Abstract
Purpose
The concept of a vibrating wristband to improve dexterous hand function of stroke survivors was recently proposed with clinical results and is referred to as “TheraBracelet” in this paper. The purpose of this study was to demonstrate feasibility of a portable, wearable TheraBracelet, and to apply usability evaluation techniques to assess potential demands of TheraBracelet and to identify critical improvement needs of the prototype.
Method
A prototype was developed with a vibrating element housed in an elastic wristband and connected to a wearable electronics box via a cable. Expectation for TheraBracelet and evaluation of the prototype were obtained from ten chronic stroke survivors using surveys before and after using the prototype and House of Quality analysis.
Results
The survey for expectation showed stroke survivors’ willingness to try out TheraBracelet at a low cost. The survey evaluating the prototype showed that the current prototype was overall satisfactory with a mean rating of 3.7 out of 5. The House of Quality analysis revealed that the priority improvement needs for the prototype are to improve clinical knowledge on long-term effectiveness, reduce cost, ease donning/doffing, and waterproof.
Conclusions
This study presents a potential for a low-cost wearable hand orthotic likable by stroke survivors.
Keywords: Hand function, House of Quality, Stroke rehabilitation, Sensory deficits, Sensory stimulation, Stochastic resonance, Tactile sensation, Usability evaluation
1. Introduction
This study developed a prototype of and evaluated usability of a wearable sensorimotor orthotic ‘TheraBracelet’. TheraBracelet represents application of imperceptible vibration to the wrist to help improve dexterous hand function for people with impaired hand function such as stroke survivors [1]. As with all medical devices, it is crucial to assess potential users’ enthusiasm and evaluation for using TheraBracelet as a hand orthotic.
The target population of this study was stroke survivors. Stroke is a leading cause of long-term disability [2] affecting more than 6 million Americans [3]. Physical disability due to stroke is particularly severe in the hand [4–8], leading to diminished vocational and self-care abilities [9, 10], affecting quality of life. Due to the importance of hand function in daily living, therapeutic devices for the hand exist such as Ness H200 (Bioness Inc., Valencia, CA) which is an arm orthosis applying electricity to forearm muscles to facilitate hand opening and closing [11] and SaeboFlex® (Saebo Inc., Charlotte, NC) which is a glove that mechanically opens the fingers to help stroke survivors release objects [12].
Similarly, TheraBracelet is to facilitate dexterous hand function for stroke survivors: application of imperceptible vibration to the wrist has been shown to improve hand function, as evidenced by the improved Nine Hole Peg Test score, Box and Block Test score, and pinch strength in chronic stroke survivors [1]. Imperceptible vibration to the wrist or dorsum of the hand also resulted in improved touch sensation of the thumb and index fingertip pads in chronic stroke survivors [13]. In healthy adults, imperceptible vibration applied to the wrist or palm improved fingertip touch sensation [14, 15] and facilitated reaction time to tactile stimuli on the hand [16]. Application of vibration whose intensity was at or below the perceptible level at the side of the fingertip resulted in improved finger tactile sensation and hand motor function in healthy adults [17]. Such improvement in tactile sensation and hand dexterity with vibration has been linked to stochastic resonance [1, 13, 14, 16, 17] in which low-level noise improves signal detection whereas high-level noise degrades signal detection by masking the original signal [18]. Compared to direct application of vibration at the fingertips [19], the possibility of applying vibration remotely from the fingertips, such as the wrist, allows less interference with finger motions and object manipulation and facilitates hand dexterity.
Based on these findings, it follows that a simple vibrating wristband could be created or vibrating function can be added to a watch or other wristband devices to facilitate hand dexterity among stroke survivors. Despite the potential of a wearable orthotic to enhance hand function, such an orthotic would not make an impact unless it is accepted and liked by users. Many assistive devices in the market are never picked up by targeted users or abandoned by users due to usability issues [20]. The common usability issues for upper-limb prostheses and orthoses include the devices being restrictive, inconvenient, frustrating, uncomfortable, not durable, and mechanically failing [21–24]. The majority of these issues could have been resolved and an assistive device would have been successful if the device were designed based on understanding targeted users’ needs and feedback through usability evaluation [25]. Product-driven manufacturing, where manufacturers introduced products into market without feedback from end-users in the development phase, is becoming obsolete [20]. For a market-oriented approach, user satisfaction is prerequisite for product development. Therefore, to meet user expectations and needs, a user-focused approach is necessary in product design.
The objectives of this study were to develop a prototype of TheraBracelet and evaluate the usability of the prototype. Stroke survivors’ expectation on such a hand orthotic and satisfaction with the prototype were assessed in surveys. In addition, House of Quality analysis was used to determine priority technical improvement needs of the current prototype. House of Quality is a widely used and recognized method to capture the ‘voice of users’ in product design from the conceptual stage [26–28]. House of Quality translates user expectations into technical requirements thereby identifying priority technical development/improvement needs to efficiently satisfy user needs.
