Abstract
Long-term prescription opioid use is associated both with new-onset and recurrence of depression. Whether chronic opioid use interferes with depression management has not been reported, therefore we determined whether patients’ longer duration of opioid use and higher opioid dose are associated with new-onset treatment resistant depression (TRD) after controlling for confounding from pain and other variables.
Data was obtained from Veteran Health Administration (VHA) de-identified patient medical records. We used a retrospective cohort design from 2000–2012. Eligible subjects (n=6,169) were 18–80 years of age, free of cancer and HIV, diagnosed with depression and opioid-free for the 24-month interval prior to the observation period. Duration of a new prescription for opioid analgesic was categorized as 1–30 days, 31–90 days and >90 days. Morphine-equivalent dose (MED) during follow-up categorized as ≤50 mg versus >50 mg per day. Pain and other sources of confounding were controlled by propensity scores and inverse probability of treatment weighting. Cox proportional hazard models were computed to estimate the association between duration and dose of opioid and onset of TRD.
After removing confounding by weighting data, opioid use for 31–90 days and for >90 days, compared to 1–30 days, was significantly associated with new onset TRD (HR=1.25; 95%CI: 1.09–1.45 and HR=1.52; 95%CI: 1.32–1.74, respectively). MED was not associated with new onset TRD.
The risk of developing TRD increased as time spent on opioid analgesics increased. Long-term opioid treatment of chronic pain may interfere with treatment of depression.
Keywords: opioids, pain, depression, epidemiology
INTRODUCTION
The opioid epidemic in the United States remains unmitigated with annual prescriptions of opioid medications exceeding 240 million in each of years 2009–2013.(1) Most research on the opioid epidemic is focused on risk of abuse, overdose and death.(1) An emerging literature now indicates risk of new onset and recurrent depression to be among the adverse effects of opioids.(2–4) This is particularly concerning because short term improvements in depression and pain following opioid therapy may lead to dose increases or continued opioid use which increases risk of adverse outcomes, including depression ((5). However, the possibility that long-term opioid use leads to depression complicates pain management in unforeseen ways. Widespread exposure to long-term opioid therapy in the U.S.(6) portends a potential increase in what may have been an otherwise avoidable depression.
Patients with pain and depression, versus those without depression, are at increased risk for receiving opioid prescriptions, using for longer periods and at higher doses, and abusing these medications.(7–9) Over the past 2 years, we have shown that opioid therapy is not only predicted by depression but independently increases the risk of new onset depression (2, 4). Longer term use, but not higher dose, is associated with 35–100% increased risk of new-onset depression,(2) and opioid exposure is related to a 100% increased risk of depression recurrence.(10)
Prescription opioids and pain may also contribute to treatment resistant depression (TRD). The literature on pain and TRD is sparse. In a study designed to characterize comorbidity in TRD and non-TRD claims data, muscle pain, joint pain, headache/migraine and back pain were twice as prevalent among patients with TRD than those without TRD.(11) Past studies provide evidence for a pain, opioid, and TRD association, but to our knowledge, no studies have investigated whether longer term use or higher opioid dose leads to TRD among patients with depression and non-cancer pain.
To determine whether long-term opioid use and/or higher opioid dose contributes to transitioning from depression to TRD, we used medical record data from a retrospective cohort of Veterans Health Administration (VA) patients. After controlling for confounding due to pain, we computed the association between increasing duration of opioid use and TRD, controlling for dose; and modeled the association between higher dose and TRD, controlling for duration.
METHODS
Patient data were obtained from administrative extracts of the VA electronic medical record. Medical record data contain all diagnoses, medications, laboratory results, type of clinics utilized, provider type, and vital signs that are collected during routine care. The data is complete in terms of capturing elements recorded during routine care. Care received outside the VA may not be included in data. We adjust for access to non-VA healthcare to account this potential bias. Our records contained clinic encounters from January 1, 2000 through December 31, 2012, ICD-9-CM diagnoses, prescription records, vital signs and demographics. This project was approved by the VA and Saint Louis University Institutional Review Boards.
Eligibility criteria
As shown in Figure 1, a random sample of 500,000 patients aged 18–80 years of age at baseline was restricted to patients free of ICD-9-CM codes for cancer or HIV. Patients were required to be regular users of VA care defined by having annual visits in 2000 and 2001. To be informative for this study, eligible patients must have had at least one visit during the follow-up period 2002–2012. All patients had a diagnosis of depression and were free of opioid prescriptions in the two years prior to the start of follow-up, 01/01/2002. We required patients to have depression at start of follow-up because this study is designed to determine if opioid use in patients with depression leads to TRD. At the start of follow-up, 47% had a current prescription for an antidepressant and 93% had filled an antidepressant prescription at some point in the prior two years. During the follow-up period, incident opioid use could occur any time prior to onset of TRD, defined below. A total of 6,223 patients met all eligibility criteria; after removing 54 patients with missing covariate data, our final analytic sample consisted of 6,169 patients.
Figure 1.
