Abstract
Humans experience a unified self that integrates our mental lives and physical bodies, but many studies focus on isolated domains of self-knowledge. We tested the hypothesis that knowledge of one’s mind and body are related by examining metamemory and interoception. We evaluated two dimensions of metamemory and interoception: subjective beliefs and the accuracy of those beliefs compared to objective criteria. We first demonstrated, in two studies, that metamemory beliefs were positively correlated with interoceptive beliefs, and this was not due to domain-general confidence. Finally, we showed that individuals with better metamemory accuracy also had better interoceptive accuracy. Taken together, these findings suggest a common mechanism subserving knowledge of our cognitive and bodily states.
Keywords: metacognition, metamemory, self-knowledge, heartbeat detection, interoception, self-awareness
1. Introduction
“Know thyself,” says the ancient Greek maxim, not “Know thy thinking self” or “Know thy feeling self.” However, studies of self-knowledge have, for the most part, been limited by domain. There are studies of how one knows thy thinking self (e.g., Fleming, Weil, Nagy, Dolan, & Rees, 2010; Kelemen, Frost, & Weaver, 2000) and studies of how one knows thy feeling self (e.g., Brackett, Rivers, Shiffman, Lerner, & Salovey, 2006; Robinson & Clore, 2002; Spain, Eaton, & Funder, 2000), but the current literature leaves open the question of whether and how these forms of self-knowledge are related (e.g., Fleming, Ryu, Golfinos, & Blackmon, 2014; Kelemen et al., 2000; Schraw, 1998; Song et al., 2011). That is, is the knowing when we recognize an acquaintance in a crowd, or that we’ve forgotten an item on the grocery list, supported by the same psychological and biological processes as knowing when we feel our hearts are beating rapidly as we wait at the arrivals gate for a long absent lover, or knowing that we sense impending doom as a deadline approaches for which we have not completed the work? As a first step to answer this question, we examined the relationship between knowledge about one’s cognitive and bodily states using measures of metamemory and interoception.
1.1 Knowledge about cognitive states: metamemory
Metamemory, a type of metacognition, is knowledge about the contents and accuracy of one’s own memory (Nelson & Narens, 1990). Metamemory is typically assessed by asking individuals to reflect on, or introspect about, their own memory (e.g., Chua, Schacter, & Sperling, 2009b; Ghetti, Mirandola, Angelini, Cornoldi, & Ciaramelli, 2011). Because introspections are fallible, we refer to self-reports about perceived memory ability as metamemory beliefs, and do not assume that these beliefs reflect accurate knowledge about memory. These beliefs can pertain to how good or bad their own memory is (e.g., “I am good at remembering names”) and can also be more broad and include general beliefs about how memory works (e.g., “studying longer will help me remember”) (Bennett-Levy & Powell, 1980; Dixon, Hultsch, & Hertzog, 1988; Gilewski, Zelinski, & Schaie, 1990). Metamemory beliefs can be assessed via questionnaires in which individuals report their overall beliefs about their own memory. Alternatively, they can also be assessed on a trial-by-trial basis by asking people to rate how confident, or certain, they are about specific memories, with high confidence ratings indicating they believe that they have retrieved correct information (e.g., Chua, Hannula, & Ranganath, 2012; Simons, Peers, Mazuz, Berryhill, & Olson, 2010). Comparing these metamemory beliefs to objective tests of memory yields information about the accuracy of these introspections, which is referred to as metamemory accuracy. In other words, metamemory accuracy provides an index of how well subjective beliefs correspond with actual memory performance. For example, an individual who is more likely to have a correct memory when he has higher confidence, and incorrect memory when he has lower confidence would have high metamemory accuracy because his confidence in his memory tracks his actual memory. In contrast, an individual who is equally likely to have a correct memory when she has high or low confidence would have low metamemory accuracy because her confidence would be a meaningless indicator of her actual memory performance. Metamemory accuracy is typically calculated using measures such as calibration, gamma, and da; these indices of metamemory accuracy include measures of confidence in combination with memory accuracy (Benjamin & Diaz, 2008; Masson & Rotello, 2009). While calibration, gamma, and da all index metamemory accuracy, their calculations are different and they tap into slightly different ways confidence can be meaningfully related to accuracy (see Section 3.1.2.1)., In examining metamemory, it is critical to evaluate and understand both of these dimensions (Table 1): 1) the metamemory beliefs, which encompass both the confidence in one’s memory for a single memory and declarative statements about one’s memory and how it works, and 2) metamemory accuracy, which is the comparison of the metamemory beliefs to actual memory performance (Chua, Pergolizzi, & Weintraub, 2014).
Table 1.
Dimensions of Metamemory and Interoception in terms of Beliefs and Accuracy, and how they relate to Studies 1–3. Modeled off of Garfinkel et al. (2015).
| Metamemory Beliefs | Metamemory Accuracy | Interoceptive Beliefs | Interoceptive Accuracy | |
|---|---|---|---|---|
| Definition | Self-perceived knowledge of one’s own memory |
Accuracy of self-perceived knowledge of one’s own memory |
Self-perceived ability to detect bodily sensations |
Accuracy of self-perceived bodily sensations |
| Example | Do you think you are good at remembering names? Will studying in spaced intervals help you remember? |
When you are highly confident in a memory, is it an accurate memory? |
Do you think that you detect internal bodily sensations? |
Can you accurately report the number of heart beats during a specific interval? |
| Mode of Assessment | Self-report about perceived memory ability |
Relationship between objective performance and self-reported beliefs |
Self-report about perceived ability to detect bodily sensations |
Relationship between objective bodily sensation and perceived bodily sensation |
| Example | Questionnaires, such as the Metamemory in Adulthood Questionnaire; Confidence in specific memories, or average confidence in memory ability |
Assessing the confidence- accuracy relationship via calibration, the gamma correlation, da, and meta d’ |
Questionnaires, such as the Body Awareness Questionnaire |
Accuracy during heartbeat counting or detection tasks |
1.2 Knowledge about bodily states: interoception
Just like we have knowledge of cognitive states (e.g., metamemory), we also have knowledge of our bodily or physiological states, referred to as interoception (Craig, 2003). Like metamemory, interoception can broadly refer to both subjective beliefs about physiological states and the accuracy of those beliefs (Table 1) (Ceunen, Van Diest, & Vlaeyen, 2013; Garfinkel & Critchley, 2013). Parallel with metamemory measures, interoceptive beliefs can be indexed via retrospective self-report measures (e.g., Body Awareness Questionnaire (BAQ); Shields, Mallory, & Simon, 1989) and when they are, typically reflect general beliefs about capacity (for review, see Mehling et al., 2009). To measure the accuracy of interoceptive beliefs about the body, participants are generally asked to report on their physiological states in the moment (Garfinkel, Seth, Barrett, Suzuki, & Critchley, 2014). As is true of metamemory accuracy, computing interoceptive accuracy involves mathematically comparing people’s beliefs about their bodies to some objective criteria about what their bodies are doing. The most common interoceptive tasks ask participants to count or monitor their heartbeats (Pollatos, Gramann, & Schandry, 2007; Schandry, 1981; Whitehead, Drescher, Heiman, & Blackwell, 1977; Whitehead & Drescher, 1980). It is important to note that less attention has been paid to the relationship between subjective beliefs and accuracy of interoception, compared to metamemory. Investigating the relationship between interoceptive beliefs and interoceptive accuracy is an active area of research (Garfinkel et al., 2014).
1.3 Linking metamemory and interoception
While cognition is often considered to be separate or distinct from bodily processes (in the spirit of Descartes) (Gilin, Maddux, Carpenter, & Galinsky, 2013; Janssen, van Osch, Lechner, Candel, & de Vries, 2012; Schlaffke et al., 2015; Swann, Griffin, Predmore, & Gaines, 1987), there is growing recognition that in fact that cognition is “embodied” such that cognitive processes rely on neural representations of the body (Barsalou, 2008). For example, evidence from neuroimaging indicates that both metamemory (Chua et al., 2006; Chua, Schacter, & Sperling, 2009b) and interoception (Critchley, Melmed, Featherstone, Mathias, & Dolan, 2002; Critchley et al., 2004; Pollatos, Schandry, Auer, & Kaufmann, 2007) recruit the insula—a key region integration of homeostatic information and visceromotor control (Craig, 2003, 2008, 2009). It is therefore possible, even probable, that metamemory and interoception are subserved by a common neural system that involves or is anchored in the insula. Before examining a possible shared neural mechanism of these effects, it is critical first to establish a relationship between metamemory and interoception.
