Abstract
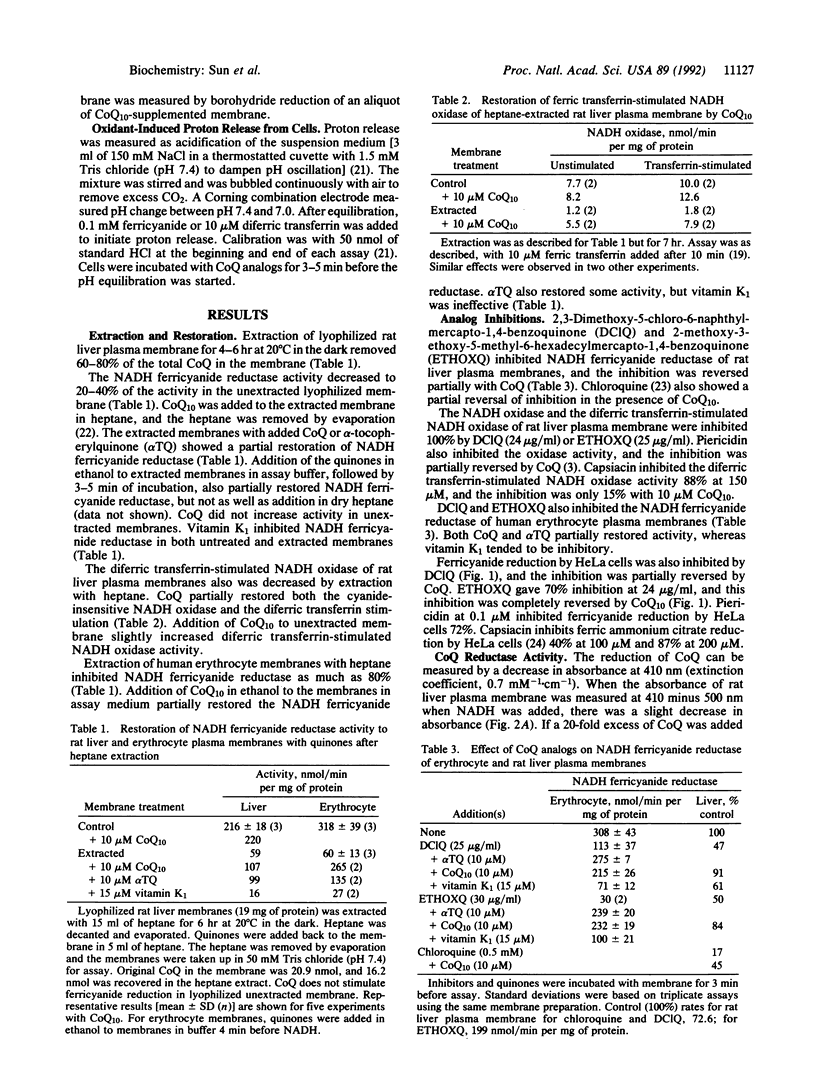
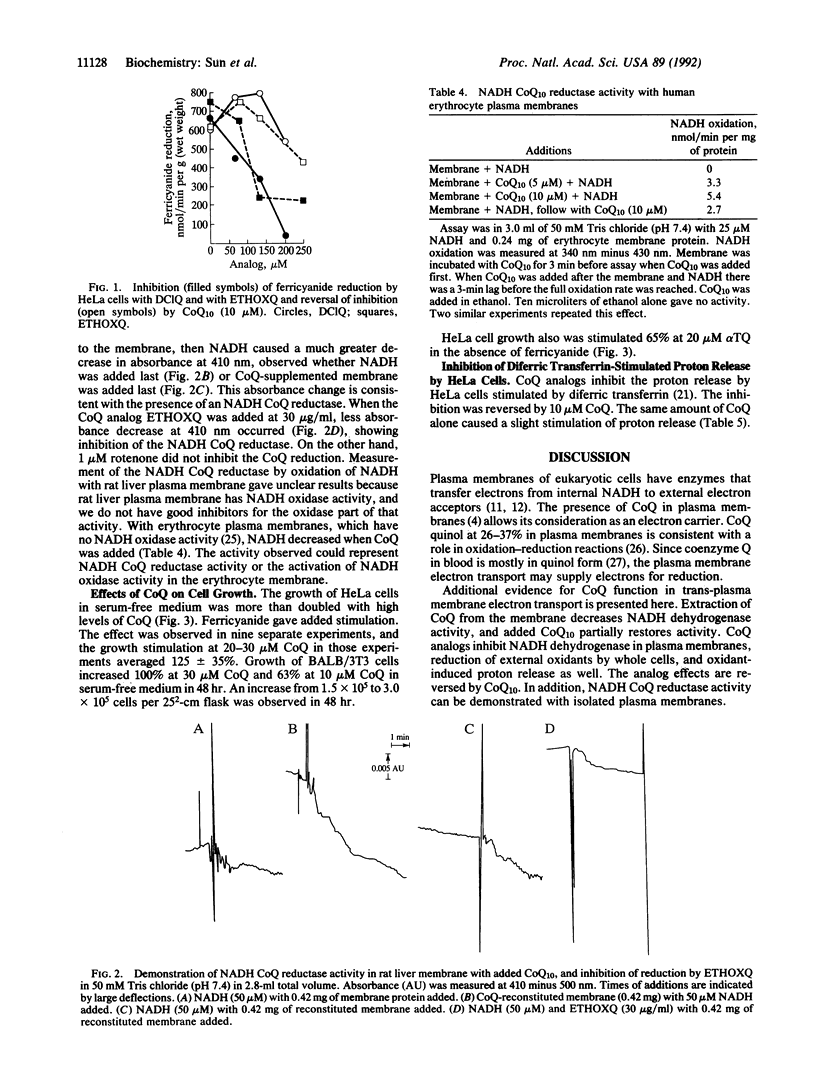
Coenzyme Q is required in the electron transport system of rat hepatocyte and human erythrocyte plasma membranes. Extraction of coenzyme Q from the membrane decreases NADH dehydrogenase and NADH:oxygen oxidoreductase activity. Addition of coenzyme Q to the extracted membrane restores the activity. Partial restoration of activity is also found with alpha-tocopherylquinone, but not with vitamin K1. Analogs of coenzyme Q inhibit NADH dehydrogenase and oxidase activity and the inhibition is reversed by added coenzyme Q. Ferricyanide reduction by transmembrane electron transport from HeLa cells is inhibited by coenzyme Q analogs and restored with added coenzyme Q10. Reduction of external ferricyanide and diferric transferrin by HeLa cells is accompanied by proton release from the cells. Inhibition of the reduction by coenzyme Q analogs also inhibits the proton release, and coenzyme Q10 restores the proton release activity. Trans-plasma membrane electron transport stimulates growth of serum-deficient cells, and added coenzyme Q10 increases growth of HeLa (human adenocarcinoma) and BALB/3T3 (mouse fibroblast) cells. The evidence is consistent with a function for coenzyme Q in a trans-plasma membrane electron transport system which influences cell growth.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CRANE F. L. Quinones in electron transport. 1. Coenzymatic activity of plastoquinone, coenzyme Q and related natural quinones. Arch Biochem Biophys. 1960 Apr;87:198–202. doi: 10.1016/0003-9861(60)90160-0. [DOI] [PubMed] [Google Scholar]
- Clark M. G., Partick E. J., Patten G. S., Crane F. L., Löw H., Grebing C. Evidence for the extracellular reduction of ferricyanide by rat liver. A trans-plasma membrane redox system. Biochem J. 1981 Dec 15;200(3):565–572. doi: 10.1042/bj2000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancis A., Roman D. G., Anderson G. J., Hinnebusch A. G., Klausner R. D. Ferric reductase of Saccharomyces cerevisiae: molecular characterization, role in iron uptake, and transcriptional control by iron. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3869–3873. doi: 10.1073/pnas.89.9.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HATEFI Y. COENZYME Q (UBIQUINONE). Adv Enzymol Relat Areas Mol Biol. 1963;25:275–328. doi: 10.1002/9780470122709.ch5. [DOI] [PubMed] [Google Scholar]
- Hall C., Wu M., Crane F. L., Takahashi H., Tamura S., Folkers K. Piericidin A: a new inhibitor of mitochondrial electron transport. Biochem Biophys Res Commun. 1966 Nov 22;25(4):373–377. doi: 10.1016/0006-291x(66)90214-2. [DOI] [PubMed] [Google Scholar]
- Harrison M. L., Rathinavelu P., Arese P., Geahlen R. L., Low P. S. Role of band 3 tyrosine phosphorylation in the regulation of erythrocyte glycolysis. J Biol Chem. 1991 Mar 5;266(7):4106–4111. [PubMed] [Google Scholar]
- Jayaraman J., Ramasarma T. Oxidation-reduction status of coenzyme Q in rat liver microsomes. Indian J Biochem. 1965 Jun;2(2):80–84. [PubMed] [Google Scholar]
- Kalén A., Norling B., Appelkvist E. L., Dallner G. Ubiquinone biosynthesis by the microsomal fraction from rat liver. Biochim Biophys Acta. 1987 Oct 8;926(1):70–78. doi: 10.1016/0304-4165(87)90183-8. [DOI] [PubMed] [Google Scholar]
- LEONHAUESER S., LEYBOLD K., KRISCH K., STAUDINGER H., GALE P. H., PAGE A. C., Jr, FOLKERS K. On the presence and significance of coenzyme Q in microsomes. Arch Biochem Biophys. 1962 Mar;96:580–582. doi: 10.1016/0003-9861(62)90340-5. [DOI] [PubMed] [Google Scholar]
- Löw H., Crane F. L., Partick E. J., Clark M. G. alpha-Adrenergic stimulation of trans-sarcolemma electron efflux in perfused rat heart. Possible regulation of Ca2+-channels by a sarcolemma redox system. Biochim Biophys Acta. 1985 Feb 21;844(2):142–148. doi: 10.1016/0167-4889(85)90084-9. [DOI] [PubMed] [Google Scholar]
- Löw H., Grebing C., Lindgren A., Tally M., Sun I. L., Crane F. L. Involvement of transferrin in the reduction of iron by the transplasma membrane electron transport system. J Bioenerg Biomembr. 1987 Oct;19(5):535–549. doi: 10.1007/BF00770036. [DOI] [PubMed] [Google Scholar]
