expression and GluA1 homomer formation, as shown for cocaine (18,45). Interestingly, epigenetic mechanisms are implicated in altered AMPAR subunit expression after non-contingent methamphetamine (46).
Based on studies of locomotor sensitization elicited by non-contingent cocaine and methamphetamine injections, it is perhaps surprising that common AMPAR adaptations in the NAc core are observed in the incubation model. Several groups have demonstrated increased surface and synaptic expression of GluA1A2-containing AMPARs in the NAc core after a week or so of withdrawal from repeated i.p. cocaine injections (for reviews, see 5,47), while this does not occur in the NAc of rats sensitized to methamphetamine (48). However, it must be kept in mind that different plasticity often results from contingent versus non-contingent drug administration (for an example pertaining to methamphetamine’s effect on glutamate levels, see ref 49). On the other hand, there can be commonalities; thus methamphetamine self-administration decreased intrinsic excitability of MSN in the NAc shell during the first few days of withdrawal (50), and the same effect has been observed shortly after discontinuing cocaine self-administration (51) and even non-contingent cocaine administration (for reviews, see 52,53). Future studies should evaluate effects of methamphetamine on intrinsic excitability in the NAc core, and examine potential relationships between methamphetamine-induced changes in intrinsic excitability and synaptic strength (53).
Incubation and CP-AMPAR plasticity occur more rapidly for methamphetamine than cocaine For cocaine, incubation of craving begins during the first week of withdrawal, continues to rise for 1–2 months and then remains high through at least WD90 before declining slowly (4,37). After a nearly identical cocaine regimen, CP-AMPAR levels in the NAc core remain at saline control levels for the first three weeks of abstinence, with an increase in CP-AMPAR levels first detected between WD25 and WD35; elevated levels are then maintained through at least WD90 (36). Once CP-AMPAR levels increase, their activation is required for expression of incubation of cocaine craving (18,23). The delayed onset of CP-AMPAR accumulation versus incubation of cocaine seeking indicates that other mechanisms must account for incubation during the first month of withdrawal, in keeping with evidence that incubation involves complex circuitry beyond the NAc (see Introduction and 3–5).
We do not fully understand why a month of withdrawal elapses before CP-AMPAR levels rise. However, the rise in CP-AMPAR levels is preceded and enabled by a decrease in mGlu1 surface expression in the NAc core (23). Activation of mGlu1 removes CP-AMPARs from NAc synapses (20), so the withdrawal-dependent decrease in cell surface mGlu1 is thought to permit CP-AMPARs to accumulate in these synapses (23,31). In contrast, the present findings show that incubation of methamphetamine craving is complete by WD7, and that CP-AMPAR accumulation follows a parallel time course. The rapidity of incubation of Meth craving is confirmed by a very recent study (54). We speculate that more rapid incubation of methamphetamine craving may contribute to methamphetamine’s highly addictive nature. Future studies will determine whether more rapid CP-AMPAR accumulation after methamphetamine reflects a more rapid loss of mGlu1 surface expression in the NAc.
