Abstract
Mid-regional pro-adrenomedullin (MR-proADM) has a good biomarker profile: its half-life is several hours, and its plasma concentrations can be determined in clinical practice, it is essentially irrelevant, but proportionally represents the levels and activity of adrenomedullin (ADM). ADM synthesis is widely distributed in tissues, including bone, adrenal cortex, kidney, lung, blood vessels and heart. Its fundamental biological effects include vasodilator, positive inotropic, diuretic, natriuretic and bronchodilator. It has been described high levels in septic patients, interacting directly with the relaxation of vascular tone, triggering hypotension of these patients. It is also found high levels in other diseases such as hypertension, heart failure, respiratory failure, renal failure, cirrhosis and cancer. MR-proADM has been identified as a prognostic marker, stratifying the mortality risk in patients with sepsis in emergency department (ED) and ICU. Evolutionary MR-proADM levels and clearance marker to the 2nd–5th days of admission help to determine the poor performance and the risk of mortality in patients with severe sepsis admitted to the ICU. The MR-proADM levels are more effective than procalcitonin (PCT) and C-reactive protein (CRP) levels to determine an unfavorable outcome and the risk of mortality in patients with sepsis admitted to the ICU. It has also proved useful in patients diagnosed with organ dysfunction of infectious etiology. MR-proADM levels are independent of the germ conversely it is related to the magnitude of organ failure and therefore severity. We consider advisable incorporating the MR-proADM the panel of biomarkers necessary for the diagnosis and treatment of critically ill patients admitted to the ICU with severe sepsis. The combined PCT and MR-proADM levels could represent a valid tool in the clinical practice to timely identify patients with bacterial infections and guide the diagnosis and treatment of sepsis and septic shock.
Keywords: Mid-regional pro-adrenomedullin (MR-proADM), adrenomedullin (ADM), biomarker, sepsis, septic shock
Introduction
Severity in septic patients is considered when perfusion disorders or organ dysfunction are present. Sepsis is becoming more and more common and, despite considerable efforts to treat it, continues to have a high mortality rate (1,2). In these patients it is essential to initiate early support measures and specific treatments, perhaps being the most effective the early administration of antibiotics. A clear relationship has been shown between the time antibiotics are started and prognosis (3,4). It is also necessary early monitoring measures and support, so they should be admitted to the ICU, and thus improve prognosis (3-6).
The profile of a good biomarker is a molecule measurable objectively, systematically and in a precise form, identifying normal and pathological levels and being able to monitor the response to the treatment. Its properties should be: establish an early diagnosis, quantify the risk (severity, mortality) and monitor the evolution of the infection and the response to the treatment (7).
A multitude of biomarkers have been studied, but no solution to this problem has been found. Of all those described, the C-reactive protein (CRP) and procalcitonin (PCT) are the most often used (8,9). Other markers, less specific, related to systemic response, organ failure (fibrinogen, antithrombin, D-dimer, lactate, N-terminal pro-B-type natriuretic peptide) or immune dysfunction (HLA-DR, ferritin) have also been shown to be useful (10). Mid-regional pro-adrenomedullin (MR-proADM) has recently been incorporated as a biomarker.
Adrenomedullin (ADM) and MR-proADM
ADM is a peptide hormone of 52 amino acids that was isolated in 1993 from extracts of a human pheochromocytoma (11). The sequences of a messenger RNA (mRNA) and the ADM gene associated have been identified in several mammalian species. The ADM belongs to the calcitonin gene peptide superfamily: calcitonin, PCT, the calcitonin gene-related peptide (CGRP), amylin and ADM. ADM molecule has a 27% similarity to CGRP (12-14).
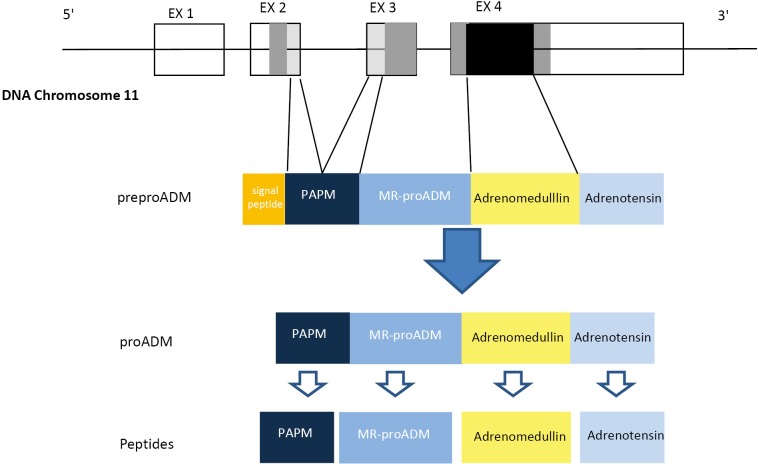
The gene for human ADM, located to a single locus on chromosome 11 consists in 4 exons and 3 introns. The mRNA encodes the information for synthesis of a preprohormone known as preproadrenomedullin, of 185-amino acids, subsequently degraded into 164-amino acids peptide called proadrenomedullin by cleaving the signal peptide. The proadrenomedullin has three vasoactive peptides, the ADM, the aminoterminal peptide of proadrenomedullin (PAMP) and adrenotensin. There is also a region without known activity, the MR-proADM (15) (Figure 1).
Figure 1.
Schematic representation of the pre-proadrenomedullin gene and biosynthesis of the peptides: aminoterminal peptide of proadrenomedullin (PAMP), the mid-regional pro-adrenomedullin (MR-proADM), adrenomedullin and adrenotensin.
ADM synthesis is widely distributed in tissues, including bone, adrenal cortex, kidney, lung, blood vessels, heart, adipose tissue, anterior pituitary, thalamus and hypothalamus. In the kidneys, it has demonstrated the presence of ADM in glomeruli, cortical and distal tubule and medullary collecting tubule (16,17). The mRNA expression of ADM in endothelial cells culture reaches concentrations 20–40 times higher than those in the adrenal gland (18,19). The ADM has been found in all epithelial surfaces separating the internal environment of the external (integument, respiratory, digestive and genito-urinary tract, etc.) and all bodily secretions such as sweat, tears, milk and bronchial washings. This distribution suggests the possibility that ADM has a function of protection against external pathogens.