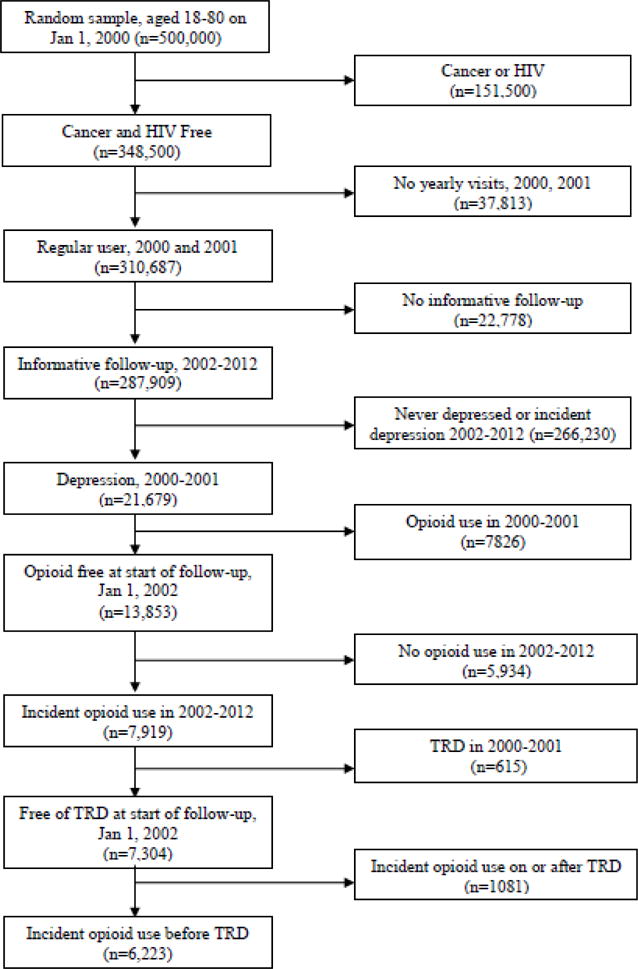
Eligibility criteria for Veterans Health Administration patient data
Opioid exposure
A prescription for any dose and duration of the following medications were included: codeine, fentanyl, hydrocodone, hydromorphone, levorphanol, meperidine, morphine, oxycodone, oxymorphone, and pentazocine. The duration of continuous use was counted by summing the days-supply of pills required to exhaust the medication if taken at the maximum dose prescribed between the end of one prescription and the start of another. Use was considered continuous if there was no gap of >30 days between fills. Duration of use was computed from date of initial opioid fill until end of follow-up or the start of a >30 day gap.
We used the same duration and dose exposure categories from our previous study of opioids and new onset depression(2) to allow us to compare results with the present study. We classified patients into three mutually exclusive categories: 1–30 days, 31–90 and >90 days continuous supply starting from their incident prescription.
Morphine equivalent dose (MED) was computed using standard morphine equivalent conversion tables that express the specific opioid medication in terms of equivalent morphine content.(12) For example, 1 mg of oral oxycodone is equal to 1.5 mg of morphine. We assigned patients to a binary variable indicating a daily MED >50 mg or ≤50 mg using the total opioid available to patients on the last day before the end of follow-up or before a >30-day gap.
Treatment-resistant depression (TRD)
Depression was defined by the presence of two outpatient or one inpatient ICD-9-CM code for depression in the same 12 months, an algorithm for prevalent depression that has excellent agreement compared to written medical records.(13) Definitions for TRD vary. These include a failure to respond to one antidepressant trial at adequate dose and duration,(14) simultaneous prescription of more than one antidepressant, augmentation with atypical antipsychotics or MAOIs and electroconvulsive therapy.(15) We followed a definition previously employed in retrospective cohort studies involving medical records.(16, 17) Patients were defined as having TRD if any of the following were recorded in the medical record: a) electroconvulsive therapy, b) MAOI prescription, c) two or more antidepressants (any SSRI, SNRI, TCA or “other” non-MAOI antidepressant) at the same time overlapping by at least 31 days, or d) augmentation therapy (i.e. prescription of a mood stabilizing or atypical antipsychotic after antidepressant treatment).
Confounders
Variables associated with pain, opioid exposure and depression were selected as potential confounders to be used in propensity score models described below. Variables included measures of pain, psychiatric and physical comorbidities, other prescription medication, health care utilization and demographics. Non-cancer pain was measured by 900 conditions for which pain could be severe enough to call for an opioid prescription,(18) and diagnoses were collapsed into five pain categories: arthritis pain, back pain, headache, musculoskeletal and neuropathic pain. The ICD-9-CM codes used for each category are reported in the supplementary material of a previous study.(4) The average maximum pain score reported any time prior to end of follow-up was obtained from patient-reported pain on a scale from 0–10. Pain scores are routinely obtained in VA care during collection of vital signs.
Psychiatric disorders were included as confounders due to evidence that they are often comorbid with depression, pain, and opioid use. (5) We used ICD-9-CM codes to identify attention deficit disorder, antisocial personality disorder, posttraumatic stress disorder and any other anxiety disorder as a composite indicator of any diagnoses for panic disorder, generalized anxiety disorder, social phobia, obsessive compulsive disorder or anxiety disorder NOS. Substance use disorder included alcohol abuse/dependence and illicit drug abuse/dependence, including opioid abuse/dependence. Data on substance use disorders and psychiatric disorders are obtained from ICD-9-CM codes which could have been assigned in specialty mental health service lines or in primary care and other settings. Tobacco use is obtained from either record indicator of smoking history or diagnoses of nicotine dependence. We did not have access to screening instrument data such as those used for alcohol and drug abuse.
We adjusted for chronic health conditions due to their association with depression(19, 20), their presence to potentially increased health care contacts and thus link to detection bias Last, we controlled for sleep apnea and low testosterone, which are known to be consequences of opioid use and related to depression(21, 22). We used ICD-9-CM codes to define the following comorbid health conditions: type 2 diabetes mellitus, hypertension, cerebrovascular disease, low testosterone, sleep apnea, and a composite cardiovascular disease variable that included hyperlipidemia, ischemic heart disease, disease of pulmonary circulation, other heart disease, hypertensive heart disease or myocardial infarction. Obesity was defined by body mass index ≥ 30 or ICD-9-CM code.
Prescription medications included fills for benzodiazepines (e.g., alprazolam, clonazepam, diazepam, lorazepam, chlordiazepoxide, and clorazepate), cardiovascular disease medications (e.g., statins and beta blockers), and prescription non-steroidal anti-inflammatory drugs, excluding ibuprofen and naproxen (e.g., celecoxib, diclofenac, diflunisal, etodolac, fenoprofen, flurbiprofen, indomethacin, ketoprofen, ketorolac, meclofenamate, mefenamic, meloxicam, nabumetone, oxaprozin, piroxicam, sulindac, and tolmetin).
