Abstract
Background
Major Depressive Disorder (MDD) and anxiety disorders often co-occur, with poorer treatment response and long-term outcomes. However, little is known about the shared and distinct neural mechanisms of comorbid MDD and anxiety (MDD+Anx). This study examined how MDD and MDD+Anx differentially impact cognitive control.
Methods
Eighteen MDD, 29 MDD+Anx, and 54 healthy controls (HC) completed the Parametric Go/No-go (PGNG) during fMRI, including Target, Commission and Rejection trials.
Results
MDD+Anx had more activation in the anterior dorsolateral prefrontal cortex, hippocampus, and caudate during Rejections, and inferior parietal lobule during correct Targets than MDD and HC. During Rejections HC had greater activation in a number of cognitive control regions compared to MDD; in the posterior cingulate compared to MDD+Anx; and in the fusiform gyrus compared to all MDD. During Commissions HC had greater activation in the right inferior frontal gyrus than all MDD. MDD had more activation in the mid-cingulate, inferior parietal lobule, and superior temporal gyrus than MDD+Anx during Commissions.
Conclusions
Despite similar performance, MDD and MDD+Anx showed distinct differences in neural mechanisms of cognitive control in relation to each other, as well as some shared differences in relation to HC. The results were consistent with our hypothesis of hypervigilance in MDD+Anx within the cognitive control network, but inconsistent with our hypothesis that there would be greater engagement of salience and emotion network regions. Comorbidity of depression and anxiety may cause increased heterogeneity in study samples, requiring further specificity in detection and measurement of intermediate phenotypes and treatment targets.
Keywords: anxiety, cognitive control, fMRI, inferior parietal lobule, Major Depressive Disorder
Introduction
Major Depressive Disorder (MDD) is a lifetime disorder for many, characterized by an insidious onset and a recurrent course. There is evidence of substantial disability and burden with the disease, including increased mortality due to suicide and morbidity due to numerous other conditions for which MDD increases risk (e.g., hypertension, obesity, diabetes1–4). Currently, this detrimental course persists even in the context of early diagnosis, effective and efficient treatments, and wellness maintenance5; 6. Personalized medicine, or the matching of sub-diagnostic specificity with targeted treatments, is a broad goal for many disorders including MDD and might result in more efficient, effective, and lasting treatments. One strategy to achieve this goal is to better identify meaningful subtypes of MDD, as it is a highly heterogeneous disorder7–12.
Within this framework, there is growing evidence that presence of a comorbid, often pre-existing, anxiety disorder can change the presentation and prognosis of MDD for our standard treatments. Historically, comorbid MDD and anxiety results in poorer response to standard treatments (e.g., STAR-D13; 14) and greater disruption in dexamethasone and metyrapone challenge of HPA axis functioning15; 16. Yet, even in the era of subdiagnostic and pandiagnostic phenotyping, championed within the Research Domain Criteria (RDoC) initiative of the NIMH, studies of comorbid anxiety as a meaningful subtype of MDD are quite limited. Furthermore, the nuances in methods design, sampling characteristics, and theoretical underpinnings make it difficult to integrate these studies. Task-based fMRI studies with emotional stimuli17; 18 report differential effects for emotional stimuli based upon diagnosis and valence. Another study showed MDD-specific mid-cingulate gyrus hyperactivation, interpreted as hyperviglance, in response to reward anticipation that was not present in MDD with Panic Disorder19. Two symptom-based neuroimaging studies evaluated depression and anxiety symptoms in relation to connectivity patterns20; 21 and demonstrated some differential patterns in salience/emotion networks and cognitive control networks. These baseline resting state connectivity networks offer an intriguing way to understand network synchronization and harmonics absent an experimental paradigm, and are powerful windows into how regions within a given network may work together to a greater or lesser extent22. Recent work is now linking these network patterns to features of illness and disease course23; 24. Overall, these initial reports suggest that differential responsiveness to emotional valence, reward, and cognitive challenge, as well as resting state connectivity patterns may be present based upon the presence or absence of anxiety disorder in the context of MDD. It is unclear whether many studies demonstrating increased activation in regions within and outside of the salience and emotional network are reflective of hypervigilance to threatening stimuli, increased depth of processing of emotionally congruent stimuli, or of increased attempts at regulation of emotional content.
To this end, one intriguing line of research includes directed manipulation of the extent of regulation of emotional content, in an explicit paradigm where study participants are directed to look passively, maintain an initial emotional response, or reappraise emotional stimuli to diminish both salience and depth of processing of these stimuli25; 26. This paradigm has lead to some interesting between group differences in regions thought to be a part of the cognitive control network27. However, manipulation checks within this design rely on both participant awareness of emotional responses, and participant ability to rate their own effectiveness in regulation. Therefore, it is unclear whether increased engagement of cognitive control regions in HC relative to MDD and/or anxiety patients relates to greater awareness, effort, or success in regulation28. Furthermore, current data does not clarify the duration of emotional responses at an individual or group level, potentially leading to confounding of control conditions within the patient groups that could lead to diminished contrast differences between explicit reappraisal and look only conditions.
One way to provide convergent evidence about the nature of weakened or diminished emotion regulation in MDD and MDD plus anxiety would be to attempt to link emotion regulation findings with cognitive control results. To our knowledge, however, few studies have investigated explicit cognitive control in these population without potentially confounding emotional stimuli29–32. Furthermore, to our knowledge no studies comparing MDD alone with MDD plus anxiety have specifically examined cognitive control, a regulatory mechanism for thoughts and emotions supported by lateral and medial prefrontal and inferior parietal regions that make up the cognitive control network. Importantly, performance and neuroimaging measures have demonstrated that disruption of this network may contribute to mood dysregulation in MDD (see33, but it is not clear if this is the case for MDD comorbid with anxiety). Given the limited research examining differences between MDD and MDD comorbid with anxiety, it is crucial that we better understand the similarities and differences in MDD and MDD comorbid with anxiety to help inform diagnostic overlaps/clarity and potentially differential treatment strategies. It is possible that these groups differ according to valence, context, and cognitive control capacity, consistent with the underlying theories and symptoms related to each diagnosis. It is also possible that results from emotion challenge task and emotion regulation paradigms could be disambiguated through the use of cognitive control tasks without emotional stimuli or explicit emotional conditions.
