Abstract
N. meningitidis (NM) is an opportunistic gram-negative human pathogen that colonizes the human nasopharyngeal epithelium. Asymptomatic carriage is common, but some meningococcal strains can invade nasopharyngeal epithelial cells and proceed to cause severe and often fatal infections. Invasion is predominantly driven by expression of bacterial virulence factors and host cell cognate receptors for bacterial recognition. Porins are among the Neisserial components involved in host cell activation and bacterial internalization processes. Similar to other virulence factors, porins present antigenic and structure variability among strains. Such sequence variability in the surface-exposed loop regions has been correlated to bacterial invasiveness and to variability in host cell responses via Toll-like receptor 2 (TLR2). Here, we examined whether TLR2 signaling by porins influences recovery of intracellular Neisseriae from epithelial cells in vitro. Our results show that TLR2 stimulation, either by the organism or exogenously, generally enhances Neisseriae internalization by epithelial cells. TLR2-driven intracellular signaling via ERK1/2, JNK and particularly NF-κB plays a role in this process. Based on these results, it is possible that expression of porin sequence variants that strongly induce TLR2 activation may be a mechanism to enhance the invasive features of pathogenic Neisseriae strains.
Keywords: PorB, intracellular bacteria, airway epithelial cells
1. Introduction
Neisseria meningitidis (NM) is a Gram-negative organism that colonizes the human nasopharyngeal epithelium. Colonization can be asymptomatic in a large number of individuals [1] and these organisms are generally referred to as carriage strains, but can also proceed to cause life-threatening infections with high morbidity and mortality in some patients or mild and banal infections in others. These organisms are referred to as invasive and, in some cases, hyper-invasive. Host cell invasion is followed by bacteria dissemination into the bloodstream and penetration of the blood–brain barrier, the causes of sepsis and meningitis, respectively.
NM express virulence factors, i.e. capsule, pili, lipo-oligosaccharide (LOS), major and minor adhesins, that promote bacterial invasion of epithelial cells by interacting with cognate host cell receptors [2, 3]. A common feature of most virulence factors is their antigenic variability and fluctuating expression levels among strains and during the bacteria life cycle (phase variability). The role of many virulence factors has been clearly defined, but invasion mechanisms independent of these have also been reported, as well as variability between bacterial clones and strains, and in vitro conditions and assays [4].
Porins are antigenically variable pan-Neisserial outer membrane proteins [5] with a trimeric structure, composed of monomers with a β-barrel core and 8 surface-exposed loop regions with high sequence variability among strains [6]. NM expresses two porins, PorA and PorB (the latter with two molecular mass variants, PorB2 and PorB3), while N. gonorrhoeae (GC) and the commensal N. lactamica (NL) only express PorB. The structure of PorB has been characterized in greater detail than that of PorA [7-10].
Porins are involved in bacterial pathogenicity. NM and GC porins promote epithelial cell invasion [11-16] while NL PorB reduces it as shown in a GC mutant strain expressing NL PorB in place of GC PorB [17]. The sequence variability of PorB has been linked to the pathogenicity of hyper-invasive and invasive meningococcal strains [18, 19] and to some of its host cell-associated functions (serum resistance, host cell survival, immune stimulation [20]). Critical residues in the surface-exposed loops of PorB influence organisms’ invasion of epithelial cells and the direct interaction of PorB with host cell receptors associated with bacterial adhesion/invasion (i.e. the laminin receptor LamR [21], the gp96 and Scavenger Receptor SREC [14]), with complement components [22] and with members of the Toll-like receptor family, specifically TLR2 and TLR1 [23]. Residues that likely mediate PorB/TLR2 interaction and subsequent host cell activation have been identified in the surface-exposed regions of loops 5 and 7 of PorB [24].
In this work, we examined the influence of PorB on Neisseriae internalization by epithelial cells and the contribution of PorB-induced TLR2 signaling to this process. We suggest that expression of PorB sequence variants by different Neisseriae strains may represent a mechanism to strengthen the virulence of certain NM organisms by rendering host cells more susceptible to bacterial internalization via stimulation of TLR2.
2. Materials and Methods
2.1 Bacterial cultures
NL strain Y92-1009 (ND:P1.ND,ND:F-ND:ST-3493, ST-613), NM serogroup B strain H44/76 (B:15;P1.7,16; L3,7,9, ST-32) Δ1Δ4 variant (lacking expression of PorA and Rmp), and the NM mutant strain expressing PorB from NL (NM-[Nlac PorB]) [24] were cultured from frozen stocks on GC agar plates containing 1% Isovitalex at 37°C in a 5% CO 2 atmosphere in candle jars. The next day, colonies were resuspended in GC liquid medium containing 1% Isovitalex and grown for approx. 2-3h to exponential phase, measured spectrophotometrically by optical density at OD660. The O.D. of the cultures was adjusted to 0.2 and used as standard condition. Bacterial suspensions were appropriately diluted prior to co-incubation with BEAS-2B and HEK cells at a multiplicity of infection (MOI) of approx. 10 and 100 bacteria/cell, confirmed by viable count of the inoculum. No differences in the growth of these strains were reported.
2.2 Cell cultures and stimulation
The human bronchial epithelial cell line, BEAS-2B cells (ATTC CRL-9609) was grown at 37°C/5% CO 2 in DMEM F-12 supplemented with 5% FBS, 2 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin in flasks coated with 0.01 mg/ml BSA, 0.03 mg/ml bovine collagen type I and 0.01 mg/ml fibronectin to favor adherence to the plastic. HEK cells stably over-expressing TLR2 and TLR4 (TLR2 HEK cells and TLR4 HEK cells, respectively) or an empty vector (pcDNA HEK cells) (24) were grown in DMEM with 5% FBS, 2mM L-glutamine and 10 g/ml ciprofloxacin. For stimulations, cells were plated at 104 cells/well in 24-well plates in antibiotic-free medium. BEAS-2B cells were always plated in FBS-free medium, unless otherwise specified. Stimulations included purified recombinant PorBWT and PorBDDE/AKR (10 μg/ml) [24], Pam3CSK4 (EMC Microcollections, Tubingen, Germany) (200 ng/ml) and phenol-purified E. coli LPS (Sigma) (200 ng/ml) overnight. Cytochalasin D (CytD) (Calbiochem) (5 μg/ml) and the inhibitors SB03580, U0126, SP600125 and parthenolide (PA) (all at 25 μM) were added for 1h using medium containing equal volumes of DMSO (diluent control) as described in the Results.
2.3 Bacterial adherence and internalization assays
To assess adherence, cells were incubated with bacterial suspension at MOIs of 10 and 100 at 37°C in 5% CO 2 in candle jars for 2h, 4h and 24h, washed with PBS to remove free and non-adherent bacteria and lysed by addition of 200 μl of a 1% sterile saponin/PBS solution for 10 minutes at room temperature followed by vigorous pipetting. To assess intracellular bacteria, cells were washed to remove non-adherent bacteria followed by addition of medium containing gentamicin (Sigma) (100 μg/ml) for 1h for killing of extracellular and adherent organisms [25] and lysed to release intracellular bacteria. Appropriately diluted cell lysate aliquots were plated on GC agar plates and incubated overnight as described above for quantification of colony-forming units (CFU) per well by colony counting. Each experiment was performed in a minimum of two individual wells and repeated at least three times. When cells were stimulated prior to infection, intracellular organisms were reported as percent relative to control wells (represented by a dashed line in the corresponding Figures). No cytotoxic effects by saponin were observed on cells or bacteria.
2.4 Cytokines ELISA
Secretion of IL-8 was quantified in cell supernatants by ELISA using OptEIA ELISA kits (BD Biosciences) as specified by the manufacturer.
2.5 Statistical Analysis
Statistical analyses were calculated using GraphPad PRISM software. Statistical significance was determined by using the Mann Whitney test (among conditions) and the one sample t test (experimental groups vs. control groups set as 100%) ± SEM. p values were assigned and considered significant when lower than 0.05.