In the present studies, we evaluated the relationship between metamemory and interoception, and indexed both beliefs and accuracy. If a common mechanism subserves metamemory and interoception then there should be consistent and related individual differences such that performance on metamemory and interoception measures is positively correlated for both beliefs and accuracy. Across three studies, we evaluated subjective beliefs and objective accuracy of metamemory and interoception, predicting that the indices would be correlated. In other words, are people who believe they have accurate knowledge of their memories also believe that they have good knowledge about reading their bodily signals, and are people who are indeed good at knowing their own memory also good at knowing their bodily signals? In Study 1, we examined the relationship between metamemory beliefs and interoceptive beliefs using self-report measures. In Study 2, we examined the relationship between metamemory beliefs related to specific memories (i.e., a performance task) and interoceptive beliefs using a self-report, while controlling for performance and confidence in other domains. Finally, in Study 3, we examined the relationship between metamemory accuracy and interoceptive accuracy. Together, these studies show a relationship between metamemory and interoception when examining both subjective beliefs and accuracy.
2. Study 1: Metamemory Beliefs & Interoceptive Beliefs
2.1 Method
2.1.1 Participants
211 Mechanical Turk (MTurk) workers (123 male and 88 female, ages 18–65) participated in this research for $1.00 remuneration. Participants were recruited via the Amazon Mechanical Turk website (www.mturk.com). MTurk workers qualified to participate if they were over 18 years of age, were located in the United States, had previously completed at least 1000 HITs that were subsequently approved, and had a least a 98% approval rate on HITs. Participant data were excluded if they failed our “catch trial”. Participants were asked “How often do you have fatal heart attacks while trying to remember something?” and were excluded if they answered anything other than “never”. Eleven participants were excluded and data from the remaining 200 participants were analyzed. Each participant provided consent by clicking a button on the computer, which was approved by the Human Research Protection Program (HRPP) of the City University of New York (CUNY).
2.1.2 Procedures
2.1.2.1 Metamemory Questionnaire
In order measure metamemory beliefs, participants completed a subset of the Metamemory in Adulthood (MIA) questionnaire (Dixon et al., 1988), and answered questions on a 5-point Likert scale related to their knowledge and use of memory strategies (Strategy subscale), knowledge about basic memory processes (Task subscale), and the relationship between anxiety and one’s own memory performance (Anxiety subscale) (Hertzog, Dixon, Schulenberg, & Hultsch, 1987). Responses were analyzed by averaging the Likert Scale responses for the Strategy, Task, and Anxiety subscales, as well as an overall average across these dimensions, which we will refer to as Overall Metamemory. Although some items were reverse scaled, scores were calculated such that: 1) for Strategy, higher numbers indicate more strategy use; 2) for Task, higher numbers indicate higher knowledge about basic memory processes; and 3) for Anxiety, higher numbers indicate higher anxiety and/or higher knowledge about how anxiety effects memory. Accordingly, higher numbers in the Overall Metamemory score indicate higher self-reported metamemory.
2.1.4 Body Awareness Questionnaire
Participants answered the 18 questions from the Body Awareness Questionnaire (Shields et al., 1989), as a measure of interoceptive beliefs. In the Body Awareness Questionnaire, participants are given several statements about their sensitivity to normal, non-emotive bodily processes. They are then asked to indicate how well the statement describes them on a 7-point Likert scale that ranges from “Not at all true of me” to “Very true of me.” The questionnaire indexes sensitivity to body cycles and rhythms, the ability to predict bodily changes, and the ability to detect small changes in bodily functioning. Bodily Awareness was calculated by averaging the 1–7 responses on the scale across the 18 questions, accounting for the reversed scale item.
2.1.5 Data Analysis
Correlations between Metamemory and Body Awareness scores were tested in SPSS 22.0 and were considered significant at p<0.05, two-tailed. Linear regression was used to examine the relationship between metamemory subscales and body awareness, and results were considered significant at p<0.05, two-tailed.
2.2 Results
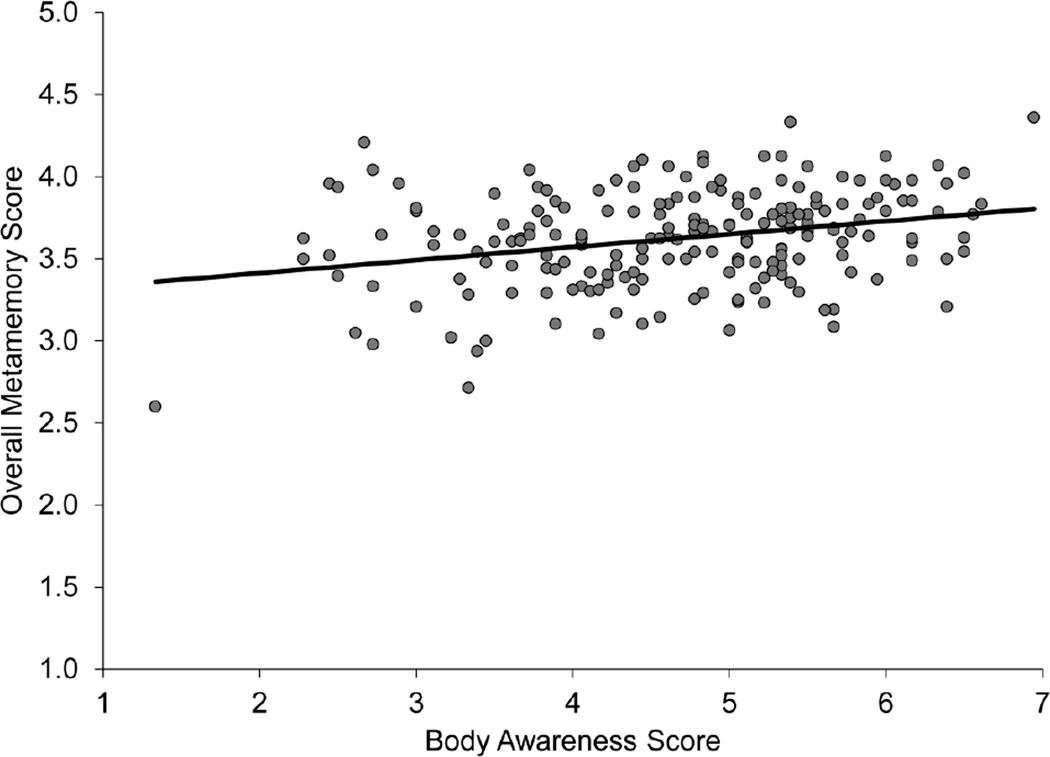
We first examined whether scores on the Body Awareness Questionnaire (Mean ± SD, 4.67 ± 1.06), a measure of interoceptive beliefs, correlated with our Overall Metamemory score (Mean ± SD, 3.62 ± 0.30), a measure of metamemory beliefs. There was a positive correlation (r=0.38, p<0.00001) such that individuals with increasing interoceptive beliefs showed increased metamemory beliefs in general (Figure 1).
Figure 1.
Scatter plot showing that metamemory beliefs and interoceptive beliefs are correlated using self-report questionnaires (Study 1). Score on the Body Awareness Questionnaire, a measure of interoceptive beliefs, positively correlated with overall metamemory, assessed by the Metamemory in Adulthood Questionnaire, a measure of metamemory beliefs (r=0.38, p<0.00001).
We next examined whether specific domains of metamemory were driving this correlation and used linear regression to determine if Strategy, Task, and/or Anxiety were predictive of interoceptive beliefs. Strategy was the only significant predictor of interoceptive beliefs (R2=0.150; R2adjusted =0.137; F(3,196)=11.50, p<0.00001; Strategy: B = 0.854, 95%CI [0.560,1.15]; Task: B = 0.233, 95%CI[−0.143,0.609]; Anxiety: B = −0.039, 95%CI[−0.241,0.164]).
2.3 Discussion
Participants who believed themselves to be better at interoception, also reported better metamemory. This relationship was driven by self-reported strategy use and/or knowledge about memory strategies, and not by general knowledge about memory, or anxiety about memory. The questionnaire responses in this study only provide insight metamemory and interoceptive beliefs, but no information about whether or not these beliefs are accurate. Our next step was to determine whether interoceptive beliefs were related to metamemory beliefs alone, or whether interoceptive beliefs were also related to metamemory accuracy. In Study 2, we used a combination of self-report questionnaires and performance tests to address this possibility, while controlling for confidence (a component of metamemory) in other domains.
3. Study 2: Metamemory Beliefs/Accuracy & Interoceptive Beliefs
3.1 Methods
3.1.1 Participants
110 MTurk workers (48 male and 62 female, ages 18–49) participated in this research for $1.00 through the Amazon Mechanical Turk website (www.mturk.com). Inclusion and exclusion criteria, and consent procedures were the same as Study 1 (Section 1.1.1). Ten people failed our ‘catch trial’, and data from the remaining 100 participants were analyzed.
