Abstract
Background
Drug addiction is defined as a chronic disease characterized by compulsive drug seeking and episodes of relapse despite prolonged periods of drug abstinence. Neurobiological adaptations, including transcriptional and epigenetic alterations in the nucleus accumbens, are thought to contribute to this life-long disease state. We previously demonstrated that transcription factor SMAD3 is increased following seven days of withdrawal from cocaine self-administration. However, it is still unknown which additional factors participate in the process of chromatin remodeling and facilitate the binding of SMAD3 to promoter regions of target genes. Here we examined the possible interaction of BRG1—also known as SMARCA4, an ATPase-containing chromatin remodeler—and SMAD3 in response to cocaine exposure.
Methods
The expression of BRG1, as well as its binding to SMAD3 and target gene promoter regions, was evaluated in the nucleus accumbens and dorsal striatum of rats using Western blotting, co-immunoprecipitation, and chromatin immunoprecipitation techniques, respectively, following withdrawal from cocaine self-administration. Rats were assessed for cocaine-seeking behaviors following either intra-accumbal injections of the BRG1 inhibitor PFI3 or viral-mediated overexpression of BRG1.
Results
Following withdrawal from cocaine self-administration, BRG1 expression and complex formation with SMAD3 are increased in the nucleus accumbens, resulting in increased binding of BRG1 to the promoter regions of Ctnnb1, Mef2d, and Dbn1. Intra-accumbal infusion of PFI3 attenuated, while viral overexpression of Brg1 enhanced, cocaine-reinstatement behavior.
Conclusion
BRG1 is a key mediator of the SMAD3-dependent regulation of cellular and behavioral plasticity mediating cocaine seeking following a period of withdrawal.
Keywords: Cocaine, Cue-induced reinstatement, Self-administration, SMAD3, BRG1, Chromatin remodeling
Introduction
Drug addiction is one of the most debilitating psychiatric disorders, and is characterized by compulsive drug seeking and episodes of relapse despite prolonged periods of abstinence. In drug addicts, relapse and craving during abstinence are often triggered by environmental cues that were previously associated with drug use (1). There are few effective treatments for drug addiction, in part due to a lack of detailed pathophysiology of the disease (2). The clinical scenario can be modeled by testing for reinstatement of drug seeking in laboratory animals after exposure to discrete cues (3–5). Such studies have shown that neurobiological adaptations leading to changes in neuronal plasticity in key brain areas, such as the nucleus accumbens (NAc), contribute to increased drug seeking and craving (6–8).
Accumulating evidence suggests that the long-term changes in cellular and behavioral function following drug exposure are mediated by alterations in gene transcription (9, 10). We previously identified SMAD3, a transcription factor within the transforming growth factor-β (TGF-β) signaling pathway, as an essential mediator of cocaine-induced plasticity and craving behavior that regulates the expression of β-catenin (Ctnnb1), adenylyl cyclase-associated protein 2 (Cap2), and drebrin (Dbn1) (11). Binding of SMAD3 to DNA and the subsequent regulation of gene expression are thought to require interactions with ATP-dependent SWI/SNF nucleosome repositioning complexes (12, 13). A core subunit of SWI/SNF family complexes is BRG1 (also known as SMARCA4), which incorporates into the transcriptional complexes formed by SMADs (12, 14, 15). Although a role of these transcriptional complexes has been documented in regulation of cognitive processes, such as memory-related synaptic plasticity (16), the role of the BRG1—SMAD3 complex in drug addiction remains unknown. The data presented here provide evidence that this complex is involved in drug seeking following a period of abstinence. Withdrawal from cocaine self-administration in rats resulted in increased expression and functional interaction of BRG1 and SMAD3 in the NAc. Furthermore, pharmacological inhibition of BRG1 attenuated its interaction with SMAD3 and suppressed cue-induced reinstatement of cocaine seeking, while overexpression of BRG1 exacerbated cocaine-seeking behavior. These results implicate the SMAD3 and BRG1 transcriptional complex in the regulation of gene expression and behavioral changes following cocaine exposure, providing a novel mechanism for potential therapeutic targets in the treatment of cocaine addiction.
Methods and materials
Subjects
Male Sprague–Dawley rats (300–375 g; Harlan, Indianapolis, IN) were allowed to habituate to the colony room for 7 d upon arrival. Rats had ad libitum access to food and water and were singly housed following surgery and for the duration of the self-administration phase of the experiments in order to protect the catheter/harness assembly. Behavioral testing took place during the dark phase of the 12-h light–dark cycle. This study was conducted in accordance with the guidelines set up by the Institutional Animal Care and Use Committee of the State University of New York at Buffalo.
Self-administration test chambers
Twenty-four standard experimental test chambers (MED Associates, Inc., St. Albans, VT) were used for self-administration and reinstatement experiments. The test chambers contain two snout-poke holes located on one wall, with a stimulus light mounted above each, and a house light mounted in the middle of the back wall. Snout pokes were monitored with infrared detectors. Test chambers were computer controlled by a MED Associates interface with MED-PC.
Drugs
(−)-Cocaine hydrochloride, generously supplied by the NIDA drug supply program, was dissolved in sterile 0.9% saline. Cocaine solutions (4.5 mg/mL) were prepared on a weekly basis, and delivered via a syringe pump. Pump durations/injection volumes were adjusted on a daily basis according to body weight in order to deliver 1.0 mg/kg/infusion. The BRG1 inhibitor PFI3 (Tocris, MN) was dissolved in a mixture of 1 part absolute ethanol, 1 part Emulphor-620 (Rhodia of Solvay, S.A., Brussels, Belgium), and 18 parts saline (17) at a concentration of 30 mM, based on a previous study (18).
Jugular catheterization and patency testing
Rats were implanted with chronic indwelling jugular catheters as previously described (19, 20). Rats were allowed 7 d to recover following surgery. The catheters were flushed daily with 0.2 mL of a solution of enrofloxacin (4 mg/mL) in heparinized saline (50 IU/mL in 0.9% sterile saline) to preserve catheter patency. At the end of behavioral testing, each animal received an i.v. infusion of ketamine hydrochloride (0.5 mg/kg in 0.05 mL), and the behavioral response was observed to verify catheter patency. Loss of muscle tone and righting reflexes served as behavioral indicators of patency.