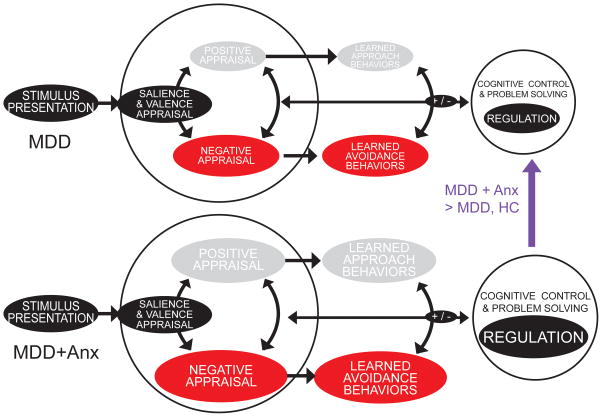
As such, we have proposed and pursued a line of research in cognitive control, with the expectation that individuals with MDD comorbid with anxiety have normative cognitive control capacity (regulation) and heightened emotion response (negative appraisal), while individuals with MDD alone have diminished cognitive control (regulation) and blunted (positive appraisal) emotion response (see Figure 1)33; 34, although only a subset of the hypotheses put forth within this model are tested here. We hypothesize that MDD comorbid with anxiety should demonstrate aspects of hypervigilance and increased activation during cognitive control, whereas MDD alone may demonstrate hypoactivation and decreased regulatory skills during a cognitive control task without any emotional context. It is important to note that in the current study we used the DSM-IV definition of MDD with comorbid anxiety, we did not use the DSM-5 definition of MDD with anxious distress, as this change in definition occurred after the participant data was collected.
Figure 1. Model for Cognitive Control and Emotion Response in MDD alone and MDD Comorbid with Anxiety.
Illustrates our hypothesis that individuals with MDD comorbid with anxiety have normative cognitive control capacity (regulation) and heightened emotion response (negative appraisal), while individuals with MDD alone have diminished cognitive control (regulation) and blunted (positive appraisal) emotion response.
Materials and Methods
Participants
Twenty-one participants with diagnosis of MDD, 32 participants with a comorbid MDD and anxiety disorder diagnosis (MDD+Anxiety), and 56 healthy controls (HC) completed the study between 2003 and 2012. Clinical assessment was conducted using the structured clinical interview for DSM-IV35. Prior to enrollment in the study, participants were unmedicated and, in order to eliminate medication and hormonal effects on functional neural activation, had been medication-free from SSRIs or SNRIs for at least 90 days and from all other medications (including birth control) for at least 30 days. All participants were right handed. Individuals who smoked cigarettes, met criteria for alcohol abuse or other drug abuse in the past two years, or reported use of illegal drugs in the past two years were excluded. In addition, HCs could not meet current or past criteria for MDD or most other Axis I or II psychiatric disorders, excluding remote history of substance use disorders (see exclusion criteria above). HCs had no first-degree relatives with a history of psychiatric illness. Participants underwent fMRI and completed several measures including the Hamilton Depression Rating Scale (HDRS36 and the Neuroticism and Extraversion Scales from the NEO-PI37.
A number of different movement parameters were evaluated to determine if, and for which individuals, BOLD signal estimates were compromised24; 38; 39. We settled on using an outlier deviation statistic, in which realignment values from MCFLIRT were used to estimate a standard deviation of the realignment required in pitch, roll and yaw, subsequently averaged across all six runs. As a result, 5 individuals were excluded for movement and an additional two were removed due to substantial signal distortion, resulting in a final sample of 18 MDD, 29 MDD+Anx, and 54 HC. Informed consent was obtained according to the guidelines of the Institutional Review Boards of The University of Michigan (UM) and consistent with the Declaration of Helsinki. Participants were compensated for their participation.
Cognitive Control Measure
Parametric Go/No-go Task (PGNG34; 40; 41)
The PGNG is a 24 minute task completed during fMRI which measures attention (Targets) and set-shifting, processing speed, and correct responses (Rejections) and incorrect responses (Commissions) to lure trials as a part of cognitive control. Participants were asked to respond with their right index finger using a button box as quickly as possible to a string of particular target letters for the “Go” condition. In the “No-Go” condition, they may only respond to one of these target letters in an alternating or non-repeating order. Scores were computed for the average correct Targets for Go items across all three levels of difficulty in the task, average correct Rejections of No-go items across the two more difficult levels of the task, and Go Response Time across all three levels of the task. For more information see41.
Data acquisition
Whole brain imaging was performed with a 3.0 Tesla GE Signa scanner (Milwaukee, Wisconsin) using a standard radio frequency coil and T2*-weighted pulse sequence. Blood-oxygen level dependent (BOLD) functional images were collected using a gradient-echo axial forward-reverse spiral sequence42 at UM between 2003 and 2012. The following parameters were used: repetition time= 2000 msec, echo time= 30 msec, flip angle= 90%, field of view= 22cm, 64 by 64 matrix, slice thickness= 4mm, 29 slices. An axial T1 SPGR structural image was obtained for each using 108–124 axial images between 1–1.5 mm in thickness for spatial normalization. During scanning, participants completed the PGNG task using a button box and the importance of remaining motionless was conveyed to each participant. There were six runs of the PGNG, each lasting 4 minutes and 20 seconds, and acquiring 120 volumes. The same scanner and acquisition sequence was used for all participants and there was no relationship between the year the fMRI was performed and extracted activation of the BOLD signal (all p-values > .05).
