Abstract
The inability to augment capillary blood volume (CBV) in response to insulin or glucose is thought to contribute to insulin resistance (IR) by limiting glucose uptake in key storage sites. Understanding the mechanisms that contribute to impaired CBV augmentation early in the onset of IR may lead to new future therapies. We hypothesized that inactivity alters the balance of vasoactive eicosanoids and contributes to microvascular IR. In ten activity-restricted (AR) and six normal-activity (NA) adult male rhesus macaques, contrast-enhanced ultrasound of skeletal muscle blood flow and CBV was performed at baseline and during intravenous glucose tolerance test (IVGTT). Plasma was analyzed for vasoconstrictor hydroxyeicosatetraenoic acids (HETEs) and the ratio of vasodilatory epoxyeicosatrienoic acids (EETs) to their less biologically active dihydroxyeicosatrienoic acids (DHETs) as an indirect measure of soluble epoxide-hydrolase (sEH) activity. AR primates were IR during IVGTT and had a 45% lower glucose-stimulated CBV response. Vasoconstrictor 18-HETE and 19-HETE and the DHET/EET ratio were markedly elevated in the AR group and correlated inversely with the CBV response. Additionally, levels of 18-HETE and 19-HETE correlated directly with microvascular IR. We conclude that a shift towards increased eicosanoid vasoconstrictor tone correlates with abnormal skeletal muscle vascular recruitment and may contribute to IR.
INTRODUCTION
In healthy subjects, euglycemic hyperinsulinemia and carbohydrate-rich meals increase skeletal muscle blood flow through insulin receptor signaling and activation of endothelial nitric-oxide synthase.1–6 This response leads to an increase in capillary blood volume (CBV) and a higher functional capillary surface area, which in-turn facilitates insulin-mediated glucose uptake.1, 6–8 Insulin resistance (IR) and obesity limit the insulin-mediated expansion of CBV, which is thought to contribute to reduced glucose uptake.8–10 Furthermore, we have demonstrated that inactivity, in the absence of obesity, reduces the CBV response to a glucose challenge and strongly correlates with the degree of IR.11
Vasoactive compounds other than nitric-oxide are thought to participate in the metabolic regulation of CBV. Metabolism of arachadonic acid through cytochrome P-450 enzymes produce epoxyeicosatrienoic acids (EETs), a family of endothelial-derived vasodilators that can influence skeletal muscle blood flow.11–15 These vasodilatory EETs are converted by soluble epoxide hydrolase (sEH) enzymes to less biologically active dihydroxyeicosatrienoic acids (DHETs).14 Inhibitors of sEH have been shown to increase skeletal muscle blood flow and CBV and to improve insulin sensitivity.12, 13, 15, 16 Alternative metabolism of arachadonic acid through separate cytochrome P-450 enzymes produce the vasoconstrictors 18, 19, and 20-hydroxyeicosatetraenoic acids (HETEs) that are associated with hypertension.17, 18 While we have previously shown that the concentration of 14,15-EET may be suppressed in inactive and IR primates,11 little is known about HETEs and measures of sEH activity in regulating skeletal muscle blood flow and their contribution to IR in a non-human primates. We hypothesized that an imbalance in vasoactive arachadonic acid metabolites toward a more vasoconstrictive pattern in activity-restricted IR primates would correlate with abnormal CBV response to glucose and measures of IR.
