Abstract
Polyunsaturated fatty acids (PUFAs) of the ω-3 and ω-6 class (e.g., α-linolenic acid, linoleic acid) are essential for maintaining biofunctions in mammalians like humans. Due to the fact that humans cannot synthesize these essential fatty acids, they must be taken up from different food sources. Classical sources for these fatty acids are porcine liver and fish oil. However, microbial lipids or single cell oils, produced by oleaginous microorganisms such as algae, fungi and bacteria, are a promising source as well. These single cell oils can be used for many valuable chemicals with applications not only for nutrition but also for fuels and are therefore an ideal basis for a bio-based economy. A crucial point for the establishment of microbial lipids utilization is the cost-effective production and purification of fuels or products of higher value. The fermentative production can be realized by submerged (SmF) or solid state fermentation (SSF). The yield and the composition of the obtained microbial lipids depend on the type of fermentation and the particular conditions (e.g., medium, pH-value, temperature, aeration, nitrogen source). From an economical point of view, waste or by-product streams can be used as cheap and renewable carbon and nitrogen sources. In general, downstream processing costs are one of the major obstacles to be solved for full economic efficiency of microbial lipids. For the extraction of lipids from microbial biomass cell disruption is most important, because efficiency of cell disruption directly influences subsequent downstream operations and overall extraction efficiencies. A multitude of cell disruption and lipid extraction methods are available, conventional as well as newly emerging methods, which will be described and discussed in terms of large scale applicability, their potential in a modern biorefinery and their influence on product quality. Furthermore, an overview is given about applications of microbial lipids or derived fatty acids with emphasis on food applications.
Keywords: single cell oil, solid-state fermentation, submerged fermentation, downstream processing, food application
Introduction
Single cell oils (SCOs) are intracellular storage lipids comprising of triacyglycerols (TAGs). SCOs are produced by oleaginous microorganisms which are able to accumulate between 20% and up to 80% lipid per dry biomass in the stationary growth phase under nutrient limitations, e.g., nitrogen or phosphor, with simultaneous excess of carbon source. Depending on the oleaginous microorganism including bacterial, yeast, microalgae or fungal species, fatty acid profile of SCOs can vary making them highly suitable for diverse industrial applications.
Considering the foreseeable depletion of crude oil, the highly controversial “food-or-fuel” discussion about using plant oils for biodiesel production, overfishing of the oceans and the urgent need for the reduction of greenhouse gas emissions, microbial SCOs seems to be intriguing substitutes for crude, plant, and fish oil. Furthermore, microbial lipid production is independent from season, climate, and location, can be realized using a wide range of carbon source, e.g., waste streams from food industry or renewable carbon sources, in case of microalgae even from CO2, does not use arable land, results in high yields and can be accomplished with genetically modified organisms changing fatty acid composition and enhancing yields. Whereas the production of very long polyunsaturated fatty acids, i.e., docosahexaenoic acid (DHA; 22:6, ω-3) and arachidonic acid (ARA; 20:4, ω-6), are commercialized using the oleaginous fungus Mortierella alpina and different oleaginous microalgae (for an overview see Ratledge, 2004), the production of biodiesel from SCO is still not economically competitive.
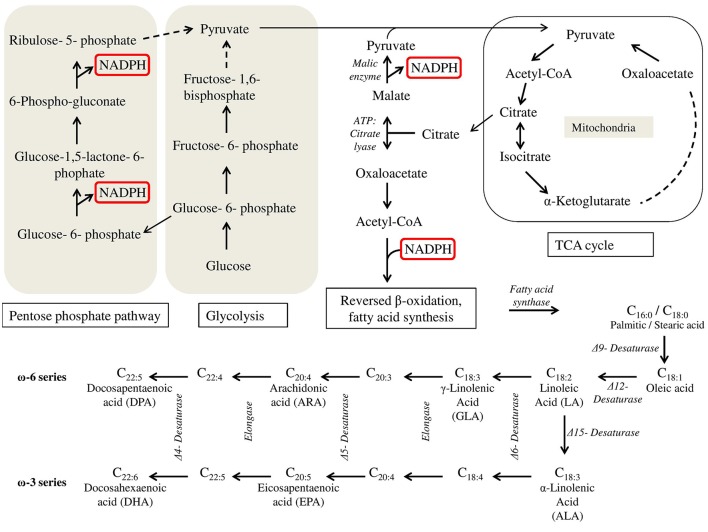
The ability of (eukaryotic) oleaginous organisms to accumulate large amount of lipids is not accounted to a difference in fatty acid biosynthesis compared to non-oleaginous species. However, a continuous supply of acetyl-CoA and NADPH for the fatty acid production by a reversed β-oxidation has to be assured under nutrient limited but carbon excess conditions. The continuous production of acetyl-CoA in oleaginous microorganisms is achieved by a cascade of enzyme reactions triggered by a nutrient limitation (in biotechnology, usually a nitrogen limitation is used) leading essentially to a citrate accumulation in the mitochondria. A unique feature of oleaginous organisms is the AMP-dependency of isocitrate dehydrogenase, an enzyme of the TCA cycle catalyzing the oxidative decarboxylation of isocitrate. In case of a nitrogen limitation, the activity of AMP deaminase, catalyzing the cleavage of AMP to IMP and ammonia, is increased considerably due to the nitrogen limitation leading to low AMP levels inside the mitochondria. As a consequence, isocitrate is not further metabolized and converted to citrate by the enzyme aconitase. Citrate is transported into the cytosol and cleaved by the enzyme ATP:citrate lyase to acetyl-CoA and oxaloacetate leading eventually to the continuous supply of acetyl-CoA for fatty acid synthesis. ATP:citrate lyase was found so far in all reported oleaginous microorganisms, however, in some non-oleaginous organisms the enzyme is also present (Botham and Ratledge, 1979; Boulton and Ratledge, 1981a,b; Evans and Ratledge, 1984, 1985; Wynn et al., 2001; Ratledge, 2002).
Besides acetyl-CoA a lot of reducing power in form of NADPH is necessary for the production of fatty acids, i.e., 16 moles of NADPH for the synthesis of stearic acid (C18). Although not finally clarified yet, malic enzyme is discussed to be mainly responsible for NADPH supply. Malic enzyme catalyzes the decarboxylation of malate (resulting from oxaloacetate) to pyruvate which is transported into the mitochondria. However, as malic enzyme activity has been reported not to be involved in NADPH regeneration in some oleaginous organisms, e.g., Yarrowia lipolytica (Zhang et al., 2013), alternative routes may also be responsible. Additionally, NADPH regeneration via the pentose phosphate pathway is also an option (Tang et al., 2015; Zhao et al., 2015). An overview about the biosynthesis is given in Figure 1.
Figure 1.
Biosynthesis of fatty acids under SCO producing conditions in oleaginous eukaryotic microorganisms (adapted and modified after Chemler et al., 2006; Tang et al., 2015).
The described fatty acid biosynthesis ends in almost every organism with the formation of palmitic (16:0) or stearic (18:0) acid. For the production of the especially desired polyunsaturated fatty acids a subsequent series of elongation and desaturation by elongases and desaturases, respectively, is necessary. Therefore, the potential of amount and type of produced PUFA is dependent on the genes of elongases and desaturases present in the genome of the respective oleaginous organism. However, only algae and fungi seem to have the ability to produce SCO containing more than 20% PUFAs making them commercially interesting.
During the last years and decades many studies and reviews dealing with SCO production have occurred. Since the commercialization of SCO production besides the mentioned PUFA production is still uneconomical, more and more researchers are focusing now on combined approaches of genetic engineering to enhance yields and productivity and the usage of low-cost substrates. However, a holistic assessment of the processes including downstream processing is often missing which is orientated on industrial scale and subsequent application of the oil. Therefore, this review aims to give an overview on processes and downstream processing methods suitable for large and industrial scale considering the limitations occurring by the final application of the product.
Microorganisms for SCO production
Lipids and oil are produced by all living macro- and microorganisms for essential structural and functional roles such as the formation of permeable membranes of cells and organelles in the form of a lipid bilayer (Dowhan and Bogdanov, 2013). However, only a relatively small number of microorganisms are able to accumulate amounts of cellular lipids over 20 or even up to 80% of their cell mass as a reserve storage material. These are termed as oleaginous microorganisms (Ratledge, 2004). The microbial production of SCO offers several advantages compared to the use of animal or plant sources. The cultivation of microorganisms is independent from geographic or climatic constraints, has short producing periods and several substrates, including industrial wastes, can be used (Ward and Singh, 2005b; Li Q. et al., 2008). The main producers of lipids are fungi, yeasts, and algae, while bacteria are bad producers (Wynn and Ratledge, 2005; Li Y. et al., 2008; Bellou et al., 2016). The lipid accumulation as a reserve storage is triggered by an excess of carbon source and one limiting nutrient, usually nitrogen. Under these conditions the carbon flux is directly channeled toward lipid synthesis and discrete oil droplets consisting of triacylglycerols are formed within the cells (Ratledge, 2004; Wynn and Ratledge, 2005). The typical course of lipid accumulation by oleaginous microorganisms is shown in Figure 2.
Figure 2.
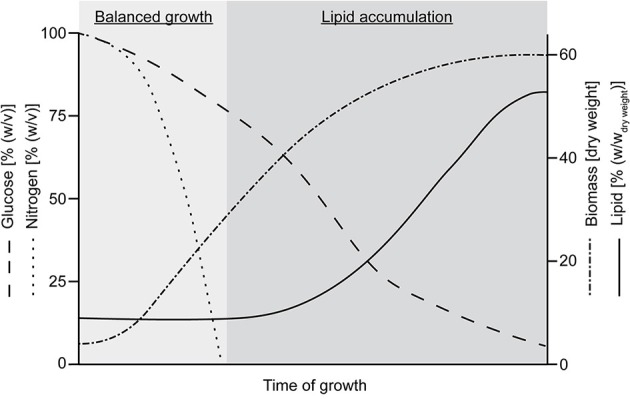
Typical course of lipid accumulation by oleaginous microorganisms (modified according to Wynn and Ratledge, 2005).
The first phase of balanced growth, where all nutrients are in excess is characterized by the production of biomass and the consumption of the carbon and nitrogen source. If the nitrogen is exhausted, the biomass production is reduced and the accumulation of lipid starts (Wynn and Ratledge, 2005). In contrast, non-oleaginous microorganisms would either stop cell division or accumulate polysaccharides, including glycogens and various mannans, glucans etc. (Ratledge, 2004). An overview on the microorganisms used for SCO production and the range of produced cellular lipids in their dried biomass is given in Table 1.
Table 1.
Overview of the genera used for the production of single cell oil (SCO) and amounts of cellular lipids accumulated per dry weight.
| Kingdom | Division | Order | Genus | SCO [% (w/wDW)] | References |
|---|---|---|---|---|---|
| Chromalveolata | Heterokontophyta | Labyrinthuales | Aurantiochytrium | 65 | Huang et al., 2012 |
| Schizochytrium | 49–67 | Chang et al., 2013; Ling et al., 2015 | |||
| Phyitales | Pythium | 76 | Cheng et al., 1999 | ||
| Fungi | Ascomycota | Eurotiales | Aspergillus | 18 | Lin et al., 2010 |
| Saccharaomycetales | Candida | 2–27 | Chatzifragkou et al., 2011 | ||
| Yarrowia | 7–43 | Papanikolaou and Aggelis, 2002; Chatzifragkou et al., 2011 | |||
| Zygosaccharomyces | 13 | Chatzifragkou et al., 2011 | |||
| Basidiomycota | Sporidiales | Rhodotorula | 22–52 | Zhao et al., 2010; Chatzifragkou et al., 2011 | |
| Sporidiobolales | Sporobolomyces | 30–50 | Matsui et al., 2011 | ||
| Tremellales | Cryptococcus | 33–78 | El-Fadaly et al., 2009; Chi et al., 2011 | ||
| Ustilaginales | Rhodosporidium | 33 | Matsakas et al., 2015 | ||
| Zygomycota | Mucorales | Cunninghamella | 21–78 | Gema et al., 2002; Fakas et al., 2009b; Chatzifragkou et al., 2010, 2011 | |
| Mucor | 18 | Chatzifragkou et al., 2011 | |||
| Thamnidium | 43 | Chatzifragkou et al., 2011 | |||
| Zygorhynchus | 42 | Chatzifragkou et al., 2011 | |||
| Mortierellas | Mortierella | 5–74 | Bajpai et al., 1991; Fakas et al., 2009b; Chatzifragkou et al., 2010; Economou et al., 2011a; Gao et al., 2013; Stressler et al., 2013; Zeng et al., 2013 |
High amounts of cellular lipids are produced by microorganisms belonging to the genera Cryptococcus, Cunninghamella, and Mortierella. The genus Mortierella is capable to produce SCO with a unique composition, containing high amounts of PUFAs (Asadi et al., 2015). M. alpina is used in an industrial process for the production of arachidonic acid (ARA, 20:4, ω-6) for food supplementation by DSM (Béligon et al., 2016).
Processes for the microbial production of SCO
The microbial production of SCO can be either conducted as submerged (SmF) or solid state fermentation (SSF).
SmF for SCO production
Table 2 summarizes culture conditions for SCO production by SmF and the cellular lipid contents obtained. The amount of lipids accumulated mainly depends on the mode of cultivation, the carbon and nitrogen source, pH and temperature.
Table 2.
SmF for the production of SCO: Species, lipid content, dry weight of biomass (DW), and cultivation conditions.
| Species | SCO [% (w/wDW)] | DW [g L−1] | Cultivation mode | Carbon source | Nitrogen source | pH [-] | Temperature [°C] | Duration | SCO Composition | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Aspergillus oryzae A-4 | 18.2 | 4.3 | Batch, flask | Glucose + cellulose | Yeast extract + (NH4)2SO4 | 5.5 | 30°C | 360 h | 27.7% PUFA | Lin et al., 2010 |
| Aurantiochytrium limacinum SR21 | 65.2 | 61.7 | Fedbatch, STR | Glycerol | Yeast extract + peptone | 6.8–7.2 | 22°C | 192 h | 66.3–87.9% PUFA | Huang et al., 2012 |
| Candida pulcherrima LFMB 1 | 1.5 | 7.3 | Batch, flask | Waste gycerol | Yeast extract + (NH4)2SO4 | 5–6 | 28°C | 63 h | 16.3% PUFA | Chatzifragkou et al., 2011 |
| Candida boidinii ATTC 32195 | 27.2 | 1.3 | Batch, flask | Waste gycerol | Yeast extract + (NH4)2SO4 | 5–6 | 28°C | 111 h | 15.6% PUFA | Chatzifragkou et al., 2011 |
| Candida curvata NRRL-Y 1511 | 6.5 | 7.9 | Batch, flask | Waste gycerol | Yeast extract + (NH4)2SO4 | 5–6 | 28°C | 137 h | 12.0% PUFA | Chatzifragkou et al., 2011 |
| Candida oleophila ATCC 20177 | 15.3 | 9.4 | Batch, flask | Waste gycerol | Yeast extract + (NH4)2SO4 | 5–6 | 28°C | 91 h | 11.4% PUFA | Chatzifragkou et al., 2011 |
| Cryptococcus curvatus NRRL-Y 1511 | 78 | 1.8 | Batch, flask | Tomato peels | NaNO3 | 5.8–6.0 | 28°C | 72 h | – | El-Fadaly et al., 2009 |
| 77 | 1.3 | Batch, flask | Glucose | Rice bran | 4.2–6.0 | 28°C | 72 h | – | El-Fadaly et al., 2009 | |
| 73 | 2.2 | Batch, flask | Potato peels | NaNO3 | 4.6–6.0 | 28°C | 72 h | – | El-Fadaly et al., 2009 | |
| 67 | 1.5 | Batch, flask | Glucose | Protelan | 4.5–6.0 | 28°C | 72 h | – | El-Fadaly et al., 2009 | |
| 64 | 2.5 | Batch, flask | Sugar cane molasses | NaNO3 | 4.9–6.0 | 28°C | 72 h | – | El-Fadaly et al., 2009 | |
| 57 | 2.8 | Batch, flask | Glucose | Corn gluten | 4.7–6.0 | 28°C | 72 h | – | El-Fadaly et al., 2009 | |
| 56 | 3.2 | Batch, flask | Sugar beet molasses | NaNO3 | 6.0–7.2 | 28°C | 72 h | – | El-Fadaly et al., 2009 | |
| 42 | 2.5 | Batch, flask | Glucose | Corn steep liquor | 5.3–6.0 | 28°C | 72 h | – | El-Fadaly et al., 2009 | |
| 34 | 3.5 | Batch, flask | Glucose | NaNO3 (control) | 5.5–6.0 | 28°C | 72 h | – | El-Fadaly et al., 2009 | |
| 33 | 3.6 | Batch, flask | Glucose (control) | NaNO3 | 5.3–6.0 | 28°C | 72 h | - | El-Fadaly et al., 2009 | |
| Cryptococcus curvatus ATCC 20509 | 75 | 168 | Fedbatch, STR | Hydrogen prduction effluent + acetic acid | NH4Cl | 7 | 30°C | 192 h | 15.6% PUFA | Chi et al., 2011 |
| Cunninghamella echinulata ATHUM 4411 | 57.7 | 7.8 | Batch, flask | Xylose | Yeast extract + (NH4)2SO4 | 5.2–6.0 | 28°C | 192 h | 6.6% GLA | Fakas et al., 2009b |
| 46.6 | 5.5 | Batch, flask | Glucose | Yeast extract | 6.0 | 28°C | 193 h | 14% GLA | Gema et al., 2002 | |
| 46 | 15 | Batch, flask | Glucose | Yeast extract + (NH4)2SO4 | 5.2–6.2 | 28°C | 360 ha | – | Fakas et al., 2009b | |
| 36.3 | 4.3 | Batch, flask | Waste gycerol | Yeast extract + (NH4)2SO4 | 5.0–6.0 | 28°C | 135 h | 26.1% PUFA | Chatzifragkou et al., 2011 | |
| 32 | 12.1 | Batch, flask | Molasse | Yeast extract + (NH4)2SO4 | 5.2–6 | 28°C | 356 h | 27.4 PUFA | Chatzifragkou et al., 2010 | |
| 30 | 12.9 | Batch, flask | Commercial glucose | Yeast extract + (NH4)2SO4 | 5.2–6 | 28°C | 309 h | 35.8% PUFA | Chatzifragkou et al., 2010 | |
| 25.6 | 7.8 | Batch, flask | Waste gycerol | Yeast extract + (NH4)2SO4 | 5.2–6.1 | 28°C | 340 h | 9.5% GLA | Chatzifragkou et al., 2011 | |
| 21 | 16.7 | Batch, flask | Commercial fructose | Yeast extract + (NH4)2SO4 | 5.2–6.0 | 28°C | 405 h | 30.6% PUFA | Chatzifragkou et al., 2010 | |