MRI Processing
Preprocessing of fMRI data was conducted using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/) and AFNI (http://afni.nimh.nih.gov/afni/). Data were despiked using AFNI. All data were then slice-time corrected in SPM8 and realigned in FSL (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) using MCFLIRT43. Anatomical and functional images were co-registered and normalized to Montreal Neurological Institute (MNI) space using SPM8. Smoothing was completed with a full width at half maximum filter of 5mm. First level models were built in SPM8 using roll, pitch and yaw realignment movement regressors from FSL for each run. The subtraction method was used to create contrast images and second level models were built in SPM8.
Statistical Procedures
Analyses for demographic and clinical characteristics were carried out using SPSS 20.0 (IBM). Group differences in demographic and clinical characteristics were assessed using analysis of variance (ANOVA) or chi-square, as appropriate. Group differences in PGNG performance was examined using one multivariate analysis of variance (MANOVA) model. Results were deemed statistically significant when p-values < .05.
fMRI data were evaluated with 3 (MDD+Anx/MDD/HC) × 1 ANCOVAs with Targets, Commissions, and Rejections as separate dependent variables and with gender, age, and task performance accuracy as covariates in each of the three models. Main effects were followed up using t-tests. Significance thresholds were derived with AlphaSim24 (p < 0.005, k > 55).
Results
Demographic and Clinical Characteristics
Participant characteristics are reported in Table 1. As expected, MDD and MDD+Anxiety had significantly higher HDRS, NEO-PI neuroticism scores, and NEO-PI extraversion scores relative to HC, but no other group differences were found.
Table 1.
Participant Characteristics
| HC n=54 | MDD n=18 | MDD+Anxiety n=29 | Group Comparisons | |
|---|---|---|---|---|
| Age | 33.80 (11.56) | 34.28 (11.69) | 33.24 (11.25) | ns |
| Sex (M/F) | 16/38 | 7/11 | 8/21 | ns |
| Education | 15.60 (1.90) | 15.33 (1.97) | 14.82 (2.09) | ns |
| Shipley IQ | 105.93 (16.67)a | 105.14 (13.78)b | 105.88 (11.24)c | ns |
| HDRS* | 0.85 (1.90) | 20.76 (7.25) | 20.71 (6.08) | HC < MDD, MDD+Anxiety |
| NEO-PI Neuroticism* | 42.27 (8.82) a | 63.17 (13.09) b | 65.94 (12.75) c | HC < MDD, MDD+Anxiety |
| NEO-PI Extraversion* | 50.33 (9.28) a | 37.33 (9.22) b | 34.89 (11.74) c | HC < MDD, MDD+Anxiety |
| PGNG Performance | ||||
| Go-Accuracy | 0.89 (0.13) | 0.85 (0.16) | 0.87 (0.14) | ns |
| No-Go Accuracy | 0.67 (0.16) | 0.70 (0.19) | 0.69 (0.18) | ns |
| Response Time to Go Targets* | 482.61 (41.43) | 524.42 (40.48) | 507.46 (56.27) | HC < MDD, MDD+Anxiety |
Note. Values are means and standard deviations unless otherwise noted; HDRS, Hamilton Depression Rating Scale;
n=46;
n=14;
n=26;
p<.05.
PGNG Performance
Groups did not differ on Go-Accuracy (percent correct Targets) or on No-Go Accuracy (percent correct inhibition; see Table 1). On the other hand, both MDD groups had significantly longer response times for Go Targets (correct Targets; see Table 1).
PGNG Task Neural Activation
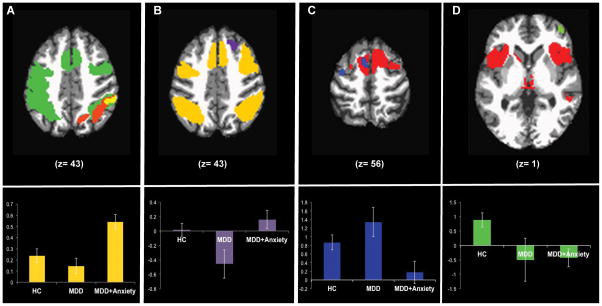
Neural activation during Targets, Commissions, and Rejections are reported in Table 2. In general, Targets and Rejections largely activated the cognitive control network (CCN), while Commissions activated paralimbic regions and parietal regions thought to be involved in visual-haptic integration and error processing (see Table 2 and Figure 2). Figure 2 includes illustration of posthoc differences for key regions within the CCN that differed between the three groups to highlight the degree and direction of differences.
Table 2.
Task Effect
| Contrast/lobe | BA | x | y | z | Z | k |
|---|---|---|---|---|---|---|
| Targets | ||||||
| Frontal | ||||||
| Middle | 9/46 | 46 | 38 | 20 | 6.51 | 1094 |
| 9/46 | −38 | 38 | 28 | 7.62 | 902 | |
| Precentral/Postcentral/Inf Parietal | 6/4/3/7/40 | −32 | −26 | 48 | Inf | 16312 |
| Mid-Cingulate/Suppl. Motor | 32/24/6 | −4 | 2 | 52 | Inf | ^ |
| Parietal | ||||||
| Postcentral | 40/7 | 60 | −16 | 18 | 4.48 | 57 |
| Inferior Parietal | 39/40/7 | 48 | −40 | 42 | 7.68 | 1921 |
| Occipital | ||||||
| Lingual | 18 | 26 | −92 | −4 | 7.53 | 96 |
| Lingual/Inferior | 17/18/19 | −26 | −92 | −2 | 7.25 | 607 |
| Middle | 19/37 | 46 | −74 | −10 | Inf | 174 |
| Subcortical | ||||||
| Cerebellum (Uncus/Culmen/Declive) | 6 | −56 | −16 | Inf | 1233 | |
| Commissions | ||||||
| Frontal | ||||||
| Anterior Cingulate/Dorso-medial | 32/24 | 8 | 30 | 28 | 7.62 | 6606 |
| Inferior, Middle, Insula | 13/47 | −34 | 16 | 6 | Inf | 3332 |
| 13/47 | 32 | 22 | 4 | 7.21 | 1484 | |
| Temporal | ||||||
| Superior | 38 | 52 | 14 | −10 | 4.8 | 73 |
| Middle | 21 | 54 | −32 | −4 | 3.07 | 77 |
| Parietal | ||||||
| Supramarginal | 40 | 58 | −48 | 28 | 6.85 | 1705 |
| 40 | −58 | −46 | 28 | 6.89 | 1854 | |
| Subcortical | ||||||
| Thalamus | 4 | −22 | 0 | 3.99 | 119 | |
| Caudate | −12 | 2 | 10 | 3.87 | 90 | |
| Cerebellum (Uncus) | 16 | −54 | −26 | 3.75 | 172 | |
| Rejections | ||||||
| Frontal | ||||||
| Inferior/Middle/Insula | 13/47/46/9 | −32 | 14 | 6 | 7.13 | 14237 |
| 13/47/46/9 | 48 | 32 | 28 | 6.68 | ^ | |
| Cingulate/Superior | 6/24/32 | 12 | 2 | 64 | 6.91 | ^ |
| Temporal | ||||||
| Middle | 21 | −58 | −28 | −6 | 3.36 | 67 |
| 21 | 60 | −34 | −6 | 4.27 | 153 | |
| Parietal | ||||||
| Postcentral | 43 | −64 | −16 | 18 | 3.96 | 86 |
| 40/7 | −30 | −66 | 44 | 5.56 | 2395 | |
| 39/40 | 60 | −46 | 32 | 6.44 | 2593 | |
| Occipital | ||||||
| Middle | 19 | −42 | −70 | −2 | 5.36 | 521 |
| 19 | 48 | −74 | −6 | 5.01 | 172 | |
Note.
