Abstract
Objective
To estimate the percentage of infants with large birth size attributable to excess gestational weight gain (GWG), independent of prepregnancy body mass index, among mothers with preexisting diabetes mellitus (PDM).
Study design
We analyzed 2004–2008 Florida linked birth certificate and maternal hospital discharge data of live, term (37–41 weeks) singleton deliveries (N = 641,857). We calculated prevalence of large-for-gestational age (LGA) (birth weight-for-gestational age ≥ 90th percentile) and macrosomia (birth weight > 4500 g) by GWG categories (inadequate, appropriate, or excess). We used multivariable logistic regression to estimate the relative risk (RR) of large birth size associated with excess compared to appropriate GWG among mothers with PDM. We then estimated the population attributable fraction (PAF) of large birth size due to excess GWG among mothers with PDM (n = 4427).
Results
Regardless of diabetes status, half of mothers (51.2%) gained weight in excess of recommendations. Large birth size was higher in infants of mothers with PDM than in infants of mothers without diabetes (28.8% versus 9.4% for LGA, 5.8% versus 0.9% for macrosomia). Among women with PDM, the adjusted RR of having an LGA infant was 1.7 (95% CI 1.5, 1.9) for women with excess GWG compared to those with appropriate gain; the PAF was 27.7% (95% CI 22.0, 33.3). For macrosomia, the adjusted RR associated with excess GWG was 2.1 (95% CI 1.5, 2.9) and the PAF was 38.6% (95% CI 24.9, 52.4).
Conclusion
Preventing excess GWG may avert over one-third of macrosomic term infants of mothers with PDM. Effective strategies to prevent excess GWG are needed.
Keywords: Gestational weight gain, LGA, Macrosomia, Preexisting diabetes
1. Introduction
The standard definition for preexisting diabetes mellitus (PDM) is any diabetes diagnosed prior to pregnancy (including both type 1 and type 2 diabetes). From 1993 to 2009, the prevalence of PDM among pregnant women increased 45%, from 0.62 to 0.90 per 100 deliveries, mainly due to the rise in type 2 diabetes (Correa et al., 1993–2009). Women with PDM are at increased risk of adverse pregnancy outcomes including stillbirth, congenital anomalies, neonatal hypoglycemia, or large birth size (i.e., large for gestational age [LGA] (birth weight at or above 90th percentile) or macrosomia (birth weight > 4500 g)) (Correa et al., 2008). Studies have shown that the risk of large birth size remains high in women with PDM even when glucose levels are well controlled and within normal limits during pregnancy (Evers et al., 2002).
Infants born too large are at greater risk for birth trauma, longer hospital stays, postpartum hemorrhage for the mother, and neonatal death (Weissmann-Brenner et al., 2012). Large birth size also elevates the risk of adverse metabolic outcomes later in life, including higher risk for metabolic diseases such as obesity, dyslipidemia, hypertension, and type 2 diabetes (Schellong et al., 2012). Further, evidence suggests that mothers who themselves were LGA are more likely to deliver an LGA offspring, perpetuating a cycle of adverse metabolic and reproductive health outcomes (Costa e Silva et al., 2015).
High prepregnancy body mass index (BMI), excess gestational weight gain (GWG) and diabetes mellitus are risk factors for delivering a large baby. In 2009, the Institute of Medicine (IOM) published revised guidelines for appropriate GWG by BMI to promote a healthy pregnancy and thereby reducing adverse health outcomes and optimizing birth weight (IOM (Institute of Medicine) & NRC (National Research Council), 2009). In 2010–2011, 47.2% of women gained gestational weight in excess of these guidelines in 28 states (Deputy et al., 2015). We have previously shown that approximately one third of LGA births may be prevented if women achieved appropriate GWG, independent of prepregnancy BMI and gestational diabetes mellitus (GDM) status (Kim et al., 2014). Since both hyperglycemia and diabetes management begin earlier during pregnancy for women with PDM, it is important to understand the burden of large birth size attributable to excess GWG among women with PDM for early intervention. The purpose of this analysis was to examine the percentage of LGA and macrosomic births attributable to excess GWG, independent of prepregnancy BMI, among women who have PDM.