Methamphetamine and mGlu5
While less is known about mGlu1, it is well established that drugs targeting mGlu5 can affect addictive behaviors in animal models, with mGlu5 negative allosteric modulators (NAMs) suppressing responses to drugs including reinstatement of drug-seeking (55) while mGlu5 PAMs exert beneficial effects by facilitating extinction of drug-seeking behavior and reversing drug-induced cognitive deficits (56). Studies conducted after methamphetamine self-administration have similarly demonstrated both pro-cognitive effects of mGlu5 PAMs (57–59) and anti-relapse effects of mGlu5 NAMs (60). The latter effect was observed following limited-access methamphetamine self-administration and extinction training (60). It is unclear whether a similar effect would be observed in the incubation model, which utilizes extended-access self-administration and abstinence rather than extinction training, based on growing evidence that different circuits and glutamatergic adaptations are engaged when methamphetamine self-administration is followed by extinction training versus abstinence. For example, inactivation of similar regions of mPFC prevented reinstatement of methamphetamine seeking after extended-access methamphetamine self-administration and extinction training (16) but did not prevent expression of incubated methamphetamine seeking (11). Other examples of different plasticity after extinction versus abstinence, related to methamphetamine’s actions on basal glutamate levels (49,61) and mGlu2/3 expression (62), are discussed in the next section. Similarly, it is well established that different neuroadaptations occur when cocaine self-administration is followed by extinction training versus abstinence (for example, 63–66). The self-administration regimen itself also determines subsequent neuroadaptations. For example, mGlu1 PAMs reduce expression of incubated cocaine seeking (23), whereas a recent study found that mGlu1 activation promoted drug primed-reinstatement of cocaine seeking after limited-access cocaine self-administration and extinction training (67). The likely explanation for the difference is that limited-access cocaine self-administration in adult rats is not sufficient to elicit CP-AMPAR accumulation (21), so there is no substrate for mGlu1-induced synaptic depression and therefore no suppression of NAc output (20,22,23).
It will also be important to examine the effect of methamphetamine self-administration on mGlu5 expression and function, given that “cocaine incubated rats” show impaired mGlu5-induced synaptic depression (20) and a small decrease in mGlu5 surface expression in the NAc after prolonged withdrawal (23; but see ref 65 for different results after limited-access cocaine self-administration). Non-contingent methamphetamine administration does not affect mGlu5 levels in the NAc, although mGlu5 expression is altered in other regions (48,68).
Methamphetamine and presynaptic glutamate function
While the present study focused on postsynaptic adaptations, methamphetamine self-administration may also alter presynaptic glutamate function. No-net flux microdialysis studies have detected changes in basal glutamate levels that are opposite in direction depending on whether methamphetamine self-administration is followed by abstinence (49) or extinction (61). A history of methamphetamine self-administration also influences glutamate efflux in response to methamphetamine re-exposure (49,61). Presynaptic mGlu2/3 receptors are also implicated in methamphetamine seeking. Their activation reduces incubated methamphetamine seeking (10), motivation to self-administer methamphetamine (69), and cue or methamphetamine-induced reinstatement of methamphetamine seeking after extinction training (70). Interestingly, extended-access methamphetamine self-administration followed by 14 days of abstinence decreased mGlu2/3 levels in the NAc, whereas this was not observed after extinction training (62).
Conclusions
The incubation of drug craving model is relevant to understanding persistent vulnerability to cue-induced drug craving and relapse in recovering addicts (4,5,37,71). The present study is the first to demonstrate a role for the NAc in the expression of incubated methamphetamine craving. Combined with previous results on the incubation of cocaine craving (23), our results suggest that recovering methamphetamine or cocaine addicts could use mGlu1 PAMs prophylactically prior to entering a situation which might provoked cue-induced drug craving. This would help them maintain abstinence for a longer period of time. No drug is presently available that provides such protection. Demonstrating that the potential utility of mGlu1 PAMs extends to both cocaine and methamphetamine addiction enhances the justification for testing this strategy in clinical studies.