3. Results
3.1 Neisseriae attachment to BEAS-2B cells and bacteria internalization
The ability of NL and NM to adhere to the human airway epithelial cell line BEAS-2B cells in vitro was examined. Cell monolayers were incubated in antibiotic-free and FBS-free medium for 2h, 4h and 24h with NL and NM at MOI of 10. Free and non-adherent bacteria were removed by washing and the total cell-associated organisms (adherent and intracellular) were quantified by colony counting. A time-dependent increase of total cell-associated NM was reported, shown in Fig. 1A. No variation of total cell-associated NL was observed at 2h and 4h, and an increase was measured at 24h (Fig. 1B), although less pronounced than NM. The 2h time-point was also examined using bacteria at MOI of 100, and demonstrated a dose-dependent increase of cell-associated NM and NL (Fig. 1C, gray and white bars, respectively). No statistical difference was detected among the two strains and no differences in their growth curves [24]. Collectively, these results indicate that both NM and NL can associate with BEAS-2B cells.
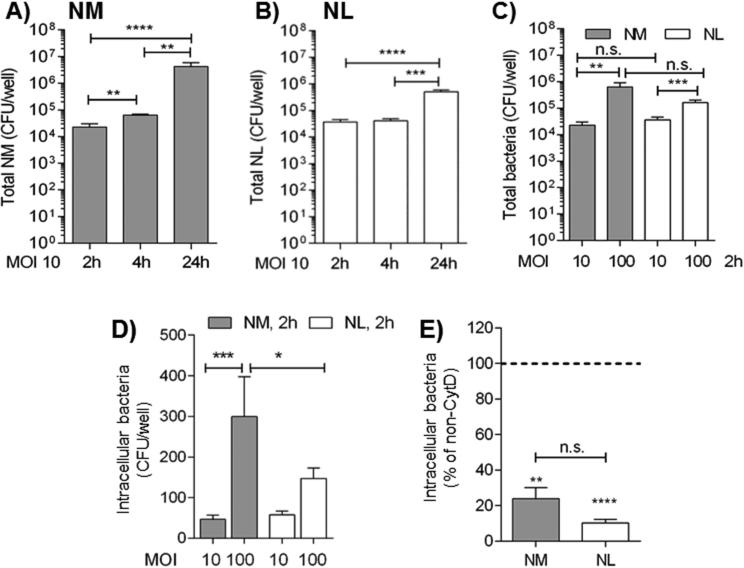
Fig. 1. Neisseriae attachment and internalization by BEAS-2B cells.
BEAS-2B cells incubated in antibiotic-free and FBS-free culture medium with A) NM MOI 10, 2h, 4h and 24h. Adherent bacteria are reported as CFU/well ± SEM. **p = 0.004 and 0.0021; ****p < 0.0001 by Mann Whitney test; B) NL MOI 10, 2h, 4h and 24h. ***p = 0.0004 and ****p < 0.0001 as above; C) NM (gray bars) and NL (white bars), MOIs of 10 and 100, 2h. **p = 0.0028 and ***p = 0.0007 as above. D) Intracellular NM (gray bars) and NL (white bars), MOIs of 10 and 100, 2h by gentamicin assay (100 μg/ml, 1h) reported as CFU/well ± SEM. ***p = 0.0004 and *p = 0.043 as above. E) Intracellular NM (gray bar) and NL (white bar), MOI 100, 2h, following cytochalasin D (CytD) treatment (5 μg/ml, 1h). Intracellular bacteria are reported as percent relative to non CytD-treated cells (dashed line) ± SEM. ***p = 0.0007 and ****p < 0.0001 by one sample t test; p = n.s. by Mann Whitney test.
Next, intracellular Neisseriae were examined using the gentamicin assay, a well-established method to evaluate intracellular organisms [25]. Cells were incubated with NM and NL at MOIs of 10 and 100 for 2h, free and non-adherent organisms were removed by washing and medium containing gentamicin (100 μg/ml) was added for 1h for killing of extracellular attached bacteria. Cells were lysed for releasing intracellular organisms, and bacteria were quantified by colony counting. A dose-dependent statistically significant increase of intracellular NM was measured (Fig. 1D, gray bars) and a more modest and non-statistically significant increase of intracellular NL (Fig. 1D, white bars). Comparison of intracellular NM and NL at MOI 10 after 2h was not statistically significant while intracellular NM at MOI 100 were significantly more than NL at the same MOI (Fig. 1D).
Actin-dependent mechanisms contribute to internalization of numerous bacteria by host cells, and inhibition of actin polymerization by cytochalasin D is known to reduce or block invasion of NM and NL [25-27]. BEAS-2B cells were treated with CytD (5 μg/ml) for 1h prior to incubation with NM or NL at MOI of 100 for 2h and intracellular bacteria were quantified as above. In Fig. 1E, a significantly lower number of intracellular bacteria was measured than in cells not treated with CytD (Fig. 1E, dashed line). No statistically significant difference was measured among intracellular NM and NL recovered in the presence of CytD, and treatment did not affect cell survival, determined by light microscopy evaluation of cell morphology and adherence to the plastic (not shown).
3.2 PorB contribution to Neisseriae internalization by BEAS-2B cells
PorB is a TLR2 ligand that induces TLR2/TLR1 and MyD88-dependent epithelial and immune cell responses [23]. Variability in cell responses to purified PorB from NM or NL has been shown, likely due to differences in the amino acid sequence and structure features of these porins that ultimately affect TLR2 activation [10, 24]. Similarly, host cell responses to a mutant NM strain in which the original PorB was replaced with NL PorB (NM-[Nlac PorB]) are also influenced by the PorB exchange.
To examine whether PorB influences bacteria/host cell interactions, BEAS-2B cells were incubated with NM-[Nlac PorB] (MOI 100, 2h) and total and intracellular bacteria were quantified as described above. No difference was observed in NM-[Nlac PorB] or NM association with BEAS-2B cells (MOI 10 for 24h) (not shown). However, an approx. 30-40% decrease of intracellular NM-[Nlac PorB] was measured relative to NM (Fig. 2A, gray dotted bar and dashed line, respectively) suggesting that PorB replacement interferes with uptake of this otherwise invasive organism, similar to previous studies using a mutant GC strain expressing NL PorB [17]. An approximately 25% higher number of intracellular NM-[Nlac PorB] was observed as compared to NL (Fig. 2A, white dotted bar and dashed line, respectively), indicating that the type of PorB that is expressed contributes, in part, to bacteria internalization regardless of the presence or absence of other invasion-related molecules.
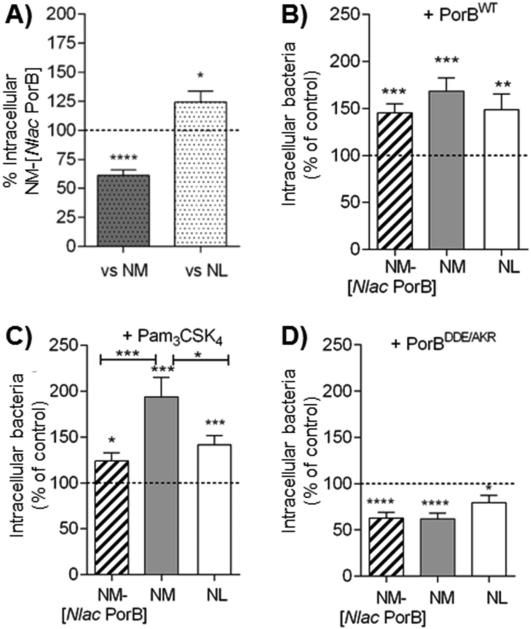
Fig. 2. PorB and TLR2-dependent Neisseriae internalization in BEAS-2B cells.