part of larger bilateral cluster for k
Figure 2. Group Differences in Cognitive Control Neural Activation During Targets (A), Rejections (B), and Commissions (C,D).
Group Differences in Cognitive Control Neural Activation During Targets (A), Rejections (B), and Commissions (C,D). The extent and relative group differences in activation are shown in each bar graph. Panel A shows task activation during Targets (green), as well as regions where HC had more activation than MDD only (orange) and regions where MDD+Anx had more activation than MDD and HC (yellow). The extracted ROI data for each group from the yellow cluster is plotted below. Panel B shows task activation during Rejections (yellow) and also a region in the anterior prefrontal cortex where MDD+Anx had more activation than MDD (purple). The extracted ROI data for each group from the anterior prefrontal cortex purple cluster is plotted below. Panel C shows task activation during Commissions (red) and regions in the prefrontal cortex where MDD only had more activation than MDD+Anx (blue). The extracted ROI data for each group from the peak blue cluster is plotted below. Panel D shows task activation during Commissions (red) and a region in the right inferior frontal gyrus where HC had more activation than all MDD (green). The extracted ROI data for each group from the green cluster is plotted below.
Group Differences in Cognitive Control Neural Activation
Targets
HC had more activation during Targets than MDD in superior temporal regions (see Table 3). However, HC had less activation during Targets than MDD+Anxiety in inferior parietal areas within the CCN (see Table 3 and Figure 2, panel A). MDD+Anxiety had greater activation during Targets than MDD in limbic and parietal regions within the CCN, as well as superior temporal regions (see Table 3 and Figure 2, panel A).
Table 3.
Group Differences for Targets
| Contrast/lobe | BA | x | y | z | Z | k |
|---|---|---|---|---|---|---|
| HC is greater than MDD only | ||||||
| Temporal | ||||||
| Superior | 22/40/39 | −56 | −42 | 14 | 3.54 | 120 |
| HC is less than MDD plus Anxiety | ||||||
| Parietal | ||||||
| Inferior | 40 | 56 | −40 | 44 | 3.86 | 111 |
| MDD only is less than MDD plus Anxiety | ||||||
| Frontal | ||||||
| Anterior Cingulate | 32/24 | 12 | 32 | 20 | 3.64 | 75 |
| Parietal | ||||||
| Inferior | 39/40 | 50 | −42 | 44 | 3.86 | 1112 |
| Temporal | ||||||
| Superior | 22/39 | −58 | −42 | 12 | 4.84 | 173 |
Rejections
HC had more activation during Rejections than MDD throughout the brain in frontal, parietal, occipital, temporal, and subcortical regions; HC had more activation during Rejections than MDD+Anxiety in the posterior cingulate; and HC had more activation during Rejections than all MDD in the fusiform gyrus (see Table 4). MDD+Anxiety had more activation during Rejections than MDD in regions outside of the CCN: the anterior prefrontal cortex, hippocampus, and caudate (see Table 4 and Figure 2, panel B).
Table 4.
Group Differences for Rejections
| Contrast/lobe | BA | x | y | z | Z | k |
|---|---|---|---|---|---|---|
| HC is greater than MDD only | ||||||
| Frontal | ||||||
| Superior | 10 | 28 | 54 | 20 | 2.99 | 64 |
| Middle | 9 | 28 | 30 | 34 | 3.07 | 105 |
| Parietal | ||||||
| Posterior Cingulate | 31 | 8 | −36 | 42 | 3.02 | 66 |
| Occipital | ||||||
| Cuneus | 17/18 | 22 | −90 | 14 | 3.65 | 149 |
| Temporal | ||||||
| Fusiform | 37 | −36 | −42 | −8 | 3.9 | 148 |
| Subcortical | ||||||
| Caudate | −18 | 22 | 8 | 4.03 | 94 | |
| HC is greater than MDD plus Anxiety | ||||||
| Parietal | ||||||
| Posterior Cingulate | 31 | 10 | −18 | 44 | 3.21 | 60 |
| MDD is less than MDD plus Anxiety | ||||||
| Frontal | ||||||
| Superior | 6 | 20 | 28 | 50 | 3.59 | 286 |
| Temporal | ||||||
| Hippocampus | 34 | −40 | 4 | 4.2 | 193 | |
| Subcortical | ||||||
| Caudate | −12 | 28 | 0 | 4.23 | 75 | |
| HC is greater than all MDD | ||||||
| Temporal | ||||||
| Fusiform Gyrus | 37 | −36 | −42 | −10 | 3.47 | 64 |
Commissions
MDD had more activation during Commissions than MDD+Anxiety within regions proposed for error processing during a visual, language based task including the mid-cingulate, inferior parietal lobule, and superior temporal gyrus, as well as within regions involved in motoric response including the precentral gyrus and supplemental motor cortex (see Table 5 and Figure 2, panel C). HC had more activation during Commissions than all MDD outside of the network activated by the task, in the right inferior frontal gyrus (see Table 5 and Figure 2, panel D).