Self-administration and cue-induced reinstatement
Self-administration procedures were conducted as previously described (11, 21, 22). Following the 7 d recovery from jugular catheter surgery, rats were assigned to self-administer either saline or 1.0 mg/kg/inf cocaine. Rats were subjected to 10 test sessions (2 h per session), during which responses to the active alternative snout-poke hole resulted in i.v. injections of cocaine or saline according to a fixed-ratio 1 (FR1) schedule of reinforcement followed by a 30-s time-out period. Infusions were accompanied by a 5 s illumination of the stimulus light above the active snout-poke hole and the house light was extinguished for the duration of the time-out period. Responses to the inactive hole resulted in no programmed consequences. The criterion for acquisition of cocaine self-administration was an average of 10 cocaine infusions per day during the 10-session test phase.
Extinction tests were initiated 24 h after the last self-administration session. Rats were exposed to multiple within-session extinction sessions, during which the chambers were dark and responses were recorded but resulted in no programmed consequences. Extinction sessions were 1 h in duration, separated by 5 min, and were continued until responding levels fell to fewer than 20 responses per session (8–10 sessions) (23, 24). Animals were then allowed an additional 6 d of abstinence, followed by 1 h test of cue-induced reinstatement, during which active responses produced cues previously paired with drug delivery.
Tissue collection
Following a 7-d withdrawal (or after cue-induced reinstatement for animals receiving PFI3 microinfusions), animals were euthanized by rapid decapitation, brains were removed and sliced into 1-mm-thick sections using a brain matrix (Braintree Scientific, Inc., Braintree, MA), and 2-mm-diameter tissue punches from the NAc and dorsal striatum/caudoputamen (CPU) were collected and rapidly frozen on dry ice.
Western blotting and immunoprecipitation
NAc or CPU tissue punches from each rat that self-administered cocaine or saline (n = 6/group) were homogenized in 25 mM Tris (pH 8.0) and 0.25 M sucrose buffer. Total protein was extracted, and 30 μg samples were loaded onto 10% Tris-SDS polyacrylamide gels for electrophoresis separation, then transferred to nitrocellulose membranes and blocked with 5% nonfat milk in PBS. Membranes were incubated overnight at 4°C with primary antibodies diluted in blocking buffer (Rockland Immunochemicals, Inc., Limerick, PA), including: rabbit anti-BRG1 (1:2,000; Abcam, Cambridge, MA), rabbit anti-phospho(p)-SMAD3 (1:500; Calbiochem of Millipore Corp., Billerica, MA), and mouse anti-β-actin (1:10,000; Cell Signaling Technologies, Inc., Danvers, MA). Membranes were incubated with IRDye secondary antibodies (1:5,000; LI-COR, Inc., Lincoln, NE) for 1 h at room temperature. The blots were imaged using the Odyssey Infrared Imaging system (LI-COR, Inc.) and quantified by densitometry using Image J. β-actin was used as a loading control.
For immunoprecipitation, NAc or CPU tissue punches from rats (n = 8 for the saline group, n = 7 for the cocaine group) were homogenized in 500 μL of homogenization buffer containing 50 mM beta-glycerol phosphate, 1 mM DTT, 2 mM EGTA, 10 mM NaF, 1 mM sodium orthovanadate, 20 mM HEPES (pH 7.4), 1% Triton X-100, 10% glycerol, 0.5% SDS, phosphatase inhibitor cocktails 2 and 3 (Sigma-Aldrich, St. Louis, MO), and protease inhibitors (Roche, Basel, Switzerland). A total of 600 μg of protein was used for immunoprecipitation with protein G beads (GE Healthcare, Little Chalfont, UK) as previously described with some modification (25). Briefly, samples were brought to a final volume of 500 μL with lysis buffer, and 5 μL of anti-BRG1 antibody (Abcam) was added followed by gentle tumbling overnight at 4°C. Next, 50 μL of protein G agarose slurry was added to each sample, followed by gentle tumbling at 4°C overnight. Beads were pelleted by centrifugation and washed three times with 1 mL of wash buffer. After the final wash, reducing sample buffer containing SDS and DTT was added to the pelleted beads and samples were heated to 95°C for 5 min and subjected to SDS-PAGE. Proteins were transferred to nitrocellulose and blotted for mouse anti-SMAD3 (1:1,000; Abcam) and anti-BRG1 (for normalization).
Quantitative (q)PCR and Chromatin immunoprecipitation (ChIP)-qPCR
Bilateral NAc punches were obtained after 7 d of withdrawal from cocaine self-administration. ChIP was performed for BRG1 as described previously (11, 26), with minor modifications. Briefly, four punches each from two rats were pooled for each sample (n = 7 samples from 14 rats for the saline group, and n = 6 from 12 rats for the cocaine group) and fixed for 12 min in 1% formaldehyde, then quenched with 2 M glycine for 5 min. The chromatin was solubilized and extracted by cell and nuclear lysis. The chromatin was sheared using a Bioruptor 300 (Diagenode Diagnostics, Seraing, Belgium) at 4°C at high sonication intensity for 30 s on/30 s off for 10 min, followed by a 10 min rest, which was repeated three times. Fragment sizes of 250–1000 bp were verified by agarose gel electrophoresis. Magnetic sheep anti-rabbit beads (Invitrogen of Thermo Fisher Scientific, Waltham, MA) were incubated with anti-BRG1 antibody at 4°C overnight on a rotator. Following the prescribed wash steps, 70 μL of the magnetic bead/antibody complex slurry was incubated with the sheared chromatin sample for 16 h at 4°C. Five percent of sheared chromatin from each sample was used as an input control. Samples were washed with LiCl and Tris-EDTA buffers. Reverse cross-linking was performed at 65°C overnight, and proteins and RNA were removed using proteinase K (Invitrogen) and RNase (Roche), respectively. DNA was purified using a DNA purification kit (Qiagen, Hilden, Germany). Additionally, an IgG control was used to test for nonspecific binding. qPCR was performed (iQ5 system; Bio-Rad Laboratories, Inc, Hercules, CA) to identify binding of BRG1 to proximal promoter regions of target genes that contain a SMAD3 binding site (11) (primers listed in Table S1). Amplification reactions were run in triplicate with iQ SYBR Green (Bio-Rad Laboratories, Inc.), and each sample was normalized to the IgG control. Fold changes were calculated as cocaine relative to saline control.
For qPCR following PFI3 microinfusions, NAc tissue punches were collected after cue-induced reinstatement, and total RNA was extracted using Trizol (Invitrogen) and the RNeasy Micro Kit (Qiagen), as previously described (11). Real-time PCR for all genes was conducted as previously reported (11) (primers used for housekeeping [GAPDH] and all other genes are listed in Table S2).