2. Methods
2.1 TheraBracelet prototype
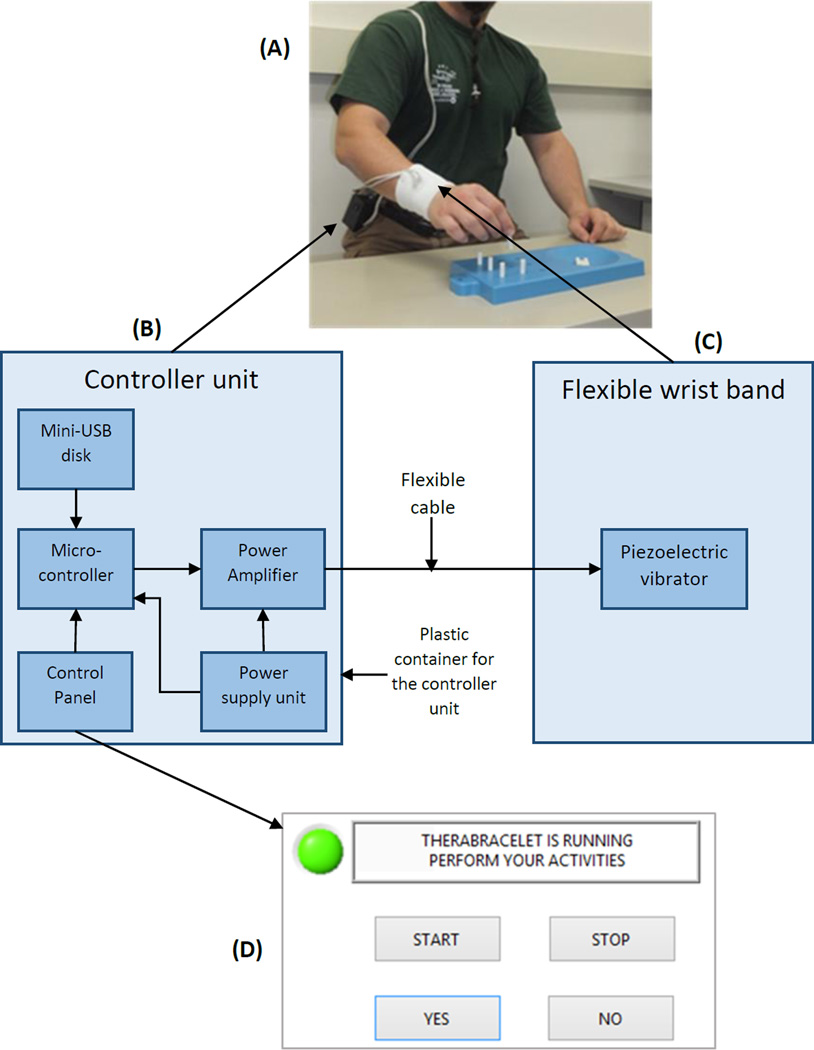
The prototype is shown in Figure 1A. A user wears a wristband with a vibrating element so that the wrist skin receives vibration. The vibrator vibrates according to the electric signal received from the controller unit that can be worn at the waist belt.
Figure 1.
TheraBracelet prototype (A) and its device architecture diagram (B–C) and user interface used in this study (D).
The device architecture is shown in Figure 1B–C. A piezoelectric vibrator (AT-1803-T-LW50-R, PUI Audio Inc, Dayton, OH) was used for its advantages of being thin and non-magnetic [14] over solenoid vibrators. The vibrator was embedded in a conventional athletic wristband. To ensure electrical insulation against the user’s skin, bare wires and solder joints were coated with nonconductive silicone sealant.
The controller unit (Figure 1B) contains five modules: mini-USB disk, micro-controller, control panel, power amplifier, and power supply unit. The mini-USB disk (SanDisk Cruzer Fit 8GB USB 2.0 Low-Profile Flash Drive SDCZ33-008G-B35) stores source signals as MP3 files to drive the vibrator. According to previous studies [1, 13, 16], white-noise low-pass filtered at 500 Hz was used as the source signal. The micro-controller (BU94603KV-E2, ROHM Semiconductor, Kyoto, Japan) reads the digital MP3 file in the mini-USB disk and converts them to an analog signal, as required by the power amplifier. This analog signal amplitude is adjusted by the input from the control panel by a user.
The power amplifier with integrated 3.3 to 105 V boost converter (DRV8662, Texas Instruments, Dallas, TX) amplifies this analog signal by a factor of 50 and produces a higher peak-to-peak voltage output. The power supply consists of a 3.7 V AA rechargeable battery, two DC-DC converters (TPS60150 5V/140mA Charge Pump DC-DC converter, Texas Instruments, Dallas, TX, to convert 3.7 V battery voltage to 5 V to feed the micro-controller, and SC630A 1 MHz Fixed 3.3 V Output Charge Pump Regulator, SEMTECH, to convert 3.7 V battery voltage to 3.3 V to feed the power amplifier) and one current-limit module (NCP380 Fixed/Adjustable Current-Limiting) to keep the micro-controller current below 1 A to prevent burning.
