Abstract
Patients with metastatic renal cell carcinoma (mRCC) have better overall survival when treated with nivolumab, a cancer immunotherapy that targets the immune checkpoint inhibitor programmed cell death 1 (PD-1), rather than everolimus (a chemical inhibitor of mTOR and immunosuppressant). Poor-risk mRCC patients treated with nivolumab seemed to experience the greatest overall survival benefit, compared to patients with favorable or intermediate-risk, in an analysis of the CheckMate-025 trial subgroup of the Memorial Sloan Kettering Cancer Center (MSKCC) prognostic risk groups. Here we explore whether tumor mutational load and RNA expression of specific immune parameters could be segregated by prognostic MSKCC risk strata and explain the survival seen in the poor-risk group. We queried whole exome transcriptome data in RCC patients (n = 54) included in The Cancer Genome Atlas that ultimately developed metastatic disease or were diagnosed with metastatic disease at presentation and did not receive immune checkpoint inhibitors. Nonsynonymous mutational load did not differ significantly by MSKCC risk group, nor was the expression of cytolytic genes –granzyme A and perforin – or selected immune checkpoint molecules different across MSKCC risk groups. In conclusion, this analysis found that mutational load and expression of markers of an active tumor microenvironment did not correlate with MSKCC risk prognostic classification in mRCC.
Advanced metastatic renal cell carcinoma (RCC) causes 14,000 deaths each year in the United States (1), but predictive biomarkers for selection of patients most likely to benefit from immune checkpoint blockade therapies have yet to be deeply explored in this cancer type. The CheckMate-025 trial was a trial in 821 patients with advanced clear-cell RCC, previously treated with vascular endothelial growth factor (VEGF)–targeted therapy (2). It demonstrated the superiority of nivolumab, a cancer immunotherapy that targets the immune checkpoint inhibitor programmed cell death 1 (PD-1), over everolimus – a mammalian target of rapamycin (mTOR) inhibitor. Nevertheless, more than one third of patients have progressive disease as their best response and have no benefit at all from nivolumab. Although responses can be durable, they encompass only 20–25% of all patients. Interestingly, subgroup analysis of Memorial Sloan Kettering Cancer Center (MSKCC) prognostic risk groups (3) – which are determined using markers of tumor aggressiveness including lactate dehydrogenase (LDH) level, serum calcium, hemoglobin, time from diagnosis, and Karnofsky performance status – showed that poor-risk mRCC patients treated with nivolumab seemed to experience greater overall survival (OS) benefit (HR 0.47 [95% CI 0.30–073]) compared to favorable (HR 0.86 [95% CI 0.59–1.32]) or intermediate-risk patients (HR 0.76 [95% CI 0.58–0.99]) (1). Immunohistochemical staining of tumor PD-1 ligand (PD-L1), the main biomarker correlate on the trial, showed that patients with high PD-L1 positivity tended to experience poorer survival whether treated with nivolumab or everolimus, suggesting PD-L1 staining has more prognostic than predictive value in mRCC and therefore this marker should not be used as a marker of treatment benefit in RCC.
Past studies in large clinical cohorts of other cancer types (metastatic melanoma and non-small-cell lung cancer) treated with immune checkpoint blockade have shown that high mutational load is associated with an improved response (4–6). Clinical benefit from anti-CTLA-4 therapy is also associated with immunoreactive RNA-based signatures in the pre-treatment tumor microenvironment (6). Although RCC is not a cancer with high mutation loads, a characteristic of melanoma and non-small-cell lung cancer (7), whole exome and whole transcriptome sequencing from tumor biopsies of mRCC patients with integrated analysis of clinical annotations may yield insights into the mechanism of action of response to immune checkpoint blockade among RCC clinical subgroups defined above, as well as help to develop predictive clinical indices for response to these drugs.
In this investigation, we sought to explore whether tumor mutational load and RNA expression of specific immune parameters segregated by prognostic MSKCC risk strata associated with greater likelihood of clinical benefit, namely OS, from nivolumab in the poor-risk group observed in CheckMate 025. We queried whole exome transcriptome data in RCC patients included in The Cancer Genome Atlas (TCGA) that ultimately developed metastatic disease or were diagnosed with metastatic disease at presentation (mRCC; n = 54, out of a total of 390 total cases) (8). This cohort of patients did not receive immune checkpoint inhibitors. This study is exploratory in nature and no pre-defined statistical assumptions were considered.