Table 5.
Group Differences for Commissions
| Contrast/lobe | BA | x | y | z | Z | k |
|---|---|---|---|---|---|---|
| MDD only is greater than MDD plus Anxiety | ||||||
| Frontal | ||||||
| Precentral | 4 | −36 | −8 | 60 | 3.46 | 60 |
| Mid-Cingulate/Suppl. Motor | 32/6 | −8 | 10 | 56 | 3.2 | 74 |
| Parietal | ||||||
| Inferior | 40 | −60 | −32 | 28 | 3.26 | 59 |
| Temporal | ||||||
| Superior | 22 | −58 | −56 | 16 | 3.75 | 93 |
| HC is greater than all MDD | ||||||
| Frontal | ||||||
| Inferior | 10 | 42 | 50 | 0 | 3.14 | 77 |
Relationships with Trait Neuroticism and Extraversion
In regions that differed between MDD and MDD+Anxiety, we further evaluated whether these differences were present independent of or in concert with trait neuroticism and extraversion. It is possible that trait neuroticism and extraversion would provide a larger effect sizes in these regions, capturing individual differences in anxiety symptoms across the lifetime as opposed to episodic experiences that could be current or remote. This can be exacerbated by known poor recall for degree, duration, and extent of past symptoms, especially during childhood44. To test this dimensional hypothesis, we extracted activation and correlated activation with trait neuroticisim and extraversion in the MDD groups, alone and together. Neuroticism and extraversion were not significantly related to activation in regions that differed between MDD and MDD+Anxiety (all p-values > .05).
Exploratory Analysis of Anxiety Subtypes
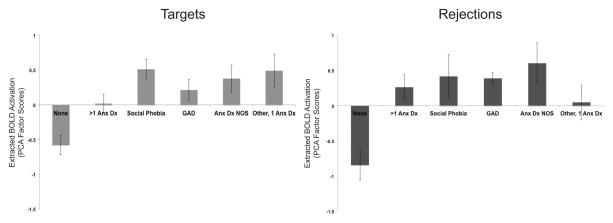
We grouped individuals with MDD and a comorbid anxiety disorder into one group due to the fact that many participants in the MDD+Anxiety group had more than one anxiety disorder diagnosis (n=12), while only small subsets had a single diagnosis including Social Phobia (n=5), Generalized Anxiety Disorder (GAD; n=4), Anxiety Disorder Not Otherwise Specified (NOS; n=4), Panic Disorder (n=2), and Simple Phobia (n=2). However, we wanted to examine whether MDD+Anxiety participants with different anxiety disorders differed in their patterns of activation in regions that MDD+Anxiety had greater activation than MDD. For data reduction purposes, we ran Principle Components Analyses (PCA) with extracted activation during Targets from three regions in which MDD+Anxiety had greater activation than MDD (see Table 3) and the three variables loaded onto one factor (81.25% variance explained, eigenvalue= 2.44). We also ran PCA with extracted activation during Rejections from four regions in which MDD+Anxiety had greater activation than MDD (see Table 4) and the four variables loaded onto one factor as well (59.30% variance explained, eigenvalue= 2.37). Due to the very small sample sizes of the Panic Disorder and Simple Phobia groups, we combined these groups together to create a single group who has one anxiety disorder. ANOVAs with anxiety subtype as the independent variable and the Target activation factor score and Rejection activation factor score as separate dependent variables with MDD and MDD+Anxiety participants found there was a significant difference between groups for the Target activation factor score (F(5,47)= 7.02, p< .001) and for the Rejection activation factor score (F(5,47)= 5.96, p< .001). Post hoc analyses using Tukey’s HSD indicated that MDD alone had lower Target activation factor scores (M= −0.58, SD= 0.59) than MDD with more than one more than one anxiety disorder diagnosis (M= 0.16, SD= 0.48), MDD with Social Phobia (M= 0.51, SD= 0.33), MDD with Anxiety Disorder NOS (M= 0.38, SD= 0.25), and MDD with one anxiety disorder (Panic Disorder or Simple Phobia; M= 0.49, SD= 0.25), but there was no difference in Target activation factor scores between MDD alone and MDD with GAD (although trending (p=.07); M= 0.21, SD= 0.25). MDD+Anxiety subgroups did not differ from one another and there were no other group differences in Target activation factor scores. Additionally, post hoc analyses using Tukey’s HSD revealed MDD alone had lower Rejection activation factor scores (M= −0.84, SD= 0.90) than MDD with more than one more than one anxiety disorder diagnosis (M= 0.27, SD= 0.62), MDD with Social Phobia (M= 0.42, SD= 0.70), MDD with GAD (M= 0.39, SD= 0.17), and MDD with Anxiety Disorder NOS (M= 0.61, SD= 0.58), but MDD alone did not differ from MDD with one anxiety disorder (Panic Disorder or Simple Phobia; M= 0.05, SD= 0.49) in Rejection activation factor scores. MDD+Anxiety subgroups did not differ from one another and there were no other group differences in Rejection activation factor scores. MDD and MDD+Anxiety subtype activation factor scores for Targets and Rejections are shown in Figure 3.
Figure 3. Exploratory Analyses of Group Differences Among MDD and MDD+Anxiety Subtypes in Cognitive Control Neural Activation During Targets and Rejections.