3.1.2 Procedures
3.1.2.1 Memory and Metamemory task
Fifty general knowledge questions were selected from a database of recently normed questions (Tauber, Dunlosky, Rawson, Rhodes, & Sitzman, 2013). In an online form, participants viewed 50 general knowledge questions and had to choose between the correct answer and an incorrect answer. Participant’s made their choice on a 6-point scale that incorporated confidence. Specifically, they were told that:
“Clicking 1–3 indicates you think it is the choice on the left and clicking 4–6 indicates you think it is the choice on the right” and “The differences between 1–3 and 4–6 indicate differences in your certainty. Clicking 1 indicates you are 100% sure that it is the choice on the left, 2 indicates you are 66% sure it is the choice on the left, 3 indicates you are 33% sure it is the choice on the left, 4 indicates you are 33% sure it is the choice on the right, 5 indicates you are 66% sure it is the choice on the right, and 6 indicates you are 100% sure it is the choice on the right.”
Memory accuracy was calculated as the proportion of correct answers. Metamemory beliefs were indexed by the proportion of high confidence responses given to all questions (“1” or “6” responses). We examined three different measures of metamemory accuracy: calibration (Jonsson & Allwood, 2003; Lichtenstein, Fischhoff, & Phillips, 1982), the Goodman-Kruskal gamma correlation (Nelson, 1984; Pannu & Kaszniak, 2005), and da (Benjamin & Diaz, 2008; Masson & Rotello, 2009). These measures are commonly used indices of metamemory accuracy, which incorporate how accurate one’s memory is with differing levels of confidence across an ordinal scale, and provide and index of how well confidence corresponds with accuracy (Chua & Solinger, 2015; Luna & Martín-Luengo, 2012; Perfect, Hollins, & Hunt, 2000; Toth, Daniels, & Solinger, 2011; Weber & Brewer, 2004). Note that metamemory accuracy describes the relationship between confidence and accuracy, and combines subjective and objective aspects of memory, whereas memory accuracy is related to objective aspects of memory and indexes whether or not memory responses were correct. One measure of metamemory accuracy, confidence-accuracy calibration, provides an absolute measure of the difference between accuracy and confidence and is tied to the rating scale. Calibration was calculated using the formula: 1/n Σ nt(rtm-ct)2 (Jonsson & Allwood, 2003; Lichtenstein et al., 1982), where n is the total number of trials, ct is the proportion of correct trials for all items in the confidence rating rt, nt is the number of times the confidence rating rt was used, and rtm is the mean of the confidence ratings in class rt. Perfect calibration is 0, and larger numbers represent more calibration errors, or a worse correspondence between confidence and accuracy. The gamma correlation is a relative measure of metamemory in that it assesses the degree to which participants can distinguish that they were more or less likely to have made an accurate memory decision. This measure is not tied to the numeric probabilities determined by the rating scale in absolute terms, but instead is sensitive to whether when an individual is more likely to be correct with higher confidence ratings. Larger gammas indicate better metamemory accuracy, with perfect metamemory accuracy being 1. We also calculated da, a signal detection theory-based measure of relative accuracy, using the formula da = √2y0/(1+m2) where y0 and m2 represent the y intercept and slope, respectively, of a normal deviate isosensitivity function (Benjamin & Diaz, 2008; Masson & Rotello, 2009). For cases in which a participant failed to use one of the confidence responses, a correction where 0 was replaced with 0.5/n (n=#trials) was applied (Stanislaw & Todorov, 1999).
3.1.2.2 Body Awareness Questionnaire
In the second part of the study, participants completed the Body Awareness Questionnaire (Shields et al., 1989; Section 2.1.4) as a measure of interoceptive beliefs.
3.1.2.3 Personal Evaluation Inventory (PEI)
As a control task, participants were given the PEI (Shrauger & Schohn, 1995) which measures domain-specific and general confidence. This is a 54-item scale that asks whether participants “Strongly Agree”, “Mainly Agree”, “Mainly Disagree”, or “Strongly Disagree” with statements that reflect common feelings, attitudes, and behaviors. The scale includes both positively and negatively worded items across several domains. Items assessed confidence in Academic, Appearance, Athletics, Romantic, Social, and Speaking domains, as well as general confidence and mood.
3.1.2.4 Dot Estimation Task
As a control, we included an additional task that allowed us to compare subjective confidence with objective performance in a domain unrelated to memory or bodily processes. Specifically, we included a dot estimation task immediately following the PEI because previous research has shown that there are individual differences related to overestimation and underestimation (Izard & Dehaene, 2008). Participants were instructed to estimate the number of dots presented on a computer screen on 6 trials. Dots were white circles on a grey background. Dots were presented in random locations on the screen for 5 seconds using Qualtrics (qualtrics.com), or until the participant advanced the screen. The trials consisted of displays of 162, 109, 81, 203, 121, and 66 dots. After each trial, participants were instructed to rate their confidence in their decision using a sliding scale ranging from 1 to 6, where 1 represented “I am the least bit confident. My estimate is probably really different from the actual number of dots” and 6 represented “I am very confident. My estimate if probably within 10 (plus or minus) dots of the number actually on the screen.”
Dot estimation accuracy was calculated using the formula: 1-[(Σ|Ai-Ri|/Ai) /6], where Ai=number of actual number of dots during trial i and Ri=number of dots estimated (reported by participant) during trial i. A value of 1 equals perfect dot estimation.
3.1.3 Data Analysis
We first ran correlation analyses to determine whether the score of the Body Awareness Questionnaire (a measure of interoceptive beliefs) related to memory accuracy, metamemory accuracy, and confidence (a measure of metamemory beliefs). We next ran correlation analyses to determine whether the score of the Body Awareness Questionnaire or the proportion of high confidence responses on the general knowledge test related to the average score on the PEI, the subscales of the PEI, dot estimation accuracy, and dot estimation confidence. Correlations were tested in SPSS 22.0 and were considered significant at p<0.05, two-tailed. Next, linear regression was used to test for effects of bodily awareness on metamemory accuracy and proportion of high confidence responses while controlling for effects of memory accuracy, trait confidence, and dot estimation confidence. In a two-step model, memory accuracy, average score on the PEI, and dot estimation confidence were entered as predictors for metamemory accuracy or proportion of high confidence responses in the first model and bodily awareness was entered as a predictor in the second model.
3.2 Results
Participants performed well above chance (50%) on the general knowledge questions with an average of 80.3 ± 9.6% (Mean ± SD) correct. Over half of their responses were made with high confidence, with an average of 61.2 ± 19% of all responses being made with high confidence. Not all participants used the full response scale, resulting in Gamma being incalculable for 6 participants and Calibration being incalculable for 10 participants. Metamemory accuracy, as measured by calibration (0.026 ± 0.018), the gamma coefficient (0.75 ± 0.26), and da (1.31 ± 0.53), was reasonably good.
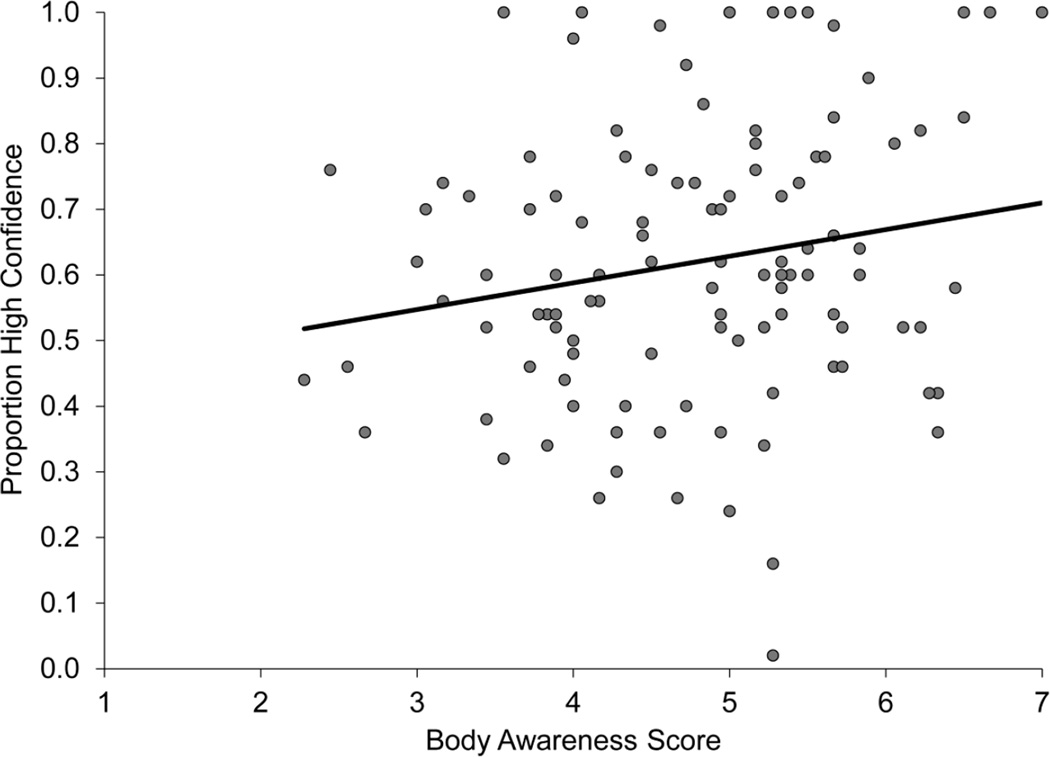
Consistent with Study 1, scores on the Body Awareness Questionnaire and proportion of high confidence responses were positively correlated (r=0.251, p<0.012; Figure 2). However, the score on the Body Awareness Questionnaire was not significantly correlated with memory accuracy (r=−0.018, p=0.861), calibration (r=0.045, p=0.675), the gamma coefficient (r=−0.126, p=0.229), or da (r=−0.083, p=0.416), suggesting that interoceptive beliefs do not correlate with memory or metamemory accuracy. For the full correlation matrix, see Table 2.