The controller unit uses a double-layer printed circuit board (PCB) with soldering chips on both sides of the board to reduce the overall size. The top layer contained the signal chips including the mini-USB disk, micro-controller, and power amplifier, while the bottom layer contained the power chips including DC-DC converters and battery, to minimize interference that any unexpected ripple and impulse from the power chips may have on the source signal.
Total current consumption was approximately 220 mA when all components were working at their maximum power. A 3.7 V AA rechargeable battery capacity is 1200–1400 mAh, which means this prototype can work for approximately 6 h after being fully charged. The total cost of the chips excluding the PCB and labor cost was approximately $50.
In this study, the control panel of the prototype was replaced by a computer-based user interface (Figure 1D) to provide automatic calibration. The user interface initially displayed “Wear the wristband and press START”. When the user wore the wristband and pressed the START button, vibration was presented at the wristband and the display in the user interface changed to “Can you feel the vibration?” The user pressed the YES or NO button based on whether or not they felt vibration. The vibration intensity was reduced when the user pressed YES and increased when the user pressed NO. Once the minimum vibration intensity that the user could feel (sensory threshold) was identified, vibration at 60% of this intensity [1, 13] was presented with the display “TheraBracelet is running. Perform your activities”.
2.2 Usability evaluation
Subjects
Ten stroke survivors (four females, ages ranging 45–82) participated. Their time since stroke ranged 2–27 years. Six stroke survivors had paralysis on the right hand, while four had paralysis on the left hand. Eight had an ischemic stroke, while two had a hemorrhagic stroke. The functional level of the affected upper extremity assessed by the Fugl-Meyer Assessment [29] ranged 13–24 (out of 24). Stroke survivors who had high scores on the Fugl-Meyer Assessment were included in the study, because manual dexterity is not thoroughly tested in the Fugl-Meyer Assessment, and TheraBracelet is expected to impact manual dexterity such as writing and handling intricate parts, even for healthy adults [14–16]. All subjects signed an informed consent form approved by the Institutional Review Board before participating in the study.
Procedure
The testing order was as follows. First, participants completed a survey regarding expectation of TheraBracelet. Then, the participants experienced the prototype. Lastly, the participants completed a survey to evaluate the prototype. The rating results from both surveys were used for the House of Quality analysis to determine priority technical improvement needs of the current prototype. Each step is detailed below.
The expectation survey entailed rating of importance for individual user expectation criteria as well as indicating expected usage. Participants were briefed on the concept of TheraBracelet before they began the survey. Participants rated importance of individual user criteria for this hand orthotic on a five-point Likert scale (1 for not important, 2 for marginally important, 3 for somewhat important, 4 for important, 5 for very important). The user expectation criteria were: easy to put on/take off, easy to control, comfortable to wear, safe to use, look, compact, lightweight, waterproof, easy to maintain, durable, his/her own improvement in hand function, evidence of clinical effectiveness provided by scientific studies, and affordable (Figure 2). These 13 criteria were pre-determined based on individual interviews with six stroke survivors prior to the survey. As for the expected usage, participants were asked regarding the likelihood of using such a hand orthotic at home if they had it, frequency and duration of use, time they would be willing to spend to put it on, likelihood of buying one if it is available for purchase, and price they would be willing to pay.
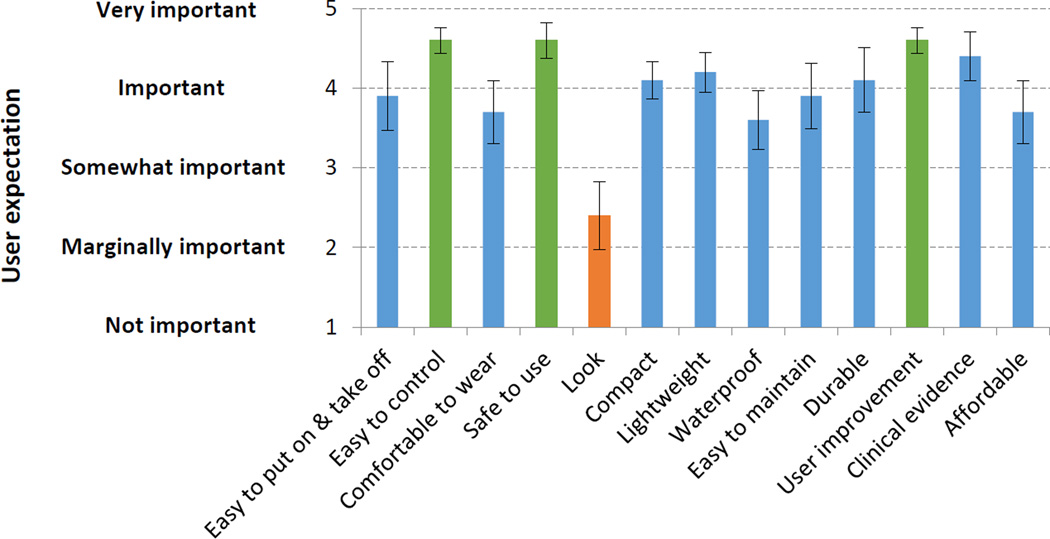
Figure 2.