A) BEAS-2B cells were incubated in antibiotic-free and FBS-free culture medium with NM-[Nlac PorB] MOI 100, 2h. Intracellular bacteria are expressed as percent of recovered intracellular NM (gray dotted bar and dashed line, respectively), or NL (white dotted bar and dashed line, respectively) ± SEM. *p = 0.02 and ****p < 0.0001 by one sample t test. B) Intracellular NM-[Nlac PorB] (striped bar), NM (gray bar) and NL (white bar) (MOI 100, 2h) recovered following cell stimulation with purified recombinant PorB (PorBWT) (10 μg/ml, 24h) and expressed as percent of non PorBWT-stimulated cells (dashed line) ± SEM.**p = 0.007; ***p = 0.0002 and 0.0001 by one sample t test. Among strains, p = n. s. by Mann Whitney test. C) Intracellular bacteria as above following stimulation with Pam3CSK4 (200 ng/ml, 24h). *p = 0.015; ***p = 0.0008 and 0.0003 as above. Among strains, *p = 0.016 and ***p = 0.0009 as above. D) Intracellular bacteria as above following stimulation with PorBDDE/AKR (10 μg/ml, 24h). **p = 0.0017 and ****p < 0.0001 as above. Among strains, p = n. s. as above.
To further evaluate the contribution of PorB to bacteria internalization, cells were stimulated overnight with purified recombinant meningococcal PorB (PorBWT) (10 μg/ml) followed by incubation with bacteria. As shown in Fig. 2B, PorBWT significantly increased the number of intracellular bacteria compared to non-PorBWT stimulated cells (Fig. 2B, dashed line). No statistically significant differences were observed among the three strains. These results suggested a potential mechanism by which host cell susceptibility to Neisseriae internalization may be enhanced by PorBWT-mediated cell stimulation.
3.3 TLR2 stimulation favors Neisseriae internalization by BEAS-2B cells
To examine whether the increased number of intracellular bacteria induced by PorBWT is due to TLR2 activation, the synthetic TLR2 ligand Pam3CSK4 was used. As shown in Fig. 2C, cell stimulation with Pam3CSK4 (200 ng/ml) also increased the number of intracellular bacteria relative to non-Pam3CSK4 stimulated cells (Fig. 2C, dashed line), particularly for NM (Fig. 2C). Next, cells were stimulated with the TLR2-inactive PorBWT loop mutant (PorBDDE/AKR) (10 μg/ml), which failed to enhance bacteria internalization relative to non-PorBDDE/AKR treated cells (Fig. 2D), and also appeared to interfere with this process, regardless of PorB expression. Overall, these results suggest that TLR2 stimulation may enhance the susceptibility of BEAS-2B cells to Neisseriae internalization. Additional experiments aimed at blocking or silencing TLR2 in these cells will clarify the direct contribution of TLR2.
The responsiveness of BEAS-2B cells to TLR2 stimulation by these ligands and whole Neisseriae has been previously established in vitro [24, 28]. Low levels of IL-8 were reported in response to NM and NL (MOI 10) at early time-points, but measurable IL-8 was assessed using bacteria at MOI 100 for 2h (Supplemental Fig. 1A). No statistically significant difference was measured among the three strains, but a trend of higher IL-8 induction by NM than NM-[Nlac PorB] and NL was observed, consistent with the previous findings.
3.4 Neisseriae internalization by HEK cells
As an alternative model to further examine the contribution of TLR2 to Neisseriae internalization, HEK cells over-expressing TLR2 (TLR2 HEK cells) were used and pcDNA HEK cells (containing an empty vector) as TLR2-negative control. Cells were incubated in antibiotic-free medium with bacteria at MOI 100 for 2h for quantification of bacteria adherence and internalization. No statistically significant difference in NM and NL association with pcDNA HEK cells and TLR2 HEK cells was observed (not shown). As shown in Fig. 3A, a generally higher percent of intracellular organisms was reported in TLR2 HEK cells than in pcDNA HEK cells, particularly for NM (Fig. 3A, gray bar), an approx. 3-fold increase for NM-[Nlac PorB] and a 2-fold increase for NL (Fig. 3A, striped and white bars, respectively). When the number of intracellular NM-[Nlac PorB] was compared to that of NM, approx. 60% less intracellular NM-[Nlac PorB] were detected (not shown), a trend similar to BEAS-2B cells.
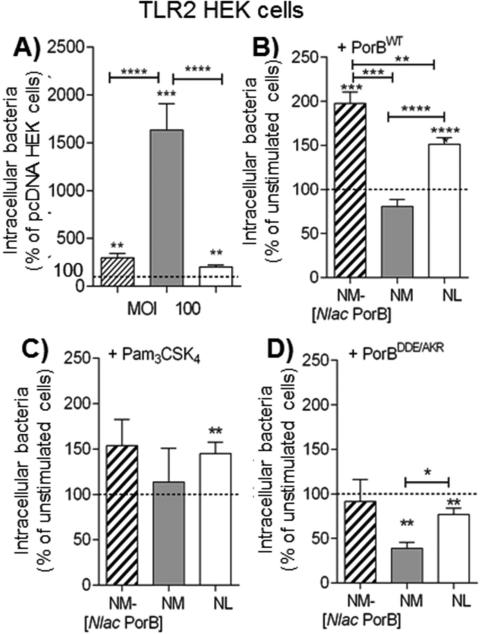
Fig. 3. Neisseriae internalization by HEK cells.
A) pcDNA and TLR2 HEK cells incubated in antibiotic-free culture medium with NM-[Nlac PorB] (striped bar), NM (gray bar) and NL (white bar), MOI 100, 2h. Intracellular bacteria are reported as percent of intracellular organisms from TLR2 HEK cells relative to pcDNA HEK cells (dashed line) ± SEM. **p = 0.0062 and ***p = 0.0001 and 0.0002 by one sample t test. Among strains, ****p < 0.0001 by Mann Whitney test. B) Intracellular NM-[Nlac PorB] (striped bar), NM (gray bar) and NL (white bar), MOI 100, 2h in TLR2 HEK cells following cell stimulation with purified recombinant PorB (PorBWT) (10 μg/ml, 24h) expressed as percent of non PorBWT-stimulated cells (dashed line) ± SEM. *** p = 0.0007 and ****p < 0.0001 by one sample t test as above. Among strains, **p = 0.0061, ***p = 0.0001 and ****p< 0.0001 by Mann Whitney test as above. C) Intracellular bacteria as above following stimulation with Pam3CSK4 (200 ng/ml, 24h). **p = 0.0063 by one sample t test as above. Among strains, p = n. s. as above. D) Intracellular bacteria as above following stimulation with PorBDDE/AKR (10 μg/ml, 24h). **p = 0.0028 and 0.0098 as above. Among strains, *p = 0.024 as above.
Next, the effect of exogenous TLR2 stimulation was examined in TLR2 HEK cells. PorBWT stimulation led to significantly higher intracellular NM-[Nlac PorB] and NL relative to non-PorBWT stimulated cells (Fig. 3B, striped and white bars, and dashed line, respectively). However, unlike in BEAS-2B cells, the number of intracellular NM was not further increased (Fig. 3B, gray bar). Similar results were obtained for Pam3CSK4 stimulation (Fig. 3C). Lastly, PorBDDE/AKR stimulation did not increase intracellular bacteria (Fig. 3D) and generally recapitulated the lower bacteria numbers observed in BEAS-2B cells. Overall, these results support the hypothesis that TLR2 stimulation enhances Neisseriae internalization by epithelial cells. However, TLR2 stimulation with PorBWT or Pam3CSK4 followed by incubation with NM (which expresses a PorB that is also a potent TLR2 agonist) did not further increase the number of intracellular NM, possibly due to a TLR2-saturating effect. HEK cell responses to PorBWT, Pam3CSK4 and Neisseriae are also well-established [24, 28]. Similar to the results in BEAS-2B cells, incubation with Neisseriae at MOI of 10 for 2h induced very low IL-8, and this was slightly increased by bacteria at MOI of 100 for 2h (not shown). A higher IL-8 secretion was induced in response to bacteria at MOI of 10 for 24h and to stimulation with PorBWT, Pam3CSK4 and PorBDDE/AKR (Supplemental Figure 1B).