Supplementary Material
Acknowledgments
This research was supported by RO1 DA009621 and DA015835 to MEW, K05 DA029099 to MEW, predoctoral NRSA DA036327 to AFS, K99 DA038110 to JAL, and postdoctoral NRSA DA036963 to DTC. We are grateful to Dr. Kuei-Yuan Tseng (Rosalind Franklin University of Medicine and Science) for advice on electrophysiological recordings, and to Dr. M. Foster Olive (University of Arizona) for his generous gift of SYN119.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures: The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Baicy K, London ED. Corticolimbic dysregulation and chronic methamphetamine abuse. Addiction. 2007;102(Suppl 1):5–15. doi: 10.1111/j.1360-0443.2006.01777.x. [DOI] [PubMed] [Google Scholar]
- 2.Salo R, Nordahl TE, Galloway GP, Moore CD, Waters C, Leamon MH. Drug abstinence and cognitive control in methamphetamine-dependent individuals. J Substance Abuse Treatment. 2009;37(3):292–297. doi: 10.1016/j.jsat.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li X, Caprioli D, Marchant NJ. Recent updates on incubation of drug craving: a mini-review. Addict Biol. 2015;20(5):872–876. doi: 10.1111/adb.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf ME. Synaptic mechanisms maintaining persistent cocaine craving. Nature Rev Neurosci. 2016 doi: 10.1038/nrn.2016.39. in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang G, Shi J, Chen N, Xu L, Li J, Li P, et al. Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PLoS One. 2013;8:e68791. doi: 10.1371/journal.pone.0068791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bedi G, Preston KL, Epstein DH, Heishman SJ, Marrone GF, Shaham Y, et al. Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol Psychiatry. 2011;69:708–711. doi: 10.1016/j.biopsych.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li P, Wu P, Xin X, Fan LY, Wang GB, Wang F, et al. Incubation of alcohol craving during abstinence in patients with alcohol dependence. Addict Biol. 2014;20:513–522. doi: 10.1111/adb.12140. [DOI] [PubMed] [Google Scholar]
- 9.Krasnova IN, Marchant NJ, Ladenheim B, McCoy MT, Panlilio LV, Bossert JM, et al. Incubation of methamphetamine and palatable food craving after punishment-induced abstinence. Neuropsychopharmacology. 2014;39(8):2008–2016. doi: 10.1038/npp.2014.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caprioli D, Venniro M, Zeric T, Li X, Adhikary S, Madangopal R, et al. Effect of the novel positive allosteric modulator of mGluR2 AZD8529 on incubation of methamphetamine craving after prolonged voluntary abstinence. Biol Psychiatry. 2015;78(7):463–473. doi: 10.1016/j.biopsych.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, Zeric T, Kambhampati S, Bossert JM, Shaham Y. The central amygdala nucleus is critical for incubation of methamphetamine craving. Neuropsychopharmacology. 2015;40(5):1297–1306. doi: 10.1038/npp.2014.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci. 2005;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- 13.Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y. Systemic and central amygdala injections of the mGluR2/3 agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Psychiatry. 2007;61:591–598. doi: 10.1016/j.biopsych.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology. 2009;56(Suppl 1):177–185. doi: 10.1016/j.neuropharm.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X, Rubio FJ, Zeric T, Bossert JM, Kambhampati S, Cates HM, et al. Incubation of methamphetamine craving is associated with selective increases in expression of Bdnf and Trkb, glutamate receptors, and epigenetic enzymes in cue-activated fos-expressing dorsal striatal neurons. J Neurosci. 2015;35:8232–8244. doi: 10.1523/JNEUROSCI.1022-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rocha A, Kalivas PW. Role of the prefrontal cortex and nucleus accumbens in reinstating methamphetamine seeking. Eur J Neurosci. 2010;31:903–909. doi: 10.1111/j.1460-9568.2010.07134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCutcheon JE, Wang X, Tseng KY, Wolf ME, Marinelli M. Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. J Neurosci. 