In mammalian blood the ADM is linked to a binding protein, ADM binding protein-1 (AMBP-1), identical to the complement factor H (20). It has been shown that ADM, once bound to its receptor, is capable of induce an increase in cyclic AMP production and depending on the target cell on which it operates, it exerts its effect through different mechanisms of action (21). In recent years there have been identified several receptors capable of recognizing the ADM and other peptides of the same family such as amylin and CGRP. A new recently identified receptor is the CRLR (calcitonin receptor-like receptor) (22).
Studies in animals and humans indicate that the pulmonary circulation is the place of clearance of ADM. Although little is known about the metabolism of ADM, it appears that plasma endopeptidases play a role in the inactivation or degradation of ADM. The ADM plasma half-life is very short, about 22 minutes, determined by rapid degradation by proteases (23). Lewis et al. (24) demonstrated that ADM was degraded in kidney, adrenal and lung and that this effect was prevented with metalloproteinase inhibitors.
Its fundamental biological effects include vasodilator, positive inotropic, diuretic, natriuretic, bronchodilator, inhibitor of insulin secretion, aldosterone inhibitor and adrenocorticotropic hormone (ACTH) inhibitor (25).
The hypotensive effect of ADM depends on at least two mechanisms: a direct effect on vascular smooth muscle cells by increasing intracellular cAMP levels and another by stimulation of calcium-dependent nitric oxide synthesis at the endothelial cells. Recently, it has been described that a third path through calcium-activated potassium channel also could mediate the vascular muscle relaxant effect of ADM. The positive inotropic effect of this peptide appears to be mediated by an increase in cytosolic calcium, independent of cyclic AMP probably via activation of protein kinase (26).
It has been described high levels in septic patients, interacting directly with the relaxation of vascular tone, triggering hypotension of these patients (27-29). It is also found high levels in other diseases such as hypertension, heart failure, respiratory failure, renal failure, cirrhosis and cancer (30-35).
They have antimicrobial and anti-inflammatory functions, participating in the defensive mechanism of the organism to bacterial invasion. It has been found that both, ADM and PAMP, have potent microbicidal action against both Gram-positive and Gram-negative bacteria and Candida albicans (17,36-39). This activity is performed by opening the hydrophilic channels, altering the membrane permeability (39,40).
Some cytokines such as tumor necrosis factor (TNF)-α/β, interleukin-1-α/β and lipopolysaccharide, stimulate the production and release of ADM. Also, hormones such as steroids, thyroid hormones, angiotensin II, norepinephrine, substance P, endothelin-1 and bradykinin also stimulate the production and secretion of ADM. Transforming growth factor (TGF)-β, interferon γ, thrombin, and forskolin and 8-bromo-cAMP inhibit the synthesis of ADM (6,21).
ADM levels are typically underestimated because the molecules are inaccessible for biochemical analysis. Some of these problems are: rapid binding to AMBP-1 and nearby receptors, fast metabolism (endopeptidases), short half-life, small concentrations, binding to the vessel walls and surfaces (20,23,24).
MR-proADM is a fragment of 48 amino acids which splits from the final proADM molecule in a ratio of 1:1 with ADM. It is essentially irrelevant, but proportionally represents the levels and activity of ADM (41,42). Its half-life is longer, several hours, and its plasma concentrations can be determined in clinical practice. It has been identified as a prognostic marker, stratifying the mortality risk in patients with sepsis.
Clinical utility of MR-proADM levels in sepsis patients
There are limited publications on the usefulness of MR-proADM in the diagnosis and prognosis of sepsis and these have been most valuable for infections and sepsis of pulmonary origin (10,43-47). The majority of the studies analyzed isolated MR-proADM levels in the emergency department (ED) and at admission; very few studies analyzed levels during evolution of the disease. Several studies make reference to the combination of this with other biomarkers, some of them conventionally used, such as CRP and PCT (44-46,48,49), and others more recently incorporated, such as pro-vasopressin (Pro-AVP), endothelin 1 (ET-1) or soluble urokinase-type plasminogen activator receptor (suPAR) (48,50,51).
The recently published TRIAGE study (52) is a multinational, prospective, observational cohort study included consecutive medical patients presenting with a medical urgency at three tertiary-care hospitals. A total of 7,132 patients were included in the final analysis. Biomarkers of three different biological pathways were studied: MR-proADM, copeptin and PCT. It was studied as primary objective the mortality within 30 days and as secondary, the admission to the ICU within 30 days and the award of priority in the triage.
The combination of clinical information on admission ED with the results of biomarkers in the blood allows early risk stratification of individual patients. This large international study found a high precision for predicting adverse outcome and high priority treatment by measuring initial biomarker. Biomarkers improved statistical models, including comprehensive clinical information and scores triage risk. MR-proADM is the best biomarker, especially for mortality prediction. MR-proADM improves triage in the emergency room and has the potential to improve early patient management and also may reduce adverse outcomes.