3.5 Intracellular signaling pathways in Neisseriae internalization
TLR2 stimulation leads to activation of intracellular signaling pathways including MAPKs and NF-κB. To verify whether these pathways contribute to Neisseriae internalization, the following chemical inhibitors were used in BEAS-2B cells: SB03580 (p38 inhibitor), U0126 (ERK1/2 inhibitor), SP600125 (JNK inhibitor) and parthenolide (PA) (NF-κB inhibitor). Cells were incubated in FBS-free and antibiotic-free medium with each inhibitor (25 μg/ml) for 1h prior to addition of bacteria MOI 100 for 2h, and intracellular bacteria were reported as percent relative to cells incubated in the absence of inhibitors or with DMSO as inhibitor diluent control (Figs. 4A-D, dashed line).
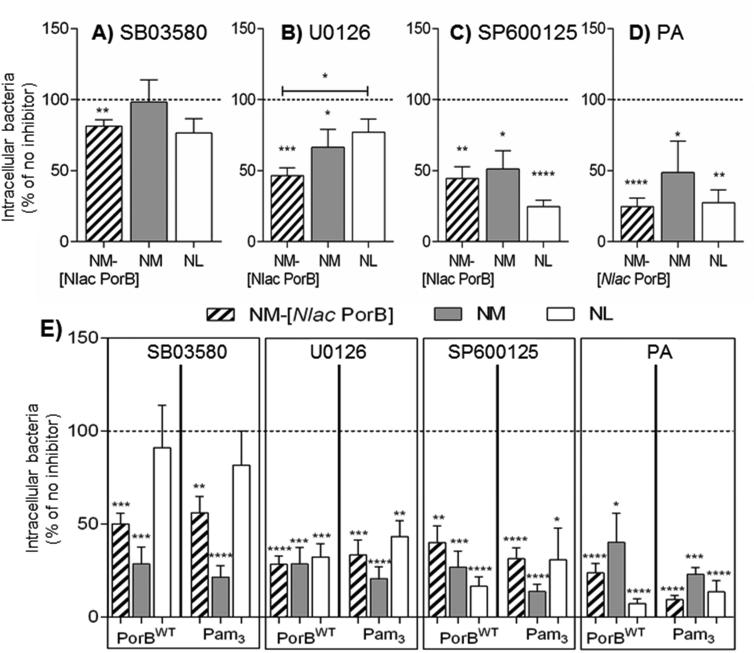
Fig. 4. Intracellular signaling pathways in Neisseriae internalization.
BEAS-2B cells incubated in antibiotic-free and FBS-free culture medium with A) SB03580 (black bars), B) U0126 (gray bars), C) SP600125 (white bars) and D) parthenolide (PA) (striped bars) (25 μg/ml) for 1h followed by addition of NM-Nlac PorB] (striped bars), NM (gray bars) and NL (white bars), MOI 100, 2h. Intracellular bacteria are expressed as percent relative to control cells in the absence of inhibitors (medium/DMSO as inhibitors diluent control) (dashed line) ± SEM. SB03580: **p = 0.0084 by one sample t test; U0126: *p = 0.045 and ***p = 0.0002 as above, and *p = 0.026 by Mann Whitney test; SP600125: *p = 0.012, **p = 0.0011 and ****p < 0.0001 as above; PA: **p = 0.0013 and ****p < 0.0001 as above. E) BEAS-2B cells incubated with inhibitors as above prior to stimulation with PorBWT (10 μg/ml, 24h) or Pam3CSK4 (200 ng/ml, 24h) and addition of NM-[Nlac PorB] (striped bars), NM (gray bars) and NL (white bars) MOI 100, 2h. Intracellular bacteria are expressed as percent relative to cells stimulated in the absence of inhibitors (dashed line) ± SEM. SB03580: ***p = 0.0003 and 0.0005 (PorBWT); **p = 0.0042 and ****p < 0.0001 (Pam3CSK4) by one sample t test. U0126: ***p = 0.0005 and 0.0007, and ****p< 0.0001 (PorBWT); **p = 0.0027, ***p = 0.0004 and ****p < 0.0001 (Pam3CSK4) as above. SP600125: **p = 0.0011, ***p = 0.0004 and ****p < 0.0001 (PorBWT); *p = 0.015 and ****p < 0.0001 (Pam3CSK4) as above. PA: *p = 0.012 and ****p < 0.0001 (PorBWT); ***p = 0.0001 and ****p < 0.0001 (Pam3CSK4) as above.
Overall, inhibition of p38 signaling by SB03580 had the least inhibitory effect on intracellular bacteria compared to inhibition of ERK1/2 and JNK. In fact, NM was not affected by SB03580 (Fig. 4A, gray bar) and only a small decrease of intracellular NM-[Nlac PorB] and NL was observed (Fig. 4A, striped and white bars, respectively). The effect of ERK1/2 and JNK inhibition was more substantial on both NM-[Nlac PorB] (Fig. 4B and 4C, striped bars) and NM (Fig. 4B and 4C, gray bars), but only inhibition of JNK had a major effect on NL (Fig. 4B and 4C, white bars). These results suggest a potential redundancy in the role of MAKPs in the cellular processes leading to Neisseriae internalization, and that inhibition of the JNK pathway has a larger effect than that of ERK1/2, while p38 inhibition has minor consequences. A consistent reduction of 50% or more in intracellular bacteria was observed by inhibition of NF-κB by PA for each organism (Fig. 4D), suggesting a relevant role for this pathway. Since all these pathways are induced by TLR2 stimulation with PorBWT and Pam3CSK4, whether preventing their induction affects bacterial internalization was also examined. Cells were treated with the inhibitors prior to stimulation with PorBWT and Pam3CSK4 and incubated with bacteria, MOI 100, 2h. Intracellular organisms from each condition were quantified and normalized to cells stimulated in the absence of inhibitors. Blockade of PorBWT-and Pam3CSK4-mediated activation of p38 strongly reduced intracellular NM and NM-[Nlac PorB] (approx. 50%) (Fig. 4E, SB03580, gray and striped bars, respectively), but did not significantly affect NL (Fig. 4E, SB03580, white bar), suggesting that blocking activation of p38 may influence both porin-dependent and porin-independent mechanisms for bacterial internalization. Inhibition of PorBWT-and Pam3CSK4-mediated ERK1/2, JNK and NF-κB signaling led to a > 50% reduction for all organisms (Fig. 4E, U0126, SP600125 and PA, respectively), confirming that multiple intracellular signaling pathways downstream of TLR2 stimulation can influence cell sensitivity to Neisseriae internalization, with a possible redundancy among MAPKs pathways.