Figure 2.
Scatter plot from Study 2 showing a significant association between interoceptive beliefs, as measured by score on the Body Awareness Questionnaire and metamemory beliefs, as measured by higher confidence on the general knowledge test (r=0.232, p<0.05).
Table 2.
The correlation matrix for all variables related to interoceptive beliefs, memory accuracy, metamemory beliefs, metamemory accuracy, the Personal Evaluation Inventory (PEI) and its subscales, and the dot estimation task from Study 2. Our primary interest was in factors that correlated with response on the Bodily Awareness Scale, which appears in bold. Individuals who believed they were more aware of their bodies, as measured by the Bodily Awareness Scale, also believed that they had better memories, as indicated by a significant correlation with the Proportion of High Confidence (CONF) responses. Cells that appear in gray represent correlations between measures of similar constructs (i.e., different measures of metamemory accuracy or different PEI subscales);
| Bodily Awareness |
Memory Accuracy |
Prop. High CONF |
Calibration | Gamma | da | Avg. PEI |
PEI: Academic |
PEI: Appearance |
PEI: Athletic |
PEI: General |
PEI: Mood |
PEI: Romantic |
PEI: Social |
PEI: Speaking |
Dot Est. |
Dot CONF |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bodily Awareness |
1 | ||||||||||||||||
| Memory Accuracy |
.005 | 1 | |||||||||||||||
| Prop. High CONF |
.232* | .238* | 1 | ||||||||||||||
| Calibration | .045 | .036 | −.257* | 1 | |||||||||||||
| Gamma | −.126 | .182 | .058 | −.274** | 1 | ||||||||||||
| da | −.053 | .622** | .202* | −.397** | .504** | 1 | |||||||||||
| Average PEI | −.004 | −.070 | −.017 | .019 | .223* | .047 | 1 | ||||||||||
| PEI: Academic |
−.113 | −.085 | −.125 | −.081 | .0106 | .124 | .409** | 1 | |||||||||
| PEI: Appearance |
.197* | −.032 | .042 | .071 | .148 | .038 | .653** | −.003 | 1 | ||||||||
| PEI: Athletic | .111 | −.003 | −.099 | .181 | −.018 | −.116 | .570** | .218* | .347** | 1 | |||||||
| PEI: General | .030 | −.058 | .064 | −.046 | .157 | .078 | .817** | .354** | .504** | .386** | 1 | ||||||
| PEI: Mood | −.130 | .041 | −.12 | .083 | .187 | −.107 | .595** | .090 | .237** | .215* | .453** | 1 | |||||
| PEI: Romantic |
−.123 | −.005 | −.77 | −.020 | .199 | −.118 | .617** | .147 | .308** | .243* | .441** | .334** | 1 | ||||
| PEI: Social | .040 | −.251* | −.037 | −.133 | .109 | .027 | .618** | .136 | .318** | .313** | .450** | .229* | .327** | 1 | |||
| PEI: Speaking |
−.054 | .076 | −.064 | .035 | .132 | .103 | .388** | .068 | .201* | .068 | .190 | .144 | .069 | .147 | 1 | ||
| Dot Estimation |
−.072 | −.235* | −.086 | −.153 | .094 | −.093 | −.030 | −.088 | .134 | −.064 | −.070 | .090 | −.160 | −.053 | .016 | 1 | |
| Dot Estimation CONF |
.008 | −.148 | .00002 | −.272** | .065 | −.010 | .113 | .011 | .192 | .007 | .058 | .081 | .069 | .079 | −.012 | .031 | 1 |
Correlation is significant at the p<0.05 level (2-tailed);
Correlation is significant at the p<0.01 level (2-tailed)
To test whether the relationship between interoceptive beliefs and metamemory beliefs was specific to memory, or related to a more general trait of self-confidence, we examined whether scores on the Body Awareness Questionnaire and proportion of high confidence responses correlated with responses to the PEI. Participant’s scores on the Body Awareness Questionnaire were not significantly correlated with the average PEI (r=−0.002, p=0.984), and the only subscale that approached significance was the appearance subscale (r=0.196, p<0.052; all other subscales |rs|<0.13, all ps>0.19). The same was true for the proportion of high confidence response to the general knowledge questions; there was no significant correlation with average PEI (r=−0.022, p=0.829), or its subscales (all 8 subscales |rs|<0.19, all ps>0.08). Thus, it appears that the relationship between interoceptive beliefs and metamemory beliefs cannot be explained by general confidence in one’s own abilities.
We next examined the specificity of the interoceptive belief and metamemory belief relationship by comparing confidence and accuracy on an unrelated task: dot estimation. The dot estimation task was indeed very different than the general knowledge task, with accuracy on the general knowledge and dot estimation tasks showing a negative correlation (r=−.262, p<0.009). Still consistent with a specific relationship between interoceptive beliefs and metamemory beliefs, scores on the Body Awareness Questionnaire did not correlate with accuracy on the dot estimation task (r=−0.068, p=0.503), or confidence on the dot estimation task (r=0.002, p=0.988). The same was true for the proportion of high confidence response to the general knowledge questions; neither dot estimation accuracy (r=−0.083, p=0.413), nor confidence on the dot estimation task (r=0.020, p=0.845) were correlated with the proportion of high confidence responses.
We used a two-step linear regression to test for effects of score on the Body Awareness Questionnaire, while controlling for memory accuracy, trait confidence using the average PEI, and task confidence using confidence in dot estimation, on the proportion of high confidence responses. The model with memory accuracy, average PEI, and dot estimation confidence was a significant predictor of proportion of high confidence responses (R2=0.336; R2adjusted =0.113; F(3,95)=4.035, p<0.01). Critically, bodily awareness explained additional unique variance (R2=0.423; R2adjusted =0.179; Fchange (1,94)=7.559, p<0.01; memory accuracy B=0.680, 95%CI [0.308,1.052]; Average PEI B=−0.008, 95%CI [−0.170,0.154]; dot estimation confidence B=0.017, 95%CI [−0.021,0.055]; bodily awareness B = 0.047, 95%CI [0.013,0.081]).
3.3 Discussion
Studies 1 & 2 showed a link between metamemory beliefs and interoceptive beliefs, but not metamemory accuracy and interoceptive beliefs. Because the link between metamemory beliefs and interoceptive beliefs replicated across studies, and there was no evidence of a relationship between metamemory accuracy and interoceptive beliefs, we next focused on the relationship between interoceptive accuracy and metamemory accuracy. This is a critical step because a previous study demonstrated that self-report measures of interoception and interoceptive accuracy did not correlate (Garfinkel et al., 2014). Given that metamemory beliefs and interoceptive beliefs were related in Studies 1 & 2, we expected that parallel aspects of interoception and metamemory would be correlated (i.e., beliefs would correlated with beliefs, and accuracy would correlate with accuracy). In Study 3, we examined the relationship between interoceptive accuracy and metamemory accuracy.
4. Study 3: Metamemory Beliefs/Accuracy & Interoceptive Accuracy
4.1 Methods
4.1.1 Participants
Thirty-six English speaking Brooklyn College students (5 male and 35 female, ages 18–35) participated in this research for course credit (1 credit for 1 hour of participation). Participants were recruited via the Brooklyn College Psychology Department Research Participation website. Participants were excluded if they had a known history of irregular heartbeats. Participants were instructed not to exercise within 2 hours of the start of their session, to refrain from drinking caffeine within 5 hours of the start of their session, and to abstain from drinking alcohol within 12 hours of the start of their session. Data from a total of 6 participants were excluded from analyses; 3 were excluded based on programming errors, 2 were excluded for failing to use the full response scale in the metamemory task, which makes the metamemory accuracy measures impossible to accurately calculate, and 1 participant was excluded for falling asleep during encoding. Thus, data from 30 participants were analyzed in this Experiment. Each participant provided written consent form in a manner approved by the Human Research Protection Program at Brooklyn College.
4.1.2 Interoceptive Accuracy
Interoceptive accuracy was measured based on the heartbeat perception task (Schandry, 1981). In this task, participants count the number of heartbeats they have during short set periods of time, without physically feeling for their pulse or heartbeat. Interoceptive accuracy is then computed by comparing participants’ reports of their heart rate to their actual heart rate. This task has been used to assess interoceptive accuracy in many studies (Dunn et al., 2007; Pollatos & Schandry, 2004; Ring & Brener, 1996), and correlates with heartbeat detection tasks (Knoll & Hodapp, 1992), such as the Whitehead task (1977).
