Abstract
Thus far the identification and functional characterization of the molecular mechanisms underlying synaptic plasticity, learning, and memory have not been particularly dissociated from the contribution of developmental changes. Brain plasticity mechanisms have been largely identified and studied using in vitro systems mainly derived from early developmental ages, yet they are considered to be general plasticity mechanisms underlying functions -such as long-term memory- that occurs in the adult brain. Although it is possible that part of the plasticity mechanisms recruited during development is then re-recruited in plasticity responses in adulthood, systematic investigations about whether and how activity-dependent molecular responses differ over development are sparse. Notably, hippocampal-dependent memories are expressed relatively late in development, and the hippocampus undergoes and extended developmental post-natal structural and functional maturation, suggesting that the molecular mechanisms underlying hippocampal neuroplasticity may actually significantly change over development. Here we quantified the relative basal expression levels of sets of plasticity, synaptic, glia and connectivity proteins in rat dorsal hippocampus, a region that is critical for the formation of long-term explicit memories, at two developmental ages, postnatal day 17 (PN17) and PN24, which correspond to a period of relative functional immaturity and maturity, respectively, and compared them to adult age. We found that the levels of numerous proteins and/or their phosphorylation, known to be critical for synaptic plasticity underlying memory formation, including immediate early genes (IEGs), kinases, transcription factors and AMPA receptor subunits, peak at PN17 when the hippocampus is not yet able to express long-term memory. It remains to be established if these changes result from developmental basal activity or infantile learning. Conversely, among all markers investigated, the phosphorylation of calcium calmodulin kinase II α (CamKII− α− and of extracellular signal-regulated kinases 2 (ERK-2), and the levels of GluA1 and GluA2 significantly increase from PN17 to PN24 and then remain similar in adulthood, thus representing correlates paralleling long-term memory expression ability.
Keywords: hippocampus, development, protein expression, synapse, myelin, connectivity, memory, plasticity
1. Introduction
The hippocampus plays a critical role in the consolidation of long-term memories including episodic, contextual, spatial, and social, which in humans comprise declarative and autobiographical memories (Eichenbaum, Yonelinas, & Ranganath, 2007; Squire, Stark, & Clark, 2004; Tulving, 2002). Consolidation is the process that stabilizes the newly learned information, and transforms it into long-lasting memories by engaging an initial phase of gene expression, which leads to long-term structural changes (Alberini, 2009; Davis & Squire, 1984; Dudai, 1996; Kandel 2001; McGaugh, 2000; Wixted & Squire, 2011). Notably, the hippocampus undergoes a relatively long post-natal developmental period to become functionally competent in long-term memory formation and expression. In fact, contextual memories are retained long-term relatively late during development, around post-natal day 21 (PN21) in rats, and 3–5 years in humans (Callaghan, Li, & Richardson, 2014; Campbell & Spear, 1972; Hayne & Herbert, 2004; Madsen & Kim, 2016). The hippocampus also shows a parallel protracted postnatal structural and functional synapse and circuitry maturation (Cotman, Matthews, Taylor, & Lynch, 1973; Coyle & Yamamura, 1976; Crain, Cotman, Taylor, & Lynch, 1973; Wilson, 1984), suggesting that the functional competence in memory expression and structural maturation are co-dependent.
Hippocampal structural maturation involves several biological processes, which include synapse and local circuitry formation, activity-dependent synaptic maturation and plasticity, myelination, and the formation of intrinsic and extrinsic connectivity to the hippocampus (Isaacson & Pribram, 1975; Jacobson, 2013; Pokorný & Yamamoto, 1981a, 1981b). It has been suggested that synaptic signaling mechanisms governing de novo synapse formation during development are recruited again in the adult brain to support structural synaptic plasticity underlying long-term memory formation (Bailey, Kandel, & Harris, 2015). However, because numerous studies on synaptic plasticity have actually been conducted using developing systems (e.g. cell culture, slices, organotypic cultures at early developmental ages), it is actually unclear which mechanisms are selectively or commonly engaged in developmental vs. adult plasticity. For example, cellular paradigms used to identify the molecular and cellular mechanisms underlying long-term potentiation (LTP) and long-term depression (LTD) have mostly employed hippocampi taken at early developmental stages (Kim, Jung, Clemens, Petralia, & Hoffman, 2007; Liao & Malinow, 1996; Muller et al., 1996), leading nevertheless to general implications about plasticity mechanisms of long-term memory formation in mature systems and animals. However, the biological composition of a hippocampus at an early stage of development is very different from that of a mature hippocampus. For example, electron microscopy analyses have shown that, during development, excitatory synapses form directly on the dendritic shafts of immature but rarely on the shafts of mature spiny hippocampal dendrites (Fiala, Feinberg, Popov, & Harris, 1998), whereas in the mature hippocampus spine volume correlates with synaptic area (Harris & Stevens, 1989). In addition, many hippocampal CA1 spines that show an apparent mature shape can form multiple synapses with different presynaptic axons during development, whereas multisynaptic CA1 spines are extremely rare in the normal mature hippocampus (Fiala et al., 1998; Harris, Jensen, & Tsao, 1992; Sorra & Harris, 2000). Also, electrophysiological responses of LTP/LTD significantly change over development (Dudek and Bear, 1993) and LTP results in opposite effects on spine density and synaptic surface area, as measured by postsynaptic density (PSD) in PN15 versus adult (P60–70) dendrites (Bailey, Kandel, & Harris, 2015).
Thus, given the variety of biological changes that the hippocampus undergoes over its extended postnatal ontogenesis, it is important to know whether, which, and how mechanisms associated to synaptic plasticity, connectivity, glia and myelination vary with development, particularly at ages when the functional competence matures.
This knowledge is still spare. In fact, despite a very informative genome-wide transcriptional profiling conducted at 5 timepoints from Embryonic day 16 to PN30 in the developing mouse hippocampus (Mody et al., 2001) thus far, the developmental changes occurring in the brain have been mostly assessed, as also mentioned above, by structural investigations. For example, electron microscopy of the dentate gyrus molecular layer revealed a 100-fold increase in number of synapse from PN4 to adulthood, whereas the number of synapses approximates adult levels by PN25 (Cotman et al., 1973; Crain et al., 1973). Moreover, ultrastructural analysis showed that in the hippocampus myelinated fibers increase during postnatal development and reach adult levels at postnatal day 25 (Meier, Bräuer, Heimrich, Nitsch, & Savaskan, 2004).
Here we compared the levels of proteins known to be critically involved in plasticity, synapse, glia, myelination, and connectivity maturation, during hippocampal development using densitometric western blot analyses in the total dorsal hippocampus (dHC) extracts of untrained rats (naïve) at two early, prepuberal developmental ages, postnatal day 17 (PN17) and PN24, and compared them to those obtained from young adults (i.e. PN80). While at PN17 rats form only short-lasting contextual memory, which is quickly lost -a phenomenon known as infantile amnesia-, at PN24, rats form, store and express long-term memory (Campbell & Spear, 1972, Travaglia et al. 2016).
2. Materials and Methods
2.1 Animals
Seventeen- and 24-day old male and female rats were obtained from pregnant Long Evans female rats (Charles River Laboratories). Rats were housed in 30.80 cm × 40.60 cm × 22.23 cm plastic cage, containing ALPHA-dri® bedding, under a 12h light/dark cycle (light on at 07.00 a.m.) with food and water ad libitum. All experiments were carried out during the light cycle. The birth date was considered PN0 and the litters were culled to 10–12. Only one male and female per litter was used in any experimental condition. Rats were weaned to at PN21. All procedures complied with the US National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the New York University Animals Care Committees.
2.2 Inhibitory avoidance
Inhibitory avoidance (IA) was carried out as previously described (Travaglia et al 2016 ). The IA chamber (Med Associates Inc., St. Albans, VT) consisted of a rectangular Perspex box divided into a safe compartment and a shock compartment (each 20.3 cm × 15.9 cm × 21.3 cm). The safe compartment was white and illuminated and the shock compartment was black and dark. The apparatus was located in a sound-attenuated, non-illuminated room. During training, each rat was placed in the safe compartment with its head facing away from the door. After 10 seconds, the door separating the compartments was automatically opened, allowing the rat access to the shock compartment. The door closed automatically when the rat entered the shock compartment with all four limbs, and a foot shock (2 s, 1 mA) was administered. Foot shocks were delivered to the grid floor of the shock chamber via a constant current scrambler circuit. Animal remained in the dark compartment for additional 10 seconds and then was returned to its home cages until testing for memory retention at designated time points. As controls, we used naïve animals (handled and remained in their home cage). Retention tests were done by placing the rat back in the safe compartment and measuring its latency to enter the dark compartment. Foot shocks were not administered during the retention tests, and testing was terminated at 900 seconds. All behavioral tests were carried out blind to training conditions.
2.3 Western blot analysis
Western blot analysis was carried out as previously reported (Chen, Bambah-Mukku, Pollonini, & Alberini, 2012). Rats were euthanized and their brains were rapidly removed and frozen in isopentane. Dorsal hippocampus punches were obtained with a neuro punch (19 gauge; Fine Science Tools, Foster City, CA) from frozen brains mounted on a cryostat. Samples were homogenized in ice cold RIPA buffer (50mM Tris base, 150mM NaCl, 0.1% SDS, 0.5% Na-Deoxycholate, 1% NP-40) with protease and phosphatase inhibitors [0.5mM PMSF, 2mM DTT, 1mM EGTA, 2mM NaF, 1µM Microcystine, 1mM Benzamidine, 1mM Sodium Orthovanadate and commercial protease and phosphatase inhibitor cocktails (Sigma Aldrich, St. Louis, MO]. Protein concentrations were determined using the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA). Equal amounts of total protein (20 µg per lane) were resolved on denaturing SDS-PAGE gels and transferred to Immobilon-FL Transfer membrane (Millipore, Billerica, MA) by electroblotting. Membranes were dried, reactivated in methanol and washed with water, before they were blocked in 5% milk and TBS for 1 h at room temperature. Membranes were then incubated with primary antibody overnight at 4 °C in solution according manufacturer’s suggestion. Primary antibodies: anti-Arc (1:10000, Synaptic System, cat# 156 003, Gottingen, Germany), anti-c-Fos (1:200, Millipore, cat# PC05, Millipore), anti-Zif268 (1:1000, Cell Signaling Technology, cat# 4153S, Danvers, MA), anti-pCREB (Ser133) (1:1000, Cell Signaling Technology, cat# 9198), anti-CREB (1:1000, Cell Signaling Technology, cat# 9104), anti-pCaMKII (Thr286) (1:1000, Cell Signaling Technology, cat# 3361S), anti-CaMKII (1:1000, Millipore, cat# 05-532), anti-pERK1/2 (pp44/42 MAPK) (Thr202/Tyr204) (1:2000, Cell Signaling Technology, cat# 9101S), anti-ERK1/2 (p44/42 MAPK) (1:2000, Cell Signaling Technology, cat# 4695S), anti-GluA1 (1:2000, Millipore, cat# AB1504), anti-GluA2 (1:1000, UC Davis/NIH NeuroMab Facility, cat# 75-002, Davis, CA), anti-pAMPA Receptor GluA1 (Ser845) (1:1000, Cell Signaling Technology, cat# 8084S), anti-pAMPA Receptor GluA1 (Ser831) (1:1000, Abcam, cat# ab109464, Cambridge, MA), anti-Synapthophysin (1:1000, Cell Signaling Technology, cat#5467), anti-PSD95 (1:1000, Cell Signaling Technology, cat#2507S), anti-Stargazin (1:1000, Cell Signaling Technology, cat#2503), anti-pcofilin (Ser3) (1:1000, Abcam, cat# ab12866), anti-cofilin (1:1000, Millipore, cat# AB3842), anti-MAP2 (1:1000, Millipore, cat#MAB3418), Anti-Growth Associated Protein43 (1:1000, Millipore, cat# AB5220), anti-Myelin Basic Protein (MBP) (1:1000, Millipore, cat# 05-675), Anti-Nogo A (1:1000, Abcam, cat#ab62024), anti-Nogo Receptor (1:1000, Abcam, cat#ab26291), Anti MAG (1:1000, Cell Signaling Technology, cat#9043S), anti-mTOR (1:1000, Cell Signaling Technology, cat# 4517S), anti-pmTOR (1:1000, Cell Signaling Technology, cat# 2971S). The membranes were then washed TBS with 0.2% Tween20 (TBST) and incubated with a species-appropriate fluorescently conjugated secondary antibody [goat anti-mouse IRCye 680LT (1:10,000) or goat anti-rabbit IRDye 800CW (1:10,000) from LI-COR Bioscience (Lincoln, NE)] for 1h at room temperature. Membranes were again washed in TBST and scanned using the Odyssey Infrared Imaging system (Li-Cor Bioscience). Data were quantified using pixel intensities with the Odyssey software (Image Studio 4.0) according to the protocols of the manufacturer (Li-Cor). Anti-actin antibody (1:20,000, Santa Cruz Biotechnology, cat# sc-47778) was used to co-stain all membranes and the relative staining was employed as loading control for all western blots. Membranes were hybridized with different antibodies targeting different molecular weight proteins and were also stripped and stained with additional antibodies to target several proteins using the same blot. Thus, the same actin control was used for the densitometric analysis of a number of proteins.