Diagnostic value of initial proADM levels
The rapid diagnosis of bacterial infection and early assessment of bad prognosis are fundamental for septic patients and are the characteristics sought in a biomarker. In our experience the 13.3% of the patients diagnosed on admission of sepsis were not considered to be septic at the time of discharge from the ICU and had other diagnoses (53). The MR-proADM levels help to identify the infectious origin in patients with systemic inflammatory response syndrome (SIRS) and organ dysfunction, since in these patients the levels at admission were 10 times higher than those in no septic patients. Predictive diagnostic power was shown with an AUC of 0.9474, with an optimal cut-off value established at 1.4256 (53). Recently, Angeletti et al. (49) comparing the MR-proADM levels in septic patients and in non-septic patients with SIRS with similar results; the median values in patients with SIRS were 0.62 nmol/L and in patients with severe sepsis were 3.13 nmol/L (P>0.0001); the AUC to determine the presence of sepsis in the studied group was 0.977 and the optimal cut-off point was 1 nmol/L. Christ-Crain (54) showed that MR-proADM is a good biomarker for differentiating between septic patients and non-septic patients with SIRS. In a group of 101 critical patients, 53 septic and 48 non-septic, the MR-proADM values rose significantly according to severity: in healthy control subjects the median was 0.4 nmol/L, in patients with SIRS 1.1 nmol/L, in septic patients 1.8 nmol/L, in severely septic patients 2.8 nmol/L, in patients with septic shock 4.5 nmol/L and in those who required noradrenalin, 5.5 nmol/L. Schuetz et al. (50) determined MR-proADM levels at admission of 95 critical patients, 48 of them septic, of whom 15 were in a state of septic shock. The mean values were below 1 nmol/L for non-septic critical patients, 2.6 nmol/L for patients with sepsis and 8 nmol/L (P<0.01) for those in a state of septic shock.
Prognostic value of initial and ongoing MR-proADM levels
The mortality in our series was high, 41.36% at 90 days. The severity of the patients was significant; the mean Acute Physiology and Chronic Health Evaluation (APACHE) II score was 24.44±8.5. The patients with severe sepsis showed a gradual decline in MR-proADM values, at the expense of the survivors, with mean values of 1.268 nmol/L at the time of discharge from the ICU. The ROC curve showed an AUC of 0.565 demonstrating that, in our patients, initial MR-proADM levels were not capable of predicting mortality (53). Christ-Crain et al. (54) found significant differences in MR-proADM levels between survivors and non-survivors (8.5 vs. 1.7 nmol/L; P<0.001). The prognostic value showed an AUC of 0.81 and an optimal cut-off point of 3.9 nmol/L. We should highlight the heterogeneity of this group of critical patients, in which almost 50% of the patients did not have sepsis. Courtais (44) found that MR-proADM values were correlated with the pneumonia severity index (PSI) and were predictor of mortality in patients with high PSI scores. Suberviola et al. (45) found an AUC of 0.72 with an optimal cut-off point for MR-proADM of 4.86 nmol/L at admission in patients with sepsis of pulmonary origin. Recently, in a study by the same author (51) of 137 septic patients admitted to the ICU, the MR-proADM levels at admission were higher in the non-survivors (5.5 vs. 3.2 nmol/L; P=0.23), but ineffective in determining risk of death, with an AUC of 0.62.
DE LA Torre-Prados et al. (55), found in 100 consecutive septic shock patients, with a mortality of 36% at 28 days, the MR-proADM, lactate levels, APACHE II as well as SOFA scores significantly higher in non-survivors patients. MR-proADM showed the best association with mortality, as well as a prognostic value (logrank test: P=0.0012). Statistical significance was also seen in the Cox regression analysis (P=0.0004) for all patients with a relative risk of 1.26 times that of the baseline for each mmol/L of increase in MR-proADM.
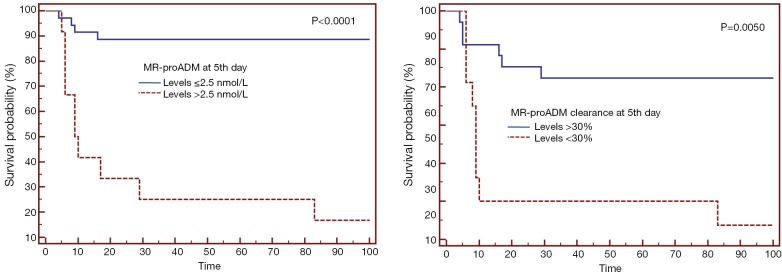
In our cohort at 48 hours following admission statistically significant differences could already be seen between survivors and non-survivors and at the 5th day following admission the differences between the two groups were greater and more significant. The ROC curve at the 5th day, with an AUC of 0.828, was enough to determine mortality. Having assessed sensitivity and specificity, the optimal cut-off point was 2.5 nmol/L with a PPV of 91% and an NPV of 92% (53) (Figure 2). Guignant et al. (48) studied the usefulness of MR-proADM and Pro-AVP at three stages of evolution: firstly during the first 1–2 days, secondly during days 3–4 and lastly during days 5–7. The greatest AUC was found during days 5–7, with a value of 0.861, similar to the values found at the 5th day in this study.
Figure 2.
Kaplan-Meier survival curve; stratification of groups of septic patients with MR-proADM levels greater or less than 2.5 nmol/L or MR-proADM clearance greater or less than 30% at the 5th day following admission in the intensive care unit (53). MR-proADM, mid-regional pro-adrenomedullin.
In the multivariate analysis, it was observed that the APACHE II score at admission, an expression of initial severity, was the only independent risk factor for mortality. The finding is similar to the results of Suberviola et al. (45) in the multivariate analysis using logistic regression, in which only APACHE II was shown to be an independent predictor of mortality after adjustment for age, SOFA, MR-proADM levels, PSI and the need for artificial respiration. At 48 hours, once antibiotic treatment had already started, the majority of surgical interventions had been performed and the patients were hemodynamically stabilized correctly. In addition to initial severity, markers of bacterial activity appeared as independent prognostic risk factors for mortality, expressed by MR-proADM levels and clearance. At a late stage of evolution in these patients, indicated in our study protocol as the 5th day, initial severity was no longer an independent prognostic factor; the predictive power of marker levels and clearance increased and factors related to comorbidity appeared, such as immune disorders and age, which could lead to exhaustion of the patient’s healing response capacity (53) (Table 1). In the multivariate analysis using a logistic regression model performed during the period of evolution during days 5–7, Guignant et al. (48) also found that MR-proADM levels and the presence of comorbidity were independent factors related to mortality.