SUBJECTS AND METHODS
This study was approved by the Animal Care and Use Committee of the Oregon National Primate Research Center and conform to the Guidelines for the Care and Use of Laboratory Animals published by the National Institutes of Health. Sixteen adult male rhesus macaques (Macaca mulatta) 9–12 yrs of age were studied. Ten animals were activity-restricted for a mean of 6 years in single-cage housing and the remaining six animals that formed the normal-activity cohort were group-housed in 1-acre corrals. All animals were fed standard chow diet (Fiber-Balanced Monkey Diet) and underwent the following procedures as previously described: (1) intravenous glucose tolerance test (IVGTT) for calculation of insulin area-under-the-curve (AUC) concentration and the homeostatic-model of IR (HOMA-IR), (2) dual X-ray absorptiometry for assessment of lean muscle mass and truncal fat percentage, (3) flow-mediated vasodilation for nitric-oxide bioavailability, (4) contrast-enhanced ultrasound of the skeletal muscle for resting and glucose-stimulated peak skeletal muscle blood flow and CBV, and (5) calculation of the microvascular IR index as the change in insulin concentration divided by the peak CBV (Δ insulin/CBV) achieved during the IVGTT.11 Plasma samples were analyzed by liquid-chromatography/mass spectrometry for freely circulating EETs cis-regioisomers (14,15-EET, 11,12-EET, 8,9-EET), their corresponding sEH metabolites (14,15-DHET, 11,12-DHET, 8,9-DHET), and for HETEs (18-HETE, 19-HETE, and 20-HETE). See supplementary section for details. The ratio of vasodilatory EETs to their less biologically active DHETs (DHET/EET) was assessed as indirect measure of sEH activity.
Statistical Analysis
Data were analyzed by Prism (v5.0, GraphPad Software, Inc.) and are expressed as ± SE. Parametric data were analyzed with unpaired student’s t-test and Pearson’s product test for correlations. Non-parametric data were analyzed with Mann-Whitney-U test and Spearman’s Rho values for correlations. One-phase decay equations were used to fit curvilinear associations. Investigators were not blinded to the cohort allocation.
RESULTS
Compared to the normal-activity cohort, activity-restricted primates were similar in age, weight, lean muscle mass, and truncal fat % (Table 1). Although basal insulin and HOMA-IR were not significantly different between groups, the activity-restricted group was IR as indicated by elevated insulin AUC concentration (Table 1).
Table 1.
Metabolic Parameters, Skeletal Muscle Capillary Blood Volume and Microvascular Blood Flow, and Endothelial Derived Vasodilators and Vasoconstrictors in Normal Activity and Activity Restricted Primates
| Normal Activity (N = 6) |
Activity Restricted (N = 10) |
|
|---|---|---|
| Age, yr | 10.1 ± 0.5 | 9.9 ± 0.2 |
| Weight, Kg | 11.5 ± 0.9 | 11.1 ± 0.6 |
| Lean muscle mass, Kg | 3.4 ± 0.4 | 3.7 ± 0.6 |
| Truncal Fat, % | 23.4 ± 3.9 | 19.5 ± 2.9 |
| Basal Glucose, mg/dL | 71 ± 7 | 58 ± 2 * |
| Basal Insulin, μIU/mL | 11 ± 2 | 17 ± 3 |
| HOMA-IR | 1.9 ± 0.4 | 2.4 ± 0.5 |
| Insulin AUC, μIU/mL×min | 2225 ± 300 | 4271 ± 812 * |
| Resting Blood Flow, mL/min/g | 0.83 ± 0.14 | 0.62 ± 0.21 |
| Resting CBV, mL/g | 0.074 ± 0.014 | 0.045 ± 0.009 * |
| Peak Blood Flow, mL/min/g | 2.55 ± 0.7 | 1.22 ± 0.33 * |
| Peak CBV, mL/g | 0.122 ± 0.017 | 0.067 ± 0.012 * |
| Microvascular IR Index | 347 ± 15 | 1986 ± 561 * |
| 8, 9-EET, pg/mL | 63 ± 2 | 66 ± 8 |
| 11, 12-EET, pg/mL | 82 ± 6 | 79 ± 9 |
| 14, 15-EET, pg/mL | 199 ± 56 | 141 ±10 |
| Average EETs, pg/mL | 115 ± 23 | 95 ± 9 |
| 8, 9-DHET, pg/mL | 73 ± 6 | 119 ± 31 |
| 11, 12-DHET, pg/mL | 183 ± 19 | 285 ± 63 |
| 14, 15-DHET, pg/mL | 245 ± 21 | 336 ± 46 * |
| Average DHETs, pg/mL | 167 ± 19 | 247 ± 32* |
| 8, 9-DHET/ETT ratio | 1.2 ± 0.1 | 1.9 ± 0.4 |
| 11, 12-DHET/ETT ratio | 2.3 ± 0.4 | 4.1 ± 1.0 |
| 14, 15-DHET/ETT ratio | 1.6 ± 0.3 | 2.5 ± 0.3 |
| Average DHET/EET ratio | 1.7 ± 0.2 | 2.8 ± 0.4 * |
| Brachial Artery FMD, % | 9.8 ± 2.3 | 4.7 ± 1.3 * |
| 18-HETE, pg/mL | 518 ± 183 | 4673 ± 1138 * |
| 19-HETE, pg/mL | 269 ± 42 | 1041 ± 215 * |
| 20-HETE, pg/mL | 497 ± 116 | 559 ± 142 |
Abbreviations: IR, insulin resistance; AUC, area under the curve concentration; FMD, flow mediated vasodilation; EETs, DHETs, HETEs, see text.