2.4 Statistical analyses
Data were analyzed with the Prism 5 (GraphPad Sofware Inc.). Statistical power calculations have been performed using the statistical software G*Power. The data were analyzed by one- or two-way analysis of variance (ANOVA) followed by post hoc tests. One-way ANOVA followed by Newman–Keuls post hoc tests was used for western blot analysis, two-way ANOVA followed by Bonferroni post hoc tests was used for behavioral experiments The significance of the results was accepted at p<0.05.
3. Results
3.1 PN17 rats show inhibitory avoidance memory only shortly after training while PN24 and PN80 rats have strong and long-lasting memories
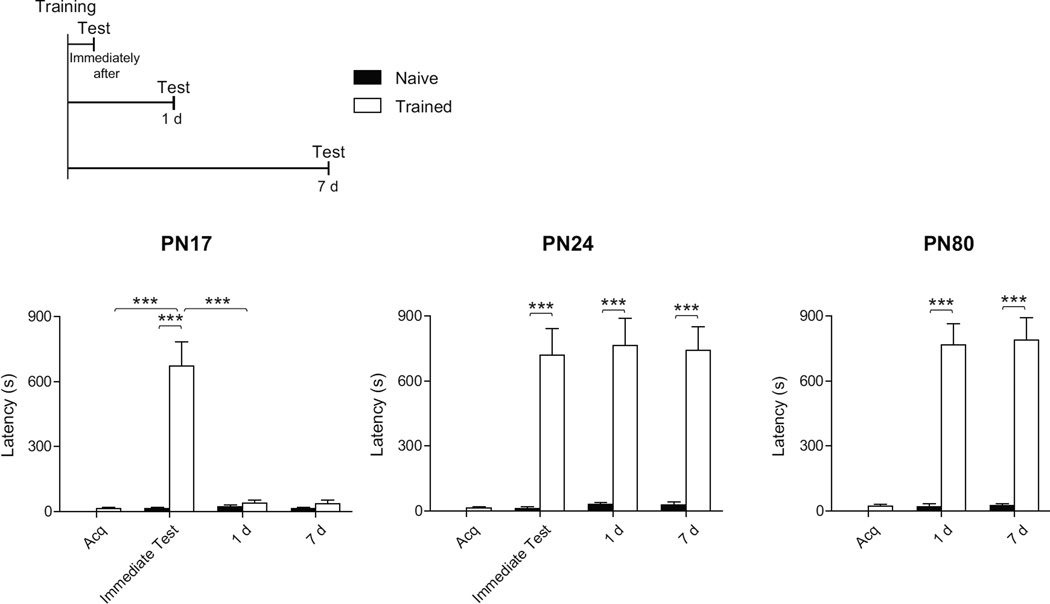
In agreement with previous studies (Campbell & Campbell, 1962; Rudy & Morledge, 1994, Travaglia et al. 2016), using the contextual fear based task inhibitory avoidance (IA) in infant rats we found that a single IA training in rats at PN17, but not at PN24, recapitulated the infantile amnesia phenomenon. Rats were trained in IA at PN17, PN24, or PN80 and tested either immediately after training, 1 day (d) or 7 d after training. Littermates left undisturbed in their home cage (naïve group) were used as a control. As shown in Figure 1, compared to both acquisition and to naïve controls, trained rats at PN17 had significant latency immediately after training (two-way ANOVA followed by Bonferroni post hoc tests; F(2, 42)= 31.74; p<0.0001). However, PN17 trained rats tested either 1 d or 7 d after training had no memory compared to their acquisition latency or to latency of naïve rats (p>0.05). In contrast, training at PN24 and PN80, compared to naïve control, elicited a strong and long-lasting associative memory at all time points tested (two-way ANOVA followed by Bonferroni post hoc tests; effect of training at PN24: F(2, 42)= 110.05; p<0.0001; effect of training at PN80: F(2, 42)= 112.06; p<0.0001). Thus, using IA and a single shock we found that rats trained at PN17 can express the memory only when tested immediately after training, whereas both PN24 and PN80 (adult) rats have strong and long lasting memories. Within the time window explored, no difference was found in memory retention between rats trained at PN24 and PN80.
Figure 1. PN17 rats show IA memory only shortly after training, PN24 and PN80 form strong and long-lasting memories.
Experimental schedule is shown above the panel. Rats were trained at PN17, PN24 or PN80 (n=8/group) and tested either immediately after training, 1 day (d) or 7 d after training. Acquisition (Acq.) and memory retention are expressed as mean latency ± s.e.m (in seconds, s). Two-way ANOVA followed by Bonferroni post hoc tests, ***p < 0.001.
Given these differences in behavior between PN17 and PN24, we next employed relative densitometric western blot analyses of dorsal hippocampal whole extracts at PN17 and PN24, and compared them to those of PN80 (young adult). The analyses focused on post-translational modification (i.e. phosphorylation) and/or relative levels of classes of proteins known to be critical for long-term plasticity, synapse maturation, and myelination. Specifically, we investigated the levels of the activity-dependent immediate-early genes c-Fos (Tischmeyer and Grimm 1999), activity-regulated-cytoskeletal-associated-protein (Arc/Arg3.1, Bramham, Worley, Moore, & Guzowski, 2008) and Zif268 (also known as EGR1 or NGFI-A, Jones et al., 2001). We analyzed the phosphorylation and level of the synaptic plasticity-coupled kinases calcium/calmodulin-dependent protein kinase II alpha (CaMKIIα) (Lisman, Schulman, & Cline, 2002), extracellular signal–regulated kinases ERK1/2 (Sweat, 2004), and of the transcription factor cAMP response element-binding protein (CREB) (Kandel 2012; Mayr & Montminy, 2001, Silva, Kogan, Frankland, & Kida, 1998; Yin & Tully, 1996 Alberini, 2009). Furthermore, because of their critical role in regulating synaptic plasticity functions, we determined the levels and critical phosphorylation of the AMPA receptor subunits GluA1 and GluA2 (Song & Huganir, 2002; Palmer, Cotton, & Henley, 2005), and the concentration of the pre- and post- synaptic maturation markers synaptophysin (Wiedenmann & Franke, 1985; Kwon & Chapman, 2011; Frick & Fernandez, 2003), postsynaptic density protein 95 (PSD-95, Cho, Hunt & Kennedy, 1992; Hunt, Schenker & Kennedy, 1996; Cline, 2005) and Stargazing (Schnell et al., 2002; Tomita et al., 2005). We also investigated the levels of the neurite connectivity markers cofilin and its phosphorylated form at Serine 3, which negatively regulate its ability to bind and de-polymerize actin filaments (Bramham, 2007; Yang et al., 1998), of microtubule-associated protein 2 (MAP2), a neuronal protein that regulates the structure and stability of microtubules and neuronal morphogenesis, (Olmsted, 1986; Sánchez, Díaz-Nido, & Avila, 2000), and of growth associated protein 43 (GAP43), a major constituent of the growth cone that regulates cytoskeletal organization in axon terminals (Aigner et al., 1995; Benowitz & Routtenberg, 1997). Finally, we determined the levels of the pleiotropic kinase mechanistic target of rapamycin (mTOR) (Brown et al., 1994; Sabatini et al., 1994; Laplante & Sabatini, 2012), and of the proteins critical for myelination myelin basic protein (MBP) (Eylar et al., 1971; Readhead et al., 1990; Boggs, 2006; Harauz & Boggs, 2013), myelin associated glycoprotein (MAG) (McKerracher et al., 1994; Mukhopadhyay et al., 1994; Quarles, 2007) and neurite outgrowth inhibitor (Nogo-A) and Nogo Receptor (Nogo-R) (Fournier, GrandPre, & Strittmatter, 2001).
3.3 The levels of activity-dependent immediate early genes are significantly higher in early development and decrease in adulthood
Immediate early genes (IEGs) are known to have a relatively low expression in basal conditions and to be transiently and rapidly induced in response to stimuli. In particular c-Fos, Zif268 and Arc/Arg3.1 have been shown to be rapidly and transiently induced following high-frequency stimulation that evoke LTP, LTD, as well as behavioral experience that leads to long-term memory (Morgan et al., 1987; Cole et al., 1989; Worley et al., 1993, Bramham et al., 2008; Jones et al., 2001; Tischmeyer & Grimm, 1999). Regulatory IEGs like c-Fos and Zif268 control the transcription of downstream targets, which ultimately drives long-term synaptic plasticity and long-term memory formation, whereas Arc plays a critical role in protein endocytosis and trafficking (Bramham et al., 2008; Lanahan & Worley, 1998; Plath et al., 2006).