| Mortierella alpina | 49 | – | Batch, STR | Glucose | Yeast extract | – | 20°C | 13 days | 38% ARA | Stressler et al., 2013 |
| 27.3 | 16.5 | Batch, flask | Glucose | Yeast extract | – | 20°C | 8–10 days | 63% PUFA, 38% ARA | Stressler et al., 2013 | |
| Mortierella alpine ATCC 32222 | 22.3 | 15.15 | Batch, flask | Glucose | Yeast extract + KNO3 | – | 11°C | 600 h | 48.1% PUFA | Bajpai et al., 1991 |
| 17.8 | 17.5 | Batch, flask | Glucose | Yeast extract + KNO3 | – | 25°C | 360 h | 63.9% PUFA | Bajpai et al., 1991 | |
| 13.2 | 6.39 | Batch, flask | Glucose | Polypeptone + yeast extract + malt extract | – | 25°C | 360 h | 21.0% PUFA | Bajpai et al., 1991 | |
| 10.4 | 9.27 | Batch, flask | Glucose | Yeast extract | – | 25°C | 360 h | 63.2% PUFA | Bajpai et al., 1991 | |
| Mortierella isabellina ATHUM 2935 | 74 | 13.2 | Batch, flask | Commercial glucose | Yeast extract + (NH4)2SO4 | 5.2–6 | 28°C | 237 h | 8.5% PUFA | Chatzifragkou et al., 2010 |
| 72 | 17.8 | Batch, STR | Commercial glucose | Yeast extract + (NH4)2SO4 | 6.0 | 28°C | 160 h | – | Chatzifragkou et al., 2010 | |
| 65.5 | 8.7 | Batch, flask | Xylose | Yeast extract + (NH4)2SO4 | 5.2–6.3 | 28°C | 216 h | 3.9% GLA | Fakas et al., 2009b | |
| 64.3 | 6a | Batch, flask | Rice hull hydrolysate | Rice hull hydrolysate | 6.0–6.4 | 28°C | 310 h | 19.5% PUFA | Economou et al., 2011b | |
| 61 | 12.1 | Batch, flask | Commercial fructose | Yeast extract + (NH4)2SO4 | 5.2–6 | 28°C | 405 h | 12.1% PUFA | Chatzifragkou et al., 2010 | |
| 54 | 9.5 | Batch, flask | Molasse | Yeast extract + (NH4)2SO4 | 5.2–6 | 28°C | 150 h | 18.7% PUFA | Chatzifragkou et al., 2010 | |
| 53.2 | 6.2 | Batch, flask | Waste gycerol | Yeast extract + (NH4)2SO4 | 5.2–6.4 | 28°C | 264 h | 1.9% GLA | Fakas et al., 2009b | |
| 44.6 | 27 | Batch, flask | Glucose | Yeast extract + (NH4)2SO4 | 5.2–6.5 | 28°C | 360 ha | – | Fakas et al., 2009b | |
| Mortierella isabellina MUCL 15102 | 33.2 | 5.6 | Batch, flask | Waste gycerol | Yeast extract + (NH4)2SO4 | 5–6 | 28°C | 186 h | 18.9% PUFA | Chatzifragkou et al., 2011 |
| Mortierella isabellina NRRL 1757 | 66.7 | 6.0 | Batch, flask | Xylose | Yeast extract + (NH4)2SO4 | 5.5 | 28°C | 360 h | 17.8% PUFA | Zeng et al., 2013 |
| 66.5 | 8.7 | Batch, flask | Glucose | Yeast extract + (NH4)2SO4 | 5.5 | 28°C | 360 h | 14.3% PUFA | Zeng et al., 2013 | |
| 62.5 | 6.1 | Batch, flask | Fructose | Yeast extract + (NH4)2SO4 | 5.5 | 28°C | 360 h | 14.0% PUFA | Zeng et al., 2013 | |
| 51.2 | 9.4 | Batch, flask | Mannose | Yeast extract + (NH4)2SO4 | 5.5 | 28°C | 360 h | 13.7% PUFA | Zeng et al., 2013 | |
| 49.1 | 8.2 | Batch, flask | Galactose | Yeast extract + (NH4)2SO4 | 5.5 | 28°C | 360 h | 15.0% PUFA | Zeng et al., 2013 | |
| 48.2 | 5.8 | Batch, flask | Arabinose | Yeast extract + (NH4)2SO4 | 5.5 | 28°C | 360 h | 22.1% PUFA | Zeng et al., 2013 | |
| 40.9 | 5.8 | Batch, flask | Cellobiose | Yeast extract + (NH4)2SO4 | 5.5 | 28°C | 360 h | 17.1% PUFA | Zeng et al., 2013 | |
| 4.8 | 0.28 | Batch, flask | CMC | Yeast extract + (NH4)2SO4 | 5.5 | 28°C | 360 h | 43.3% PUFA | Zeng et al., 2013 | |
| 30.2 | 3.4 | Batch, flask | Ribose | Yeast extract + (NH4)2SO4 | 5.5 | 28°C | 360 h | 19.9% PUFA | Zeng et al., 2013 | |
| 16.9 | 3.7 | Batch, flask | Sucrose | Yeast extract + (NH4)2SO4 | 5.5 | 28°C | 360 h | 22.0% PUFA | Zeng et al., 2013 | |
| 64.2 | 28.8 | Batch, flask | Xylose | Yeast extract | 6.0 | 28°C | 540 h | 13.4% PUFA | Gao et al., 2013 | |
| 34 | 12.6 | Batch, flask | Straw hydrolysate | Yeast extract + (NH4)2SO5 | 5.5 | 28°C | 360 h | 11.6% PUFA | Zeng et al., 2013 | |
| Mortierella ramanniana MUCL 9235 | 37.1 | 7.3 | Batch, flask | Waste gycerol | Yeast extract + (NH4)2SO4 | 5.0–6.0 | 28°C | 216 h | 22.4% PUFA | Chatzifragkou et al., 2011 |
| Mucor sp. LGAM 365 | 18.1 | 5.3 | Batch, flask | Waste gycerol | Yeast extract + (NH4)2SO4 | 5.0–6.0 | 28°C | 237 h | 31.8% PUFA | Chatzifragkou et al., 2011 |
| Pythium irregular ATCC 10951 | 76 | 35 | Batch, flask | Glucose + Soybean oil | (NH4)2SO4 | 6.0 | 18°C | 10 days | 2.0% EPA, 1.0% ARA | Cheng et al., 1999 |
| Rhodosporidium toruloides CCT 0783 | 33 | 40 a | Batch, flask | Sorghum stalks | – | 30°C | 250 h | Matsakas et al., 2015 | ||
| Rhodotorula mucilaginosa TJY15a | 52.2 | 19.5 | Fedbatch, STR | Artichoke tuber extract hydrolysate | Yeast extract + (NH4)2SO4 | 6.0 | 30°C | 108 h | 11.3% PUFA | Zhao et al., 2010 |
| 48.8 | 14.8 | Batch, flask | Inulin hydrolysate | Yeast extract + (NH4)2SO4 | 6.0 | 28°C | 72 h | Zhao et al., 2010 | ||
| 48.6 | 14.5 | Batch, flask | Artichoke tuber extract hydrolysate | Yeast extract + (NH4)2SO4 | 6.0 | 28°C | 72 h | Zhao et al., 2010 | ||
| Rhodotorula sp. LFMB 22 | 22 | 8 | Batch, flask | Waste gycerol | Yeast extract + (NH4)2SO4 | 5.0–6.0 | 28°C | 168 h | 12.4% PUFA | Chatzifragkou et al., 2011 |
| Schizochytrium sp. LU310 | 67.3 | 60.8 | Batch, flask | Glucose | Corn steep powder + MSG | 6.5 | 28°C | 120 h | 41% DHA | Ling et al., 2015 |
| 54.6 | 46.8 | Batch, unbaffeld flasks | Glucose | Corn steep powder + MSG | 6.5 | 28°C | 120 h | 33% DHA | Ling et al., 2015 | |
| Schizochytrium sp. S31 | 49.1 | 40 a | Batch, flask | Glycerol | Yeast extract + (NH4)2SO4 | 6.8 | 28°C | 80 h | Chang et al., 2013 | |
| Sporobolomyces carnicolor O33 | 50 | 3.3 | Batch, flask | Glucose | Urea | 5.6 | – | 10 h | Matsui et al., 2011 | |
| 30 | 1.6 | Batch, flask | Glucose | (NH4)2SO4 | 5.6 | – | 7 h | Matsui et al., 2011 | ||
| Thamnidium elegans CCF 1465 | 42.6 | 6.8 | Batch, flask | Waste gycerol | Yeast extract + (NH4)2SO4 | 5.0–6.0 | 28°C | 271 h | 15.7% PUFA | Chatzifragkou et al., 2011 |
| Yarrowia lipolytica LFMB 19 | 6.8 | 6.2 | Batch, flask | Waste gycerol | Yeast extract + (NH4)2SO4 | 5.0–6.0 | 28°C | 72 h | 27.7% PUFA | Chatzifragkou et al., 2011 |
| Yarrowia lipolytica LGAM S(7)1 | 43.0 | 8.1 | Continous, STR | Glycerol | Yeast extract + (NH4)2SO4 | 6.0 | 28°C | – | – | Papanikolaou and Aggelis, 2002 |
| Zygorhynchus moelleri MUCL 1430 | 42.4 | 3.7 | Batch, flask | Waste gycerol | Yeast extract + (NH4)2SO4 | 5.0–6.0 | 28°C | 192 h | 51.2% PUFA | Chatzifragkou et al., 2011 |
| Zygosaccharomyces rouxii LFMB 3 | 12.5 | 5.5 | Batch, flask | Waste gycerol | Yeast extract + (NH4)2SO4 | 5.0–6.0 | 28°C | 168 h | 15.4% PUFA | Chatzifragkou et al., 2011 |
Estimated from figure.
The most frequently used carbon source is glucose (compare Table 2). Various mono- or disaccharides and carboxymethyl-cellulose (CMC) were tested as carbon source for Mortierella isabellina by Zeng et al. (2013). In this study cellular lipid contents above 60% were generated with xylose, glucose and fructose as substrates. CMC was a poor substrate, implying the absence of a cellulase system (Zeng et al., 2013). Cultivation of Cunninghamella echinulata and M. isabellina showed that the carbon source is a crucial parameter for the production of SCO as well as γ-linolenic acid (GLA, 18:3, ω-6). Both fungi showed satisfactory growth on glucose, fructose, and molasse, while M. isabellina failed to grow on saccharose (Chatzifragkou et al., 2010). Due to the high amounts of carbon source necessary to trigger the lipid accumulation it is economically effective to use low-cost raw materials, such as glycerol (Fakas et al., 2009b; Chatzifragkou et al., 2011; Tchakouteu et al., 2015), commercial sugars (Chatzifragkou et al., 2010), plant material (Lin et al., 2010; Economou et al., 2011b; Zeng et al., 2013; Matsakas et al., 2015), and lignocellulosic materials (Zeng et al., 2013). Glycerol, a waste product of the biodiesel production was tested for example by (Chatzifragkou et al., 2011) as a carbon source for 15 eukaryotic microorganisms. The tested yeasts accumulated up to 22% (w/w) lipids. On the contrary, the tested fungi showed cellular lipid contents of 18 to 43% (w/w). No difference between the oil accumulation of C. echinulata and M. isabellina were observed when either using raw glycerol or pure glycerol as carbon source. The successful application of plant wastes like tomato, potato or orange peels was shown by El-Fadaly et al. (2009), Gema et al. (2002), Zhao et al. (2010) resulting in cellular lipid concentrations above 50%.
Besides the selection of a suitable carbon source, the nitrogen source influences the accumulation of SCO. As well organic and inorganic nitrogen sources are used individually or in combination in the literature. These include yeast extract, urea, peptone, glycine, KNO3, NH4NO3, and (NH4)2SO4 (compare Table 2). Gao et al. (2013) investigated the influence of the nitrogen source when cultivating M. isabellina on xylose. The highest lipid accumulation (64.2%) was achieved with yeast extract. The influence of nitrogen compounds from tomato waste hydrolysate on the uptake of glucose was shown by Fakas et al. (2008). The removal of some quantities of organic nitrogen resulted in reduced glucose uptake and large amounts of biomass with low lipid content. The lipid accumulation was not effected when using glycerol as carbon source (Fakas et al., 2008). In addition to the selection of a suitable nitrogen source, the C/N ratio influences the lipid accumulation. Reported ratios range from 35 to 340 mol mol−1 (Papanikolaou et al., 2004; Fakas et al., 2009b; Ruan et al., 2012).
In principal, oleaginous microorganisms can be cultivated as batch, fed-batch or continuous cultures. Most of the reported experiments in literature are based on batch shaking flask cultivations (compare Table 2). A comparison of baffeled and unbaffeled flasks for Schizochytrium sp. showed that the use of baffled flaks increases the biomass production, the lipid accumulation and the concentration of docosahexaenoic acid (DHA, 22:6, ω-3; Ling et al., 2015). The cultivation of M. alpina in a stirred tank reactor resulted in an increase of lipid accumulated in the cells compared to shaking flasks (Stressler et al., 2013). In contrast, the lipid accumulation of M. isabellina was not affected by using a stirred tank reactor or shaking flasks, only the biomass production increased (Chatzifragkou et al., 2010).
An interesting feasibility study of an integrated process combining a heterotrophic cultivation of yeast with CO2 recycling in a phototrophic process was published by Dillschneider et al. (2014). The oleaginous yeast Cryptococcus curvatus and the oleaginous microalgae Phaeodactylum tricornutum were used and both processes revealed in lipid contents of about 40–45% (w/w).
SSF for SCO production
SSF reproduces the natural microbiological processes such as food production, composting, and ensiling (Pandey et al., 1999). In general the advantages of SSF are a higher productivity, the possibility to use low cost media, reduced energy, and waste water costs. The disadvantages are for example difficulties in scale-up, in the control of process parameters and increasing cost for product recovery (Couto and Sanromán, 2006; Asadi et al., 2015).
An overview on SSF processes for the production of SCO is shown in Table 3. The amounts of produced SCO are given as weight percent based on the dried mixture consisting of substrate and biomass and are therefore difficult to compare. The achieved values range from 1.7 to 15.8% SCO per fermented mass. High amount of nutritionally valuable PUFA were produced in SSFs using Mortierella species (Fakas et al., 2009a; Stressler et al., 2013). In order to reduce costs, agro-industrial product, or residues such as wheat straw, cereal based products (bran, straw), wastes (orange peel, pear pomace, press cake, brewers spent grain) are used as substrates for SSF processes (Conti et al., 2001; Gema et al., 2002; Fakas et al., 2009a; Jacobs et al., 2010; Lin et al., 2010; Stressler et al., 2013). In dependence on the substrate and the enzymatic activity of the production strain a pretreatment of the substrate (chemically or enzymatically) is needed to break down insoluble material into available monomers (Peng and Chen, 2008). Lin et al. (2010) showed the direct microbial conversion of cellulose and wheat straw by Aspergillus oryzae. Furthermore, the substrate is often supplemented with nitrogen to obtain an ideal C/N ratio (Table 3).
Table 3.
SSF for the production of SCO: Species, lipid content, and cultivation conditions.
| Species | SCO in fermented mass [% (w/wDW)] | Cultivation mode | Carbon source | Nitrogen source | pH [–] | Temperature [°C] | Duration | SCO Composition | References |
|---|---|---|---|---|---|---|---|---|---|
| Aspergillus oryzae A-4 | 6.3 | Batch, petri dishes | Wheat straw + wheat bran | (NH4)2SO4 | – | 30°C | 360 h | – | Lin et al., 2010 |
| Cunninghamella elegans CCF 1318 | 15.8 | Batch, flaks | Barley | Yeast extract + peptone | – | 28°C | 420 h | – | Conti et al., 2001 |
| 14.9 | Batch, flaks | Millet | Yeast extract + peptone | – | 28°C | 420 h | – | Conti et al., 2001 | |
| 9.7 | Batch, flaks | Wheat | Yeast extract + peptone | – | 28°C | 420 h | – | Conti et al., 2001 | |
| 13.8 | Batch, flaks | Rice | Yeast extract + peptone | – | 28°C | 420 h | – | Conti et al., 2001 | |
| Cunninghamella echinulata ATHUM 4411 | 1.72 | Batch, flaks | Orange peel | 4.1–6.4 | 28°C | 240 h | 5.2% GLA | Gema et al., 2002 | |
| Cunninghamella echinulata ATHUM 4411 | 2.39 | Batch, flaks | Orange peel + glucose | (NH4)2SO4 | 4.1–6.4 | 28°C | 240 h | 5.1% GLA | Gema et al., 2002 |
| Mortierella alpina | 16.4 | Batch, tablet reactor | Oat bran | – | 20°C | 360 h | 52% ARA, 74% PUFA | Stressler et al., 2013 | |
| Mortierella isabellina ATHUM 2935 | 12 | Batch, petri dishes | Pear pomace | 6.5 | 28°C | 212 h | ~27% PUFA | Fakas et al., 2009a | |
| 9–11 | Batch, petri dishes | Crusted sweet sorghum | 6 | 28°C | 192 h | – | Economou et al., 2010 | ||
The genus Mortierella for PUFA production
As aforementioned, isolates of the genus Mortierella are excellent producers of SCO with high amounts of PUFAs (Bajpai et al., 1991; Stressler et al., 2013; Asadi et al., 2015). For the use in human nutrition PUFAs are of a special interest due to the high nutritional value (see Section Human Nutrition and Food Application). The genus Mortierella can be divided into two subgenera Mortierella (M. alpina, M. hyalina, M. elongata) and Micromucor (M. ramanniana, M. isabellina, M. vinaces), varying in their composition of SCO (Dyal and Narine, 2005). High amounts of ARA up to 70% are produced by M. alpina. The genera M. hyalina and M. elongata produce up to 23% ARA and tend to have higher concentrations of oleic acid (18:1; Dyal and Narine, 2005). Isolates belonging to the subgenera Micromucor accumulate high proportions of linoleic acid (LA; 18:2, ω-6) (up to 25%) and GLA (up to 31%). However, they tend to the production of high concentrations of oleic acid (Dyal and Narine, 2005). Strains of M. alpina were investigated by several researchers. Bajpai et al. (1991) achieved in a submerged fermentation of M. alpina ATCC 32222 9.6 g ARA per 100 g dried biomass (54% of total SCO) when using glucose and yeast extract as carbon and nitrogen source, respectively. The cultivation of M. alpina ATCC 32222 on soluble starch as carbon source ended up in 14.3 g ARA per 100 g dried biomass (66.4% of total PUFAs). Stressler et al. (2013) used an own isolated strain of M. alpina for the production of PUFAs by submerged fermentation. After 10 days of cultivation on glucose and yeast extract 10.3 g ARA per 100 g biodrymass (38% of SCO) were received.
Microalgae as PUFA producers
Besides Mortierella species microalgae are also important producers of valuable PUFAs essential for human nutrition like EPA, DHA, and ARA (an overview is given by Yap and Chen, 2001; Huang et al., 2013). The most prominent DHA producer amongst microalgae is the heterotrophic dinoflaggelate Crypthecodinium cohnii containing more than 50% (w/w) DHA of total fatty acids (Jiang and Chen, 2000; Ratledge et al., 2001; de Swaaf et al., 2003a,b) and is also used commercially (DHASCO™; see Section Human Nutrition and Food Application). Other significant DHA producers are green microalgae of the genus Schizochytrium, e.g., Schizochytrium sp. S31 (Wu et al., 2005), Schizochytrium G13/2S (Ganuza and Izquierdo, 2007), and Schizochytrium limacinum (Chi et al., 2007).