Pharmacological inhibition of BRG1
Rats received jugular catheters and implantation of bilateral guide cannulae (C235G-2.4; Plastics One, Inc., Roanoke, VA) that were aimed at the NAc [coordinates from bregma according to Paxinos & Watson (27): AP: +1.7, ML: +1.2, DV: −6.5]. Animals were assigned to receive microinjections (1 μL/hemisphere) of PFI3 (n = 7) or vehicle (n = 8, counterbalanced based on their performance during cocaine self-administration and extinction) infused at a rate of 0.5 μL/min; injectors were left in place for an additional 3 min to allow for diffusion. Rats received microinjections once per day for 4 d during the withdrawal period, beginning on the fourth day after the last cocaine exposure. Thirty minutes after the last microinjection, animals were tested for cue-induced reinstatement for 1 h, and then immediately sacrificed for tissue collection.
Food reinforcement
Commercially available two-lever operant chambers located within sound-attenuating, ventilated enclosures (Coulbourn Instruments, LLC, Whitehall, PA) were used for all food reinforcement experiments. Rats were trained to lever press for a food reward (45 mg pellet; Bio-Serv, Flemington, NJ). During the daily 1 h training sessions, rats could press either lever (both active) under an FR1 schedule and earn up to 50 food pellets. The response requirement was gradually increased to FR10 over a period of 10 d. Following food training, rats were implanted with bilateral guide cannulae aimed at the NAc for PFI3 or vehicle (n = 7/group) infusions as described above. Rats were tested for the response rate 30 min after the last microinjection.
Locomotor activity
Locomotor activity was recorded by an infrared motion-sensor system (AccuScan Instruments, Inc., Columbus, OH) fitted outside transparent plastic cages (40 × 40 × 30 cm). The Versa Max animal activity software monitors the distance the animal travelled in 1 h. Locomotor testing was performed 30 min after the final microinfusion for animals receiving PFI3 or vehicle (n = 8/group), or following complete expression of virus after viral microinjection (n = 8/group).
Viral overexpression of BRG1 and dnSmad3 in the NAc
The generation of the dnSmad3 construct has been described in detail previously (11). Briefly, serines in the SSXS motif at the C-terminus of SMAD3 (which confer activation when phosphorylated and allow for translocation) were mutated to alanines (SAXA) (28, 29). Wild-type Brg1 (Addgene, Cambridge, MA) (12) and dnSmad3 were cloned into a p1005 herpes simplex virus (HSV) transcription cassette. Such HSV vectors exhibit maximal expression 3–5 d post-infection (30). Viruses were validated both in vitro and in vivo prior to use in behavioral experiments.
Intra-accumbal viral injections were performed after the 7-d withdrawal period following within-session extinction from self-administration. Rats were counterbalanced according to self-administration and extinction performances and assigned to receive bilateral injections of HSV-dnSmad3 (n = 9), HSV-Brg1 (n = 8), or HSV-GFP (as a control: for HSV-dnSmad3 control, n = 8; for HSV-Brg1 control, n = 10) aimed at the NAc; injectors were set at a 10° angle; Paxinos & Watson (27) coordinates: AP: +1.7, ML: +2.45, DV: −6.7 (31). Viruses (1 μL/hemisphere) were infused at a rate of 0.2 μL/min, and injectors were left in place for an additional 10 min to allow for diffusion. Three days later, when the transgene overexpression is maximal, rats were tested for cue-induced reinstatement for 1 h.
Statistical analyses
Statistical analyses were conducted using Prism software (GraphPad Software, Inc., La Jolla, CA). Performance during self-administration and extinction was analyzed using repeated measures two-way within-subject analyses of variance (ANOVAs) followed by Bonferroni’s post-hoc tests. Student’s t tests were conducted for analysis of cue-induced reinstatement, food reinforcement, locomotor activity, and immunoblotting. One-way ANOVAs were conducted on ChIP data followed by Fisher’s LSD post-hoc tests. Significance was set at P < 0.05, and data are presented as the mean ± standard error of the mean.
Results
Upregulation of p-SMAD3 and BRG1 following abstinence from cocaine self-administration
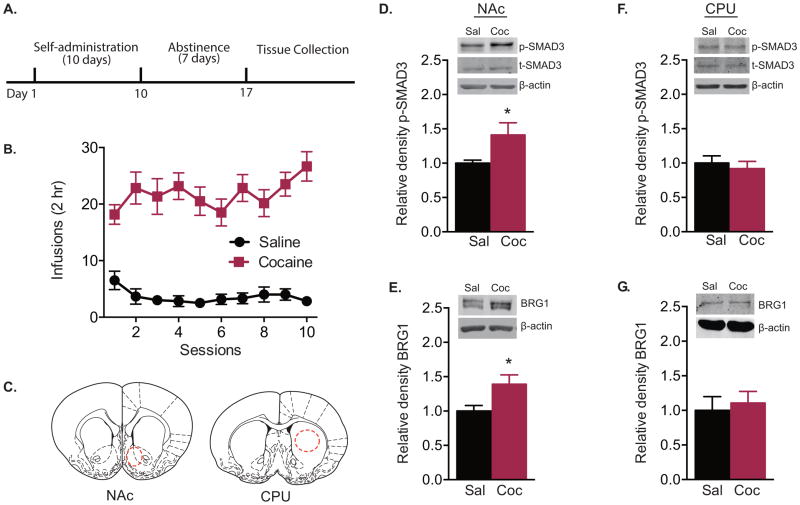
Rats self-administered significantly more infusions of cocaine than saline during the 10-session training paradigm (Fig. 1A, B; two-way repeated-measures ANOVA, interaction effect F(9,90) = 2.559, P = 0.011; drug effect F(1,10) = 95.21, P < 0.001; session effect F(9,90) = 1.237, P = 0.282). Tissue punches from NAc and CPU (Fig. 1C) were taken 7 d after the last cocaine exposure. Following this withdrawal period, levels of p-SMAD3 (t test, t(10) = 2.241, P = 0.049) and BRG1 (t test, t(10) = 2.470, P = 0.033) were significantly increased in the NAc (Fig. 1D, E), while there was no change in total SMAD3 protein (saline: 1.000 ± 0.040; cocaine: 0.949 ± 0.082; t test, t(10) = 0.552, P = 0.592). In contrast, there were no significant changes in the CPU (Fig. 1F, G).
