Abstract
Socioeconomic status (SES) is a complex construct of multiple indicators, known to impact cancer outcomes, but has not been adequately examined among pediatric AML patients. This study aimed to identify the patterns of co-occurrence of multiple community-level SES indicators and to explore associations between various patterns of these indicators and pediatric AML mortality risk. A nationally representative US sample of 3,651 pediatric AML patients, aged 0–19 years at diagnosis was drawn from 17 Surveillance, Epidemiology, and End Results (SEER) database registries created between 1973 and 2012. Factor analysis, cluster analysis, stratified univariable and multivariable Cox proportional hazards models were used. Four SES factors accounting for 87% of the variance in SES indicators were identified: F1) economic/educational disadvantage, less immigration; F2) immigration-related features (foreign-born, language-isolation, crowding), less mobility F3) housing instability; and, F4) absence of moving. F1 and F3 showed elevated risk of mortality, adjusted hazards ratios (aHR) (95% CI): 1.07(1.02–1.12) and 1.05(1.00–1.10), respectively. Seven SES-defined cluster groups were identified. Cluster 1: (low economic/educational disadvantage, few immigration-related features, and residential-stability) showed the minimum risk of mortality. Compared to Cluster 1, Cluster 3: (high economic/educational disadvantage, high-mobility) and Cluster 6: (moderately-high economic/educational disadvantages, housing-instability and immigration-related features) exhibited substantially greater risk of mortality, aHR(95% CI) = 1.19(1.0–1.4) and 1.23 (1.1–1.5), respectively. Factors of correlated SES-indicators and their pattern-based groups demonstrated differential risks in the pediatric AML mortality indicating the need of special public-health attention in areas with economic-educational disadvantages, housing-instability and immigration-related features.
Keywords: pediatrics, acute myeloid leukemia, mortality, social class, immigrants, residential mobility
1. Introduction
In the United States (US) acute myeloid leukemia (AML) accounts for less than 20% of pediatric leukemia cases but 50% of pediatric leukemia deaths [1] with a 5-year survival rate of 62.8% for those under age 19 [2]. Given this high mortality rate, it is important to understand factors associated with AML mortality risk. Socioeconomic status (SES), is a known contributor to pediatric cancer mortality risk [3–9]. While often an individually-based measure of economic, sociological, educational and/or cultural indicators [5], census-based community-level SES measures are also useful predictors of mortality risk [3,5,8,9] and may point toward communities in need of resources to reduce SES-related survival disparities. Specifically within the US, children living in communities with high poverty rates have been found to have greater mortality risk [5,9]. Race/ethnicity, age at diagnosis, AML subtype and region of the country are also prognostic indicators in US population-based data.
While the mechanism through which SES exerts effects on mortality are likely complex and indirect [10–12], evidence does suggest that compounding SES risk factors (e.g., housing instability, financial strain, limited transportation) can interfere with health status [13], healthcare delivery [14] and subsequent survival. Negative community-level factors (e.g., limited resource access, chronic stress exposure, limited healthcare access) likely amplify family and individual-level mortality risk factors (e.g, family stress, biological vulnerability) [15,16].
SES indicators are interrelated in complex ways [17,18]. Within pediatric leukemia samples, for example, lower household income is associated with greater residential mobility [13]. SES indicators such as poverty and unemployment rates, educational status, housing instability, immigration, and language isolation may exist in isolation or cluster within specific communities.
A first step in examining associations between community-level SES indicators and outcomes is to identify how these variables naturally co-occur. Analyses constructing formal latent variables may better capture patterns of association among these variables compared to other methods of combining the data such as summing across indicators or entering multiple correlated indicators simultaneously in predictive models. A second step is to explore how the confluence of correlations of these many indicators impacts health and disease outcomes.
The purpose of the current study is to build upon the current research investigating community-level SES and pediatric AML survival with a large US population-based dataset examining: a) how multiple community-level SES indicators are associated with one another; and b) how the confluence of these indicators is associated with mortality of pediatric AML patients. Exploratory factor analysis was used to form latent variables (i.e. ‘factor scores’), based on the pattern of correlation among SES indicators, then cluster analysis of these latent variables was conducted to group pediatric AML patients based upon the pattern of SES indicators in their community. Finally, the mortality risk was investigated as a function of SES latent variables and SES cluster group. The large Surveillance, Epidemiology and End Results (SEER) database provided the opportunity to explore these associations with adequate precision even though there is substantial heterogeneity in the disease and treatment response of AML, as well as in the correlation of community-level SES indicators.
2. Methods
2.1 Data Source
Data were drawn from the US population-based SEER 17 registry and included 3,651 pediatric (0–19 years) AML patients diagnosed between 1973 and 2012 [19]. A written approval was received to use SEER data. The SEER registry collects demographic, clinical treatment and outcome data on cancer patients representing approximately 28% of the US population. The SEER database includes community-level SES indicators collected by the Census American Community Survey (ACS). Detailed descriptions of the SEER data are available in the SEER Program Coding and Staging Manual [20].
2.2 Variables Investigated
2.2.1 Individual and County Demographics
Sex, race-ethnicity (Non-Hispanic Caucasian, Non-Hispanic African American, Hispanic, Other) and US geographic region (Eastern seaboard, Pacific Coast (including Alaska), Northern Plains, and Southwest) were the individual prognostic indicators examined. The community composition of typically non-working age groups (i.e., percentage < 18 years and > 65 years of age) were also examined.
2.2.2 Clinical Characteristics
Age at diagnosis and AML subtype were the clinical variables examined. Age at diagnosis was coded in years and grouped within SEER data as <1, 1–4, 5–9, 10–14, and 15–19. Following World Health Organization (WHO) 2008 classification, AML subtype was classified as one 16 options as listed in Table 2 [21].
Table 2.