Demographics included age, gender, race, marital status and insurance. Insurance was defined as VA care only vs. VA plus other sources. Controlling for non-VA health care coverage adjusts for detection bias because patients with non-VA coverage may not use the VA as often. This variable is also a proxy for higher socioeconomic status among those with private insurance. We further controlled for detection bias by including a measure of health service utilization that was computed by quartiles of average number of clinic visits per month. The highest quartile of use was considered high health care utilization.
Control for pain and other confounding was accomplished by computing propensity scores (PS) and applying PS to inverse probability of treatment weighting (IPTW). The PS is the conditional probability that a patient will receive a treatment. Separate PS models were computed to predict opioid duration and opioid MED. The PS score was computed using a multivariable, multinomial logistic regression model for opioid duration and a binary logistic regression model for MED, predicting exposure groups as a function of the covariates listed in Table 1. We used standard IPTW approaches(23–25) to equate the distribution of pain and other confounders across each category of opioid duration and across the two levels of MED. IPTW generated two pseudo-populations, one for duration and one for dose. Successful weighting results in balanced confounders which are no longer significantly associated with the opioid treatment variable and are similarly distributed across categories of opioid duration and MED. For example, as shown in tables 2 and 3, differences in the distribution of pain variables will not be significantly associated with levels of duration and dose if IPTW is successful. This controls for confounding associated with receiving an opioid for a given duration or dose.
Table 1.
Association of opioid exposure and covariates with new onset treatment resistant depression in Veterans Health Administration patients (2002–2012; N=6,169)
| Treatment Resistant Depression | ||||
|---|---|---|---|---|
| Exposure/covariates, n (%) | Overall (n=6,169) |
NO (n=4,623) |
YES (n=1,546) |
p-value |
| Opioid duration (days) | <.0001 | |||
| 1–30 | 4631 (75.1) | 3541 (76.6) | 1090 (70.5) | |
| 31–90 | 802 (13.0) | 578 (12.5) | 224 (14.5) | |
| > 90 | 736 (11.9) | 504 (10.9) | 232 (15.0) | |
| Opioid dose > 50 mg | 431 (7.0) | 312 (6.7) | 119 (7.7) | .205 |
| Psychiatric comorbidities# | ||||
| PTSD | 2280 (37.0) | 1638 (35.4) | 642 (41.5) | <.0001 |
| Other anxiety* | 2346 (38.0) | 1751 (37.9) | 595 (38.5) | .669 |
| ADD | 132 (2.1) | 101 (2.2) | 31 (2.0) | .673 |
| Antisocial Personality Disorder | 317 (5.1) | 250 (5.4) | 67 (4.3) | .098 |
| Nicotine abuse/dependence | 3385 (54.9) | 2579 (55.8) | 806 (52.1) | .013 |
| Alcohol abuse/dependence | 2568 (41.6) | 1977 (42.8) | 591 (38.2) | .002 |
| Any illicit drug abuse/dependence | 2040 (33.1) | 1568 (33.9) | 472 (30.5) | .014 |
| Metabolic/Cardiovascular comorbidities# | ||||
| Diabetes Type II | 2318 (37.6) | 1770 (38.3) | 548 (35.5) | .046 |
| Hypertension | 4661 (75.6) | 3549 (76.8) | 1112 (71.9) | <.001 |
| Cardiovascular disease@ | 5170 (83.8) | 3939 (85.2) | 1231 (79.6) | <.0001 |
| Cerebrovascular disease | 1079 (17.5) | 864 (18.7) | 215 (13.9) | <.0001 |
| Obesity diagnosis | 2856 (46.3) | 2133 (46.1) | 723 (46.8) | .669 |
| Other comorbidities# | ||||
| Low Testosterone | 243 (3.9) | 195 (4.2) | 48 (3.1) | .051 |
| Sleep apnea | 940 (15.2) | 711 (15.4) | 229 (14.8) | .591 |
| Medications# | ||||
| NSAIDs | 3432 (55.6) | 2569 (55.6) | 863 (55.8) | .863 |
| Benzodiazepines | 3359 (54.4) | 2485 (53.7) | 874 (56.5) | .057 |
| Statins/Beta Blockers | 4459 (72.3) | 3408 (73.7) | 1051 (68.0) | <.0001 |
| Painful conditions# | ||||
| Arthritis | 5212 (84.5) | 3936 (85.1) | 1276 (82.5) | .014 |
| Back pain | 4615 (74.8) | 3457 (74.8) | 1158 (74.9) | .922 |
| Headaches | 2076 (33.7) | 1539 (33.3) | 537 (34.7) | .298 |
| Musculoskeletal pain | 4328 (70.2) | 3276 (70.9) | 1052 (68.1) | .036 |
| Neuropathic pain | 2237 (36.3) | 1676 (36.3) | 561 (36.3) | .981 |
| Maximum pain score, mean (sd) | 9.0 (1.6) | 8.9 (1.7) | 9.3 (1.3) | <.0001 |
| Healthcare utilization: Top 25th percentile | 2915 (47.3) | 2004 (43.4) | 911 (58.9) | <.0001 |
| Age | 49.3 (11.4) | 49.6 (11.7) | 48.3 (10.6) | <.0001 |
| Gender: male | 5336 (86.5) | 4043 (87.5) | 1293 (83.6) | <.001 |
| Race: White | 4736 (76.8) | 3498 (76.7) | 1238 (80.1) | <.001 |
| Insurance: VA Only | 4569 (74.1) | 3409 (73.7) | 1160 (75.0) | .316 |
| Marital status: Married | 2564 (41.6) | 1862 (40.3) | 702 (45.4) | <.001 |
Comorbidities and medications occurring before TRD
Other anxiety disorders = panic disorder, OCD, social phobia, GAD, Anxiety NOS
Cardiovascular disease = hyperlipidemia, ischemic heart disease, diseases of pulmonary circulation, other heart disease, hypertensive heart disease, myocardial infarction
Table 2.