An indisputable advantage of autotrophic microalgae is the ability to produce lipids from CO2 and sunlight. In non-sterile open pond systems microalgae can be cultivated in large scale under natural growth conditions and minimal costs for construction and operation. However, suboptimal cultivation conditions like low dissolved CO2 concentrations and inconsistent light intensities result in low cell densities making downstream processing cost-intensive. Furthermore, open pond systems are restricted to a limited number of species, either very robust e.g., toward high salinity or very fast-growing to be successful against competitive species (Yap and Chen, 2001; Ratledge and Cohen, 2008). Usually, bulk products for human diet supplement such as carotenoids or biomass, sold as powdered algae/cyanobacteria (e.g., Spirulina) are produced in open pond systems. However, high-value products like highly purified PUFAs for human nutrition can also be produced economically in well-controlled environments like photobioreactors. The high achievable prizes of the product justify higher production costs. In photobioreactor higher cell-densities can be reached while using significantly less space compared to open pond systems (Menetrez, 2012).
Downstream processing
Downstream processing costs are one of the major obstacles to be solved for full economic efficiency of microbial lipids. Because single cell oils are formed intracellular for storage purposes, they have to be extracted upon further applications as long as production strains are not engineered to excrete TAGs or free fatty acids which would drastically simplify downstream processing. However, metabolic engineering efforts for secretion of TAGs from oleaginous strains have not been reported yet, but for engineered Escherichia coli (Lu et al., 2008; Lennen and Pfleger, 2012; Liu et al., 2012; Meng et al., 2013; Xu et al., 2013) and Sacharomyces cerevisiae (Michinaka et al., 2003; Leber et al., 2015) strains secreting free fatty acids.
For the extraction of lipids from microbial biomass cell disruption is most important, because efficiency of cell disruption directly influences subsequent downstream operations and overall extraction efficiencies (Senanayake and Fichtali, 2006). A multitude of cell disruption and lipid extraction methods are available which can be roughly divided in mechanical and non-mechanical methods. Nevertheless, depending on microorganism, scale, economics, and lipid application the method spectrum is narrowed to a few. Consequently, microbial lipids applied in food industry cannot be extracted with toxic solvents or should in the best case avoid any solvents to prevent solvent residues in food or contaminations with heavy metals (Uematsu et al., 2002; Sahena et al., 2009). Also, some methods may be highly suitable for analytical purposes but may not be applicable in industrial large scale operations due to high costs or simply a non-scalable extraction set-up. Additionally, the optimal method in terms of recovery can vary with each production strain and has to be elucidated for each strain separately as shown in comparative studies with microalgae (Lee et al., 2010; Li et al., 2014). Considering all limitations the optimal extraction method should enable a rapid, reproducible, quantitative, cost-effective, and non-toxic removal of lipids under mild conditions to prevent oxidative damage to polyunsaturated fatty acids.
Literature comparing systematically large scale cell disruption and extraction methods is scarce. Nevertheless, as microalgae oil production seems to receive more and more attention during the last years several recent comparative studies are now available dealing with downstream processing. For oleaginous filamentous fungi literature is even less found. Most of the studies are comparing different solvent extraction methods but pretreat the in liquid N2-frozen mycelium mechanically with mortar and pestle on bench scale which cannot be adapted to larger scales but is maybe somehow comparable to bead milling.
In the following an overview is given about common cell disruption and extraction methods (summarized also in Table 4).
Table 4.
Summary and comparison of different cell disruption and extraction methods.
| Method | Advantages | Disadvantages | Scalability | Remarks |
|---|---|---|---|---|
| CELL DISRUPTION METHODS | ||||
| Mechanical methods | Species independent, effective, no product contamination | |||
| Bead milling | Simple and efficient | Less efficient for bacteria | From lab to industrial scale | |
| Homogenization | Well-established in industry for other applications | Less suitable for filamentous fungi | To industrial scale | |
| Ultrasound | Continous operation possible | Heat generation and radical formation | Large scale not possible | |
| Physical methods | Limited scalability | |||
| Decompression | Gentle technique, minimizes chemical and physical stresses, and heat development | Less suitable for cell with tough cell wall, e.g., yeast, fungi and spores | Potentially larger scales | |
| Osmotic shock | Gentle technique, microorganims with cell walls are only weakened, not destroyed | High costs of additives | Smale scale only | |
| Microwaves | No drying necessary, quick, and inexpensive | Heat development, free radicals | Industrial scale for other applications | |
| Pulsed electrical field | Cell suspension has to be free of ions, cell disruption decreases gradually | Potentially larger scales | ||
| Drying | Easily scalable | Energy demands depend on method, potentially very energy intensive, yeasts and plant cell only poorly affected | Industrial scale for other applications | Crucial for effective downstream processing, conservative effect |
| Chemical methods | Contamination of the products, unsuitable for some applications | |||
| Solvents | Possibly combines cell disruption and extraction | Cell walls of most microorganisms are usually impermeable to most solvents, large amounts of solvents necessary | Industrial scale | |
| In situ transesterifica-tion | Combines cell disruption, lipid extraction and transesterification | Chemical modification of the product –> suitable for analytical means or biodiesel production | Easily scalable | |
| Enyzmes | Mild reaction conditions, substrate specific, environmental friendly, safe for food applications | Specific enzyme cocktails needed for every microorganism, possible very expensive | Large scale application possible but dependent on enzyme costs | |
| EXTRACTION METHODS | ||||
| Classical methods | Established procedures | Requires high amounts on solvents | Analytical to industrial scale | |
| Soxhlet | automated systems available, combinable with other methods | requires a lot of time and high amounts of solvents, not suitable for thermosensitive compounds | Analytical to large scale | |
| Bligh and Dyer | Requires less solvents than Folch methods, also wet samples extractable | The unmodified method underestimates significantly lipid content for samples with < 2% lipid content | Analytical to large scale | |
| Folch | Standard technique for total lipid extraction, very liable | Needs dry samples, higher amounts of solvents than Bligh and Dyer | Analytical to large scale | |
| Pressurized liquid extraction | Enhanced extraction performance due to enhanced solubility and mass transfer properties, 5-fold faster and requires 20-fold less solvent than Bligh and Dyer, automated | High investment costs | Potentially large scale | |
| Supercritical fluid extraction (CO2) | Extraction can be performed at low temperatures, enabling a gentle extraction of thermosensitive compounds, protection against oxidation, environmental friendly | Moisture content of the sample hinders extraction efficiency, high investment costs | Industrial scale for other applications | |
Mechanical disruption methods
Cell disruption by mechanical methods are achieved by high stress to the cells due to cavitation, shear and/or impingement using high pressure, abrasion, or ultrasound resulting in non-specific cell wall breakdown (Chisti and Moo-Young, 1986; Harrison, 1991; Middelberg, 1995; Geciova et al., 2002). They are often combined with solvent extraction. In terms of cell disruption effectiveness, mechanical methods have great industrial potential as these methods seem to be less dependent on species (Klimek-Ochab et al., 2011; Lee et al., 2012) and many are applicable on industrial scale. Contamination with chemicals of the product lipid is unlikely as long as no chemicals are used for pretreatment. However, employing mechanical methods result in heat generation making cooling necessary in order to prevent damage to heat sensitive lipids (Geciova et al., 2002; Lee et al., 2010; Lee et al., 2012). Most of the currently used equipment was originally designed for other commercial purposes such as the homogenization and size reduction of paint and milk, but has been successfully adapted for cell disruption means (Chisti and Moo-Young, 1986). Oil recovery by mechanical pressing as it is commonly used for oilseeds like sunflower or rapeseed is not effective for microorganisms due to their small size enabling bypassing the press. Furthermore, the obligatory drying process prior to pressing requires a lot of energy (de Boer et al., 2012).
Bead milling
Cell disruption by bead milling is simple, effective, and suitable for a wide range of microorganisms. Cells are disintegrated by the impact of grinding beads and biomass as well as by compaction and shearing actions and the resulting energy transfer (Middelberg, 1995). Disruption efficiency is further depending on bead size and type, agitator velocity, flow rate, cell concentrations, bead loading, and microorganism (Chisti and Moo-Young, 1986; Middelberg, 1995; Doucha and Lívanský, 2008). Various types of bead mills are available both for lab-scale and industrial-scale. Effective cell disruption have been successfully demonstrated for yeast species, e.g., S. cerevisiae, S. carlsbergensis, Candida boidinii (Limon-Lason et al., 1979; Schütte et al., 1983; Hummel and Kula, 1989; van Gaver and Huyghebaert, 1991; Heim et al., 2007), algal species, e.g., Chlorella P12, Botryococcus sp., Chlorella vulgaris, Scenedesmus sp. (Doucha and Lívanský, 2008; Lee et al., 2010), fungal species, e.g., Rhodotorula gracilis, Aspergillus fumigatus, Penicillium citrinum (Klimek-Ochab et al., 2011), as well as for bacterial species, e.g., Bacillus cereus, E. coli, Rhodococcus sp. (Hummel and Kula, 1989). However, disruption efficiency for bacteria is considerably lower, due to the smaller cell dimension (Schütte et al., 1983). In terms of energy consumption, bead milling is more efficient, when high biomass concentrations are processed and the extracted products can be easily separated afterwards (Greenwell et al., 2010). To recover microbial oils, solvent extraction is generally performed subsequently to the bead milling process. Bead milling prior to solvent extraction has been shown to enhance oil recovery for the oleaginous yeast C. curvatus compared to treatment with microwaves or autoclaving. For the oleaginous fungus M. isabellina bead milling resulted in acceptable oil recovery but was surpassed by direct Soxhlet extraction of the mycelium (Yu et al., 2015).
Homogenization
Beside the original purpose of high-pressure homogenizers to mix, disperse, and reduce particle size in emulsions and suspensions, the adaption to cell disruption in biotechnology for the release of intracellular products has been successfully realized and can be applied on large scale. During the homogenization process, biomass is forced under high pressure through an orifice (Chisti and Moo-Young, 1986; Middelberg, 1995). The exact mechanisms and causes for cell disruption are still conversely discussed and were summarized by Clarke et al. (2010). Cell disruption efficiency is dependent on applied pressure, number of passes and organisms (Chisti and Moo-Young, 1986) and has been used successfully for yeast species, e.g., S. cervisiae (Spiden et al., 2013), algae species, e.g., Chlorococcum sp. (Halim et al., 2012), bacterial species, e.g., E. coli (Ling et al., 1997), and also some filamentous fungi, e.g., Rhizopus nigricans (Keshavarz et al., 1990). However, disruption of highly filamentous fungi by high-pressure homogenization have been found not very suitable due to blocking and clogging of the homogenizing valve by mycelium and fungal pellets (Chisti and Moo-Young, 1986; Hopkins, 1994; Middelberg, 1995) which can be prevented by using low biomass concentrations (Keshavarz et al., 1990). As a pretreatment for microbial lipid extraction high-pressure homogenization was applied to the oleaginous yeasts C. curvatus (Thiru et al., 2011) and Pichia kudriavzevii (Sankh et al., 2013), but was not compared to other pretreatment methods for its efficiency in SCO recovery. It has to be considered, however, that homogenization was originally applied to form emulsions. Therefore, the mixture of wet cells, SCOs and other intracellular lipids, especially phospholipids, is forming a very stable emulsion which is not easy to break complicating considerably lipid recovery.
Ultrasound
Ultrasound using frequencies around 25 kHz is another liquid-shear method which is frequently used in other industries, e.g., emulsification, degassing, or defoaming, and was found to be suitable for cell disruption. The most relevant effects of ultrasound on microorganisms are mechanical and can be attributed to cavitation, meaning the formation, growth, and collapse of gas bubbles. The collapse of gas bubbles results in shock waves creating liquid shear forces which disrupt cells (Chisti and Moo-Young, 1986; Thompson and Doraiswamy, 1999). However, the cavitation process generates high temperatures making on the one hand cooling necessary and on the other hand promotes radical formation enabling a wide range of chemical reactions with possible damage to the product (Suslick, 1989; Lee et al., 2012). Although it is possible to operate sonicators batch-wise or even continuously with minimal heat production (James et al., 1972; Borthwick et al., 2005), application for large-scale cell disruption is not feasible due to the difficulty of energy transmission to larger volumes (Chisti and Moo-Young, 1986). For an efficient cell disruption optimization of sonication time, cell density, power input, cycle number, and operation mode (batch-wise or continuous) has to be performed for the respective microorganisms. By optimizing sonication conditions an effective cell disruption was shown for the microalgae Scenedesmus dimorphus and Nannochloropsis oculata (McMillan et al., 2013; Wang and Yuan, 2015), the yeast S. cerevisiae (Jayakar and Singhal, 2012), the bacterium E. coli (Ho et al., 2006), and the filamentous fungi P. citrinum and A. fumigatus (Klimek-Ochab et al., 2011). However, due to the different definitions and measurements of the respective studies regarding cell disruption a general statement about the disruption suitability and efficiency of sonication is difficult. In some studies disruption efficiency is measured by the release of specific enzymes or proteins in general or other metabolites, in other studies it is evaluated by examination of the cells under the microscope as done by McMillan et al. (2013). An evaluation of cell morphology under the microscope might be better suited for a conclusion regarding cell disruption, but the release of metabolites or the activity of a specific enzyme might be better suited for the evaluation whether sonication is a suitable method for the extraction of the respective product. However, to enhance product release from a cell, a complete cell disruption might not be necessary. Permeabilisation of the cell in combination with another extraction step might be sufficient. In case of lipid extraction sonication is used in combination with solvent extraction and often called ultrasonic-assisted extraction. For C. curvatus ultrasonic assisted extraction of SCO has been shown to be beneficial in comparison to other methods, e.g., microwave-assisted, but for M. isabellina or Chlorella sorokiniana sonication proved not to be suitable (Yu et al., 2015). Ultrasonic pretreatment for lipid extraction can be problematic as it may affect the quality of lipids negatively for example by lipid oxidation of polyunsaturated fatty acids by free radicals (Gerde et al., 2012).
Non-mechanical disruption methods
Cell disruption in a gentler way with the possibility of a more specific targeting of cell wall components can be achieved by non-mechanical methods. These methods require less energy but their application is often restricted to small scale processes due to limitations in process economy and efficiency (Klimek-Ochab et al., 2011).
Physical methods
Several physical disruption methods are available, including decompression, osmotic shock, microwave-treatment, pulsed electrical fields, and (freeze)-drying. However, applicability to process scale is limited for most of these methods.
Cell disruption by decompression is achieved by mixing cell suspension with pressurized supercritical gas and subsequent release of the pressure. The gas which has entered the cells expands upon pressure release and causes cell disruption due to the high pressure. It is a gentle technique, minimizing chemical and physical stresses as well as heat development and has the potential to be applied to larger scales (Middelberg, 1995; Simpson, 2010). Different gases can be used, e.g., nitrogen or carbon dioxide. Decompression with nitrogen has been shown effective for cell disruption of mammalian cells, plant cells, or bacteria, but less suitable for cell with tough cell wall, e.g., yeast, fungi, and spores (Simpson, 2010). For the disruption of wet yeast cells decompression with carbon dioxide was highly efficient, whereas under the same optimized conditions (pressure, temperature, and duration) dry cells were only poorly destroyed as well as when applying nitrogen for decompression. The low suitability of nitrogen was explained by its limited solubility in water (Nakamura et al., 1994). However, the inert gas nitrogen does not influence pH in contrast to carbon dioxide; furthermore, labile cell components are protected from oxidation (Simpson, 2010). Supercritical carbon dioxide can also be used for lipid extraction and will be discussed in Section Extraction Methods.
Osmotic shock is applied by exposing cells to a medium containing high concentration of a solute, e.g., salt or sugar exerting a high osmotic pressure and the subsequent sudden dilution of the medium resulting in an increase in intracellular pressure. Microorganisms with cell walls are not destroyed by osmotic shock, but weakened. Due to high costs of additives this methods is restricted to small scale applications (Middelberg, 1995).
Microwaves are oscillating non-ionizing electromagnetic waves with frequencies between 300 MHz and 300 GHz generating heat in dielectric or polar material by electric field-induced polarization and reorientation of molecules causing friction. The most effective range of dielectric heating are microwaves with frequencies between 915 MHz (for industrial application with greater penetration depth) and 2450 MHz (domestic microwave ovens and extraction applications; Leonelli and Mason, 2010; Routray and Orsat, 2012). The high cell disruption potential of microwaves is based on their interaction with the abundant free water molecules within cells resulting in localized heating, i.e., a sudden non-uniform temperature rise especially e.g., in vacuoles where free water is available in larger portions. Due to the volume expansion of the heated water intracellular pressure increases followed by spontaneously cell rupture (Chuanbin et al., 1998). The combination of microwave treatment with solvent extraction (microwave-assisted solvent extraction) has the potential of a quick and inexpensive method of lipid extraction as amounts of solvents can be reduced, drying of biomass prior to extraction is not necessary and yield can be increased compared to a simple thermal treatment (Cravotto et al., 2008; Balasubramanian et al., 2011; Mercer and Armenta, 2011; Rakesh et al., 2015). As microwave chemistry is already applied in many fields in industrial scale, e.g., drying, defrosting, food lyophilization, or devulcanization of rubber (Leonelli and Mason, 2010) application of microwave assisted solvent extraction of microbial lipids should be possible in large scales. However, similar to ultrasonic-assisted extraction, development of heat, and free-radicals as well as possible chemical conversions might damage polyunsaturated fatty acids influencing product quality (Yoshida et al., 1990; Günerken et al., 2015).
Cell disruption using a pulsed electrical field treatment is essentially accomplished by pore formation in cell membrane and cell wall as used for DNA transformation experiments via electroporation (Ho and Mittal, 1996). Besides cell destruction, pulsed electrical field treatment also leads to a temperature increase effecting and destroying intracellular molecules as well as an increase in lipid extraction (Sheng et al., 2011a; Zbinden et al., 2013; Lai et al., 2014). Pulsed electrical field treatment is promising in combination of extraction methods to recover lipids with the potential of large scale application (10,000 L/h capacity, Diversified Technologies, 2010) as well as continuous lipid extraction systems (Flisar et al., 2014). Like microwave treatment, wet cells can be used, however, cell suspension has to be free of ions, making washing steps, and/or deionization steps necessary. Additionally, cell disruption decreases gradually due to an increase of conductivity by the release of intracellular compounds (Günerken et al., 2015).
Drying of biomass is often done prior to other cell disruption and/or extraction techniques. In fact, most studies in this review use dry biomass for further downstream processing or analytics and drying is seen as crucial for effective downstream processing with very few exceptions. However, the drying methods (sun drying, oven drying, or freeze drying) had very little effect on the subsequent cell disruption technique when recovering lipid from Scenedesmus sp. as reported by Guldhe et al. (2014). In combination of thawing, drying itself can be a cell disruption method, though. During the process of slow freezing, large ice crystals are formed within the cell causing the rupture of intracellular membrane compartments. This method is inexpensive and easily scalable; however, plant cells need a large number of freeze-and-thaw-cycles, whereas yeast cells are only poorly affected (Hopkins, 1994).
Chemical methods
Cell disruption or permeabilization can be accomplished by a variety of chemicals, e.g., antibiotics, chelating agents, chaotropes, detergents, solvents, alkalis, and acids with different selectivity, efficiency and mode on action to the respective cell wall components of different microorganisms. Many chemical treatments are excluded in food applications or make at least an intensive downstream-processing necessary as the chemicals contaminate the products and are often of non-food grade (Middelberg, 1995; Geciova et al., 2002). Harsh chemical conditions may also damage the product.