Distribution of Within Cluster Pediatric AML Patients by Known Prognostic Factors of Pediatric AML Survival
| Variables | Cluster 1 |
Cluster 2 |
Cluster 3 |
Cluster 4 |
Cluster 5 |
Cluster 6 |
Cluster 7 |
|---|---|---|---|---|---|---|---|
|
n (% within) |
n (% within) |
n (% within) |
n (% within) |
n (% within) |
n (% within) |
n (% within) |
|
| Age at Diagnosis | |||||||
| < 1 year | 62 (9.2) |
50 (10.2) |
27 (6.5) |
38 (11.3) |
62 (7.9) |
27 (7.5) |
59 (10) |
| 1 – 4 years | 137 (20.4) |
113 (23) |
99 (23.9) |
87 (25.8) |
197 (25.2) |
84 (23.2) |
139 (23.6) |
| 5 – 9 years | 106 (15.8) |
76 (15.5) |
60 (14.5) |
39 (11.6) |
120 (15.4) |
48 (13.3) |
98 (16.6) |
| 10 – 14 years | 174 (25.9) |
118 (24) |
87 (21) | 86 (25.5) |
159 (20.4) |
93 (25.7) |
132 (22.4) |
| 15 – 19 years | 193 (28.7) |
134 (27.3) |
142 (34.2) |
87 (25.8) |
243 (31.1) |
110 (30.4) |
161 (27.3) |
| Sex | |||||||
| Male | 308 (45.8) |
231 (47) |
201 (48.4) |
158 (46.9) |
383 (49) |
192 (53) |
276 (46.9) |
| Female | 364 (54.2) |
260 (53) |
214 (51.6) |
179 (53.1) |
398 (51) |
170 (47) |
313 (53.1) |
| Race/Ethnicity | |||||||
| Caucasian | 483 (71.9) |
101 (20.6) |
219 (52.8) |
127 (37.7) |
520 (66.6) |
140 (38.7) |
286 (48.6) |
| African American | 70 (10.4) |
37 (7.5) |
92 (22.2) |
15 (4.5) |
119 (15.2) |
44 (12.2) |
57 (9.7) |
| Hispanic | 90 (13.4) |
306 (62.3) |
77 (18.6) |
61 (18.1) |
85 (10.9) |
149 (41.2) |
145 (24.6) |
| Other | 29 (4.3) |
47 (9.6) |
27 (6.5) |
134 (39.8) |
57 (7.3) |
29 (8) | 101 (17.1) |
| US Region | |||||||
| Eastern | 478 (71.1) |
0 (0) | 135 (32.5) |
16 (4.7) |
289 (37) |
56 (15.5) |
25 (4.2) |
| Pacific Coast, Alaska | 8 (1.2) | 491 (100) |
61 (14.7) |
321 (95.3) |
162 (20.7) |
306 (84.5) |
476 (80.8) |
| Northern Plains | 144 (21.4) |
0 (0) | 162 (39) |
0 (0) | 117 (15) |
0 (0) | 88 (14.9) |
| South West | 42 (6.3) |
0 (0) | 57 (13.7) |
0 (0) | 213 (27.3) |
0 (0) | 0 (0) |
| AML Subtypes | |||||||
| 9866/3: Acute Promyelocytic Leukemia (APL) | 65 (9.7) |
68 (13.8) |
47 (11.3) |
33 (9.8) |
75 (9.6) |
51 (14.1) |
71 (12.1) |
| 9861/3: Acute Myeloid Leukemia (AML) | 416 (61.9) |
181 (36.9) |
243 (58.6) |
164 (48.7) |
458 (58.6) |
150 (41.4) |
306 (52) |
| 9840/3: Acute Erythroid Leukemia (AEL) | 4 (0.6) | 8 (1.6) | 6 (1.4) | 14 (4.2) |
14 (1.8) |
10 (2.8) |
3 (0.5) |
| 9865/3: AML with t(6;9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.1) | 0 (0) | 0 (0) |
| 9867/3: Acute Myelomonocytic Leukemia (AMML) |
49 (7.3) |
73 (14.9) |
34 (8.2) |
27 (8) | 43 (5.5) |
34 (9.4) |
55 (9.3) |
| 9871/3: AML with inv(16) | 9 (1.3) | 7 (1.4) | 5 (1.2) | 4 (1.2) | 14 (1.8) |
7 (1.9) | 9 (1.5) |
| 9872/3: AML with minimal differentiation | 20 (3) | 14 (2.9) |
9 (2.2) | 10 (3) | 16 (2) | 13 (3.6) |
27 (4.6) |
| 9873/3: AML without maturation | 12 (1.8) |
24 (4.9) |
10 (2.4) |
4 (1.2) | 29 (3.7) |
27 (7.5) |
18 (3.1) |
| 9874/3: AML with maturation | 19 (2.8) |
44 (9) | 18 (4.3) |
30 (8.9) |
39 (5) | 21 (5.8) |
26 (4.4) |
| 9895/3: AML with myelodysplasia-related changes (AML w/ MDS) |
9 (1.3) | 3 (0.6) | 2 (0.5) | 5 (1.5) | 2 (0.3) | 3 (0.8) | 9 (1.5) |
| 9896/3: AML t(8;21) | 13 (1.9) |
9 (1.8) | 7 (1.7) | 6 (1.8) | 15 (1.9) |
11 (3) | 8 (1.4) |
| 9897/3: AML with t(9;11) | 7 (1) | 6 (1.2) | 3 (0.7) | 4 (1.2) | 6 (0.8) | 7 (1.9) | 9 (1.5) |
| 9898/3: Myeloid Leukemia with Down Syndrome (AML w/ DS) |
1 (0.1) | 2 (0.4) | 2 (0.5) | 5 (1.5) | 7 (0.9) | 3 (0.8) | 4 (0.7) |
| 9910/3: Acute Megakaryoblastic Leukemia (AMKL) |
34 (5.1) |
48 (9.8) |
23 (5.5) |
27 (8) | 54 (6.9) |
19 (5.2) |
33 (5.6) |
| 9911/3: AML with t(1;22) | 0 (0) | 1 (0.2) | 0 (0) | 0 (0) | 0 (0) | 1 (0.3) | 0 (0) |
| 9920/3: Therapy-related Acute Myeloid Neoplasm (t-AML) |
14 (2.1) |
3 (0.6) | 6 (1.4) | 4 (1.2) | 8 (1) | 5 (1.4) | 11 (1.9) |
Note. Not all columns round to 100% due to rounding
2.2.3 County-level SES indicators
The following county-level SES indicators drawn from the ACS County Attributes data from 2009–2013 were examined. Education (3 variables): the percentage of individuals over the age of 25 years with an education of less than 9th grade, less than high school graduate, and at least a bachelor’s degree. Poverty (4 variables): the percentage of persons below poverty, families below poverty, persons below 150% poverty, and persons below 200% poverty in the county of residence. Crowding (1 variable): the percentage of households with more than one person per room. Income and employment (3 variables): unemployment (the percentage of persons over age 16 years who were unemployed) and median family and median household income measured in 2013 inflation adjusted dollars. Relocation (8 variables): the percentage of individuals in the county age 5 years old older and the percentage of individuals in the county age 1 year and older who remained in the same house, moved within the county, moved to a different county within the same state, and moved to a different state. Immigration (4 variables): the percentage of persons who were foreign born (from the 2008–2012 Census), percentage of households linguistically isolated (no household member age 14 years or older speaking English or speaking a language other than English and speaking English “very well”) and percentages (ages ≥1 years) and (ages ≥5 years) within the county that moved to the US from another nation.