Table 1. Endpoint 90-day mortality.
| Variable | Hazard ratio (95% CI) | Regression coefficient | Standard error | P value |
|---|---|---|---|---|
| At admission | ||||
| APACHE II | 1.1231 (1.077–1.170) | 0.116100 | 0.021190 | <0.0001 |
| At 48 hours | ||||
| APACHE II | 1.0983 (1.039–1.160) | 0.093790 | 0.028120 | 0.0009 |
| MR-proADM 48 hours | 1.0756 (1.008–1.147) | 0.729000 | 0.033310 | 0.0286 |
| At 5th day | ||||
| Age | 1.0557 (1.056–1.108) | 0.054250 | 0.024950 | 0.0297 |
| Immunodeficiency | 4.2387 (1.257–14.29) | 1.444300 | 0.623300 | 0.0205 |
| MR-proADM 5th day | 1.2199 (1.102–1.350) | 0.198800 | 0.052040 | 0.0001 |
| CI MR-proADM 5th day | 0.9959 (0.993–0.998) | −0.004091 | 0.004091 | 0.0046 |
Multivariable analysis (Cox proportional hazards models): endpoint 90-day mortality (53). APACHE II, Acute Physiology and Chronic Health Evaluation II; MR-proADM, mid-regional pro-adrenomedullin; CI MR-proADM, mid-regional pro-adrenomedullin clearance.
This disparity in the initial prognostic value could be due to the time of evolution, the treatment time and the heterogeneity of the severity of patients. At this initial stage of the process there was a difference in severity due to the extent of the infection and the delay in treatment, which will manifest as organ dysfunction expressed by high scores in the severity variables (APACHE II, SAPS II and SOFA) and which will influence the final outcome; but the therapeutic effect has still not appeared, since the treatment was only recently started and the biomarker needs time to differentiate between favorable or non-favorable evolution.
Marker clearance
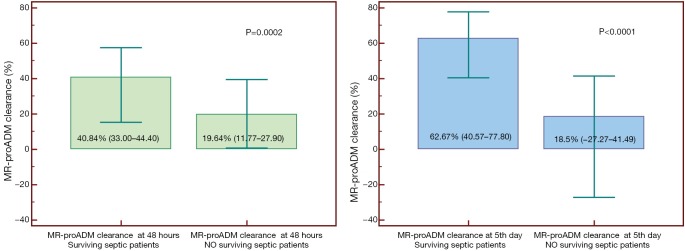
We evaluated the value of MR-proADM clearance at 48 hours and 5 days (53). This is the first study in which MR-proADM clearance has been evaluated. Other authors have shown interesting results for the clearance of other markers such as PCT (56). MR-proADM clearance at 48 hours practically doubles in survivors; In our data, not yet published, by increasing the sample to 419 patients at doubles the difference persists in survivors, statistically significant (40.84% vs. 19.64%; P=0.0002) (Figure 3). The difference in clearance of the biomarker at the 5th day is statistically significant, with an AUC of 0.718. The cut-off point of 30% showed a PPV of 58.8% and an NPV of 84.4% (53) (Figure 2).
Figure 3.
MR-proADM clearance values at 48 hours and at 5th day following admission in the ICU in a sample of 419 patients (our unpublished data). MR-proADM, mid-regional pro-adrenomedullin.
The difference in PCT clearance was greater according to the evolution of the septic patients. Ruiz-Rodríguez et al. (56) found that, at 48 hours, median PCT clearance was 70.5% in survivors and −7.29% in non-survivors (P<0.05), with an AUC of 0.86. PCT and MR-proADM have different metabolisms; the half-life of PCT is longer than that of MR-proADM (over 24 hours), whereas MR-proADM has a half-life of just a few hours; the possible elimination of infection and persistence of active organ dysfunction may be the key to the difference in biomarkers clearance.
The persistence of high values or the reduction in clearance of the biomarker at 48 hours or 5th day following admission may indicate persistence of its synthesis and release due to an active organ dysfunction, although infection has been eliminated. Also the persistence of low clearances of both biomarkers (MR-proADM and PCT) may play a key role in decision-making with regard to changing the initial antibiotic therapy and in some cases seeking other types of solution, particularly diagnostic or surgical measures. On the other hand, an increase in clearance and above all a reduction in levels at the 5th day indicate favorable evolution and would therefore help us to reduce the amount of time on antibiotics and organic support measures. There cannot be a biomarker that is useful in prognostic stratification which is not evolutionary. Theoretically, its clinical significance is only possible if it assesses treatment efficacy as well as initial severity.
MR-proADM in immunosuppressed groups and cancer
Infection in immunocompromised cancer patients could show different systemic response and therefore may change the synthesis of certain molecules involved, including biomarkers. In our series, the incidence of cancer was of 23%. The values of MR-proADM in this subgroup are similar to the global group. These results are similar to several studies in patients with hematological and solid cancer (57-59). In an extensive study of hematological patients, Al Shuaibi et al. (57) demonstrated the value of initial MR-proADM levels of septic patients and non-septic patients with SIRS; however, PCT levels were not useful in this sense. In a recent study Debiane et al. (60), investigate the utility of MR-proADM, PCT and CRP as diagnostic and prognostic biomarkers in 114 febrile critically ill patients with cancer (55 hematologic malignancy, 48.2%; 59 solid tumor, 51.8%). MR-proADM and PCT levels at follow-up (4–7 d after fever onset) were both significantly higher in patients who died during that time period than those who did not, whereas CRP level at follow-up period showed no statistical difference. The AUC for mortality prediction was 0.82 for MR-proADM and 0.77 for PCT. They concluded that MR-proADM and PCT both have a promising role in predicting bloodstream infections in a more helpful manner than CRP. These two biomarkers were superior to CRP in the prognostic analysis of response to antimicrobial therapy for those patients with documented infections. However, MR-proADM was superior to PCT in predicting response in all febrile patients and was unique in showing increased levels among no responders.