p < 0.05.
On contrast-enhanced ultrasound imaging under basal/fasting conditions there was a trend for lower skeletal muscle microvascular blood flow and a significantly lower CBV in the activity-restricted versus normal-activity primates (Table 1). During the IVGTT, there was a reduction in the microvascular response to the glucose bolus in the activity-restricted group manifest by a lower peak microvascular blood flow that was attributable to a lower peak CBV (Table 1). The microvascular IR index was nearly 6-fold higher in activity-restricted primates.
The endothelial-derived eicosanoid vasodilators (8,9-EET, 11,12-EET, and 14, 15-EET) as well as the mean concentration of these vasodilators tended to be higher in the normal-activity primates but was not different between the two cohorts (Table 1). Conversely, each of the DHETs regio-isomers was elevated in the activity-restricted cohort and the combined average of the DHETs metabolites were significantly elevated compared to the normal-activity primates (Table 1). The overall production of vasodilatory eicosanoids, assessed as the sum of all EETs and DHETs regio-isomers, was not different between the normal-activity (845 ± 79 pg/mL) and the activity-restricted primates (1026 ± 141 pg/mL) p=0.25, suggesting that the increase in DHETs concentrations were not due to an increased production of vasodilatory eicosanoids. As such, the DHET/ETT ratio was assessed as an indirect measure of sEH activity. Each of the DHET/EET ratios was elevated in the activity-restricted cohort and the average DHET/EET ratio was significantly elevated (Table 1) suggesting an up-regulation of sEH activity. The eicosanoid vasoconstrictor concentrations of 18-HETE,19-HETE, and 20-HETE were elevated in the activity-restricted primates, though significant elevations were noted in 18 and 19-HETE (Table 1).
The eicosanoid vasoconstrictors 18, 19, and 20-HETE and the DHET/EET ratio all correlated inversely with the peak CBV during IVGTT (Figure 1). Conversely, both 18-HETE and 19-HETE correlated linearly with the microvascular IR index (Figure 1). Although brachial artery FMD was significantly reduced in the activity-restricted cohort (Table 1), suggesting reduced nitric-oxide bioavailability, no correlations were noted with microvascular blood flow or measures of IR.
Figure 1.