Application of solvents, however, offers the possibility of combining cell disruption and lipid extraction without further pretreatment. Classical methods like Bligh and Dyer (1959) and Folch et al. (1957) can be used for wet and dry biomass but uses large amounts of organic hazardous solvents, like chloroform and methanol. Adaptations of these methods using less toxic solvents, like hexane/isopropanol, have been developed, though (Hara and Radin, 1978). Lipid extraction from microalgae using aqueous isopropanol (Yao et al., 2013) and ethanol (Yang et al., 2014) has been successfully shown. However, cell walls of most microorganisms are usually impermeable to most solvents (Sobus and Homlund, 1976; Jacob, 1992; Lee et al., 1998; Ryckebosch et al., 2012), therefore, at least a cell conditioning or pre-treatment has to be applied prior to solvent treatment to enhance solvent contact and extraction efficiency (Jacob, 1992). Different solvent extraction methods will be explained in detail in Section Extraction Methods.
Acid catalyzed in situ transesterification of either wet (Johnson and Wen, 2009; Cerón-García et al., 2013; Kim et al., 2015; Macías-Sánchez et al., 2015) or dry biomass (Liu and Zhao, 2007; Johnson and Wen, 2009; D'Oca et al., 2011; Li et al., 2011; Wahlen et al., 2011; Xu and Mi, 2011) combines cell disruption, lipid extraction, and transesterification to fatty acid methyl or ethyl esters (FAME or FAEE, respectively) for biodiesel production. Although studied extensively with oleaginous microalgae, in situ transesterification as also applied to oleaginous yeast, e.g., Lipomyces starkeyi, Rhodosporidium toruloides (Liu and Zhao, 2007), and fungi, e.g., M. isabellina, Aspergillus candidus (Liu and Zhao, 2007; Kakkad et al., 2015).
Already in 1985 Harrington and D'Arcy-Evans compared “conventional and in situ methods of transesterification” on sunflower seed oil. They concluded and were confirmed afterwards by several other studies dealing with oleaginous microorganisms (e.g., Chlorella species, Ehimen et al., 2010) that the fatty ester composition from in situ transesterification did not differ from that of pre-extracted oils reaching even higher yields when using the in situ method. However, when using H2SO4 as the acid catalyst moisture decreases heavily the yield of transesterification, making drying of biomass absolutely necessary (Harrington and D'Arcy-Evans, 1985; Ehimen et al., 2010). Drying, however, consumes a lot of energy and accounts considerably to the total production costs of low-value products like biodiesel (Molina Grima et al., 2003; Mata et al., 2010). Nevertheless, some studies showed, that by changing the catalyst to either HCl (Kim et al., 2015) or acetyl chloride (Macías-Sánchez et al., 2015) even wet biomass can be used for efficient direct in situ transesterification.
Use of enzymes
Cell disruption with lytic enzymes possess several advantages, e.g., mild reaction conditions and therefore prevention of shear and other stresses, substrate specificity, environmental friendly, and safe for food applications. Enzymes attack specifically cell wall components leading to a release of intracellular products. Due to their specificity, different enzymatic cocktails are needed for different microorganisms and the effectiveness of cell disruption is dependent on the enzyme type (Zheng et al., 2011). Enzymatic lysis as a tool for cell disruption has been extensively studied, especially for yeast and E. coli cells and several enzymes are available for all kinds of applications (reviewed by Salazar and Asenjo, 2007). The effectiveness of an enzymatic treatment in combination with solvent extraction, pressing, or ultrasound has been demonstrated for the lipid extraction of microalgae (Zheng et al., 2011) as well as for oilseeds (Shah et al., 2004; Soto et al., 2007) and the oleaginous yeast R. toruloides (Jin et al., 2012). Application on large scale is possible but depends heavily on the costs. When expensive enzyme cocktails are required for an effective cell destruction of certain organisms, enzyme immobilization may be a solution to lower enzyme costs due to the possibility of enzyme recycling. The feasibility of (re-)using immobilized cellulase to hydrolyze microalgae with subsequent lipid extraction was shown by Fu et al. (2010).
Extraction methods
Lipid extraction (as every other extraction procedure) aims to separate efficiently and specifically the desired class of lipids from other cellular components, i.e., proteins, polysaccharides, non-desired lipids, and other small molecules. For an efficient and specific lipid extraction the characteristics of the particular microorganisms regarding cell wall structure and lipid content (sample matrix) have to be taken into account as well as the chemical structure, characteristics and location of the desired lipid (analyte; Mustafa and Turner, 2011). Furthermore, extracted lipids have to be preserved against oxidation and degradation by enzymes. Additionally, the future application of the lipids has to be considered when choosing extraction methods and solvents, especially when aiming for food applications, non-toxic and non-harmful solvents which are easily removable and recoverable are preferred.
The choice of solvents depends on the polarity of lipids to be extracted. Storage lipids, like triacylglycerols, are neutral lipids, and are therefore extracted with rather non-polar solvents, e.g., chloroform or hexane, whereas more polar lipids, like phospholipdis and glycolipids can be extracted with more polar solvents, e.g., alcohols. Mixtures of solvents, especially non-polar and polar systems, can enhance the extraction efficiency (Lee et al., 1998; Ryckebosch et al., 2012; Li et al., 2014; Ramluckan et al., 2014; Byreddy et al., 2015) due to the better release of lipids from protein-lipid complexes by the polar solvent and subsequent dissolving of the lipids in the non-polar solvent.
Classical extraction methods
The Soxhlet Extraction was invented by Franz Soxhlet in 1879 for the lipid extraction from milk powder (Soxhlet, 1879) and is one of the most common semi-continuous methods for lipid extraction from solid food samples. The sample is dried, ground to a fine powder and placed on a porous thimble inside the extraction chamber. The sample is extracted by several washing rounds with an organic solvent (originally petroleum ether) under reflux. After extraction, the solvent is evaporated and the residue is weighed, giving the total dry mass of extracted lipid. Several automated Soxhlet extraction systems were developed and are commercially available, e.g., Büchi extraction system B-811, however, the Soxhlet extraction is time consuming and requires high amounts of solvents. Nevertheless, in literature Soxhlet extraction is often used as standard method when comparing different extraction methods. It can be modified to enhance extraction convenience, for example by combining with microwaves (microwave-assisted Soxhlet extraction, García-Ayuso et al., 2000) which reduced considerably extraction time and solvent consumption. Soxhlet extraction is a highly useful extraction method for analytical purposes as it effectively extracts total lipids for class analysis. However, as stated by McNichol et al. (2012), it is not useful in extracting single lipid classes, e.g., TAG content for judging biodiesel potential, as it significantly overestimates the yield for total fatty acids due to the presence of several moderately polar lipid classes and other co-extracted compounds. Furthermore, Soxhlet extraction might not be suitable for extraction of lipids containing unsaturated fatty acids due to their instability at higher temperatures under reflux (Guckert et al., 1988).
The Folch extraction method (Folch et al., 1957) is generally accepted as a standard technique for recovering total lipids. It was originally invented as a simple method for the extraction of total lipids from animal tissues (brain, liver, and muscle) and uses the chloroform:methanol (2:1) solvent system and addition of salts to the crude extract. By washing the crude extract with water or a salt solution, a biphasic system is formed, with the lipid fraction in the lower phase and the non-lipid fraction in the upper (watery) phase. The Folch method is the most reliable extraction method for total lipids and is often used as a standard technique in extracting microbial lipids, similar to the Bligh and Dyer (1959) extraction method which is also used very often for this purpose. The difference between both methods is the ratio between the amounts of solvents and sample and the washing procedure. Originally developed as a fast and economic method to extract total lipids from large wet samples (frozen fish), it uses a minimal amount of chloroform:methanol (2:1) solvent mixture. However, in samples containing more than 2% of lipids, the Bligh and Dyer method underestimates significantly lipid content with raising inaccuracy with increasing lipid content (Iverson et al., 2001), therefore, it has to be modified when aiming to recover lipids from oleaginous microorganisms.
As stated above, other solvent systems have been evaluated in order to exchange toxic chloroform and methanol mixtures. Hexane is often applied as unpolar substitute and gives good results when extracting triacylglycerols. However, when aiming for total lipid extraction, other solvent systems than chloroform-methanol mixtures result in lower extraction efficiency, and are more sensitive to the water content of the sample (Fishwick and Wright, 1977; Hara and Radin, 1978; Guckert et al., 1988; Sheng et al., 2011b).
It has further been noted, that due to the high variation between organisms, pre-treatment, and extraction methods has to be adapted individually, resulting in a large variety of different procedures. Frequently, also new or simplified extraction methods are introduced in literature, (e.g., Ryckebosch et al., 2012; Axelsson and Gentili, 2014), however, these methods are mainly tested and applied to a very limited range of organisms or tissues, making comparability and transferability to other microorganisms very hard. As stated by Christie (1993) for consistent results with any method, it is inevitable to adopt a strict protocol following the principles of the original inventors.
Pressurized liquid extraction
Pressurized liquid extraction (PLE) is similar to Soxhlet extraction but uses liquid solvents at elevated temperatures and pressures resulting in an enhanced extraction performance due to enhanced solubility and mass transfer properties compared to methods at room temperature and without increased pressures (Richter et al., 1996, introduced as accelerated solvent extraction). As for Soxhlet extraction, solid or semi-solid samples are extracted but much less time and solvents are needed. Briefly, the sample is placed into an extraction cell which is heated to 80–200°C. The solvent is pumped into the extraction cell and remains a certain time, usually 5–10 min, under elevated pressure (10–20 MPa). Subsequently, fresh solvent is introduced and the extract is stored in a collection vial. Finally, the whole solvent is pushed out into the collection vial using pressurized nitrogen.
Originally introduced for the extraction of contaminants from diverse environmental samples and animal tissues, it is now applied for all kinds of bioactive compounds and nutraceuticals from wide varieties of samples, e.g., polyphenols, lignans, carotenoids, oils, and lipids, antioxidants etc. (see Mustafa and Turner, 2011 and citations within for a general overview).
In case of lipid extraction the efficiency as well as the effect of temperature and pressure on the extraction of polar and non-polar lipids from corn and oat was tested by Moreau et al. (2003). They revealed that for triacylglyceride extraction methylene chloride was best suited at an extraction temperature of 100°C. Microbial lipids have also been extracted successfully using PLE. In the studies of Macnaughton et al. (1997) and White et al. (2009) Phospholipids and neutral lipids were extracted as microbial lipid biomarkers as indicators for viable biomass and community structure of different environmental samples (soil, water, air). They concluded that PLE is more efficient in extracting some sorts of phospholipids from bacteria and fungi as well as in extracting eukaryotic neutral lipids, lipids from spores, and air-borne samples. PLE has also been applied in extracting total lipids from the oleaginous yeast Rhodotorula glutinis (Cescut et al., 2011). In their study, they optimized their PLE method using the response surface method by testing the influence of cycle duration, extraction temperature, and effect of dispersant. The optimized method was as efficient in extracting total lipids as the Bligh and Dyer method, but was 5-fold faster and required 20-fold less solvent with the advantage to be entirely automated. PLE equipment is commercially available (Dionex, ASE). Applying sequential PLE approaches, different lipid classes can be extracted separately. In a first step the sample is treated with n-hexane/acetone at 50°C to extract neutral lipids, and in a second round extraction is performed with chloroform/methanol at 110°C to obtain polar lipids. Combining sequential PLE with an “in-cell-fractionation” using silica-based sorbents, enables a highly efficient separation of neutral lipids and polar phospholipids (Poerschmann and Carlson, 2006).
Supercritical fluid extraction (SFE)
Supercritical fluids are defined as any substance above its critical temperature and pressure. In supercritical state, substances have highly desirable properties making them suitable for extractions: they can penetrate into and effuse through solids like a gas, but dissolve lipids or any other analyte like a fluid. By altering temperature and pressure above the critical point the density of the supercritical fluid and therefore the solubility of the analyte is enhanced. By adjusting temperature and pressure, the most suitable combination between penetration of the sample and solubility of the analyte can be achieved.
For extracting lipids or lipid-soluble compounds, supercritical CO2 is good solvent enabling a high level of recovery (Fattori et al., 1988). It also has several other advantages compared to organic solvents as it dissolves non-polar or slightly polar compounds, with a low solubility for water and no solubility of proteins, polysaccharides, sugar and salts (del Valle and Aguilera, 1989; Sahena et al., 2009). Due to the relatively low critical temperature of CO2 (31.1°C), extraction can be performed at low temperatures, enabling a gentle extraction of thermosensitive compounds, e.g., polyunsaturated fatty acids (Dron et al., 1997; Sahena et al., 2009). These compounds are also protected against oxidation due to the absence of oxygen during supercritical CO2 extraction (Bernardo-Gil et al., 2002). CO2 can be easily removed from the extract by simple decompression and can be recycled afterwards, therefore, it minimizes waste. Furthermore, it is non-toxic, non-flammable, non-polluting, inexpensive and inert, making it an ideal substance in food applications. Effectively, it is applied industrially in food processing, e.g., for decaffeination of coffee or tea and for several other applications (Raventós et al., 2002 and citations within). Using only CO2 as solvent in SFE, mostly non-polar lipids are extracted. If the extraction of more polar lipids or substances is desired, it can be achieved by addition of co-solvents like water, methanol or ethanol (Barth et al., 1995; Da Porto et al., 2014; Radzali et al., 2014). However, water content of the sample can negatively influences extraction efficiency, therefore, samples are usually dried or freeze-dried (Spanos et al., 1993; Berset et al., 1999) or have to be treated with a special surfactant (Walker et al., 1999).
Some studies also showed the suitability of SFE for microbial oil extraction, especially PUFAs, from oleaginous microorganisms. Walker et al. (1999) optimized a SFE method for lipid extraction of the filamentous fungus Pythium irregulare and showed the influence of moisture content and extraction efficiency on the solubility of lower- and higher molecular fatty acids. Certik and Horenitzky (1999) compared supercritical CO2 extraction of SCO containing GLA produced by C. echinulata with chloroform/methanol extraction according to Folch and a two-step Soxhlet extraction using hexane and ethanol in both lab-scale and semi-scale. They showed that although oil recovery by supercritical CO2 extraction was comparable to Soxhlet extraction, GLA content was about 10% higher than achieved with Soxhlet extraction. Furthermore, they revealed that oil extractability was mainly controlled by the oil solubility capacity of supercritical CO2 and is dependent on used CO2 volume. Sakaki et al. (1990) compared different supercritical fluids for the extraction of fungal SCO from Mortierella ramanniana var. angulispora. Oil solubility was found to be best in supercritical N2O followed by supercritical CO2 at different tested temperatures and pressures. Although both N2O and CO2 possess similar physical properties, e.g., molecular weight, critical temperature and pressure, oil solubility in supercritical N2O was nearly five times higher than in supercritical CO2. However, calculations for oil solubility in supercritical CO2 from Certik and Horenitzky (1999) were two times higher than from Sakaki et al. (1990) apparently due to different sample preparation and extraction conditions. In the study of Couto et al. (2010) supercritical CO2 extraction was found highly suitable for the extraction of lipids from the heterotrophic microalga C. cohnii which is rich in DHA. While the extraction according to Bligh and Dyer yielded in a mixture of lipid compound with different polarities, supercritical CO2 extraction was more selective and enriched PUFAs resulting in an extract containing 72% of DHA.
Human nutrition and food application
Polyunsaturated fatty acids (PUFAs) affect many different physiological functions in the human body and are therefore important for the human health (Dyal and Narine, 2005; Bellou et al., 2016). PUFAs of the ω-3 and ω-6 class [e.g., α-linolenic acid (ALA; 18:3, ω-3), linoleic acid (LA; 18:2, ω-3)] play important roles not only as structural components of membrane phospholipids but also as precursors of the eicosanoids, which are essential for all mammals (Sakuradani et al., 2009; Stressler et al., 2013). Eicosanoids are hormone-like substances and influence the cardiovascular system, the immune system, the central nervous system, the brain and are involved by inflammatory reactions (Shinmen et al., 1989; Ward and Singh, 2005b; Arjuna, 2014; Bellou et al., 2016). In addition, for example, the cerebral cortex as well as the retina have a high amount of PUFAs such as arachidonic acid (ARA; 20:4, ω-6) and docosahexaenoic acid (DHA; 22:6, ω-3; Fontani et al., 2005; Ward and Singh, 2005b; Bellou et al., 2016). Furthermore, PUFAs are components of thrombocytes, neuronal and muscle cells as well as immunocompetent cells such as neutrophils and monocytes (Simopoulos, 1999; Ward and Singh, 2005b). Because mammals lack the ability to synthesize essential fatty acids (LA, ALA), these must be supplied by the diet (Laoteng and Certik, 2010). In humans these fatty acids can be converted to higher PUFAs, but this conversion often occurs with extremely low rates and therefore an external PUFA uptake is necessary (Bellou et al., 2016). It is postulated, that a balance in the uptake of ω-3 and ω-6 fatty acids in a ratio of 1:4 is important for the health and physical wellbeing of humans (Gill and Valivety, 1997; Simopoulos, 1999). In the food industry, lipids rich in PUFAs are highly demanded and used as food additives in order to enhance and/or supplement the fatty acid composition of specific foods such as infant food (Bellou et al., 2016). It is to be mention, that using fish oils as supplements is discussed critically, expressly for infants, due to the presence of environmental pollutants like dioxins, heavy metals and polychlorinated biphenyls (PCBs; Béligon et al., 2016). These components can be taken up from the fish oils and concentrated in the liver and other organs (Béligon et al., 2016). On the other hand, DHA and ARA are essential for the neural development and visual acuity of new-borns. Normally, the infants took up these PUFAs by the mother's milk (Granot et al., 2016). But if the mother's milk is replaced by cow's milk, the new-borns have a deficiency of these PUFA caused by their absent in cow's milk. Thus, ARA and DHA have to be added to the diet of infants to ensure a normal development (Béligon et al., 2016). In general, the enrichment of food with PUFAs can be realized by different ways: (i) The direct addition of PUFAs into food, (ii) the supplementation of food with PUFA-producing edible microorganisms, and (iii) the usage of animal feeds, rich in PUFAs, which results in animal products (e.g., eggs, meat) with increased PUFA contents (Bellou et al., 2016). Specially, microbial lipids, which are rich in PUFAs, can be extracted and added to various foods as pure oil or as stable emulsions (Bellou et al., 2016). As an alternative, some agricultural products (e.g., cereals) and by-products (e.g., orange peels, apple or pear pomace, sweet sorghum, etc.) can be enriched in PUFAs through solid or semi-solid state fermentation with PUFA producing microorganisms and directly used as food and/or feed supplements (Gema et al., 2002; Fakas et al., 2009a; Economou et al., 2010, 2011b; Čertík et al., 2013; Bača et al., 2014; Bellou et al., 2016).
The first commercial microbial oil was produced in 1985 and was rich in γ-linolenic acid (GLA; 18:3, ω-6). The oil was aimed at being an alternative source of the oil from seeds of evening primrose (Oenothera biennis; Wynn and Ratledge, 2005). The oil was produced by Mucor circinelloides, also known as Mucor javanicus and sold under the trade name of “Oil of Javanicus” (Wynn and Ratledge, 2005). Previous studies already showed that GLA exists in most, if not all, species in the group of Mucorales (Shaw, 1965, 1966; Wynn and Ratledge, 2005). However, in 1990 the production of Oil of Javanicus was folded because it could no longer compete against borage oil (Borago officinalis), which was developed during the second half of the 1980's, sold under the name “starflower oil” and had an even higher content of GLA than oil of Javanicus (Wynn and Ratledge, 2005).