2. Methods
We analyzed full-term (37–41 weeks) live, singleton deliveries occurring from March 2004 through December 2008 to women aged 20 or more years using Florida’s revised 2003 U.S. Standard Certificate of Live Birth linked to the state’s Hospital Inpatient Discharge Database (N = 746,328). Birth records were linked to maternal inpatient hospitalizations through a multistage, stepwise approach that has been described elsewhere (Salemi et al., 2013). All hospitals in Florida, except for military and Veterans Administration institutions, are represented. The Florida Department of Health transferred de-identified data to the Centers for Disease Control and Prevention (CDC) for analysis. Because there were no identifiers, an Institutional Review Board of the CDC determined approval was not needed because CDC was not engaged in human subjects’ research.
2.1. Maternal characteristics
Diabetes status in pregnancy was determined by using both the birth certificate and the hospital discharge data. On the birth certificate, diabetes is recorded as prepregnancy (diagnosis before this pregnancy), gestational (diagnosis during this pregnancy), or none. Only one selection is allowed. On the hospital discharge record, diabetes is identified by the following International Classification of Diseases, 9th Revision, Clinical Modification (ICD9-CM) codes: 648.8 (abnormal glucose tolerance (gestational diabetes)); 648.0 (diabetes mellitus); or 250.0–250.9 (diabetes mellitus (excludes gestational diabetes)). We used a previous medical record review of a small subset of the pregnancies to formulate rules for assigning PDM status (Kim et al., 2012): PDM cases were defined as deliveries in which the hospital discharge record included the ICD9-CM codes for preexisting diabetes (648.0 and 250.0–250.9) regardless of birth certificate status as that gave the highest specificity. Pregnancies without diabetes were those for which both the hospital discharge record and birth certificate indicated no diabetes (neither preexisting nor gestational).
We used birth certificate data to obtain additional information on maternal characteristics including age, educational attainment, marital status, race/ethnicity, insurance status, parity, smoking status, birth country, height, prepregnancy weight, weight at delivery, and enrollment in the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC). Prepregnancy BMI (prepregnancy weight in kilograms/height in meters2) was calculated, and women were classified as underweight (BMI < 18.5 kg/m2), normal weight (BMI 18.5–24.9 kg/m2), overweight (BMI 25.0–29.9 kg/m2), class I obese (BMI 30.0–34.9 kg/m2), class II obese (BMI 35–39.9 kg/m2), or class III obese (BMI ≥ 40.0 kg/m2). GWG, calculated as the difference between weight at delivery and prepregnancy weight, was used to categorize women with inadequate, appropriate, or excess GWG based on the 2009 IOM recommendations (IOM (Institute of Medicine) & NRC (National Research Council), 2009). Appropriate GWG was defined as 28–40 lb for underweight women, 25–35 lb for normal weight women, 15–25 lb for overweight women, and 11–20 lb for obese women (regardless of obesity class).
LGA was defined as birthweight >90th percentile for gestational age based on the distribution of birth weights in Florida recorded on birth certificates from 2004 to 2008. Gestational age was calculated using the obstetric estimate also as recorded on the birth certificate. Macrosomia was defined as birth weight >4500 g.
2.2. Inclusion and exclusion criteria
Records with no missing data on diabetes status and indicated either no diabetes in both data sources or PDM on hospital discharge were eligible for the study (n = 697,872). Births where hospital discharge indicated GDM (n = 37,620), birth certificate indicated PDM or GDM but hospital discharge indicated no diabetes (n = 8503), or hospital discharge records indicated both PDM and GDM (n = 147) were not eligible.
We also excluded records missing values on birth weight or extreme values (<1000 or >7257 g) (n = 50), missing prepregnancy BMI including those with implausible or extreme maternal height (<4′2″ or >6′5″), weight (<75 lb) (n = 42,071), missing GWG (n = 48,998), or nativity (n = 280). Our final analytic sample included 92.0% of our eligible study population, or 641,857 births. There were differences between those eligible but excluded compared to those included; notably, those excluded had a lower proportion of LGA and macrosomia but no difference in the proportion of PDM (Supplemental Table 1). Although the proportion of inadequate gestational weight gain was higher, the majority of women were missing data on this variable.