Prognostic value of MR-proADM levels according to the germ causing
It is known that the PCT levels in patients with sepsis modify depending on the seed type being the highest levels with gram negative and being lower in yeast and intracellular germs (59,61). However MR-proADM levels are independent of the germ conversely it is related to the magnitude of organ failure and therefore severity. Angeletti et al. (49), stratified 200 patients with sepsis by germs: Gram-positive, Gram-negative, yeast and polymicrobial sepsis; the AUC for MR-proADM for each were 0.967 (P<0.0001), 0.943 (P<0.0001), 0.993 (P<0.0001) and 0.983 (P<0.0001), respectively. The difference between the AUC for MR-proADM and PCT was significant in the Gram-positive and yeast, 0.0819 (P=0.0076) and 0.188 (P=0.0062).
Although the utility of this biomarker in sepsis virus has not been previously studied, recently we reported that MR-proADM plasma levels measured in the ED in adults with influenza a virus (IAV) pneumonia, without bacterial co-infection, was an effective tool to anticipate the risk of mortality and need for mechanical ventilation, being even more useful than PCT or CRP (62,63).
MR-proADM and other biomarkers
In several studies it has purchased the usefulness of biomarkers in both initial and evolutionary prognosis in septic patients, showing as superior the MR-proADM. We evaluate the usefulness of MR-proADM, PCT and CRP levels in the prognosis of severe sepsis patients admitted to the ICU. CRP, PCT and MR-proADM levels at 48 hours following admission are related to the prognosis, and MR-proADM levels are the most effective to determine unfavorable evolution and the risk of mortality in patients with severe sepsis admitted to the ICU. In the multivariate analysis (Cox proportional-hazards regression), MR-proADM levels at 48 hours following admission, were statistically significant predictive factors for mortality (53,64).
Andaluz-Ojeda et al. (65) measured PCT, lactate, CRP and MR-proADM levels at first 24 h following admission to the ICU, and again on the 3rd day and on the 7th. MR-proADM was the only biomarker showing significant differences between survivors and non-survivors for predicting mortality in the ICU. MR-proADM showed a significant association with organ failure extent at all the time points evaluated, assessed by SOFA score.
Utility of PCT and MR-proADM combined in the diagnosis and prognosis of the sepsis
The early diagnosis of bacterial infection and detection of incipient organ failure, factor for severity and poor prognosis, is crucial to start treatment and ICU admission.
Angeletti et al. (66) studied the behavior of PCT and MR-proADM at income and evolutionarily biomarkers in a group of patients with sepsis and one with localized infection. They evaluated the diagnostic capacity, response to antibiotics and prognostic value of these biomarkers individually and combined. The combined use of PCT and MR-proADM increased the post-test probability of the diagnosis of bacterial infections compared to PCT alone. In conclusion, PCT and MR-proADM combination improves the diagnosis of bacterial infection and contribute to prognosis and antibiotic therapy effectiveness.
The same authors developed a combined score derived from the combination of PCT and MR-proADM markers in patients with sepsis and localized infections and evaluate its role in the diagnosis and prognosis of these infections. PCT and MR-proADM levels concentrations in 168 septic patients, 148 patients with severe sepsis/septic shock and 66 patients with localized infections and 90 patients with SIRS due to other causes. They defined scores from 0 to 4 according to the values of PCT and MR-proADM (67,68).
The combined score was statistically significant higher in patients with severe sepsis/septic shock patients than in patients with sepsis and the SIRS patients. In localized infections, the combined score was significantly higher in comparison to patients with SIRS and healthy subjects but it was lower than patients with sepsis. In the ROC curve analysis the combined score showed an AUC of 0.96, 0.99 and 0.88 in patients with sepsis, severe sepsis/septic shock patients and localized infections, respectively, confirmed the usefulness of PCT and MR-proADM in the diagnosis and prognosis of bacterial infections, as previously reported. In conclusion, the combined score could represent a valid tool in the clinical practice to timely identify patients with bacterial infections and guide the diagnosis of sepsis and severe sepsis, conditions that require a prompt treatment.
Conclusions
The initial levels of MR-proADM help in the diagnosis of infectious origin of patients with SIRS and organ dysfunction. Evolutionary MR-proADM levels and it clearance on the 2nd–5th days of admission help us to determine the poor outcome and the risk of mortality in patients with severe sepsis admitted to the ICU. The MR-proADM levels are more effective than PCT and CRP levels to determine an unfavorable outcome and the risk of mortality in patients with sepsis admitted to the ICU.