In the mid of the 1960's an oil rich in ARA from a microbial source was investigated. The initial interest on this fatty acid was the misapprehension that it has the potential as chicken flavor additive (Wynn and Ratledge, 2005). A number of potentially useful organisms were identified by the research group of Bob Shaw at Unilever Ltd., (Shaw, 1965, 1966; Wynn and Ratledge, 2005). Beside the potential application of such an oil rich in ARA for the food industry, a non-dietetic application was as a cosmetic additive. The Lion Corp of Japan developed and patenting a process in 1988 for the production of ARA-rich oil (Wynn and Ratledge, 2005). In respect for a commercial production of ARA-rich single cell oil, only two fungi Mortierella alpina and Pythium sp. seem to be preferable (Kyle, 1997; Wynn and Ratledge, 2005). The following developments focused on M. alpina because it was considered as the most productive species (Shinmen et al., 1989; Stredanská and Šajbidor, 1992) and all current commercial processes for ARA-rich single cell oil used this fungus (Wynn and Ratledge, 2005). According to Wynn and Ratledge (2005) there are currently at least three commercial processes to produce ARA-rich single cell oils. Two of them are operating in the Far East (Suntory Corp., Japan; Wuhan Alking Bioengineering Co. Ltd., China) and the ARA-rich single cell oils are used for infant formula supplementation and as health supplements. But both processes do not constitute as a major global source for ARA-rich single cell oil. The largest proportion (>95% in 2005; Wynn and Ratledge, 2005) of the global ARA-rich single cell oil is produced by DSM Co. in Italy under contract for Martek Biosciences Corp. The DSM product is sold under the trade name ARASCO™ as part of the infant formula additive Formulaid™.
As mentioned above, DHA is important for infants to ensure a normal development and fish oil has significant quantities of it. However, due to the presence of environmental pollutants, the inclusion of fish oil into infant milk formula is not recommended (Béligon et al., 2016). Two heterotrophic microalgae, a dinoflagellate (C. cohnii), and a stramenopile (Schizochytrium) have been selected as production organisms for commercial DHA production (Wynn and Ratledge, 2005). C. cohnii has a total fatty acid content up to 50% and DHA corresponds to 95% of all PUFAs (Wynn and Ratledge, 2005). In Schizochytrium a significant amount (approximately one third of DHA) of docosapentaenoic acid (DPA; 22:5, ω-6) is also present and thus the appearance of this fatty acid in humans was associated with a deficiency of DHA, it was thought that this fatty acid should be avoided in infant formula (Wynn and Ratledge, 2005). In summary, while the C. cohnii oil was developed as an infant formula additive, the Schizochytrium oil was developed as a general food additive and as an animal food ingredient aimed at increasing the amount of DHA in the human diet in the form of meat and eggs (Barclay, 1991; Wynn and Ratledge, 2005). Since April 2002, Martek and OmegaTech have been a single company and both DHA-rich oils remain under development (Wynn and Ratledge, 2005). The product derived from C. cohnii is sold under the trade name DHASCO™.
From a commercial point of view, single cell oil became successful in the twenty-first century although the first SCO was launched on the market in 1985. The commercial breakthrough was based on the supplementation of infant formula with a mixture of ARASCO™ and DHASCO™ in many countries in Europe, Australia, and the Far East (Wynn and Ratledge, 2005). In May 2001, also the Food and Drug Administration (FDA) gave the GRAS status for DHA and ARA single cell oil for its inclusion in infant formula in the USA. Since the launch of single cell oil containing formula in February 2002 in the US market, the single cell oil fortified formula have captured over 50% of the US formula sales (Wynn and Ratledge, 2005). Since 1994 Martek's oils (also called life'sDHA™) have been used in infant formula and until 2010 it has been estimated that over 24 million babies, including more than 500000 preterm infants, have been fed infant formulas containing life'sDHA™ (Fichtali and Senanayake, 2010). Until 2010, life'sDHA™ oils are licensed and sold to 24 infant formula manufacturers, which represent more than 70% of the worldwide wholesale infant formula market (Table 5; Fichtali and Senanayake, 2010).
Table 5.
List of companies that produce DHA-supplemented infant formula (modified Fichtali and Senanayake, 2010).
| Company | Country |
|---|---|
| Abbott Laboratories | USA |
| Aspen Pharmacare | South Africa |
| Heinz Wattie's Limited – a subsidiary of the H.J. Heinz Company | New Zealand |
| Royal Numico | The Netherlands |
| Laboratotios Ordesa | Spain |
| Materna Ltd | Israel |
| Mead Johnson Nutritionals – Bristol-Myers Squibb | USA |
| Medici Medical | Italy |
| Nestle | Switzerland |
| Nutrition and Sante Iberia, S.L. | Spain |
| Pasteur Milk | South Korea |
| PT Sanghiang Perkasa | Indonesia |
| Semper AB | Sweden |
| Synutra, Inc. | China |
| Wyeth Ayerst | USA |
| PBM Products, LLC | USA |
| Arla Foods | Denmark |
| Murray Goulburn | Australia |
| Namyang Dairy Products Co., Ltd | South Korea |
| Parmalat Colombia | Colombia |
| Hain Celestial Group | USA |
| Alter Farmacia | Spain |
| Nature's One | USA |
| Earth's Best | USA |
| Vermont Organics | USA |
Before microbial single cell oil was used for infant formula, it was first explored in the 1980's for preparation of cocoa butter substitutes since cocoa butter was in short supply (Ward and Singh, 2005a). Therefore, C. curvatus was mutated to partially block the Δ-9 desaturase, which converts stearate (18:0) to oleate (18:1, ω-9), to increase the amount of stearate at the expense of oleate (Ward and Singh, 2005a). The oil obtained contained the fatty acids palmitic acid (16:0), stearic acid, and oleic acid in a ratio of 24:21:30 (%, w/w), which was quite similar to cocoa butter (28:35:35; %, w/w; Moreton, 1988; Verwoert et al., 1989; Davies, 1992; Ratledge, 2001; Ward and Singh, 2005a). However, the process became uncompetitive when the world price of cocoa butter dropped from $8000/ton to < $2500/ton (Ratledge, 2001; Ward and Singh, 2005a; Wynn and Ratledge, 2005).
Besides the infant formula, also commercial dairy products such as liquid milk and yogurt are currently fortified with ω-3 PUFAs obtained from flaxseed, fish oil, or even marine microalgae (Ganesan et al., 2014). In addition, vegetable and marine microalgae oils have been used to supply substantial amounts of ω-3 PUFAs in order to produce ω-3 PUFA-enriched meat products (Jiménez-Colmenero, 2007). Examples for products formulated with non-meat fats from algal are ground turkey patties, fresh pork sausage, and restricted hams (Lee et al., 2006a,b). Here, the oils were incorporated as oil-in-water emulsions with whey protein isolates as protein source. The advantage of such an emulsion is, that it is stable against oxidation and physically stable at pH 3.0 (Djordjevic et al., 2004). In general, unsaturated fatty acids are unstable and will oxidize quickly leading to formation of unpleasant smelling and tasting aldehydes and ketons (Kralovec et al., 2012). Various types of antioxidants have been used for the chemical stabilization of e.g., DHA, but these are not adequate to enable sensory stabilization of such oils for addition to many foods and beverages (Kralovec et al., 2012). In previously published articles, the usage of antioxidants to improve the stability of oils containing unsaturated fatty acids, were discussed (Shahidi and Zhong, 2010a,b). Beside the previously named emulsification, also microencapsualtion can improve the oxidative stability of fatty acids (Kralovec et al., 2012). Microencapsulation is a unique process that has been used not only to “convert” liquids to solids, but also to add functionalities or even to improve oxidative stability to ingredients (Kralovec et al., 2012).
One of the most important aspects of the design of potential functional foods is the scale of the alterations needed to achieve potential health-promoting functions. One of the possible limitations affecting fortified products is that large quantities may need to be consumed to assure recommended intake levels (Garg et al., 2006; Jiménez-Colmenero, 2007). For example, as mentioned above, non-meat fat (algal oil) was used to improve the lipid composition of various meat products. The effort required to replace animal fat varies widely depending on the fat content of the meat product. In products containing low fat (< 10%), regardless of the percentage of fat replaced, the presence of non-meat fat in the product is limited (≤ 5 g/100 g). In medium- to high-fat products, more animal fat is replaced and hence there is a larger intake of healthier non-meat lipids when the new product is consumed in its normal form (Jiménez-Colmenero, 2007). In general, one fundamental requirement of the design and reformulation of products with a view to potential health benefits is to assure that the lipid content and profile are optimum. The final product should contain enough of these beneficial compounds so that the quantity of the product that a person can reasonably be expected to consume supplies enough of the nutrient to produce the nutritional or physiological effect claimed on the basis of generally accepted scientific data (Jiménez-Colmenero, 2007).
Other applications of microbial oils
Besides the well-established application of microbial oils as food supplements, other applications are thinkable and desired for a successful establishment of a more sustainable or biobased economy.
Biodiesel
The idea of using SCO for the production of fatty acid methyl or ethyl esters, also known as biodiesel seems tempting and offers several advantages compared to biodiesel produced from plant or animal lipids. Fuel demands cannot be satisfied by using plant oil or animal lipids due to the large areas of production land required and the competition to food production (IEA, 2006). In contrast, microbial oil production is characterized by the short life cycle of microbes and the possibility of a production process not influenced by external factors such as venue, season, or climate (Thiru et al., 2011). The production of single cell oil in large scale is suited to avoid the conflict with plants used for energy and material industry, and to avoid the appearance of mono cultures and exhaustion of the soils. Furthermore, less land is needed for microbial production than for conventional agricultural production (Ratledge and Cohen, 2008). The production of biodiesel and its advantages and shortcomings has been exhaustingly reviewed in the last years (e.g., Ma and Hanna, 1999; Molina Grima et al., 2003; Chisti, 2007; Li Y. et al., 2008; Meng et al., 2009; Brennan and Owende, 2010; Mata et al., 2010; Liang and Jiang, 2013) with different focus on diverse aspects. However, the general conclusion of all authors is that the high costs of using SCO for biodiesel production hinder the commercial production. Unsurprisingly, the additional efforts needed for microbial oil production compared to biodiesel production from plant oil (including production in a bioreactor, substrate costs, and downstream processing as reviewed in this article) are responsible for the higher costs of a very cheap product. In the opinion of the authors biodiesel production from SCO will not be economically competitive as long as cheap fossil alternatives are available.
Chemicals (“oleochemicals”)
Oleochemicals are usually defined as chemical products derived from plant or animal triacylglycerols (Rupilius and Ahmad, 2006). However, in the opinion of the authors, also microbial sources should be included to the definition. Basic oleochemicals include fatty acids, fatty alcohols, and methyl ester. Especially fatty alcohols and ester between a fatty acid and a fatty alcohol (wax ester) can be applied in various industries, e.g., in soaps, detergents, cosmetic additives, pheromones, and flavors (Noweck and Grafahrend, 2006; Steen et al., 2010). Fatty alcohols and wax ester are obtained either from natural source, i.e., plant and animal oil, by hydrogenation of triacylglycerides or directly from jojoba plant oil which consists of wax esters. Alternatively, the chemical synthesis using petrochemical feedstocks can also be used (Noweck and Grafahrend, 2006). According to Steen et al. (2010) the market value for fatty alcohols, aldehydes, and wax esters was ~3 billion $ in 2004 with a prize of 1500 $/per ton. The microbial production of tailor-made fatty acid derivatives offers therefore a huge market and an economic alternative for the production of biodiesel. The principal feasibility of this approach was demonstrated by Steen et al. (2010) and Youngquist et al. (2013). Using metabolic engineering of the non-oleaginous organism E. coli fatty alcohols and wax esters were produced from sugar. The microbial production of oleochemicals is also subject of several patents, e.g., Schirmer et al. (2012) (US 8,268,599 B2), Hu (2011) (EP 2 367 947 B1), and Osterhout and Burgard (2014) (US20140127765 A1).
Conclusions and outlook
SCOs have been proven to be a highly interesting class of biotechnological products ranging from bulk chemicals to high-value products. Especially the occurrence of PUFAs in SCO similar as in plant oils classifies them to be very valuable resources with the advantages of short cultivation times of the producing oleaginous microorganisms and high product purity. However, major obstacles are still the extraction of the intracellular SCOs being difficult and cost-intensive. Therefore, commercialization has only occurred for high-value products, i.e., PUFAs in food applications. A general transfer of established oil extraction methods for plant oils is usually not applicable for oleaginous microorganisms due to the small size and the risk of emulsification. A step toward better profitability might be the realization of high cell densities achieved in short time, product purity, i.e., the enrichment of single desired fatty acids in SCO achieved by metabolic engineering, and the development of an universal and cost-efficient method for SCO recovery.
Author contributions
KO is corresponding author, and has written Sections Introduction, Downstream Processing, and Other Applications of Microbial Oils of the manuscript. CG was responsible for literature aquisition and has written Sections Microorganisms for SCO Production and Processes for the Microbial Production of SCO of the manuscript. TS has written Section Human Nutrition and Food Application of the manuscript. LF and CS have been responsible for critical review of the manuscript as well as discussion and overall improvement. Together, they have written the Section Conclusion and Outlook.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge support by Deutsche Forschungsgemeinschaft and Open Access Publishing Fund of Karlsruhe Institute of Technology.
References
- Arjuna A. (2014). Production of polyunsaturated fatty acids by fungi: a review. Int. J. Pharma Bio Sci. 5, 931–954. [Google Scholar]
- Asadi S. Z., Khosravi-Darani K., Nikoopour H., Bakhoda H. (2015). Evaluation of the effect of process variables on the fatty acid profile of single cell oil produced by Mortierella using solid-state fermentation. Crit. Rev. Biotechnol. 35, 94–102. 10.3109/07388551.2013.804805 [DOI] [PubMed] [Google Scholar]
- Axelsson M., Gentili F. (2014). A single-step method for rapid extraction of total lipids from green microalgae. PLoS ONE 9:e89643. 10.1371/journal.pone.0089643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bača M., Marcinčák S., Čertík M., Popelka P., Marcinčáková D., Guothová L., et al. (2014). Effect of adding prefermented cereal product containing gamma-linolenic acid to broiler feed on production indicators and fatty acid profile of chicken breast. Acta Vet. Brno 83, 379–384. 10.2754/avb201483040379 [DOI] [Google Scholar]
- Bajpai P. K., Bajpai P., Ward O. P. (1991). Production of arachidonic acid by Mortierella alpina ATCC 32222. J. Ind. Microbiol. 8, 179–185. 10.1007/BF01575851 [DOI] [PubMed] [Google Scholar]
- Balasubramanian S., Allen J. D., Kanitkar A., Boldor D. (2011). Oil extraction from Scenedesmus obliquus using a continuous microwave system – design, optimization, and quality characterization. Bioresour. Technol. 102, 3396–3403. 10.1016/j.biortech.2010.09.119 [DOI] [PubMed] [Google Scholar]
- Barclay W. R. (1991). Process for the Heterotrophic Production of Products with High Concentrations of Omega-3 Highly Unsaturated Fatty Acids. Available online at: https://www.google.com/patents/WO1991007498A1?cl=en22
- Barth M. M., Zhou C., Kute K. M., Rosenthal G. A. (1995). Determination of optimum conditions for supercritical fluid extraction of carotenoids from carrot (Daucus carota L.) tissue. J. Agric. Food Chem. 43, 2876–2878. 10.1021/jf00059a019 [DOI] [Google Scholar]
- Béligon V., Christophe G., Fontanille P., Larroche C. (2016). Microbial lipids as potential source to food supplements. Curr. Opin. Food Sci. 7, 35–42. 10.1016/j.cofs.2015.10.002 [DOI] [Google Scholar]