2011;31:5737–5743. doi: 10.1523/JNEUROSCI.0350-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCutcheon JE, Loweth JA, Ford KA, Marinelli M, Wolf ME, Tseng KY. Group I mGluR activation reverses cocaine-induced accumulation of calcium-permeable AMPA receptors in nucleus accumbens synapses via a protein kinase C-dependent mechanism. J Neurosci. 2011;31:14536–14541. doi: 10.1523/JNEUROSCI.3625-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purgianto A, Scheyer AF, Loweth JA, Ford KA, Tseng KY, Wolf ME. Different adaptations in AMPA receptor transmission in the nucleus accumbens after short vs long access cocaine self-administration regimens. Neuropsychopharmacology. 2013;38:1789–1797. doi: 10.1038/npp.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheyer AF, Wolf ME, Tseng KY. A protein synthesis-dependent mechanism sustains calcium-permeable AMPA receptor transmission in nucleus accumbens synapses during withdrawal from cocaine self-administration. J Neurosci. 2014;34(8):3095–3100. doi: 10.1523/JNEUROSCI.4940-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loweth JA, Scheyer AF, Milovanovic M, LaCrosse AL, Flores-Barrera E, Werner CT, et al. Synaptic depression via mGluR1 positive allosteric modulation suppresses cue-induced cocaine craving. Nat Neurosci. 2014;17:73–80. doi: 10.1038/nn.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hollander JA, Carelli RM. Abstinence from cocaine self-administration heightens neural encoding of goal-directed behaviors in the accumbens. Neuropsychopharmacology. 2005;30:1464–1474. doi: 10.1038/sj.npp.1300748. [DOI] [PubMed] [Google Scholar]
- 25.Hollander JA, Carelli RM. Cocaine-associated stimuli increase cocaine seeking and activate accumbens core neurons after abstinence. J Neurosci. 2007;27:3535–3539. doi: 10.1523/JNEUROSCI.3667-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guillem K, Ahmed SH, Peoples LL. Escalation of cocaine intake and incubation of cocaine seeking are correlated with dissociable neuronal processes in different accumbens subregions. Biol Psychiatry. 2014;76:31–39. doi: 10.1016/j.biopsych.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 27.Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Ciano P, Everitt BJ. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J Neurosci. 2004;24:7167–7173. doi: 10.1523/JNEUROSCI.1581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci U S A. 2002;99(12):8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheyer AF, Wolf ME, Tseng KY. Emergence of endocytosis-dependent mGluR1 LTD at nucleus accumbens synapses during withdrawal from cocaine self-administration. Soc Neurosci Abstr. 2015;41:778.21. doi: 10.3389/fnsyn.2018.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loweth JA, Tseng KY, Wolf ME. Using metabotropic glutamate receptors to modulate cocaine’s synaptic and behavioral effects: mGluR1 finds a niche. Curr Opin Neurobiol. 2013;23:500–506. doi: 10.1016/j.conb.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Amsterdam: Elsevier Academic Press; 2005. [Google Scholar]
- 33.Ngomba RT, Santolini I, Biagioni F, Molinaro G, Simonyi A, van Rijn CM, et al. Protective role for type-1 metabotropic glutamate receptors against spike and wave discharges in the WAG/Rij rat model of absence epilepsy. Neuropharmacology. 2011;60:1281–1291. doi: 10.1016/j.neuropharm.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Vieira E, Huwyler J, Jolidon S, Knoflach F, Mutel V, Wichmann J. Fluorinated 9H-xanthene-9-carboxlic acid oxazol-2-yl-amides as potent, orally available mGlu1 receptor enhances. Bioorganic Medicinal Chem Lett. 2009;19:1666–1669. doi: 10.1016/j.bmcl.2009.01.108. [DOI] [PubMed] [Google Scholar]
- 35.Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 36.Wolf ME, Tseng KY. Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how, and why? Front Mol Neurosci. 2012;5:72–98. doi: 10.3389/fnmol.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47(Suppl 1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 38.Bellone C, Lüscher C. mGluRs induce a long-term depression in the ventral tegmental area that involves a switch of the subunit composition of AMPA receptors. Eur J Neurosci. 2005;21:1280–1288. doi: 10.1111/j.1460-9568.2005.03979.x. [DOI] [PubMed] [Google Scholar]