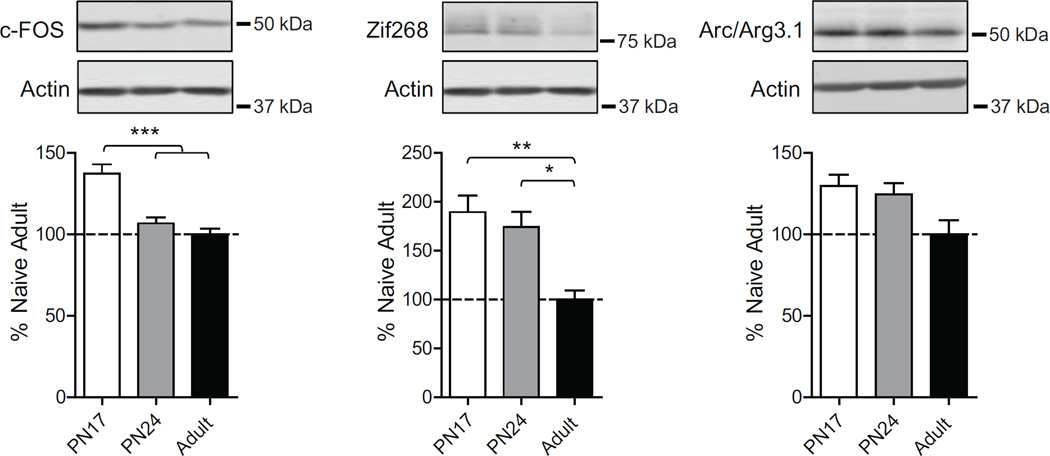
As shown in Fig. 2, one-way ANOVA followed by Newman–Keuls post hoc tests revealed that PN17 rats had a significant higher level of c-Fos (F(2, 19)=16.09; p=0.0001) compared to either PN24 (p<0.001) or adult rats (p<0.001), and that Zif268 was significantly higher at both PN17 and PN24 compared to adult (F(2, 19)=6.12; p=0.0099), without significant difference between PN17 and PN24 (p>0.05). Finally, only a trend toward an increase in Arc levels at both PN17 and PN24 compared to adult age was found, without statistically significant differences (F(2, 19)=3.248; p=0.0639). Thus, the dorsal hippocampus at PN17 has the highest levels of Arc, c-Fos and Zif268 expression, which decrease with age, reaching lower levels at PN24 and significant lower levels in adulthood. These data suggest a state of higher activation of the infant hippocampus compared to the functionally competent, but still developing hippocampus at PN24, and even more so when compared to a fully developed, adult hippocampus.
Figure 2. The levels of immediate early genes c-Fos, Zif268 and Arc are higher in early development and decrease in adulthood.
Examples and densitometric western blot analyses of dHC total extracts from naïve rats euthanized at PN17 (white), PN24 (gray) or PN80 (adult; black) (n=4–8). Data are expressed as mean percentage ± s.e.m. of adult naïve rats. One-way ANOVA followed by Newman–Keuls post hoc tests, *p < 0.05, **p < 0.01, and ***p < 0.001.
3.4. Opposite trajectory in developmental changes between CREB phosphorylation and phosphorylation of CaMKIIα and ERK
Hippocampus-dependent long-term synaptic plasticity and memory formation critically involve the activation of signaling cascades, including those regulated by CaMKIIα and ERK1/2. Both signaling can converge onto the activation of the transcription factor CREB, which has been found to play a critical and evolutionarily conserved role in long-term plasticity and memory formation (Alberini, 2009; Frank & Greenberg, 1994; Silva, Kogan, Frankland, & Kida, 1998; Yin & Tully, 1996). In addition to their relative concentrations, we investigated the autophosphorylation site of CaMKIIα in Threonine (Thr) 286, (pCamKTTα), known to be crucial for the enzymatic persistent activation and LTP induction (Lisman, Schulman, & Cline, 2002; Lisman, Yasuda, & Raghavachari, 2012), the phosphorylation of ERK1 and ERK2 in Thr 202/Tyrosine (Tyr) 204 (pERK1/2) which activate the enzymatic activity, leading to the phosphorylation and activation of downstream targets and transcription factors, including CREB (Sweatt, 2004; Thomas & Huganir, 2004), and finally the phosphorylation in Serine (Ser) 133 of CREB (pCREB), which is critical for the recruitment of co-activators leading to the transcription of the target genes (Mayr & Montminy, 2001).
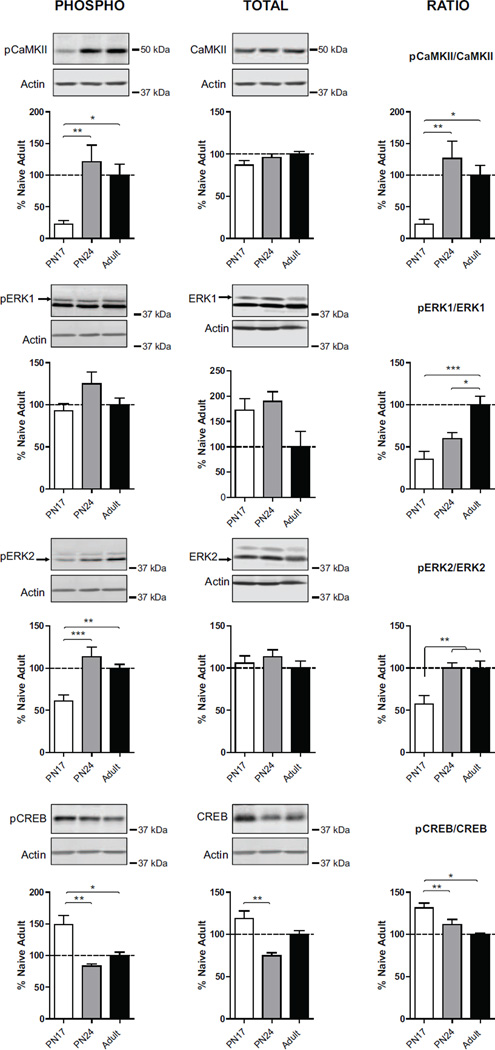
As shown in Fig. 3, one-way ANOVA followed by Newman–Keuls post hoc tests revealed that the dorsal hippocampus of PN17 rats had a striking and significant lower level of pCaMKIIα (F(2, 19)=7.82; p=0.0039) compared with both PN24 (p<0.01) and adult rats (p<0.05), with no significant difference in pCaMKIIα between PN24 and adult rats (p>0.05). No significant difference was found in the level of total CaMKIIα among the three age groups (F(2, 19)=1,607; p=0.23) (Fig.3). Thus, PN17 rats had a significant lower pCaMKIIα/CaMKIIα ratio (F(2, 21)=8.07; p=0.0034) compared with both PN24 (p<0.01) and to adult rats (p<0.05), with no significant difference between PN24 and adult rats (p>0.05) (Fig.3).
Figure 3. Opposite developmental correlation between CREB vs. CaMKIIα and ERK1/2 phosphorylation.
Examples and densitometric western blot analyses of dHC total extracts from untrained rats euthanized at PN17 (white), PN24 (gray) or PN80 (adult; black) (n=4–8). Data are expressed as mean percentage ± s.e.m. of adult naïve rats. One-way ANOVA followed by Newman–Keuls post hoc tests, *p < 0.05, **p < 0.01, and ***p < 0.001.
Similarly, PN17 rats had also a significant lower level of the pERK2 (F(2, 21)=11,67; p=0.0005) compared with both PN24 (p<0.001) and adult rats (p<0.01), while no significant difference was found between PN24 and adult rats (p>0.05) (Fig.3). Also, no significant difference was found in the level of pERK1 (F(2, 21)=2.53; p=0.10), nor in the level of total ERK1 (F(2, 15)=3.63; p=0.07) and ERK2 (F(2, 15)=0.54; p=0.60) among the age groups. Of note, both the ratio pERK1/ERK1 and pERK2/ERK2 were significantly lower at PN17 when compared with adult rats (For pERK1/ERK1: F(2, 15)=11,59; p=0.0013; for pERK2/ERK2: F(2, 15)=9.15; p=0.0033) (Fig.3).
In contrast, one-way ANOVA followed by Newman–Keuls post hoc tests revealed that PN17 rats had a significantly higher level of pCREB (F(2, 21)=11,57; p=0.0007) compared to both PN24 (p<0.001) and adult rats (p<0.05) (Fig.3). Again, no significant difference was found between PN24 and adult rats (p>0.05). However, also the level of total CREB was significantly higher in the hippocampus of PN17 compared to that of PN24 (p<0.01) (Fig.3). Regardless of the change in total CREB level, the ratio pCREB/CREB remained significantly higher at PN17 compared to PN24 and adult rats (F(2, 21)=6.62; p=0.0075), without a significant difference between PN24 and adult rats (p>0.05) (Fig.3).
Together these data suggest that: i) there is a general uncoupling between the activation of the signaling pathways mediated by pCaMKIIα and pERK1/2, which are significantly reduced in development compared to adulthood, and the activation of CREB, which, conversely, is significantly higher in the immature PN17 hippocampus compared to adult hippocampus; ii) the hippocampus of PN17 rats is significantly different from those of the other two groups, with higher levels of pCREB and lower levels of pCamKIIα and pERK2, suggesting an important developmental regulation of these molecular markers; and iii) the dorsal hippocampi of PN24 rat, an age when long-term memories are formed, stored and expressed like in adulthood, have levels of pCaMKIIα, pERK1/2 and pCREB similar to adult rats.
3.5. The levels of GluA1 and GluA2 AMPA receptor subunits significantly increase from PN17 to PN24, while their phosphorylation significantly decreases
AMPA receptor subunits GluA1 and GluA2 control the majority of excitatory synaptic transmission, mediating most of the depolarizing effect of glutamate. Furthermore, activity-dependent phosphorylation of GluA1 at Ser831 delivers AMPARs to synapses (Hayashi et al., 2000) whereas its phosphorylation at Ser845 regulates channel open probability (Barria, Muller, Derkach, Griffith, & Soderling, 1997), thus controlling synaptic maturation and strength.
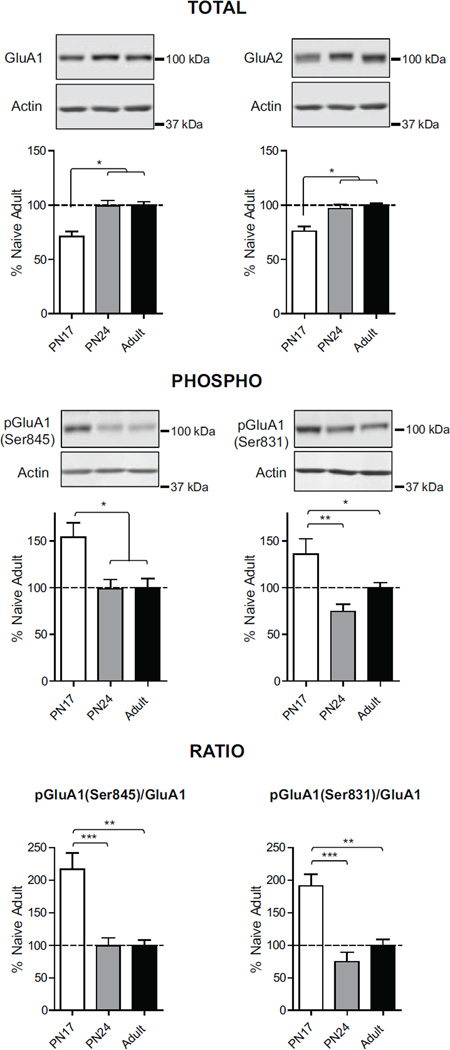
One-way ANOVA followed by Newman–Keuls post hoc tests revealed that the dorsal hippocampus of PN17 rats had a significant lower level of both GluA1 (F(2, 23)=15.01; p=0.0001) and GluA2 (F(2, 23)=14.86; p=0.0001) compared to the hippocampus of both PN24 (p<0.001) and adult rats (p<0.001) (Fig.4). No significant difference was found between PN24 and adult rats (p>0.05). Conversely, there was a significant higher level of phosphorylation of GluA1 at both Ser845 (F(2, 23)=6.16; p=0.0097) and Ser831 (F(2, 23)=8.12; p=0.0024) in PN17 rats, compared to both PN24 and adult rats (Fig.4). The differences among age groups were even stronger when comparing the ratios pGluA1 (Ser845)/GluA1 and pGluA1(Ser831)/GluA1 [One way ANOVA followed by Newman Keuls post hoc test, (F(2, 19)=12.38; p=0.0005 compared to PN24 and F(2, 19)=15.84, p=0.0001 compared to adult, respectively; p<0.0001)]. PN24 and adult hippocampi were very similar (Fig.4).