Heartbeat data were collected using a PowerLab 8/30 system by ADInstruments with an electric pulse transducer using Chart 5 software (Dunedin, New Zealand). The session began with the participant sitting in an armchair resting for 5–10 minutes. Participants were instructed to turn their focus inward and to sense their heartbeat without using their hands to feel their heart or pulse. Participants were instructed to begin counting their heartbeats at the start signal and end counting at the stop signal. Trials were 25, 35, and 45 seconds long. After each stop signal, participants reported the number of heartbeats they believed to have occurred during the previous period to the experimenter.
Actual heartbeat counts were calculated from the pulse data using LabChart7 (ADInstruments, Dunedin New Zealand). Integrity and accuracy of the pulse data was assured based on visual inspection of the data for any artifacts that would alter the pulse counts. In this experiment, all participants had adequate pulse data for accurate pulse counts. Interoceptive accuracy was calculated using the formula: 1-[(Σ|Ai-Ri|/Ai)/3], where Ai=number of actual heartbeats during trial i and Ri=number of heartbeats counted (reported by participant) during trial i. A value of 1 equals perfect heartbeat detection.
4.1.3 Metamemory Accuracy
Stimuli consisted of 150 photographs of faces presented on a neutral background with neutral facial expressions (Minear & Park, 2004). There were equal numbers of male and female faces that represented a range of ages from young to older adults with a variety of racial and ethnic backgrounds. Faces were paired with 150 first names selected from the Social Security website indexing popular names by year. During the study phase, participants viewed faces on a grey background paired with a first name written in white underneath the face. Each face-name pair was presented for 4 seconds. The memory test consisted of a 3-alternative forced choice recognition task for the name associated with the face. Participants viewed the face with 3 different names underneath (1 correct, 2 incorrect). The 2 incorrect names had been paired with other faces during study so that participants could not solve the task using name familiarity alone. Following recognition, participants indicated their confidence that they had chosen the correct name on a 3-point scale (high, medium, and low). Participants were tested on one-third of the face-name pairs that they studied so that there were no repeated names during test, for a total of 50 test trials. The specific face-name pairs seen at test were counterbalanced across participants. The memory test was self-paced. Stimuli were presented and behavioral responses were collected using PsychoPy 1.73 (Peirce, 2007) on a Dell Optiplex GX620.
Metamemory accuracy was calculated by relating confidence and accuracy on the recognition test using the same methods as Study 2 (Section 3.1.2.1).
4.1.4 Data Analysis
Our primary interest was in examining whether interoceptive accuracy and metamemory accuracy measures were related. Correlations were tested in SPSS 22.0 and were considered significant at p<0.05, two-tailed. Next, linear regression was used to test for effects of interoceptive accuracy on metamemory above and beyond effects of memory accuracy. In a two-step model, memory accuracy was entered as a predictor for metamemory accuracy in the first model and interoceptive accuracy was entered as a predictor for metamemory accuracy in the second model.
4.2 Results
Overall, participants performed above chance (33%) on the memory task. There was a satisfactory range of scores on interoceptive accuracy, memory accuracy, confidence-accuracy calibration, gamma, and da, suggesting that this sample’s memory processes were comparable to previous samples (Chua et al., 2006; Chua, Schacter, & Sperling, 2009b). See Table 3.
Table 3.
Descriptive statistics for interoceptive, memory, and metamemory measures.
| Mean | SD | Min | Max | |
|---|---|---|---|---|
| Average
Heart Rate (beats/min) |
81.12 | 10.73 | 62.17 | 104.72 |
| Interoceptive Accuracy |
0.68 | 0.17 | 0.39 | 0.97 |
| Memory Accuracy |
0.50 | 0.10 | 0.34 | 0.72 |
| Confidence- Accuracy Calibration |
0.072 | 0.057 | 0.0082 | 0.26 |
| Gamma | .48 | .25 | −0.07 | .92 |
| da | 0.67 | .40 | −0.09 | 1.35 |
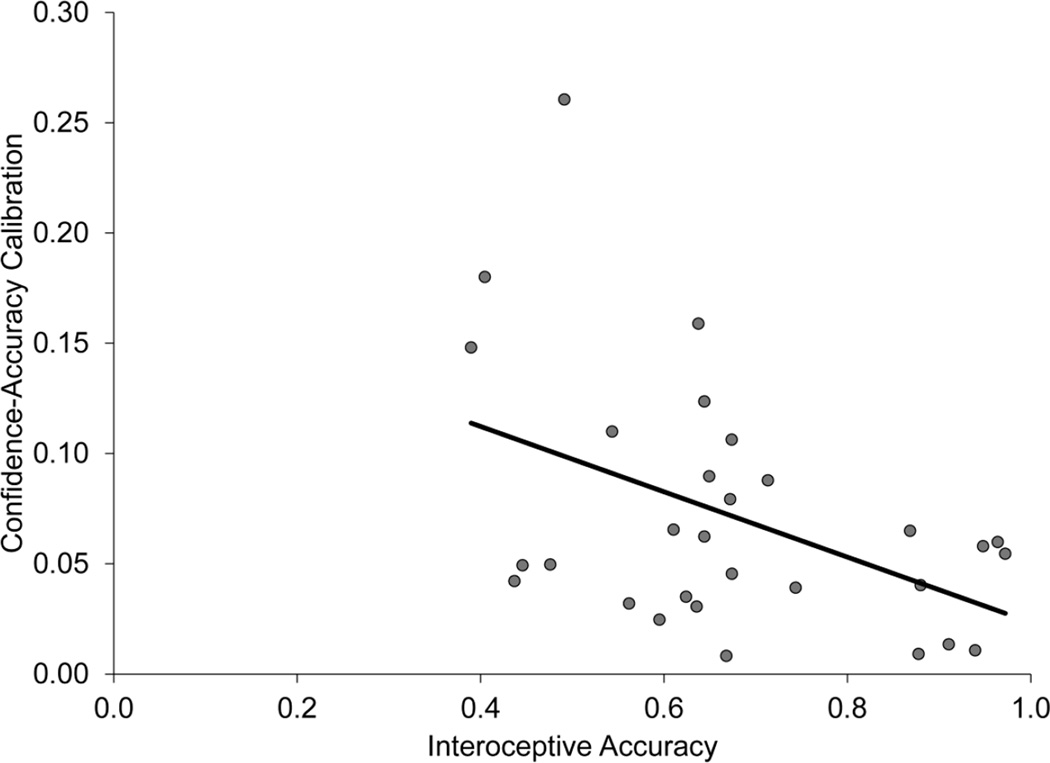
Our primary interest was whether interoceptive accuracy was related to metamemory accuracy, or memory accuracy. Table 4 shows the correlation matrix for interoceptive accuracy, memory accuracy, calibration, gamma and da. Of our metamemory and memory measures, only calibration was significantly correlated with interoceptive accuracy (Figure 3). Participants who had higher confidence-accuracy calibration (lower numbers) had higher interoceptive accuracy (higher numbers).
Table 3.
Correlation matrix for measures of interoception, memory accuracy, and metamemory accuracy.
| Interoceptive Accuracy |
Recognition Accuracy |
Calibration | Gamma | da | |
|---|---|---|---|---|---|
| Interoceptive Accuracy |
1 | ||||
| Memory Accuracy |
0.282 | 1 | |||
| Calibration | −0.45* | −0.71** | 1 | ||
| Gamma | 0.34* | 0.61** | −0.74** | 1 | |
| da | 0.22 | 0.52** | −0.66** | 0.91** | 1 |
Correlation is significant at the 0.05 level (2-tailed).
Correlation is significant at the 0.001 level (2-tailed).
Figure 3.
Scatter plots showing that interoceptive accuracy is negatively correlated with metamemory accuracy, as measured by confidence-accuracy calibration (r=−0.45, p<0.05). Note perfect calibration is zero, so the negative relationship is expected.
Because interoceptive accuracy and memory accuracy both correlated with calibration, we used a two-step linear regression to test for effects of interoceptive accuracy, while controlling for memory accuracy, on confidence-accuracy calibration. Memory accuracy was a significant predictor of confidence-accuracy calibration (R2=0.504; R2adjusted =0.486; F(1,28)=28.45, p<0.0001), and interoceptive accuracy explained additional variance (R2=0.574; R2adjusted =0.542; Fchange (1,27)=4.43, p<0.05; memory accuracy B=−0.38, 95%CI for B [−.54,−.22]; interoceptive accuracy B = −0.09, 95%CI[−0.18.,−0.002]).