Exploratory Analyses of Group Differences Among MDD and MDD+Anxiety Subtypes in Cognitive Control Neural Activation During Targets and Rejections. The extent and relative group differences in factor scores of activation are shown in each bar graph. The MDD alone group is shown as “None” (n=18), while MDD+Anxiety subtypes are shown as “>1 Anx Dx” (more than one anxiety disorder diagnosis; n=12), “Social Phobia” (n=5), “GAD” (Generalized Anxiety Disorder; n=4), “Anx Dx NOS” (Anxiety Disorder Not Otherwise Specified; n=4), and “Other, 1 Anx Dx” (Panic Disorder (n=2) and Simple Phobia (n=2)).
Discussion
The present report highlights one potential way to reduce heterogeneity in the study of MDD, by investigating MDD alone in relation to MDD with comorbid anxiety. We found group differences between all MDD participants and the HC group, as might be expected based upon prior work, including decreased activation within right inferior frontal gyrus when participants were unable to demonstrate cognitive control (Commission errors)45. Notably, and consistent with other biological markers and reports, the presence of comorbid anxiety, even in the context of equal depression symptoms, resulted in differential activation patterns for attention and cognitive control processes, as measured by Targets, correct Rejections, and Commission errors. Typically the pattern was of greater activation in comorbid MDD and anxiety relative to MDD alone.
There are a number of important results of the present study. First, it is an event-related design, based upon performance. As such, there can be separation of subgroup by behavior activation differences, with activation differences observed within the correct Target, correct Rejection, and incorrect Commission analyses. First, this allows us to separate out elements of the CCN that are engaged for correct, successful regulation compared to those that are engaged within the context of failure28. Errors result in more extensive engagement of midline cingulate and anterior insula (sometimes referred to as salience network46), whereas successful regulation results in more extensive engagement of ventrolateral and dorsolateral prefrontal cortex (often referred to as CCN proper). Within this framework, greater confidence might be ascribed to ventro and dorsolateral prefrontal engagement for emotion regulation paradigms as being reflective of successful regulation, and salience network as reflective of failure to do so effectively and efficiently.
Moreover, for dissociating MDD and MDD plus anxiety, there are nuances in results that are aided by the ability to separate out different event types. In one case, this results in greater activation within the CCN during Targets in the comorbid group, consistent with our hypothesis of diminished cognitive control engagement in the MDD alone group. The results were also consistent with our hypothesis of hypervigilance during Targets within the comorbid group, with more activation in the inferior parietal lobule relative to both other groups47; 48. There was not, however, any evidence of differential engagement of typical salience and emotion network regions within the comorbid group (e.g., amygdala, subgenual anterior cingulate, anterior insula). Importantly, exploratory analyses of MDD+Anxiety subtypes generally supported that these results were true across different anxiety disorders, supportive of the broader RDoC hypothesis about some shared dimensions across disorders49.
In addition, and contrary to expectation, for Commission errors the MDD alone group exhibited increased activation within regions proposed for error processing (and also cognitive control) during a visual, language based task including the mid-cingulate (putatively within salience network), inferior parietal lobule, and superior temporal gyrus. This increase in activation was in comparison to the comorbid group, emphasizing the potential value and specificity gained in studying MDD alone separate from the comorbid condition. While some of these regions are not within the salience and emotional networks, these results are contrary to the idea that reactivity to errors is somehow exaggerated within the comorbid group, and is not related to or consistent with trait anxiety levels as measured by neuroticism (or inversely with extraversion)50. We do note that the sample was unmedicated, allowing us to avoid common concerns about activation differences that might result from treatment. Moreover, different treatments might be entertained and engaged based upon the presence of a comorbid anxiety disorder, so we were able to avoid potential treatment by subtype medication effects.
There are also limitations that are important to review. First, while the sample of MDD subjects recruited for the study was large (N=55), after dividing into subtypes and removing those with significant movement and distortion, the subgroup samples were more modest. The MDD alone group was only 18 individuals. Second, there was a relatively broad age range studied, from 18–57, which might mask significant comorbidity based differences that are influenced by age. Third, there were no performance differences between the MDD subgroups, although both were slower in Go response time relative to the HC group. However, it is important to note that a key dimensional marker of anxiety, trait neuroticism, was elevated in both MDD groups, and only to a nominally greater extent in the comorbid MDD group. Furthermore, other measures of negative and positive affect (e.g., PANAS, BIS/BAS, MASQ) were not captured in the whole sample and therefore we were not able to more thoroughly examine how negative and positive affect may contribute to some of the group differences found21. Moreover, although exploratory analyses generally found that anxiety disorders included in the MDD+Anxiety group had similar overall patterns of activation during Targets and Rejections, each subtype of anxiety disorders examined had small sample sizes, limiting our ability to better understand how specific anxiety disorders may differ from one another. It will be important for future studies to examine potential differences in different anxiety disorders, alone and in combination with MDD.
Conclusion
In summary, the presence of a comorbid anxiety disorder within the context of MDD may obscure or accentuate differences in relation to HC groups, based upon activation results reported herein. Additionally, more refined subtyping strategies have been employed with self-reported anxiety scales that further strengthen this line of inquiry. The average earlier onset of anxiety disorders relative to MDD51, as well as the shared factor structure for these disorders52 in many self-report measures, may have glossed over some nuanced differences between these groups. Cognitive control, although relatively heavily emphasized within emotion challenge and regulation paradigms, has been understudied in isolation and may provide a context for more clearly distinguishing between these groups and also in pursuing dimensions highlighted within the RDoC initative29–32; 53. Furthermore, cognitive control paradigms without emotional stimuli/challenges can then be integrated with emotion challenge and regulation paradigms in future studies to better disambiguate independent and interactive components of cognitive systems and negative valence systems.
Acknowledgments
Funding: This study was supported by the National Institute of Mental Health (NIMH) (K23MH074459, PI: SAL), a NARSAD Young Investigator Award (SAL); and the National Institute on Drug Abuse (F31DA038388, PI: NAC). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIMH, NIDA, or the National Institutes of Health.
Footnotes
Conflicts of Interest: SAL reports consulting work with Easter Seals, Inc. that was not related to the study. The authors declare no other real or potential conflicts of interest.