2.3. Statistical analysis
We examined maternal demographic characteristics and birth outcomes by diabetes status. We also examined the prevalence of LGA and macrosomia by GWG adequacy among women who had PDM. We present LGA prevalence by GWG adequacy and BMI categories. Cell sizes <20 could not be reported; therefore, we only present prevalence of macrosomia among women with excess GWG by BMI category. We computed relative risks (RR) and 95% confidence intervals (CI) for LGA among those who gained excess and inadequate GWG according to methods described by Flanders and Rhodes (Flanders & Rhodes, 1987) with appropriate GWG as the referent group. This RR estimate produces a marginal RR based on logistic regression fit to the data. Using the adjusted logistic regression methods described by Graubard & Fears (2005), we then estimated the adjusted population attributable fraction (PAF) of LGA and macrosomic births to PDM mothers with excess GWG. We interpreted each PAF estimate to be the reduction in LGA or macrosomia prevalence that would be expected if all women who had excess GWG had an LGA risk equal to that of women who had appropriate GWG, assuming that the risk for LGA among those with appropriate GWG remained unchanged (Levine, 2008). We adjusted for prepregnancy BMI, maternal age, race/ethnicity and parity in the logistic models and PAF because they have been shown to be independently associated with GWG and LGA (Weissmann-Brenner et al., 2012; Deputy et al., 2015).
3. Results
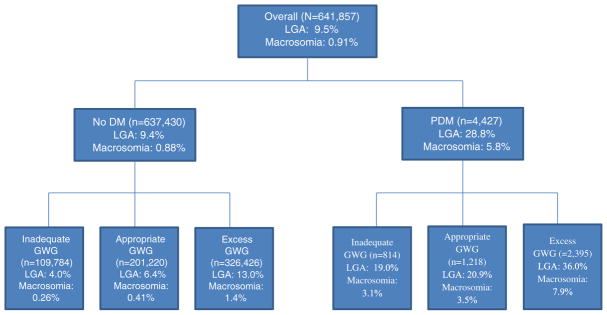
Maternal characteristics and birth outcomes by diabetes status are shown in Table 1. Overall, excess GWG was 51.2%. Compared to women without diabetes, a higher proportion of women with PDM were older, less educated, non-Hispanic black, enrolled in WIC, parous, obese, and had GWG outside of recommendations (Table 1). LGA and macrosomia prevalence overall was 9.5% and 0.9%, respectively (Fig. 1); stratified by diabetes status, LGA and macrosomia prevalence among women with no diabetes was 9.4% and 0.9% and among women with PDM was 28.8% and 5.8%, respectively. Among women with no PDM, LGA prevalence by GWG adequacy was 4.0%, 6.4%, and 13.0% for inadequate, appropriate, and excess GWG, respectively, whereas, among women with PDM, LGA prevalence by GWG adequacy was 19.0%, 20.9%, and 36.0% (Fig. 1).
Table 1.
Maternal characteristics and birth outcomes by diabetes statusa, Florida, 2004–2008, %.
| No diabetes (N = 637,430) | Preexisting diabetes mellitus (N = 4427) | P-value | |
|---|---|---|---|
| Overall % | 99.3 | 0.7 | |
| Age | <0.0001 | ||
| 20–29 | 60.6 | 43.4 | |
| 30–39 | 36.6 | 50.8 | |
| 40+ | 2.7 | 5.8 | |
| Education | <0.0001 | ||
| <12 | 12.9 | 14.4 | |
| 12 | 30.9 | 32.8 | |
| >12 | 55.9 | 52.5 | |
| Race/ethnicity | <0.0001 | ||
| Non-Hispanic White | 52.6 | 46.1 | |
| Non-Hispanic Black | 19.6 | 28.8 | |
| Hispanic | 25.1 | 21.8 | |
| Asian/Pacific Islander | 2.7 | 3.3 | |
| WIC status | <0.0001 | ||
| Yes | 40.1 | 49.5 | |
| No | 59.0 | 50.1 | |
| Insurance status | <0.0001 | ||
| Medicaid | 40.8 | 43.3 | |
| Private insurance | 51.6 | 51.7 | |
| Self-pay | 5.8 | 3.5 | |
| Other | 1.6 | 1.2 | |
| Parity | <0.0001 | ||
| 0 | 38.1 | 33.8 | |
| 1 | 34.7 | 34.1 | |
| 2+ | 26.8 | 31.8 | |
| Smoking during pregnancy | 7.6 | 7.7 | 0.5825 |
| Nativity | <0.0001 | ||
| U.S. | 73.1 | 75.8 | |
| Foreign | 27.0 | 24.2 | |
| BMI | <0.0001 | ||
| <18.5 | 4.9 | 1.2 | |
| 18.5–24.9 | 52.0 | 23.5 | |
| 25.0–29.9 | 24.0 | 22.4 | |
| 30.0–34.9 | 11.3 | 21.4 | |
| 35.0–39.9 | 4.8 | 14.8 | |
| ≥40.0 | 3.0 | 16.7 | |
| Gestational weight gain | <0.0001 | ||
| Inadequate | 17.2 | 18.4 | |
| Appropriate | 31.6 | 27.5 | |
| Excess | 51.2 | 54.1 | |
| LGA >90th percentile | 9.4 | 28.8 | <0.0001 |
| Macrosomia 4500 g | 0.9 | 5.8 | <0.0001 |
Included only mothers 20 years or older with a full-term (37–41 weeks) live, singleton birth.