3.6 TLR4 stimulation does not enhance intracellular Neisseriae
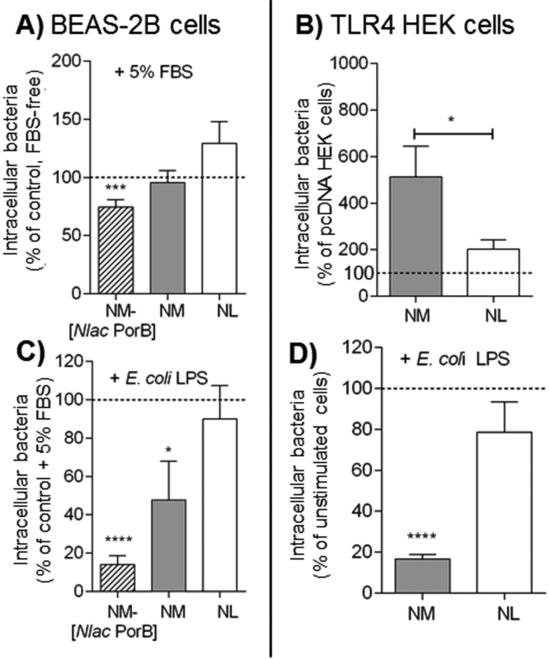
The role of TLR4 on internalization of Neisseriae by epithelial cells was also examined. In BEAS-2B cells, this receptor is scarcely expressed on the cell surface and has an abundant intracellular localization, but upon cell stimulation, it is re-directed to the cell surface. However, these cells lack expression of MD-2 and are intrinsically hypo-responsive to TLR4 signaling, as shown by the low levels of IL-8 measured in response to E. coli LPS stimulation (200 ng/ml, 24h) (Supplemental Fig. 1C, white bars). By providing exogenous MD-2 using FBS-supplemented medium (TLR4-permissive condition), LPS stimulation leads to higher levels of IL-8 (Supplemental Fig. 1C, black bars) [24, 28]. However, in TLR4-permissive conditions, stimulation with bacteria at MOI 100 for 2h only slightly increased IL-8 in response to NM-[Nlac PorB] and NL (Supplemental Fig. 1D, striped and white bars) (**p = 0.043 by Mann Whitney test, not shown) than in FBS-free medium, but not NM (Supplemental Fig. 1D, gray bar), suggesting that at this early time point, TLR4 signaling may not be a major driver of IL-8 induction by Neisseriae in BEAS-2B cells.
Intracellular bacteria were evaluated in BEAS-2B cells in TLR4-permissive conditions relative to FBS-free conditions (Fig. 5A, dashed line). A non-statistically significant small increase was observed for NL (Fig. 5A, white bar), no effect on NM and even a reduction of NM-[Nlac PorB] were observed (Fig. 5A, gray and striped bars, respectively), indicating that TLR4-permissive conditions did not generally favor bacteria internalization. It is possible that, due to the nature of TLR4 expression and signaling in these cells, its potential role in Neisseriae internalization may be difficult to detect. Thus, intracellular NM and NL were evaluated in HEK cells over-expressing TLR4 (since NM and NM-[Nlac PorB] have the same lipid composition and no effect of PorB is expected in TLR4 HEK cells). As shown in Fig. 5B, an increase in intracellular NL was measured in TLR4 HEK cells compared to pcDNA HEK cells, similar to the previous results in TLR2 HEK cells (Figs. 5B and 3A, white bars); however, approx. one third less intracellular NM were recovered from TLR4 HEK cells than in TLR2 HEK cells, (Figs. 5B and 3A, gray bars), suggesting a less prominent role for TLR4 than TLR2 in this process.
Fig 5. Intracellular Neisseriae in TLR4-permissive conditions.
A) BEAS-2B cells incubated in antibiotic-free medium supplemented with 5% FBS with NM-[Nlac PorB] (striped bars), NM (gray bars) and NL (white bars), MOI 100, 2h. Intracellular bacteria are expressed as percent relative to cells incubated with bacteria in FBS-free medium (dashed line) ± SEM. ***p = 0.0009 by one sample t test. B) Intracellular NM (gray bar) and NL (white bar), MOI 100, 2h in TLR4 HEK cells, expressed as percent relative to pcDNA HEK cells (dashed line) ± SEM. p = n.s. by one sample t test (not shown), and *p = 0.039 by Mann Whitney test. C) Intracellular bacteria NM-[Nlac PorB] (striped bar), NM (gray bar) and NL (white bar) (MOI 100, 2h) in BEAS-2B cells in medium containing 5% FBS following stimulation with E. coli LPS (200 ng/ml, 24h) is expressed as percent of non LPS-stimulated cells (dashed line) ± SEM. *p = 0.0 and ****p < 0.0001 by one sample t test. D) Intracellular NM (gray bar) and NL (white bar), MOI 100, 2h, in TLR4 HEK cells following stimulation with E. coli LPS as above. ****p < 0.0001 by one sample t test as above.
Since exogenous TLR2 stimulation increased the number of intracellular bacteria, the effect of exogenous TLR4 stimulation by E. coli LPS (200 ng/ml) was also examined. In BEAS-2B cells in TLR4-permissive conditions, the number of intracellular NM-[Nlac PorB] and NM were significantly reduced by cell stimulation with LPS (Fig. 5C, striped and gray bars, respectively) while NL was not quite affected (Fig. 5C, white bar). Similar results were observed in TLR4 HEK cells (Fig. 5D, gray and white bars), suggesting that TLR4 stimulation may not represent a sensitizing event, but rather inhibitory for Neisseriae internalization by epithelial cells. Whether inhibition of LPS-induced intracellular signaling enhanced or reversed this effect was also examined using the MAPKs and NF-κB inhibitors. In TLR4-permissive BEAS-2B cells, NF-κB inhibition caused a > 50% reduction of intracellular bacteria (not shown), supporting a central role for this pathway. MAPKs inhibition had a variable effect: ERK1/2, JNK and also p38 inhibition substantially reduced intracellular NM and NM-[Nlac PorB] (>50%, not shown), regardless of porin expression, suggesting that blocking MAPKs in TLR4-permissive conditions affects both porin-dependent and porin-independent mechanisms. In contrast, internalization of NL in TLR4-permissive conditions was not susceptible to ERK1/2 and JNK inhibition and was even increased by p38 inhibition (not shown). Thus, while TLR2 and TLR4 signaling converge on NF-κB for influencing cell susceptibility to Neisseriae uptake, MAPKs likely play different roles in this process depending on different TLRs and their downstream effectors. Nevertheless, since TLR2 signaling in BEAS-2B cells in TLR4-permissive conditions is not precluded, the contribution of each individual pathway may not be easy to tease out at this time.
4. Discussion
The duality of human infections by Neisseriae carriage strains and invasive/hyper-invasive strains is remarkable and, while the mechanisms of such different outcomes are not completely clear, it is well accepted that both the microbe and the host cell play important roles in Neisseriae infections. At the site of colonization, the nasopharynx, strain-specific and environmentally-regulated phase- and antigenically-variable virulence factors interact with host cell cognate receptors [3], which are also variable in expression, cellular localization (i.e. intracellular vs. extracellular, basal vs. apical) and tissue-dependence (i.e. in epithelial and endothelial cells or immune cells). Cells with high surface receptor levels are likely more vulnerable than cells with absent, insufficient or restricted interactor molecules. Therefore, meningococcal pathogenicity may reflect the sum of expression and cooperative interaction of both virulence factors and their host cell receptors. In this study, to avoid bias due to Opa-mediated cell invasion processes and to the scarce information on their cellular receptors in BEAS-2B cells (CEACAM1 gene up-regulation is reported upon cristobalite silica and asbestos exposure [29]), no specific Opa phenotype was selected for NM and NL. However, BEAS-2B cells can sustain bacteria adhesion and infection, for example, by Moraxella [30] or E. coli mutants expressing meningococcal outer membrane adhesins (App or MspA [31]). In HEK cells, CEACAMs are often experimentally over-expressed [32].
Direct and indirect mechanisms that contribute to cellular internalization of Neisseriae do not solely rely on conventional virulence factors/host cell receptors, but also include those mediated by Neisserial porins [11-16]. Porins also favor bacterial survival following host infection by inhibiting phagocyte functions [33, 34] and modulating host cell death [35, 36]. It has been established that criticalr esidues in the surface-exposed loop regions of PorB influence organisms’ internalization by host cells [9, 13, 17] and may be involved in the hyper-invasive features of some NM strains [18]. Our group has recently reported a prevalence of negative surface charges is reported in loops 1, 4, 6 and 7 of PorB from invasive meningococcal serogroup B clinical isolates, while overall positive charges are reported in the corresponding PorB loops of invasive meningococcal serogroup C clinical isolates [19]. The sequence variability of PorB also influences interactions with TLR2 and the subsequent intracellular signaling pathways [23, 24, 28]. In contrast, NL porin reduces the number of intracellular organisms [17]. It is possible that PorB sequence variability also affects internalization of Neisseriae by airway epithelial cells, where initial establishment of host colonization occurs.