- 39.Bellone C, Lüscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci. 2006;9:636–641. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- 40.Clem RL, Huganir RL. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science. 2010;330:1108–1112. doi: 10.1126/science.1195298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly L, Farrant M, Cull-Candy SG. Synaptic mGluR activation drives plasticity of calcium permeable AMPA receptors. Nat Neurosci. 2009;12:593–601. doi: 10.1038/nn.2309. [DOI] [PubMed] [Google Scholar]
- 42.Clem RL, Huganir RL. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science. 2010;330:1108–1112. doi: 10.1126/science.1195298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mameli M, Balland B, Luján R, Lüscher C. Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science. 2007;317:530–533. doi: 10.1126/science.1142365. [DOI] [PubMed] [Google Scholar]
- 44.Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R, Lüscher C. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat Neurosci. 2009;12:1036–1041. doi: 10.1038/nn.2367. [DOI] [PubMed] [Google Scholar]
- 45.Ferrario CR, Loweth JA, Milovanovic M, Ford KA, Galiñanes GL, Heng L-J, Tseng KY, Wolf ME. Alterations in AMPA receptor subunits and TARPs in the rat nucleus accumbens related to the formation of Ca2+-permeable AMPA receptors during the incubation of cocaine craving. Neuropharmacology. 2011;61:1141–1151. doi: 10.1016/j.neuropharm.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jayanthi S, McCoy MT, Chen B, Britt JP, Kourrich S, Yau HJ, Ladenheim B, Krasnova IN, Bonci A, Cadet JL. Methamphetamine downregulates striatal glutamate receptors via diverse epigenetic mechanisms. Biol Psychiatry. 2014;76(1):47–56. doi: 10.1016/j.biopsych.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolf ME, Ferrario CR. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci & Biobehav Rev. 2010;35(2):185–211. doi: 10.1016/j.neubiorev.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herrold AA, Persons AL, Napier TC. Cellular distribution of AMPA receptor subunits and mGlu5 following acute and repeated administration of morphine or methamphetamine. J Neurochem. 2013;126(4):503–517. doi: 10.1111/jnc.12323. [DOI] [PubMed] [Google Scholar]
- 49.Lominac KD, Sacramento AD, Szumlinski KK, Kippin TE. Distinct neurochemical adaptations within the nucleus accumbens produced by a history of self-administered vs non-contingently administered intravenous methamphetamine. Neuropsychopharmacology. 2012;37:707–722. doi: 10.1038/npp.2011.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Graves SM, Clark MJ, Traynor JR, Hu XT, Napier TC. Nucleus accumbens shell excitability is decreased by methamphetamine self-administration and increased by 5-HT2C receptor inverse agonism and agonism. Neuropharmacology. 2015;89:113–121. doi: 10.1016/j.neuropharm.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mu P, Moyer JT, Ishikawa M, Zhang Y, Panksepp J, Sorg BA, et al. Exposure to cocaine dynamically regulates the intrinsic membrane excitability of nucleus accumbens neurons. J Neurosci. 2010;30:3689–3699. doi: 10.1523/JNEUROSCI.4063-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolf ME. The Bermuda Triangle of cocaine-induced neuroadaptations. Trends Neurosci. 2010;33:391–398. doi: 10.1016/j.tins.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kourrich S, Calu DJ, Bonci A. Intrinsic plasticity: an emerging player in addiction. Nat Rev Neurosci. 2015;16:173–184. doi: 10.1038/nrn3877. [DOI] [PubMed] [Google Scholar]
- 54.Adhikary S, Caprioli D, Venniro M, Kallenberger P, Shaham Y, Bossert JM. Incubation of extinction responding and cue-induced reinstatement, but not context-or drug priming-induced reinstatement, after withdrawal from methamphetamine. Addict Biol. 2016 Mar 14; doi: 10.1111/adb.12386. 2016. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Olive MF. Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr Drug Abuse Rev. 2009;2:83–98. doi: 10.2174/1874473710902010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olive MF. Cognitive effects of group I metabotropic glutamate receptor ligands in the context of drug addiction. Eur J Pharmacol. 