Fig. 1.
Prevalence of large for gestational age (LGA) and macrosomia infants overall, by diabetes status, and by gestational weight gain status (GWG), Florida, 2004–2008. Large for gestational age (LGA); diabetes mellitus (DM); preexisting diabetes mellitus (PDM); gestational weight gain (GWG). LGA: defined as ≥90th percentile; macrosomia defined as >4500 g. Includes only mothers with a full-term (37–41 weeks) singleton, live birth.
LGA prevalence was highest among PDM women who experienced excess GWG and were obese (Table 2). A similar pattern was observed for macrosomia (data not shown). Among women with no diabetes and excess GWG, the prevalence of macrosomia was 1.1%, 1.5%, and 2.0% for normal weight, overweight, and obese women, respectively. Prevalence of macrosomia increased to 5.8%, 7.3%, and 8.9%, respectively, for women with PDM and excess GWG.
Table 2.
Prevalence (cell count) of large-for-gestational age (LGA) by body mass index (BMI), preexisting diabetes mellitus (PDM) status, and gestational weight gain for births of gestational age 37–41 weeks, Florida, 2004–2008.
| Percent LGA (birthweight ≥ 90th percentile)
|
||||||
|---|---|---|---|---|---|---|
| No DM
|
PDM
|
|||||
| Gestational weight gain categories | ||||||
|
|
|
|||||
| BMI (kg/m2) | Inadequate | Appropriate | Excess | Inadequate | Appropriate | Excess |
| Underweight | 1.1 (90) | 2.9 (401) | 7.5 (651) | –a | –a | –a |
| Normal | 3.1 (1966) | 5.7 (7077) | 11.5 (16,578) | 9.9 (22) | 17.9 (66) | 32.6 (147) |
| Overweight | 4.4 (702) | 7.0 (2500) | 13.5 (13,725) | 20.8 (26) | 21.6 (60) | 34.5 (203) |
| Obese | 7.5 (1678) | 10.3 (2797) | 15.9 (11,476) | 23.3 (106) | 23.7 (128) | 38.1 (512) |
| Class I | 5.7 (549) | 8.6 (1301) | 15.1 (7139) | 18.9 (21) | 20.4 (44) | 36.9 (228) |
| Class II | 8.2 (550) | 11.5 (851) | 17.0 (2775) | 22.7 (27) | 26.6 (41) | 36.9 (141) |
| Class III | 9.5 (579) | 13.8 (645) | 18.9 (1562) | 25.8 (58) | 25.3 (43) | 41.6 (143) |
| Overall | 4.0 (4436) | 6.3 (12775) | 13.0 (42,430) | 19.0 (155) | 20.9 (255) | 36.0 (863) |
Tables are based on Florida data for 2004–2008.
Birth weight percentiles are computed from Florida 2004–2008 data after excluding births with BW < 1000 g and births with BW > 7257 g (16 lb).
Birth certificate (BC)-hospital discharge (HD); gestational diabetes mellitus (GDM); diabetes mellitus (DM).
BC-HD combinations of PDM-PDM, GDM-PDM, No DM-PDM are classified as ‘PDM’.
BC-HD combination No DM-No DM is classified as ‘No DM’.
All other BC-HD combinations are excluded from the tables.
Cells are <20 and cannot be reported.