Association of new onset TRD and covariates with opioid duration before and after applying inverse probability of treatment weighting among 6,169 Veterans Health Administration patients (2002–2012)
| Unweighted data Duration of opioid use |
Weighted data Duration of opioid use |
|||||
|---|---|---|---|---|---|---|
|
| ||||||
| Outcome/covariates, % | 1–30 days (n=4631) |
31–90 days (n=802) |
> 90 days (n=736) |
1–30 days | 31–90 days | > 90 days |
| New onset TRD | 23.5 | 27.9 | 31.5*** | – | – | – |
| Opioid dose > 50 mg | 6.7 | 7.0 | 9.0 | 7.0 | 7.0 | 7.2 |
| Psychiatric comorbidities# | ||||||
| PTSD | 37.3 | 36.0 | 35.6 | 37.0 | 36.3 | 38.2 |
| Other anxiety* | 37.9 | 36.5 | 40.4 | 38.0 | 37.3 | 39.1 |
| ADD** | 2.2 | 1.9 | 2.2 | 2.1 | 1.9 | 1.8 |
| Antisocial Personality Disorder | 5.1 | 5.7 | 4.5 | 5.2 | 5.0 | 5.5 |
| Nicotine abuse/dependence | 53.6 | 58.0 | 59.8** | 54.9 | 54.7 | 54.6 |
| Alcohol abuse/dependence | 41.8 | 43.5 | 38.6 | 41.6 | 40.5 | 40.9 |
| Any illicit drug abuse/dependence | 33.0 | 33.3 | 33.3 | 33.0 | 32.3 | 31.5 |
| Metabolic/Cardiovascular comorbidities# | ||||||
| Diabetes Type II | 37.4 | 36.8 | 39.8 | 37.6 | 37.3 | 38.3 |
| Hypertension | 74.7 | 77.7 | 78.7* | 75.6 | 74.5 | 75.3 |
| Cardiovascular disease@ | 83.6 | 84.2 | 84.9 | 83.8 | 83.5 | 82.7 |
| Cerebrovascular disease | 17.3 | 18.2 | 18.1 | 17.5 | 18.1 | 16.7 |
| Obesity diagnosis | 46.3 | 47.8 | 44.8 | 46.3 | 45.6 | 46.8 |
| Other comorbidities # | ||||||
| Low Testosterone | 3.8 | 4.5 | 4.4 | 3.9 | 3.9 | 4.1 |
| Sleep apnea | 15.7 | 14.2 | 13.7 | 15.2 | 15.2 | 16.5 |
| Medications# | ||||||
| NSAIDs | 53.3 | 60.9 | 64.4*** | 55.7 | 55.8 | 57.1 |
| Benzodiazepines | 52.0 | 59.1 | 64.8*** | 54.4 | 54.8 | 54.9 |
| Statins/Beta Blockers | 71.4 | 73.6 | 76.6** | 72.3 | 72.0 | 71.6 |
| Painful conditions# | ||||||
| Arthritis | 83.3 | 88.4 | 87.6*** | 84.5 | 84.2 | 85.6 |
| Back pain | 72.4 | 79.3 | 85.3*** | 74.8 | 74.6 | 75.4 |
| Headaches | 33.6 | 34.0 | 33.3 | 33.7 | 34.1 | 34.1 |
| Musculoskeletal pain | 69.9 | 72.2 | 69.4 | 70.2 | 69.0 | 73.5 |
| Neuropathic pain | 34.6 | 40.8 | 41.9*** | 36.3 | 36.3 | 36.6 |
| Maximum pain score, mean (sd) | 9.0 (1.7) | 9.2 (1.3) | 9.2 (1.3)*** | 9.0 (1.6) | 9.0 (1.7) | 9.1 (1.4) |
| Healthcare utilization: Top 25th percentile | 47.4 | 49.6 | 43.8 | 47.3 | 47.4 | 47.7 |
| Age, mean (sd) | 49.3 (11.6) | 50.0 (11.4) | 49.8 (10.7)* | 49.3 (11.5) | 49.4 (11.5) | 49.4 (11.4) |
| Gender: male | 86.0 | 88.7 | 87.2 | 86.5 | 86.4 | 86.2 |
| Race: White | 75.2 | 79.2 | 83.8*** | 76.8 | 77.2 | 78.0 |
| Insurance: VA Only | 73.6 | 76.7 | 74.1 | 74.0 | 73.8 | 73.9 |
| Marital status: Married | 41.0 | 41.2 | 45.7 | 41.6 | 42.0 | 41.6 |
Comorbidities and medications occurring before TRD
Other anxiety disorders = panic disorder, OCD, social phobia, GAD, Anxiety NOS,
ADD – attention deficit disorder
Cardiovascular disease = hyperlipidemia, ischemic heart disease, diseases of pulmonary circulation, other heart disease, hypertensive heart disease, myocardial infarction
p<0.05,
p<0.01.
p<0.001
Table 3.