2.2.4. Outcome: Mortality
The SEER variable “vital status recode” was used to determine overall mortality. Patients who were alive on the last follow-up day were reported as alive. The follow-up time was documented as the duration from the time of diagnosis to death or the last day of the available survival information in the SEER registry. The last follow-up day is the December 31, 2012.
2.3 Statistical Analysis
The 23 county-level socioeconomic variables available in the SEER data were subject to an exploratory factor analysis with varimax rotation for data reduction and identification of the structure of these complex correlated variables. Decisions regarding the final factor solution were based upon scree plot, total explained variance, amount of variance extracted from each individual item and factor loadings. Each patient in the dataset was then assigned factor scores, a linear combination of their observed values on each variable accomplished through multiple regressions. These factors scores were then subject to a two-stage agglomerative hierarchical clustering method with Gaussian mixture model-based distance measure in order to form distinct groups within the SEER database based on heterogeneity of the SES factor structures. The Schwarz Bayesian Criterion (BIC) associated with different cluster solutions was used to determine the optimal number of clusters. The resulting cluster groups were then characterized in regard to the original SES variables, patients’ demographics, clinical characteristics and vital status. Quantitative variables were summarized by mean and SD or median and inter-quartile range (IQR), as appropriate. Categorical variables were summarized by frequencies and percentages of patients in the corresponding groups.
Univariable stratified Cox proportional hazards models were then used to determine the association of the SES factors, SES cluster groups and other possible prognostic indicators (i.e., age at diagnosis, sex, race-ethnicity, AML subtypes and US region) with AML mortality. Finally, separate stratified multivariable Cox proportional hazards model were used to determine associations between the SES factors and SES cluster groups with AML mortality adjusting for the significant prognostic factors identified in the univariable models. All models were stratified by year of diagnosis to account for the time-varying survival pattern and other heterogeneity that arises in long-term population-based registry data. The rationale and method of the stratified Cox proportional hazards model are previously described [9]. All analyses were two-tailed with the level of significance of 0.05. The statistical software SPSS version 22 (IBM, Chicago, IL) and SAS 9.3 (SAS Institute, Cary, NC) were used for data analyses.
3. Results
3.1 Co-occurrence of county-level SES variables: Characterization of SES factors
Factor analysis of the 23 SES variables resulted in a four factor solution that explained 87% of the variance in the original variables. The correlation coefficients between the variables and factors (factor loadings) and the proportion of information extracted from each variable are presented in Supplementary Table 1. Factor 1 captured variables related to economic and educational disadvantage but not immigration: greater rates of community-level poverty, lower income, lack of higher education, higher unemployment and lower rates of immigration within the past year. Factor 2 captured variables related to immigration but recent residential stability: greater rates of community-level language isolation, foreign-born status, crowding, education at or below the high school level, but higher rates of residential stability within the state during the past year. Factor 3 captured items related to housing instability: lower rates of community-members staying within the same house in the past year, higher rates of moving within the county, and moving to the US from outside the country in the past year. Factor 4 reflected low rates of community members changing residences within the state.
3.2 Formation and description of SES profiles: Confluence of SES indicators
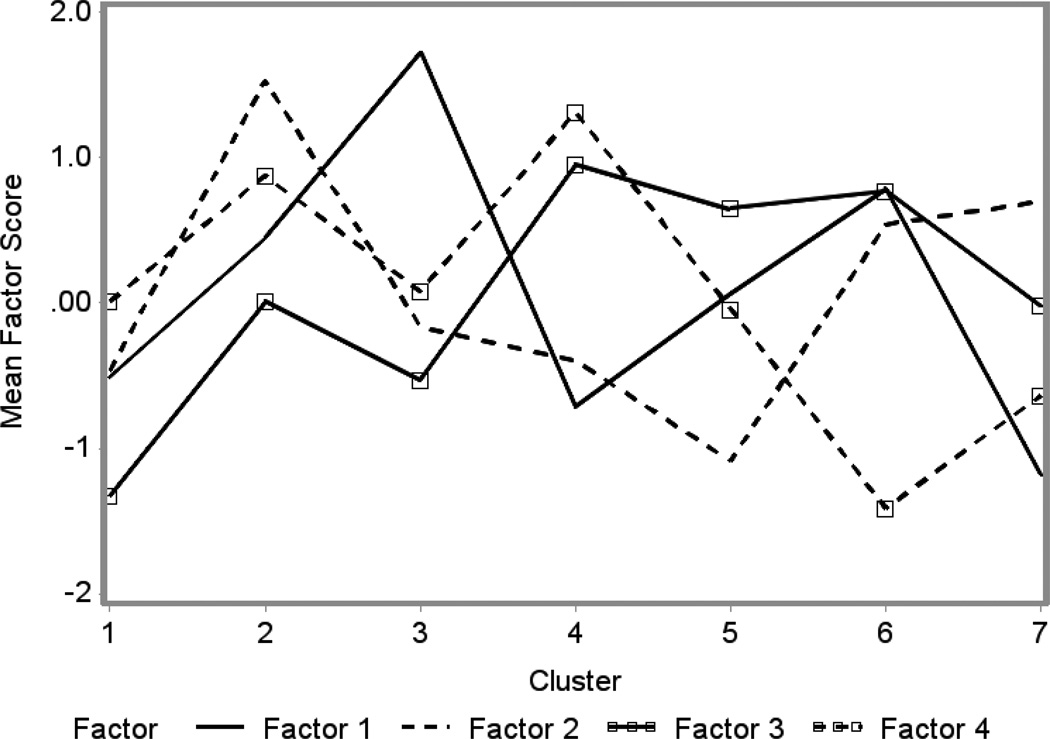
Cluster analysis of the SES factor scores resulted in seven distinct SES groups. Figure 1 illustrates the pattern of factor scores across the clusters and Table 1 presents the county level socio-economic characteristics of the pediatric AML patients falling into each of these seven cluster groups. Cluster 1 was distinguished by low community rates of economic and educational disadvantage (Factor 1), immigration-related features (Factor 2), and residential instability (Factor 3). Cluster 2 was distinctive for a high proportion of immigration-related features (Factor 2) and residential instability (Factors 3 and 4reversed) with moderately high rates of economic and educational disadvantage (Factor 1). Cluster 3 was distinguished by the highest proportion of economic and educational disadvantage (Factor 1) and low rates of residential instability (Factor 3). Cluster 4 had low rates of economic and educational disadvantage (Factor 1) and immigration-related features (Factor 2) with the greatest rates of housing instability within the county or from another country (Factor 3) but low rates of moving within the state across counties (Factor 4). Cluster 5 was characterized by the lowest levels of immigration-related factors within the community (Factor 2) with high rates of housing instability (Factor 3). Cluster 6 was distinguished by high rates of economic and educational disadvantage (Factor 1), immigration-related features (Factor 2) and housing instability (Factors 3 and 4reversed). Cluster 7 was distinguished by the lowest rates of economic and educational disadvantage (Factor 1), but high rates of immigration-related features (Factor 2) and moderate rates of housing instability (Factor 3) and changing residents within the state (Factor 4). Table 2 displays the demographic and clinical characteristics of the patients within each cluster group. The distributions of patients differed across groups with respect to all SES indicators, race/ethnicity, AML subtypes and US region (p < 0.001). No significant differences emerged in regard to sex (p = 0.42) or age at diagnosis (p = 0.10).