We consider advisable to incorporate the MR-proADM to the panel of biomarkers necessary for the diagnosis and treatment of critically ill patients admitted to the ICU with severe sepsis. The combination of PCT and MR-proADM levels could represent a valid tool in the clinical practice to identify in a timely manner patients with bacterial infections and to guide the diagnosis and treatment of sepsis and septic shock.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Blanco J, Muriel-Bombín A, Sagredo V, et al. Incidence, organ dysfunction and mortality in severe sepsis: a Spanish multicenter study. Crit Care 2008;12:R158. 10.1186/cc7157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esteban A, Frutos-Vivar F, Fergusson ND, et al. Sepsis incidence and outcome: contrasting the intensive care unit with the hospital ward. Crit Care Med 2007;35:1284-9. 10.1097/01.CCM.0000260960.94300.DE [DOI] [PubMed] [Google Scholar]
- 3.Garnacho-Montero J, García-Garmendía JL, Barrero-Almodóvar AE, et al. Impact of adequate empirical antibiotic therapy on the outcome in patients admitted to the ICU with sepsis. Crit Care Med 2003;31:2742-51. 10.1097/01.CCM.0000098031.24329.10 [DOI] [PubMed] [Google Scholar]
- 4.Raman K, Nailor MD, Nicolau DP, et al. Early antibiotic discontinuation in patients with clinically suspected ventilator-associated pneumonia and negative quantitative bronchoscopy cultures. Crit Care Med 2013;41:1656-63. 10.1097/CCM.0b013e318287f713 [DOI] [PubMed] [Google Scholar]
- 5.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006;34:1589-96. 10.1097/01.CCM.0000217961.75225.E9 [DOI] [PubMed] [Google Scholar]
- 6.Julián-Jiménez A, Candel-González FJ, González Del Castillo J. Usefulness of inflammation and infection biomarkers in the Emergency Department. Enferm Infecc Microbiol Clin 2014;32:177-90. [DOI] [PubMed] [Google Scholar]
- 7.Reinhart K, Meisner M. Biomarkers in the Critically ill Patient: Procalcitonin. Crit Care Clin 2011;27:253-63. 10.1016/j.ccc.2011.01.002 [DOI] [PubMed] [Google Scholar]
- 8.Matthaiou DK, Ntani G, Kontogiorgi M, et al. An ESICM systematic review and meta-analysis of procalcitonin-guided antibiotic therapy algorithms in adult critically ill patients. Intensive Care Med 2012;38:940-49. 10.1007/s00134-012-2563-7 [DOI] [PubMed] [Google Scholar]
- 9.Casserly B, Read R, Levy MM. Multimarker panels in sepsis. Crit Care Clin 2011;27:391-405. 10.1016/j.ccc.2010.12.011 [DOI] [PubMed] [Google Scholar]
- 10.Christ-Crain M, Opal SM. Clinical review: The role of biomarkers in the diagnosis and management of community acquired pneumonia. Crit Care 2010;14:203. 10.1186/cc8155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitamura K, Kangawa K, Kawamoto M, et al. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun 1993;192:553-60. 10.1006/bbrc.1993.1451 [DOI] [PubMed] [Google Scholar]
- 12.Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Crit Care 2010;14:R15. 10.1186/cc8872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LaRosa SP, Opal SM. Biomarker: the future. Crit Care Clin 2011;27:407-19. 10.1016/j.ccc.2010.12.012 [DOI] [PubMed] [Google Scholar]
- 14.Kitamura K, Sakata J, Kangawa K, et al. Cloning and characterization of cDNA encoding a precursor for human adrenomedullin. Biochem Biophys Res Commun 1993;194:720-5. 10.1006/bbrc.1993.1881 [DOI] [PubMed] [Google Scholar]
- 15.Ishimitsu T, Kojima M, Kangawa K, et al. Genomic structure of human adrenomedullin gene. Biochem Biophys Res Commun 1994;203:631-9. 10.1006/bbrc.1994.2229 [DOI] [PubMed] [Google Scholar]
- 16.Linscheid P, Seboek D, Zulewski H, et al. Autocrine/paracrine role of inflammation-mediated calcitonin gene-related peptide and adrenomedullin expression in human adipose tissue. Endocrinology 2005;146:2699-708. 10.1210/en.2004-1424 [DOI] [PubMed] [Google Scholar]
- 17.Hinson JP, Kapas S, Smith DM. Adrenomedullin, a multifunctional regulatory peptide. Endocr Rev 2000;21:138-67. [DOI] [PubMed] [Google Scholar]
- 18.Sugo S, Minamino N, Kangawa K, et al. Endothelial cells actively synthetize and secrete adrenomedullin. Biochem Biophys Res Commun 1994;201:1160-6. 10.1006/bbrc.1994.1827 [DOI] [PubMed] [Google Scholar]
- 19.Sugo S, Minamino N, Shogi H, et al. Production and secretion of adrenomedullin from vascular smooth muscle cells: augmented production by tumor necrosis factor. Biochem Biophys Res Commun 1994;203:719-26. 10.1006/bbrc.1994.2241 [DOI] [PubMed] [Google Scholar]
- 20.Pio R, Martinez A, Unsworth EJ, et al. Complement factor H is a serum-binding protein for adrenomedullin, and the resulting complex modulates the bioactivities of both partners. J Biol Chem 2001;276:12292-300. 10.1074/jbc.M007822200 [DOI] [PubMed] [Google Scholar]
- 21.Sekine N, Takano K, Kimata-Hayashi N, et al. Adrenomedullin inhibits insulin exocytosis via pertussis toxin-sensitive G protein-coupled mechanism. Am J Physiol Endocrinol Metab 2006;291:E9-14. 10.1152/ajpendo.00213.2005 [DOI] [PubMed] [Google Scholar]
- 22.Wimalawansa SJ. Amylin, calcitonin gene-related peptide, calcitonin, and adrenomedullin: a peptide superfamily. Crit Rev Neurobiol 1997;11:167-239. 10.1615/CritRevNeurobiol.v11.i2-3.40 [DOI] [PubMed] [Google Scholar]
- 23.Meeran K, O’Shea D, Upton PD, et al. Circulating adrenomedullin does not regulate systemic blood pressure but increases plasma prolactin after intravenous infusion in humans: a pharmacokinetic study. J Clin Endocrinol Metab 1997;82:95-100. [DOI] [PubMed] [Google Scholar]