- Bellou S., Triantaphyllidou I.-E., Aggeli D., Elazzazy A. M., Baeshen M. N., Aggelis G. (2016). Microbial oils as food additives: recent approaches for improving microbial oil production and its polyunsaturated fatty acid content. Curr. Opin. Biotechnol. 37, 24–35. 10.1016/j.copbio.2015.09.005 [DOI] [PubMed] [Google Scholar]
- Bernardo-Gil M. G., Grenha J., Santos J., Cardoso P. (2002). Supercritical fluid extraction and characterisation of oil from hazelnut. Eur. J. Lipid Sci. Technol. 104, 402–409. 10.1002/1438-9312(200207)104:73.0.CO;2-N [DOI] [Google Scholar]
- Berset J. D., Ejem M., Holzer R., Lischer P. (1999). Comparison of different drying, extraction and detection techniques for the determination of priority polycyclic aromatic hydrocarbons in background contaminated soil samples. Anal. Chim. Acta 383, 263–275. 10.1016/S0003-2670(98)00817-4 [DOI] [Google Scholar]
- Bligh E. G., Dyer W. J. (1959). A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917. 10.1139/o59-099 [DOI] [PubMed] [Google Scholar]
- Borthwick K. A. J., Coakley W. T., McDonnell M. B., Nowotny H., Benes E., Gröschl M. (2005). Development of a novel compact sonicator for cell disruption. J. Microbiol. Methods 60, 207–216. 10.1016/j.mimet.2004.09.012 [DOI] [PubMed] [Google Scholar]
- Botham P. A., Ratledge C. (1979). A biochemical explanation for lipid accumulation in Candida 107 and other oleaginous micro-organisms. J. Gen. Microbiol. 114, 361–375. 10.1099/00221287-114-2-361 [DOI] [PubMed] [Google Scholar]
- Boulton C. A., Ratledge C. (1981a). Correlation of lipid accumulation in yeasts with possession of ATP: citrate lyase. J. Gen. Microbiol. 127, 169–176. [Google Scholar]
- Boulton C. A., Ratledge C. (1981b). ATP: Citrate Lyase - The regulatory enzyme for lipid biosynthesis in Lipornyces starkeyi? J. Gen. Microbiol. 127, 423–426. 10.1099/00221287-127-2-423 [DOI] [Google Scholar]
- Brennan L., Owende P. (2010). Biofuels from microalgae - A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 14, 557–577. 10.1016/j.rser.2009.10.009 [DOI] [Google Scholar]
- Byreddy A. R., Gupta A., Barrow C. J., Puri M. (2015). Comparison of cell disruption methods for improving lipid extraction from Thraustochytrid strains. Mar. Drugs 13, 5111–5127. 10.3390/md13085111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerón-García M. C., Macías-Sánchez M. D., Sánchez-Mirón A., García-Camacho F., Molina-Grima E. (2013). A process for biodiesel production involving the heterotrophic fermentation of Chlorella protothecoides with glycerol as the carbon source. Appl. Energy 103, 341–349. 10.1016/j.apenergy.2012.09.054 [DOI] [Google Scholar]
- Čertík M., Adamechová Z., Guothová L. (2013). Simultaneous enrichment of cereals with polyunsaturated fatty acids and pigments by fungal solid state fermentations. J. Biotechnol. 168, 130–134. 10.1016/j.jbiotec.2013.03.016 [DOI] [PubMed] [Google Scholar]
- Certik M., Horenitzky R. (1999). Supercritical CO2 extraction of fungal oil containing γ-linolenic acid. Biotechnol. Tech. 13, 11–15. 10.1023/A:1008853214591 [DOI] [Google Scholar]
- Cescut J., Severac E., Molina-Jouve C., Uribelarrea J.-L. (2011). Optimized pressurized liquid extraction of microbial lipids using the response surface method. J. Chromatogr. A 1218, 373–379. 10.1016/j.chroma.2010.12.003 [DOI] [PubMed] [Google Scholar]
- Chang G., Luo Z., Gu S., Wu Q., Chang M., Wang X. (2013). Fatty acid shifts and metabolic activity changes of Schizochytrium sp. S31 cultured on glycerol. Bioresour. Technol. 142, 255–260. 10.1016/j.biortech.2013.05.030 [DOI] [PubMed] [Google Scholar]
- Chatzifragkou A., Fakas S., Galiotou-Panayotou M., Komaitis M., Aggelis G., Papanikolaou S. (2010). Commercial sugars as substrates for lipid accumulation in Cunninghamella echinulata and Mortierella isabellina fungi. Eur. J. Lipid Sci. Technol. 112, 1048–1057. 10.1002/ejlt.201000027 [DOI] [Google Scholar]
- Chatzifragkou A., Makri A., Belka A., Bellou S., Mavrou M., Mastoridou M., et al. (2011). Biotechnological conversions of biodiesel derived waste glycerol by yeast and fungal species. Energy 36, 1097–1108. 10.1016/j.energy.2010.11.040 [DOI] [Google Scholar]
- Chemler J. A., Yan Y., Koffas M. A. G. (2006). Biosynthesis of isoprenoids, polyunsaturated fatty acids and flavonoids in Saccharomyces cerevisiae. Microb. Cell Fact. 5:20. 10.1186/1475-2859-5-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M. H., Walker T. H., Hulbert G. J., Raman D. R. (1999). Fungal production of eicosapentaenoic and arachidonic acids from industrial waste streams and crude soybean oil. Bioresour. Technol. 67, 101–110. 10.1016/S0960-8524(98)00113-8 [DOI] [Google Scholar]
- Chi Z., Pyle D., Wen Z., Frear C., Chen S. (2007). A laboratory study of producing docosahexaenoic acid from biodiesel-waste glycerol by microalgal fermentation. Process Biochem. 42, 1537–1545. 10.1016/j.procbio.2007.08.008 [DOI] [Google Scholar]
- Chi Z., Zheng Y., Ma J., Chen S. (2011). Oleaginous yeast Cryptococcus curvatus culture with dark fermentation hydrogen production effluent as feedstock for microbial lipid production. Int. J. Hydrogen Energy 36, 9542–9550. 10.1016/j.ijhydene.2011.04.124 [DOI] [Google Scholar]
- Chisti Y. (2007). Biodiesel from microalgae. Biotechnol. Adv. 25, 294–306. 10.1016/j.biotechadv.2007.02.001 [DOI] [PubMed] [Google Scholar]
- Chisti Y., Moo-Young M. (1986). Disruption of microbial cells for intracellular products. Enzyme Microb. Technol. 8, 194–204. 10.1016/0141-0229(86)90087-6 [DOI] [Google Scholar]
- Christie W. W. (ed.). (1993). Preparation of lipid extracts tissues, in Advances in Lipid Methodology - Two (Dundee: Oily Press; ), 195–213. [Google Scholar]
- Chuanbin L., Jian X., Fengwu B., Zhiguo S. (1998). Trehalose extraction from Saccharomyces cerevisiae after microwave treatment. Biotechnol. Tech. 12, 914–943. 10.1023/A:1008881915907 [DOI] [Google Scholar]
- Clarke A., Prescott T., Khan A., Olabi A. B. (2010). Causes of breakage and disruption in a homogenizer. Appl. Energy 87, 3680–3690. 10.1016/j.apenergy.2010.05.007 [DOI] [Google Scholar]
- Conti E., Stredansky M., Stredanska S., Zanetti F. (2001). γ-Linolenic acid production by solid-state fermentation of Mucorales strains on cereals. Bioresour. Technol. 76, 283–286. 10.1016/S0960-8524(00)00097-3 [DOI] [PubMed] [Google Scholar]
- Couto R. M., Simões P. C., Reis A., Da Silva T. L., Martins V. H., Sánchez-Vicente Y. (2010). Supercritical fluid extraction of lipids from the heterotrophic microalga Crypthecodinium cohnii. Eng. Life Sci. 10, 158–164. 10.1002/elsc.200900074 [DOI] [Google Scholar]
- Couto S. R., Sanromán M. Á. (2006). Application of solid-state fermentation to food industry—A review. J. Food Eng. 76, 291–302. 10.1016/j.jfoodeng.2005.05.022 [DOI] [Google Scholar]
- Cravotto G., Boffa L., Mantegna S., Perego P., Avogadro M., Cintas P. (2008). Improved extraction of vegetable oils under high-intensity ultrasound and/or microwaves. Ultrason. Sonochem. 15, 898–902. 10.1016/j.ultsonch.2007.10.009 [DOI] [PubMed] [Google Scholar]
- D'Oca M. G. M., Viêgas C. V., Lemões J. S., Miyasaki E. K., Morón-Villarreyes J. A., Primel E. G., et al. (2011). Production of FAMEs from several microalgal lipidic extracts and direct transesterification of the Chlorella pyrenoidosa. Biomass Bioener. 35, 1533–1538. 10.1016/j.biombioe.2010.12.047 [DOI] [Google Scholar]
- Da Porto C., Decorti D., Natolino A. (2014). Water and ethanol as co-solvent in supercritical fluid extraction of proanthocyanidins from grape marc: a comparison and a proposal. J. Supercrit. Fluids 87, 1–8. 10.1016/j.supflu.2013.12.019 [DOI] [Google Scholar]
- Davies R. J. (1992). Scale up of yeast technology, in Industrial Applications of Single Cell Oils, eds Kyle D. J., Ratledge C. (Champaign, IL: AOCS; ), 196–218. [Google Scholar]
- de Boer K., Moheimani N. R., Borowitzka M. A., Bahri P. A. (2012). Extraction and conversion pathways for microalgae to biodiesel: a review focused on energy consumption. J. Appl. Phycol. 24, 1681–1698. 10.1007/s10811-012-9835-z [DOI] [Google Scholar]
- del Valle J. M., Aguilera J. M. (1989). Effects of substrate densification and CO2 conditions on supercritical extraction of mushroom oleoresins. J. Food Sci. 54, 135–141. 10.1111/j.1365-2621.1989.tb08586.x [DOI] [Google Scholar]
- de Swaaf M. E., Pronk J. T., Sijtsma L. (2003a). Fed-batch cultivation of the docosahexaenoic-acid-producing marine alga Crypthecodinium cohnii on ethanol. Appl. Microbiol. Biotechnol. 61, 40–43. 10.1007/s00253-002-1118-1 [DOI] [PubMed] [Google Scholar]
- de Swaaf M. E., Pronk J. T., Sijtsma L. (2003b). High-cell-density fed-batch cultivation of the docosahexaenoic acid producing marine alga Crypthecodinium cohnii. Biotechnol. Bioeng. 81, 666–672. 10.1002/bit.10513 [DOI] [PubMed] [Google Scholar]
- Dillschneider R., Schulze I., Neumann A., Posten C., Syldatk C. (2014). Combination of algae and yeast fermentation for an integrated process to produce single cell oils. Appl. Microbiol. Biotechnol. 98, 7793–7802. 10.1007/s00253-014-5867-4 [DOI] [PubMed] [Google Scholar]
- Diversified Technologies (2010). Pulsed Electric Field Pre-treatment of Algae for Oil Extraction. Available online at: http://www.divtecs.com/data/File/papers/PDF/pef_algae_10_web_nb.pdf
- Djordjevic D., McClements D. J., Decker E. A. (2004). Oxidative stability of whey protein-stabilized oil-in-water emulsions at pH 3: potential ω-3 fatty acid delivery systems (Part B). J. Food Sci. 69, C356–C362. 10.1111/j.1365-2621.2004.tb10697.x [DOI] [Google Scholar]
- Doucha J., Lívanský K. (2008). Influence of processing parameters on disintegration of Chlorella cells in various types of homogenizers. Appl. Microbiol. Biotechnol. 81, 431–440. 10.1007/s00253-008-1660-6 [DOI] [PubMed] [Google Scholar]
- Dowhan W., Bogdanov M. (2013). Functional roles of lipids in membranes, in Encyclopedia of Biophysics, ed Roberts G. C. K. (Berlin; Heidelberg: Springer Berlin Heidelberg; ), 868–875. [Google Scholar]
- Dron A., Guyeru D. E., Gage D. A., Lira C. T. (1997). Yield and quality of onion flavor oil obtained by supercritical fluid extraction and other methods. J. Food Process Eng. 20, 107–124. 10.1111/j.1745-4530.1997.tb00414.x [DOI] [Google Scholar]
- Dyal S. D., Narine S. S. (2005). Implications for the use of Mortierella fungi in the industrial production of essential fatty acids. Food Res. Int. 38, 445–467. 10.1016/j.foodres.2004.11.002 [DOI] [Google Scholar]
- Economou C. N., Aggelis G., Pavlou S., Vayenas D. V. (2011a). Modeling of single-cell oil production under nitrogen-limited and substrate inhibition conditions. Biotechnol. Bioeng. 108, 1049–1055. 10.1002/bit.23026 [DOI] [PubMed] [Google Scholar]
- Economou C. N., Aggelis G., Pavlou S., Vayenas D. V. (2011b). Single cell oil production from rice hulls hydrolysate. Bioresour. Technol. 102, 9737–9742. 10.1016/j.biortech.2011.08.025 [DOI] [PubMed] [Google Scholar]
- Economou C. N., Makri A., Aggelis G., Pavlou S., Vayenas D. V. (2010). Semi-solid state fermentation of sweet sorghum for the biotechnological production of single cell oil. Bioresour. Technol. 101, 1385–1388. 10.1016/j.biortech.2009.09.028 [DOI] [PubMed] [Google Scholar]
- Ehimen E. A., Sun Z. F., Carrington C. G. (2010). Variables affecting the in situ transesterification of microalgae lipids. Fuel 89, 677–684. 10.1016/j.fuel.2009.10.011 [DOI] [Google Scholar]
- El-Fadaly H., El-Ahmady El-Naggar N., Marwan E.-S. M. (2009). Single cell oil production by an oleaginous yeast strain in a low cost cultivation medium. Res. J. Microbiol. 4, 301–313. 10.3923/jm.2009.301.313 [DOI] [Google Scholar]
- Evans C. T., Ratledge C. (1984). Effect of nitrogen source on lipid accumulation in oleaginous yeasts. Microbiology 130, 1693–1704. 10.1099/00221287-130-7-1693 [DOI] [Google Scholar]
- Evans C. T., Ratledge C. (1985). Possible regulatory roles of ATP:citrate lyase, malic enzyme, and AMP deaminase in lipid accumulation by Rhodosporidium toruloides CBS 14. Can. J. Microbiol. 31, 1000–1005. 10.1139/m85-189 [DOI] [Google Scholar]
- Fakas S., Makri A., Mavromati M., Tselepi M., Aggelis G. (2009a). Fatty acid composition in lipid fractions lengthwise the mycelium of Mortierella isabellina and lipid production by solid state fermentation. Bioresour. Technol. 100, 6118–6120. 10.1016/j.biortech.2009.06.015 [DOI] [PubMed] [Google Scholar]
- Fakas S., Papanikolaou S., Batsos A., Galiotou-Panayotou M., Mallouchos A., Aggelis G. (2009b). Evaluating renewable carbon sources as substrates for single cell oil production by Cunninghamella echinulata and Mortierella isabellina. Biomass Bioener. 33, 573–580. 10.1016/j.biombioe.2008.09.006 [DOI] [Google Scholar]
- Fakas S., Papanikolaou S., Galiotou-Panayotou M., Komaitis M., Aggelis G. (2008). Organic nitrogen of tomato waste hydrolysate enhances glucose uptake and lipid accumulation in Cunninghamella echinulata. J. Appl. Microbiol. 105, 1062–1070. 10.1111/j.1365-2672.2008.03839.x [DOI] [PubMed] [Google Scholar]
- Fattori M., Bulley N. R., Meisen A. (1988). Carbon dioxide extraction of canola seed: oil solubility and effect of seed treatment. J. Am. Oil Chem. Soc. 65, 968–974. 10.1007/BF02544522 [DOI] [Google Scholar]
- Fichtali J., Senanayake S. P. J. N. (2010). Development and commercialization of microalgae-based functional lipids, in Functional Food Product Development, eds Smith J., Charter E. (Oxford, UK: Wiley-Blackwell; ), 206–225. [Google Scholar]
- Fishwick M. J., Wright A. J. (1977). Comparison of methods for the extraction of plant lipids. Phytochemistry 16, 1507–1510. 10.1016/0031-9422(77)84011-9 [DOI] [Google Scholar]
- Flisar K., Meglic S. H., Morelj J., Golob J., Miklavcic D. (2014). Testing a prototype pulse generator for a continuous flow system and its use for E. coli inactivation and microalgae lipid extraction. Bioelectrochemistry 100, 44–51. 10.1016/j.bioelechem.2014.03.008 [DOI] [PubMed] [Google Scholar]
- Folch J., Lees M., Sloane Stanley G. H. (1957). A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226, 497–509. [PubMed] [Google Scholar]
- Fontani G., Corradeschi F., Felici A., Alfatti F., Migliorini S., Lodi L. (2005). Cognitive and physiological effects of Omega-3 polyunsaturated fatty acid supplementation in healthy subjects. Eur. J. Clin. Invest. 35, 691–699. 10.1111/j.1365-2362.2005.01570.x [DOI] [PubMed] [Google Scholar]
- Fu C.-C., Hung T.-C., Chehn J.-Y., Su C.-H., Wu W.-T. (2010). Hydrolysis of microalgae cell walls for production of reducing sugar and lipid extraction. Bioresour. Technol. 101, 8750–8754. 10.1016/j.biortech.2010.06.100 [DOI] [PubMed] [Google Scholar]
- Ganesan B., Brothersen C., McMahon D. J. (2014). Fortification of foods with omega-3 polyunsaturated fatty acids. Crit. Rev. Food Sci. Nutr. 54, 98–114. 10.1080/10408398.2011.578221 [DOI] [PubMed] [Google Scholar]
- Ganuza E., Izquierdo M. (2007). Lipid accumulation in Schizochytrium G13/2S produced in continuous culture. Appl. Microbiol. Biotechnol. 76, 985–990. 10.1007/s00253-007-1019-4 [DOI] [PubMed] [Google Scholar]
- Gao D., Zeng J., Zheng Y., Yu X., Chen S. (2013). Microbial lipid production from xylose by Mortierella isabellina. Bioresour. Technol. 133, 315–321. 10.1016/j.biortech.2013.01.132 [DOI] [PubMed] [Google Scholar]
- García-Ayuso L. E., Velasco J., Dobarganes M. C., Luque de Castro M. D. (2000). Determination of the oil content of seeds by focused microwave-assisted soxhlet extraction. Chromatographia 52, 103–108. 10.1007/BF02490801 [DOI] [Google Scholar]
- Garg M. L., Wood L. G., Singh H., Moughan P. J. (2006). Means of delivering recommended levels of long chain n-3 polyunsaturated fatty acids in human diets. J. Food Sci. 71, R66–R71. 10.1111/j.1750-3841.2006.00033.x [DOI] [Google Scholar]
- Geciova J., Bury D., Jelen P. (2002). Methods for disruption of microbial cells for potential use in the dairy industry - a review. Int. Dairy J. 12, 541–553. 10.1016/S0958-6946(02)00038-9 [DOI] [Google Scholar]
- Gema H., Kavadia A., Dimou D., Tsagou V., Komaitis M., Aggelis G. (2002). Production of gamma-linolenic acid by Cunninghamella echinulata cultivated on glucose and orange peel. Appl. Microbiol. Biotechnol. 58, 303–307. 10.1007/s00253-001-0910-7 [DOI] [PubMed] [Google Scholar]
- Gerde J. A., Montalbo-Lomboy M., Yao L., Grewell D., Wang T. (2012). Evaluation of microalgae cell disruption by ultrasonic treatment. Bioresour. Technol. 125, 175–171. 10.1016/j.biortech.2012.08.110 [DOI] [PubMed] [Google Scholar]
- Gill I., Valivety R. (1997). Polyunsaturated fatty acids, part 1: occurrence, biological activities and applications. Trends Biotechnol. 15, 401–409. 10.1016/S0167-7799(97)01076-7 [DOI] [PubMed] [Google Scholar]
- Granot E., Ishay-Gigi K., Malaach L., Flidel-Rimon O. (2016). Is there a difference in breast milk fatty acid composition of mothers of preterm and term infants? J. Matern. Fetal Neonatal Med. 29, 832–835. 10.3109/14767058.2015.1020785 [DOI] [PubMed] [Google Scholar]
- Greenwell H. C., Laurens L. M., Shields R. J., Lovitt R. W., Flynn K. J. (2010). Placing microalgae on the biofuels priority list: a review of the technological challenges. J. R. Soc. Interface 7, 703–726. 10.1098/rsif.2009.0322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guckert J. B., Cooksey K. E., Jackson L. L. (1988). Lipid solvent systems are not equivalent for analysis of lipid classes in the microeukaryotic green alga, Chlorella. J. Microbiol. Methods 8, 139–149. 10.1016/0167-7012(88)90015-2 [DOI] [Google Scholar]