We have shown that Neisseriae attach to and are internalized by the human airway epithelial cell line BEAS-2B cells in a dose-dependent and time-dependent fashion, with NM being more efficient than NL. Although bacterial internalization is not exclusively actin-dependent, actin cytoskeleton rearrangements strongly contribute to Neisseriae internalization and a role for porins in these events has been shown. Our results confirm that the number of intracellular NM and NL is reduced by inhibiting actin polymerization with cytochalasin D in BEAS-2B cells. In these cells, internalization of a NM strain expressing NL PorB (NM-[Nlac PorB]) was lower than that of NM but it remained higher than that of NL, suggesting that the type of PorB expressed by the organisms contributes to this process, as previously shown for NL PorB expression in GC. It is possible that NM PorB expression into NL may enhance internalization, but introduction of a bacterial pathogen product into a commensal organism may not be a safe experimental choice. Therefore, as an alternative approach in vitro, purified PorB from NM was used to stimulate cells prior to incubation with bacteria. This not only restored the number of intracellular NM-[Nlac PorB] to levels comparable to NM but also increased that of NL, suggesting an enhancement of cell susceptibility to Neisseriae uptake.
Since PorB is a TLR2 ligand, this effect may be driven by TLR2 stimulation, and, in fact, a similar increase of intracellular bacteria is induced by the TLR2 agonist Pam3CSK4; in converse, the TLR2-inactive PorB mutant PorBDDE/AKR reduced intracellular bacteria, suggesting that TLR2 engagement and activation provide a positive signal for bacteria internalization. BEAS-2B cells express low, constitutive TLR2 surface levels (a potential mechanism to confer hypo-responsiveness to commensal bacteria). TLR2 is upregulated by pathogen-induced cell activation and pro-inflammatory cytokines, representing a potential cause of cell responses variability depending on the stimulus. While future experiments should be aimed at silencing TLR2 mRNA expression in these cells, HEK cells over-expressing TLR2 were used to validate our findings and to further examine the effect of TLR2 stimulation on internalization of Neisseriae. In TLR2 HEK cells, an increased susceptibility to NM-[Nlac PorB], NL and particularly NM internalization was reported than in HEK cells lacking TLR2. Similarly, TLR2 HEK cells stimulation by PorBWT and Pam3CSK4 recapitulated the increase of intracellular NL and NM-[Nlac PorB] - (both NL PorB-expressing organisms) shown in BEAS-2B cells, but did not affect intracellular NM. It is possible that simultaneous stimulation with potent TLR2 ligands and NM (which expresses a PorB that also induces robust TLR2 stimulation) leads to a TLR2 signaling saturation. In toto, these results indicate a role of PorB on host cell susceptibility to Neisseriae internalization and link TLR2 expression and stimulation to this process.
Previously, protein tyrosine kinases (PTKs), Src-mediated and NF-κB signaling have been shown to participate in Neisseriae internalization processes [26, 27, 37]. Since PorB from NM and NL induce TLR2 cell activation via similar, but not identical signaling mechanisms, we examined individual pathways downstream of TLR2 using specific inhibitors of MAPKs (p38, ERK1/2 and JNK) and NF-κB pathways [24, 38]. While inhibition of NF-κB strongly reduced intracellular organisms, variability was observed for MAPKs: inhibition of JNK signaling generally led to lower intracellular bacteria, the effect of ERK1/2 inhibition was less considerable and that of p38 inhibition was rather dubious, consistent with previous observations [26] and with a potential function redundancy among these pathways. Blocking signaling downstream of TLR2 stimulation was similarly variable: inhibition of p38 strongly reduced intracellular NM, partially reduced intracellular NM-[Nlac PorB] and did not affect intracellular NL, regardless of the TLR2 ligand used. This could be attributed to a competitive effect of NL PorB, which may counteract TLR2 stimulation and ultimately result in dampened cell activation. This may also explain the partial inhibition of internalized NM-[Nlac PorB], an organism that expresses the same virulence factors as NM (except for PorB). Blocking TLR2-inducible ERK1/2, JNK and NF-κB activation consistently reduced the number of intracellular organisms, and confirmed a role for these pathways in Neisseriae internalization by epithelial cells.
TLR2 is not the only cellular receptor responding to Neisseriae and the role of TLR4 is well established. BEAS-2B cells normally express TLR4 within the Golgi compartment and lack expression of MD-2, resulting in TLR4 hypo-responsiveness; however, when TLR4 is translocated to the cell surface and exogenous MD-2 is provided, signaling is permitted. However, “TLR4-permissive” BEAS-2B cells did not show overall increased intracellular bacteria. When TLR4 HEK cells were used to validate these results, comparable intracellular NL were reported than in TLR2 HEK cells, but intracellular NM were 3-fold lower, likely because the sensitizing effect of PorB was lacking. The central role for NF-κB in Neisseriae internalization was confirmed in TLR4-permissive conditions, as well as the variability of the MAPKs pathways contribution. Interestingly, exogenous TLR4 stimulation by LPS did not enhance intracellular bacteria in either type of cell, and had a rather (likely PorB-independent) inhibitory effect on NM-[Nlac PorB] and NM. Thus, unlike TLR2, TLR4 stimulation does not appear a sensitizing event for Neisseriae internalization.
The reason for such differences in the recovered intracellular Neisseriae in TLR2 and TLR4 stimulation remains unclear. It is possible that, while both receptors induce similar patterns of intracellular signaling, the components of each cascade (i.e. NF-κB subunits and MAPKs phosphorylation patterns) may differ, or that multiple signaling complexes may be formed, and even that temporal differences in signaling may ultimately influence the epithelial cells vulnerability to bacteria internalization. It is also accepted that the local inflammatory cytokine milieu influences epithelial tissues susceptibility to bacterial invasion [39]. It may be possible that Neisseriae strains expressing PorB molecules with certain sequences, structure and TLR2-dependent features predispose airway epithelial cells to infection by inducing a particular inflammatory environment. In contrast, PorB variants from carriage or commensal strains may not equally sensitize target cells. To this end, severe and fatal meningococcal disease correlate with high levels of IL-6, IL-8, TNF-α and IFN-γ production [40-42], cytokines that are all are highly induced by NM and its PorB and not by NL and its PorB [24, 28]. Expression of bacterial recognition receptors on airway epithelial cells may be also driven by TLR2-dependent molecular mechanisms and further favor cell vulnerability to infection in a predetermined inflammatory environment [43]. For example, expression of CEACAMs is upregulated in airway epithelial cells by TLR3 signaling [44] and that of PAFr (platelet activating factor receptor, involved in airway epithelial cell interaction with Neisseriae and other pathogens [45, 46]) is linked to TLR2 [47]. In addition, TLR2 surface expression itself is up-regulated by NM and its PorB but not by NL and its PorB, providing an additional feedback loop for enhancing cell activation and possibly internalization of invasive strains, but not carriage and commensal strains that express other PorB variants. Lastly, analysis of TLR polymorphisms incidence in humans indicates a potential correlation between TLR signaling and meningococcal pathology, by increased risk of infection and susceptibility to NM with TLR2, TLR4 and TLR9 polymorphisms [48-51].
In conclusion, the cooperative interaction of bacterial virulence factors and host cell components, the specific cell signaling cascades and the inflammatory responses induced in host cells are all events that contribute to Neisseriae pathogenicity. We have shown that TLR2 stimulation enhances cellular internalization of Neisseriae and propose that expression of PorB molecules with distinct loop residues, structure and functional features that promote TLR2 signaling may represent a mechanism to strengthen the virulence of pathogenic NM strains. Such a mechanism may be particularly relevant for the disease caused by serogroup B strains that may express PorBs that are potent TLR2 activators.