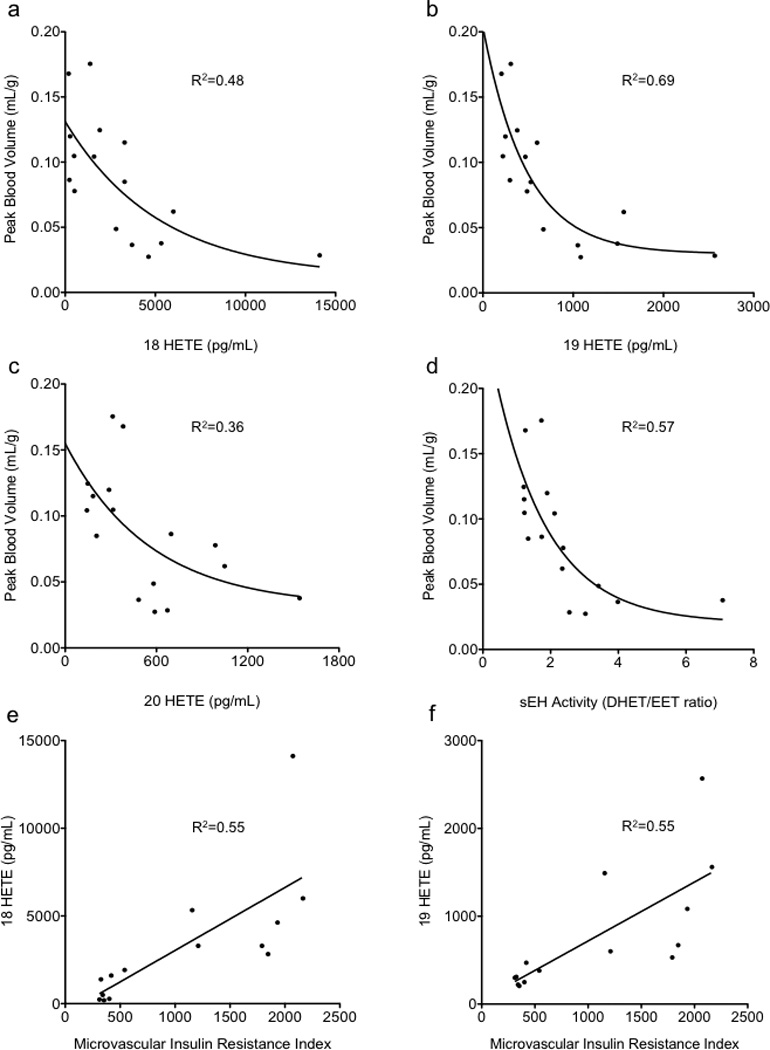
Correlations of vasoconstrictor HETEs (a,b,c) and (d) Soluble Epoxide-Hydrolase (sEH) Activity (DHET/ETT ratio) with peak CBV during IVGTT. Correlations of microvascular insulin resistance index to 18-HETE (e) and 19-HETE (f).
DISCUSSION
Eicosanoids are endothelial-derived vasoactive substances that can influence skeletal muscle blood flow and functional capillary recruitment.15 Dynamic changes in CBV are thought to be important for facilitating skeletal muscle glucose uptake.6, 7, 15 Our hypothesis that eicosanoid-mediated changes in CBV influence glucose handling are based on findings that sEH inhibitors improve blood glucose concentrations, modulate CBV, and ameliorate IR in small animal models of disease.12, 15, 16 The purpose of this study was to examine the arachadonic acid derived vasodilator and vasoconstrictor pathways in concert with skeletal muscle blood flow in a primate model of IR produced by inactivity. Our data indicate that elevated eicosanoid vasoconstrictors (18,19, & 20-HETE) and the indirect measurement of sEH activity (elevated DHET/EET ratio) are contributors in the pathophysiology of impaired capillary recruitment and microvascular IR in lean activity-restricted primates. Furthermore, this study supports the notion of a reflexive shift between reduced physical activity and alterations in vascular tone as a potential pathophysiologic link between reduced activity, impaired microvascular capillary function, and IR.11, 17, 19, 20
We studied a subset of ten activity-restricted primates that were part of a larger cohort of activity-restricted animals in which we first demonstrated that inactivity, in the absence of obesity, limits the CBV response to a glucose challenge and correlates with the degree of IR.11 In this original report, a complete assessment of all vasoactive arachadonic acid metabolites was not performed. It was the initial finding of a lower concentration of 14,15 EET that lead us to a more complete evaluation of EETs, DHETs, and HETEs and their relationship to CBV and microvascular IR. Thus, a full evaluation of arachadonic acid vasoactive metabolites was performed in ten out of the original thirteen primates previously described in which there was a sufficient amount of plasma available to complete testing. The plasma samples were tested for freely circulating ETTs, DHETs, and HETEs. However, it should be noted that these vasoactive metabolites can be esterified and reincorporated into membrane phospholipid pools with future release into the circulation through phospholipase activity. Thus, circulating concentrations of these metabolites do not reflect the complete vasoactive eicosanoid pool.18
An important limitation of the study is that we have not provided mechanistic proof that HETEs or an altered DHET/EET ratio are responsible for abnormal CBV responses or contribute to IR. Instead, we have provided compelling evidence for a relationship between elevated vasoconstrictors to a reduced CBV and elevated degree of microvascular IR. Additionally, we have provided evidence for relationship between an elevated DHET/ETT ratio as an indirect marker of sEH activity with reduced CBV. These relationships support the notion that a shift in vasoactive arachadonic acid metabolites towards a more vasoconstrictive profile limit functional capillary recruitment and contribute to vascular IR. Further long-term studies will be needed to understand the pathophysiologic consequences that may arise in the mediators of microvascular tone and the impact they have on skeletal muscle capillary function during the development of obesity.