Among women with PDM, the relative risk of LGA was 1.7 (95% CI 1.5, 1.9) for excess GWG and 0.9 (95% CI 0.8, 1.1) for inadequate GWG compared to appropriate gain, controlling for prepregnancy BMI and other covariates (Table 3). The PAF of LGA attributable to excess GWG was 27.7% (95% CI 22.0, 33.3). Similarly, the adjusted relative risk of macrosomia was 2.1 (95% CI 1.5, 2.9) for excess GWG and 0.8 (95% CI 0.5, 1.3) for inadequate GWG compared to appropriate gain. The PAF of macrosomia attributable to excess GWG was 38.6% (95% CI 24.9, 52.4). Adjustments did not change any of the crude estimates.
Table 3.
Relative risk and population attributable fraction of LGA and macrosomia for excess gestational weight gain.
| LGA | Macrosomia | |
|---|---|---|
| Relative risk (95% CI)a | 1.7 (1.5, 1.9) | 2.1 (1.5, 2.9) |
| Population attributable fraction (95% CI)a | 27.7% (22.0, 33.3) | 38.6% (24.9, 52.4) |
Adjusted for prepregnancy BMI, maternal age, race/ethnicity, and parity.
4. Discussion
Our study demonstrates that independent of prepregnancy BMI, as much as 27.7% of LGA and 38.6% of macrosomia among children born to women with PDM might be prevented if these women gained gestational weight within IOM recommendations. More than half of women in the study had excess GWG, which is similar to other population-based reports in the US (Deputy et al., 2015). Among women with PDM, prevalence of LGA was 29% and increased to 36% among women who gained excess GWG. Others have reported similar LGA estimates ranging from 30.1% to 42.1% among women with PDM who had excess GWG (Scifres et al., 2014; Siegel et al., 2015b). Prevalence of macrosomia among women with PDM in our study was 5.8% and increased to 7.9% in those with excess GWG.
The positive association between GWG and fetal growth is well documented (IOM (Institute of Medicine) & NRC (National Research Council), 2009) and confirmed in our study. Current GWG guidelines are based on balancing the risks of too little or too much GWG in the average healthy population but these guidelines do not differ by diabetes status (IOM (Institute of Medicine) & NRC (National Research Council), 2009). There is limited evidence on the range of GWG that balances risk of small for gestational age (SGA) and LGA among women with PDM. A recent, albeit small, study of women with both obesity and type 2 diabetes found that those who gained 5 kg or less, compared to those who gained >5 kg, had fewer LGA infants and no difference in SGA infants, regardless of gestational age (Asbjornsdottir et al., 2013). Another study examining women with obesity and any diabetes found a significant reduction of LGA risk among term (≥39 weeks gestation) infants, but only in women with GWG less than the recommended 11–20 lb and class III obesity. Term SGA risk increased, but was not statistically significant. Among the women with class I and II obesity and any diabetes, lower gestational weight gains did not significantly reduce the risk of LGA or increase the risk of SGA (Gavard & Artal, 2014). These two studies are similar to our results in that we did not observe a statistically significant lower risk of LGA with inadequate gain compared to appropriate gain (Asbjornsdottir et al., 2013; Gavard & Artal, 2014). However, more evidence is needed to determine if a lower range of GWG is appropriate for women specifically with PDM within each BMI category. Nonetheless, it is important for women with diabetes to not have excess GWG.
In 2009–2012, among women 20–44 year of age in the U.S., 1.6% have diagnosed diabetes with an additional 1.7% having undiagnosed diabetes (National Center for Health Statistics, 2014). Among pregnant women, <1% had PDM (Bardenheier et al., 2015). Although the prevalence of preexisting diabetes is low, the maternal and fetal implications are concerning given the risk of stillbirth, congenital anomalies, and excess fetal growth. Maintaining glycemic control before and during pregnancy is important as studies have shown a positive association between maternal glucose levels and fetal growth (Metzger et al., 2008). Therefore, providing appropriate preconception care to women with diabetes is an important opportunity to help them achieve optimal glucose control prior to becoming pregnant. In addition, screening for diabetes early in pregnancy among high risk women is another opportunity to diagnose diabetes and provide appropriate interventions early to maintain glycemic control and limit GWG. The American College of Obstetricians and Gynecologists (ACOG) recommends early pregnancy screening for undiagnosed type 2 diabetes in women with risk factors such as prior history of GDM and obesity (Anon., 2013a). The Endocrine Society recommends universal screening for undiagnosed diabetes in early pregnancy with either a fasting plasma glucose, untimed random plasma glucose, or hemoglobin A1C (Blumer et al., 2013).