Importance of each of 13 criteria for a wearable hand orthotic rated by participants. Mean ± standard error from 10 stroke survivors’ ratings are shown.
The participants experienced the developed prototype by performing dexterous hand tasks with and without the prototype, upon completion of the expectation survey. Specifically, participants were asked to write with a pen, type on a keyboard, pour water into a cup, add/stir sugar in the cup, and complete manual dexterity tests of the Box and Block [30] and Purdue Pegboard Tests [31]. The purpose of these tasks was for the participants to feel and formulate an idea of how it would be to go about their day-to-day activities with the prototype and the way the prototype affected their performance. A written manual on how to wear and use the prototype was provided. The manual also showed preliminary evidence for immediate effectiveness in the Box and Block Test score and pinch strength among chronic stroke survivors from a previous study [1].
The participants’ satisfaction with the prototype was assessed in the evaluation survey. Satisfaction for each of the 13 criteria was rated on a five-point Likert scale (1 for very unsatisfactory, 2 for unsatisfactory, 3 for OK, 4 for satisfactory, 5 for exceeding expectation). Satisfaction for affordability was rated for a cost of $100 which was based on the off-the-shelf part cost of $50 and potential PCB/labor cost. In addition to the satisfaction rating, any additional comments were asked.
House of Quality analysis
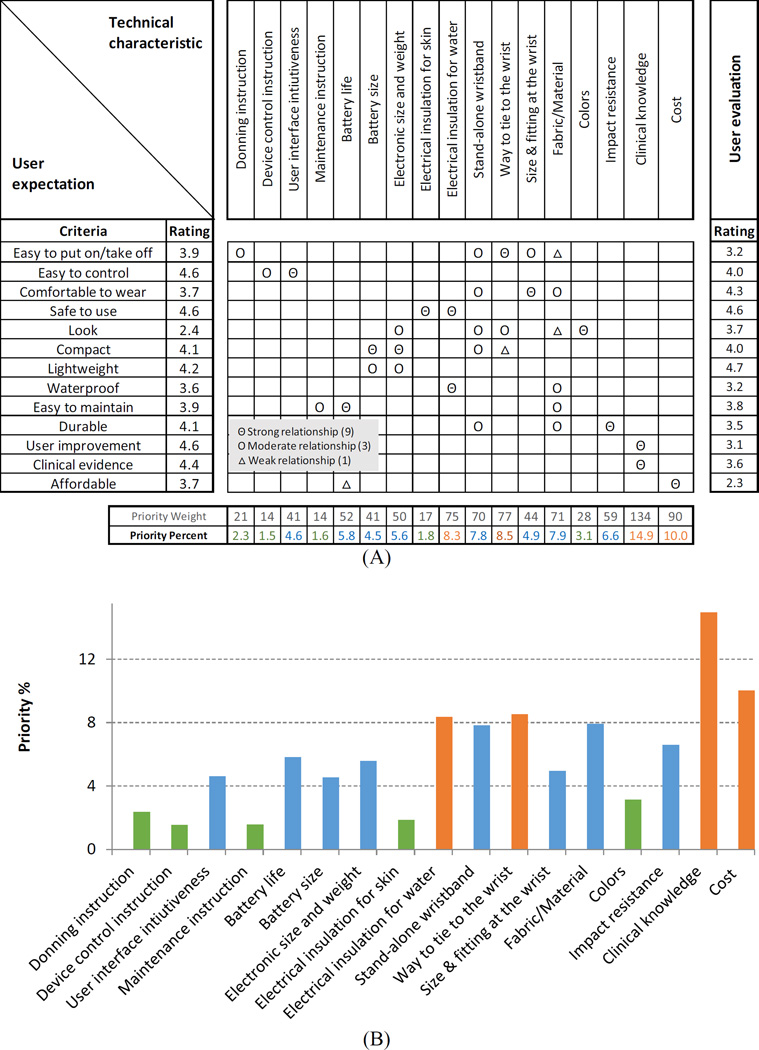
House of Quality analysis was performed to determine priority technical improvement needs of the current prototype. The analysis used the rating results for the 13 criteria from the expectation and evaluation surveys. In addition, a list of technical characteristics and an inter-relationship matrix describing the relationship between individual criteria and technical characteristics were developed by engineers (Figure 5A top row and center matrix, respectively). Priority weights for each technical characteristic (Figure 5A second to the bottom row) were calculated as the sum of the multiplication of the user expectation rating, relationship score (9, 3, or 1), and 5 less user evaluation rating for individual criteria. The priority percentage (Figure 5A bottom row) was the priority weight normalized to the sum of all priority weights across all technical characteristics. High priority percentages note high technical improvement priority to efficiently increase user satisfaction.
Figure 5.
House of Quality matrix (A) and the priority percent showing the relative priority of improvement needs in a plot (B).