Figure 4. Significant increase of GluA1 and GluA2 AMPA receptor subunit concentrations and significant decrease of their phosphorylation over development.
Examples and densitometric western blot analyses of dHC total extracts from naïve rats euthanized at PN17 (white), PN24 (gray) or PN80 (adult; black) (n=4–8). Data are expressed as mean percentage ± s.e.m. of adult naïve rats. One-way ANOVA followed by Newman–Keuls post hoc tests, *p < 0.05, **p < 0.01, and ***p < 0.001.
Together, these data suggest that PN17 rat dorsal hippocampus has reduced amount of AMPA receptors, despite their phosphorylation in Ser 845 and 831 is remarkably high, while at PN24 AMPA receptor subunit and phosphorylation levels have already reached adult concentration. Thus, similarly to what was seen for the activity/plasticity markers, the major significant difference was found between PN17 and PN24, whereas PN24 and adult hippocampi were biochemically very similar.
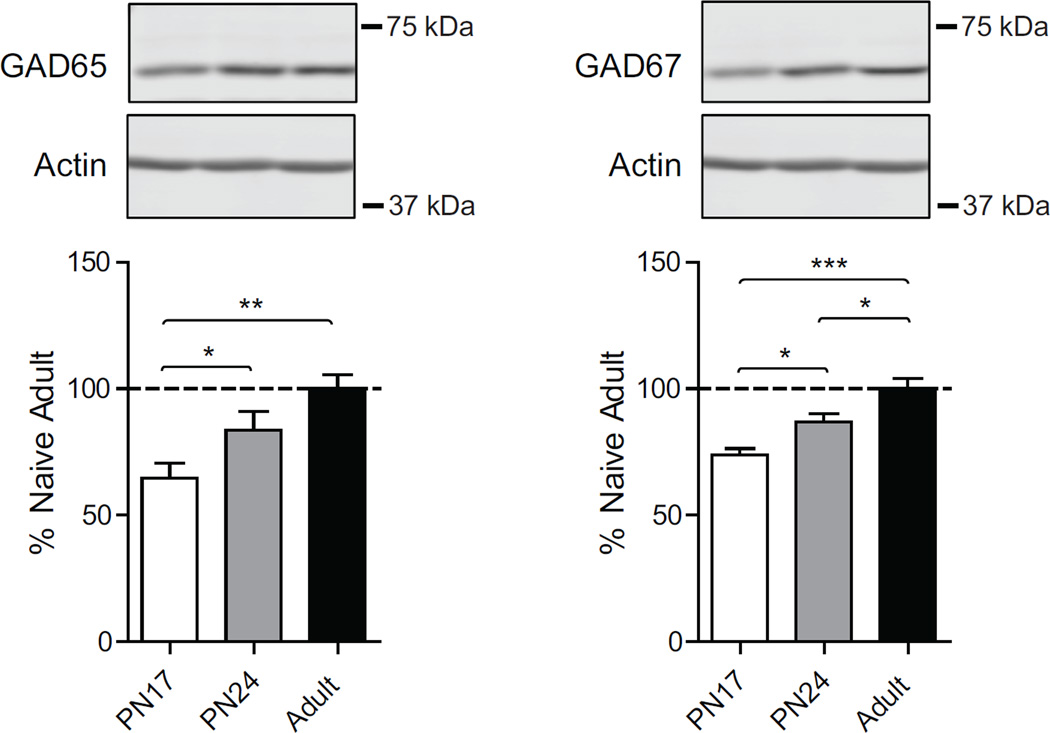
3.6. GAD65 and GAD67 levels significantly increase in the rat hippocampus during postnatal development
γ-Aminobutyric acid (GABA) is the main inhibitory neurotransmitter in the adult brain. Early in development (before PN10 in rats), GABAergic synaptic transmission is excitatory, and brain maturation is associated with a developmental switch in the role of GABA from an excitatory transmitter to an inhibitory transmitter (Ben-Ari, 2002). GABAergic transmission is necessary for neuronal development and shapes excitatory/inhibitory circuitry (Ben-Ari, 2002; Chamberland & Topolnik, 2012). GABA is synthesized by glutamic acid decarboxylase (GAD), which exists in two isoforms, referred to as GAD65 and GAD67 (Bu et al., 1992; Erlander, Tillakaratne, Feldblum, Patel, & Tobin, 1991; Soghomonian & Martin, 1998). GAD65 is mainly concentrated in axon terminals and bound to synaptic vesicles, whereas GAD67 is widely distributed throughout the cell, (Bu et al., 1992; Erlander et al., 1991; Soghomonian & Martin, 1998). Moreover, GAD67 catalyzes GABA synthesis under resting conditions, whereas GAD65 catalyzes GABA synthesis under evoked neuronal activity (Bu et al., 1992; Erlander et al., 1991; Soghomonian & Martin, 1998). It has been shown that both mRNA and protein levels of GAD65 and GAD67 increased gradually during postnatal development (Frahm & Draguhn, 2001; Popp et al., 2009; Soghomonian & Martin, 1998). In agreement with these studies, one-way ANOVA followed by Newman–Keuls post hoc tests revealed that the dorsal hippocampus of PN17 rats had a significant lower level of both GAD65 (F(2, 15)=6.8; p=0.0095) and GAD67 (F(2, 16)=14.81; p=0.0004) compared to both PN24 and adult rats (Fig.5). Furthermore, PN24 rat dorsal hippocampus had a significant lower level of GAD67 compared to that of adults (p<0.05), whereas there was no significant difference in the level of GAD65 (p>0.05) (Fig.5).
Figure 5. GAD65 and GAD67 increase over development.
Examples and densitometric western blot analyses of dHC total extracts from untrained rats euthanized at PN17 (white), PN24 (gray) or PN80 (adult; black) (n=4–8). Data are expressed as mean percentage ± s.e.m. of adult naïve rats. One-way ANOVA followed by Newman–Keuls post hoc tests, *p < 0.05, **p < 0.01, and ***p < 0.001.
Thus, there is a significant, gradual increase in the levels of inhibitory synapse maturation markers, GAD65 and GAD67, over postnatal development.
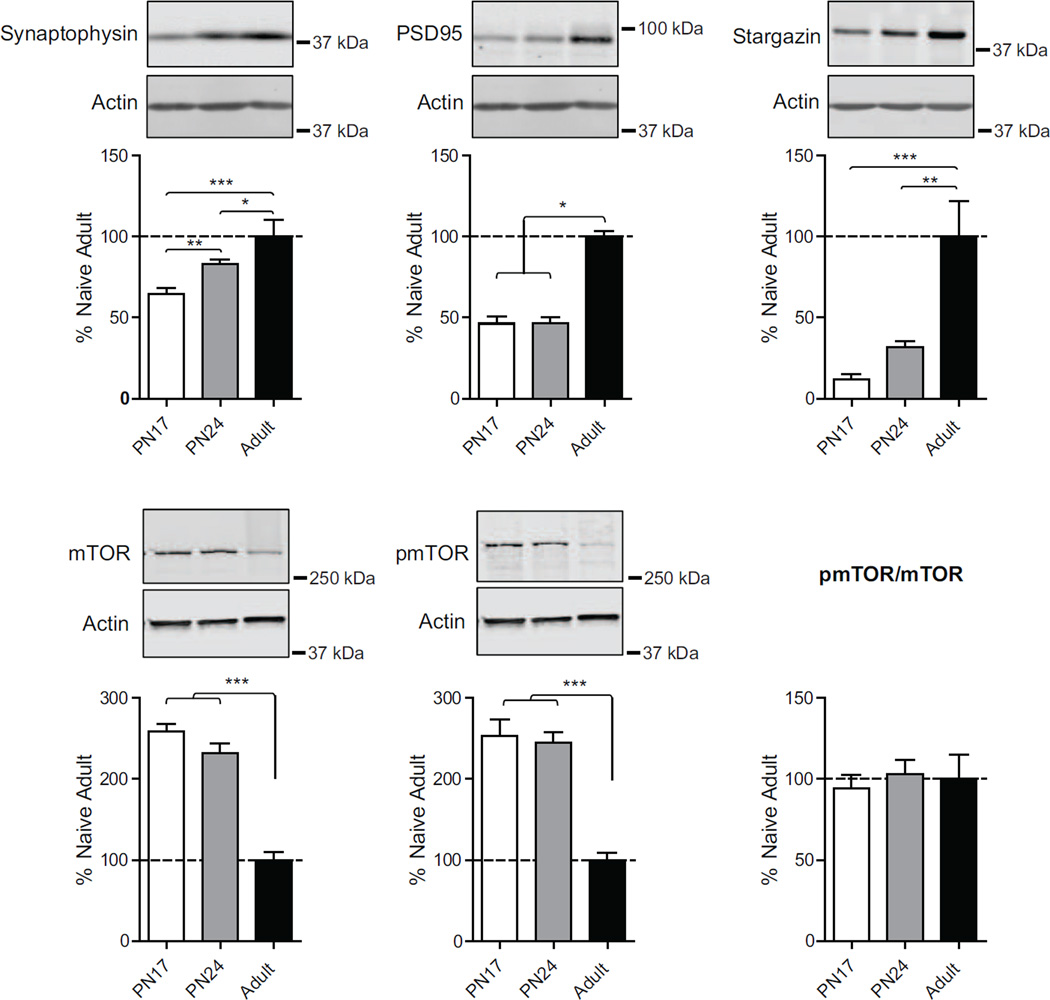
3.7. Levels of proteins critical for synapse maturation significantly increase over development and into adulthood
Glutamate receptors associate with several proteins that constitute a scaffold, which also modulate their signal transduction. These scaffolding proteins, which include PSD-95, the most abundant member of the postsynaptic density (Cline, 2005), and stargazing, which facilitates the trafficking and clustering of AMPA receptors (AMPARs) (Tomita et al., 2005), are involved in assembling, clustering and recycling AMPARs and exert allosteric control over their partners (Good, Zalatan, & Lim, 2011). Furthermore, the presynaptic proteins synaptophysin and synapsin regulates exo- and endo-cytosis of neurotransmitter vesicles (Bähler, Benfenati, Valtorta, & Greengard, 1990; Kwon & Chapman, 2011), and their presence reflects the maturation of functional synapses (Wiedenmann & Franke, 1985; Kwon & Chapman, 2011; Frick & Fernandez, 2003). The quantification of these pre- and post-synaptic proteins is generally used as a measure of synapse functional maturation.
As shown in Fig. 6, one-way ANOVA followed by Newman–Keuls post hoc tests revealed that the dorsal hippocampus of PN17 rats had a significant lower level of synaptophysin (F(2, 19)=12.02; p=0.0006) compared to both PN24 (p<0.01) and adult rats (p<0.001); furthermore, PN24 rat dorsal hippocampus had a significant lower level of synaptophysin compared to that of adults (p<0.05). There was no significant difference in the level of PSD95 (F(2, 19)=39.28; p<0.0001) and stargazin (F(2, 19)=12.90; p=0.0002) between PN17 and PN24 rats (p<0.05), whereas both age groups had a significantly lower level compared to adult rats (for PSD95: PN17 and PN24 vs. Adults p<0.001; for stargazin: PN17 vs. adult p<0.001, PN24 vs. Adults p<0.01) (Fig.6).
Figure 6. Structural synapse maturation proteins increase with development.
Examples and densitometric western blot analyses of dHC total extracts from naïve rats euthanized at PN17 (white), PN24 (gray) or PN80 (adult; black) (n=4–8). Data are expressed as mean percentage ± s.e.m. of adult naïve rats. One-way ANOVA followed by Newman–Keuls post hoc tests, *p < 0.05, **p < 0.01, and ***p < 0.001.