Although we cannot prevent PDM in pregnancy, excess GWG among women with PDM can be prevented. ACOG recommends that clinicians educate all women on the importance of appropriate GWG and counsel women on proper dietary behaviors and physical activity to achieve recommended GWG (Anon., 2013b). Furthermore, it is recommended that management of diabetes in pregnancy is focused on proper glucose control using a careful combination of diet, exercise, and insulin therapy (ACOG Practice Bulletin, 2005). ACOG encourages PDM patients to keep a log of diet, insulin dosages, exercise, and glucose values to help them manage their glucose control (ACOG Practice Bulletin, 2005). Since weight gain has been shown to be a common problem with intensive insulin regimens (Scholl TO & Chen, 2002), women with PDM may benefit from additional care by a registered dietician who can provide an individualized nutrition program to achieve the appropriate carbohydrate and insulin balance needed to avoid excess GWG. We have limited population-level estimates on how much information patients receive from clinicians regarding GWG using the most recent IOM guidelines. However, recent research has shown that improving clinician knowledge and effort to address appropriate GWG has been effective in helping women gain within IOM recommendations (Lindberg et al., 2016; Wilkinson et al., 2016). In addition, strategies such as meeting dietary and physical activity goals have been shown to help women gain within IOM recommendations (Muktabhant et al., 2015). There are limited studies assessing whether interventions targeting patient education on GWG can lead to measurable improvements. Therefore, it is important for healthcare providers to provide advice consistent with current IOM guidelines and be knowledgeable in proper referrals to develop individualized physical activity and nutrition plans.
Our study used a large, racially and ethnically diverse, population-based database to examine the population burden of LGA and macrosomia attributable to excess GWG. However, the analysis has limitations. Although our database was large and diverse, we still had a small sample-size to examine macrosomia by GWG and BMI. GWG was calculated using prepregnancy weight and weight at delivery from the birth certificate. Since self-reported prepregnancy weight may be underreported (Park et al., 2011), both BMI and GWG may be misclassified with BMI being underestimated and GWG being overestimated. Second, we may have underestimated the prevalence of PDM, given that sensitivity for ascertaining PDM hospital discharge data may be as low as 47.1% (Devlin et al., 2008). Furthermore, although our study is observational, we assumed a causal association between GWG and large birth size in order to calculate the PAF, as there is a large body of evidence that is strong and shows a large magnitude of association (IOM (Institute of Medicine) & NRC (National Research Council), 2009). By definition, the PAF is estimated to be the reduction in LGA or macrosomia prevalence that would occur if all women who had excess GWG had an LGA or macrosomia risk equal to that of women who gained appropriate GWG. Third, we do not have data on glycemic control, which may confound the relationship between GWG (through insulin use) and large birth size. However, a recent study indicated that the increase in LGA and macrosomia risk among women with PDM who had excess GWG was not explained by glycemic control (Siegel et al., 2015a). Fourth, we did not have any information on diet, physical activity, and other behavior and lifestyle factors that may influence gestational weight gain. Future studies should include these factors if available. Finally, our findings may not be generalizable to women outside of Florida. However, Florida is the fourth most populous U.S. state and is racially and ethnically diverse. Further, the LGA prevalence was similar to national average (Donahue et al., 2010).
5. Conclusion
Infants born too large increase risk for adverse maternal and infant health outcomes including delivery and neonatal complications as well as long-term morbidities in the infant such as obesity and diabetes (Johnsson et al., 2015). As much as one-third of macrosomia among women with PDM may be explained by excess GWG; thus, targeted prevention efforts to reduce excess GWG are needed.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ypmed.2016.08.026.
Supplementary Material
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors report no conflict of interest.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Transparency document
The Transparency document associated with this article can be found, in the online version.