3. Results
3.1 Expectation for TheraBracelet
Participants’ rating of importance for individual criteria for TheraBracelet hand orthotic is shown in Figure 2. Participants responded that it is very important (with mean rating>4.5) for the hand orthotic to be easy to control, safe to use, and effectively improving their hand function. Other important criteria (with mean rating>3.5) were easy to put on/take off, comfortable to wear, compact, lightweight, waterproof, easy to maintain, durable, clinically proven with evidence, and affordable. Look/appearance was not considered important (mean rating<2.5).
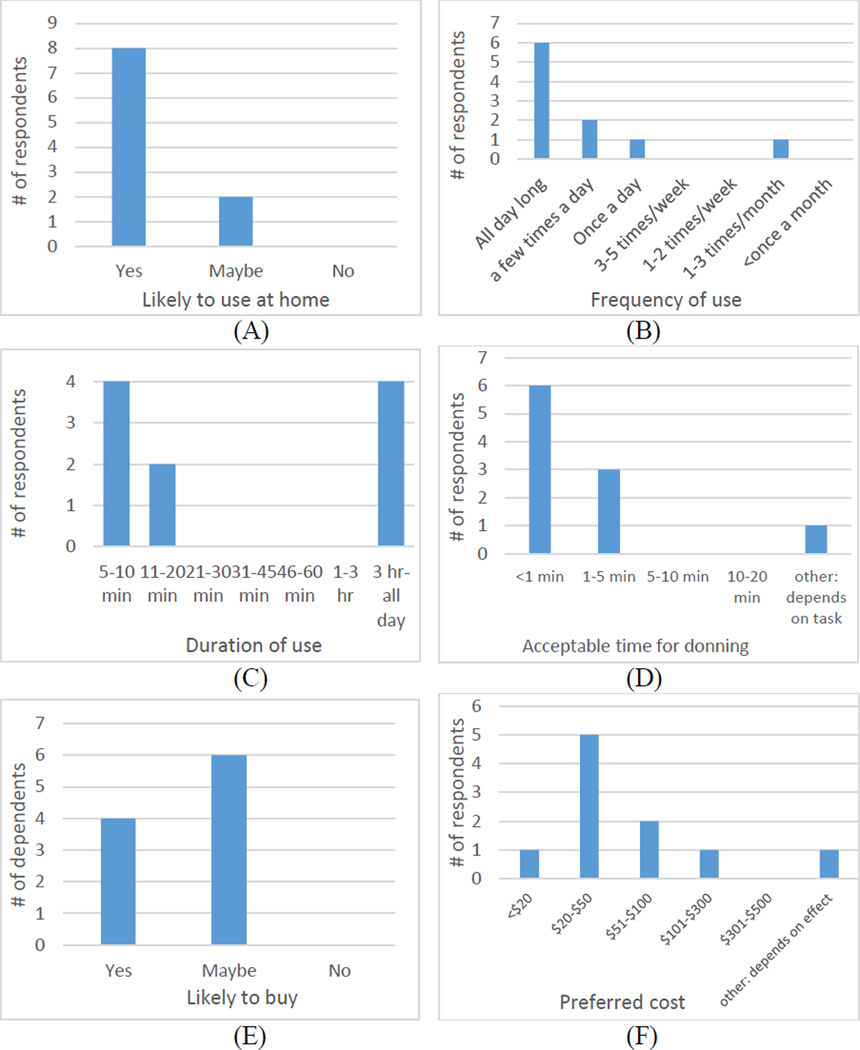
As for expected usage of TheraBracelet, most participants responded that they would use it if they have it at home (Figure 3A). Most participants responded that they would use it all day long (Figure 3B) with 5–10 min blocks or continuously for more than 3 h at a time (Figure 3C). Most participants wanted to spend less than 1 min to put on the orthotic (Figure 3D). Four of 10 participants responded that they would likely buy it if available for purchase and 6 participants responded as maybe (Figure 3E). Most participants responded that they would be willing to pay $20–$50 for such an orthotic (Figure 3F).
Figure 3.
Histograms showing stroke survivors’ responses regarding expected usage of TheraBracelet, including the likelihood of using it at home (A), expected frequency of use (B), duration of each use (C), acceptable time to put on the wearable (D), likelihood of buying one (E), and price they would be willing to pay (F).