Figure 1. Withdrawal from cocaine self-administration increases p-SMAD3 and BRG1 protein levels and interaction.
(A) Timeline of behavioral testing and tissue collection. (B) Mean number of saline and cocaine (1 mg/kg/inf) infusions per session during self-administration training (n = 6/group). (C) Representative anatomical location of tissue punches taken from rat nucleus accumbens (NAc) and caudoputamen (CPU). (D–G) Relative p-SMAD3 and BRG1 expression in the NAc (D, E; n = 6/group) and CPU (F, G; n = 5–6/group). Data are expressed as mean ± SEM; *P < 0.05 vs. saline. Sal = saline; Coc = cocaine.
Increased BRG1-SMAD3 interaction following abstinence from cocaine self-administration
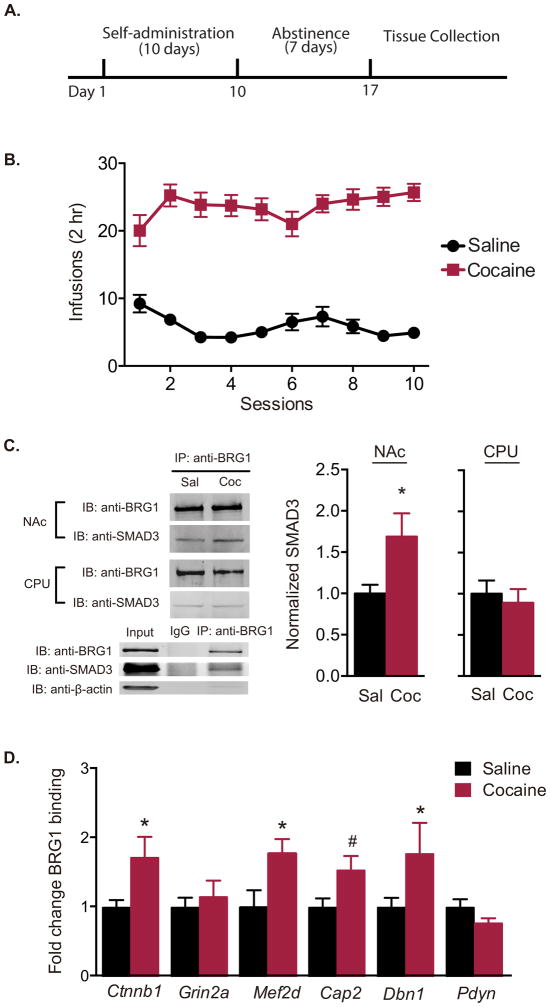
To evaluate protein interactions, another cohort of rats was trained to self-administer cocaine (Fig. 2A, B; two-way repeated-measures ANOVA, interaction effect F(9,351) = 3.389, P < 0.001; drug effect F(1,39) = 168.1, P < 0.001; session effect F(9,351) = 1.340, P = 0.214). A significantly greater amount of SMAD3 co-immunoprecipitated with BRG1 in the NAc (t test, t(13) = 2.399, P = 0.032) but not the CPU (t test, t(8) = 0.4731, P = 0.649) after 7 d of withdrawal (Fig. 2C). Using ChIP assay, there was increased BRG1 at the promoter regions of several SMAD3-dependent target genes that have previously been implicated in actin dynamics and cocaine-induced synaptic plasticity (11), including Ctnnb1 (P = 0.026), Mef2d (P = 0.016), and Dbn1 (P = 0.012), with a small increase in binding to Cap2 (P = 0.080), but not Grin2a (P = 0.610) or Pdyn (P = 0.477) (Fig. 2D; one-way ANOVA, F(11,62) = 2.573, P = 0.009).
Figure 2. Withdrawal from cocaine self-administration increases functional BRG1—SMAD3 interaction.
(A) Timeline of behavioral testing and tissue collection. (B) Mean number of saline and cocaine (1 mg/kg/inf) infusions per session during self-administration training (n = 19–22/group). (C) Protein lysates from rat nucleus accumbens (NAc) and caudoputamen (CPU) collected following withdrawal from cocaine or saline self-administration were immunoprecipitated with anti-BRG1 antibody and probed for SMAD3 binding (n = 5–8/group). (D) Occupancy of BRG1 on the promoter regions of β-catenin (Ctnnb1), NMDA receptor 2A (Grin2a), myocyte enhancer factor 2d (Mef2d), adenylyl cyclase-associated protein 2 (Cap2), drebrin (Dbn1), and prodynorphin (Pdyn) in the NAc as measured by quantitative chromatin immunoprecipitation following 7-d withdrawal from saline or cocaine self-administration (n = 5–7 samples/group (two animals count as one sample)). Data are expressed as mean ± SEM; *P < 0.05, #P < 0.10 vs. saline. Sal = saline; Coc = cocaine.