Association of TRD and covariates with morphine equivalent dose (MED) before and after applying inverse probability of treatment weighting among 6,169 Veterans Health Administration patients (2002–2012)
| Unweighted data Morphine equivalent dose |
Weighted data Morphine equivalent dose |
|||
|---|---|---|---|---|
|
| ||||
| Outcome/covariates, % | ≤ 50 mg (n=5738) |
> 50 mg (n=431) |
≤ 50 mg | > 50 mg |
| New onset TRD | 24.9 | 27.6 | – | – |
| Opioid duration (days) | ||||
| 1–30 | 75.3 | 71.7 | 75.1 | 74.8 |
| 31–90 | 13.0 | 13.0 | 13.0 | 12.9 |
| > 90 | 11.7 | 15.3 | 11.9 | 12.3 |
| Psychiatric comorbidities# | ||||
| PTSD | 36.8 | 39.4 | 37.0 | 38.3 |
| Other anxiety* | 38.0 | 39.0 | 38.0 | 37.4 |
| ADD** | 2.1 | 3.0 | 2.1 | 2.0 |
| Antisocial Personality Disorder | 5.1 | 5.3 | 5.1 | 5.4 |
| Nicotine abuse/dependence | 54.4 | 60.8* | 54.9 | 55.9 |
| Alcohol abuse/dependence | 41.1 | 48.5** | 41.6 | 41.7 |
| Any illicit drug abuse/dependence | 32.7 | 38.5* | 33.1 | 33.1 |
| Metabolic/Cardiovascular comorbidities# | ||||
| Diabetes Type II | 37.4 | 39.4 | 37.6 | 38.2 |
| Hypertension | 75.5 | 76.1 | 75.6 | 75.6 |
| Cardiovascular disease@ | 83.8 | 84.2 | 83.8 | 81.9 |
| Cerebrovascular disease | 17.4 | 19.0 | 17.5 | 17.5 |
| Obesity diagnosis | 46.4 | 44.6 | 46.3 | 45.5 |
| Other comorbidities# | ||||
| Low Testosterone | 4.0 | 3.5 | 3.9 | 3.4 |
| Sleep apnea | 15.2 | 15.3 | 15.2 | 15.5 |
| Medications# | ||||
| NSAIDs | 55.3 | 59.4 | 55.6 | 56.4 |
| Benzodiazepines | 54.0 | 61.0** | 54.4 | 54.9 |
| Statins/Beta Blockers | 72.2 | 73.6 | 72.3 | 70.5 |
| Painful conditions# | ||||
| Arthritis | 84.2 | 87.9* | 84.5 | 85.1 |
| Back pain | 74.5 | 78.9* | 74.8 | 76.4 |
| Headaches | 33.7 | 32.5 | 33.7 | 34.0 |
| Musculoskeletal pain | 70.0 | 71.9 | 70.2 | 70.0 |
| Neuropathic pain | 36.2 | 37.1 | 36.3 | 35.5 |
| Maximum pain score, mean (sd) | 9.0 (1.6) | 9.3 (1.4)*** | 9.0 (1.6) | 9.1 (1.5) |
| Healthcare utilization: Top 25th percentile | 46.5 | 57.1*** | 47.3 | 48.6 |
| Age, mean (sd) | 49.3 (11.5) | 49.0 (10.9) | 49.3 (11.4) | 49.1 (11.2) |
| Gender: male | 86.3 | 88.6 | 86.5 | 86.8 |
| Race: White | 76.6 | 79.1 | 76.8 | 75.7 |
| Insurance: VA Only | 74.1 | 74.0 | 74.1 | 73.9 |
| Marital status: Married | 41.6 | 41.1 | 41.6 | 42.3 |
Comorbidities and medications occurring before TRD
Other anxiety disorders = panic disorder, OCD, social phobia, GAD, Anxiety NOS** ADD – attention deficit disorder
Cardiovascular disease = hyperlipidemia, ischemic heart disease, diseases of pulmonary circulation, other heart disease, hypertensive heart disease, myocardial infarction
p<0.05,
p<0.01.
p<0.001
Analysis
We computed bivariate analyses including ANOVA and t-tests for continuous variables and chi–square tests for categorical variables, and we re-computed bivariate analyses after data weighting to determine whether variables balanced across opioid duration and MED. To measure the association between opioid prescription and TRD, we computed Cox proportional hazards models first in unweighted data and then in weighted data. All Cox models treated opioid duration and MED as time-dependent variables. To account for the potential contribution of changing pain after the start of opioid use, we further adjusted Cox models by adding time-dependent pain conditions and pain scores. We used the PHREG procedure in SAS version 9.4 (SAS Institute, Cary, NC) with α set at 0.05 for the Cox regression models. Evaluation of hazard trends over time confirmed that proportional hazard assumptions were met for both duration of opioid use (p=0.83) and MED (p=0.74). Two-tailed tests were conducted to allow for both risk factors and protective effects.
RESULTS
During follow-up, 25.1% developed TRD. Among patients with TRD, 48.8% of patients met TRD criteria by dual antidepressant use, 49.5% by augmentation therapy, 0.7% by MAOI prescription and 1.0% by receipt of ECT. In unweighted data, the incidence rates for TRD were 43.6/1000 person-years among patients with 1–30 days of use, 53.8/1000 person-years among 31–90 day users, and 62.0/1000 person-years among those using opioid for >90 days. The incidence rates for TRD by dose were 46.5/1000 person-years among those with a dose of 1–50 mg MED and 53.7/1000 person-years among those with a dose of >50 mg MED.
Table 1 shows exposure and covariates overall and by TRD, Table 2 by opioid duration, and Table 3 by dose. There was a significant association between duration of opioid use and TRD with a higher percentage of 31–90 day and >90 day users developing TRD while a smaller proportion of 1–30 day users developed TRD (Table 2). There was no significant association between MED and TRD as shown in row one of Table 3.
As shown in Table 1, patients with TRD were more likely to have PTSD than patients without TRD (p<0.0001). Substance use disorders were all significantly less prevalent among patients with TRD compared to those without TRD (p<0.05 to p<0.01). Similarly, hypertension, cardiovascular disease, cerebrovascular disease (stroke), arthritis, musculoskeletal pain, and fills for statins/beta blockers were significantly less prevalent among patients with than without TRD (p<0.05 to p<0.0001). Average maximum pain score was significantly greater among patients with TRD than without (p<0.0001). Last, patients with TRD used significantly more health services, were significantly younger, more likely to be female and more often white and married (p<0.001).