Figure 1. Patterns of SES Factor Scores Across SES Clusters.
Factor 1: Community-level greater rates of poverty, lower income, lack of higher education, greater unemployment and lower rates of immigration within the past year. Factor 2: Greater rates of language isolation, foreign-born status, crowding, education at or below the high school level, but higher rates of residential stability within the state during the past year. Factor 3: Greater rates of housing instability and country in the past year. Factor 4: Greater rates of housing stability.
Cluster 1: low economic and educational disadvantage (low poverty, higher education), immigration-related features (language isolation, foreign-born status, crowding) and residential instability; Cluster 2: high proportion of immigration-related features, residential instability, moderately high rates of economic and educational disadvantage; Cluster 3: highest proportion of economic and educational disadvantage, low rates of residential instability; Cluster 4: low rates of economic and educational disadvantage, immigration-related features, greatest rates of housing instability, immigration, but low rates of moving within the state across counties; Cluster 5: lowest levels of immigration-related factors, high rates of housing instability, moderately low economic and educational disadvantage; Cluster 6: high economic and educational disadvantage, immigration-related features, housing instability; Cluster 7: the lowest economic and educational disadvantage, high immigration-related features, moderate housing instability.
Table 1.
Characterization of County-level Socioeconomic Variables by Cluster
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | Cluster 5 | Cluster 6 | Cluster 7 | |
|---|---|---|---|---|---|---|---|
| Variables | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| Age | |||||||
| % < 18 Years |
24.11 (2.86) | 24.11 (0.63) | 25.71 (2.74) | 22.07 (0.79) | 25.31 (3.58) | 26.74 (2.87) | 22.43 (2.8) |
| % > 65 Years |
14.2 (2.83) | 11.22 (0.05) | 13.24 (2.47) | 12.67 (1.66) | 11.39 (3.09) | 11 (1.98) | 12.71 (1.27) |
| Education | |||||||
| % < 9th Grade |
4.46 (1.8) | 13.93 (1.22) | 9.04 (5.01) | 5.32 (1.93) | 3.89 (1.51) | 9.82 (3.44) | 6.56 (2.23) |
| % < High School |
11.05 (3.18) | 23.69 (1.39) | 21.19 (5.92) | 10.93 (3.08) | 10.33 (2.67) | 20.21 (4.89) | 12.50 (2.99) |
| % at least Bachelor’ s |
31.34 (9.69) | 29.31 (1.72) | 17.79 (4.49) | 36.68 (6.75) | 31.64 (8.19) | 20.82 (5.78) | 41.91 (5.27) |
| Poverty | |||||||
| % Families Below |
8.08 (2.58) | 14.14 (0.5) | 19.47 (3.42) | 8.64 (1.91) | 10.68 (2.89) | 14.66 (3.22) | 7.57 (1.22) |
| % Persons Below |
10.78 (3) | 17.82 (0.39) | 24.5 (3.8) | 12.32 (1.86) | 14.7 (3.44) | 19.09 (3.8) | 11.02 (1.71) |
| % Persons < 150% |
18.03 (4.55) | 29.84 (0.59) | 36.82 (4.84) | 19.8 (3.12) | 23.83 (4.79) | 30.69 (4.86) | 18.28 (2.62) |
| % Persons < 200% |
25.77 (6) | 40.38 (0.76) | 47.51 (5.38) | 27.54 (4.17) | 33.21 (5.73) | 41.36 (5.5) | 25.53 (3.27) |
| % Crowding |
2.27 (1.64) | 12.11 (0.17) | 4.1 (3.12) | 6.13 (2.44) | 2.64 (1.15) | 6.4 (2.3) | 5.68 (2.5) |
| Income | |||||||
| % Unemploy ed |
9.31 (2.56) | 11.46 (0.46) | 14.32 (3.77) | 8.23 (1.46) | 9.14 (2.28) | 14.11 (2.16) | 9.6 (0.8) |
| Median Family ($) |
80,389 (16,285) |
62,236 (1,109) |
48,798 (5,898) |
81,423 (8,678) |
67,345 (8,727) |
59,483 (7,869) |
91,484 (8,522) |
| Median Househol d ($) |
65,493 (13,515) |
55,999 (1,131) |
39,802 (5,164) |
67,452 (4,298) |
55,357 (7,787) |
51,950 (7,273) |
76,794 (8,436) |
|
Relocatio n |
|||||||
|
Age 5+ years |
|||||||
| % No Moving |
89.07 (1.73) | 86.50 (0.50) | 86.7 (2.45) | 83.81 (1.51) | 82.78 (2.78) | 81.60 (1.93) | 85.48 (1.25) |
| % Move within County |
6.20 (1.38) | 10.44 (0.17) | 9.22 (2.52) | 10.39 (1.35) | 9.97 (1.72) | 11.89 (1.82) | 8.82 (1.31) |
| % Move within State |
2.54 (1.19) | 1.33 (0.39) | 2.48 (1.15) | 1.37 (0.64) | 3.35 (1.26) | 4.66 (1.65) | 3.47 (0.76) |
| % Move within Country |
1.69 (0.66) | 1.04 (0.26) | 1.27 (0.74) | 3.22 (0.78) | 3.22 (1.07) | 1.34 (0.55) | 1.31 (0.31) |
|
Age 1+ years |
|||||||
| % No Moving |
88.75 (1.77) | 86.32 (0.51) | 86.25 (2.52) | 83.61 (1.47) | 82.48 (2.72) | 81.13 (1.83) | 85.26 (1.20) |
| % Move within County |
6.40 (1.43) | 10.64 (0.17) | 9.59 (2.62) | 10.56 (1.33) | 10.21 (1.69) | 12.28 (1.83) | 9.02 (1.34) |
| % Move within State |
2.60 (1.24) | 0.32 (0.37) | 2.53 (1.16) | 1.36 (0.63) | 3.37 (1.27) | 4.71 (1.61) | 3.48 (0.75) |
| % Move within Country |
1.75 (0.69) | 1.03 (0.27) | 1.29 (0.74) | 3.26 (0.81) | 3.25 (1.09) | 1.35 (0.57) | 1.31 (0.30) |