4.3 Discussion
Study 3 showed that interoceptive accuracy correlates with metamemory accuracy, when controlling for memory accuracy. Although this sample is somewhat small, post-hoc power analyses in R (R Core, 2013) using the package pwr (Champely, 2015) indicated that the present study was powered at 82.70% for a one sided test and 73.27% power for a two-sided test. Future work should replicate this effect using a larger sample.
Only one metamemory accuracy measure, confidence-accuracy calibration, correlated with interoceptive accuracy. However, it is worth noting that gamma correlated with calibration, and the correlation between gamma and interoceptive accuracy approached significance (p<0.065). One possible explanation for why calibration was the only measure of metamemory accuracy that correlated with interoceptive accuracy might be that interoceptive accuracy and confidence-accuracy calibration are absolute measures (i.e., they reflect how well the subjective ratings estimate objective performance and are tied to the rating scale), while gamma and da are not tied to the rating scale. Although this study did not include additional control tasks (such as the dot estimation task), the relationship between metamemory accuracy and interoceptive accuracy remained when controlling for memory accuracy, suggesting a distinct relationship between metamemory accuracy and interoceptive accuracy. Furthermore, in Study 2, there was no relationship between dot estimation accuracy, memory accuracy, and metamemory accuracy, leading us to infer that it is unlikely metamemory accuracy and interoceptive accuracy are related because of a general ability to succeed at all tasks. Nevertheless, future work should include additional non-memory control measures, as well as questionnaire measures of metamemory and interoception.
5. General Discussion
Three studies provide initial evidence that knowledge of our minds and bodies are related, using both self-report and performance methods. These findings suggest that there may be a common mechanism underlying different aspects of self-knowledge: knowing the content of your mental processes, in the case of metamemory, and your peripheral physiological state, in the case of interoception. Using questionnaire measures, Study 1 demonstrated that interoceptive beliefs were positively correlated with metamemory beliefs. Converging evidence emerged using an interoception questionnaire and a trial-by-trial metamemory test in Study 2; interoceptive beliefs again correlated with metamemory beliefs, and remained when controlling for a general confidence effect. Finally, in Study 3, interoceptive accuracy and metamemory accuracy, as measured by confidence-accuracy calibration, were related such that individuals with higher interoceptive accuracy also had better calibration. Taken together, the effect documented in these studies suggests that parallel dimensions of metamemory and interoceptive are related, and therefore, may share a common underlying mechanism.
Work on metamemory (Chua, Pergolizzi, & Weintraub, 2014) and interoception (Garfinkel & Critchley, 2013; Garfinkel et al., 2014) have independently emphasized the need to understand multiple dimensions of these constructs, and our current work ties them together in a novel way. For metamemory, two dimensions are: 1) metamemory beliefs, which are self-perceptions about one’s memory, often measured by the level of confidence expressed or general beliefs about capacity, and 2) metamemory accuracy, which is the accuracy of those beliefs (Chua et al., 2014). For interoception, two dimensions are: 1) interoceptive beliefs, which are self-perceptions about one’s sense of the body, and 2) interoceptive accuracy, which is how well an individual performs on objective interoceptive tasks (Garfinkel & Critchley, 2013; Garfinkel et al., 2014). Across our different studies, there were significant correlations between our interoception measures and our metamemory measures, and these were seen only within the corresponding dimensions (i.e., beliefs correlated with beliefs, but not accuracy; accuracy correlated with accuracy), and were not reducible to a general confidence effect. These results indicate that metamemory and interoception are related, and that this relationship is specific to the dimension of the construct being measured.
Our findings are also consistent with classic theories of self-awareness. Classic theories of self-awareness – reflective, conscious awareness of one’s self – posit that awareness arises from the comparison of one’s own actions, beliefs, and experiences to a criterion or standard (Carver & Scheier, 1981; Duval & Wicklund, 1972; Silvia & Gendolla, 2001; Wicklund, 1980). Such self-awareness likely emerges from the coordinating activity of multiple neural regions that subserve complementary roles in the processing. One candidate anatomical hub for this mechanism is the insula. Insula activity has correlated with both interoception (Critchley et al., 2004; Pollatos, Gramann, et al., 2007; Zaki et al., 2012) and metamemory (Chua et al., 2006; Kikyo & Miyashita, 2004; Moritz, Gläscher, Sommer, Büchel, & Braus, 2006) and is thought to play a broad role in the representation of affective information (Craig, 2009). Another possible hub is the anterior cingulate cortex (ACC), which like the insula, has been implicated in interoception (Critchley et al., 2002, 2004; Pollatos, Schandry, et al., 2007) and metacognition (Chua et al., 2006, 2009a). Ventral medial prefrontal cortex (vmPFC) is also a candidate brain region for the network because it has shown to be critical for accurate metamemory in lesion studies (Modirrousta & Fellows, 2008; Schnyer et al., 2004) and which shows greater activity with greater metamemory accuracy in fMRI studies (Schnyer, Nicholls, & Verfaellie, 2005). The vmPFC has also shown to be critical in using affective information to guide behavior (Damasio, 1996). Furthermore, fMRI studies have also shown that activity in the mPFC correlates with self-referential thinking (Jenkins & Mitchell, 2011; Saxe, Moran, Scholz, & Gabrieli, 2006) and is implicated if a broad array of affective experiences (for a meta-analytic review: Lindquist, Wager, Kober, Bliss-Moreau, & Barrett, 2012). With these three anatomical hubs as starting places, future work will investigate the neural mechanisms that subserve the relationship between metamemory and interoception.
One interpretation of our data – that knowledge of one’s cognitive and bodily processes are related – is that both processes rely on a common mechanism. However, our data cannot rule out the alternative explanation that metamemory signals are based, at least in part, on peripheral psychophysiological signals. In this view, participants would use bodily signals to make decisions about whether their memories are accurate. Indeed, there is some evidence that metamemory may be embodied in this way. For example, in one study used physical weight as an embodied cue for importance, and showed that stimuli with greater weights were given higher metacognitive ratings (Alban & Kelley, 2013). Another embodied metacognition study showed a similar effect -contracting one’s eyebrows embodied a feeling of effort and led to lower subjective ratings (Koriat & Nussinson, 2009), again showing that the bodily state can impact metacognitive judgments. Similarly, Goldinger & Hansen (2005) showed that a subliminal somatic cue (a buzz) led to increased confidence in false alarms and decreased confidence in hits, suggesting that peripheral physiological signals in combination with mnemonic information give rise to confidence. In this view, interoception and metamemory are correlated because metamemory relies on embodied processing that is made possible by interoceptive processes.
6. Conclusion
The present report takes the first step in understanding how unified self-knowledge emerges, by documenting the relationship between cognitive and bodily self-knowledge. Future work will investigate the causal nature of this relationship – whether, in fact, metamemory and interoception share a mechanism or whether one process is driven by the other. Understanding common mechanisms that shape self-knowledge may be important for understanding diseases processes that relate to self-knowledge of memory and affect, such as anosognosia in dementias (Cosentino, Metcalfe, Steffener, Holmes, & Stern, 2011; Cosentino, Metcalfe, Butterfield, & Stern, 2007) and emotion-related psychopathology (Paulus & Stein, 2006), respectively. Future work will pursue investigations of these mechanisms.
Highlights.
We examined metamemory and interoception in terms of beliefs and accuracy.
Metamemory beliefs and interoceptive beliefs correlated.
Metamemory and interoceptive beliefs did not correlate with other beliefs.
Metamemory accuracy and interoceptive accuracy were correlated.
A common mechanism subserves metamemory and interoception.
Acknowledgments
The authors thank Suzanne Conning Mayers for help with data collection and Deborah Walder for use of her psychophysiology equipment in Study 3. EFC was funded by the National Institute of General Medical Sciences and National Institutes on Aging (SC2AG046910) during data collection and preparation of this manuscript. EBM was funded by the National Institutes of Mental Health (F32MH087067 and K99MH10138) during data collection and the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Elizabeth F. Chua, Brooklyn College of the City University of New York, The Graduate Center of the City University of New York
Eliza Bliss-Moreau, University of California, Davis.