Supplementary Material
Acknowledgments
Funding Information
This work was supported by the NIH/NIAID grant R01 AI40944-18
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflict of interests
Reference List
- 1.Cartwright KA, Stuart JM, Jones DM, Noah ND. The Stonehouse survey: nasopharyngeal carriage of meningococci and Neisseria lactamica. Epidemiol Infect. 1987;99:591–601. doi: 10.1017/s0950268800066449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carbonnelle E, Hill DJ, Morand P, Griffiths NJ, Bourdoulous S, Murillo I, et al. Meningococcal interactions with the host. Vaccine. 2009;27(Suppl 2):B78–B89. doi: 10.1016/j.vaccine.2009.04.069. [DOI] [PubMed] [Google Scholar]
- 3.Pizza M, Rappuoli R. Neisseria meningitidis: pathogenesis and immunity. Curr Opin Microbiol. 2015;23:68–72. doi: 10.1016/j.mib.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Townsend R, Goodwin L, Stevanin TM, Silcocks PB, Parker A, Maiden MC, et al. Invasion by Neisseria meningitidis varies widely between clones and among nasopharyngeal mucosae derived from adult human hosts. Microbiology. 2002;148:1467–74. doi: 10.1099/00221287-148-5-1467. [DOI] [PubMed] [Google Scholar]
- 5.Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev. 2003;67:593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derrick JP, Urwin R, Suker J, Feavers IM, Maiden MC. Structural and evolutionary inference from molecular variation in neisseria porins. Infect Immun. 1999;67:2406–13. doi: 10.1128/iai.67.5.2406-2413.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanabe M, Nimigean CM, Iverson TM. Structural basis for solute transport, nucleotide regulation, and immunological recognition of Neisseria meningitidis PorB. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.0912115107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen A, Seifert HS. Structure-function studies of the Neisseria gonorrhoeae major outer membrane porin. Infect Immun. 2013;81:4383–91. doi: 10.1128/IAI.00367-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeth K, Kozjak-Pavlovic V, Faulstich M, Fraunholz M, Hurwitz R, Kepp O, et al. Structure and function of the PorB porin from disseminating Neisseria gonorrhoeae. Biochem J. 2013;449:631–42. doi: 10.1042/BJ20121025. [DOI] [PubMed] [Google Scholar]
- 10.Kattner C, Toussi DN, Zaucha J, Wetzler LM, Ruppel N, Zachariae U, et al. Crystallographic analysis of Neisseria meningitidis PorB extracellular loops potentially implicated in TLR2 recognition. J Struct Biol. 2014;185:440–7. doi: 10.1016/j.jsb.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giardina PC, Williams R, Lubaroff D, Apicella MA. Neisseria gonorrhoeae induces focal polymerization of actin in primary human urethral epithelium. Infect Immun. 1998;66:3416–9. doi: 10.1128/iai.66.7.3416-3419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorby GL, Ehrhardt AF, Apicella MA, Elkins C. Invasion of human fallopian tube epithelium by Escherichia coli expressing combinations of a gonococcal porin, opacity-associated protein, and chimeric lipooligosaccharide. J Infect Dis. 2001;184:460–72. doi: 10.1086/322784. [DOI] [PubMed] [Google Scholar]
- 13.Kuhlewein C, Rechner C, Meyer TF, Rudel T. Low-phosphate-dependent invasion resembles a general way for Neisseria gonorrhoeae to enter host cells. Infect Immun. 2006;74:4266–73. doi: 10.1128/IAI.00215-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rechner C, Kuhlewein C, Muller A, Schild H, Rudel T. Host glycoprotein Gp96 and scavenger receptor SREC interact with PorB of disseminating Neisseria gonorrhoeae in an epithelial invasion pathway. Cell Host Microbe. 2007;2:393–403. doi: 10.1016/j.chom.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 15.van Putten JP, Duensing TD, Carlson J. Gonococcal invasion of epithelial cells driven by P.IA, a bacterial ion channel with GTP binding properties. J Exp Med. 1998;188:941–52. doi: 10.1084/jem.188.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wen KK, Giardina PC, Blake MS, Edwards J, Apicella MA, Rubenstein PA. Interaction of the gonococcal porin P.IB with G- and F-actin. Biochemistry. 2000;39:8638–47. doi: 10.1021/bi000241j. [DOI] [PubMed] [Google Scholar]
- 17.Bauer FJ, Rudel T, Stein M, Meyer TF. Mutagenesis of the Neisseria gonorrhoeae porin reduces invasion in epithelial cells and enhances phagocyte responsiveness. Mol Microbiol. 1999;31:903–13. doi: 10.1046/j.1365-2958.1999.01230.x. [DOI] [PubMed] [Google Scholar]
- 18.Yang L, Zhang X, Peng J, Zhu Y, Dong J, Xu J, et al. Distribution of surface-protein variants of hyperinvasive meningococci in China. J Infect. 2009;58:358–67. doi: 10.1016/j.jinf.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 19.Stefanelli P, Neri A, Tanabe M, Fazio C, Massari P. Typing and surface charges of the variable loop regions of PorB from Neisseria meningitidis. IUBMB Life. 2016;68:488–95. doi: 10.1002/iub.1508. [DOI] [PubMed] [Google Scholar]
- 20.Wetzler LM. Innate immune function of the neisserial porins and the relationship to vaccine adjuvant activity. Future Microbiol. 2010;5:749–58. doi: 10.2217/fmb.10.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abouseada NM, Assafi MS, Mahdavi J, Oldfield NJ, Wheldon LM, Wooldridge KG, et al. Mapping the laminin receptor binding domains of Neisseria meningitidis PorA and Haemophilus influenzae OmpP2. PLoS ONE. 2012;7:e46233. doi: 10.1371/journal.pone.0046233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ram S, McQuillen DP, Gulati S, Elkins C, Pangburn MK, Rice PA. Binding of complement factor H to loop 5 of porin protein 1A: A molecular mechanism of serum resistance of nonsialylated neisseria gonorrhoeae. J Exp Med. 1998;188:671–80. doi: 10.1084/jem.188.4.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massari P, Visintin A, Gunawardana J, Halmen KA, King CA, Golenbock DT, et al. Meningococcal porin PorB binds to TLR2 and requires TLR1 for signaling. J Immunol. 2006;176:2373–80. doi: 10.4049/jimmunol.176.4.2373. [DOI] [PubMed] [Google Scholar]
- 24.Toussi DN, Carraway M, Wetzler LM, Lewis LA, Liu X, Massari P. The amino acid sequence of Neisseria lactamica PorB surface-exposed loops influences Toll-like receptor 2-dependent cell activation. Infect Immun. 2012;80:3417–28. doi: 10.1128/IAI.00683-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Virji M, Makepeace K, Ferguson DJ, Achtman M, Sarkari J, Moxon ER. Expression of the Opc protein correlates with invasion of epithelial and endothelial cells by Neisseria meningitidis. Mol Microbiol. 1992;6:2785–95. doi: 10.1111/j.1365-2958.1992.tb01458.x. [DOI] [PubMed] [Google Scholar]
- 26.Sokolova O, Heppel N, Jagerhuber R, Kim KS, Frosch M, Eigenthaler M, et al. Interaction of Neisseria meningitidis with human brain microvascular endothelial cells: role of MAP- and tyrosine kinases in invasion and inflammatory cytokine release. Cell Microbiol. 2004;6:1153–66. doi: 10.1111/j.1462-5822.2004.00422.x. [DOI] [PubMed] [Google Scholar]