In summary, we have found that IR with inactivity shifts the plasma eicosanoids to a more vasoconstrictive profile and these findings may lead to new therapeutic vascular targets for the treatment of IR.
Supplementary Material
Acknowledgments
Dr. Chadderdon was funded by a Fellow-to-Faculty Award (0875005N) from the American Heart Association and grant 5KL2TR000152 from the National Institutes of Health (NIH). Dr. Lindner is supported by grants, R01-HL-078610 and R01-HL-111969 from the NIH. Dr. Grove is supported by grant R01-DK-79194 from the NIH. The Oregon National Primate Research Center is supported by a National Center for Research Resources grant (S10-RR-024585) and a Research Program Projects and Centers grant (P51 DK011092) from the NIH. This publication was made possible with support from the Oregon Clinical and Translational Research Institute (OCTRI), grant number UL1 RR024140 from the National Center for Advancing Translational Sciences, a component of the NIH and NIH Roadmap for Medical Research.
Footnotes
Conflict of Interest
There are no conflicts for any of the authors of this study.
REFERENCES
- 1.Coggins M, Lindner J, Rattigan S, Jahn L, Fasy E, Kaul S, et al. Physiologic hyperinsulinemia enhances human skeletal muscle perfusion by capillary recruitment. Diabetes. 2001;50:2682–2690. doi: 10.2337/diabetes.50.12.2682. [DOI] [PubMed] [Google Scholar]
- 2.Vincent MA, Clerk LH, Lindner JR, Price WJ, Jahn LA, Leong-Poi H, et al. Mixed meal and light exercise each recruit muscle capillaries in healthy humans. Am J Physiol Endocrinol Metab. 2006;290:E1191–E1197. doi: 10.1152/ajpendo.00497.2005. [DOI] [PubMed] [Google Scholar]
- 3.Scherrer U, Randin D, Vollenweider P, Vollenweider L, Nicod P. Nitric oxide release accounts for insulin's vascular effects in humans. J Clin Invest. 1994;94:2511–2515. doi: 10.1172/JCI117621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solomon TP, Haus JM, Li Y, Kirwan JP. Progressive hyperglycemia across the glucose tolerance continuum in older obese adults is related to skeletal muscle capillarization and nitric oxide bioavailability. J Clin Endocrinol Metab. 2011;96:1377–1384. doi: 10.1210/jc.2010-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest. 1994;94:1172–1179. doi: 10.1172/JCI117433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vincent MA, Barrett EJ, Lindner JR, Clark MG, Rattigan S. Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am J Physiol Endocrinol Metab. 2003;285:E123–E129. doi: 10.1152/ajpendo.00021.2003. [DOI] [PubMed] [Google Scholar]
- 7.Vincent MA, Clerk LH, Lindner JR, Klibanov AL, Clark MG, Rattigan S, et al. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes. 2004;53:1418–1423. doi: 10.2337/diabetes.53.6.1418. [DOI] [PubMed] [Google Scholar]