|
Immigrati on |
|||||||
| % Foreign Born |
12.17 (7.93) | 34.88 (1.09) | 8.51 (6.71) | 21.55 (4.88) | 9.9 (5.12) | 18.48 (6) | 27.04 (8.71) |
| % Language Isolation |
4.34 (3.13) | 14.46 (0.41) | 4.13 (3.97) | 7.35 (2.4) | 3.24 (1.83) | 6.94 (2.77) | 8.31 (3.24) |
| % Moved to US (5+ yrs) |
0.50 (0.23) | 0.68 (0.02) | 0.33 (0.18) | 1.2 (0.14) | 0.68 (0.32) | 0.51 (0.23) | 0.93 (0.34) |
| % Moved to US (1+ yrs) |
0.50 (0.23) | 0.68 (0.17) | 0.33 (0.18) | 1.20 (0.14) | 0.68 (0.32) | 0.51 (0.23) | 0.93 (0.34) |
Note. All SES variables with percentages represent the percentage of persons living within a county with that characteristics calculated by the Census American Community Survey (ACS) 2009 – 2013; Median Family Income and Median Household income are in dollars; ANOVA p value < .001 for all variables
3.3 Identification of other important prognostic indicators
Table 3 provides hazard ratios (HR), 95% confidence intervals (CI) and p-values to illustrate the association between known prognostic factors and AML mortality from univariable Cox proportional hazards models. Age at diagnosis, race/ethnicity, regional location, and AML subtype were all associated with mortality and were used for adjustment in the analysis determining the association between SES and pediatric AML mortality.
Table 3.
Distribution of Pediatric AML Mortality and Hazard Risk Associated with Various Prognostic Factors, SEER 1973–2012
| Variable | Number of Death n (%) | HR (95% CI) | p-value |
|---|---|---|---|
| Age at Diagnosis | |||
| < 1 year | 150 (46) | 1.00 | - |
| 1 – 4 years | 365 (43) | 0.85 (0.7, 1.02) | 0.08 |
| 5 – 9 years | 246 (45) | 0.77 (0.63, 0.95) | 0.01 |
| 10 – 14 years | 429 (51) | 0.98 (0.81, 1.18) | 0.79 |
| 15 – 19 years | 572 (53) | 1.1 (0.92, 1.32) | 0.31 |
| Sex | |||
| Female | 831 (48) | 1.00 | - |
| Male | 931 (49) | 1.05 (0.96, 1.16) | 0.27 |
| Race/Ethnicity | |||
| Caucasian | 911 (49) | 1.00 | |
| African American | 243 (56) | 1.29 (1.12, 1.49) | <0.001 |
| Hispanic | 508 (56) | 1.17 (1.04, 1.32) | 0.01 |
| Other | 224 (53) | 1.05 (0.9, 1.22) | 0.54 |
| US Region | |||
| Eastern | 428 (43) | 1.00 | - |
| Pacific Coast, Alaska | 843 (46) | 1.08 (0.96, 1.22) | 0.20 |
| Northern Plains | 310 (61) | 1.06 (0.91, 1.24) | 0.44 |
| South West | 181 (57) | 1.23 (1.03, 1.46) | 0.02 |
| AML Subtypes | |||
| 9866/3: APL | 125 (30) | 1.00 | - |
| 9861/3: AML | 1071 (56) | 1.7 (1.41, 2.06) | <0.001 |
| 9840/3: AEL | 36 (61) | 2.2 (1.52, 3.2) | <0.001 |
| 9865/3: AML with t(6;9) | - | 0 (0, 0) | 0.91 |
| 9867/3: AMML | 157 (50) | 1.93 (1.52, 2.44) | <0.001 |
| 9871/3: AML with inv(16) | 9 (16) | 0.64 (0.32, 1.25) | 0.19 |
| 9872/3: AML w/ min diff | 63 (58) | 2.39 (1.76, 3.25) | <0.001 |
| 9873/3: AML w/o mat | 55 (44) | 1.79 (1.3, 2.46) | <0.001 |
| 9874/3: AML w/ mat | 76 (39) | 1.41 (1.06, 1.88) | 0.02 |
| 9895/3: AML w/ MDS | 11 (33) | 1.46 (0.79, 2.71) | 0.23 |
| 9896/3: AML t(8;21) | 17 (25) | 1 (0.6, 1.66) | 0.99 |
| 9897/3: AML with t(9;11) | 13 (31) | 1.52 (0.86, 2.69) | 0.15 |
| 9898/3: AML w/ DS | 1 (4) | 0.3 (0.04, 2.17) | 0.23 |
| 9910/3: AMKL | 99 (42) | 1.57 (1.2, 2.04) | 0.001 |
| 9911/3: AML with t(1;22) | 1 (50) | 7.5 (1.04, 54.15) | 0.05 |
| 9920/3: t-AML | 28 (55) | 4.26 (2.8, 6.48) | <0.001 |
Note: AML: Acute Myeloid Leukemia; HR: Hazards Ratio; CI: Confidence Interval; ;9866/3: Acute Promyelocytic Leukemia (APL); 9861/3: Acute Myeloid Leukemia (AML); 9840/3: Acute Erythroid Leukemia (AEL); 9865/3: AML with t(6;9); 9867/3: Acute Myelomonocytic Leukemia (AMML); 9871/3: AML with inv(16); 9872/3: AML with minimal differentiation; 9873/3: AML without maturation; 9874/3: AML with maturation; 9895/3: AML with myelodysplasia-related changes (AML w/MDS); 9896/3: AML t(8;21); 9897/3: AML with t(9;11); 9898/3: Myeloid Leukemia with Down Syndrome (AML w/ DS); 9910/3: Acute Megakaryoblastic Leukemia (AMKL); 9911/3: AML with t(1;22); 9920/3: Therapy-related Acute Myeloid Neoplasm (t-AML)
3.4 SES factors, SES profiles and AML mortality