References
- 1.Greenberg PE, Kessler RC, Birnbaum HG, et al. The economic burden of depression in the United States: how did it change between 1990 and 2000? J Clin Psychiatry. 2003;64(12):1465–75. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Akiskal HS, Ames M, et al. Prevalence and effects of mood disorders on work performance in a nationally representative sample of U.S. workers. Am J Psychiatry. 2006;163(9):1561–8. doi: 10.1176/appi.ajp.163.9.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musselman DL, Betan E, Larsen H, Phillips LS. Relationship of depression to diabetes types 1 and 2: epidemiology, biology, and treatment. Biol Psychiatry. 2003;54(3):317–29. doi: 10.1016/s0006-3223(03)00569-9. [DOI] [PubMed] [Google Scholar]
- 4.Nemeroff CB, Goldschmidt-Clermont PJ. Heartache and heartbreak--the link between depression and cardiovascular disease. Nat Rev Cardiol. 2012;9(9):526–39. doi: 10.1038/nrcardio.2012.91. [DOI] [PubMed] [Google Scholar]
- 5.Blazer DG, Kessler RC, McGonagle KA, Swartz MS. The prevalence and distribution of major depression in a national community sample: The National Comorbidity Survey. Am J Psychiatry. 1994;151(7):979–986. doi: 10.1176/ajp.151.7.979. [DOI] [PubMed] [Google Scholar]
- 6.Yanagita M, Willcox BJ, Masaki KH, et al. Disability and depression: investigating a complex relation using physical performance measures. Am J Geriatr Psychiatry. 2006;14(12):1060–1068. doi: 10.1097/01.JGP.0000224364.70515.12. [DOI] [PubMed] [Google Scholar]
- 7.Agosti V, Stewart J. Atypical and non-atypical subtypes of depression: comorbidity of social functioning, symptoms, course of illness, co-morbidity and demographic features. Journal of Affective Disorders. 2001;65:75–79. doi: 10.1016/s0165-0327(00)00251-2. [DOI] [PubMed] [Google Scholar]
- 8.Cassano GB, Musetti L, Perugi G. Family history and stressors in subtypes of depression. Clin Neuropharmacol. 1992;15(Suppl 1 Pt A):570A–571A. doi: 10.1097/00002826-199201001-00297. [DOI] [PubMed] [Google Scholar]
- 9.Joffe RT, Bagby RM, Levitt A. Anxious and nonanxious depression 1. Am J Psychiatry. 1993;150(8):1257–1258. doi: 10.1176/ajp.150.8.1257. [DOI] [PubMed] [Google Scholar]
- 10.Schatzberg AF, Posener JA, DeBattista C, et al. Neuropsychological deficits in psychotic versus nonpsychotic major depression and no mental illness. Am J Psychiatry. 2000;157(7):1095–1100. doi: 10.1176/appi.ajp.157.7.1095. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan P, Prescott P, Kendler K. The subtypes of major depression in a twin registry. J Affect Disord. 2002;68:273–284. doi: 10.1016/s0165-0327(00)00364-5. [DOI] [PubMed] [Google Scholar]
- 12.Winokur G. The development and validity of familial subtypes in primary unipolar depression. J Am Acad Child Adolesc Psychiatry. 1982;15:142–146. doi: 10.1055/s-2007-1019527. [DOI] [PubMed] [Google Scholar]
- 13.Fava M, Rush AJ, Alpert JE, et al. Difference in Treatment Outcome in Outpatients With Anxious Versus Nonanxious Depression: A STAR*D Report. Am J Psychiatry. 2008 doi: 10.1176/appi.ajp.2007.06111868. [DOI] [PubMed] [Google Scholar]
- 14.Fava M, Rush AJ, Alpert JE, et al. What clinical and symptom features and comorbid disorders characterize outpatients with anxious major depressive disorder: a replication and extension. Can J Psychiatry. 2006;51(13):823–835. doi: 10.1177/070674370605101304. [DOI] [PubMed] [Google Scholar]
- 15.Lopez JF, Liberzon I, Vazquez DM, et al. Serotonin 1A receptor messenger RNA regulation in the hippocampus after acute stress. Biol Psychiatry. 1999;45(7):934–7. doi: 10.1016/s0006-3223(98)00224-8. [DOI] [PubMed] [Google Scholar]
- 16.Young EA, Abelson JL, Cameron OG. Effect of comorbid anxiety disorders on the hypothalamic-pituitary-adrenal axis response to a social stressor in major depression. Biol Psychiatry. 2004;56(2):113–120. doi: 10.1016/j.biopsych.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton JP, Chen MC, Waugh CE, et al. Distinctive and common neural underpinnings of major depression, social anxiety, and their comorbidity. Soc Cogn Affect Neurosci. 2015;10(4):552–60. doi: 10.1093/scan/nsu084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beesdo K, Lau JY, Guyer AE, et al. Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Arch Gen Psychiatry. 2009;66(3):275–85. doi: 10.1001/archgenpsychiatry.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorka SM, Huggins AA, Fitzgerald DA, et al. Neural response to reward anticipation in those with depression with and without panic disorder. J Affect Disord. 2014;164:50–6. doi: 10.1016/j.jad.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oathes DJ, Patenaude B, Schatzberg AF, Etkin A. Neurobiological signatures of anxiety and depression in resting-state functional magnetic resonance imaging. Biol Psychiatry. 2015;77(4):385–93. doi: 10.1016/j.biopsych.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spielberg JM, Miller GA, Warren SL, et al. Transdiagnostic dimensions of anxiety and depression moderate motivation-related brain networks during goal maintenance. Depress Anxiety. 2014;31(10):805–13. doi: 10.1002/da.22271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeo BTT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobs RH, Barba A, Gowins JR, et al. Decoupling of the Amygdala to Other Salience Network Regions in Adolescent Onset Recurrent Major Depressive Disorder. Psychol Med. 2016;46:1055–1067. doi: 10.1017/S0033291715002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobs RH, Jenkins LM, Gabriel LB, et al. Increased coupling of intrinsic networks in remitted depressed youth predicts rumination and cognitive control. PLoS One. 2014;9(8):e104366. doi: 10.1371/journal.pone.0104366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ochsner KN, Ray RD, Cooper JC, et al. For better or worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 26.Robertson E, Ochsner K, Ray R, et al. An fMRI investigation of emotion and emotion regulation in depression. J Cogn Neurosci. 2005:198–198. [Google Scholar]