References
- Alban MW, Kelley CM. Embodiment Meets Metamemory: Weight as a Cue for Metacognitive Judgments. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2013;39(5):1628–1634. doi: 10.1037/a0032420. [DOI] [PubMed] [Google Scholar]
- Benjamin AS, Diaz M. Measurement of relative metamnemoic accuracy. In: Dunlosky J, Bjork RA, editors. Handbook of memory and metamemory. New York: Psychology Press; 2008. pp. 73–94. [Google Scholar]
- Bennett-Levy J, Powell GE. The Subjective Memory Questionnaire (SMQ). An investigation into the self-reporting of “real-life” memory skills. British Journal of Social and Clinical Psychology. 1980;19(2):177–188. [Google Scholar]
- Brackett MA, Rivers SE, Shiffman S, Lerner N, Salovey P. Relating emotional abilities to social functioning: a comparison of self-report and performance measures of emotional intelligence. Journal of Personality and Social Psychology. 2006;91(4):780–795. doi: 10.1037/0022-3514.91.4.780. [DOI] [PubMed] [Google Scholar]
- Carver CS, Scheier MF. The self-attention-induced feedback loop and social facilitation. Journal of Experimental Social Psychology. 1981;17(6):545–568. [Google Scholar]
- Ceunen E, Van Diest I, Vlaeyen JWS. Accuracy and awareness of perception: related, yet distinct (commentary on Herbert et al., 2012) Biological Psychology. 2013;92(2):426–427. doi: 10.1016/j.biopsycho.2012.09.012. [DOI] [PubMed] [Google Scholar]
- Champely S. pwr: Basic functions for power analysis. R package version 1.1–3. Vienna, Austria: The R Foundation; 2015. [Google Scholar]
- Chua EF, Hannula DE, Ranganath C. Distinguishing highly confident accurate and inaccurate memory: insights about relevant and irrelevant influences on memory confidence. Memory (Hove, England) 2012;20(1):48–62. doi: 10.1080/09658211.2011.633919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua EF, Pergolizzi D, Weintraub RR. The Cognitive Neuroscience of Metamemory Monitoring: Understanding Metamemory Processes, Subjective Levels Expressed, and Metacognitive Accuracy. In: Fleming SM, Frith CD, editors. The Cognitive Neuroscience of Metacognition. Berlin Heidelberg: Springer; 2014. pp. 267–291. [Google Scholar]
- Chua EF, Schacter DL, Rand-Giovannetti E, Sperling RA. Understanding metamemory: neural correlates of the cognitive process and subjective level of confidence in recognition memory. NeuroImage. 2006;29(4):1150–1160. doi: 10.1016/j.neuroimage.2005.09.058. [DOI] [PubMed] [Google Scholar]
- Chua EF, Schacter DL, Sperling RA. Neural basis for recognition confidence in younger and older adults. Psychology and Aging. 2009a;24(1):139–153. doi: 10.1037/a0014029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua EF, Schacter DL, Sperling RA. Neural correlates of metamemory: a comparison of feeling-of-knowing and retrospective confidence judgments. Journal of Cognitive Neuroscience. 2009b;21(9):1751–1765. doi: 10.1162/jocn.2009.21123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua EF, Solinger LA. Building metamemorial knowledge over time: insights from eye tracking about the bases of feeling-of-knowing and confidence judgments. Frontiers in Psychology. 2015;6 doi: 10.3389/fpsyg.2015.01206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino S, Metcalfe J, Butterfield B, Stern Y. Objective metamemory testing captures awareness of deficit in Alzheimer’s disease. Cortex. 2007;43(7):1004–1019. doi: 10.1016/s0010-9452(08)70697-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino S, Metcalfe J, Steffener J, Holmes B, Stern Y. Finding the self in Metacognitive evaluations: metamemory and agency in non-demented elders. Neuropsychology. 2011;25(5):602–612. doi: 10.1037/a0023972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Current Opinion in Neurobiology. 2003;13(4):500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception and emotion: a neuroanatomical perspective. In: Lewis MD, Haviland-Jones JM, Barrett LF, editors. Handbook of emotions. Third. 06. 2008. pp. 272–288. [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nature Reviews. Neuroscience. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Melmed RN, Featherstone E, Mathias CJ, Dolan RJ. Volitional control of autonomic arousal: a functional magnetic resonance study. NeuroImage. 2002;16(4):909–919. doi: 10.1006/nimg.2002.1147. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7(2):189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 1996;351(1346):1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Dixon RA, Hultsch DF, Hertzog C. The Metamemory in Adulthood (MIA) questionnaire. Psychopharmacology Bulletin. 1988;24(4):671–688. [PubMed] [Google Scholar]
- Dunn BD, Dalgleish T, Ogilvie AD, Lawrence AD. Heartbeat perception in depression. Behaviour Research and Therapy. 2007;45(8):1921–1930. doi: 10.1016/j.brat.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Duval S, Wicklund RA. A theory of objective self awareness. Oxford, England: Academic Press; 1972. [Google Scholar]
- Fleming SM, Ryu J, Golfinos JG, Blackmon KE. Domain-specific impairment in metacognitive accuracy following anterior prefrontal lesions. Brain: A Journal of Neurology. 2014;137(Pt 10):2811–2822. doi: 10.1093/brain/awu221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Weil RS, Nagy Z, Dolan RJ, Rees G. Relating introspective accuracy to individual differences in brain structure. Science (New York, N.Y.) 2010;329(5998):1541–1543. doi: 10.1126/science.1191883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel SN, Critchley HD. Interoception, emotion and brain: new insights link internal physiology to social behaviour. Commentary on:: “Anterior insular cortex mediates bodily sensibility and social anxiety” by Terasawa et al. (2012) Social Cognitive and Affective Neuroscience. 2013;8(3):231–234. doi: 10.1093/scan/nss140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel SN, Seth AK, Barrett AB, Suzuki K, Critchley HD. Knowing your own heart: Distinguishing interoceptive accuracy from interoceptive awareness. Biological Psychology. 2014;104:65–74. doi: 10.1016/j.biopsycho.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Ghetti S, Mirandola C, Angelini L, Cornoldi C, Ciaramelli E. Development of subjective recollection: understanding of and introspection on memory states. Child Development. 2011;82(6):1954–1969. doi: 10.1111/j.1467-8624.2011.01645.x. [DOI] [PubMed] [Google Scholar]
- Gilewski MJ, Zelinski EM, Schaie KW. The Memory Functioning Questionnaire for assessment of memory complaints in adulthood and old age. Psychology and Aging. 1990;5(4):482–490. doi: 10.1037//0882-7974.5.4.482. [DOI] [PubMed] [Google Scholar]
- Gilin D, Maddux WW, Carpenter J, Galinsky AD. When to use your head and when to use your heart: the differential value of perspective-taking versus empathy in competitive interactions. Personality & Social Psychology Bulletin. 2013;39(1):3–16. doi: 10.1177/0146167212465320. [DOI] [PubMed] [Google Scholar]
- Goldinger SD, Hansen WA. Remembering by the seat of your pants. Psychological Science. 2005;16(7):525–529. doi: 10.1111/j.0956-7976.2005.01569.x. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Dixon RA, Schulenberg JE, Hultsch DF. On the differentiation of memory beliefs from memory knowledge: the factor structure of the Metamemory in Adulthood Scale. Experimental Aging Research. 1987;13(1–2):101–107. doi: 10.1080/03610738708259308. [DOI] [PubMed] [Google Scholar]
- Izard V, Dehaene S. Calibrating the mental number line. Cognition. 2008;106(3):1221–1247. doi: 10.1016/j.cognition.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Janssen E, van Osch L, Lechner L, Candel M, de Vries H. Thinking versus feeling: differentiating between cognitive and affective components of perceived cancer risk. Psychology & Health. 2012;27(7):767–783. doi: 10.1080/08870446.2011.580846. [DOI] [PubMed] [Google Scholar]
- Jenkins AC, Mitchell JP. Medial prefrontal cortex subserves diverse forms of self-reflection. Social Neuroscience. 2011;6(3):211–218. doi: 10.1080/17470919.2010.507948. [DOI] [PubMed] [Google Scholar]
- Jonsson A-C, Allwood CM. Stability and variability in the realism of confidence judgments over time, content domain, and gender. Personality and Individual Differences. 2003;34(4):559–574. [Google Scholar]
- Kelemen WL, Frost PJ, Weaver CA. Individual differences in metacognition: Evidence against a general metacognitive ability. Memory & Cognition. 2000;28(1):92–107. doi: 10.3758/bf03211579. [DOI] [PubMed] [Google Scholar]
- Kikyo H, Miyashita Y. Temporal lobe activations of “feeling-of-knowing” induced by face-name associations. NeuroImage. 2004;23:1348–1357. doi: 10.1016/j.neuroimage.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Knoll JF, Hodapp V. A Comparison between Two Methods for Assessing Heartbeat Perception. Psychophysiology. 1992;29(2):218–222. doi: 10.1111/j.1469-8986.1992.tb01689.x. [DOI] [PubMed] [Google Scholar]
- Koriat A, Nussinson R. Attributing study effort to data-driven and goal-driven effects: Implications for metacognitive judgments. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2009;35(5):1338–1343. doi: 10.1037/a0016374. [DOI] [PubMed] [Google Scholar]