3.8. Levels of mTOR and pmTOR decreases over development and into adulthood
The mechanistic target of rapamycin (mTOR) is a master growth regulator that senses cellular nutrients and energy levels, and it is involved in a vast variety of cellular function, including activity-dependent synaptic protein translation (Costa-Mattioli & Monteggia, 2013; Hoeffer & Klann, 2010) lipid synthesis, autophagy, cell survival and proliferation, and oligodendrocyte differentiation and myelination (Narayanan, Flores, Wang, & Macklin, 2009; Wahl, McLane, Bercury, Macklin, & Wood, 2014; Laplante & Sabatini, 2009, 2012; Mizushima, Levine, Cuervo, & Klionsky, 2008), just to name a few. MTOR also regulates growth in the developing brain and the turnover of misfolded proteins in developed neurons (Graber, McCamphill, & Sossin, 2013). Thus, the expression and regulation of mTOR may reflect synaptic and neurite functional development and maturation. Furthermore, phosphorylation of mTOR in Ser2448 correlates with mTOR activity (Scott et al., 1998; Reynolds et al., 2002; Nave et al., 2009; Inoki et al., 2002).
One way ANOVA followed Newman–Keuls post hoc tests revealed no significant difference in mTOR and pmTOR levels when comparing PN17 and PN24 rats, whereas both age groups showed a significantly higher mTOR and pmTOR levels when compared to adult rats (mTOR: F(2, 15)=53.51; p<0.0001; pmTOR: F(2, 15)=23.18; p<0.0001) (Fig.5). Overall, no statistical differences were found among the age groups in the pmTOR/mTOR ratio (F(2, 15)=0.20; p<0.82).
These data together with the changes in synapse maturation markers shown above suggest that at PN17 synapses are significantly immature, and that process of synapse maturation in the dHC is more pronounced after PN24 and toward adulthood.
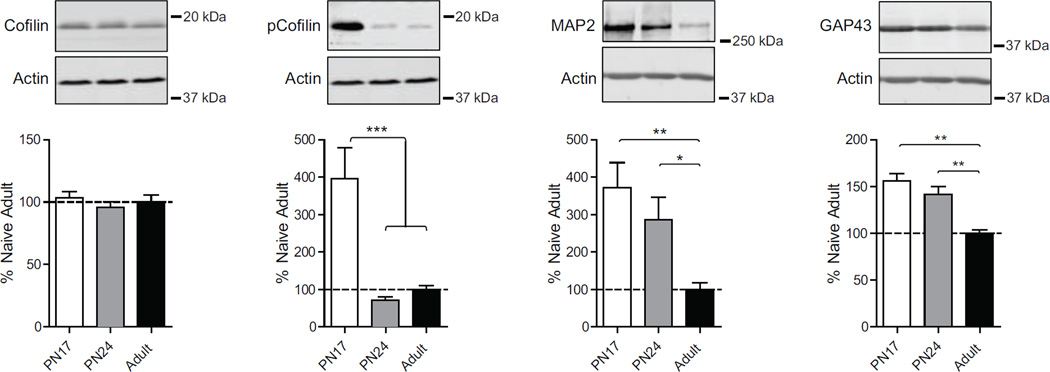
3.9. Neurite connectivity protein levels decrease over development and into adulthood
Neuronal morphogenesis has a critical role in the developing brain. Many proteins provide physical support to shape the fine structure of neuronal processes. These include: i) cofilin, a ubiquitous actin-binding factor, which via phosphorylation at Ser3 regulates actin filament polimerization (Bramham, 2007); ii) microtubule-associated protein 2 (MAP2), a neuronal protein that regulates the structure and stability of microtubules and neuronal morphogenesis (Sánchez, Díaz-Nido, & Avila, 2000) and iii) growth associated protein 43, (GAP43), major constituent of the growth cone that regulates cytoskeletal organization in axon terminals and in neurite formation (Benowitz & Routtenberg, 1997).
As shown in Figure 7, one way ANOVA followed by Newman-Keuls post hoc tests revealed no significant difference in the level of cofilin among the three age groups (F(2, 23)=0.54; p=0.59), but a remarkable and significant higher levels of pcofilin at PN17 (F(2, 23)=14.00; p=0.0001) compared to both PN24 (p<0.001) and adult rats (p<0.001), whereas no significant difference was found between PN24 and adult rats (p>0.05).
Figure 7. Neurite connectivity marker levels decrease over development.
Examples and densitometric western blot analyses of dHC total extracts from naïve rats euthanized at PN17 (white), PN24 (gray) or PN80 (adult; black) (n=4–8). Data are expressed as mean percentage ± s.e.m. of adult naïve rats. One-way ANOVA followed by Newman–Keuls post hoc tests, *p < 0.05, **p < 0.01, and ***p < 0.001.
Furthermore, the hippocampus of PN17 rats had a significantly higher level of MAP2 (F(2, 19)=3.76; p=0.044) and GAP43 (F(2, 19)=9.21; p=0.0020), compared to and adult rats, and PN24 rats had a significant higher level of GAP43 compared to adult rats (p<0.01) (Fig.7).
Together these data suggest that the high activity of the PN17 brain, as indicated by the levels of the immediate early genes, is paralleled by a significant neuronal growth and remodeling.
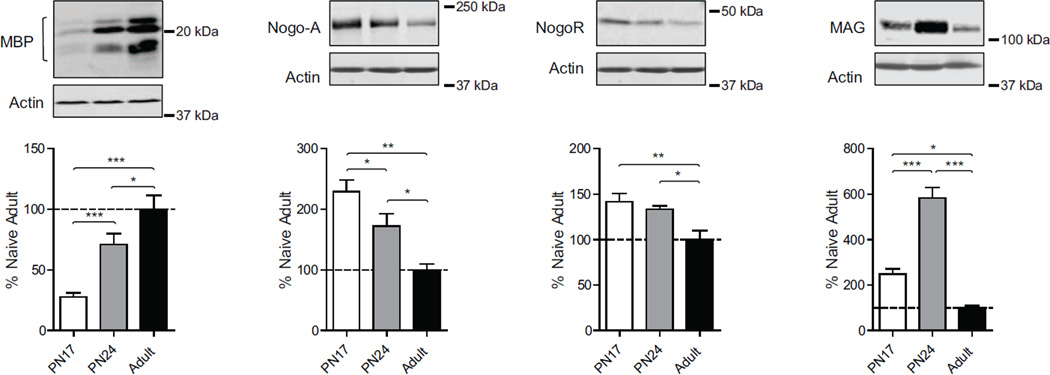
3.10. Levels of myelination markers significantly change over development
Myelin sheaths are multi-layered membranes with a critical role to stabilize axonal projections and increase the conduction velocity of axonal impulses. Several lipids and proteins are present in the myelin sheath. Among them, myelin basic protein (MBP) is an essential constituent of myelin (Harauz & Boggs, 2013). The formation of myelin sheaths is also regulated by other myelin components such as myelin associated glycoprotein (MAG) (Quarles, 2007), and neurite outgrowth inhibitor (Nogo-A) and its receptor (NogoR), which inhibit axon and neurite outgrowth (Fournier et al., 2001).
As shown in figure 8, we found a dramatic increase in MBP over the developmental window investigated. One-way ANOVA followed by Newman–Keuls post hoc tests revealed that PN17 rats had a significant lower level of MBP (F(2, 19)=20.07; p<0.0001) compared with both PN24 (p<0.001) and adult rats (p<0.001) (Fig.8). Moreover, PN24 rats had a significant lower level of MBP compared to adults (p<0.05). The opposite was found for Nogo A and NogoR (Fig.8). Statistical analysis revealed that both PN17 and PN24 rats had a significant higher level of Nogo A and NogoR when compared with adult rats (for Nogo A: F(2, 15)=10.55; p<0.0019; for NogoR: F(2, 15)=7.16; p<0.008). PN17 rats had higher level of Nogo A (p<0.05) but not of NogoR (p>0.05) compared with PN24 rats. Both PN17 and PN24 rats had a significant higher level of MAG compared with adults (F(2, 15)=50.45; p<0.0001). Of note, the level of MAG in the PN24 hippocampus was significantly elevated compared to that of PN17 rats (p<0.001).
Figure 8. Changes in myelination markers over development.
Examples and densitometric western blot analyses of dHC total extracts from naïve rats euthanized at PN17 (white), PN24 (gray) or PN80 (adult; black) (n=4–8). Data are expressed as mean percentage ± s.e.m. of adult naïve rats. One-way ANOVA followed by Newman–Keuls post hoc tests, *p < 0.05, **p < 0.01, and ***p < 0.001.
Together these results suggest that hippocampal myelination drastically increase during postnatal development between PN17 and PN24.
4. Discussion
Identifying the biological changes occurring in the hippocampus over development is key for understanding the acquisition of its functional abilities. The hippocampus, a region critical for the formation and consolidation of explicit memories undergoes a protracted postnatal developmental phase, as also shown by the infantile amnesia phenomenon (Campbell & Spear, 1972; Hayne & Herbert, 2004, Travaglia et al. 2016). In this study, we reported that molecules known to be critical for synaptic plasticity underlying memory formation in mature brains and systems, including the expression levels of IEGs, phosphorylation of kinases and transcription factors as well as of AMPA receptor subunits, have significant -and in some case remarkable- differential basal expression levels in the developing dorsal hippocampus compared to the adult dorsal hippocampus. The data are summarized in Figure 9. Our investigation compared two developmental phases in rats that, although close in time, correspond to very different ability to form and retain long-term memories: PN17, which is an age of infantile amnesia, and PN24 when the rats already acquired the ability to form and store long-term memory, similarly to adults. We compared both ages to adulthood to identify possible correlates of infantile amnesia.
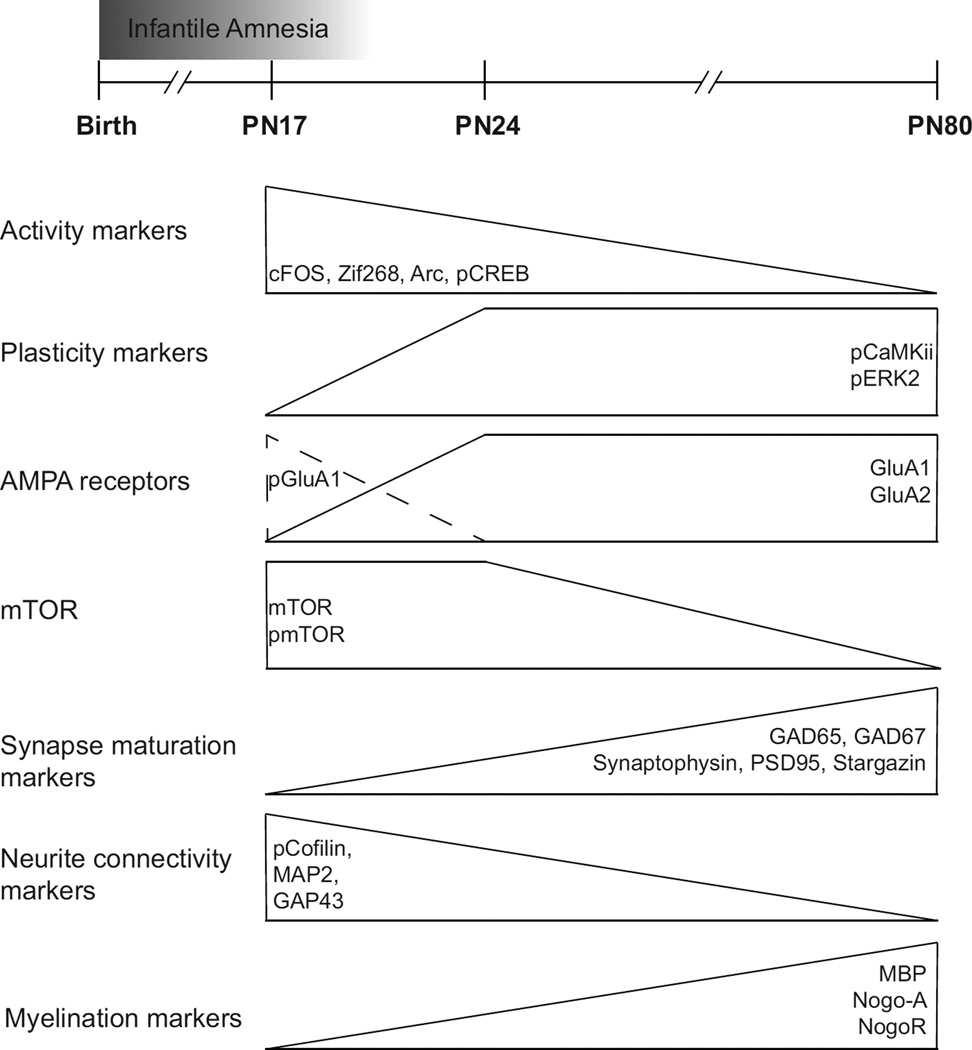
Figure 9. Synopsis of plasticity, synaptic, glia and connectivity protein levels in rat dorsal hippocampus over development.