Out of 3651 AML pediatric patients, 1762 (48.3%) died. Table 4 presents the unadjusted and adjusted hazard risk of pediatric AML mortality associated with each SES factor and cluster using univariable and multivariable Cox proportional hazards models stratified by year of diagnosis. A one unit increase in the average score on Factor 1 (economic/educational disadvantage) and Factor 3 (housing instability) are associated with an 8% and 5% increase in the hazard risk of death; HR (95% CI): 1.08 (1.03, 1.13) and 1.05 (1.00, 1.10), respectively. After mutual adjustment for factors and known prognostic variables, an increased score on Factor 1 and Factor 3 were still associated with a substantially higher risk of mortality, adjusted HR (95% CI): 1.07 (1.02, 1.12) and 1.05 (1.00, 1.10), respectively. In comparison to the patients in Cluster 1 who had the lowest risk of mortality, patients in Cluster 3 (high economic and educational disadvantage) and Cluster 6 (moderately high economic and education disadvantage in conjunction with residential instability and immigration-related factors) demonstrated an average of 23% and 26% increased risk of mortality in the univariable model, HR (95% CI): 1.23 (1.04, 1.46) and 1.26 (1.03, 1.54), respectively. The increased mortality risk associated with each of these two clusters persisted after adjustment for known prognostic factors, HR (95% CI): 1.19 (1.00, 1.42) and 1.23 (1.01, 1.51), respectively. No substantial difference was observed in results after stratification of the model by age at diagnosis and sex in addition to year of diagnosis except the marginally loss of significance in the difference of mortality between Cluster 1 and Cluster 3 (Table S2). In addition, the variables in their descending order of predictive death risk of pediatric AML is provided in the Supplementary Table S3.
Table 4.
Unadjusted and Adjusted Hazard Risk of Pediatric AML Mortality Associated with Socio-economic Factors and Clusters, SEER 1973–2012
| Unadjusted Analysis | Adjusted Analysis | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | p-value | HR (95% CI) | p-value |
| SES Factors | ||||
| Factor 1 | 1.08 (1.03, 1.13) | 0.002 | 1.07 (1.02, 1.12) | 0.005 |
| Factor 2 | 1.00 (0.95, 1.05) | 0.95 | 0.99 (0.94, 1.04) | 0.69 |
| Factor 3 | 1.05 (1.00, 1.10) | 0.04 | 1.05 (1.00, 1.10) | 0.06 |
| Factor 4 | 0.99 (0.95, 1.04) | 0.82 | 0.98 (0.93, 1.03) | 0.48 |
| SES Clusters | ||||
| Cluster 1 | 1.00 | - | 1.00 | - |
| Cluster 2 | 1.15 (0.96, 1.37) | 0.13 | 1.13 (0.94, 1.37) | 0.20 |
| Cluster 3 | 1.23 (1.04, 1.46) | 0.02 | 1.19 (1.00, 1.42) | 0.05 |
| Cluster 4 | 1.09 (0.9, 1.32) | 0.36 | 1.10 (0.9, 1.34) | 0.37 |
| Cluster 5 | 1.11 (0.96, 1.29) | 0.16 | 1.13 (0.97, 1.31) | 0.10 |
| Cluster 6 | 1.26 (1.03, 1.54) | 0.02 | 1.23 (1.01, 1.51) | 0.04 |
| Cluster 7 | 1.05 (0.89, 1.23) | 0.59 | 1.04 (0.88, 1.23) | 0.64 |
Note: AML: Acute Myeloid Leukemia; HR: Hazards Ratio; CI: Confidence Interval; Adjusted models controlled for the following prognostic indicators: age at diagnosis, race-ethnicity, sex, US region and AML subtypes
4. Discussion
This analysis of associations between community-level SES indicators and pediatric AML mortality risk found two high-risk groups: a) those with the highest levels of economic and educational disadvantage in their communities (Cluster 3) and b) those with moderately high levels of economic and educational disadvantage in conjunction with residential instability and immigration-related features (i.e., language isolation, foreign-born status and crowding; Cluster 6). These findings held after controlling for other well-known prognostic indicators (e.g., age, race/ethnicity, AML subtype, regional area). This study advances knowledge related to the naturally occurring associations between community-level SES indicators and specific patterns of these variables that constitute elevated risk of pediatric AML mortality.
Prior research has linked various indices of SES to mortality among pediatric leukemia patients. These studies have examined individually-based indices such as parental occupation, maternal education and household income [4,6,7] and community-level variables such as poverty and unemployment rates, educational attainment, median household income and crowding [5,8, 22]. Typically such indices are either examined separately, in some form of composite (e.g., summed, averaged), or simultaneously in predictive models, ignoring correlations among variables. Our approach to these data greatly improves upon the analytic methods used in past research. We first used factor analysis to isolate latent factors within a large set of community-based SES indicators. This approach reduced the number of variables and empirically disentangled the complex underlying relationships between them. Next, cluster analysis was conducted with factor scores on these latent constructs to identify distinct patterns of community-level SES indicators. This approach acknowledges that, although these SES constructs often overlap, they may not all co-occur within specific communities and their specific patterns of co-occurrence may put children with AML at greater risk for mortality.