References
- Anonymous. Practice Bulletin No. 137: Gestational diabetes mellitus. Obstet Gynecol. 2013a;122(2 Pt 1):406–416. doi: 10.1097/01.AOG.0000433006.09219.f1. [DOI] [PubMed] [Google Scholar]
- Anonymous. ACOG Committee Opinion no. 548: weight gain during pregnancy. Obstet Gynecol. 2013b;121(1):210–212. doi: 10.1097/01.aog.0000425668.87506.4c. [DOI] [PubMed] [Google Scholar]
- ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 60, March 2005 Pregestational diabetes mellitus. Obstet Gynecol. 2005;105(3):675–685. doi: 10.1097/00006250-200503000-00049. [DOI] [PubMed] [Google Scholar]
- Asbjornsdottir B, Rasmussen SS, Kelstrup L, Damm P, Mathiesen ER. Impact of restricted maternal weight gain on fetal growth and perinatal morbidity in obese women with type 2 diabetes. Diabetes Care. 2013;36(5):1102–1106. doi: 10.2337/dc12-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardenheier BH, Imperatore G, Devlin HM, Kim SY, Cho P, Geiss LS. Trends in pre-pregnancy diabetes among deliveries in 19 U.S. States, 2000–2010. Am J Prev Med. 2015;48(2):154–161. doi: 10.1016/j.amepre.2014.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumer I, Hadar E, Hadden DR, et al. Diabetes and pregnancy: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(11):4227–4249. doi: 10.1210/jc.2013-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa A, Bardenheier B, Elixhauser A, Geiss LS, Gregg E. Trends in prevalence of diabetes among delivery hospitalizations, United States, 1993–2009. Matern Child Health J. 2014 doi: 10.1007/s10995-014-1553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa A, Gilboa SM, Besser LM, et al. Diabetes mellitus and birth defects. Am J Obstet Gynecol. 2008;199(3):237–239. doi: 10.1016/j.ajog.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa e Silva LI, Gomes FM, Valente MH, Escobar AM, Brentani AV, Grisi SJ. The intergenerational effects on birth weight and its relations to maternal conditions, Sao Paulo, Brazil. BioMed Res Int. 2015;2015:615034. doi: 10.1155/2015/615034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deputy NP, Sharma AJ, Kim SY, Hinkle SN. Prevalence and characteristics associated with gestational weight gain adequacy. Obstet Gynecol. 2015;125(4):773–781. doi: 10.1097/AOG.0000000000000739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin HM, Desai J, Walaszek A. Reviewing performance of birth certificate and hospital discharge data to identify births complicated by maternal diabetes. Matern Child Health J. 2008;13(5):660–666. doi: 10.1007/s10995-008-0390-9. [DOI] [PubMed] [Google Scholar]
- Donahue SM, Kleinman KP, Gillman MW, Oken E. Trends in birth weight and gestational length among singleton term births in the United States: 1990–2005. Obstet Gynecol. 2010;115(2 Pt 1):357–364. doi: 10.1097/AOG.0b013e3181cbd5f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers IM, de Valk HW, Mol BW, ter Braak EW, Visser GH. Macrosomia despite good glycaemic control in Type I diabetic pregnancy; results of a nationwide study in The Netherlands. Diabetologia. 2002;45(11):1484–1489. doi: 10.1007/s00125-002-0958-7. [DOI] [PubMed] [Google Scholar]
- Flanders WD, Rhodes PH. Large sample confidence intervals for regression standardized risks, risk ratios, and risk differences. J Chronic Dis. 1987;40(7):697–704. doi: 10.1016/0021-9681(87)90106-8. [DOI] [PubMed] [Google Scholar]
- Gavard JA, Artal R. The association of gestational weight gain with birth weight in obese pregnant women by obesity class and diabetic status: a population-based historical cohort study. Matern Child Health J. 2014;18(4):1038–1047. doi: 10.1007/s10995-013-1356-0. [DOI] [PubMed] [Google Scholar]
- Graubard BI, Fears TR. Standard errors for attributable risk for simple and complex sample designs. Biometrics. 2005;61(3):847–855. doi: 10.1111/j.1541-0420.2005.00355.x. [DOI] [PubMed] [Google Scholar]
- IOM (Institute of Medicine), NRC (National Research Council) Weight Gain During Pregnancy: Reexamining the Guidelines. The National Academies Press; Washington, DC: 2009l. [Google Scholar]