Graphic representations of the identified temporal molecular changes occurring in the rat dorsal hippocampus from PN17 to PN24 and to PN80 (adult). The ontogenic progression is characterized by a decrease in markers of neural activation and an increase in proteins supporting structural and functional maturations, in particular those involved in growth of dendrites, axons and synapses. The distinct molecular background of PN17 compared to PN24 and adult rat dorsal hippocampus may reflect the period of infantile amnesia.
We found that the relative concentrations of the IEGs c-Fos, Arc and Zif268 are higher at PN17, and for Zif268 and Arc also at PN24 compared to adult. Another plasticity correlate, the phosphorylation of CREB in Ser133 (pCREB) followed a similar trajectory: it is significantly elevated at PN17 compared to both PN24 and adult, which show similar relative concentrations. Likewise, other molecular correlates of synaptic plasticity underlying long-term memory, the increase in GluA1 subunit levels, and GluA1 phosphorylations at Ser845 and Ser831, which reflect AMPARs activity and subcellular trafficking, were found significantly higher at PN17 compared to PN24, whereas the PN24 levels once again were similar to adult’s levels. These phosphorylation changes are critical for LTP expression and the retention of spatial memories (Lee et al., 2003); however, like for the induction of IEGs and pCREB their highest levels at the early developmental age of PN17 likely reflect a high state of activation, which, however, does not correlate with the ability to express long-term memories. This dissociation between highest levels of synaptic activation and hippocampal functional competence suggests two opposite speculations: first that these molecular activations are not learning-specific, but rather reflect “developmental” basal activity, perhaps necessary to promote growth. Second, that all these activations at PN17 are actually learning-dependent changes, precisely because a great deal of novel hippocampal-dependent learning occurs at this early developmental age, although memories are still not expressed long-term. It is also possible that developmental basal activity mechanisms are re-recruited in learning. Furthermore, perhaps other mechanisms, or their lack of, preclude long-term memory storage/expression.
In contrast to all these activations that peak at PN17, we found an opposite trajectory for pCaMKIIα activation: while the levels of CaMKIIα were similar at the three ages, its phosphorylation in Thr286, dramatically increased -approximately 500%- from PN17 to PN24, while PN24 was similar to adult. This highly significant, remarkable difference in pCaMKIIα at PN24 compared to PN17, together with the fact that phosphorylation of CamKIIα is further induced in the adult hippocampus following learning, lead us to speculate that CaMKIIα phosphorylation in Thr286 may be a correlative molecular signature of the acquisition of hippocampal functional competence. Similar changes, although much less dramatic, were seen for pERK, and particularly pERK2.
The synapse maturation markers synaptophysin, PSD95 and stargazin changed significantly to a very high degree between PN17 and PN24, and only synaptophysin showed a significant increase between PN24 and adult, while the post-synaptic marker levels at PN24 were similar to those of the adult. These results together with the pCaMKIIα increase over development suggest that important steps of excitatory synapse maturation largely takes place between PN17 and PN24, and therefore correlates with hippocampal competence acquisition of long-term memory expression. In parallel, we found that also the levels of inhibitory synapse maturation markers GAD65 and GAD67 increased significantly from PN17 and PN24 and from PN24 to adulthood, suggesting that inhibitory synapse maturation occurs throughout the investigated developmental phase. It is tempting to speculate that a low inhibition, as measured by GAD65 and GAD67 early in development and gradually increasing from PN17 to PN24 and then to adulthood may contribute to the high activation of the PN17 hippocampus. However, these changes do not correlate with functional competence in long-term memory expression but rather seem to be more in line with an extended and gradual developmental process of synapse maturation.
The mTOR level and its phosphorylation in Ser2448 were significantly elevated in development at both PN17 and PN24, compared to adult age, suggesting that mTOR expression and activation may correlate with synaptic functional development. The pmTOR/mTOR ratio did not change among the age groups suggesting that the expression is the main regulating factor. Nevertheless this kinase has been shown to be critical for multiple processes, thus implying that it is associated to growth in general. In fact, mTOR regulates protein synthesis, lipid synthesis, autophagy, cell survival and proliferation (Laplante & Sabatini, 2009, 2012; Mizushima et al., 2008), as well as the turnover of misfolded proteins in developed neurons (Graber et al., 2013). Our data on mTOR and pmTOR appear to be in disagreement with a previous study showed that there were no significant differences between levels of hippocampal mTOR and pmTOR of PN16, PN21 and adult rats (Talos et al., 2012). This study analyzed hippocampal protein levels, whereas our data reflect changes occurring in the dorsal hippocampus, thus the apparent discrepancy could be due to region specific differences or protein extraction procedures.
The expression levels of connectivity proteins showed an opposite trajectory compared to that of synapse maturation: in fact, pcofilin was significantly higher at PN17 compared to PN24 and adult, which did not significantly differ. MAP2 and GAP43 were significantly higher at both PN17 and PN24 compared to adult hippocampus. These results confirm previous knowledge that connectivity development proceeds at a higher speed at both early developmental ages compared to adult (Benowitz & Routtenberg, 1997; Biranowska et al., 2000).
Finally we showed that the myelination markers MBP and NOGO-A/NOGOR change in opposite directions, which is in agreement with their roles in promoting myelination and inhibiting axonal growth, respectively (Deber & Reynolds, 1991; Fournier et al., 2001; Harauz & Boggs, 2013). Notably, the axon growth inhibitory protein MAG peaked at PN24, with an approximately 600% higher level compared to the adult hippocampus, and an approximately 300% higher level compared to PN17. Hence, PN24 seems to correspond to a critical phase of MAG-regulated changes in myelination.
Overall our biochemical analyses reveal a dramatic increase in proteins and posttranslational modification supporting synaptogenesis and synapse maturation with development (Figure 9). Most of our data on protein levels are in agreement with what the Allen Brain Atlas reported for the mRNA levels in mouse brain development, -although these have been investigated at slightly different ages (PN14, PN28 and PN56 instead of PN17, PN24 and PN80)- suggesting that translation follows transcription changes.
It is interesting to note that there are significant differences between PN24 and adult rats in the levels of markers of synapse maturations (synaptophisyn, PSD95, stargazin), and myelination (MBP, Nogo-A, NogoR, MAG), despite both age groups can form long-term memories. These changes may reflect the acquisition of hippocampal complex functions, such as pattern completion and separation, and more studies are needed to understand the changes associated to the extended phase of hippocampal development. On the other hand, at PN24 the levels of markers of synaptic plasticity underlying long-term memory such as pCREB, pERK and pCaMKIIα, as well as AMPA receptor and their phosphorylations, were similar to those of the adult. This might suggest that at PN24 the hippocampus is not physically and structurally mature and it is still growing, but has already acquired some of the adult functional competence. In agreement with these idea, Campbell and Campbell (1962), using a different paradigm and multiple shocks, showed that PN23 rats form long term memories, but not as robustly as adult rats. Thus, it is possible that the differences seen at PN23/24 in our study vs. the Campbell and Campbell’ study may be due to the long maturation process of the hippocampus, which includes relative biological changes. This long process of functional, thus biological and structural maturations, likely mirrors the acquisition of different functional abilities, and it is consistent with our findings that the level of some structural proteins, such as stargazin and MBP (Figure 9), significantly increase from PN24 to adulthood. These changes might provide some explanation for why memories at PN24 are not as robust as in adulthood.
In sum, our results show that numerous activity-dependent molecular correlates of long-term synaptic plasticity, including transcription regulation as well as synaptic maturation and AMPA receptor activation are significantly elevated at the highest level at the early developmental age investigated, PN17, when the hippocampus is not yet functionally able to express contextual memories long-term. Thus, it remains to be understood how these high basal levels differentially contribute to learning-dependent changes, and/or to what extent these are learning-dependent changes in a system that is not yet functionally competent, yet is undergoing functional maturation. In relation to these questions, recently Travaglia et al. (2016) showed that at PN17, during the infantile amnesia period, learning induces critical period maturation changes in the hippocampus to form latent memory traces. On the other hand, here we found that levels of pCaMKIIα, pERK2, GluA1 and GluA2 seem to correlate with the acquisition of functional competence in forming a mature long-term memory. These correlative changes may be permissive rather than instructive, and further studies are needed to address this question.
Nevertheless it is clear from our data that levels of proteins and posttranslational modification critical for synaptic plasticity are significantly and in many cases drastically different in the dorsal hippocampus at different developmental ages compared to adult, and are highly regulated over development. Thus, studies of hippocampal synaptic plasticity and memory should take into consideration that the basal biological composition of the brain greatly differs during development compared to adulthood, in other words that a pup’s brain is not a small adult brain, but a very distinct biological system.
Highlights.