For example, our findings indicate that immigration-related features within the community (i.e., language isolation, foreign-born status, crowding) were only associated with high mortality risk in the context of moderately high levels of economic and educational disadvantage and housing instability. Communities with similar levels of immigration-related features were not associated with increased AML mortality risk when coupled with modest median family incomes ($62,236) and low rates of housing instability (Cluster 2) or when coupled with low rates of economic/educational disadvantage, even with moderate rates of housing instability (Cluster 7). This pattern of results may explicate divergent findings related to health outcomes among immigrant populations [23] and illustrates SES-related heterogeneity among immigrant communities related to factors such as duration of US residence and education levels [24]. Similarly, pediatric AML patient in counties with moderate to high housing instability but low rates of economic and educational disadvantage (Cluster 4) or low rates of immigration-related features (Cluster 5) were not at risk for increased mortality.
Pediatric AML patients with the highest mortality risk were located in communities with the greatest level of economic and educational disadvantage (Cluster 3). In the USA, income, education and health insurance coverage influence access to appropriate care, impacting early detection, treatment and palliative care [25]. Pathways from poverty to health outcomes are complex. They are partially determined by differential cumulative exposure to health harming situations (e.g., environmental toxins [26], trauma, chronic stress, and nutritional deprivation [27]) hypothesized to result in prolonged endocrine, immune, and central nervous system over-functioning [28] and biological ‘wear and tear.’ As in other countries [8, 22], mortality risk may be higher for those living in economic/educationally disadvantaged communities in the US due to poor access to healthcare, lack of appropriate pediatric oncology subspecialists, lack of knowledge of cancer diagnosis, treatment and inability to access clinical trials. It is well documented in the US that uninsured children have poorer access to healthcare and poorer outcomes [25, 29–31].
Pediatric AML patients living in communities with many immigration-related features (language isolation, crowding and foreign-born status) and housing instability in the context of moderately high economic/educational disadvantage also demonstrated elevated mortality risk. In addition to the various possible mechanisms listed above linking SES to mortality risk, these individuals may experience additional challenges to accessing appropriate care related to language barriers and their racial/ethnic minority status [28]. Additionally, children living in families who relocate frequently (≥ 3 times) have poorer overall physical health, report periods of no medical coverage, postpone medical care, and experience social network and family instability [17,18]. The intensity of AML treatment demands (e.g., active treatment adherence, long-term follow up) and the great financial burden of cancer care [29] may differentially impact these poor, immigrant families [18].
Strengths and Limitations
This study used an advanced statistical approach to investigate the associations of community-level SES indicators with mortality risk of pediatric AML patients in a large, nationally-representative sample and the findings have implications for planning the infrastructure of our healthcare system.
The results of the study do need to be considered in the context of its limitations. Individual-level SES variables were not assessed and should be examined in future research. A recent meta-analysis indicates that such research is scarce [5]. Economic indicators were derived from the Census American Community Survey County Attributes Survey, 2009–2013, and were not directly linked to time of diagnosis for the patients in the sample. Additionally, variables such as co-morbidities, access to care (insurance status; proximity to treatment center), and treatment received were not available for analysis. Our findings likely cannot be generalized to adult AML patients. Lastly, SEER data facilitate essential population-level research, but do not address event-free survival.
Conclusion
Our analyses indicate that the majority of variance in SEER county-level SES indicators can be captured by four latent factors. Analysis of patterns of these four factors revealed two significant AML mortality risk profiles. Pediatric patients with AML residing in communities with greater economic and educational disadvantage and in communities with a confluence of moderately high economic and educational disadvantage, housing instability, and immigration-related features are at increased risk of mortality; these children are not benefiting equally from the survival advances that are being made in pediatric AML research. Children with AML in these communities may have difficulties accessing adequate healthcare and the deployment of healthcare resources in these communities could reduce their increased mortality risk. Future research should examine the association of SES factors with access to healthcare within these communities to reduce the survival disparities that were uncovered.
Supplementary Material
Highlights.
Four SES factors surmised 23 county SES measures in SEER pediatric AML data.
Cluster analysis of 4 factor-scores revealed 7 distinct county-level SES patterns.
Mortality risk was higher in economic and educationally disadvantaged counties.
Housing instability and immigration-related aspects increased pediatric AML mortality.
Consideration of county-level SES patterns could reduce pediatric AML mortality
Acknowledgments
The authors express their special appreciation to the anonymous reviewers for their valuable comments and critiques. The salary of Md Jobayer Hossain, the senior author of this manuscript, was partially supported by the National Institutes of Health (NIH) COBRE Grant 8P20GM103464-9 (PI: Shaffer) and an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the NIH under Grant no. U54-GM104941 (PI: Binder-Macleod).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship Contribution Statement:
All authors contributed significantly to the preparation of this manuscript, and all approved the final draft of the manuscript.
Naomi B. Knoble, PhD contributed to conceptualization, data interpretation, drafting and final approval of the completed manuscript.
Melissa A. Alderfer, PhD contributed to conceptualization, data interpretation, drafting and final approval of the completed manuscript.
Jobayer Hossain, PhD contributed to the conception and design; acquisition and analysis of data; interpretations; and drafting and final approval of the submitted manuscript.
Conflict of Interest
Authors declared no conflict of interest.