BRG1-SMAD3 interaction regulates cue-induced reinstatement of cocaine seeking
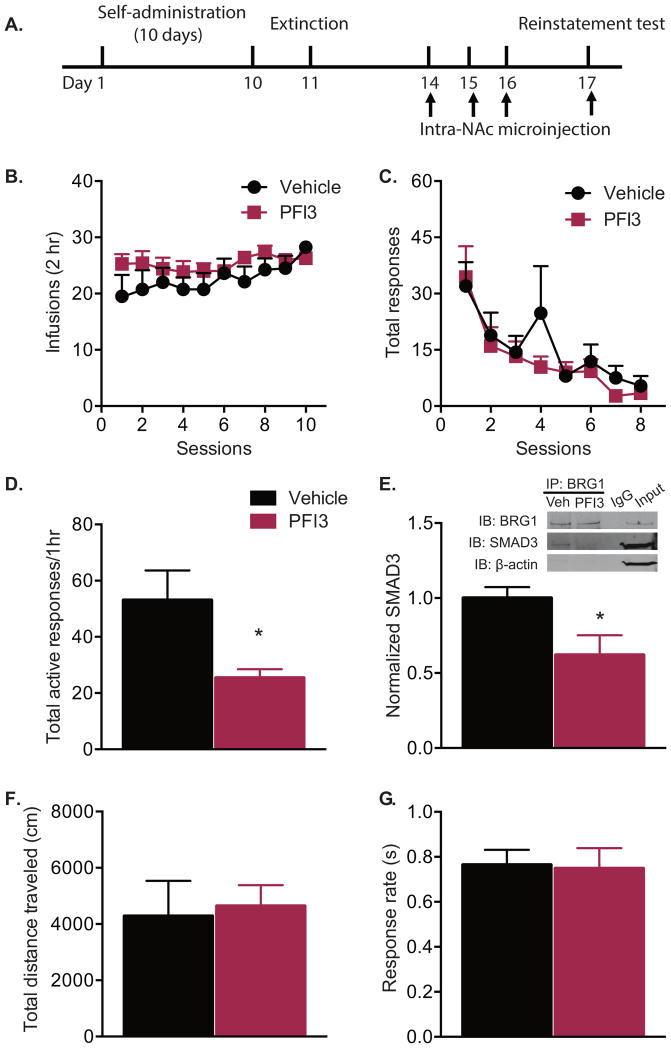
To test whether the BRG1 activity is required for cocaine-seeking behavior, rats received intra-accumbal microinfusions of PFI3 or vehicle during the withdrawal period following self-administration (Fig. 3A). Rats were assigned to vehicle or PFI3 groups such that there was no difference in cocaine self-administration training (Fig. 3B, two-way repeated-measures ANOVA, treatment effect F(1,13) = 0.388, P > 0.05) or extinction (Fig. 3C, two-way repeated-measures ANOVA, treatment effect F(1,13) = 0.342, P > 0.05) prior to BRG1 inhibition. Rats receiving microinfusions of PFI3 had significantly fewer total active responses during cue-induced reinstatement (Fig. 3D, t test, t(13) = 2.374, P = 0.033). Tissue samples from the NAc were collected. Co-immunoprecipitation experiments revealed reduced binding of SMAD3 to BRG1 following PFI3 infusion (Fig. 3E, t test, t(10) = 2.519, P = 0.030). Repeated intra-accumbal infusions resulted in decreased expression of several BRG1—SMAD3 target genes, including Mef2d, Cap2, and Dbn1 (Figure S1). The decreased responses during cue-induced reinstatement was not due to reduced overall activity, as there was no difference between groups in total distance traveled during a locomotor test (Fig. 3F, t test, t(14) = 0.082, P = 0.935). Importantly, PFI3 treatment did not alter the response rate for a food reinforcer (Fig. 3G, t test, t(12) = 0.139, P = 0.891), demonstrating that the effect of PFI3 was specific to cue-induced reinstatement.
Figure 3. Pharmacological inhibition of BRG1 in the nucleus accumbens decreases cue-induced reinstatement of cocaine seeking.
(A) Timeline of behavioral testing and intra-accumbal microinjections. (B) Mean number of infusions per session during cocaine self-administration (1 mg/kg/inf) and (C) total responses during extinction did not differ between groups assigned to receive BRG1 inhibitor PFI3 or vehicle. (D) Number of active responses during cue-induced reinstatement following microinjection (1 μL/hemisphere) of vehicle or PFI3 (30 mM) (n = 7–8/group). (E) SMAD3 and BRG1 co-immunoprecipitation following microinjection of vehicle or PFI3 (n = 6/group). (F) Total distance traveled in a locomotor test and (G) responding for a food reward did not differ between groups (n = 7–8/group). Data are expressed as mean ± SEM; *P < 0.05 vs. vehicle.
To confirm the importance of the SMAD3 transcriptional complex in cue-mediated cocaine-seeking behaviors, HSV constructs overexpressing dnSmad3 or a GFP control were microinjected into the NAc. Consistent with our previous work (11), blockade of SMAD3 via viral overexpression of dnSmad3 significantly reduced cue-induced reinstatement as measured by the attenuation of the total active responses (HSV-GFP group: 47.50 ± 7.693; HSV-dnSmad3 group: 28.56 ± 4.197; t test, t(15) = 2.231, P = 0.041). Taken together, these data demonstrate that SMAD3 and BRG1 activity are essential for cocaine seeking after a period of withdrawal, as inhibition of either suppresses reinstatement behaviors.
Overexpression of BRG1 potentiates cue-induced reinstatement of cocaine seeking
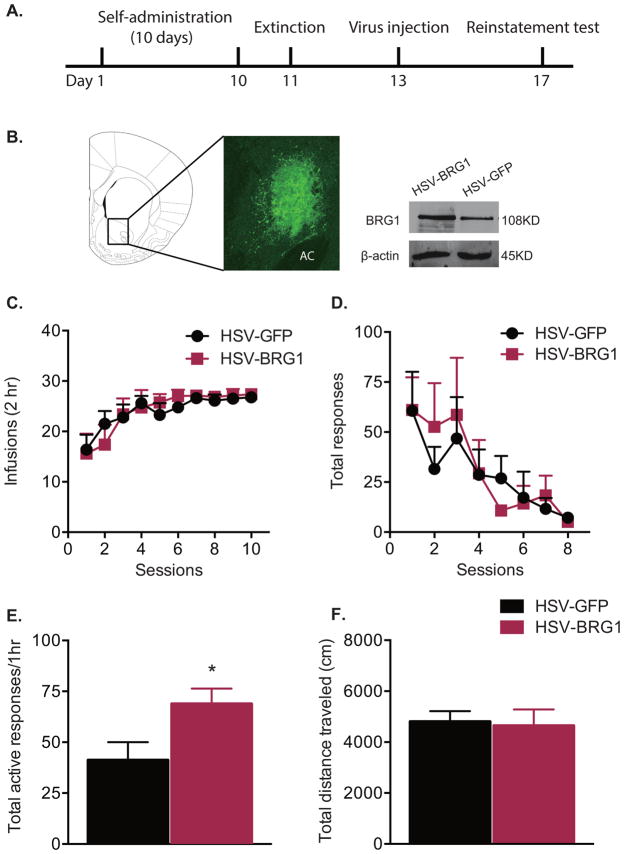
To determine if increased expression of BRG1 could further enhance cocaine-seeking behavior, rats received intra-accumbal injections of HSV-Brg1 during the period of withdrawal from cocaine self-administration (Fig. 4A, B). As in the above experiments with PFI3 infusions, groups receiving either HSV-Brg1 or HSV-GFP did not differ with respect to the amount of cocaine taken during self-administration (Fig 4C; two-way repeated-measures ANOVA, treatment effect F(1, 16) = 0.013, P > 0.05) or extinction responses (Fig. 4 D; two-way repeated-measures ANOVA, treatment effect F(1, 16) = 0.024, P > 0.05) prior to viral injection. Viral-mediated overexpression of BRG1 in the NAc significantly increased the number of active responses in the cue-induced reinstatement test (Fig. 4E, t test, t(16) = 2.312, P = 0.034) compared to HSV-GFP controls. This increase in responding was not due to differences in general locomotor activity, as overexpression of BRG1 in the NAc had no effect on total distance traveled during a locomotor test (Fig. 4F, t test, t(14) = 0.279, P = 0.783).