- 24.Lewis LK, Smith MW, Yandle TG, et al. Adrenomedullin(1-52) measured in human plasma by radioimmunoassay: plasma concentration, adsorption, and storage. Clin Chem 1998;44:571-7. [PubMed] [Google Scholar]
- 25.Kitamura K, Kangawa K, Eto T. Adrenomedullin and PAMP: discovery, structures, and cardiovascular functions. Microsc Res Tech 2002;57:3-13. 10.1002/jemt.10052 [DOI] [PubMed] [Google Scholar]
- 26.Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev 2004;84:903-34. 10.1152/physrev.00037.2003 [DOI] [PubMed] [Google Scholar]
- 27.Struck J, Tao C, Morgenthaler NG, et al. Identification of an adrenomedullin precursor fragment in plasma of sepsis patients. Peptides 2004;25:1369-72. 10.1016/j.peptides.2004.06.019 [DOI] [PubMed] [Google Scholar]
- 28.Eto T. A review of the biological properties and clinical implications of adrenomedullin and proadrenomedullin N-terminal 20 peptide (PAMP), hypotensive and vasodilating peptides. Peptides 2001;22:1693-711. 10.1016/S0196-9781(01)00513-7 [DOI] [PubMed] [Google Scholar]
- 29.Nishio K, Akai Y, Murao Y, et al. Increased plasma concentrations of adrenomedullin correlate with relaxation of vascular tone in patients with septic shock. Crit Care Med 1997;25:953-7. 10.1097/00003246-199706000-00010 [DOI] [PubMed] [Google Scholar]
- 30.Herrero Puente P, Fernández García D, Gil Román JJ, et al. Estudio piloto de la utilidad de la región medial de la proadrenomedulina (RM-proADM) en la valoración de la disnea de origen respiratorio en urgencias. Emergencias 2012;24:357-65. [Google Scholar]
- 31.Fábrega E, Casafont F, Crespo J, et al. Plasma adrenomedullin levels in patients with hepatic cirrhosis. Am J Gastroenterol 1997;92:1901-4. [PubMed] [Google Scholar]
- 32.Guevara M, Ginès P, Jiménez W, et al. Increased adrenomedullin levels in cirrhosis: relationship with hemodynamic abnormalities and vasoconstrictor systems. Gastroenterology 1998;114:336-43. 10.1016/S0016-5085(98)70486-X [DOI] [PubMed] [Google Scholar]
- 33.Kojima H, Tsujimoto T, Uemura M, et al. Significance of increased plasma adrenomedullin concentration in patients with cirrhosis. J Hepatol 1998;28:840-6. 10.1016/S0168-8278(98)80235-3 [DOI] [PubMed] [Google Scholar]
- 34.Pousset F, Masson F, Chavirovskaia O, et al. Plasma adrenomedullin, a new independent predictor of prognosis in patients with chronic heart failure. Eur Heart J 2000;21:1009-14. 10.1053/euhj.1999.1904 [DOI] [PubMed] [Google Scholar]
- 35.Turker Y, Aslantas Y, Turker Y, et al. A novel indicator for assessment of mitral regurgitation severity: Pro-adrenomedullin. Int J Cardiol 2013;168:2998-3000. 10.1016/j.ijcard.2013.04.007 [DOI] [PubMed] [Google Scholar]
- 36.Allaker RP, Grosvenor PW, McAnerney DC, et al. Mechanisms of adrenomedullin antimicrobial action. Peptides 2006;27:661-6. 10.1016/j.peptides.2005.09.003 [DOI] [PubMed] [Google Scholar]
- 37.Brell B, Temmesfeld-Wollbrück B, Altzschner I, et al. Adrenomedullin reduces Staphylococcus aureus alpha-toxin-induced rat ileum microcirculatory damage. Crit Care Med 2005;33:819-26. 10.1097/01.CCM.0000159194.53695.7A [DOI] [PubMed] [Google Scholar]
- 38.Allaker RP, Zihni C, Kapas S. An investigation into the antimicrobial effects of adrenomedullin on members of the skin, oral, respiratory tract and gut microflora. FEMS Immunol Med Microbiol 1999;23:289-93. 10.1111/j.1574-695X.1999.tb01250.x [DOI] [PubMed] [Google Scholar]
- 39.Walsh TJ, Martinez A, Peter J, et al. Antimicrobial activity of adrenomedullin and its gene-related peptides. Clin Infect Dis 1998;23:96. [Google Scholar]
- 40.Martínez A, Garayoa M, Pío R, et al. Adrenomedullin: a new peptide with many clinical implications. An Sist Sanit Navar 1999;22:307-15. [DOI] [PubMed] [Google Scholar]
- 41.Morgenthaler NG, Struck J, Alonso C, et al. Measurement of midregional proadrenomedullin in plasma with an immunoluminometric assay. Clin Chem 2005;51:1823-9. 10.1373/clinchem.2005.051110 [DOI] [PubMed] [Google Scholar]
- 42.Stolz D, Christ-Crain M, Morgenthaler NG, et al. Plasma pro-adrenomedullin but not plasma pro-endothelin predicts survival in exacerbations of COPD. Chest 2008;134:263-72. 10.1378/chest.08-0047 [DOI] [PubMed] [Google Scholar]
- 43.Quenot JP, Luyt CE, Roche N, et al. Role of biomarkers in the management of antibiotic therapy: an expert panel review II: clinical use of biomarkers for initiation or discontinuation of antibiotic therapy. Ann Intensive Care 2013;3:21. 10.1186/2110-5820-3-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Courtais C, Kuster N, Dupuy AM, et al. Proadrenomedullin, a useful tool for risk stratification in high Pneumonia Severity Index score community acquired pneumonia. Am J Emerg Med 2013;31:215-21. 10.1016/j.ajem.2012.07.017 [DOI] [PubMed] [Google Scholar]
- 45.Suberviola B, Castellanos-Ortega A, Llorca J, et al. Prognostic value of proadrenomedullin in severe sepsis and septic shock patients with community acquired pneumonia. Swiss Med Wkly 2012;142:w13542. [DOI] [PubMed] [Google Scholar]
- 46.Bereciartua Urbieta E, Mar Medina C, Capelastegui Sáiz A, et al. Proteína C reactiva, procalcitonina y proadrenomedulina en la evolución de neumonías hospitalizadas. Rev Lab Clin 2011;4:23-9. [Google Scholar]
- 47.Schuetz P, Wolbers M, Christ-Crain M, et al. Prohormones for prediction of adverse medical outcome in community-acquired pneumonia and lower respiratory tract infections. Crit Care 2010;14:R106. 10.1186/cc9055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guignant C, Voirin N, Venet F, et al. Assessment of pro-vasopressin and proadrenomedullin as predictors of 28-day mortality in septic shock patients. Intensive Care Med 2009;35:1859-67. 10.1007/s00134-009-1610-5 [DOI] [PubMed] [Google Scholar]