The median nonsynonymous mutational load in the aggregate mRCC cohort was 54 mutations/sample (range: 1 – 99) (Table 1). This translates to a median of 1.42 mutations/Mb (range: 0.035 – 2.77) and is comparable to previous reports (Kidney Renal Clear Cell Carcinoma-TCGA). MSKCC risk groups did not significantly differ in nonsynonymous mutational load (P = 0.17; Kruskal-Wallis chi-squared) (Table 1, Fig. 1A). We repeated the analysis classifying our 54 patients according to the more contemporary International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk criteria (9) and again mutational loads did not differ across different risk strata (P = 0.39; Kruskal-Wallis chi-squared).
Table 1.
Mutational load analysis by MSKCC risk groups
| MSKCC risk group |
Sample size |
Median nonsynonymous mutation load (Range) |
Median nonsynonymous mutation load / Mb (Range) |
|---|---|---|---|
| Poor | 8 | 43.5 (21–61) | 1.18 (0.49–1.77) |
| Intermediate | 38 | 55 (1–99) | 1.46 (0.035–2.77) |
| Favorable | 8 | 54.5 (2–77) | 1.42 (0.035–2.18) |
| Total | 54 | 54 (1–99) | 1.42 (0.035–2.77) |
Figure 1.
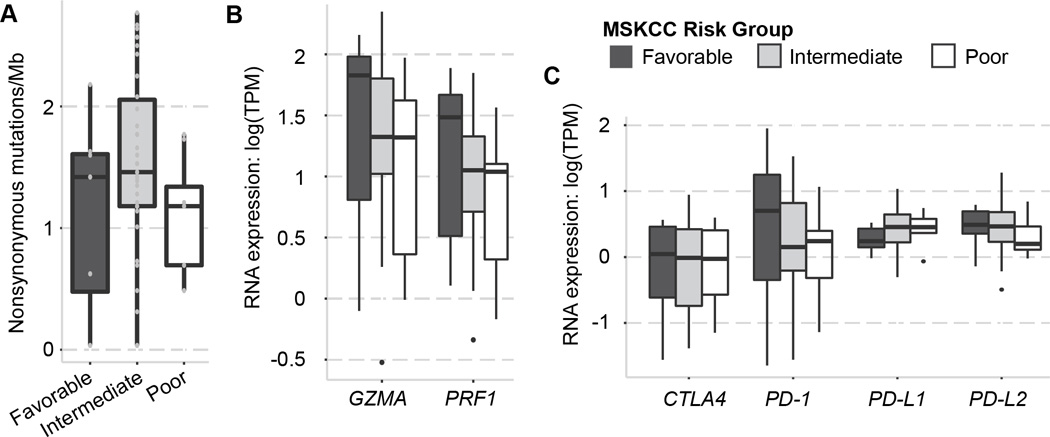
(A) Plots show the overall mutational load across different MSKCC prognostic subroups. Nonsynonymous mutational load was not associated with MSKCC prognostic risk categories. (B) Immune cytolytic activity (GZMA and PRF1) did not correlate with MSKCC prognostic risk categories. (C) RNA-seq expression of selected immune checkpoints CTLA-4, PD-1, PD-L1, and PD-L2 did not show a correlation with MKSCC prognostic risk categories.
Although RCC has lower mutational rates compared to other tumors in which immune checkpoint blockade has been successful, such as melanoma, bladder, and non-small-cell lung cancer (7, 9–11), its cytolytic activity is one of the highest of all cancer types (12), suggesting that an immunoreactive tumor microenvironment may be involved in mediating response to immunotherapies. Whole transcriptome data for the same set of 54 TCGA patients, revealed no differences in expression of cytolytic genes – granzyme A (GZMA) and perforin (PRF1) – or in selected immune checkpoint molecules (PD-1, PD-L1, PD-L2, CTLA-4) across MSKCC risk groups (P > 0.05 for all; Kruskal-Wallis chi-squared) (Fig. 1B–C).