Figure 4. Overexpression of Brg1 in the nucleus accumbens enhances cue-induced reinstatement of cocaine seeking.
(A) Timeline of behavioral testing and intra-accumbal virus injection. (B) Representative picture of a coronal section of the rat brain (1.7 mm from bregma), depicting GFP-infected cells in the nucleus accumbens (AC: anterior commissure). Representative Western blots showing increased BRG1 protein levels in the nucleus accumbens following HSV-Brg1 overexpression. (C) Mean number of infusions per session during cocaine self-administration (1 mg/kg/inf) and (D) total responses during extinction procedure did not differ between groups assigned to receive HSV-Brg1 or HSV-GFP. (E) Number of active responses during cue-induced reinstatement following overexpression of HSV-GFP or HSV-Brg1 (n = 8–10/group). (F) Total distance traveled in a locomotor test (n = 8/group). Data are expressed as mean ± SEM, *P < 0.05 vs. HSV-GFP.
Discussion
Accumulating evidence suggests that alterations in gene regulation contribute substantially to the long-term changes in brain structure and function after drug exposure (reviewed in 7). Transcription factors shown to be involved in cocaine-induced behavioral and synaptic plasticity include ΔFosB (32, 33), CREB (33, 34), and EGR3 (35). In addition, our previous work identified SMAD3 as another important regulator in the expression of genes that are heavily implicated in neuronal plasticity (11). The results presented here confirm the importance of SMAD3, and demonstrate an important and novel role for BRG1 and its interaction with SMAD3 in cocaine-mediated behavioral adaptations. Following a seven-day withdrawal from cocaine self-administration, the interaction between SMAD3 and BRG1 in the NAc was significantly increased, and was associated with increased binding of BRG1 to the promoter regions of SMAD3-regulated genes. Moreover, pharmacological or viral-mediated disruption of this interaction bidirectionally altered cue-induced reinstatement in a model of cocaine seeking.
It is well accepted that transcription factors control gene expression by interacting with other chromatin-remodeling factors (36). Abnormalities in components of chromatin remodeling complexes have been implicated in many psychiatric diseases. For example, mutations of SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin proteins are involved in psychiatric disorders such as schizophrenia (37) and autism (38, 39). In addition, the ATP-utilizing chromatin assembly and remodeling factor is a critical component of a chromatin-remodeling complex involved in the development of susceptibility to depression and in regulating stress-related behaviors (40). Recent data also suggest an underlying role of chromatin remodeling factors in the neurobiology of drug abuse. For example, chronic cocaine exposure results in increased expression of the bromodomain and extraterminal protein BRD4, an epigenetic reader protein (41), and PSMC5, an ATPase-containing subunit of the 19s proteasomal complex (42). We recently demonstrated that an accessory ATPase subunit of the ISWI family of chromatin remodeling complexes, bromodomain adjacent to zinc finger domain 1B, is also upregulated in the NAc (22).
In line with the emerging evidence, our data implicate BRG1, a key component of SWI/SNF chromatin remodeling complex, in addiction and drug abuse. In addition to its role in regulating differentiation and development in the central nervous system (43–46), the data implicate BRG1 in long-term neuroplasticity. BRG1 was found to interact with SMAD3 in vivo and modulate behaviors in response to withdrawal from cocaine treatment. Importantly, inhibition of BRG1 decreased, whereas overexpression increased, behavioral responses in a cocaine-seeking test. We propose that the inhibitor PFI3 disrupts the BRG1—SMAD3 interaction, as the same effect was observed by interfering with SMAD3 activity via dnSmad3 overexpression. Thus, the expression levels of SMAD3 and BRG1, as well as the integrity of the BRG1—SMAD3 transcriptional complex, play important roles in regulating cocaine-seeking behavior.
Withdrawal from cocaine exposure increased the binding of BRG1 to DNA targets, which is consistent with previous in vivo work demonstrating that BRG1 predominantly interacts with SMAD3 on target promoters to facilitate transforming growth factor-β-mediated gene transcription (12). BRG1 has also been shown to interact with other transcription factors, such as ΔFosB and CREB-binding protein, in response to cocaine exposure (42, 47). These data suggest that BRG1 may be an essential component of multiple transcriptional complexes mediating gene expression following cocaine exposure. In vitro studies demonstrate that BRG1 is involved in the organization of actin filaments (48–50). Similarly, BRG1 binding was increased on genes involved in synaptic and morphological adaptation, including drebrin (51, 52) and adenylyl cyclase-associated protein 2 (53, 54). The binding of BRG1 to the promoter regions of these genes may in turn alter the synaptic plasticity in NAc medium spiny neurons, and thus regulate the behavioral response to cocaine (8, 55).
Identifying effective treatments for addiction has been a subject of intensive investigation. Our data establish that the SWI/SNF chromatin-remodeling component BRG1 in the NAc is involved in SMAD3-dependent gene regulation in withdrawal after cocaine self-administration. Furthermore, pharmacological disruption of the BRG1—SMAD3 interaction by the BRG1 inhibitor PFI3 in the NAc was sufficient to decrease cocaine-seeking behavior. Here we demonstrate the essential role of BRG1 and SMAD3 interactions in the NAc following cocaine self-administration. The ability of the BRG1 inhibitor to alter drug-seeking behavior offers potential therapeutic value, however, future studies will be necessary to determine the ability of PFI3 to readily cross the blood brain barrier in addition to fully elucidating BRG1-SMAD3 regulation in other mesolimbic dopamine brain regions. Together, these findings provide new insight into epigenetic mechanisms underlying cocaine addiction, and provide a new direction for the design of improved treatments for drug addiction.
Supplementary Material
Acknowledgments
This work was supported by R01DA037257 (D.M.D).