3.2 Prototype evaluation
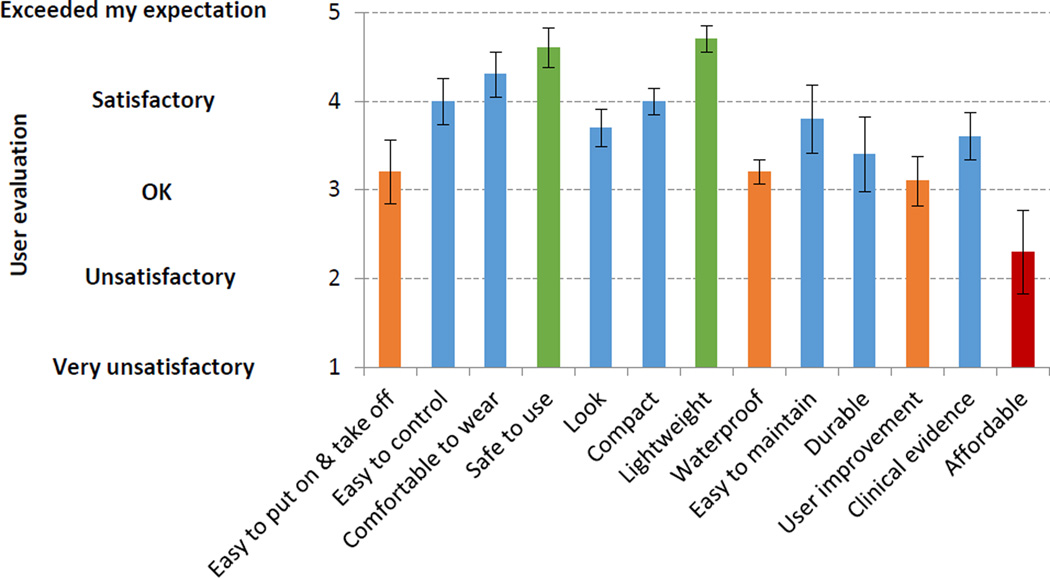
Rating of satisfaction obtained after the participants used the prototype is shown for the 13 criteria in Figure 4. The most unsatisfactory criterion was affordability, indicating $100 was considered not affordable to the participants. Other criteria that were below satisfactory (with mean rating<3.5) were easy to put on/take off, waterproof, and user improvement. The rest criteria were satisfactory or above satisfactory (mean rating>3.5). Specifically, safety and lightness of the prototype almost exceeded expectation (mean rating>4.5) (Figure 4). Additional comments from the participants included wire-free, washable, Velcro wrist strap as opposed to the athletic wristband, and a device that improves hand function over time such that eventually it is no longer needed.
Figure 4.
User satisfaction with the prototype for individual criteria. Mean ± standard error from 10 stroke survivors are shown.
The House of Quality matrix was created (Figure 5A) based on the user expectation and evaluation (Figure 2 and Figure 4 applied to the left and right columns of Figure 5A, respectively). The top priority improvement need was found in clinical knowledge, followed by cost, the way to tie to the wrist, and electrical insulation against water (Figure 5A bottom row, plotted in Figure 5B).
4. Discussions
4.1 Feasibility for TheraBracelet
This study demonstrated feasibility for a portable, wearable, battery-operated TheraBracelet that applies small vibration to the wrist with intent to affect hand dexterity. The prototype uses a rechargeable battery that can last up to 6 h. Off-the-shelf components totaled at approximately $50.
The current prototype has the vibrating wristband connected to the controller unit via a cable (Figure 1). A future prototype may aim for a stand-alone wristband containing electronics, similar to a watch, by reducing the controller unit size. The controller unit size may be reduced by replacing the AA battery with a coin-cell battery and removing the mini-USB disk. The mini-USB disk offers the ability to change the vibration source signal, which is useful for scientific investigation but unnecessary for a user product. Removal of the mini-USB disk may also reduce the part cost. The cost may further be reduced by mass production.
4.2 Expectation for TheraBracelet
Appearance of a hand orthotic was regarded as the least important, while ease of control, safety, and effectiveness in its intended purpose of improving hand function were regarded as the most important (Figure 2). Most stroke survivors wanted to try a hand orthotic all day long or frequently in 5–10 min blocks at a cost of $20–$50 (Figure 3).
4.3 Prototype evaluation
Stroke survivors evaluated the prototype with a mean rating of 3.7 out of 5 (close to satisfactory). The three most satisfying aspects about the prototype were: lightweight, safe to use, and comfortable (Figure 4). The criteria of lightweight and safe to use almost exceeded expectation (mean rating>4.5).
The unsatisfying criterion about the prototype was affordability (mean rating=2.3). Criteria that were rated OK only (with mean rating<3.5) were user improvement, don/doff, and waterproof (Figure 4). These dissatisfactions may be addressed by focusing future development efforts toward the top priority improvement needs in the cost, clinical knowledge, way to tie to the wrist, and electrical insulation against water (Figure 5B), as detailed below.
Dissatisfaction with the $100 cost is understandable based on the preferred cost of $20–$50 (Figure 3F). Accordingly, the prototype cost was identified as one of the highest priority improvement needs in the House of Quality analysis (Figure 5). The preferred cost may be achievable via design simplification involving removal of the mini-USB disk as described earlier, mass production, and potential insurance coverage.