- Johnsson IW, Haglund B, Ahlsson F, Gustafsson J. A high birth weight is associated with increased risk of type 2 diabetes and obesity. Pediatr Obes. 2015;10(2):77–83. doi: 10.1111/ijpo.230. [DOI] [PubMed] [Google Scholar]
- Kim SY, England L, Sappenfield W, et al. Racial/ethnic differences in the percentage of gestational diabetes mellitus cases attributable to overweight and obesity, Florida, 2004–2007. Prev Chronic Dis. 2012;9:E88. doi: 10.5888/pcd9.110249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Sharma AJ, Sappenfield W, Wilson HG, Salihu HM. Association of maternal body mass index, excessive weight gain, and gestational diabetes mellitus with large-for-gestational-age births. Obstet Gynecol. 2014;123(4):737–744. doi: 10.1097/AOG.0000000000000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine BJ. The other causality question: estimating attributable fractions for obesity as a cause of mortality. Int J Obes. 2008;32(Suppl 3):S4–S7. doi: 10.1038/ijo.2008.81. [DOI] [PubMed] [Google Scholar]
- Lindberg SM, DeBoth A, Anderson CK. Effect of a best practice alert on gestational weight gain, health services, and pregnancy outcomes. Matern Child Health J. 2016 doi: 10.1007/s10995-016-2052-7. (Epub Ahead of Print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- Muktabhant B, Lawrie TA, Lumbiganon P, Laopaiboon M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database Syst Rev. 2015 Jun;:6. doi: 10.1002/14651858.CD007145.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Health Statistics. [Last accessed 10/21/15];Data Interactive. 2014 PrFont34Bin0BinSub0Frac0Def1Margin0Margin0Jc1Indent1440Lim0Lim1 www.cdc.gov/nchs/hdi.htm.
- Park S, Sappenfield WM, Bish C, Bensyl DM, Goodman D, Menges J. Reliability and validity of birth certificate prepregnancy weight and height among women enrolled in prenatal WIC program: Florida, 2005. Matern Child Health J. 2011;15(7):851–859. doi: 10.1007/s10995-009-0544-4. [DOI] [PubMed] [Google Scholar]
- Salemi JL, Tanner JP, Bailey M, Mbah AK, Salihu HM. Creation and evaluation of a multi-layered maternal and child health database for comparative effectiveness research. J Registry Manag. 2013;40(1):14–28. [PubMed] [Google Scholar]
- Schellong K, Schulz S, Harder T, Plagemann A. Birth weight and long-term overweight risk: systematic review and a meta-analysis including 643,902 persons from 66 studies and 26 countries globally. PLoS One. 2012;7(10):e47776. doi: 10.1371/journal.pone.0047776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl TO, Chen X. Insulin and the “thrifty” woman: the influence of insulin during pregnancy on gestational weight gain and postpartum weight retention. Matern Child Health J. 2002;6(4):255–261. doi: 10.1023/a:1021162117177. [DOI] [PubMed] [Google Scholar]
- Scifres CM, Feghali MN, Althouse AD, Caritis SN, Catov JM. Effect of excess gestational weight gain on pregnancy outcomes in women with type 1 diabetes. Obstet Gynecol. 2014;123(6):1295–1302. doi: 10.1097/AOG.0000000000000271. [DOI] [PubMed] [Google Scholar]
- Siegel AM, Tita A, Biggio JR, Harper LM. Evaluating gestational weight gain recommendations in pregestational diabetes. Am J Obstet Gynecol. 2015a;213(4):563–565. doi: 10.1016/j.ajog.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel AM, Tita A, Biggio JR, Harper LM. Evaluating gestational weight gain recommendations in pregestational diabetes. 2015b doi: 10.1016/j.ajog.2015.07.030. http://dx.doi.org/10.1016/j.ajog.2015.07.030 (LID S0002-9378(15)00780-2 [pii], 1097-6868 (Electronic)) [DOI] [PMC free article] [PubMed]
- Weissmann-Brenner A, Simchen MJ, Zilberberg E, et al. Maternal and neonatal outcomes of macrosomic pregnancies. Med Sci Monit. 2012;18(9):H77–H81. doi: 10.12659/MSM.883340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson SA, Donaldson E, Beckmann M, Stapleton H. Service-wide management of healthy gestational weight gain following an implementation scient approach. Matern Child Nutr. 2016 doi: 10.1111/mcn.12266. (Epub Ahead of Print) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.