Separate PS models for duration and for dose produced a narrow range of weights, with means nearing 1.0 (see e-tables 1a and 1b) indicating that the models performed well in predicting both opioid exposure variables. As shown in Tables 2 and 3, IPTW was successful in removing all significant differences in the distribution of covariates across categories of opioid duration and MED.
Results of Cox proportional hazard models estimating the association between duration of opioid use and new onset TRD are shown in Table 4. Prior to controlling for confounding in unweighted data, patients who used opioids for 31–90 days compared to 1–30 days were significantly more likely to develop TRD (HR=1.25; 95%CI:1.09–1.45) as were patients who used for >90 days (HR=1.46; 95%CI:1.27–1.69). The association between duration of use and new onset TRD was similar after weighting data in model 2. After adjusting for painful conditions and pain scores that may have occurred after opioid initiation, the association between duration of use and new onset TRD remained significant. Compared to 1–30 day users, patients receiving opioids for 31–90 days were 1.25 (95%CI:1.09–1.45) times more likely to develop TRD and those using >90 days were 1.52 (95%CI:1.32–1.74) times more likely to develop TRD.
Table 4.
Association between opioid duration and treatment resistant depression in Veterans Health Administration patients, unweighted and weighted by inverse probability opioid duration (n=6,169)
| Model 1–Crude | Model 2–Weighted | Model 3–Weighted + Pain | |
|---|---|---|---|
| Variable | HR (95% CI) | HR (95% CI) | HR (95% CI) |
| Opioid duration (days) | |||
| 1–30 | 1.00 | 1.00 | 1.00 |
| 31–90 | 1.25 (1.09–1.45) | 1.27 (1.10–1.47) | 1.25 (1.09–1.45) |
| > 90 | 1.46 (1.27–1.69) | 1.57 (1.36–1.81) | 1.52 (1.32–1.74) |
| Arthritis | 1.22 (1.06–1.40) | ||
| Back pain | 1.35 (1.20–1.53) | ||
| Headache | 1.25 (1.13–1.39) | ||
| Musculoskeletal pain | 1.29 (1.15–1.45) | ||
| Neuropathy | 1.32 (1.19–1.47) | ||
| Pain Score | 1.06 (1.02–1.10) |
HR=hazard ratio; CI=confidence interval
As shown in Table 5, results of Cox proportional hazard models computed using unweighted data indicated no significant association between patients receiving >50 mg MED and new onset TRD. After balancing data in model 2, the magnitude of the association decreased and remained non-significant (HR=1.08; 95%CI:0.89–1.30). The hazard ratio estimating the association between higher MED and TRD remained non-significant after adding pain variables in model 3 (HR=1.07; 95%CI:0.88–1.30).
Table 5.
Association between opioid dose and treatment resistant depression, unweighted and weighted by inverse probability opioid dose (n=6,169)
| Model 1 – Crude | Model 2–Weighted | Model 3–Weighted + Pain | |
|---|---|---|---|
| Variable | HR (95% CI) | HR (95% CI) | HR (95% CI) |
| Opioid dose > 50 mg | 1.15 (0.96–1.39) | 1.08 (0.89–1.30) | 1.07 (0.88–1.30) |
| Arthritis | 1.21 (1.05–1.39) | ||
| Back pain | 1.45 (1.28–1.63) | ||
| Headache | 1.25 (1.12–1.39) | ||
| Musculoskeletal pain | 1.24 (1.11–1.40) | ||
| Neuropathy | 1.34 (1.20–1.49) | ||
| Pain Score | 1.08 (1.04–1.12) |
HR=hazard ratio; CI=confidence interval
DISCUSSION
In a cohort of 6,169 patients with depression at baseline, those who initiated and remained on an opioid for 31–90 days were 25% more likely, and those who remained users for >90 days were 52% more likely, to transition to TRD compared to patients who used for only 1–30 days. This finding remained after controlling for confounding due to pain associated with opioid initiation, pain that could occur after opioid initiation and for opioid dose reached before end of follow-up. The present results are consistent with our prior findings that patients initially free of depression at baseline were at increased risk of new onset depression when use increased beyond 31 days and risk of new onset depression continued to increase when use reached >90 days.(2) In the present study, dose was not associated with TRD which is consistent with our prior study revealing no association between dose and new onset depression(2).
These results suggest that long-term opioid therapy complicates rather than eases treatment of concurrent depression. Treatment of chronic pain with these opioids may provide short-term mood elevation but is likely to worsen depression over the long term. Several clinical trials suggest buprenorphine, an antagonist of the kappa opioid receptor, has been shown to improve depression in patients with TRD (26, 27). The present study suggests mu opioid receptor agonists (hydrocodone, oxycodone, morphine etc) worsen depression and lead to TRD. This is consistent with evidence that no benefit has been found when testing a mu opioid antagonist for TRD (28) and converges with our previous studies revealing that mu opioid agonists lead to new onset depression and depression re-occurrence. Transition to TRD adds to the depressogenic consequences we have observed in patients initially without depression. In these studies, patients longer duration of opioid use was associated with new onset depression in VA patient samples and in two private sector patient samples from large health systems serving the general population in their respective geographic regions.(2) In a second study of VA and private sector patients, opioid use vs. no opioid use in patients with remitted depression was associated with increased risk of depression recurrence(29) In sum, evidence now indicates that (a) longer term use, but not higher dose, leads to new-onset depression in persons without depression in the prior two years, (b) opioid use contributes to increased risk of depression relapse among patients who were in a period of depression remission, and (c) longer opioid use is associated with greater risk of TRD as shown in the present report. These findings point to a public health concern because low back pain and depression are the 2013 global leading causes of years lived in disability(30) and TRD is associated with even greater costs and morbidity.(31)
Limitations should be considered when interpreting our results. VA patients have more comorbid conditions than those in the private sector(2, 29); however our previous studies of opioid use and new onset depression in VA samples have been replicated in private sector settings providing a template that may be used in the TRD investigation. Second, we only know that prescriptions were filled, but not whether they were taken as prescribed. We may have underestimated risk of TRD if the true duration of continuous use was less than the duration we computed from prescription fill dates. Third, the definition of TRD does not necessarily equate to severity of depression and it is possible that some patients might have been misclassified as TRD when augmentation was prescribed for a comorbid psychiatric disorder such as bipolar or PTSD. Such misclassification might result in over-estimating the total incidence of TRD, however, this should not bias our survival models because misclassification is not specific to opioid duration or dose and would increase the outcome in all levels of the exposure variables. Last, opioid use was measured from the incident period, and the present design does not provide information on intervals between opioid cessation and TRD nor the potential effect of intermittent opioid use.