User improvement with the prototype was the second least satisfactory criterion, although rated as OK (mean rating=3.1). Interestingly, secondary analyses examining correlations between the impairment level (Fugl-Meyer Assessment score) and the user improvement rating and between the impairment level and the overall rating showed no correlation (R2 < 0.01 and R2 = 0.02, respectively). This lack of correlation suggests that the perceived change in the manual dexterity and overall evaluation of the prototype were not dependent upon the upper limb impairment level within this subject group. With the user improvement rated as the second least satisfactory, correspondingly, improved knowledge on clinical effectiveness for specific individuals’ characteristics was identified as the top priority improvement need (Figure 5). The currently available clinical knowledge is from studies that showed statistically significant improvements in hand function immediately with imperceptible vibration [1, 13]. Yet, long-term effectiveness of a hand orthotic is of main interest for people with persistent hand impairment, as exemplified by the participant comment of being able to improve hand function over time so as not to need the orthotic any more. Thus, future clinical studies on effectiveness in the long run with repeated use are needed.
Ease of donning/doffing was also rated as below satisfactory (Figure 4). The House of Quality suggests that ease of donning/doffing may be addressed chiefly by improving the way to tie the bracelet to the wrist, followed by improving fabrics/materials with different tightness/sizes and condensing the electronics into a stand-alone wristband to eliminate the loose cable getting in the way (Figure 5). While the participants tried the prototype, it was anecdotally observed that the participants had difficulty putting the athletic band around the paretic fingers without getting it caught between fingers and sliding it to the wrist, using only the nonparetic hand. This barrier in donning needs to be addressed, especially because most participants indicated that they would like to spend less than 1 min for donning (Figure 3D). Alternative design solutions such as a cuff-bangle bracelet to bypass the fingers and directly access the wrist may be considered, in addition to the Velcro strap suggested in the participant comments.
The last criterion below satisfactory was waterproof (Figure 4). Most importantly, the prototype’s electronics were not waterproofed, which needs to be improved as identified in the House of Quality. The fabric/material may also be replaced to be non-water-absorbent (Figure 5). Waterproof is desired for washing/cleaning of the orthotic for maintenance as suggested by the participant comments. Waterproof is also desired given the frequent needs of washing hands or washing objects with the hands in daily living, especially when people wish to wear the device all day long (Figure 3B).
5. Conclusions
Feasibility for a battery-powered portable, wearable vibrating wristband to improve hand function, i.e., TheraBracelet, was demonstrated. The survey for expectation on such a hand orthotic showed stroke survivors’ willingness to try it out at a low cost. In addition, the current prototype was well received by stroke survivors with a mean rating of 3.7 out of 5 (close to satisfactory). Evaluation of the current prototype using the House of Quality analysis revealed that the priority improvement needs for the prototype are to improve clinical knowledge on long-term effectiveness, reduce cost, ease donning/doffing, and waterproof. In summary, this study presents a potential for a low-cost wearable hand orthotic likable by stroke survivors.
Implications for rehabilitation.
Feasibility for a portable wearable wristband-type hand orthotic was demonstrated.
The survey showed stroke survivors are willing to try such an orthotic at low cost.
The current prototype was rated as satisfactory overall by stroke survivors.
This study provides a potential for a low-cost wearable hand orthotic likable by stroke survivors.
Acknowledgments
This project was supported by the WiSys Applied Research Grant and the National Center for Advancing Translational Sciences (NIH 8UL1TR000055).
Footnotes
Declaration of Interest
The corresponding author is an inventor for a pending patent regarding imperceptible vibration to improve hand function. Others report no declarations of interest.
Contributor Information
Kishor Lakshminarayanan, Email: lakshmi4@uwm.edu.
Fa Wang, Email: fwang57@wisc.edu.
John G. Webster, Email: john.webster@wisc.edu.
Na Jin Seo, Email: seon@musc.edu.
References
- 1.Seo NJ, et al. Effect of Remote Sensory Noise on Hand Function Post Stroke. Front Hum Neurosci. 2014;8:934. doi: 10.3389/fnhum.2014.00934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Prevalence and most common causes of disability among adults--United States, 2005. MMWR Morb Mortal Wkly Rep. 2009;58(16):421–426. [PubMed] [Google Scholar]
- 3.Go AS, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trombly CA. Occupational therapy for physical dysfunction. Baltimore: Williams & Wilkins; 1989. Stroke; pp. 454–471. [Google Scholar]