- Guldhe A., Singh B., Rawat I., Ramluckan K., Bux F. (2014). Efficacy of drying and cell disruption techniques on lipid recovery from microalgae for biodiesel production. Fuel 128, 46–52. 10.1016/j.fuel.2014.02.059 [DOI] [Google Scholar]
- Günerken E., D'Hondt E., Eppink M. H. M., Garcia-Gonzalez L., Elst K., Wijffels R. H. (2015). Cell disruption for microalgae biorefineries. Biotechnol. Adv. 33, 243–260. 10.1016/j.biotechadv.2015.01.008 [DOI] [PubMed] [Google Scholar]
- Halim R., Harun R., Danquah M. K., Webley P. A. (2012). Microalgal cell disruption for biofuel development. Appl. Energy 91, 116–121. 10.1016/j.apenergy.2011.08.048 [DOI] [Google Scholar]
- Hara A., Radin N. S. (1978). Lipid extraction of tissues with a low-toxicity solvent. Anal. Biochem. 90, 420–426. 10.1016/0003-2697(78)90046-5 [DOI] [PubMed] [Google Scholar]
- Harrington K. J., D'Arcy-Evans C. (1985). A comparison of conventional and in situ methods of transesterification of seed oil from a series of sunflower cultivars. J. Am. Oil Chem. Soc. 62, 1009–1013. 10.1007/BF02935703 [DOI] [Google Scholar]
- Harrison S. T. L. (1991). Bacterial cell disruption: a key unit operation in the recovery of intracellular products. Biotechnol. Adv. 9, 217–240. 10.1016/0734-9750(91)90005-G [DOI] [PubMed] [Google Scholar]
- Heim A., Kamionowska U., Solecki M. (2007). The effect of microorganism concentration on yeast cell disruption in a bead mill. J. Food Eng. 83, 121–128. 10.1016/j.jfoodeng.2007.02.047 [DOI] [Google Scholar]
- Ho C. W., Chew T. K., Ling T. C., Kamaruddina S., Tan W. S., Tey B. T. (2006). Efficient mechanical cell disruption of Escherichia coli by an ultrasonicator and recovery of intracellular hepatitis B core antigen. Process Biochem. 41, 1829–1834. 10.1016/j.procbio.2006.03.043 [DOI] [Google Scholar]
- Ho S. Y., Mittal G. S. (1996). Electroporation of cell membranes: a review. Crit. Rev. Biotechnol. 16, 349–362. 10.3109/07388559609147426 [DOI] [PubMed] [Google Scholar]
- Hopkins T. R. (1994). Physical and chemical cell disruption for the recovery of intracellular proteins, in Purification and Analysis of Recombinant Proteins, eds Seetharam R., Sharma S. K. (New York, NY: Marcel Dekker; ), 57–83. [PubMed] [Google Scholar]
- Hu Z. (2011). Methods for Producing Fatty Alcohols. European Patent EP 2 367 947 B1. Munich; The Hague; Brussels; Vienna; Berlin: European Patent Office. [Google Scholar]
- Huang C., Chen X.-F., Xiong L., Chen X.-D., Ma L.-L., Chen Y. (2013). Single cell oil production from low-cost substrates: the possibility and potential of its industrialization. Biotechnol. Adv. 31, 129–139. 10.1016/j.biotechadv.2012.08.010 [DOI] [PubMed] [Google Scholar]
- Huang T. Y., Lu W. C., Chu I. M. (2012). A fermentation strategy for producing docosahexaenoic acid in Aurantiochytrium limacinum SR21 and increasing C22:6 proportions in total fatty acid. Bioresour. Technol. 123, 8–14. 10.1016/j.biortech.2012.07.068 [DOI] [PubMed] [Google Scholar]
- Hummel W., Kula M.-R. (1989). Simple method for small-scale disruption of bacteria and yeasts. J. Microbiol. Methods 9, 201–209. 10.1016/0167-7012(89)90037-7 [DOI] [Google Scholar]
- International Energy Agency(IEA) (2006). World Energy Outlook 2006. Available online at: http://www.worldenergyoutlook.org/media/weowebsite/2008-1994/weo2006.pdf
- Iverson S. J., Lang S. L. C., Cooper M. H. (2001). Comparison of the Bligh and Dyer and Folch methods for total lipid determination in a broad range of marine tissue. Lipids 36, 1283–1287. 10.1007/s11745-001-0843-0 [DOI] [PubMed] [Google Scholar]
- Jacob Z. (1992). Yeast lipids: extraction, quality analysis, and acceptability. Crit. Rev. Biotechnol. 12, 463–491. 10.3109/07388559209114236 [DOI] [Google Scholar]
- Jacobs A., Botha A., Van Zyl W. H. (2010). Sunflower press cake as a substrate for eicosapentaenoic acid production by representatives of the genus Mortierella. Bioresources 5, 1232–1243. 10.15376/biores.5.2.1232-1243 [DOI] [Google Scholar]
- James C. X., Coakley W. T., Hughes D. E. (1972). Kinetics of protein release from yeast sonicated in batch and flow systems at 20 kHz. Biotechnol. Bioeng. 14, 33–42. 10.1002/bit.260140105 [DOI] [Google Scholar]
- Jayakar S. S., Singhal R. S. (2012). Development of an efficient cell disruption method for release of lipoic acid from Saccharomyces cerevisiae. Glob. J. Biotechnol. Biochem. 7, 90–99. 10.5829/idosi.gjbb.2012.7.4.6546 [DOI] [Google Scholar]
- Jiang Y., Chen F. J. (2000). Effects of temperature and temperature shift on docosahexaenoic acid production by the marine microalge Crypthecodinium cohnii. Amer. Oil. Chem. Soc. 77, 613–617. 10.1007/s11746-000-0099-0 [DOI] [Google Scholar]
- Jiménez-Colmenero F. (2007). Healthier lipid formulation approaches in meat-based functional foods. Technological options for replacement of meat fats by non-meat fats. Trends Food Sci. Technol. 18, 567–578. 10.1016/j.tifs.2007.05.006 [DOI] [Google Scholar]
- Jin G., Yang F., Hu C., Shen H., Zhao Z. K. (2012). Enzyme-assisted extraction of lipids directly from the culture of the oleaginous yeast Rhodosporidium toruloides. Bioresour. Technol. 111, 378–382. 10.1016/j.biortech.2012.01.152 [DOI] [PubMed] [Google Scholar]
- Johnson M. B., Wen Z. (2009). Production of biodiesel fuel from microalga Schizochytrium limacinum by direct transesterificaton of algal biomass. Energy Fuels 23, 5179–5183. 10.1021/ef900704h [DOI] [Google Scholar]
- Kakkad H., Khot M., Zinjarde S., RaviKumar A. (2015). Biodiesel production by direct in situ transesterification of an oleaginous tropical mangrove fungus grown on untreated agro-residues and evaluation of its fuel properties. Bioener. Res. 8, 1788–1799. 10.1007/s12155-015-9626-x [DOI] [Google Scholar]
- Keshavarz E., Bonnerjea J., Hoare M., Dunnill P. (1990). Disruption of a fungal organism, Rhizopus nigricans, in a high-pressure homogenizer. Enzyme Microb. Technol. 12, 494–498. 10.1016/0141-0229(90)90064-W [DOI] [Google Scholar]
- Kim B., Im H., Lee J. W. (2015). In situ transesterification of highly wet microalgae using hydrochloric acid. Bioresour. Technol. 185, 421–425. 10.1016/j.biortech.2015.02.092 [DOI] [PubMed] [Google Scholar]
- Klimek-Ochab M., Brzezińska-Rodak M., Zymańczyk-Duda E., Lejczak B., Kafarski P. (2011). Comparative study of fungal cell disruption - scope and limitations of the methods. Folia Microbiol. 56, 469–475. 10.1007/s12223-011-0069-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralovec J. A., Zhang S., Zhang W., Barrow C. J. (2012). A review of the progress in enzymatic concentration and microencapsulation of omega-3 rich oil from fish and microbial sources. Food Chem. 131, 639–644. 10.1016/j.foodchem.2011.08.085 [DOI] [Google Scholar]
- Kyle D. J. (1997). Arachidonic Acid and Methods for the Production and Use Thereof. Available online at: http://www.google.com/patents/US5658767
- Lai Y. S., Parameswaran P., Li A., Baez M., Rittmann B. E. (2014). Effects of pulsed electric field treatment on enhancing lipid recovery from the microalga, Scenedesmus. Bioresour. Technol. 173, 457–461. 10.1016/j.biortech.2014.09.124 [DOI] [PubMed] [Google Scholar]
- Laoteng K., Certik M. (2010). Biotechnological production and application of high-value microbial oils, in Industrial Fermentation: Food Processes, Nutrient Sources and Production Strategies (Hauppauge, NY: Nova Science Publishers, Inc.), 187–215. [Google Scholar]
- Leber C., Polson B., Fernandez-Moya R., Da Silva N. A. (2015). Overproduction and secretion of free fatty acids through disrupted neutral lipid recycle in Saccharomyces cerevisiae. Metab. Eng. 28, 54–62. 10.1016/j.ymben.2014.11.006 [DOI] [PubMed] [Google Scholar]
- Lee A. K., Lewis D. M., Ashman P. J. (2012). Disruption of microalgal cells for the extraction of lipids for biofuels: processes and specific energy requirements. Biomass Bioener. 46, 89–101. 10.1016/j.biombioe.2012.06.034 [DOI] [Google Scholar]
- Lee J. Y., Yoo C., Jun S. Y., Ahn C. Y., Oh H. M. (2010). Comparison of several methods for effective lipid extraction from microalgae. Bioresour. Technol. 101, 575–577. 10.1016/j.biortech.2009.03.058 [DOI] [PubMed] [Google Scholar]
- Lee S., Faustman C., Djordjevic D., Faraji H., Decker E. A. (2006a). Effect of antioxidants on stabilization of meat products fortified with n-3 fatty acids. Meat Sci. 72, 18–24. 10.1016/j.meatsci.2005.05.022 [DOI] [PubMed] [Google Scholar]
- Lee S., Hernandez P., Djordjevic D., Faraji H., Hollender R., Faustman C., et al. (2006b). Effect of antioxidants and cooking on stability of n-3 fatty acids in fortified meat products. J. Food Sci. 71, C233–C238. 10.1111/j.1365-2621.2006.tb15623.x [DOI] [Google Scholar]
- Lee S. J., Yoon B.-Y., Oh H.-M. (1998). Rapid method for the determination of lipid from the green alga Botryococcus braunii. Biotechnol. Tech. 12, 553–556. 10.1023/A:1008811716448 [DOI] [Google Scholar]
- Lennen R. M., Pfleger B. F. (2012). Engineering Escherichia coli to synthesize free fatty acids. Trends Biotechnol. 30, 659–667. 10.1016/j.tibtech.2012.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonelli C., Mason T. J. (2010). Microwave and ultrasonic processing: now a realistic option for industry. Chem. Eng. Process. 49, 885–900. 10.1016/j.cep.2010.05.006 [DOI] [Google Scholar]
- Li P., Miao X., Li R., Zhong J. (2011). In situ biodiesel production from fast-growing and high oil content Chlorella pyrenoidosa in rice straw hydrolysate. J. Biomed. Biotechnol. 2011:141207. 10.1155/2011/141207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Du W., Liu D. (2008). Perspectives of microbial oils for biodiesel production. Appl. Microbiol. Biotechnol. 80, 749–756. 10.1007/s00253-008-1625-9 [DOI] [PubMed] [Google Scholar]
- Li Y., Ghasemi Naghdi F., Garg S., Adarme-Vega T. C., Thurecht K. J., Ghafor W. A., et al. (2014). A comparative study: the impact of different lipid extraction methods on current microalgal lipid research. Microb. Cell Fact. 13:14. 10.1186/1475-2859-13-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Horsman M., Wu N., Lan C. Q., Dubois-Calero N. (2008). Biofuels from microalgae. Biotechnol. Prog. 24, 815–820. 10.1021/bp070371k [DOI] [PubMed] [Google Scholar]
- Liang M. H., Jiang J. G. (2013). Advancing oleaginous microorganisms to produce lipid via Metab. Eng. technology. Prog. Lipid Res. 52, 395–408. 10.1016/j.plipres.2013.05.002 [DOI] [PubMed] [Google Scholar]
- Limon-Lason J., Hoare M., Orsborn C. B., Doyle D. J., Dunnill P. (1979). Reactor properties of a high-speed bead mill for microbial cell rupture. Biotechnol. Bioeng. 21, 745–774. 10.1002/bit.260210503 [DOI] [Google Scholar]
- Lin H., Cheng W., Ding H., Chen X., Zhou Q., Zhao Y. (2010). Direct microbial conversion of wheat straw into lipid by a cellulolytic fungus of Aspergillus oryzae A-4 in solid-state fermentation. Bioresour. Technol. 101, 7556–7562. 10.1016/j.biortech.2010.04.027 [DOI] [PubMed] [Google Scholar]
- Ling X., Guo J., Liu X., Zhang X., Wang N., Lu Y., et al. (2015). Impact of carbon and nitrogen feeding strategy on high production of biomass and docosahexaenoic acid (DHA) by Schizochytrium sp. LU310. Bioresour. Technol. 184, 139–47. 10.1016/j.biortech.2014.09.130 [DOI] [PubMed] [Google Scholar]
- Ling Y., Wong H. H., Thomas C. J., Williams D. R. G., Middelberg A. P. J. (1997). Pilot-scale extraction of PHB from recombinant E. coli by homogenization and centrifugation. Bioseparation 7, 9–15. 10.1023/A:1007900416356 [DOI] [PubMed] [Google Scholar]
- Liu B., Zhao Z. (2007). Biodiesel production by direct methanolysis of oleaginous microbial biomass. J. Chem. Technol. Biotechnol. 82, 775–780. 10.1002/jctb.1744 [DOI] [Google Scholar]
- Liu H., Yu C., Feng D., Cheng T., Meng X., Liu W., et al. (2012). Production of extracellular fatty acid using engineered Escherichia coli. Microb. Cell Fact. 11:41. 10.1186/1475-2859-11-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Vora H., Khosla C. (2008). Overproduction of free fatty acids in E. coli: Implications for biodiesel production. Metab. Eng. 10, 333–339. 10.1016/j.ymben.2008.08.006 [DOI] [PubMed] [Google Scholar]
- Macías-Sánchez M. D., Robles-Medina A., Hita-Peña E., Jiménez-Callejón M. J., Estéban-Cerdán L., González-Moreno P. A., et al. (2015). Biodiesel production from wet microalgal biomass by direct transesterification. Fuel 150, 14–20. 10.1016/j.fuel.2015.01.106 [DOI] [PubMed] [Google Scholar]
- Macnaughton S. J., Jenkins T. L., Wimpee M. H., Cormiér M. R., White D. C. (1997). Rapid extraction of lipid biomarkers from pure culture and environmental samples using pressurized accelerated hot solvent extraction. J. Microbiol. Methods 31, 19–27. 10.1016/S0167-7012(97)00081-X [DOI] [Google Scholar]
- Ma F., Hanna M. A. (1999). Biodiesel production: a review. Bioresour. Technol. 70, 1–15. 10.1016/S0960-8524(99)00025-5 [DOI] [Google Scholar]
- Mata T. M., Martins A. A., Caetano N. S. (2010). Microalgae for biodiesel production and other applications: a review. Renew. Sustain. Energy Rev. 14, 217–232. 10.1016/j.rser.2009.07.020 [DOI] [Google Scholar]
- Matsakas L., Bonturi N., Miranda E., Rova U., Christakopoulos P. (2015). High concentrations of dried sorghum stalks as a biomass feedstock for single cell oil production by Rhodosporidium toruloides. Biotechnol. Biofuels 8, 6. 10.1186/s13068-014-0190-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T., Otsuka K., Sato S. (2011). Microbial oil production from carbohydrates using Sporobolomyces carnicolor strain O33. Ann. Microbiol. 62, 861–864. 10.1007/s13213-011-0316-4 [DOI] [Google Scholar]
- McMillan J. R., Watson I. A., Ali M., Jaafar W. (2013). Evaluation and comparison of algal cell disruption methods: microwave, waterbath, blender, ultrasonic and laser treatment. Appl. Energy 103, 128–134. 10.1016/j.apenergy.2012.09.020 [DOI] [Google Scholar]
- McNichol J., MacDougall K. M., Melanson J. E., McGinn P. J. (2012). Suitability of Soxhlet extraction to quantify microalgal fatty acids as determined by comparison with in situ transesterification. Lipids 47, 195–207. 10.1007/s11745-011-3624-3 [DOI] [PubMed] [Google Scholar]
- Menetrez M. Y. (2012). An overview of algae biofuel production and potential environmental impact. Environ. Sci. Technol. 46, 7073−7085. 10.1021/es300917r [DOI] [PubMed] [Google Scholar]
- Meng X., Shang H., Zheng Y., Zhang Z. (2013). Free fatty acid secretion by an engineered strain of Escherichia coli. Biotechnol. Lett. 35, 2099–2103. 10.1007/s10529-013-1305-4 [DOI] [PubMed] [Google Scholar]
- Meng X., Yang J., Xu X., Zhang L., Nie Q., Xian M. (2009). Biodiesel production from oleaginous microorganisms. Renew. Energy 34, 1–5. 10.1016/j.renene.2008.04.014 [DOI] [Google Scholar]
- Mercer P., Armenta R. E. (2011). Developments in oil extraction from microalgae. Eur. J. Lipid Sci. Technol. 113, 539–547. 10.1002/ejlt.201000455 [DOI] [Google Scholar]
- Michinaka Y., Shimauchi T., Aki T., Nakajima T., Kawamoto S., Shigeta S., et al. (2003). Extracellular secretion of free fatty acids by disruption of a fatty acyl-CoA synthetase gene in Saccharomyces cerevisiae. J. Biosci. Bioeng. 95, 435–440. 10.1016/S1389-1723(03)80041-5 [DOI] [PubMed] [Google Scholar]
- Middelberg A. P. J. (1995). Process-scale disruption of microorganisms. Biotechnol. Adv. 13, 491–551. 10.1016/0734-9750(95)02007-P [DOI] [PubMed] [Google Scholar]
- Molina Grima E., Belarbi E. H., Acién Fernández F. G., Robles Medina A., Chisti Y. (2003). Recovery of microalgal biomass and metabolites: process options and economics. Biotechnol. Adv. 20, 491–515. 10.1016/S0734-9750(02)00050-2 [DOI] [PubMed] [Google Scholar]
- Moreau R. A., Powell M. J., Singh V. (2003). Pressurized liquid extraction of polar and nonpolar lipids in corn and oats with hexane, methylene chloride, isopropanol, and ethanol. J. Am. Oil Chem. Soc. 80, 1063–1067. 10.1007/s11746-003-0821-y [DOI] [Google Scholar]
- Moreton R. S. (1988). Physiology of lipid accumulating yeasts, in Single Cell Oil, ed Moreton R. S. (London; New York, NY: Longman-Wiley; ), 1–32. [Google Scholar]
- Mustafa A., Turner C. (2011). Pressurized liquid extraction as a green approach in food and herbal plants extraction: a review. Anal. Chim. Acta 703, 8–18. 10.1016/j.aca.2011.07.018 [DOI] [PubMed] [Google Scholar]
- Nakamura K., Enomoto A., Fukushima H., Nagai K., Hakoda M. (1994). Disruption of microbial cells by the flash discharge of high-pressure carbon dioxide. Biosci. Biotechnol. Biochem. 58, 1297–1301. 10.1271/bbb.58.1297 [DOI] [Google Scholar]
- Noweck K., Grafahrend W. (2006). Fatty alcohols, in Ullmann's Encyclopedia of Industrial Chemistry, ed Elvers B. (Weinheim: Wiley-VCH; ), 117–141. [Google Scholar]
- Osterhout R. E., Burgard A. P. (2014). Microorganisms and Methods for Production of Specific Length Fatty Alcohols and Related Compounds. US Patent US 20140127765 A1. Washington, DC: U.S. Patent and Trademark Office. [Google Scholar]
- Pandey A., Selvakumar P., Soccol C. R., Nigam P. (1999). Solid state fermentation for the production of industrial enzymes. Curr. Sci. 77, 149–162. [Google Scholar]