- 49.Angeletti S, Battistoni F, Fioravanti M, et al. Procalcitonin and mid-regional pro-adrenomedullin test combination in sepsis diagnosis. Clin Chem Lab Med 2013;51:1059-67. 10.1515/cclm-2012-0595 [DOI] [PubMed] [Google Scholar]
- 50.Schuetz Ph, Christ-Crain M, Morgenthaler NC, et al. Circulating precursor levels of endothelin-1 and adrenomedullin, two endothelium-derived, counteracting substances, in sepsis. Endothelium 2007;14:345-51. 10.1080/10623320701678326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suberviola B, Castellanos-Ortega A, Ruiz Ruiz A, et al. Hospital mortality prognostication in sepsis using the new biomarkers suPAR and proADM in a single determination on ICU admission. Intensive Care Med 2013;39:1945-52. 10.1007/s00134-013-3056-z [DOI] [PubMed] [Google Scholar]
- 52.Schuetz Ph, Hausfater P, Amin D, et al. TRIAGE Study group . Biomarkers from distinct biological pathways improve early risk stratification in medical emergency patients: the multinational, prospective, observational TRIAGE study. Critical Care 2015;19:377. 10.1186/s13054-015-1098-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Valenzuela Sanchez F, Valenzuela Méndez B, Bohollo de Austria R, et al. Diagnostic and prognostic usefulness of mid-regional pro-adrenomedullin levels in patients with severe sepsis. Intensive Care Med Exp 2015;3:A306 10.1186/2197-425X-3-S1-A306 [DOI] [Google Scholar]
- 54.Christ-Crain M, Morgenthaler NG, Struck J, et al. Mid-regional proadrenomedullin as a prognostic marker in sepsis: an observational study. Crit Care 2005;9:R816-24. 10.1186/cc3885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.DE LA Torre-Prados MV , Garcia-DE LA Torre A, Enguix A, et al. Mid-regional pro-adrenomedullin as prognostic biomarker in septic shock. Minerva Anestesiol 2016;82:760-6. [PubMed] [Google Scholar]
- 56.Ruiz-Rodríguez JC, Caballero J, Ruiz-Sanmartin A, et al. Usefulness of procalcitonin clearance as a prognostic biomarker in septic shock. A prospective pilot study. Med Intensiva 2012;36:475-80. 10.1016/j.medin.2011.11.024 [DOI] [PubMed] [Google Scholar]
- 57.Al Shuaibi M, Bahu RR, Chaftari AM, et al. Pro-adrenomedullin as a Novel Biomarker for Predicting Infections and Response to Antimicrobials in Febrile Patients With Hematologic Malignancies. Clin Infect Dis 2013;56:943-50. 10.1093/cid/cis1029 [DOI] [PubMed] [Google Scholar]
- 58.Hachem R, Debiane L, Shomali W, et al. Comparing biomarkers procalcitonin (PCT) and pro-adrenomedullin (proADM) as a predictor of response to antibiotics in critically ill cancer patients with bacteremia and sepsis. 49th Annual Meeting of the Infectious Diseases Society of America. Boston. October 20-23, 2011. [Google Scholar]
- 59.Jeong S, Park Y, Cho Y, et al. Diagnostic utilities of procalcitonin and C-reactive protein for the prediction of bacteremia determined by blood culture. Clin Chim Acta 2012;413:1731-6. 10.1016/j.cca.2012.06.030 [DOI] [PubMed] [Google Scholar]
- 60.Debiane L, Hachem RY, Al Wohoush I, et al. The Utility of Proadrenomedullin and Procalcitoninin Comparison to C-Reactive Protein as Predictors of Sepsis and Bloodstream Infections in Critically Ill Patients With Cancer. Crit Care Med 2014;42:2500-07. 10.1097/CCM.0000000000000526 [DOI] [PubMed] [Google Scholar]
- 61.Dou YH, Du JK, Liu HL, et al. The role of procalcitonin in the identification of invasive fungal infection-a systemic review and meta-analysis. Diagn Microbiol Infect Dis 2013;76:464-9. 10.1016/j.diagmicrobio.2013.04.023 [DOI] [PubMed] [Google Scholar]
- 62.Valenzuela Sanchez F, Valenzuela Méndez B, Rodríguez Gutierrez JF, et al. Initial levels of mr-proadrenomedullin: a predictor of severity in patients with influenza a virus pneumonia. Intensive Care Med Exp 2015;3:A832 10.1186/2197-425X-3-S1-A832 [DOI] [Google Scholar]
- 63.Valenzuela-Sánchez F, Valenzuela-Méndez B, Rodríguez-Gutiérrez JF, et al. Personalized medicine in severe influenza. Eur J Clin Microbiol Infect Dis 2016;35:893-7. 10.1007/s10096-016-2611-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Valenzuela Sanchez F, Valenzuela Méndez B, Bohollo de Austria R, et al. pro-Adrenomedullin, procalcitonin, C-reactive protein as prognostic markers of mortality in patients with severe sepsis. Intensive Care Med 2014;40:S43. [Google Scholar]
- 65.Andaluz-Ojeda D, Cicuéndez R, Calvo D, et al. Sustained value of proadrenomedullin as mortality predictor in severe sepsis. J Infect 2015;71:136-9. 10.1016/j.jinf.2015.02.002 [DOI] [PubMed] [Google Scholar]
- 66.Angeletti S, Spoto S, Fogolari M, et al. Diagnostic and prognostic role of procalcitonin (PCT) and MR-pro-Adrenomedullin (MR-proADM) in bacterial infections. APMIS 2015;123:740-8. 10.1111/apm.12406 [DOI] [PubMed] [Google Scholar]
- 67.Angeletti S, Dicuonzo G, Fioravanti M, et al. Procalcitonin, MR-Proadrenomedullin, and Cytokines Measurement in Sepsis Diagnosis: Advantages from Test Combination. Dis Markers 2015;2015:951532. [DOI] [PMC free article] [PubMed]
- 68.Angeletti S, Ciccozzi M, Fogolari M, et al. Procalcitonin and MR-proAdrenomedullin combined score in the diagnosis and prognosis of systemic and localized bacterial infections. J Infect 2016;72:395-8. 10.1016/j.jinf.2015.12.006 [DOI] [PubMed] [Google Scholar]