It is also possible that the clinical observation of nivolumab providing superior benefit to poor-risk patients is due to a lower activity of everolimus in these patients. This speculation is not supported by the historical perspective in which temsirolimus –another sirolimus analog – showed a preferential benefit in patients with multiple poor prognostic features (13).
Intriguing data shows that anti-PD-L1 therapy may be associated with superior responses in tumors with Fuhrman grade 4 and/or sarcomatoid features (14). To avoid this potentially cofounding factor, we obtained Fuhrman grade information in our series and did not find an association between patients with low, intermediate, and high grades with MSKCC risk categories (P = 0.17).
In conclusion, this analysis in 54 clinically annotated whole-exome– and whole-transcriptome–sequenced RCC samples found that mutational load and expression of markers of an active tumor microenvironment, does not correlate with MSKCC risk prognostic classification in mRCC and, therefore cannot explain the superior benefit of nivolumab over everolimus in the poor-risk subgroup. We cannot exclude the fact that a real difference could not be detected because of the smaller size of our cohort. Other hypotheses to explore include the role of specific neoantigens in mediating response to mRCC: although mRCC may not be highly mutated on average, the presentation of a small number of immunogenic tumor-specific peptides may be sufficient to engender strong antitumor responses following release of immunosuppression using a cancer immunotherapy. Similarly, more complex interactions of tumors and the immune microenvironment may inform the subtype-specific response associations observed clinically that may be revealed through T-cell receptor sequencing (15). Given the large number of immunotherapies in clinical trials or preclinical development for mRCC, identifying patients most likely to benefit – or not – from these new treatments is a clinical priority for the future of cancer precision medicine. Biomarker-based studies with ample baseline and on-therapy tissue collection may lead to new biologic insights (16).
Acknowledgments
Acknowledgments/Funding Sources
The Trust family, Loker Pinard, and Michael Brigham Funds for Kidney Cancer Research (to T.K. Choueiri) at Dana-Farber Cancer Institute, the Dana-Farber/Harvard Cancer Center Kidney Cancer Program, and the Dana-Farber/Harvard Cancer. Center Kidney Cancer SPORE P50 CA101942-01. Spanish Society of Medical Oncology/ CRIS Cancer Foundation (to G.de Velasco). 2015 Kure It-AACR Research Grant for Immunotherapy in Kidney Cancer (to EVA and TKC). The funding source had no role in study design, analysis, interpretation or writing the manuscript.
Footnotes
Conflicts of Interest and Financial Disclosures
No potential conflicts of interest involving the work under consideration for publication
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol Off J Am Soc Clin Oncol. 1999;17:2530–2540. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 4.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350:207–211. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SAJR, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Creighton CJ, Morgan M, Gunaratne PH, Wheeler DA, Gibbs RA, Gordon Robertson A, et al. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013;499:43–49. doi: 10.1038/nature12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 11.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160:48–61. doi: 10.1016/j.cell.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 14.McDermott DF, Sosman JA, Sznol M, Massard C, Gordon MS, Hamid O, et al. Atezolizumab, an Anti-Programmed Death-Ligand 1 Antibody, in Metastatic Renal Cell Carcinoma: Long-Term Safety, Clinical Activity, and Immune Correlates From a Phase Ia Study. [cited 2016 Feb 13];J Clin Oncol [Internet] 2016 doi: 10.1200/JCO.2015.63.7421. Available from: http://jco.ascopubs.org/cgi/doi/10.1200/JCO.2015.63.7421. [DOI] [PubMed] [Google Scholar]
- 15.Cha E, Klinger M, Hou Y, Cummings C, Ribas A, Faham M, et al. Improved Survival with T Cell Clonotype Stability After Anti-CTLA-4 Treatment in Cancer Patients. Sci Transl Med. 2014;6:238ra70–238ra70. doi: 10.1126/scitranslmed.3008211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choueiri TK, Fishman M, Escudier B, McDermott DF, Drake CG, Kluger HM, et al. Immunomodulatory Activity of Nivolumab in Metastatic Renal Cell Carcinoma. Clin Cancer Res. 2016 May 11; doi: 10.1158/1078-0432.CCR-15-2839. pii: clincanres.2839.2015. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]