-
-
Ontogenic molecular changes in the rat dorsal hippocampus at PN17, PN24 and PN80
-
-
IEGs and pCREB decrease, while pCaMKIIα and pERK2 increase from PN17 to PN24
-
-
mTOR and pmTOR decrease between PN24 and PN80
-
-
Gradual increase in synaptic and myelination and decrease in connectivity markers
-
-
Developing and adult hippocampus are different biological systems
Acknowledgments
This work was supported by R01-MH074736 and Agalma Foundation grants to CMA. A.T. was supported by a fellowship from Agalma Foundation. R.B. was supported by a fellowship from the Swiss National Science Foundation. E.C. was supported by 5T32MH019524-23.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aigner L, Arber S, Kapfhammer JP, Laux T, Schneider C, Botteri F, Brenner HR, Caroni P. Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell. 1995;83(2):269–278. doi: 10.1016/0092-8674(95)90168-x. [DOI] [PubMed] [Google Scholar]
- Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiological Reviews. 2009;89(1):121–145. doi: 10.1152/physrev.00017.2008. http://doi.org/10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähler M, Benfenati F, Valtorta F, Greengard P. The synapsins and the regulation of synaptic function. BioEssays: News and Reviews in Molecular, Cellular and Developmental Biology. 1990;12(6):259–263. doi: 10.1002/bies.950120603. http://doi.org/10.1002/bies.950120603. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Kandel ER, Harris KM. Structural Components of Synaptic Plasticity and Memory Consolidation. Cold Spring Harbor Perspectives in Biology. 2015;7(7):a021758. doi: 10.1101/cshperspect.a021758. http://doi.org/10.1101/cshperspect.a021758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barco A, Pittenger C, Kandel ER. CREB, memory enhancement and the treatment of memory disorders: promises, pitfalls and prospects. Expert Opin Ther Targets. 2003;7(1):101–114. doi: 10.1517/14728222.7.1.101. [DOI] [PubMed] [Google Scholar]
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276(5321):2042–2045. doi: 10.1126/science.276.5321.2042. http://doi.org/10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nature Reviews Neuroscience. 2002;3(9):728–739. doi: 10.1038/nrn920. http://doi.org/10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, Routtenberg A. GAP-43: An intrinsic determinant of neuronal development and plasticity. Trends in Neurosciences. 1997 doi: 10.1016/s0166-2236(96)10072-2. http://doi.org/10.1016/S0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- Biranowska J, Berdel B, Ludkiewicz B, Dziewiatkowski J, Jagalska-Majewska H, Moryś J. Developmental changes of MAP2 immunoreactivity in the hippocampus proper and dentate gyrus of the rat. Folia Neuropathologica / Association of Polish Neuropathologists and Medical Research Centre, Polish Academy of Sciences. 2000;38(1):1–6. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11057027. [PubMed] [Google Scholar]
- Boggs JM. Myelin basic protein: a multifunctional protein. Cell Mol Life Sci. 2006;63(17):1945–1961. doi: 10.1007/s00018-006-6094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR. Control of synaptic consolidation in the dentate gyrus: mechanisms, functions, and therapeutic implications. Progress in Brain Research. 2007;163:453–471. doi: 10.1016/S0079-6123(07)63025-8. http://doi.org/10.1016/S0079-6123(07)63025-8. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Worley PF, Moore MJ, Guzowski JF. The immediate early gene arc/arg3.1: regulation, mechanisms, and function. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2008;28(46):11760–11767. doi: 10.1523/JNEUROSCI.3864-08.2008. http://doi.org/10.1523/JNEUROSCI.3864-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, Schreiber SL. A mammalian protein targeted by G1 -arresting rapamycin-receptor complex. Nature. 369(6483):756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- Bu DF, Erlander MG, Hitz BC, Tillakaratne NJ, Kaufman DL, Wagner-McPherson CB, Tobin AJ. Two human glutamate decarboxylases, 65-kDa GAD and 67-kDa GAD, are each encoded by a single gene. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(6):2115–2119. doi: 10.1073/pnas.89.6.2115. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=48607&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BL, Li S, Richardson R. The elusive engram: What can infantile amnesia tell us about memory? Trends in Neurosciences. 2014 doi: 10.1016/j.tins.2013.10.007. http://doi.org/10.1016/j.tins.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Campbell BA, Spear NE. Ontogeny of memory. Psychological Review. 1972;79(3):215–236. doi: 10.1037/h0032690. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/4341445. [DOI] [PubMed] [Google Scholar]
- Campbell BA, Campbell EH. Retention and extinction of learned fear in infant and adult rats. J Comp Physiol Psychol. 1962;55:1–8. doi: 10.1037/h0049182. [DOI] [PubMed] [Google Scholar]
- Chamberland S, Topolnik L. Inhibitory control of hippocampal inhibitory neurons. Frontiers in Neuroscience. 2012;6:165. doi: 10.3389/fnins.2012.00165. http://doi.org/10.3389/fnins.2012.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DY, Bambah-Mukku D, Pollonini G, Alberini CM. Glucocorticoid receptors recruit the CaMKIIα-BDNF-CREB pathways to mediate memory consolidation. Nature Neuroscience. 2012;15(12):1707–1714. doi: 10.1038/nn.3266. http://doi.org/10.1038/nn.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KO, Hunt CA, Kennedy MB. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron. 1992;9(5):929–942. doi: 10.1016/0896-6273(92)90245-9. [DOI] [PubMed] [Google Scholar]
- Cline H. Synaptogenesis: a balancing act between excitation and inhibition. Current Biology: CB. 2005;15(6):R203–R205. doi: 10.1016/j.cub.2005.03.010. http://doi.org/10.1016/j.cub.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Cole AJ, Saffen DW, Baraban JM, Worley PF. Rapid increase of an immediate early gene messenger RNA in hippocampal neurons by synaptic NMDA receptor activation. Nature. 1989;340(6233):474–476. doi: 10.1038/340474a0. [DOI] [PubMed] [Google Scholar]
- Costa-Mattioli M, Monteggia LM. mTOR complexes in neurodevelopmental and neuropsychiatric disorders. Nature Neuroscience. 2013;16(11):1537–1543. doi: 10.1038/nn.3546. http://doi.org/10.1038/nn.3546. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Matthews DA, Taylor D, Lynch G. Synaptic rearrangement in the dentate gyrus: histochemical evidence of adjustments after lesions in immature and adult rats. Proceedings of the National Academy of Sciences of the United States of America. 1973;70(12):3473–3477. doi: 10.1073/pnas.70.12.3473. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=427262&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT, Yamamura HI. Neurochemical aspects of the ontogenesis of cholinergic neurons in the rat brain. Brain Research. 1976;118(3):429–440. doi: 10.1016/0006-8993(76)90310-3. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1009427. [DOI] [PubMed] [Google Scholar]
- Crain B, Cotman C, Taylor D, Lynch G. A quantitative electron microscopic study of synaptogenesis in the dentate gyrus of the rat. Brain Research. 1973;63:195–204. doi: 10.1016/0006-8993(73)90088-7. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/4764297. [DOI] [PubMed] [Google Scholar]
- Davis HP, Squire LR. Protein Synthesis and Memory : A Review. Psychological Bulletin. 1984;96(3):518–559. http://doi.org/10.1037/0033-2909.96.3.518. [PubMed] [Google Scholar]
- Deber CM, Reynolds SJ. Central nervous system myelin: structure, function, and pathology. Clinical Biochemistry. 1991;24(2):113–134. doi: 10.1016/0009-9120(91)90421-A. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1710177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y. Consolidation: Fragility on the road to the engram. Neuron. 1996 doi: 10.1016/s0896-6273(00)80168-3. http://doi.org/10.1016/S0896-6273(00)80168-3. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. Bidirectional long-term modification of synaptic effectiveness in the adult and immature hippocampus. J Neurosci. 1993;13:2910–2918. doi: 10.1523/JNEUROSCI.13-07-02910.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. http://doi.org/10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlander MG, Tillakaratne NJ, Feldblum S, Patel N, Tobin AJ. Two genes encode distinct glutamate decarboxylases. Neuron. 1991;7(1):91–100. doi: 10.1016/0896-6273(91)90077-d. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2069816. [DOI] [PubMed] [Google Scholar]
- Eylar EH, Brostoff S, Hashim G, Caccam J, Burnett P. Basic A1 protein of the myelin membrane. The complete amino acid sequence. J Biol Chem. 1971;246(18):5770–5784. [PubMed] [Google Scholar]
- Fiala JC, Feinberg M, Popov V, Harris KM. Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1998;18(21):8900–8911. doi: 10.1523/JNEUROSCI.18-21-08900.1998. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9786995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409(6818):341–346. doi: 10.1038/35053072. http://doi.org/10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- Frank DA, Greenberg ME. CREB: a mediator of long-term memory from mollusks to mammals. Cell. 1994;79(1):5–8. doi: 10.1016/0092-8674(94)90394-8. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7923377. [DOI] [PubMed] [Google Scholar]
- Frahm C, Draguhn A. GAD and GABA transporter (GAT-1) mRNA expression in the developing rat hippocampus. Brain Res Dev Brain Res. 2001;132(1):1–13. doi: 10.1016/s0165-3806(01)00288-7. [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM. Enrichment enhances spatial memory and increases synaptophysin levels in aged female mice. Neurobiology of Aging. 2003;24(4):615–626. doi: 10.1016/s0197-4580(02)00138-0. http://doi.org/10.1016/S0197-4580(02)00138-0. [DOI] [PubMed] [Google Scholar]
- Good MC, Zalatan JG, Lim WA. Scaffold proteins: hubs for controlling the flow of cellular information. Science (New York, N.Y.) 2011;332(6030):680–686. doi: 10.1126/science.1198701. http://doi.org/10.1126/science.1198701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber TE, McCamphill PK, Sossin WS. A recollection of mTOR signaling in learning and memory. Learning & Memory (Cold Spring Harbor, N.Y.) 2013;20(10):518–530. doi: 10.1101/lm.027664.112. http://doi.org/10.1101/lm.027664.112. [DOI] [PubMed] [Google Scholar]
- Harauz G, Boggs JM. Myelin management by the 18.5-kDa and 21.5-kDa classic myelin basic protein isoforms. Journal of Neurochemistry. 2013 doi: 10.1111/jnc.12195. http://doi.org/10.1111/jnc.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Jensen FE, Tsao B. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1992;12(7):2685–2705. doi: 10.1523/JNEUROSCI.12-07-02685.1992. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1613552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Stevens JK. Dendritic spines of CA 1 pyramidal cells in the rat hippocampus: serial electron microscopy with reference to their biophysical characteristics. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1989;9(8):2982–2997. doi: 10.1523/JNEUROSCI.09-08-02982.1989. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2769375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban Ja, Piccini a, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science (New York, N.Y.) 2000;287(5461):2262–2267. doi: 10.1126/science.287.5461.2262. http://doi.org/10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Hayne H, Herbert J. Verbal cues facilitate memory retrieval during infancy. Journal of Experimental Child Psychology. 2004;89(2):127–139. doi: 10.1016/j.jecp.2004.06.002. http://doi.org/10.1016/j.jecp.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends in Neurosciences. 2010;33(2):67–75. doi: 10.1016/j.tins.2009.11.003. http://doi.org/10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt CA, Schenker LJ, Kennedy MB. PSD-95 is associated with the postsynaptic density and not with the presynaptic membrane at forebrain synapses. J Neurosci. 1996;16(4):1380–1388. doi: 10.1523/JNEUROSCI.16-04-01380.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson RL, Pribram KH. Developmental Neurobiology | Scott Lipnick |. Springer; 1975. Retrieved April 27, 2016, from http://www.springer.com/us/book/9780306483301. [Google Scholar]
- Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4(9):648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- Jacobson M. The Hippocampus, Volume 4. Springer Science & Business Media; 2013. Retrieved from https://books.google.com/books?hl=en&lr=&id=ZOnTBwAAQBAJ&pgis=1. [Google Scholar]