- Papanikolaou S., Aggelis G. (2002). Lipid production by Yarrowia lipolytica growing on industrial glycerol in a single stage continuous culture. Bioresour. Technol. 82, 43–49. 10.1016/S0960-8524(01)00149-3 [DOI] [PubMed] [Google Scholar]
- Papanikolaou S., Komaitis M., Aggelis G. (2004). Single cell oil (SCO) production by Mortierella isabellina grown on high-sugar content media. Bioresour. Technol. 95, 287–291. 10.1016/j.biortech.2004.02.016 [DOI] [PubMed] [Google Scholar]
- Peng X., Chen H. (2008). Single cell oil production in solid-state fermentation by Microsphaeropsis sp. from steam-exploded wheat straw mixed with wheat bran. Bioresour. Technol. 99, 3885–3889. 10.1016/j.biortech.2007.08.015 [DOI] [PubMed] [Google Scholar]
- Poerschmann J., Carlson R. (2006). New fractionation scheme for lipid classes based on “in-cell-fractionation” using sequential pressurized liquid extraction. J. Chromatogr. A 1127, 18–25. 10.1016/j.chroma.2006.07.063 [DOI] [PubMed] [Google Scholar]
- Radzali S. A., Baharin B. S., Othman R., Markom M., Rahman R. A. (2014). Co-solvent selection for supercritical fluid extraction of astaxanthin and other carotenoids from Penaeus monodon waste. J. Oleo Sci. 63, 769–777. 10.5650/jos.ess13184 [DOI] [PubMed] [Google Scholar]
- Rakesh S., Dhar D. W., Prasanna R., Saxena A. K., Saha S., Shukla M., et al. (2015). Cell disruption methods for improving lipid extraction efficiency in unicellular microalgae. Eng. Life Sci. 15, 443–447. 10.1002/elsc.201400222 [DOI] [Google Scholar]
- Ramluckan K., Moodley K. G., Bux F. (2014). An evaluation of the efficacy of using selected solvents for the extraction of lipids from algal biomass by the soxhlet extraction method. Fuel 116, 103–108. 10.1016/j.fuel.2013.07.118 [DOI] [Google Scholar]
- Ratledge C. (2001). Microbial lipids, in Biotechnology Set, 2nd Edn., eds Rehm H.-J., Reed G. (Weinheim: Wiley-VCH Verlag GmbH; ), 133–197. 10.1002/9783527620999.ch4g [DOI] [Google Scholar]
- Ratledge C. (2002). Regulation of lipid accumulation in oleaginous micro-organisms. Biochem. Soc. Trans. 30, 1047–1050. 10.1042/bst0301047 [DOI] [PubMed] [Google Scholar]
- Ratledge C. (2004). Fatty acid biosynthesis in microorganisms being used for single cell oil production. Biochimie 86, 807–815. 10.1016/j.biochi.2004.09.017 [DOI] [PubMed] [Google Scholar]
- Ratledge C., Cohen Z. (2008). Microbial and algal oils: do they have a future for biodiesel or as commodity oils? Lipid Technol. 20, 155–160. 10.1002/lite.20080004421109668 [DOI] [Google Scholar]
- Ratledge C., Kanagachandran C., Anderson A. J., Grantham D. J., Stephenson J. C. (2001). Production of docosahexaenoic acid by Crypthecodinium cohnii grown in a pH-auxostat culture with acetic acid as principal carbon source. Lipids 36, 1241–1246. 10.1007/s11745-001-0838-x [DOI] [PubMed] [Google Scholar]
- Raventós M., Duarte S., Alarcón R. (2002). Application and possibilities of supercritical CO2 extraction in food processing industry: an overview. Food Sci. Technol. Int. 8, 269–284. 10.1106/108201302029451 [DOI] [Google Scholar]
- Richter B. E., Jones B. A., Ezzell J. I., Porter N. L., Avdalovic N., Pohl C. (1996). Accelerated solvent extraction; a technique for sample preparation. Anal. Chem. 68, 1033–1039. 10.1021/ac9508199 [DOI] [Google Scholar]
- Routray W., Orsat V. (2012). Microwave-assisted extraction of flavonoids: a review. Food Bioproc. Tech. 5, 409–424. 10.1007/s11947-011-0573-z [DOI] [Google Scholar]
- Ruan Z., Zanotti M., Wang X., Ducey C., Liu Y. (2012). Evaluation of lipid accumulation from lignocellulosic sugars by Mortierella isabellina for biodiesel production. Bioresour. Technol. 110, 198–205. 10.1016/j.biortech.2012.01.053 [DOI] [PubMed] [Google Scholar]
- Rupilius W., Ahmad S. (2006). The changing world of oleochemicals. Palm Oil Dev. 44, 15–28. [Google Scholar]
- Ryckebosch E., Muylaert K., Foubert I. (2012). Optimization of an analytical procedure for extraction of lipids from microalgae. J. Am. Oil Chem. Soc. 89, 189–198. 10.1007/s11746-011-1903-z [DOI] [Google Scholar]
- Sahena F., Zaidul I. S. M., Jinap S., Karim A. A., Abbas K. A., Norulaini N. A. N., et al. (2009). Application of supercritical CO2 in lipid extraction – A review. J. Food Eng. 95, 240–253. 10.1016/j.jfoodeng.2009.06.026 [DOI] [Google Scholar]
- Sakaki K., Yokochi T., Suzuki O., Hakuta T. (1990). Supercritical fluid extraction of fungal oil using CO2, N2O, CHF3 and SF6. J. Am. Oil Chem. Soc. 67, 553–557. 10.1007/BF02540765 [DOI] [Google Scholar]
- Sakuradani E., Ando A., Ogawa J., Shimizu S. (2009). Improved production of various polyunsaturated fatty acids through filamentous fungus Mortierella alpina breeding. Appl. Microbiol. Biotechnol. 84, 1–10. 10.1007/s00253-009-2076-7 [DOI] [PubMed] [Google Scholar]
- Salazar O., Asenjo J. A. (2007). Enzymatic lysis of microbial cells. Biotechnol. Lett. 29, 985–994. 10.1007/s10529-007-9345-2 [DOI] [PubMed] [Google Scholar]
- Sankh S., Thiru M., Saran S., Rangaswamy V. (2013). Biodiesel production from a newly isolated Pichia kudriavzevii strain. Fuel 106, 690–696. 10.1016/j.fuel.2012.12.014 [DOI] [Google Scholar]
- Schirmer A., Rude M., Brubaker S. (2012). Method for Producing a Fatty Alcohol or Fatty Aldehyde. US Patent US 8,268,599 B2. Washington, DC: U.S. Patent and Trademark Office. [Google Scholar]
- Schütte H., Kroner K. H., Hustedt H., Kula M.-R. (1983). Experiences with a 20 litre industrial bead mill for the disruption of microorganisms. Enzyme Microb. Technol. 5, 143–148. 10.1016/0141-0229(83)90050-9 [DOI] [Google Scholar]
- Senanayake S. P. J. N., Fichtali J. (2006). Single-cell oils as sources of nutraceutical and specialty lipids: processing technologies and applications, in Nutraceutical and Specialty Lipids and their Co-Products, ed Shahidi F. (Boca Raton, FL: CRC Press; ), 251–280. [Google Scholar]
- Shah S., Sharma A., Gupta M. N. (2004). Extraction of oil from Jatropha curcas L. seed kernels by enzyme assisted three phase partitioning. Ind. Crops Prod. 20, 275–279. 10.1016/j.indcrop.2003.10.010 [DOI] [Google Scholar]
- Shahidi F., Zhong Y. (2010a). Lipid oxidation and improving the oxidative stability. Chem. Soc. Rev. 39, 4067–4079. 10.1039/b922183m [DOI] [PubMed] [Google Scholar]
- Shahidi F., Zhong Y. (2010b). Novel antioxidants in food quality preservation and health promotion. Eur. J. Lipid Sci. Technol. 112, 930–940. 10.1002/ejlt.201000044 [DOI] [Google Scholar]
- Shaw R. (1965). The occurrence of γ-linolenic acid in fungi. Biochim. Biophys. Acta 98, 230–237. 10.1016/0005-2760(65)90117-7 [DOI] [PubMed] [Google Scholar]
- Shaw R. (1966). The polyunsaturated fatty acids of microorganisms. Adv. Lipid Res. 4, 107–174. 10.1016/B978-1-4831-9940-5.50011-9 [DOI] [PubMed] [Google Scholar]
- Sheng J., Vannela R., Rittmann B. E. (2011a). Evaluation of cell-disruption effects of pulsed-electric-field treatment of Synechocystis PCC 6803. Environ. Sci. Technol. 45, 3795–3802. 10.1021/es103339x [DOI] [PubMed] [Google Scholar]
- Sheng J., Vannela R., Rittmann B. E. (2011b). Evaluation of methods to extract and quantify lipids from Synechocystis PCC 6803. Bioresour. Technol. 102, 1697–1703. 10.1016/j.biortech.2010.08.007 [DOI] [PubMed] [Google Scholar]
- Shinmen Y., Shimizu S., Akimoto K., Kawashima H., Yamada H. (1989). Production of arachidonic acid by Mortierella fungi - Selection of a potent producer and optimization of culture conditions for large-scale production. Appl. Microbiol. Biotechnol. 31, 11–16. [Google Scholar]
- Simopoulos A. P. (1999). Essential fatty acids in health and chronic disease. Am. J. Clin. Nutr. 70, 560–569. [DOI] [PubMed] [Google Scholar]
- Simpson R. J. (2010). Disruption of cultured cells by nitrogen cavitation. Cold Spring Harb. Protoc. 11:pdb.prot5513. 10.1101/pdb.prot5513 [DOI] [PubMed] [Google Scholar]
- Sobus M. T., Homlund C. E. (1976). Extraction of lipids from yeast. Lipids 11, 341–348. 10.1007/BF02544064 [DOI] [PubMed] [Google Scholar]
- Soto C., Chamy R., Zúñiga M. E. (2007). Enzymatic hydrolysis and pressing conditions effect on borage oil extraction by cold pressing. Food Chem. 102, 834–840. 10.1016/j.foodchem.2006.06.014 [DOI] [Google Scholar]
- Soxhlet F. (1879). Die gewichtsanalytische Bestimmung des Milchfettes: von Dr. F. Soxhlet. Dinglers Polytechnisches J. 232, 461–465. [Google Scholar]
- Spanos G. A., Chen H., Schwartz S. J. (1993). Supercritical CO2 extraction of β-carotene from sweet potatoes. J. Food Sci. 58, 817–820. 10.1111/j.1365-2621.1993.tb09366.x [DOI] [Google Scholar]
- Spiden E. M., Scales P. J., Kentish S. E., Martin G. J. O. (2013). Critical analysis of quantitative indicators of cell disruption applied to Saccharomyces cerevisiae processed with an industrial high pressure homogenizer. Biochem. Eng. J. 70, 120–126. 10.1016/j.bej.2012.10.008 [DOI] [Google Scholar]
- Steen E. J., Kang Y., Bokinsky G., Hu Z., Schirmer A., McClure A., et al. (2010). Microbial production of fatty-acid-derived fuels and chemicals from plant biomass. Nature 463, 559–562. 10.1038/nature08721 [DOI] [PubMed] [Google Scholar]
- Stredanská S., Šajbidor J. (1992). Oligounsaturated fatty acid production by selected strains of micromycetes. Folia Microbiol. (Praha). 37, 357–359. 10.1007/BF02815662 [DOI] [PubMed] [Google Scholar]
- Stressler T., Eisele T., Rost J., Haunschild E. M., Kuhn A., Fischer L. (2013). Production of polyunsaturated fatty acids by Mortierella alpina using submerse and solid state fermentation. Chem. Ingenieur Tech. 85, 318–322. 10.1002/cite.201200094 [DOI] [Google Scholar]
- Suslick K. S. (1989). The chemical effects of ultrasound. Sci. Am. 260, 80–86. 10.1038/scientificamerican0289-80 [DOI] [Google Scholar]
- Tang X., Chen H., Chen Y. Q., Chen W., Garre V., Song Y., et al. (2015). Comparison of biochemical activities between high and low lipid-producing strains of Mucor circinelloides: an explanation for the high oleaginicity of strain WJ11. PLoS ONE 10:e0128396. 10.1371/journal.pone.0128396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchakouteu S. S., Kalantzi O., Gardeli C., Koutinas A. A., Aggelis G., Papanikolaou S. (2015). Lipid production by yeasts growing on biodiesel-derived crude glycerol: strain selection and impact of substrate concentration on the fermentation efficiency. J. Appl. Microbiol. 118, 911–927. 10.1111/jam.12736 [DOI] [PubMed] [Google Scholar]
- Thiru M., Sankh S., Rangaswamy V. (2011). Process for biodiesel production from Cryptococcus curvatus. Bioresour. Technol. 102, 10436–10440. 10.1016/j.biortech.2011.08.102 [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Doraiswamy L. K. (1999). Sonochemistry: science and engineering. Ind. Eng. Chem. Res. 38, 1215–1249. 10.1021/ie9804172 [DOI] [Google Scholar]
- Uematsu Y., Hirata K., Suzuki K., Iida K., Kamata K. (2002). Survey of residual solvents in natural food additives by standard addition head-space GC. Food Addit. Contam. 19, 335–342. 10.1080/02652030110088301 [DOI] [PubMed] [Google Scholar]
- van Gaver D., Huyghebaert A. (1991). Optimization of yeast cell disruption with a newly designed bead mill. Enzyme Microb. Technol. 13, 665–671. 10.1016/0141-0229(91)90082-L [DOI] [Google Scholar]
- Verwoert I. G. S., Ykema A., Valkenburg J. C., Verbree E., John H., Nijkamp J., et al. (1989). Modification of the fatty-acid composition in lipids of the oleaginous yeast Apiotrichum curvatum by intraspecific spheroplast fusion. Appl. Microbiol. Biotechnol. 32, 327–333. 10.1007/BF00184984 [DOI] [Google Scholar]
- Wahlen B. D., Willis R. M., Seefeldt L. C. (2011). Biodiesel production by simultaneous extraction and conversion of total lipids from microalgae, cyanobacteria, and wild mixed-cultures. Bioresour. Technol. 102, 2724–2730. 10.1016/j.biortech.2010.11.026 [DOI] [PubMed] [Google Scholar]
- Walker T. H., Cochran H. D., Hulbert G. J. (1999). Supercritical carbon dioxide extraction of lipids from Pythium irregulare. J. Am. Oil Chem. Soc. 76, 595–602. 10.1007/s11746-999-0009-3 [DOI] [Google Scholar]
- Wang M., Yuan W. (2015). Microalgal cell disruption in a high-power ultrasonic flow system. Bioresour. Technol. 193, 171–177. 10.1016/j.biortech.2015.06.040 [DOI] [PubMed] [Google Scholar]
- Ward O. P., Singh A. (2005a). Microbial production of polyunsaturated fatty acids, in Biotechnological Applications of Microbes, eds Varma A., Podila G. K. (New Delhi: I. K. International Pvt Ltd.), 199–220. [Google Scholar]
- Ward O. P., Singh A. (2005b). Omega-3/6 fatty acids: alternative sources of production. Process Biochem. 40, 3627–3652. 10.1016/j.procbio.2005.02.020 [DOI] [Google Scholar]
- White P. M., Potter T. L., Strickland T. C. (2009). Pressurized liquid extraction of soil microbial phospholipid and neutral lipid fatty acids. J. Agric. Food Chem. 57, 7171–7177. 10.1021/jf901257n [DOI] [PubMed] [Google Scholar]
- Wu S.-T., Yu S.-T., Lin L.-P. (2005). Effect of culture conditions on docosahexaenoic acid production by Schizochytrium sp. S31. Process Biochem. 40, 3103–3108. 10.1016/j.procbio.2005.03.007 [DOI] [Google Scholar]
- Wynn J. P., Hamid A. A., Li Y., Ratledge C. (2001). Biochemical events leading to the diversion of carbon into storage lipids in the oleaginous fungi Mucor circinelloides and Mortierella alpina. Microbiology 147, 2857–2864. 10.1099/00221287-147-10-2857 [DOI] [PubMed] [Google Scholar]
- Wynn J. P., Ratledge C. (2005). Microbial production of oils and fats, in Food Biotechnology, Second Edition Food Science and Technology, eds Shetty K., Paliyath G., Pometto A., Levin R. E. (Boca Raton, FL: CRC Press; ), 443–472. [Google Scholar]
- Xu P., Gu Q., Wang W., Wong L., Bower A. G., Collins C. H., et al. (2013). Modular optimization of multi-gene pathways for fatty acids production in E. coli. Nat. Commun. 4:1409. 10.1038/ncomms2425 [DOI] [PubMed] [Google Scholar]
- Xu R., Mi Y. (2011). Simplifying the process of microalgal biodiesel production through in situ transesterification technology. J. Am. Oil Chem. Soc. 88, 91–99. 10.1007/s11746-010-1653-3 [DOI] [Google Scholar]
- Yang F., Xiang W., Sun X., Wu H., Li T., Long L. (2014). A novel lipid extraction method from wet microalga Picochlorum sp. at room temperature. Mar. Drugs 12, 1258–1270. 10.3390/md12031258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L., Lee S.-L., Wang T., Gerde J. A. (2013). Comparison of lipid extraction from microalgae and soybeans with aqueous isopropanol. J. Am. Oil Chem. Soc. 90, 571–578. 10.1007/s11746-012-2197-5 [DOI] [Google Scholar]
- Yap C. Y., Chen F. (2001). Polyunsaturated fatty acids: biological significance, biosynthesis, and production by microalgae and microalgae-like organisms, in Algae and Their Biotechnological Potential, eds Chen F., Jiang Y. (Dordrecht: Springer Netherlands; ), 1–32. [Google Scholar]
- Yoshida H., Hiroka N., Kajimoto G. (1990). Microwave energy effects on quality of some seed oils. J. Food Sci. 55, 1412–1416. 10.1111/j.1365-2621.1990.tb03947.x [DOI] [Google Scholar]
- Youngquist J. T., Schumacher M. H., Rose J. P., Raines T. C., Politz M. C., Copeland M. F., et al. (2013). Production of medium chain length fatty alcohols from glucose in Escherichia coli. Metab. Eng. 20, 177–186. 10.1016/j.ymben.2013.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Dong T., Zheng Y., Miao C., Chen S. (2015). Investigations on cell disruption of oleaginous microorganisms: hydrochloric acid digestion is an effective method for lipid extraction. Eur. J. Lipid Sci. Technol. 117, 730–737. 10.1002/ejlt.201400195 [DOI] [Google Scholar]
- Zbinden M. D., Sturm B. S., Nord R. D., Carey W. J., Moore D., Shinogle H., et al. (2013). Pulsed electric field (PEF) as an intensification pretreatment for greener solvent lipid extraction from microalgae. Biotechnol. Bioeng. 110, 1605–1615. 10.1002/bit.24829 [DOI] [PubMed] [Google Scholar]
- Zeng J., Zheng Y., Yu X., Yu L., Gao D., Chen S. (2013). Lignocellulosic biomass as a carbohydrate source for lipid production by Mortierella isabellina. Bioresour. Technol. 128, 385–391. 10.1016/j.biortech.2012.10.079 [DOI] [PubMed] [Google Scholar]
- Zhang H., Zhang L., Chen H., Chen Y. Q., Ratledge C., Song Y., et al. (2013). Regulatory properties of malic enzyme in the oleaginous yeast, Yarrowia lipolytica, and its non-involvement in lipid accumulation. Biotechnol. Lett. 35, 2091–2098. 10.1007/s10529-013-1302-7 [DOI] [PubMed] [Google Scholar]
- Zhao C.-H., Zhang T., Li M., Chi Z.-M. (2010). Single cell oil production from hydrolysates of inulin and extract of tubers of Jerusalem artichoke by Rhodotorula mucilaginosa TJY15a. Process Biochem. 45, 1121–1126. 10.1016/j.procbio.2010.04.002 [DOI] [Google Scholar]
- Zhao L., Zhang H., Wang L., Chen H., Chen Y. Q., Chen W., et al. (2015). (13)C-metabolic flux analysis of lipid accumulation in the oleaginous fungus Mucor circinelloides. Bioresour. Technol. 197, 23–29. 10.1016/j.biortech.2015.08.035 [DOI] [PubMed] [Google Scholar]
- Zheng H., Yin J., Gao Z., Huang H., Ji X., Dou C. (2011). Disruption of Chlorella vulgaris cells for the release of biodiesel-producing lipids: a comparison of grinding, ultrasonication, bead milling, enzymatic lysis, and microwaves. Appl. Biochem. Biotechnol. 164, 1215–1224. 10.1007/s12010-011-9207-1 [DOI] [PubMed] [Google Scholar]