Footnotes
Financial Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O’Brien CP, Childress AR, McLellan AT, Ehrman R. Classical conditioning in drug-dependent humans. Ann N Y Acad Sci. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- 2.Renthal W, Nestler EJ. Epigenetic mechanisms in drug addiction. Trends Mol Med. 2008;14:341–350. doi: 10.1016/j.molmed.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meil WM, See RE. Conditioned cued recovery of responding following prolonged withdrawal from self-administered cocaine in rats: an animal model of relapse. Behav Pharmacol. 1996;7:754–763. [PubMed] [Google Scholar]
- 4.Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 5.Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology (Berl) 2013;229:453–476. doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nestler EJ. Epigenetic mechanisms of drug addiction. Neuropharmacology. 2014;76(Pt B):259–268. doi: 10.1016/j.neuropharm.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. 2011;12:623–637. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010;33:267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maze I, Russo SJ. Transcriptional mechanisms: underlying addiction-related structural plasticity. Mol Interv. 2010;10:219–230. doi: 10.1124/mi.10.4.5. [DOI] [PubMed] [Google Scholar]
- 10.Lull ME, Freeman WM, Vrana KE, Mash DC. Correlating human and animal studies of cocaine abuse and gene expression. Ann N Y Acad Sci. 2008;1141:58–75. doi: 10.1196/annals.1441.013. [DOI] [PubMed] [Google Scholar]
- 11.Gancarz AM, Wang ZJ, Schroeder GL, Damez-Werno D, Braunscheidel KM, Mueller LE, et al. Activin receptor signaling regulates cocaine-primed behavioral and morphological plasticity. Nat Neurosci. 2015;18:959–961. doi: 10.1038/nn.4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xi Q, He W, Zhang XH, Le HV, Massague J. Genome-wide impact of the BRG1 SWI/SNF chromatin remodeler on the transforming growth factor beta transcriptional program. J Biol Chem. 2008;283:1146–1155. doi: 10.1074/jbc.M707479200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Massague J. TGFbeta signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ross S, Cheung E, Petrakis TG, Howell M, Kraus WL, Hill CS. Smads orchestrate specific histone modifications and chromatin remodeling to activate transcription. EMBO J. 2006;25:4490–4502. doi: 10.1038/sj.emboj.7601332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen MM. Nodal signaling: developmental roles and regulation. Development. 2007;134:1023–1034. doi: 10.1242/dev.000166. [DOI] [PubMed] [Google Scholar]
- 16.Vogel-Ciernia A, Matheos DP, Barrett RM, Kramar EA, Azzawi S, Chen Y, et al. The neuron-specific chromatin regulatory subunit BAF53b is necessary for synaptic plasticity and memory. Nat Neurosci. 2013;16:552–561. doi: 10.1038/nn.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu JF, Thorn DA, Zhang Y, Li JX. Effects of Trace Amine-associated Receptor 1 Agonists on the Expression, Reconsolidation, and Extinction of Cocaine Reward Memory. Int J Neuropsychopharmacol. 2016 doi: 10.1093/ijnp/pyw009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vangamudi B, Paul TA, Shah PK, Kost-Alimova M, Nottebaum L, Shi X, et al. The SMARCA2/4 ATPase Domain Surpasses the Bromodomain as a Drug Target in SWI/SNF-Mutant Cancers: Insights from cDNA Rescue and PFI-3 Inhibitor Studies. Cancer Res. 2015;75:3865–3878. doi: 10.1158/0008-5472.CAN-14-3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandra R, Lenz JD, Gancarz AM, Chaudhury D, Schroeder GL, Han MH, et al. Optogenetic inhibition of D1R containing nucleus accumbens neurons alters cocaine-mediated regulation of Tiam1. Front Mol Neurosci. 2013;6:13. doi: 10.3389/fnmol.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gancarz AM, Kausch MA, Lloyd DR, Richards JB. Between-session progressive ratio performance in rats responding for cocaine and water reinforcers. Psychopharmacology (Berl) 2012;222:215–223. doi: 10.1007/s00213-012-2637-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gancarz-Kausch AM, Schroeder GL, Panganiban C, Adank D, Humby MS, Kausch MA, et al. Transforming growth factor beta receptor 1 is increased following abstinence from cocaine self-administration, but not cocaine sensitization. PLoS One. 2013;8:e83834. doi: 10.1371/journal.pone.0083834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun H, Martin JA, Werner CT, Wang ZJ, Damez-Werno DM, Scobie KN, et al. BAZ1B in Nucleus Accumbens Regulates Reward-Related Behaviors in Response to Distinct Emotional Stimuli. J Neurosci. 2016;36:3954–3961. doi: 10.1523/JNEUROSCI.3254-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gancarz-Kausch AM, Adank DN, Dietz DM. Prolonged withdrawal following cocaine self-administration increases resistance to punishment in a cocaine binge. Sci Rep. 2014;4:6876. doi: 10.1038/srep06876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cahill ME, Bagot RC, Gancarz AM, Walker DM, Sun H, Wang ZJ, et al. Bidirectional Synaptic Structural Plasticity after Chronic Cocaine Administration Occurs through Rap1 Small GTPase Signaling. Neuron. 2016;89:566–582. doi: 10.1016/j.neuron.2016.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferguson D, Koo JW, Feng J, Heller E, Rabkin J, Heshmati M, et al. Essential role of SIRT1 signaling in the nucleus accumbens in cocaine and morphine action. J Neurosci. 2013;33:16088–16098. doi: 10.1523/JNEUROSCI.1284-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paxinos G, Watson C. The Rat Brain In Stereotaxic Coordinates. Elselvier; Burlington, MA: 2009. [Google Scholar]
- 28.Chipuk JE, Cornelius SC, Pultz NJ, Jorgensen JS, Bonham MJ, Kim SJ, et al. The androgen receptor represses transforming growth factor-beta signaling through interaction with Smad3. J Biol Chem. 2002;277:1240–1248. doi: 10.1074/jbc.M108855200. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Song K, Sponseller TL, Danielpour D. Novel function of androgen receptor-associated protein 55/Hic-5 as a negative regulator of Smad3 signaling. J Biol Chem. 2005;280:5154–5162. doi: 10.1074/jbc.M411575200. [DOI] [PubMed] [Google Scholar]