2010;639:47–58. doi: 10.1016/j.ejphar.2010.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reichel CM, Schwendt M, McGinty JF, Olive MF, See RE. Loss of object recognition memory produced by extended access to methamphetamine self-administration is reversed by positive allosteric modulation of metabotropic glutamate receptor 5. Neuropsychopharmacology. 2011;36:782–792. doi: 10.1038/npp.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kufahl PR, Hood LE, Nemirovsky NE, Barabas P, Halstengard C, Villa A, et al. Positive allosteric modulation of mGluR5 accelerates extinction learning but not relearning following methamphetamine self-administration. Front Pharmacol. 2012;3:194. doi: 10.3389/fphar.2012.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peters J, Scofield MD, Ghee SM, Heinsbroek JA, Reichel CM. Perirhinal cortex mGlu5 receptor activation reduces relapse to methamphetamine seeking by restoring novelty salience. Neuropsychopharmacology. 2015 Sep 14; doi: 10.1038/npp.2015.283. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gass JT, Osborne MP, Watson NL, Brown JL, Olive MF. mGluR5 antagonism attenuates methamphetamine reinforcement and prevents reinstatement of methamphetamine-seeking behavior in rats. Neuropsychopharmacology. 2009;34:820–833. doi: 10.1038/npp.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parsegian A, See RE. Dysregulation of dopamine and glutamate release in the prefrontal cortex and nucleus accumbens following methamphetamine self-administration and during reinstatement in rats. Neuropsychopharmacology. 2014;39:811–822. doi: 10.1038/npp.2013.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schwendt M, Reichel CM, See RE. Extinction-dependent alterations in corticostriatal mGluR2/3 and mGluR7 receptors following chronic methamphetamine self-administration in rats. PLoS ONE. 2012;7:e34299. doi: 10.1371/journal.pone.0034299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Self DW, Choi KH, Simmons D, Walker JR, Smagula CS. Extinction training regulates neuroadaptive responses to withdrawal from chronic cocaine self-administration. Learn Mem. 2004;11:648–57. doi: 10.1101/lm.81404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci. 2006;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW. Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. J Neurosci. 2010;30:7984–7992. doi: 10.1523/JNEUROSCI.1244-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ghasemzadeh MB, Vasudevan P, Mueller C, Seubert C, Mantsch JR. Neuroadaptations in the cellular and postsynaptic group 1 metabotropic glutamate receptor mGluR5 and Homer proteins following extinction of cocaine self-administration. Neurosci Lett. 2009;452:167–171. doi: 10.1016/j.neulet.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmidt HD, Kimmey BA, Arreola AC, Pierce RC. Group I metabotropic glutamate receptor-mediated activation of PKC gamma in the nucleus accumbens core promotes the reinstatement of cocaine seeking. Addiction Biol. 2015;20:285–296. doi: 10.1111/adb.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Herrold AA, Voigt RM, Napier TC. Brain region-selective cellular redistribution of mGlu5 but not GABA(B) receptors following methamphetamine-induced associative learning. Synapse. 2011;65:1333–1343. doi: 10.1002/syn.20968. [DOI] [PubMed] [Google Scholar]
- 69.Crawford JT, Roberts DC, Beveridge TJ. The group II metabotropic glutamate receptor agonist, LY379268, decreases methamphetamine self-administration in rats. Drug Alcohol Depend. 2013;132:414–419. doi: 10.1016/j.drugalcdep.2013.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kufahl PR, Watterson LR, Nemirovsky NE, Hood LW, Villa A, Halstengard C, et al. Attenuation of methamphetamine seeking by the mGluR2/3 agonist LY379268 in rats with histories of restricted and escalated self-administration. Neuropharmacol. 2013;66:290–301. doi: 10.1016/j.neuropharm.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reichel CM, Bevins RA. Forced abstinence model of relapse to study pharmacological treatments of substance use disorder. Curr Drug Abuse Rev. 2009;2:184–194. doi: 10.2174/1874473710902020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