- 5.Gray CS, et al. Motor recovery following acute stroke. Age Ageing. 1990;19(3):179–184. doi: 10.1093/ageing/19.3.179. [DOI] [PubMed] [Google Scholar]
- 6.Nakayama H, et al. Recovery of upper extremity function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil. 1994;75(4):394–398. doi: 10.1016/0003-9993(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 7.Parker VM, Wade DT, Langton Hewer R. Loss of arm function after stroke: measurement, frequency, and recovery. Int Rehabil Med. 1986;8(2):69–73. doi: 10.3109/03790798609166178. [DOI] [PubMed] [Google Scholar]
- 8.Kamper DG, Rymer WZ. Impairment of voluntary control of finger motion following stroke: role of inappropriate muscle coactivation. Muscle Nerve. 2001;24(5):673–681. doi: 10.1002/mus.1054. [DOI] [PubMed] [Google Scholar]
- 9.Hartman-Maeir A, et al. Activities, participation and satisfaction one-year post stroke. Disabil Rehabil. 2007;29(7):559–566. doi: 10.1080/09638280600924996. [DOI] [PubMed] [Google Scholar]
- 10.Woodson AM. Stroke. In: Trombly CA, editor. Occupational therapy for physical dysfunction. Philadelphia: Lippincott, Williams & Wilkins; 2002. pp. 817–853. [Google Scholar]
- 11.Alon G, Levitt AF, McCarthy PA. Functional electrical stimulation enhancement of upper extremity functional recovery during stroke rehabilitation: a pilot study. Neurorehabil Neural Repair. 2007;21(3):207–215. doi: 10.1177/1545968306297871. [DOI] [PubMed] [Google Scholar]
- 12.Barry JG, et al. Effects of the SaeboFlex® orthosis and a home exercise program on upper extremity recovery in individuals with chronic. Journal of Neurologic Physical Therapy. 2006;30(4):207. [Google Scholar]
- 13.Enders LR, et al. Remote vibrotactile noise improves light touch sensation in stroke survivors' fingertips via stochastic resonance. J Neuroeng Rehabil. 2013;10(1):105. doi: 10.1186/1743-0003-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang F, et al. An MRI-compatible hand sensory vibrotactile system. Physiol Meas. 2015;36(1):N15–N21. doi: 10.1088/0967-3334/36/1/N15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lakshminarayanan K, et al. Application of Vibration to Wrist and Hand Skin Affects Fingertip Tactile Sensation. Physiological Reports. 2015;3(7):e12465. doi: 10.14814/phy2.12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hur P, Wan Y-H, Seo NJ. Investigating the Role of Vibrotactile Noise in Early Response to Perturbation. IEEE Transactions on Biomedical Engineering. 2014;61(6):1628–1633. doi: 10.1109/TBME.2013.2294672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurita Y, Shinohara M, Ueda J. Wearable Sensorimotor Enhancer for Fingertip Based on Stochastic Resonance Effect. IEEE Transactions on Human-Machine Systems. 2013;43(3):333–337. [Google Scholar]
- 18.Wells C, et al. Touch noise increases vibrotactile sensitivity in old and young. Psychological Science. 2005;16(4):313–320. doi: 10.1111/j.0956-7976.2005.01533.x. [DOI] [PubMed] [Google Scholar]
- 19.Collins JJ, Imhoff TT, Grigg P. Noise-mediated enhancements and decrements in human tactile sensation. Physical Review E. 1997;56(1):923. [Google Scholar]
- 20.Batavia AI, Hammer GS. Toward the development of consumer-based criteria for the evaluation of assistive devices. J Rehabil Res Dev. 1990;27(4):425–436. doi: 10.1682/jrrd.1990.10.0425. [DOI] [PubMed] [Google Scholar]
- 21.Biddiss EA, Chau TT. Upper limb prosthesis use and abandonment: a survey of the last 25 years. Prosthet Orthot Int. 2007;31(3):236–257. doi: 10.1080/03093640600994581. [DOI] [PubMed] [Google Scholar]
- 22.Glynn MK, et al. Management of the upper-limb-deficient child with a powered prosthetic device. Clin Orthop Relat Res. 1986;(209):202–205. [PubMed] [Google Scholar]
- 23.Bhaskaranand K, Bhat AK, Acharya KN. Prosthetic rehabilitation in traumatic upper limb amputees (an Indian perspective) Arch Orthop Trauma Surg. 2003;123(7):363–366. doi: 10.1007/s00402-003-0546-4. [DOI] [PubMed] [Google Scholar]
- 24.Atkins DJ, Heard DCY, Donovan WH. Epidemiologic Overview of Individuals with Upper-Limb Loss and Their Reported Research Priorities. JPO: Journal of Prosthetics and Orthotics. 1996;8(1):2–11. [Google Scholar]
- 25.Engelbrektsson P. Effects of product experience and product representations in focus group interviews. Journal of Engineering Design. 2002;13(3):215–221. [Google Scholar]
- 26.Hauser JR, Clausing D. The house of quality. Harvard Business Review. 1988;66:63–73. [Google Scholar]
- 27.Durga Prasad KG, et al. Development of total quality engineering education model using QFD. The Journal of Engineering Education. 2008;21(4):1–6. [Google Scholar]
- 28.Seo NJ, et al. Usability evaluation of low-cost hand and arm virtual reality rehabilitation games. Journal of Rehabilitation Research & Development. doi: 10.1682/JRRD.2015.03.0045. in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fugl-Meyer AR, et al. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7(1):13–31. [PubMed] [Google Scholar]
- 30.Mathiowetz V, et al. Adult norms for the Box and Block Test of manual dexterity. Am J Occup Ther. 1985;39(6):386–391. doi: 10.5014/ajot.39.6.386. [DOI] [PubMed] [Google Scholar]
- 31.Desrosiers J, et al. The Purdue Pegboard Test: normative data for people aged 60 and over. Disabil Rehabil. 1995;17(5):217–224. doi: 10.3109/09638289509166638. [DOI] [PubMed] [Google Scholar]