- 30.Neve RL, Neve KA, Nestler EJ, Carlezon WA., Jr Use of herpes virus amplicon vectors to study brain disorders. Biotechniques. 2005;39:381–391. doi: 10.2144/05393PS01. [DOI] [PubMed] [Google Scholar]
- 31.Robison AJ, Vialou V, Mazei-Robison M, Feng J, Kourrich S, Collins M, et al. Behavioral and structural responses to chronic cocaine require a feedforward loop involving DeltaFosB and calcium/calmodulin-dependent protein kinase II in the nucleus accumbens shell. J Neurosci. 2013;33:4295–4307. doi: 10.1523/JNEUROSCI.5192-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nestler EJ. Review. Transcriptional mechanisms of addiction: role of DeltaFosB. Philos Trans R Soc Lond B Biol Sci. 2008;363:3245–3255. doi: 10.1098/rstb.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McClung CA, Nestler EJ. Regulation of gene expression and cocaine reward by CREB and DeltaFosB. Nat Neurosci. 2003;6:1208–1215. doi: 10.1038/nn1143. [DOI] [PubMed] [Google Scholar]
- 34.Carlezon WA, Jr, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, et al. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- 35.Chandra R, Francis TC, Konkalmatt P, Amgalan A, Gancarz AM, Dietz DM, et al. Opposing role for Egr3 in nucleus accumbens cell subtypes in cocaine action. J Neurosci. 2015;35:7927–7937. doi: 10.1523/JNEUROSCI.0548-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narlikar GJ, Fan HY, Kingston RE. Cooperation between complexes that regulate chromatin structure and transcription. Cell. 2002;108:475–487. doi: 10.1016/s0092-8674(02)00654-2. [DOI] [PubMed] [Google Scholar]
- 37.Loe-Mie Y, Lepagnol-Bestel AM, Maussion G, Doron-Faigenboim A, Imbeaud S, Delacroix H, et al. SMARCA2 and other genome-wide supported schizophrenia-associated genes: regulation by REST/NRSF, network organization and primate-specific evolution. Hum Mol Genet. 2010;19:2841–2857. doi: 10.1093/hmg/ddq184. [DOI] [PubMed] [Google Scholar]
- 38.Neale BM, Kou Y, Liu L, Ma’ayan A, Samocha KE, Sabo A, et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature. 2012;485:242–245. doi: 10.1038/nature11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP, et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012;485:246–250. doi: 10.1038/nature10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun H, Damez-Werno DM, Scobie KN, Shao NY, Dias C, Rabkin J, et al. ACF chromatin-remodeling complex mediates stress-induced depressive-like behavior. Nat Med. 2015;21:1146–1153. doi: 10.1038/nm.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sartor GC, Powell SK, Brothers SP, Wahlestedt C. Epigenetic Readers of Lysine Acetylation Regulate Cocaine-Induced Plasticity. J Neurosci. 2015;35:15062–15072. doi: 10.1523/JNEUROSCI.0826-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohnishi YH, Ohnishi YN, Nakamura T, Ohno M, Kennedy PJ, Ohkawa Y, et al. PSMC5, a 19S Proteasomal ATPase, Regulates Cocaine Action in the Nucleus Accumbens. PLoS One. 2015;10:e0126710. doi: 10.1371/journal.pone.0126710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xia CY, Zhang S, Gao Y, Wang ZZ, Chen NH. Selective modulation of microglia polarization to M2 phenotype for stroke treatment. Int Immunopharmacol. 2015;25:377–382. doi: 10.1016/j.intimp.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 44.Yu Y, Chen Y, Kim B, Wang H, Zhao C, He X, et al. Olig2 targets chromatin remodelers to enhancers to initiate oligodendrocyte differentiation. Cell. 2013;152:248–261. doi: 10.1016/j.cell.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Narayanan R, Tuoc TC. Roles of chromatin remodeling BAF complex in neural differentiation and reprogramming. Cell Tissue Res. 2014;356:575–584. doi: 10.1007/s00441-013-1791-7. [DOI] [PubMed] [Google Scholar]
- 46.Bischof M, Weider M, Kuspert M, Nave KA, Wegner M. Brg1-dependent chromatin remodelling is not essentially required during oligodendroglial differentiation. J Neurosci. 2015;35:21–35. doi: 10.1523/JNEUROSCI.1468-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 48.Rando OJ, Zhao K, Janmey P, Crabtree GR. Phosphatidylinositol-dependent actin filament binding by the SWI/SNF-like BAF chromatin remodeling complex. Proc Natl Acad Sci U S A. 2002;99:2824–2829. doi: 10.1073/pnas.032662899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asp P, Wihlborg M, Karlen M, Farrants AK. Expression of BRG1, a human SWI/SNF component, affects the organisation of actin filaments through the RhoA signalling pathway. J Cell Sci. 2002;115:2735–2746. doi: 10.1242/jcs.115.13.2735. [DOI] [PubMed] [Google Scholar]
- 50.Nishimoto N, Watanabe M, Watanabe S, Sugimoto N, Yugawa T, Ikura T, et al. Heterocomplex formation by Arp4 and beta-actin is involved in the integrity of the Brg1 chromatin remodeling complex. J Cell Sci. 2012;125:3870–3882. doi: 10.1242/jcs.104349. [DOI] [PubMed] [Google Scholar]
- 51.Merriam EB, Millette M, Lumbard DC, Saengsawang W, Fothergill T, Hu X, et al. Synaptic regulation of microtubule dynamics in dendritic spines by calcium, F-actin, and drebrin. J Neurosci. 2013;33:16471–16482. doi: 10.1523/JNEUROSCI.0661-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reissner KJ, Uys JD, Schwacke JH, Comte-Walters S, Rutherford-Bethard JL, Dunn TE, et al. AKAP signaling in reinstated cocaine seeking revealed by iTRAQ proteomic analysis. J Neurosci. 2011;31:5648–5658. doi: 10.1523/JNEUROSCI.3452-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peche V, Shekar S, Leichter M, Korte H, Schroder R, Schleicher M, et al. CAP2, cyclase-associated protein 2, is a dual compartment protein. Cell Mol Life Sci. 2007;64:2702–2715. doi: 10.1007/s00018-007-7316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Freeman WM, Lull ME, Patel KM, Brucklacher RM, Morgan D, Roberts DC, et al. Gene expression changes in the medial prefrontal cortex and nucleus accumbens following abstinence from cocaine self-administration. BMC Neurosci. 2010;11:29. doi: 10.1186/1471-2202-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dietz DM, Sun H, Lobo MK, Cahill ME, Chadwick B, Gao V, et al. Rac1 is essential in cocaine-induced structural plasticity of nucleus accumbens neurons. Nat Neurosci. 2012;15:891–896. doi: 10.1038/nn.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.