- Maguire J. J., Kagan V., Ackrell B. A., Serbinova E., Packer L. Succinate-ubiquinone reductase linked recycling of alpha-tocopherol in reconstituted systems and mitochondria: requirement for reduced ubiquinone. Arch Biochem Biophys. 1992 Jan;292(1):47–53. doi: 10.1016/0003-9861(92)90049-3. [DOI] [PubMed] [Google Scholar]
- Morré D. J., Brightman A. O. NADH oxidase of plasma membranes. J Bioenerg Biomembr. 1991 Jun;23(3):469–489. doi: 10.1007/BF00771015. [DOI] [PubMed] [Google Scholar]
- Navas P., Nowack D. D., Morré D. J. Isolation of purified plasma membranes from cultured cells and hepatomas by two-phase partition and preparative free-flow electrophoresis. Cancer Res. 1989 Apr 15;49(8):2147–2156. [PubMed] [Google Scholar]
- Norling B., Glazek E., Nelson B. D., Ernster L. Studies with ubiquinone-depleted submitochondrial particles. Quantitative incorporation of small amounts of ubiquinone and its effects on the NADH and succinate oxidase activities. Eur J Biochem. 1974 Sep 16;47(3):475–482. doi: 10.1111/j.1432-1033.1974.tb03715.x. [DOI] [PubMed] [Google Scholar]
- Okamoto T., Matsuya T., Fukunaga Y., Kishi T., Yamagami T. Human serum ubiquinol-10 levels and relationship to serum lipids. Int J Vitam Nutr Res. 1989;59(3):288–292. [PubMed] [Google Scholar]
- SESHADRI SASTRY P., JAYARAMAN J., RAMASARMA T. Distribution of coenzyme Q in rat liver cell fractions. Nature. 1961 Feb 18;189:577–577. doi: 10.1038/189577a0. [DOI] [PubMed] [Google Scholar]
- Shimomura Y., Kawada T., Suzuki M. Capsaicin and its analogs inhibit the activity of NADH-coenzyme Q oxidoreductase of the mitochondrial respiratory chain. Arch Biochem Biophys. 1989 May 1;270(2):573–577. doi: 10.1016/0003-9861(89)90539-0. [DOI] [PubMed] [Google Scholar]
- Skelton F. S., Pardini R. S., Heidker J. C., Folkers K. Inhibition of coenzyme Q systems by chloroquine and other antimalarials. J Am Chem Soc. 1968 Sep 11;90(19):5334–5336. doi: 10.1021/ja01021a084. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Kant J. A. Preparation of impermeable ghosts and inside-out vesicles from human erythrocyte membranes. Methods Enzymol. 1974;31:172–180. doi: 10.1016/0076-6879(74)31019-1. [DOI] [PubMed] [Google Scholar]