- 8.Laakso M, Edelman SV, Brechtel G, Baron AD. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man. A novel mechanism for insulin resistance. J Clin Invest. 1990;85:1844–1852. doi: 10.1172/JCI114644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clerk LH, Vincent MA, Barrett EJ, Lankford MF, Lindner JR. Skeletal muscle capillary responses to insulin are abnormal in late-stage diabetes and are restored by angiotensin-converting enzyme inhibition. Am J Physiol Endocrinol Metab. 2007;293:E1804–E1809. doi: 10.1152/ajpendo.00498.2007. [DOI] [PubMed] [Google Scholar]
- 10.Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, Barrett EJ. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes. 2006;55:1436–1442. doi: 10.2337/db05-1373. [DOI] [PubMed] [Google Scholar]
- 11.Chadderdon SM, Belcik JT, Smith E, Pranger L, Kievit P, Grove KL, et al. Activity restriction, impaired capillary function, and the development of insulin resistance in lean primates. Am J Physiol Endocrinol Metab. 2012;303:E607–E613. doi: 10.1152/ajpendo.00231.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iyer A, Kauter K, Alam MA, Hwang SH, Morisseau C, Hammock BD, et al. Pharmacological inhibition of soluble epoxide hydrolase ameliorates diet-induced metabolic syndrome in rats. Exp Diabetes Res. 2012;2012:1–11. doi: 10.1155/2012/758614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luria A, Bettaieb A, Xi Y, Shieh GJ, Liu HC, Inoue H, et al. Soluble epoxide hydrolase deficiency alters pancreatic islet size and improves glucose homeostasis in a model of insulin resistance. Proc Natl Acad Sci U S A. 2011;108:9038–9043. doi: 10.1073/pnas.1103482108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mustafa S, Sharma V, McNeill JH. Insulin resistance and endothelial dysfunction: Are epoxyeicosatrienoic acids the link? Exp Clin Cardiol. 2009;14:e41–e50. [PMC free article] [PubMed] [Google Scholar]
- 15.Shim CY, Kim S, Chadderdon S, Wu M, Qi Y, Xie A, et al. Epoxyeicosatrienoic Acids Mediate Insulin-Mediated Augmentation in Skeletal Muscle Perfusion and Blood Volume. Am J Physiol Endocrinol Metab. 2014;307:E1097–E1104. doi: 10.1152/ajpendo.00216.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roche C, Besnier M, Cassel R, Harouki N, Coquerel D, Guerrot D, et al. Soluble epoxide hydrolase inhibition improves coronary endothelial function and prevents the development of cardiac alterations in obese insulin-resistant mice. Am J Physiol Heart Circ Physiol. 2015;308:H1020–H1029. doi: 10.1152/ajpheart.00465.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleming I. Cytochrome p450 and vascular homeostasis. Circ Res. 2001;89:753–762. doi: 10.1161/hh2101.099268. [DOI] [PubMed] [Google Scholar]
- 18.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 19.Hamburg NM, McMackin CJ, Huang AL, Shenouda SM, Widlansky ME, Schulz E, et al. Physical inactivity rapidly induces insulin resistance and microvascular dysfunction in healthy volunteers. Arterioscler Thromb Vasc Biol. 2007;27:2650–2656. doi: 10.1161/ATVBAHA.107.153288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nosova EV, Yen P, Chong KC, Alley HF, Stock EO, Quinn A, et al. Short-term physical inactivity impairs vascular function. J Surg Res. 2014;190:672–682. doi: 10.1016/j.jss.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