This latter limitation leaves open the question of how chronic opioid use may contribute to TRD. One possibility is interference with treatment either due to decreased adherence or mitigating the impact of antidepressant medication. For example, up to 75% of patients on chronic opioid therapy will develop an androgen deficiency(21, 32) which alone leads to low mood and thus, could impair recovery from depression. A second possibility is opioids worsen depression by promoting physical and social deactivation leading to treatment resistance to first-line treatments and need for a second antidepressant or augmentation, or for some, ECT. Additional research incorporating patient-reported symptom scores is needed to determine if patients’ depression worsens with longer and/or higher dose opioid treatment.
The lack of association between maximum MED and risk of TRD requires further investigation. Future studies should consider the starting dose of MED, model different patterns of rate of MED increase and total MED consumed while controlling for duration of use. These other definitions for dose are beyond the pre-defined variables used in this study but warrant additional investigation for associations with new onset depression, depression recurrence and TRD.
Conclusions
TRD should be discussed as a risk of long-term opioid therapy when considering opioid initiation in patients with comorbid depression and non-cancer pain. Our results extend the rationale for depression screening both at the time of opioid initiation and repeatedly thereafter to determine whether depression is worsening. Informed patient-provider discussion of the risk-benefit balance should address the impact of opioids on depression, especially among patients currently depressed.
Highlights.
Longer opioid duration but not dose associated with new onset depression
Treatment resistant depression (TRD) is associated with more pain
We used a large patient cohort to test if opioid use led to TRD
Longer opioid use, but not dose, associated with incident TRD
Acknowledgments
Funding Support: This study was supported by the National Institute of Mental Health, Prescription Opioid Analgesics and Risk of Depression, R21MH101389. Funding sources had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Prior presentations: none
Names and affiliations of all authors or individuals who conducted data analysis: Joanne Salas1,2 1) Department of Family and Community Medicine, Saint Louis University School of Medicine, St. Louis MO. 63104, 2) Harry S. Truman Veterans Administration Medical Center. Columbia, MO.
Joanne Salas and Jeffrey Scherrer had full access to all of the VA data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosures and Conflict of Interest: The authors report no financial conflicts of interest with respect to the content of this manuscript. The views expressed in this paper are not necessarily those of the Veterans Administration.
References
- 1.Dart RC, Surratt HL, Cicero TJ, Parrino MW, Severtson G, Bucher-Bartelson B, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372:241–8. doi: 10.1056/NEJMsa1406143. [DOI] [PubMed] [Google Scholar]
- 2.Scherrer JF, Salas J, Copeland LA, Stock EM, Ahmedani BK, Sullivan M, et al. Prescription opioid duration, dose, and increased risk of depression in 3 large patient populations. Ann Fam Med. 2016;14:54–62. doi: 10.1370/afm.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scherrer JF, Salas J, Lustman PJ, Burge S, Schneider FD, Texas RRNo Change in opioid dose and change in depression in a longitudinal primary care patient cohort. Pain. 2015;156(2):348–55. doi: 10.1097/01.j.pain.0000460316.58110.a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scherrer JF, Svrakic DM, Freedland KE, Chrusciel T, Balasubramanian S, Bucholz KK, et al. Prescription opioid analgesics increase the risk of depression. Journal of General Internal Medicine. 2014;29(3):491–9. doi: 10.1007/s11606-013-2648-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howe CQ, S MD. The missing ‘P’ in pain management: how the current opioid epidemic highlights the need for psychiatric services in chronic pain care. Gen Hosp Psychiatry. 2014;36(1):99–104. doi: 10.1016/j.genhosppsych.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Informatics IIfH, editor. The use of medicines in the United States: Review of 2010. 2011. [Google Scholar]
- 7.Grattan A, Sullivan MD, Saunders KW, Campbell CI, Von Korff MR. Depression and prescription opioid misuse among chronic opioid therapy recipients with no history of substance abuse. Ann Fam Med. 2012;10(4):304–11. doi: 10.1370/afm.1371. Epub 2012/07/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sullivan MD. Who gets high-dose opioid therapy for chronic non-cancer pain? Pain. 2010;151(3):567–8. doi: 10.1016/j.pain.2010.08.036. Epub 2010/09/10. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan MD, Edlund MJ, Zhang L, Unutzer J, Wells KB. Association between mental health disorders, problem drug use, and regular prescription opioid use. Arch Intern Med. 2006;166(19):2087–93. doi: 10.1001/archinte.166.19.2087. Epub 2006/10/25. [DOI] [PubMed] [Google Scholar]
- 10.Scherrer JF, Salas J, Copeland LA, Stock EM, Schneider FD, Sullivan MD, et al. Increased risk of depression recurrence after initiation of prescription opioids in non-cancer pain patients. Pain. doi: 10.1016/j.jpain.2015.12.012. In review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubitz N, Mehra M, Potluri RC, Garg N, Cossrow N. Characterization of treatment resistant depression episodes in a cohort of patients from a U.S. commercial claims database. PLOS One. 2013;8:e76882. doi: 10.1371/journal.pone.0076882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hygiene NYCDoHaM. Morphine milligram equivalent (MME) calculator. Available from: http://www.nyc.gov/html/doh/html/mental/MME.html.