- Jones MW, Errington ML, French PJ, Fine A, Bliss TV, Garel S, Davis S. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nature Neuroscience. 2001;4(3):289–296. doi: 10.1038/85138. http://doi.org/10.1038/85138. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001 Nov 2;294(5544):1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol Brain. 2012;5:14. doi: 10.1186/1756-6606-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Jung S-C, Clemens AM, Petralia RS, Hoffman DA. Regulation of dendritic excitability by activity-dependent trafficking of the A-type K+ channel subunit Kv4.2 in hippocampal neurons. Neuron. 2007;54(6):933–947. doi: 10.1016/j.neuron.2007.05.026. http://doi.org/10.1016/j.neuron.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon SE, Chapman ER. Synaptophysin regulates the kinetics of synaptic vesicle endocytosis in central neurons. Neuron. 2011;70(5):847–854. doi: 10.1016/j.neuron.2011.04.001. http://doi.org/10.1016/j.neuron.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanahan a, Worley P. Immediate-early genes and synaptic function. Neurobiology of Learning and Memory. 1998;70(1-2):37–43. doi: 10.1006/nlme.1998.3836. http://doi.org/10.1006/nlme.1998.3836. [DOI] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling at a glance. Journal of Cell Science. 2009;122(Pt 20):3589–3594. doi: 10.1242/jcs.051011. http://doi.org/10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293. doi: 10.1016/j.cell.2012.03.017. http://doi.org/10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-K, Takamiya K, Han J-S, Man H, Kim C-H, Rumbaugh G, Huganir RL. Phosphorylation of the AMPA Receptor GluR1 Subunit Is Required for Synaptic Plasticity and Retention of Spatial Memory. Cell. 2003;112(5):631–643. doi: 10.1016/s0092-8674(03)00122-3. http://doi.org/10.1016/S0092-8674(03)00122-3. [DOI] [PubMed] [Google Scholar]
- Liao D, Malinow R. Deficiency in induction but not expression of LTP in hippocampal slices from young rats. Learning & Memory (Cold Spring Harbor, N.Y.) 1996;3(2–3):138–149. doi: 10.1101/lm.3.2-3.138. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10456084. [DOI] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nature Reviews. Neuroscience. 2002;3(3):175–190. doi: 10.1038/nrn753. http://doi.org/10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Lisman J, Yasuda R, Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nature Reviews. Neuroscience. 2012;13(3):169–182. doi: 10.1038/nrn3192. http://doi.org/10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen HB, Kim JH. Ontogeny of memory: An update on 40 years of work on infantile amnesia. Behavioural Brain Research. 2016;298(Pt A):4–14. doi: 10.1016/j.bbr.2015.07.030. http://doi.org/10.1016/j.bbr.2015.07.030. [DOI] [PubMed] [Google Scholar]
- Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nature Reviews. Molecular Cell Biology. 2001;2(8):599–609. doi: 10.1038/35085068. http://doi.org/10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory--a century of consolidation. Science (New York, N.Y.) 2000;287(5451):248–251. doi: 10.1126/science.287.5451.248. http://doi.org/10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13(4):805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- Meier S, Bräuer AU, Heimrich B, Nitsch R, Savaskan NE. Myelination in the hippocampus during development and following lesion. Cellular and Molecular Life Sciences. 2004;61(9):1082–1094. doi: 10.1007/s00018-004-3469-5. http://doi.org/10.1007/s00018-004-3469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451(7182):1069–1075. doi: 10.1038/nature06639. http://doi.org/10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody M, Cao Y, Cui Z, Tay KY, Shyong A, Shimizu E, Tsien JZ. Genome-wide gene expression profiles of the developing mouse hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(15):8862–8867. doi: 10.1073/pnas.141244998. http://doi.org/10.1073/pnas.141244998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JI, Cohen DR, Hempstead JL, Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987;237(4811):192–197. doi: 10.1126/science.3037702. [PubMed] [Cross Ref] [DOI] [PubMed] [Google Scholar]
- Muller D, Wang C, Skibo G, Toni N, Cremer H, Calaora V, Kiss JZ. PSA-NCAM is required for activity-induced synaptic plasticity. Neuron. 1996;17(3):413–422. doi: 10.1016/s0896-6273(00)80174-9. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8816705. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13(3):757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Nave BT, Ouwens M, Withers DJ, Alessi DR, Shepherd PR. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J. 2009;344:427–431. [PMC free article] [PubMed] [Google Scholar]
- Narayanan SP, Flores AI, Wang F, Macklin WB. Akt signals through the mammalian target of rapamycin pathway to regulate CNS myelination. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2009;29(21):6860–6870. doi: 10.1523/JNEUROSCI.0232-09.2009. http://doi.org/10.1523/JNEUROSCI.0232-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted JB. Microtubule-associated proteins. Annu. Rev. Cell Biol. 1986;2:421–457. doi: 10.1146/annurev.cb.02.110186.002225. [DOI] [PubMed] [Google Scholar]
- Palmer CL, Cotton L, Henley JM. The molecular pharmacology and cell biology of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. Pharmacological Reviews. 2005;57(2):253–277. doi: 10.1124/pr.57.2.7. http://doi.org/10.1124/pr.57.2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Kuhl D. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52(3):437–444. doi: 10.1016/j.neuron.2006.08.024. http://doi.org/10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Pokorný J, Yamamoto T. Postnatal ontogenesis of hippocampal CA1 area in rats. I. Development of dendritic arborisation in pyramidal neurons. Brain Research Bulletin. 1981a;7(2):113–120. doi: 10.1016/0361-9230(81)90075-7. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7272792. [DOI] [PubMed] [Google Scholar]
- Pokorný J, Yamamoto T. Postnatal ontogenesis of hippocampal CA1 area in rats. II. Development of ultrastructure in stratum lacunosum and moleculare. Brain Research Bulletin. 1981b;7(2):121–130. doi: 10.1016/0361-9230(81)90076-9. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7272793. [DOI] [PubMed] [Google Scholar]
- Popp A, Urbach A, Witte OW, Frahm C. Adult and Embryonic GAD Transcripts Are Spatiotemporally Regulated during Postnatal Development in the Rat Brain. PLoS ONE. 2009;4(2):e4371. doi: 10.1371/journal.pone.0004371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarles RH. Myelin-associated glycoprotein (MAG): past, present and beyond. Journal of Neurochemistry. 2007;100(6):1431–1448. doi: 10.1111/j.1471-4159.2006.04319.x. http://doi.org/10.1111/j.1471-4159.2006.04319.x. [DOI] [PubMed] [Google Scholar]
- Readhead C, Takasashi N, Shine HD, Saavedra R, Sidman R, Hood L. Role of myelin basic protein in the formation of central nervous system myelin. Ann. NY Acad. Sci. 1990;605:280–285. doi: 10.1111/j.1749-6632.1990.tb42401.x. [DOI] [PubMed] [Google Scholar]
- Reynolds IT, Bodine SC, Lawrence JC., Jr Control of Ser 2448 phosphorylation in the mammalian target of rapamycin by insulin and skeletal muscle load. J. Biol. Chem. 2002;277:17657–17662. doi: 10.1074/jbc.M201142200. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Morledge P. Ontogeny of contextual fear conditioning in rats: implications for consolidation, infantile amnesia, and hippocampal system function. Behav Neurosci. 1994;108:227–234. doi: 10.1037//0735-7044.108.2.227. [DOI] [PubMed] [Google Scholar]
- Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994;78(1):35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- Sánchez C, Díaz-Nido J, Avila J. Phosphorylation of microtubule-associated protein 2 (MAP2) and its relevance for the regulation of the neuronal cytoskeleton function. Progress in Neurobiology. 2000;61(2):133–168. doi: 10.1016/s0301-0082(99)00046-5. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10704996. [DOI] [PubMed] [Google Scholar]
- Schnell E, Sizemore M, Karimzadegan S, Chen L, Bredt DS, Nicoll RA. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc Natl Acad Sci U S A. 2002;99(21):13902–13907. doi: 10.1073/pnas.172511199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott PH, Brunn GJ, Kohn AD, Roth RA, Lawrence JC., Jr Evidence of insulin-stimulated phosphorylation and activation of the mammalian target of rapamycin mediated by a protein kinase B signaling pathway. Proc. Natl. Acad. Sci. USA. 1998;95:7772–7777. doi: 10.1073/pnas.95.13.7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annual Review of Neuroscience. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. http://doi.org/10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- Soghomonian JJ, Martin DL. Two isoforms of glutamate decarboxylase: why? Trends in Pharmacological Sciences. 1998;19(12):500–505. doi: 10.1016/s0165-6147(98)01270-x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9871412. [DOI] [PubMed] [Google Scholar]
- Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002;25(11):578–588. doi: 10.1016/s0166-2236(02)02270-1. [DOI] [PubMed] [Google Scholar]
- Sorra KE, Harris KM. Overview on the structure, composition, function, development, and plasticity of hippocampal dendritic spines. Hippocampus. 2000;10(5):501–511. doi: 10.1002/1098-1063(2000)10:5<501::AID-HIPO1>3.0.CO;2-T. http://doi.org/10.1002/1098-1063(2000)10:5<501::AID-HIPO1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CEL, Clark RE. The medial temporal lobe. Annual Review of Neuroscience. 2004;27(1):279–306. doi: 10.1146/annurev.neuro.27.070203.144130. http://doi.org/10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14(3):311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Talos DM, Sun H, Zhou X, Fitzgerald EC, Jackson MC, Klein PM, Jensen FE. The interaction between early life epilepsy and autistic-like behavioral consequences: a role for the mammalian target of rapamycin (mTOR) pathway. PloS One. 2012;7(5):e35885. doi: 10.1371/journal.pone.0035885. http://doi.org/10.1371/journal.pone.0035885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nature Reviews. Neuroscience. 2004;5(3):173–183. doi: 10.1038/nrn1346. http://doi.org/10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- Tischmeyer W, Grimm R. Activation of immediate early genes and memory formation. Cellular and Molecular Life Sciences: CMLS. 1999;55(4):564–574. doi: 10.1007/s000180050315. http://doi.org/10.1007/s000180050315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Adesnik H, Sekiguchi M, Zhang W, Wada K, Howe JR, Bredt DS. Stargazin modulates AMPA receptor gating and trafficking by distinct domains. Nature. 2005;435(7045):1052–1058. doi: 10.1038/nature03624. http://doi.org/10.1038/nature03624. [DOI] [PubMed] [Google Scholar]
- Travaglia A, Bisaz R, Sweet ES, Blitzer RD, Alberini CM. Infantile amnesia reflects a developmental critical period for hippocampal learning. Nature Neuroscience. 2016 doi: 10.1038/nn.4348. In press, http://dx.doi.org/10.1038/nn.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Episodic memory: from mind to brain. Annual Review of Psychology. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. http://doi.org/10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- Wahl SE, McLane LE, Bercury KK, Macklin WB, Wood TL. Mammalian target of rapamycin promotes oligodendrocyte differentiation, initiation and extent of CNS myelination. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2014;34(13):4453–4465. doi: 10.1523/JNEUROSCI.4311-13.2014. http://doi.org/10.1523/JNEUROSCI.4311-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA. A comparison of the postnatal development of post-activation potentiation in the neocortex and dentate gyrus of the rat. Brain Research. 1984;318(1):61–68. doi: 10.1016/0165-3806(84)90063-4. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/6488055. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B, Franke WW. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985;41(3):1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- Wixted JT, Squire LR. The medial temporal lobe and the attributes of memory. Trends in Cognitive Sciences. 2011;15(5):210–217. doi: 10.1016/j.tics.2011.03.005. http://doi.org/10.1016/j.tics.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley PF, Bhat RV, Baraban JM, Erickson CA, McNaughton BL, Barnes CA. Thresholds for synaptic activation of transcription factors in hippocampus: correlation with long-term enhancement. Journal of Neuroscience. 1993;13(11):4776–4786. doi: 10.1523/JNEUROSCI.13-11-04776.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Higuchi O, Ohashi K, Nagata K, Wada A, Kangawa K, Mizuno K. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998;393(6687):809–812. doi: 10.1038/31735. http://doi.org/10.1038/31735. [DOI] [PubMed] [Google Scholar]
- Yin JC, Tully T. CREB and the formation of long-term memory. Current Opinion in Neurobiology. 1996;6(2):264–268. doi: 10.1016/s0959-4388(96)80082-1. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8725970. [DOI] [PubMed] [Google Scholar]