- 27.Slanina H, Hebling S, Hauck CR, Schubert-Unkmeir A. Cell invasion by Neisseria meningitidis requires a functional interplay between the focal adhesion kinase, Src and cortactin. PLoS ONE. 2012;7:e39613. doi: 10.1371/journal.pone.0039613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X, Wetzler LM, Nascimento LO, Massari P. Human airway epithelial cell responses to Neisseria lactamica and purified porin via Toll-like receptor 2-dependent signaling. Infect Immun. 2010;78:5314–23. doi: 10.1128/IAI.00681-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perkins TN, Shukla A, Peeters PM, Steinbacher JL, Landry CC, Lathrop SA, et al. Differences in gene expression and cytokine production by crystalline vs. amorphous silica in human lung epithelial cells. Part Fibre Toxicol. 2012;9:6. doi: 10.1186/1743-8977-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slevogt H, Seybold J, Tiwari KN, Hocke AC, Jonatat C, Dietel S, et al. Moraxella catarrhalis is internalized in respiratory epithelial cells by a trigger-like mechanism and initiates a. Cell Microbiol. 2007;9:694–707. doi: 10.1111/j.1462-5822.2006.00821.x. [DOI] [PubMed] [Google Scholar]
- 31.Turner DP, Marietou AG, Johnston L, Ho KK, Rogers AJ, Wooldridge KG, et al. Characterization of MspA, an immunogenic autotransporter protein that mediates adhesion to epithelial and endothelial cells in Neisseria meningitidis. Infect Immun. 2006;74:2957–64. doi: 10.1128/IAI.74.5.2957-2964.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuespert K, Roth A, Hauck CR. Neisseria meningitidis has two independent modes of recognizing its human receptor CEACAM1. PLoS ONE. 2011;6:e14609. doi: 10.1371/journal.pone.0014609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bjerknes R, Guttormsen HK, Solberg CO, Wetzler LM. Neisserial porins inhibit human neutrophil actin polymerization, degranulation, opsonin receptor expression, and phagocytosis but prime the neutrophils to increase their oxidative burst. Infect Immun. 1995;63:160–7. doi: 10.1128/iai.63.1.160-167.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mosleh IM, Huber LA, Steinlein P, Pasquali C, Gunther D, Meyer TF. Neisseria gonorrhoeae porin modulates phagosome maturation. J Biol Chem. 1998;273:35332–8. doi: 10.1074/jbc.273.52.35332. [DOI] [PubMed] [Google Scholar]
- 35.Massari P, Ho Y, Wetzler LM. Neisseria meningitidis porin PorB interacts with mitochondria and protects cells from apoptosis. Proc Natl Acad Sci U S A. 2000;97:9070–5. doi: 10.1073/pnas.97.16.9070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Binnicker MJ, Williams RD, Apicella MA. Gonococcal porin IB activates NF-kappaB in human urethral epithelium and increases the expression of host antiapoptotic factors. Infect Immun. 2004;72:6408–17. doi: 10.1128/IAI.72.11.6408-6417.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Besbes A, Le GS, Antunes A, Terrade A, Hong E, Giorgini D, et al. Hyperinvasive Meningococci Induce Intra-nuclear Cleavage of the NF-kappaB Protein p65/RelA by Meningococcal IgA Protease. PLoS Pathog. 2015;11:e1005078. doi: 10.1371/journal.ppat.1005078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Macleod H, Bhasin N, Wetzler LM. Role of protein tyrosine kinase and Erk1/2 activities in the Toll-like receptor 2-induced cellular activation of murine B cells by neisserial porin. Clin Vaccine Immunol. 2008;15:630–7. doi: 10.1128/CVI.00435-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahdavi J, Royer PJ, Sjolinder HS, Azimi S, Self T, Stoof J, et al. Pro-inflammatory cytokines can act as intracellular modulators of commensal bacterial virulence. Open Biol. 2013;3:130048. doi: 10.1098/rsob.130048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carrol ED, Thomson AP, Jones AP, Jeffers G, Hart CA. A predominantly anti-inflammatory cytokine profile is associated with disease severity in meningococcal sepsis. Intensive Care Med. 2005;31:1415–9. doi: 10.1007/s00134-005-2787-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holub M, Scheinostova M, Dzupova O, Fiserova A, Beran O, Kalmusova J, et al. Neisseria meningitidis strains from patients with invasive meningococcal disease differ in stimulation of cytokine production. Folia Microbiol (Praha) 2007;52:525–8. doi: 10.1007/BF02932114. [DOI] [PubMed] [Google Scholar]
- 42.van DM, van d, V, Bartelink AK, van DR, Sauerwein RW, Van der Meer JW. Correlation between proinflammatory cytokines and antiinflammatory mediators and the severity of disease in meningococcal infections. J Infect Dis. 1995;172:433–9. doi: 10.1093/infdis/172.2.433. [DOI] [PubMed] [Google Scholar]
- 43.Griffiths NJ, Bradley CJ, Heyderman RS, Virji M. IFN-gamma amplifies NFkappaB-dependent Neisseria meningitidis invasion of epithelial cells via specific upregulation of CEA-related cell adhesion molecule 1. Cell Microbiol. 2007;9:2968–83. doi: 10.1111/j.1462-5822.2007.01038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klaile E, Klassert TE, Scheffrahn I, Muller MM, Heinrich A, Heyl KA, et al. Carcinoembryonic antigen (CEA)-related cell adhesion molecules are co-expressed in the human lung and their expression can be modulated in bronchial epithelial cells by non-typable Haemophilus influenzae, Moraxella catarrhalis, TLR3, and type I and II interferons. Respir Res. 2013;14:85. doi: 10.1186/1465-9921-14-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suri R, Periselneris J, Lanone S, Zeidler-Erdely PC, Melton G, Palmer KT, et al. Exposure to welding fumes and lower airway infection with Streptococcus pneumoniae. J Allergy Clin Immunol. 2016;137:527–34. doi: 10.1016/j.jaci.2015.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jen FE, Warren MJ, Schulz BL, Power PM, Swords WE, Weiser JN, et al. Dual pili post-translational modifications synergize to mediate meningococcal adherence to platelet activating factor receptor on human airway cells. PLoS Pathog. 2013;9:e1003377. doi: 10.1371/journal.ppat.1003377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cook EB, Stahl JL, Esnault S, Barney NP, Graziano FM. Toll-like receptor 2 expression on human conjunctival epithelial cells: a pathway for Staphylococcus aureus involvement in chronic ocular proinflammatory responses. Ann Allergy Asthma Immunol. 2005;94:486–97. doi: 10.1016/S1081-1206(10)61120-9. [DOI] [PubMed] [Google Scholar]
- 48.Faber J, Henninger N, Finn A, Zenz W, Zepp F, Knuf M. A toll-like receptor 4 variant is associated with fatal outcome in children with invasive meningococcal disease. Acta Paediatr. 2009;98:548–52. doi: 10.1111/j.1651-2227.2008.01163.x. [DOI] [PubMed] [Google Scholar]
- 49.Sanders MS, van Well GT, Ouburg S, Morre SA, van Furth AM. Toll-like receptor 9 polymorphisms are associated with severity variables in a cohort of meningococcal meningitis survivors. BMC Infect Dis. 2012;12:112. doi: 10.1186/1471-2334-12-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Telleria-Orriols JJ, Garcia-Salido A, Varillas D, Serrano-Gonzalez A, Casado-Flores J. TLR2-TLR4/CD14 polymorphisms and predisposition to severe invasive infections by Neisseria meningitidis and Streptococcus pneumoniae. Med Intensiva. 2014;38:356–62. doi: 10.1016/j.medin.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 51.van Well GT, Sanders MS, Ouburg S, van Furth AM, Morre SA. Polymorphisms in Toll-like receptors 2, 4, and 9 are highly associated with hearing loss in survivors of bacterial meningitis. PLoS ONE. 2012;7:e35837. doi: 10.1371/journal.pone.0035837. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.