References
- 1.Linabery AM, Ross JA. Childhood and adolescent cancer survival in the US by race and ethnicity for the diagnostic period 1975–1999. Cancer. 2008;113:2575–2596. doi: 10.1002/cncr.23866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, et al. Cancer treatment and survivorship statistics, 2014. CA. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 3.Gupta S, Wilejto M, Pole JD, Guttmann A, Sung L. Low socioeconomic status is associated with worse survival in children with cancer: A systematic review. PLoS One. 2014;9:1–13. doi: 10.1371/journal.pone.0089482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Njoku K, Basta N, Mann KD, McNally RJQ, Pearce MS. Socioeconomic variation in survival from childhood leukaemia in northern England, 1968–2010. Br J Cancer. 2013;108:2339–2345. doi: 10.1038/bjc.2013.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petridou ET, Sergentanis TN, Perlepe C, Papathoma P, Tsilimidos G, Kontogeorgi E, et al. Socioeconomic disparities in survival from childhood leukemia in the United States and globally: A meta-analysis. Ann Oncol. 2015;26:589–597. doi: 10.1093/annonc/mdu572. [DOI] [PubMed] [Google Scholar]
- 6.Sergentanis TN, Dessypris N, Kavavidis P, Skalkidis I, Baka M, Polychronopoulou s, et al. Socioeconomic status, area remoteness, and survival from childhood leukemia: REsults from the Nationwide Registry for Childhood Hematological Malignancies in Greece. Eur J Cancer Prev. 2013;22:473–479. doi: 10.1097/CEJ.0b013e32835c7f69. [DOI] [PubMed] [Google Scholar]
- 7.Syse A, Lyngstad TH, Kravdal O. Is mortality after childhood cancer dependent on social or economic resources of parents? A population-based study. Int J Cancer. 130:1870–1878. doi: 10.1002/ijc.26186. [DOI] [PubMed] [Google Scholar]
- 8.Youlden DR, Baade PD, Valery PC, Ward LJ, Green AC, Aitken JF. Differentials in survival for childhood cancer in Australia by remoteness of residence and area disadvantage. Cancer Epidemiol Biomarkers Prev. 2011;20:1649–1656. doi: 10.1158/1055-9965.EPI-11-0432. [DOI] [PubMed] [Google Scholar]
- 9.Hossain MJ, Xie L, Caywood EH. Prognostic factors of childhood and adolescent acute myeloid leukemia (AML) survival: Evidence from four decades of US population data. Cancer Epidemiol. 2015;39:720–726. doi: 10.1016/j.canep.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adler N, Newman K. Socioeconomic disparities in health: Pathways And policies. Health Aff. 2002;21:60–76. doi: 10.1377/hlthaff.21.2.60. [DOI] [PubMed] [Google Scholar]
- 11.Braveman PA, Cubbin C, Egerter S, Chideya S, Marchi KS, Metzler M, et al. Socioeconomic status in health research: One size does not fit all. JAMA. 2005;294:2879–2888. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- 12.Braveman P, Gottlieb L. The social determinants of health: It’s time to consider the causes of the causes. Public Health Rep. 2014;129(Suppl):19–31. doi: 10.1177/00333549141291S206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Busacker A, Kasehagen L. Association of residential mobility with child health: An analysis of the 2007 National Survey of Children’s Health. Matern Child Health J. 2012;16(Suppl 1):S78–S87. doi: 10.1007/s10995-012-0997-8. [DOI] [PubMed] [Google Scholar]
- 14.Costas-Muniz R, Leng J, Aragones A, Ramirez J, Roberts N, Mujawar MI, et al. Association of socioeconomic and practical unmet needs with self-reported nonadherence to cancer treatment appointments in low-income Latino and Black cancer patients. Ethn Health. 2015:1–11. doi: 10.1080/13557858.2015.1034658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schreier HMC, Chen E. Socioeconomic status and the health of youth: A multilevel, multidomain approach to conceptualizing pathways. Psychol Bull. 2012;139:606–654. doi: 10.1037/a0029416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conger RD, Conger KJ, Martin MJ. Socioeconomic status, family processes, and individual development. J Marriage Fam. 2010;72:685–704. doi: 10.1111/j.1741-3737.2010.00725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jelleyman T, Spencer N. Residential mobility in childhood and health outcomes: A systematic review. J Epidemiol Community Heal. 2008;62:584–592. doi: 10.1136/jech.2007.060103. [DOI] [PubMed] [Google Scholar]
- 18.Reid KW, Vittinghoff E, Kushel MB. Association between the level of housing instability, economic standing and health care access: A meta-regression. J Health Care Poor Underserved. 2008;19:1212–1228. doi: 10.1353/hpu.0.0068. [DOI] [PubMed] [Google Scholar]
- 19.Park HS, Lloyd S, Decker RH, Wilson LD, Yu JB. Overview of the Surveillance, Epidemiology, and End Results database: evolution, data variables, and quality assurance. Curr Probl Cancer. 2012;36:183–190. doi: 10.1016/j.currproblcancer.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Dickie L. SEER Program Coding and Staging Manual. Bethesda, MD: National Cancer Institute; 2015. pp. 20850–29765. [Google Scholar]
- 21.Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le B, eau MM, Hellström-Lindberg E, Tefferi A, Bloomfield CD. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009 Jul 5;114:937. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 22.Schillinger JA, Gosclaude PC, Honjo S, Quinn MJ, Sloggett A, Coleman MP. Survival after acute lymphocytic leukemia: Effects of socioeconomic status and geographic region. Arch Dis Child. 1999;80:311–317. doi: 10.1136/adc.80.4.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duncan B, Trejo SJ. Assessing the socioeconomic mobility and integration of U.S. immigrants and their descendants. Ann Am Acad Pol Soc Sci. 2015;657:108–135. [Google Scholar]
- 24.Gupta S, Sutradhar R, Guttmann A, Sung L, Pole JD. Socioeconomic status and event free survival in pediatric acute lymphoblastic leukemia: A population-based cohort study. Leuk Res. 2014;38:1407–1412. doi: 10.1016/j.leukres.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 25.Bhatia S. Disparities in cancer outcomes: Lessons learned from children with cancer. Pediatr Blood Cancer. 2011;56:994–1002. doi: 10.1002/pbc.23078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evans GW, Kim P. Childhood poverty, chronic stress, self-regulation, and coping. Child Dev Perspect. 2013;7:43–48. [Google Scholar]
- 27.Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav. 2012;106:29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 28.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. JAMA. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 29.DeVoe JE, Tillotson CJ, Wallce LS. Receipt of health care services and family health insurance patterns. Ann Fam Med. 2009;7:406–413. doi: 10.1370/afm.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fry-Johnson YW, Daniels EC, Levine R, Rust G. Being uninsured:impact on children's healthcare and health. Curr Opin Pediatr. 2005;17:753–758. doi: 10.1097/01.mop.0000187455.17077.94. [DOI] [PubMed] [Google Scholar]
- 31.Skinner AC, Mayer ML. Effects of insurance status on children's access to specialtycare: a systematic review of the literature. BMC Health Services Research. 2007;7:194. doi: 10.1186/1472-6963-7-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Price JH, Khubchandani J, McKinney M, Braun R. Racial/ethnic disparities in chronic diseases of youths and access to health care in the United States. Biomed Res Int. 2013;2013 doi: 10.1155/2013/787616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warner BEL, Kirchhoff AC, Nam GE, Fluchel M. Financial burden of pediatric cancer for patients and their families. J Oncol Pract. 2012:1–7. doi: 10.1200/JOP.2014.001495. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.