- 13.Solberg LI, Engebretson KI, Sperl-Hillen JM, Hroscikoski MC, O’Connor PG. Are claims data accurate enough to identify patients for performance measures or quality improvement? The case of diabetes, heart disease and depression. Am J Med Qual. 2006;21:238–45. doi: 10.1177/1062860606288243. [DOI] [PubMed] [Google Scholar]
- 14.Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am. 1996;19:180–203. doi: 10.1016/s0193-953x(05)70283-5. [DOI] [PubMed] [Google Scholar]
- 15.Thase ME, Rush AJ. When at first you don’t succeed: sequential strategies for antidepressant nonresponders. J Clin Psychiatry. 1997;58(supple 13):23–9. [PubMed] [Google Scholar]
- 16.Corey-Lisle PK, Brinbam HG, Greenberg PE, Marynchenko MB, Claxton AJ. Identification of a claims data “signature’ and economic consequences for treatment-resistant depression. J Clin Psychiatry. 2002;63:717–26. doi: 10.4088/jcp.v63n0810. [DOI] [PubMed] [Google Scholar]
- 17.Scherrer JF, Chrusciel T, Garfield LD, Freedland KE, Carney RM, Lustman PJ, et al. Treatment-resistant and insufficiently treated depresion and all-cause mortality following myocardial infarction. Br J Psychiatry. 2012;200:137–42. doi: 10.1192/bjp.bp.111.096479. [DOI] [PubMed] [Google Scholar]
- 18.Seal KH, Shi Y, Cohen G, Cohen BE, Maguen S, Krebs EE, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US Veterans of Iraq and Afghanistan. JAMA. 2012;307:940–7. doi: 10.1001/jama.2012.234. [DOI] [PubMed] [Google Scholar]
- 19.Eaton WW. Medical and Psychiatric Comorbidity Over the Course of Life. American Psychiatric Publishing, Inc; Arlington VA: 2006. [Google Scholar]
- 20.Glassman A, Maj M, Satrorius N. Depression and Heart Disease. John Wiley and Sons Ltd; West Sussex, UK: 2011. [Google Scholar]
- 21.Smith HS, Elliott JA. Opioid-induced androgen deficiency (OPIAD) Pain Physician. 2012;15:ES145–ES56. [PubMed] [Google Scholar]
- 22.Onen SH, Onen F, Courpron P, Dubray C. How pain and analgesics disturb sleep. Clin J Pain. 2005;21:422–31. doi: 10.1097/01.ajp.0000129757.31856.f7. [DOI] [PubMed] [Google Scholar]
- 23.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168:656–64. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curtis LH, Hammill BG, Eisenstein EL, Kramer JM, Anstrom KJ. Using inverse probability-weighted estimators in comparative effectiveness analysis with observational databases. Med Care. 2007;45:S103–S7. doi: 10.1097/MLR.0b013e31806518ac. [DOI] [PubMed] [Google Scholar]
- 25.Kilpatrick RD, G D, Brookhart MA, Polley E, Rothman KJ, Bradbury BD. Exploring large weight deletion and the ability to balance confounders when using inverse probability of treatment weighting in the presence of rate treatment decisions. Pharmacoepidemiol Drug Safety. 2013;22:111–21. doi: 10.1002/pds.3297. [DOI] [PubMed] [Google Scholar]
- 26.Karp JF, Butters MA, Begley AE, Miller MD, Lenze EJ, Blumberger DM, et al. Safety, tolerability, and clinical effect of low-dose buprenorphine for treatment-resistant depression in midlife and older adults. J Clin Psychiatry. 2014;75:e785–e93. doi: 10.4088/JCP.13m08725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fava M, Memisoglu A, Thase ME, Bodkin JA, Trivedi MH, de Somer M, et al. Opioid modulation with burprenorphine/samidorphan as adjuntive treatment for inadequate response to antidepressants: a randomized double-blind placebo controlled trial. Am J Psychiatry. 2016;173:499–508. doi: 10.1176/appi.ajp.2015.15070921. [DOI] [PubMed] [Google Scholar]
- 28.Terenius L, Wahlstrom A, Agren H. Naloxone (Narcan) treatment in depression: Clinical observations and effects on CSF endorphins and monoamine metabolites. Psychopharmacology (Berl) 1977;54:31–3. doi: 10.1007/BF00426537. [DOI] [PubMed] [Google Scholar]
- 29.Scherrer JF, Salas J, Copeland LA, Stock EM, Schneider FD, Sullivan MD, et al. Increased risk of depression recurrence after initiation of prescription opioids in non-cancer pain patients. J Pain. 2016;17:473–82. doi: 10.1016/j.jpain.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vos T, Barber RM, Bell B, Bertozzi-Villa A, Biryukov S, Bollinger I, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russell JM, Hawkins K, Ozminkowski RJ, Orsini L, Crown WH, Kennedy S, et al. The cost of treatment-resistant depression. J Clin Psychiatry. 2004;65:341–7. doi: 10.4088/jcp.v65n0309. [DOI] [PubMed] [Google Scholar]
- 32.Daniell HW. Opioid endocrinopathy in women consuming prescribed sustained-action opioids for control of nonmalignant pain. The Journal of Pain. 2008;9:28–36. doi: 10.1016/j.jpain.2007.08.005. [DOI] [PubMed] [Google Scholar]