- Stocker R., Bowry V. W., Frei B. Ubiquinol-10 protects human low density lipoprotein more efficiently against lipid peroxidation than does alpha-tocopherol. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1646–1650. doi: 10.1073/pnas.88.5.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun I. L., Crane F. L., Grebing C., Löw H. Properties of a transplasma membrane electron transport system in HeLa cells. J Bioenerg Biomembr. 1984 Dec;16(5-6):583–595. doi: 10.1007/BF00743247. [DOI] [PubMed] [Google Scholar]
- Sun I. L., Crane F. L., Löw H., Grebing C. Inhibition of plasma membrane NADH dehydrogenase by adriamycin and related anthracycline antibiotics. J Bioenerg Biomembr. 1984 Jun;16(3):209–221. doi: 10.1007/BF00751050. [DOI] [PubMed] [Google Scholar]
- Sun I. L., Navas P., Crane F. L., Morré D. J., Löw H. NADH diferric transferrin reductase in liver plasma membrane. J Biol Chem. 1987 Nov 25;262(33):15915–15921. [PubMed] [Google Scholar]
- Sun I. L., Toole-Simms W., Crane F. L., Morré D. J., Löw H., Chou J. Y. Reduction of diferric transferrin by SV40 transformed pineal cells stimulates Na+/H+ antiport activity. Biochim Biophys Acta. 1988 Feb 8;938(1):17–23. doi: 10.1016/0005-2736(88)90117-4. [DOI] [PubMed] [Google Scholar]
- Takada M., Ikenoya S., Yuzuriha T., Katayama K. Studies on reduced and oxidized coenzyme Q (ubiquinones). II. The determination of oxidation-reduction levels of coenzyme Q in mitochondria, microsomes and plasma by high-performance liquid chromatography. Biochim Biophys Acta. 1982 Feb 17;679(2):308–314. doi: 10.1016/0005-2728(82)90301-2. [DOI] [PubMed] [Google Scholar]
- Toole-Simms W., Sun I. L., Morré D. J., Crane F. L. Transplasma membrane electron and proton transport is inhibited by chloroquine. Biochem Int. 1990;21(4):761–769. [PubMed] [Google Scholar]