- 27.Burklund LJ, Craske MG, Taylor SE, Lieberman MD. Altered emotion regulation capacity in social phobia as a function of comorbidity. Soc Cogn Affect Neurosci. 2015;10(2):199–208. doi: 10.1093/scan/nsu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langenecker SA, Kennedy SE, Guidotti LM, et al. Frontal and limbic activation during inhibitory control predicts treatment response in major depressive disorder. Biol Psychiatry. 2007;62(11):1272–80. doi: 10.1016/j.biopsych.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitterschiffthaler MT, Williams SC, Walsh ND, et al. Neural basis of the emotional Stroop interference effect in major depression. Psychol Med. 2008;38(2):247–256. doi: 10.1017/S0033291707001523. [DOI] [PubMed] [Google Scholar]
- 30.Siegle G, Steinhauer S, Carter C, Thase M. Sustained processing on the Stroop task in depression 2003. City: Blackwell Publishing; pp. S79–S79. [Google Scholar]
- 31.Videbech P, Ravnkilde B, Gammelgaard L, et al. The Danish PET/depression project: Performance on Stroop’s test linked to white matter lesions in the brain. Psychiatry Res-Neuroim. 2004;130:117–130. doi: 10.1016/j.pscychresns.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Wagner G, Sinsel E, Sobanski T, et al. Cortical inefficiency in patients with unipolar depression: An event-related MRI study with the Stroop task. Biol Psychiatry. 2006;59(10):958–965. doi: 10.1016/j.biopsych.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 33.Langenecker SA, Jacobs RH, Passarotti AM. Current Neural and Behavioral Dimensional Constructs across Mood Disorders. Curr Behav Neurosci Rep. 2014;1(3):144–153. doi: 10.1007/s40473-014-0018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langenecker SA, Bieliauskas LA, Rapport LJ, et al. Face emotion perception and executive functioning deficits in depression. J Clin Exp Neuropsychol. 2005;27(3):320–33. doi: 10.1080/13803390490490515720. [DOI] [PubMed] [Google Scholar]
- 35.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV® Axis I Disorders (SCID-I), Clinician Version, Administration Booklet. 2012. [Google Scholar]
- 36.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costa PTJ, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI) professional manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- 38.Jo HJ, Gotts SJ, Reynolds RC, et al. Effective Preprocessing Procedures Virtually Eliminate Distance-Dependent Motion Artifacts in Resting State FMRI. J Appl Math. 2013;2013 doi: 10.1155/2013/935154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Power JD, Barnes KA, Snyder AZ, et al. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–54. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langenecker SA, Zubieta JK, Young EA, et al. A task to manipulate attentional load, set-shifting, and inhibitory control: convergent validity and test-retest reliability of the Parametric Go/No-Go Test. J Clin Exp Neuropsychol. 2007;29(8):842–53. doi: 10.1080/13803390601147611. [DOI] [PubMed] [Google Scholar]
- 41.Votruba KL, Langenecker SA. Factor structure, construct validity, and age- and education-based normative data for the Parametric Go/No-Go Test. J Clin Exp Neuropsychol. 2013;35(2):132–46. doi: 10.1080/13803395.2012.758239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glover GH, Thomason ME. Improved combination of spiral-in/out images for BOLD fMRI. Magn Reson Med. 2004;51(4):863–8. doi: 10.1002/mrm.20016. [DOI] [PubMed] [Google Scholar]
- 43.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 44.Beesdo K, Knappe S, Pine DS. Anxiety and Anxiety Disorders in Children and Adolescents: Developmental Issues and Implications for DSM-V. Psychiatric Clinics of North America. 2009;32(3):483–524. doi: 10.1016/j.psc.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hampshire A, Chamberlain SR, Monti MM, et al. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage. 2010;50(3):1313–9. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105(34):12569–74. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nitschke JB, Heller W, Palmieri PA, Miller GA. Contrasting patterns of brain activity in anxious apprehension and anxious arousal. Psychophysiology. 1999;36(5):628–637. [PubMed] [Google Scholar]
- 48.Gold AL, Morey RA, McCarthy G. Amygdala–Prefrontal Cortex Functional Connectivity During Threat-Induced Anxiety and Goal Distraction. Biol Psychiatry. 2015;77(4):394–403. doi: 10.1016/j.biopsych.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cuthbert BN. Dimensional models of psychopathology: research agenda and clinical utility. J Abnorm Psychol. 2005;114(4):565–9. doi: 10.1037/0021-843X.114.4.565. [DOI] [PubMed] [Google Scholar]
- 50.Aarts K, Pourtois G. Anxiety not only increases, but also alters early error-monitoring functions. Cogn Affect Behav Neurosci. 2010;10(4):479–492. doi: 10.3758/CABN.10.4.479. [DOI] [PubMed] [Google Scholar]
- 51.Kendler KS, Kessler RC, Walters EE, et al. Stressful Life Events, Genetic Liability, and Onset of An Episode of Major Depression in Women 2 31. Am J Psychiatry. 1995;152(6):833–842. doi: 10.1176/ajp.152.6.833. [DOI] [PubMed] [Google Scholar]
- 52.Vollebergh WA, Iedema J, Bijl RV, et al. The structure and stability of common mental disorders: the NEMESIS study. Arch Gen Psychiatry. 2001;58(6):597–603. doi: 10.1001/archpsyc.58.6.597. [DOI] [PubMed] [Google Scholar]
- 53.Raynor G, Jackson G, Wilson S. Cognition-related brain networks underpin the symptoms of unipolar depression: Evidence from a systematic review. Neurosci Biobehav Rev. 2016;61:53–65. doi: 10.1016/j.neubiorev.2015.09.022. [DOI] [PubMed] [Google Scholar]