- Lichtenstein S, Fischhoff B, Phillips LD. Calibration of probabilities: the state of the art to 1980. In: Kahneman D, Slovic P, Tverski A, editors. Judgment under uncertainty: Heuristics and biases. Cambridge, UK: Cambridge University Press; 1982. pp. 306–334. [Google Scholar]
- Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. The brain basis of emotion: a meta-analytic review. The Behavioral and Brain Sciences. 2012;35(3):121–143. doi: 10.1017/S0140525X11000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna K, Martín-Luengo B. Confidence-Accuracy Calibration with General Knowledge and Eyewitness Memory Cued Recall Questions. Applied Cognitive Psychology. 2012 Aug;26:289–295. [Google Scholar]
- Masson MEJ, Rotello CM. Sources of Bias in the Goodman – Kruskal Gamma Coefficient Measure of Association?: Implications for Studies of Metacognitive Processes. 2009;35(2):509–527. doi: 10.1037/a0014876. [DOI] [PubMed] [Google Scholar]
- Mehling WE, Gopisetty V, Daubenmier J, Price CJ, Hecht FM, Stewart A. Body awareness: construct and self-report measures. PloS One. 2009;4(5):e5614. doi: 10.1371/journal.pone.0005614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minear M, Park DC. A lifespan database of adult facial stimuli. Behavior Research Methods, Instruments, & Computers. 2004;36(4):630–633. doi: 10.3758/bf03206543. [DOI] [PubMed] [Google Scholar]
- Modirrousta M, Fellows LK. Medial prefrontal cortex plays a critical and selective role in “feeling of knowing” meta-memory judgments. Neuropsychologia. 2008;46(12):2958–2965. doi: 10.1016/j.neuropsychologia.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Moritz S, Gläscher J, Sommer T, Büchel C, Braus DF. Neural correlates of memory confidence. NeuroImage. 2006;33(4):1188–1193. doi: 10.1016/j.neuroimage.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Nelson TO. A comparison of current measures of the accuracy of feeling-of-knowing predictions. Psychological Bulletin. 1984;95(1):109. [PubMed] [Google Scholar]
- Nelson TO, Narens L. Metamemory: a theoretical framework and new findings. In: Bower GH, editor. The psychology of learning and motivation. 26th. Vol. 26. San Diego: Academic Press; 1990. pp. 125–141. [Google Scholar]
- Pannu JK, Kaszniak AW. Metamemory experiments in neurological populations: a review. Neuropsychology Review. 2005;15(3):105–130. doi: 10.1007/s11065-005-7091-6. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biological Psychiatry. 2006;60(4):383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Peirce JW. PsychoPy--Psychophysics software in Python. Journal of Neuroscience Methods. 2007;162(1–2):8–13. doi: 10.1016/j.jneumeth.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfect TJ, Hollins TS, Hunt a L. Practice and feedback effects on the confidence-accuracy relation in eyewitness memory. Memory (Hove, England) 2000;8(4):235–244. doi: 10.1080/096582100406793. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Gramann K, Schandry R. Neural systems connecting interoceptive awareness and feelings. Human Brain Mapping. 2007;28(1):9–18. doi: 10.1002/hbm.20258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollatos O, Schandry R. Accuracy of heartbeat perception is reflected in the amplitude of the heartbeat-evoked brain potential. Psychophysiology. 2004;41(3):476–482. doi: 10.1111/1469-8986.2004.00170.x. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Schandry R, Auer DP, Kaufmann C. Brain structures mediating cardiovascular arousal and interoceptive awareness. Brain Research. 2007;1141:178–187. doi: 10.1016/j.brainres.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Ring C, Brener J. Influence of beliefs about heart rate and actual heart rate on heartbeat counting. Psychophysiology. 1996;33:541–546. doi: 10.1111/j.1469-8986.1996.tb02430.x. Retrieved from http://onlinelibrary.wiley.com/doi/10.1111/j.1469-8986.1996.tb02430.x/abstract. [DOI] [PubMed] [Google Scholar]
- Robinson MD, Clore GL. Belief and feeling: Evidence for an accessibility model of emotional self-report. Psychological Bulletin. 2002;128(6):934–960. doi: 10.1037/0033-2909.128.6.934. [DOI] [PubMed] [Google Scholar]
- Saxe R, Moran JM, Scholz J, Gabrieli J. Overlapping and non-overlapping brain regions for theory of mind and self reflection in individual subjects. Social Cognitive and Affective Neuroscience. 2006;1(3):229–234. doi: 10.1093/scan/nsl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schandry R. Heart Beat Perception and Emotional Experience. Psychophysiology. 1981;18(4):483–488. doi: 10.1111/j.1469-8986.1981.tb02486.x. [DOI] [PubMed] [Google Scholar]
- Schlaffke L, Lissek S, Lenz M, Juckel G, Schultz T, Tegenthoff M, Brüne M. Shared and nonshared neural networks of cognitive and affective theory-of-mind: A neuroimaging study using cartoon picture stories. Human Brain Mapping. 2015;36(1):29–39. doi: 10.1002/hbm.22610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnyer DM, Nicholls L, Verfaellie M. The role of VMPC in metamemorial judgments of content retrievability. Journal of Cognitive Neuroscience. 2005;17(5):832–846. doi: 10.1162/0898929053747694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnyer DM, Verfaellie M, Alexander MP, LaFleche G, Nicholls L, Kaszniak AW. A role for right medial prefontal cortex in accurate feeling-of-knowing judgements: evidence from patients with lesions to frontal cortex. Neuropsychologia. 2004;42(7):957–966. doi: 10.1016/j.neuropsychologia.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Schraw G. Promoting general metacognitive awareness. Instructional Science. 1998;26(1–2):113–125. [Google Scholar]
- Shields SA, Mallory ME, Simon A. The Body Awareness Questionnaire: Reliability and Validity. Journal of Personality Assessment. 1989;53(4):802–815. [Google Scholar]
- Shrauger JS, Schohn M. Self-Confidence in College Students: Conceptualization, Measurement, and Behavioral Implications. Assessment. 1995;2(3):255–278. [Google Scholar]
- Silvia P, Gendolla G. On introspection and self-perception: Does self-focused attention enable accurate self-knowledge? Review. Review of General Psychology. 2001;5:241–269. [Google Scholar]
- Simons JS, Peers PV, Mazuz YS, Berryhill ME, Olson IR. Dissociation between memory accuracy and memory confidence following bilateral parietal lesions. Cerebral Cortex (New York, N.Y.: 1991) 2010;20(2):479–485. doi: 10.1093/cercor/bhp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Kanai R, Fleming SM, Weil RS, Schwarzkopf DS, Rees G. Relating inter-individual differences in metacognitive performance on different perceptual tasks. Consciousness and Cognition. 2011;20(4):1787–1792. doi: 10.1016/j.concog.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain JS, Eaton LG, Funder DC. Perspective on Personality: The Relative Accuracy of Self Versus Others for the Prediction of Emotion and Behavior. Journal of Personality. 2000;68(5):837–867. doi: 10.1111/1467-6494.00118. [DOI] [PubMed] [Google Scholar]
- Stanislaw H, Todorov N. Calculation of signal detection theory measures. Behavior Research Methods, Instruments, & Computers: A Journal of the Psychonomic Society, Inc. 1999;31(1):137–149. doi: 10.3758/bf03207704. [DOI] [PubMed] [Google Scholar]
- Swann WB, Griffin JJ, Predmore SC, Gaines B. The cognitive-affective crossfire: When self-consistency confronts self-enhancement. Journal of Personality and Social Psychology. 1987;52(5):881–889. doi: 10.1037//0022-3514.52.5.881. [DOI] [PubMed] [Google Scholar]
- Tauber SK, Dunlosky J, Rawson Ka, Rhodes MG, Sitzman DM. General knowledge norms: updated and expanded from the Nelson and Narens (1980) norms. Behavior Research Methods. 2013;45(4):1115–1143. doi: 10.3758/s13428-012-0307-9. [DOI] [PubMed] [Google Scholar]
- Toth JP, Daniels KA, Solinger LA. What you know can hurt you: Effects of age and prior knowledge on the accuracy of judgments of learning. Psychology and Aging. 2011;26(4):919–931. doi: 10.1037/a0023379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber N, Brewer N. Confidence-accuracy calibration in absolute and relative face recognition judgments. Journal of Experimental Psychology. Applied. 2004;10(3):156–172. doi: 10.1037/1076-898X.10.3.156. [DOI] [PubMed] [Google Scholar]
- Whitehead WE, Drescher V. Perception of gastric contractions and self-control of gastric motility. Psychophysiology. 1980;17(6):552–558. doi: 10.1111/j.1469-8986.1980.tb02296.x. [DOI] [PubMed] [Google Scholar]
- Whitehead WE, Drescher VM, Heiman P, Blackwell B. Relation of heart rate control to heartbeat perception. Biofeedback and Self-Regulation. 1977;2(4):371–392. [PubMed] [Google Scholar]
- Wicklund RA. Group contact and self-focused attention. In: Paulus P, editor. Psychology of group influence. Hillsdale, NJ: Erlbaum; 1980. pp. 189–208. [Google Scholar]
- Zaki J, Davis JI, Ochsner KN. Overlapping activity in anterior insula during interoception and emotional experience. NeuroImage. 2012;62(1):493–499. doi: 10.1016/j.neuroimage.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]